Abstract
Obesity is associated with risk of colorectal adenoma (CRA) and colorectal cancer (CRC). The signaling pathway activated by metformin (LKB1/AMPK/mTOR) is implicated in tumor suppression in ApcMin/+ mice via metformin-induced reduction in polyp burden, increased ratio of pAMPK/AMPK, decreased pmTOR/mTOR ratio, and decreased pS6Ser235/S6Ser235 ratio in polyps. We hypothesized that metformin would affect colorectal tissue S6Ser235 among obese patients with recent history of CRA. A phase IIa clinical biomarker trial was conducted via the U.S. National Cancer Institute-Chemoprevention Consortium. Non-diabetic, obese subjects (BMI ≥30) age 35–80 with recent history of CRA, were included. Subjects received 12 weeks of oral metformin 1000mg twice daily. Rectal mucosa biopsies were obtained at baseline and end-of-treatment (EOT) endoscopy. Tissue S6Ser235 and Ki-67 immunostaining were analyzed in a blinded fashion using Histo score (Hscore) analysis. Among 32 eligible subjects, the mean baseline BMI was 34.9. Comparing EOT to baseline tissue S6Ser235 by IHC, no significant differences were observed. Mean (SD) Hscore at baseline was 1.1 (0.57) and 1.1 (0.51) at EOT; median Hscore change was 0.034 (p=0.77). Similarly, Ki-67 levels were unaffected by the intervention. The adverse events were consistent with metformin’s known side effect profile. Among obese CRA patients, 12 weeks of oral metformin does not reduce rectal mucosa pS6 or Ki-67 levels. Further research is needed to determine what effects metformin has on the target tissue of origin as metformin continues to be pursued as a CRC chemopreventive agent.
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosis among men and women and the second most common cancer cause of death in the U.S. (1). Accumulation of genetic and epigenetic alterations contributes to the progression of normal colorectal tissue to an adenoma and subsequently into cancer, via the well-defined adenoma-carcinoma sequence (2). Among other factors, obesity is implicated in colorectal adenoma (CRA) risk, risk of adenoma recurrence (3), CRC development (4), all-cause mortality from CRC (along with many other malignancies) (5), and high risk of disease recurrence and mortality among CRC survivors (6,7). Identifying obese individuals with history of CRAs as a high-risk group has generated interest among chemoprevention clinical trials researchers, including members of our study team (8). Obesity is rising in the U.S., with prevalence estimates ranging from 34%−50% among adults – disproportionately affecting Hispanics, and non-Hispanic black individuals. The magnitude of the problem and associated health disparities have prompted the American Society of Clinical Oncology to create an Energy Balance Workgroup to develop a policy statement on obesity and cancer, highlighting the importance of diet and physical activity in controlling obesity as a means of cancer prevention. Currently, there are considerable efforts to “repurpose” the diabetes medication metformin for cancer prevention (9), particularly among the obese due to 1) its role in tumor-suppressive and growth-inhibitory pathways, and 2) favorable metabolic effects in obese individuals.
While agreement in the literature is not uniform (10,11), a growing evidence base of population-based studies (12–17) shows reduced cancer (including CRC (18,19)) incidence and cancer-specific death among diabetics using metformin versus other treatments. Two non-mutually exclusive mechanisms have been proposed: reduction of host insulin levels by metformin, and the direct action of metformin as an AMPK activator and mTOR inhibitor in neoplastic cells (Supplemental Figure). A key action of metformin is activation of the LKB1/AMPK pathway (20). One pivotal study demonstrated that the in vivo action of metformin is severely attenuated in liver-specific LKB1 knockout mice (21). There is evidence that hyperinsulinemia stimulates aggressive cancer behavior. Metformin has important insulin-lowering and glucose lowering activity in hyperinsulinemic patients with the metabolic syndrome, obesity, and/or type II diabetes (22). Metformin has a direct growth inhibitory action (23,24), requiring AMPK activation that leads to inhibition of mTOR activation and protein synthesis (23,24), and reduced proliferation. Multiple investigations suggest specific relevance of these hypotheses to CRC (25–33). In CRC mouse models, 10-weeks treatment with metformin favorably alters mTOR pathway intermediates in colorectal polyps and results in decreased intestinal polyp formation. However, it is not known how metformin affects the mTOR pathway in colorectal tissues among humans.
Multiple investigations (25–34) suggest specific relevance of metformin action on the mTOR pathway to CRC. mTOR inhibition has been associated with decreased colorectal carcinogenesis in mice (35). Metformin induced intestinal polyp suppression in ApcMin/+ mice, along with an increased ratio of pAMPK/AMPk, decreased ratio of pmTOR/mTOR, and decreased ratio of pS6Ser235/S6Ser235 in the polyp specimens (28). Metformin also suppressed azoxymethane-induced colorectal aberrant crypt foci (ACF) by activating AMP-activated protein kinase in murine models (36). A small trial of metformin as a CRC chemopreventive agent in humans was first reported in 2010 (37). Twenty three individuals with ACF were randomized to receive 1 month of metformin 250mg/day (n=9) vs. no treatment (n=14). The number of ACF per individual was significantly reduced after metformin treatment, as was the proliferating cell nuclear antigen index.
Despite well-characterized effects of metformin inhibiting colorectal polyps in CRC mouse models via effects on the mTOR pathway, this has not been validated in humans at risk for CRC. We therefore performed a phase IIa clinical trial to test whether oral metformin affects rectal tissue S6Ser235 levels among obese CRA patients.
Methods
Study Design
This was a multicenter phase IIa study of oral metformin on colorectal mucosa tissue biomarkers among individuals with a history of colorectal adenomas and a BMI ≥ 30 (ClinicalTrials.gov Identifier NCT01312467). Participants were enrolled at 3 sites (University of California Irvine Medical Center, Orange, CA, VA Long Beach Healthcare System, Long Beach, CA, and Kaiser Permanente Sacramento, Sacramento, CA) during the period June 2011 to Dec. 2013.
Eligibility Criteria
Obese individuals age 35–80 with history of colorectal adenomas within the prior 3 years were eligible for enrollment (adenomas must have been endoscopically removed). Documentation of colorectal adenomas was established via review of pathology reports. Obesity was defined as having a body mass index (BMI) ≥ 30, rounded to the nearest whole integer. Individuals <35 years of age were excluded as these may represent an unusual presentation for colorectal adenomas or hereditary condition. Individuals with diabetes mellitus were excluded, as were individuals with vitamin B12 deficiency, or history of liver or kidney disorders, lactic acidosis, metabolic acidosis, or eating disorder (anorexia nervosa, bulimia, or nausea). Participants were required to have excellent to good performance status (defined as Eastern Cooperative Oncology Group, ECOG performance status 0–1) and normal organ function.
Intervention & On-study Assessments
Treatment was initiated with metformin at 500 mg (Extended Release tablets) daily for Week 1, with a dose escalation of 500 mg each week until the final dose of 2000 mg a day (1000 mg twice daily) was reached by Week 4. This schedule of two 500 mg tablets in the morning and two 500 mg tablets in the evening was continued for the remaining duration of the intervention period, from Week 4 through Week 12 (± 1 week), including on the day of the end-of-treatment (EOT) endoscopy procedure.
Subjects were evaluated at Weeks 0, 4, 8, and 12 during treatment, and at Week 16 post-treatment for toxicity assessment, laboratory review, and compliance.
Procedures and Laboratory Analyses
Rectal Biopsy:
Participants were scheduled for a flexible sigmoidoscopy or colonoscopy with biopsy, performed according to standard protocol. The procedure was done in a manner that allowed tissue to be collected, fixed in formalin and embedded in paraffin in the same day. A universal bowel prep was utilized by all institutions for flexible sigmoidoscopy or colonoscopy procedures as follows: for colonoscopy procedures, all patients used Golytely plus 2 Fleets enemas. Bowel preparation was initiated 14 to 18 hours before the procedure. Participants arrived in clinic having fasted after midnight of the day of the procedure. For flexible sigmoidoscopy procedures, 2 Fleets enemas were used 1–2 hours before the procedure. No fasting was required for participants undergoing flexible sigmoidoscopy.
Flexible sigmoidoscopy with biopsy was performed as an outpatient procedure. Colonoscopy was performed under standard conditions including conscious sedation. Eight (8) normal rectal mucosal biopsies were obtained 10 cm from the anal verge or at the first rectal valve using large (3.4 mm) forceps (38), yielding approximately 15.5mg tissue per biopsy and which has been associated with low risk of complications. Biopsies were obtained at baseline and at week 12 of metformin treatment by endoscopy using standard procedures.
Following the validated methods of Tabernero et al. (39), tissue specimens were immediately placed into a 4°C pre-cooled 4% neutral buffered formalin solution and fixed for 8–16 hours, with a maximum duration of 24 hours. Fixed specimens were further processed through routine specimen dehydration using graded ethanols to xylene. Tissue specimens were embedded in paraffin wax under vacuum at 60°C and stored at room temperature until analysis at the lead site. 4μm tissue sections were mounted onto positively charged glass slides. For each subject, 2 slides from the same tissue block were stained with individual positive and negative controls. Immunohistochemical staining was performed using an automated Ventana BenchMark ULTRA immunostainer and according to the manufacturer’s protocol. The antigen-retrieval was applied as needed for each antibody. Two antibodies were utilized: pS6Ser235 (Ser235/236, 1:200, Cell Signaling, Danvers, MA) and Ki-67 (30–9, Ventana, Tucson, AZ). The Ki-67 antibody was pre-diluted and ready to use (RTU) by the manufacturer. Histologic assessment was done by two pathologists. In order to carefully quantitate immunostaining levels in epithelial (as compared to stromal) cells we used two experienced Pathologists (Drs. Rezk and Carpenter) rather than an automated scoring system for scoring. The study pathologists were blinded with respect to the pre/post metformin status of the biopsy material. Ten high-power fields per sample were assessed for immunostaining and the lead pathologist (Dr. Rezk) assigned a numeric score representing the proportion of cells staining positive. Qualitative changes in marker expression were assessed in a blinded fashion. For quantitative analysis, the Histo score (Hscore) was calculated to evaluate complete biopsy sections at high magnification using a light microscopy, as reported previously (39): The Hscore is determined by estimation of the percentage of tissue cells positively stained with low, medium, or high staining intensity. The final score is determined by weighted estimate, as follows: Hscore = (low %) × 1 + (medium %) × 2 + (high %) × 3. Scoring of the proliferation marker Ki-67 was assessed by estimation of a ratio of tumor cells positively stained for Ki-67 versus the total number of tumor cells. This result is expressed as a percent of tumor cells stained. For IHC endpoints and a positive score, cytoplasmic staining is required for pS6serine235, and nuclear staining for Ki-67.
Toxicity Evaluation
All subjects were evaluated for toxicity assessment from the time of first dose of metformin, using Common Terminology for Adverse Events v4.0 (CTCAE, from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.02_2009-0915_QuickReference_8.5x11.pdf). Since toxicities in this study are measured as categorical data, primary analysis was done using tests of binomial proportions (e.g., Mantel-Haenszel chi-squared statistic).
Response Evaluation
Analyses of primary and secondary endpoints included all subjects for whom tissue was accessible for both pre and post treatment quantitative immunohistochemistry. Participants taking metformin on ≥70% of days (as assessed by pill count) were prospectively defined as “good compliers”.
Statistical Considerations
Distributions were determined from blood and tissue samples at baseline. Biomarker Endpoints: A paired t-test was used to examine the effect of short-term (12-week duration) oral metformin on rectal mucosa biomarkers (pS6Ser235, Ki-67) as assessed by immunostaining. The distribution of the difference between post- and pre-treatment values was examined. Measures of central tendency and variability were computed. We tested the assumption of normality of the differences and if violated we sought transformations that most closely satisfy the assumption. Power calculations were based using a paired t-test to demonstrate at least a 35% decrease in the rectal mucosa pS6Ser235 level (primary endpoint) based on a similar reduction in the pS6Ser235/pS6Ser235 ratio in polyp specimens observed in prior murine studies after metformin treatment (28). Such calculations indicated that a sample size of 32 subjects will have power = 0.80. 45 subjects were accrued to account for attrition. Each subject had 2 slides evaluated for cell proliferation at pre-treatment, and 2 slides at post-treatment. The mean percentage of positive nuclei staining for Ki-67 at the same time point was obtained. A 2-sided paired t-test was used to examine the effect of short-term (12-week duration) oral metformin on percentage of positive nuclei staining for Ki-67.The descriptive statistics and profile plot in percentage of positive nuclei staining for Ki-67 were generated. The Pearson correlation coefficient was obtained between the difference (Post – Pre) in Hscore and the difference (Post – Pre) in percentage of positive nuclei staining for Ki-67. The scatter plots also were constructed.
Analyses of the time to side effect development were done by the proportional hazards model. The latter analysis used a time-dependent covariate to explore the cumulative dose effect and a dosage group effect. A sensitivity analysis was conducted from the subset of subjects determined to be “good compliers”.
Reporting and Exclusions
Dropouts and those lost to follow-up were not analyzed for primary, or secondary endpoints. Subject compliance was monitored at each follow-up visit. Non-compliance was determined based on the participant adherence to at least 70% of the study medication. Data were analyzed for all patients completing the endoscopy exams with biopsy. Subjects were asked to keep a diary/calendar to document consumption of medication and to bring their diary to each visit. Pill counts were used as a secondary measure to validate self-reports.
Data and Safety Monitoring Plan
In accordance with the policies and procedures of Phase I-II Cancer Prevention Consortia: Southern California Chemoprevention Consortium (University of California, Irvine Chao Family Comprehensive Cancer Center) and the NIH and NCI policies for Data and Safety Management of clinical trials, all Consortium clinical trials are monitored to insure the safety of human participants, the validity and integrity of the data, and appropriate termination. The UC Irvine Chao Family Comprehensive Cancer Center (CFCCC) Data Safety Monitoring Board (DSMB) was responsible for monitoring the study.
Ethical Considerations and Institutional Review Board Approval
Prior to initiating the study and receiving agent, the Investigators at the Lead Organization and the Participating Organization(s) obtained written approval to conduct the study from the local Institutional Review Boards (IRBs). Studies were conducted in accordance with the Belmont report. Written informed consent was obtained from all participants, after a full discussion of risks and benefits.
Results
Between 2011 and 2013, 45 obese CRA individuals were accrued at 3 sites in order to attain 32 evaluable subjects (UC Irvine, n=10, Kaiser Permanente, n=10, VA Long Beach Healthcare System, n=12). Among the 45 individuals initially enrolled, 4 were deemed ineligible, 4 came off study due to adverse events (AEs, including diarrhea, insomnia, headache), 1 was lost to follow-up, 2 withdrew consent, and 2 were removed for other reasons. Baseline demographic data are listed in Table 1. Among the evaluable subjects, the median age was 59.1 years. The study population was predominately male (71.9%) and White race (84%); patients with Hispanic ethnicity comprised 9.4% of patients. The median baseline weight was 105.2kg and median BMI was 34.9 kg/m2.
Table 1.
Baseline characteristics.
| Baseline characteristics | Registered to the study (N = 45) |
Completed the study (N = 32) |
|---|---|---|
| Age, yr mean (SD) | 59.6 (6.8) | 59.1 (7.3) |
| Male, n (%) | 30 (67%) | 23 (72%) |
| Female | 15 (33%) | 9 (28%) |
| Ethnicity | ||
| Hispanic or Latino, n (%) | 5 (11%) | 3 (9%) |
| Not Hispanic or Latino, n (%) | 39 (87%) | 29 (91%) |
| Unknown, n (%) | 1 (2%) | 0 |
| Race | ||
| White, n (%) | 38 (84%) | 27 (84%) |
| Black/African American, n (%) | 4 (9%) | 4 (13%) |
| Not reported/Unknown, n (%) | 2 (4%) | 1 (3%) |
| Native Hawaiian/Other Pacific Islander, n (%) | 1 (2%) | 0 |
| Weight, kg mean (SD) | 103.25 (15.94) | 105.16 (17.42) |
| BMI, Mean (SD) | 34.28 (5.04) | 34.92 (5.57) |
Efficacy
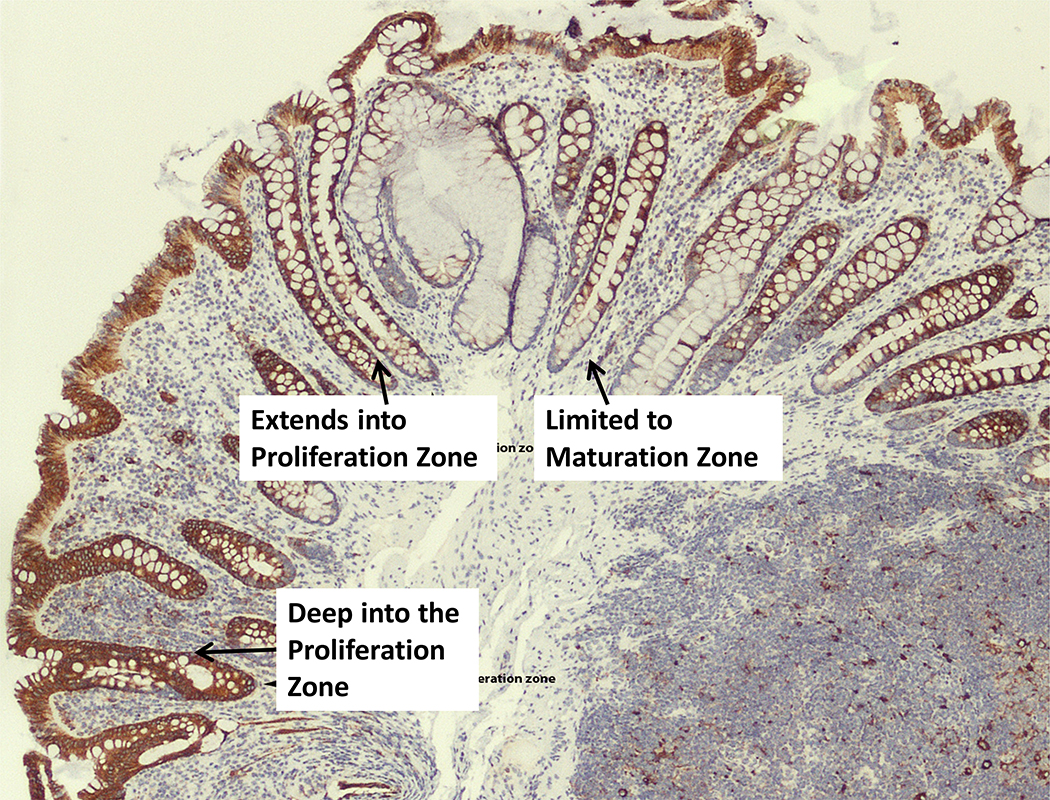
The primary endpoint was to assess levels of activated pS6Ser235/236 in colorectal mucosa pre- and post- metformin. As seen in representative Fig. 1, immunostaining patterns were variable by crypt location. In order to examine inter-rater variability, a second pathologist read 10 random slides for analysis of inter-rater variability of pS6serine235 (non-blinded fashion, by the “agree” vs. “disagree” method). Based on these 10 slides, there was 80% (8/10, 95% exact CI 44% to 97%) agreement between the two pathologists’ evaluations. Immunostaining revealed no significant differences in pre- vs. post- metformin pS6Ser235 in the rectal mucosa. The median difference in pS6Ser235 Hscores in paired analyses of Week 12 vs. baseline rectal mucosa samples was 0.034 ± 0.44SD, p=0.77 (NS). Among the 32 evaluable subjects, 17 had rectal mucosa pS6Ser235 Hscores that were lower at end-of-study than at baseline. Conversely, 15 patients had rectal mucosa pS6Ser235 Hscores that were greater at Week 12 than at baseline.
Figure 1.
pS6ser235 by immunohistochemistry in the rectal mucosa of an obese colorectal adenoma patient on-study, showing differential staining patterns throughout the colorectal crypts.
In the analyses stratified by study site, the means and standard errors of Hscores of pS6Ser235 at baseline were 1.531 and 0.105 respectively for Kaiser Permanente at Sacramento, 0.521 and 0.096 respectively for Long Beach VAMC, and 1.400 and 0.105 respectively for UC Irvine. This stratification indicated that there was a statistically significant difference in Hscore of pS6Ser235 at baseline among the three study sites with a p-value < 0.001. Means and standard deviations of the change in the Hscores of pS6Ser235 from baseline were 0.321 and 0.488 respectively for Kaiser Permanente at Sacramento (nominal p-value, 0.068), −0.201 and 0.289 respectively for Long Beach VAMC (nominal p-value, 0.035) and −0.006 and 0.416 for UC Irvine (nominal p-value, 0.964). There was no statistically significant change in the Hscore of pS6serine235 from baseline at each study site after the Bonferroni-Holm adjustment method was applied.
Ki-67 levels in colorectal mucosa were assessed by immunostaining, as a secondary endpoint. Pearson correlation coefficients were calculated between the difference (Post – Pre) in Hscore and the difference (Post – Pre) in percentage of positive nuclei staining for Ki-67. The Pearson correlation coefficient was 0.15 (p-value = 0.43, NS).
Safety Assessment (evaluable cohort, N = 32)
Table 2 shows the type and number of reported attributable adverse events (possible, probable, and definitely related) among the 32 evaluable subjects, using CTCAE v4.0. Twenty-three subjects (72%) reported at least one possible, probable or definite study related adverse event on or after the treatment began. The most common study related events that were reported include diarrhea (N = 15 (47%) subjects), anorexia (N = 6 (19%) subjects), flatulence (N = 9 (28%) subjects), nausea (N = 6 (19%) subjects), and abdominal/stomach pain (N = 11 (34%) subjects; 4 events of cramping). No grade 3 or 4 adverse events were observed.
Table 2.
Study related adverse events (AE’s) that occurred on or after treatment began.
| Adverse Event | Number of Unique Patients | |||||
|---|---|---|---|---|---|---|
| Mild (Grade1) | Moderate (Grade 2) | Sub-Total | Mild (Grade1) | Moderate (Grade 2) | Sub-Total | |
| ABDOMINAL PAIN | 2 | 0 | 2 | 2 | 0 | 2 |
| ANOREXIA | 6 | 0 | 6 | 6 | 0 | 6 |
| BLOATING | 1 | 0 | 1 | 1 | 0 | 1 |
| CONSTIPATION | 1 | 0 | 1 | 1 | 0 | 1 |
| DIARRHEA | 16 | 1 | 17 | 15 | 1 | 16 |
| DIZZINESS | 3 | 0 | 3 | 3 | 0 | 3 |
| DRY MOUTH | 1 | 0 | 1 | 1 | 0 | 1 |
| DYSPEPSIA | 5 | 1 | 6 | 3 | 1 | 4 |
| FATIGUE | 3 | 0 | 3 | 3 | 0 | 3 |
| FEVER | 1 | 0 | 1 | 1 | 0 | 1 |
| FLATULENCE | 9 | 0 | 9 | 9 | 0 | 9 |
| HYPERHIDROSIS | 1 | 0 | 1 | 1 | 0 | 1 |
| HYPOGLYCEMIA | 1 | 1 | 2 | 1 | 1 | 2 |
| INSOMNIA | 2 | 0 | 2 | 2 | 0 | 2 |
| METABOLISM AND NUTRITION DISORDERS - OTHER, SPECIFY | 1 | 0 | 1 | 1 | 0 | 1 |
| MYALGIA | 2 | 0 | 2 | 2 | 0 | 2 |
| NAUSEA | 6 | 0 | 6 | 6 | 0 | 6 |
| STOMACH PAIN | 15 | 4 | 19 | 8 | 2 | 10 |
| TREMOR | 2 | 0 | 2 | 1 | 0 | 1 |
| VOMITING | 1 | 1 | 2 | 1 | 1 | 2 |
| Total | 79 | 8 | 87 | |||
Note: Study related was defined as Possible, Probable, and Definite related to the study regimen. For the number of participants, column percent was calculated. Rows are not mutually exclusive. Of note, no Grade 3 or Grade 4 AE’s were observed. Shaded cells indicate the data is not applicable.
Comparing Week 12 (end-of-treatment, EOT) with baseline values, metformin treatment for 12 weeks did not statistically alter hematologic (white blood cell count, hemoglobin, hematocrit, platelet count) or blood chemistry profiles (including electrolyte values, renal or liver function values). Of note, metformin treatment resulted in significantly reduced serum Vitamin B12 levels decreased by 46.7ng/L (95% CI −73.2 to −20.2). Vitamin B12 levels were significantly reduced at EOT vs. baseline (see Supplemental Table for clinical laboratory data). Metformin treatment resulted in significant weight loss after 12 weeks on-study (−2.15kg, 95% CI −3.26 to −1.04).
Discussion
In chemoprevention clinical trials-based research, utmost import is placed on 1) identifying high-risk individuals who can be targeted for prevention, 2) investigating agents with acceptable toxicity profiles commensurate with the risk level in a target population, 3) utilizing agents that have demonstrated activity in the target tissue of origin, and 4) ushering potential agents through the clinical trials development process only when there is extensive supportive preclinical evidence to corroborate epidemiological associations of risk reduction (40). Considering metformin as a potential chemopreventive agent, our goal was to fill remaining gaps in this paradigm by demonstrating activity of metformin in the target tissue of origin among a special population at risk for CRC. A better understanding of metformin as a chemopreventive agent in the clinical trials setting is important not only for CRC research but also for research focused on other solid tumor malignancies, particularly obesity-associated malignancies (e.g., endometrial, breast, pancreas). Our unique, high-risk population (obese individuals with CRAs) was suited as a target for any chemopreventive effects, in addition to the known favorable metabolic effects of metformin. However, in this clinical trial, we did not detect any differences in rectal tissue pS6Ser235/236 or Ki67 immunostaining levels after 12 weeks metformin treatment among obese CRA patients. Similar negative tissue effects have been observed after metformin use in Barrett’s esophagus (41). In a randomized placebo-controlled trial of 74 individuals with Barrett’s esophagus, no significant biomarker differences were seen for pS6Ser235/236, proliferation (by Ki67 labeling index), or apoptosis (caspase 3) levels after 12 weeks of treatment with either metformin or placebo (41). However, in three small clinical trials involving endometrial cancer patients, metformin treatment prior to surgery resulted in decreased mTOR pathway biomarkers as predicted, and reduced cellular proliferation (as determined by percent Ki-67 staining) (42–44).
Our results (examining metformin effects on the normal rectal mucosa in humans) differ substantially from those observed in the prior murine experiments (which focused on metformin-induced changes in polyp specimens). Evidence for modulation of mTOR signaling in normal rectal mucosa has been established. In a small study of patients given aspirin 600mg/daily for 1 week, Din et al. reported decreased phosphorylation of S6 and S6K1 in rectal mucosal samples (45). While our clinical trial was designed to assess tissue pS6Ser235/236, it is now known that pS6Ser235/236 can be mTOR independent, however pS6Ser240/244 is always mTOR-dependent (46). Furthermore, validated antibodies now exist that may better reflect metformin activity (e.g., pAMPK). It is possible that the choice of endpoint, antibody used, immunohistochemical technique, and short trial duration may not have adequately captured metformin’s tissue effects. Given the complex nature of carcinogenesis, analysis of a limited number of tissue biomarkers on proliferation or relevant signaling pathways (as reported here) may not offer a comprehensive assessment of metformin tissue effects.
It is important to acknowledge here the results of a landmark phase III trial from Japan, where Higurashi et al. randomized 151 non-diabetic individuals with colorectal adenomas (after polypectomy) to treatment with either metformin 250mg daily or placebo for 12 months (47). Approximately 70% of participants had multiple or advanced adenomas or carcinoma in situ at baseline. The result was a 40% risk reduction of adenomas in individuals receiving metformin vs. placebo (RR=0.6, 95% CI 0.39–0.92). Adverse events in this trial were low (11% AE’s reported, all of which were grade 1), as expected with the low dose of metformin (250mg daily) utilized in the trial.
There are substantial differences between the participants and the intervention in the prior phase III clinical trial by Higurachi et al. (47) and this phase IIB clinical trial. Study participants in the phase III clinical trial were Japanese, compared to primarily U.S. Caucasians in the current Phase IIB study. All patients in the phase IIB study were obese (median BMI was 34.9 kg/m2, as a BMI > 30 kg/m2 was required for study entry), compared with patients in the phase III trial who had an average BMI of 23 kg/m2. The duration of treatment was much longer for in the Phase III clinical trial (12 months) compared to our study (12 weeks), and the metformin dose was much lower in the phase III trial (250mg/day vs. 1000mg twice daily). The Higurashi study (47). is a chemopreventive study with a clinical primary endpoint (adenoma recurrence) whereas our study focused on a biomarker endpoint. Furthermore, we did not assess tissue biomarker changes in adenomas, rather our focus was on the target tissue of origin: normal rectal mucosa.
In our study, metformin treatment at 1000mg twice daily was met with substantial toxicity- particularly gastrointestinal toxicity (diarrhea, abdominal cramping, flatulence). After 12-weeks intervention, 72% of patients reported at least one adverse event. 47% of patients developed Grade I or Grade II diarrhea, and 34% reported Grade I/II stomach pain. Of note, these relatively high rates of adverse events are despite the relatively slow and deliberate upward titration of metformin to achieve 1000mg twice daily dose by week 4. Certain patients will not tolerate full dose metformin in this setting, evidenced by the fact that 4 patients came off study due to adverse events. As such, though metformin is considered safe from a therapeutic standpoint at 1000mg twice daily, the dosage in the setting of cancer prevention, at least among obese non-diabetic Western patients appears to be too high, and not recommended for future colorectal cancer prevention clinical trials.
In the phase III trial of metformin vs. placebo by Higurashi et al. (47), significant colorectal adenoma reduction was reported. Interestingly, this effect was seen after 12-months treatment duration at a metformin dose of just 250mg/day. Of note, this was the same metformin dose used in by members of the same research group in their prior phase IIB trial of metformin vs. placebo demonstrating colorectal aberrant crypt foci (ACF) reduction (Hosono et al, 2010). As mentioned, the adverse event rate in the phase III trial was very low at 11%, all being grade 1 adverse events. Given the balance of efficacy against clinical endpoints (colorectal adenomas, ACFs) and with a favorable safety profile, it appears that low-dose metformin (250mg/day) is the optimal dose for testing in future colorectal cancer prevention clinical trials. This preferred metformin dose (250mg/d for colorectal cancer prevention) is lower than the dose used in major North American clinical trials, including the clinical trial of metformin (850mg twice daily) vs. placebo in early stage breast cancer (NCIC Clinical Trials Group MA.32; ClinicalTrials.gov Identifier: NCT01101438).
In addition to the aforementioned gastrointestinal side effects observed after metformin 1000mg twice daily dose in our study, patients experienced weight loss (which is beneficial in the setting of obesity), and a decrease in serum vitamin B12 levels. These latter differences shed light on non-diabetic populations that may not be suitable for metformin-based clinical trials (i.e., patients with low BMI, or individuals with baseline low-normal serum B12 levels). Other limitations include a small sample size related to the phase IIa clinical trial design, where we had insufficient statistical power to detect small biomarker effects, the relatively short duration of intervention, and the limited number of biomarkers assessed.
Despite our negative results, given the recent positive clinical results of the aforementioned phase III clinical trial of metformin for colorectal adenoma reduction, we believe future research is needed to elucidate potentially chemopreventive actions of metformin in the obese population. The field is developing rapidly – with a growing body of clinical trials-based research soon to emerge. We await the result of the large phase III breast cancer post-adjuvant clinical trial of metformin vs. placebo led by the NCI Canada (“MA.32”) – which will not be available until after 2020 (48). Smaller clinical studies may help to elucidate the role of metformin as an anti-cancer agent, such as the METEOR phase II study (investigating tumor size after neoadjuvant treatment with or without metformin in hormone-receptor positive breast cancer patients) (49). For colorectal cancer, large scale clinical trials in the U.S. have been discussed and there is renewed optimism (50), however no large scale phase III clinical trials are currently active. Of note, the ongoing Diabetes Prevention Program Outcomes Study-3 (DPPOS3) aims to look at cancer (among other) outcomes in their cohort of patients originally assigned to metformin, placebo, or lifestyle modification (https://clinicaltrials.gov/ct2/show/NCT00038727), with results expected in the next five years. It is anticipated that such emerging clinical, translational, and basic experimental data will help to clarify many of the perplexing issues related to metformin’s potential role in cancer chemoprevention.
Supplementary Material
Acknowledgments:
The study team acknowledges Phil Carpenter, MD, for independent pathology slide review and scoring.
Funding: NCI-DCP N01-CN-35160, NIH-NCI 3P30CA062203-15, and supported by the UC Irvine Institute for Clinical Translational Science (ICTS) via Public Health Service research grant M01 RR00827 from the National Center for Research Resources
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61(5):759–67 doi 0092-8674(90)90186-I [pii]. [DOI] [PubMed] [Google Scholar]
- 3.Bonithon-Kopp C, Piard F, Fenger C, Cabeza E, O’Morain C, Kronborg O, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum 2004;47(3):323–33 doi 10.1007/s10350-003-0054-1. [DOI] [PubMed] [Google Scholar]
- 4.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16(12):2533–47 doi 16/12/2533, 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine 2003;348(17):1625–38 doi 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clinical cancer research : an official journal of the American Association for Cancer Research 2010;16(6):1884–93 doi 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer 2013;119(8):1528–36 doi 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zell JA, Lin BS, Madson N, McLaren CE, Gerner EW, Meyskens FL . Role of obesity in a randomized placebo-controlled trial of difluoromethylornithine (DFMO) + sulindac for the prevention of sporadic colorectal adenomas. Cancer causes & control : CCC 2012;23(10):1739–44 doi 10.1007/s10552-012-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017;60(9):1639–47 doi 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev 2012;21(2):280–6 doi 10.1158/1055-9965.EPI-11-0992-T. [DOI] [PubMed] [Google Scholar]
- 11.Fransgaard T, Thygesen LC, Gogenur I. Association between metformin use after surgery for colorectal cancer and oncological outcomes: A nationwide register-based study. International journal of cancer 2018;143(1):63–72 doi 10.1002/ijc.31305. [DOI] [PubMed] [Google Scholar]
- 12.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330(7503):1304–5 doi bmj.38415.708634.F7, 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29(2):254–8 doi 29/2/254 [pii]. [DOI] [PubMed] [Google Scholar]
- 14.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009. doi dc08–2175, 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Chubak J, Boudreau DM, Barlow WE, Weiss NS, Li CI. Diabetes Treatments and Risks of Adverse Breast Cancer Outcomes among Early-Stage Breast Cancer Patients: A SEER-Medicare Analysis. Cancer Res 2017;77(21):6033–41 doi 10.1158/0008-5472.CAN-17-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin W, Fang L, Fang Q, Zheng X, Huang P. Effects of metformin on survival outcomes of pancreatic cancer patients with diabetes: A meta-analysis. Molecular and clinical oncology 2018;8(3):483–8 doi 10.3892/mco.2017.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos JF, Qudsi M, Samara A, Rahim MM, Al-Bayedh SA, Ahmed H. Metformin for lung cancer prevention and improved survival: a novel approach. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation 2018. doi 10.1097/CEJ.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 18.Sehdev A, O’Neil BH. The Role of Aspirin, Vitamin D, Exercise, Diet, Statins, and Metformin in the Prevention and Treatment of Colorectal Cancer. Current treatment options in oncology 2015;16(9):43 doi 10.1007/s11864-015-0359-z. [DOI] [PubMed] [Google Scholar]
- 19.Cardel M, Jensen SM, Pottegard A, Jorgensen TL, Hallas J. Long-term use of metformin and colorectal cancer risk in type II diabetics: a population-based case-control study. Cancer medicine 2014;3(5):1458–66 doi 10.1002/cam4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108(8):1167–74 doi 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310(5754):1642–6 doi 1120781, 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler JE, Beer NA, Jakubowicz DJ, Beer RM. Effects of a reduction in circulating insulin by metformin on serum dehydroepiandrosterone sulfate in nondiabetic men. J Clin Endocrinol Metab 1994;78(3):549–54. [DOI] [PubMed] [Google Scholar]
- 23.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67(22):10804–12 doi 67/22/10804, 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 24.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66(21):10269–73 doi 0008–5472.CAN-06–1500, 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 25.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, et al. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev 2006;15(4):750–5 doi 15/4/750, 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 26.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 2005;14(4):850–5 doi 14/4/850, 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst 2004;96(7):546–53. [DOI] [PubMed] [Google Scholar]
- 28.Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci 2008;99(11):2136–41 doi CAS933, 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila Pa) 2008;1(5):369–75 doi 1/5/369, 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto K, Onodera H, Kondo S, Kan S, Ikeuchi D, Maetani S, et al. Expression of insulin-like growth factor-2 can predict the prognosis of human colorectal cancer patients: correlation with tumor progression, proliferative activity and survival. Oncology 1998;55(3):242–8 doi ocl55242 [pii]. [DOI] [PubMed] [Google Scholar]
- 31.Oshima T, Akaike M, Yoshihara K, Shiozawa M, Yamamoto N, Sato T, et al. Clinicopathological significance of the gene expression of matrix metalloproteinase-7, insulin-like growth factor-1, insulin-like growth factor-2 and insulin-like growth factor-1 receptor in patients with colorectal cancer: insulin-like growth factor-1 receptor gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol Rep 2008;20(2):359–64. [PubMed] [Google Scholar]
- 32.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol 1999;30(10):1128–33. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham MP, Essapen S, Thomas H, Green M, Lovell DP, Topham C, et al. Coexpression of the IGF-IR, EGFR and HER-2 is common in colorectal cancer patients. Int J Oncol 2006;28(2):329–35. [PubMed] [Google Scholar]
- 34.Kabat GC, Kim MY, Strickler HD, Shikany JM, Lane D, Luo J, et al. A longitudinal study of serum insulin and glucose levels in relation to colorectal cancer risk among postmenopausal women. British journal of cancer 2012;106(1):227–32 doi 10.1038/bjc.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YJ, Tian XQ, Sun DF, Zhao SL, Xiong H, Fang JY. Combined inhibition of MEK and mTOR signaling inhibits initiation and progression of colorectal cancer. Cancer Invest 2009;27(3):273–85 doi 908490891, 10.1080/07357900802314893. [DOI] [PubMed] [Google Scholar]
- 36.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Molecular carcinogenesis 2010;49(7):662–71 doi 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 37.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer prevention research 2010;3(9):1077–83 doi 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 38.Bronner MP, Skacel M, Crispin DA, Hoff PD, Emond MJ, Lai LA, et al. Array-based comparative genomic hybridization in ulcerative colitis neoplasia: single non-dysplastic biopsies distinguish progressors from non-progressors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2010;23(12):1624–33 doi 10.1038/modpathol.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008;26(10):1603–10 doi JCO.2007.14.5482, 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 40.Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clinical cancer research : an official journal of the American Association for Cancer Research 2006;12(12):3661–97 doi 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 41.Chak A, Buttar NS, Foster NR, Seisler DK, Marcon NE, Schoen R, et al. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett’s esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2015;13(4):665–72 e1–4 doi 10.1016/j.cgh.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laskov I, Drudi L, Beauchamp MC, Yasmeen A, Ferenczy A, Pollak M, et al. Anti-diabetic doses of metformin decrease proliferation markers in tumors of patients with endometrial cancer. Gynecologic oncology 2014;134(3):607–14 doi 10.1016/j.ygyno.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: a preoperative prospective trial. Cancer 2014;120(19):2986–95 doi 10.1002/cncr.28853. [DOI] [PubMed] [Google Scholar]
- 44.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer medicine 2015;4(2):161–73 doi 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012;142(7):1504–15 e3 doi 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meric-Bernstam F, Akcakanat A, Chen H, Do KA, Sangai T, Adkins F, et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(6):1777–89 doi 10.1158/1078-0432.CCR-11-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. The Lancet Oncology 2016;17(4):475–83 doi 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 48.Goodwin PJ, Parulekar WR, Gelmon KA, Shepherd LE, Ligibel JA, Hershman DL, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst 2015;107(3) doi 10.1093/jnci/djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Lim W, Kim EK, Kim MK, Paik NS, Jeong SS, et al. Phase II randomized trial of neoadjuvant metformin plus letrozole versus placebo plus letrozole for estrogen receptor positive postmenopausal breast cancer (METEOR). BMC cancer 2014;14:170 doi 10.1186/1471-2407-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan AT. Metformin for cancer prevention: a reason for optimism. The Lancet Oncology 2016;17(4):407–9 doi 10.1016/S1470-2045(16)00006-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.