Abstract
The augmenter of liver regeneration protein (ALR) is critical for lipid homeostasis and mitochondrial function. We investigated high fat/high carbohydrate diet (HF/HC)-induced nonalcoholic fatty liver disease (NAFLD) in wild-type, and hepatocyte-specific ALR-knockout (ALR-H-KO) and ALR-heterozygous (ALR-H-HET) mice. ALR was measured in serum of human nonalcoholic steatohepatitis (NASH), and NASH-induced cirrhosis (serum and liver). HF/HC feeding decreased ALR expression in all groups of mice. The otherwise normal ALR-H-HET mice gained more weight and steatosis than wild-type mice when challenged metabolically with HF/HC diet; ALR-H-KO mice gained least weight and had least steatosis. These findings were consistent with correspondingly increased triglycerides and cholesterol, and altered CPT1a, SREBP1c, ACACA and FASN expressions. All HF/HC-fed mice developed insulin resistance, magnitude being lower in ALR-H-KO mice. HF/HC-fed ALR-H-HET mice were more resistant to glucose challenge than wild-type or ALR-H-KO mice. The frequency of TNFα-, IL6- and IL17-producing cells was greater in ALR-H-KO than ALR-H-HET and lowest in wild-type mice. HF/HC feeding did not increase their number in ALR-H-KO mice, and the increase in ALR-H-HET was greater than wild-type mice except for IL17-cells. CD25+FoxP3+CD4+ regulatory T cell (Treg) frequency was lower in ALR-H-HET than wild-type mice and further reduced in ALR-H-KO mice; HF/HC reduced Treg only in wild-type mice. HF/HC-fed ALR-H-HET but not wild-type mice developed fibrosis and ALR-H-KO mice progressed to cirrhosis. White adipose tissue of HF/HC-fed ALR-deficient mice developed strong inflammation indicating bidirectional interactions with the liver. Hepatic and serum ALR levels were significantly reduced in patients with NASH-cirrhosis. Serum ALR was also significantly lower in patients with NASH. In conclusion, hepatic ALR deficiency may be a critical predisposing factor for aggressive NAFLD progression.
Keywords: Hepatocytes, inflammation, insulin, NAFLD, steatosis
INTRODUCTION
Augmenter of liver regeneration (ALR) protein (encoded by Gfer gene) is highly expressed in the liver, predominantly in hepatocytes, and found in cytosol, nuclei and mitochondria1,2. Hepatocytes secrete ALR constitutively and are its primary source in the serum. ALR’s mitochondrial function is critical as its depletion causes rapid loss of ATP leading to cell death3. Originally isolated from weanling rat liver and characterized4, rat, mouse and human ALR gene and protein show remarkable (~90%) homology5,6. ALR’s global knockout in mouse is embryonically lethal indicating its critical importance in embryonic development; mice with hepatocyte-specific knockout of ALR (ALRflox/flox;Alb-Cre) (ALR-H-KO) are normal at birth, but develop excessive steatosis, mitochondrial dysfunction and degeneration, and robust apoptosis between 1 and 2 weeks7. ALR-expressing cells then regenerate from the residual hepatocytes, and presumably from the oval cell compartment. Although steatosis resolves in ALR-H-KO mice, continued cell death, regeneration and inflammation lead to modest fibrosis and eventually hepatocellular carcinoma at 1 year7.
Increasing incidence of nonalcoholic fatty liver disease (NAFLD) is a global clinical challenge. NAFLD is inclusive of simple hepatic steatosis (fatty liver) that progresses to nonalcoholic steatohepatitis (NASH) in about 44% of subjects, and significant NASH subjects develop cirrhosis and hepatocellular carcinoma8,9. Diet rich in saturated fat and simple carbohydrates, obesity and sedentary life style are considered responsible for NAFLD10. Although NAFLD is associated with diabetes mellitus, and environmental and genetic factors are implicated in progressive advanced disease, precise mechanisms of why a subpopulation is predisposed to the aggressive form of NASH are unclear11. Because mitochondrial dysfunction is a major player in the development of NAFLD12–14, and ALR has critical mitochondrial function2,3,7,15,16, we hypothesized that ALR-deficiency may be an important predisposing factor for NASH and its silent progression to fibrosis/cirrhosis in subjects with underlying undiagnosed pathology. Indeed, our findings show high fat/high carbohydrate diet-induced NASH/fibrosis in otherwise normal hepatocyte-specific ALR-heterozygous (ALR-H-HET) mice, and cirrhosis in ALR-H-KO mice with underlying modest pathology; WT mice only developed steatosis. Both serum and hepatic ALR levels were also reduced in human NASH-induced end-stage liver disease (ESLD), and serum ALR in NASH.
MATERIALS AND METHODS
Animals:
8-week old female ALR-H-KO (ALRflox/flox;Alb-Cre), ALR-H-HET (ALR+/flox;Alb-Cre) and littermate WT (ALRflox/flox) mice on B6.SV129 background (bred in the animal facilities at University of Cincinnati or Cincinnati Children’s Hospital Medical Center)7 were placed on high fat diet (60% kcal) (Product number D12492- Research Diet, Inc., New Brunswick, NJ) plus 2.3% fructose and 1.9% sucrose in drinking water (high fat/high carbohydrate: HF/HC-diet)17,18; control mice continued to receive laboratory chow-diet and water (n=6–8 each). Mice were maintained in 12h/12h dark/light cycle at 22ºC (standard vivarium environment). Natural comfortable temperature for mice is 32–36ºC. Mice were weighed weekly, their food consumption measured, and euthanized at the end of 16 weeks. Blood was drawn, and livers excised, washed in ice-cold PBS, weighed and portions saved in 10% buffered formalin, 2% paraformaldehyde or snap frozen in liquid nitrogen. Formalin-fixed and paraffin-embedded tissue sections were stained with hematoxylin/eosin for histopathological examination by JW who was blinded to treatment groups. Steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2) were graded, and fibrosis was scored separately on a scale of 0–419. ALR was measured using kits from Biomatik (Wilmington, DE) or Cusabio Technology LLC (Houston, TX).
Human study:
Informed consent was obtained from patients undergoing liver transplantation for NASH-ESLD at University of Cincinnati College of Medicine (IRB-approved study #2015–4407). Blood was obtained at the time of consent (prior to surgery). A portion of the liver tissue was obtained shortly after excision of the organ. Controls were blood and normal portion of the liver tissue from consented patients undergoing partial liver resection for focal damage. Deidentified tissue samples were also obtained from the Health Sciences Tissue Bank, University of Pittsburgh Medical Center. Likewise serum was obtained from the consented biopsy-proven NASH patients from the Fatty Liver Clinic at the University of Pittsburgh Medical Center (IRB-approved study #MOD09100075–04).
All other methods which are standard and published are described in Supplemental Material Section.
Statistical Analysis:
Data were analyzed with Sigma Plot 12.0 (San Jose, CA). Statistical significance between the groups was determined by one–way ANOVA followed by Bonferroni multiple comparison tests. Kruskal–Wallis tests were conducted if the data did not have a normal distribution. All data are presented as means ±SD for each group. Probability values of <0.05 were considered significant.
RESULTS
Differential susceptibility of ALR-H-HET and ALR-H-KO mice to HF/HC-induced gain in body and liver weight
The food (caloric) consumption of chow-fed WT, ALR-H-HET and ALR-H-KO mice was similar throughout the experimental period (Figure 1A). Caloric consumption increased in all groups fed HF/HC diet, but was greater in ALR-H-HET mice than the WT and ALR-H-KO mice.
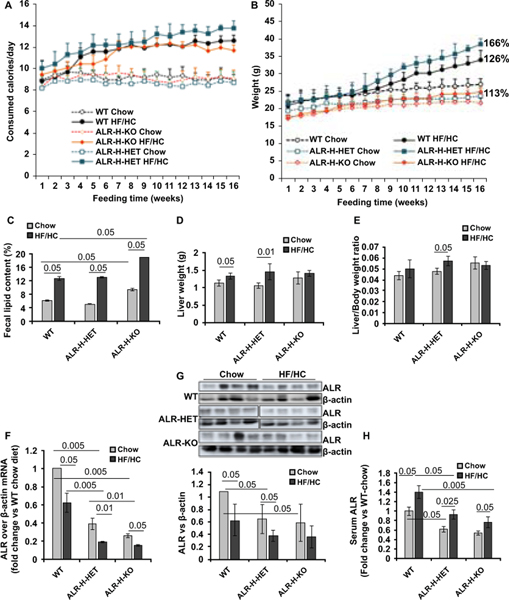
Figure 1: Caloric consumption, weight gain and ALR levels in HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice.
(A, B) Weekly caloric consumption and body weight gain of mice fed chow or HF/HC diet for 16 weeks. Numbers against the filled symbols indicate total % weight gain at the end of HF/HC vs chow feeding in mice of respective ALR-genotype. (C) Fecal lipid, (D) liver weight and (E) liver/body weight ratio at the end of HF/HC feeding. (F) Hepatic ALR mRNA (qRT-PCR) and (G) protein (Upper panel shows Western blot); bar graph in lower panel shows ALR protein expression relative to β-actin. (H) Serum ALR in chow- or HF/HC-fed mice; values are expressed as fold-change vs serum ALR in chow-fed WT mice, the concentration being 1523 ± 136 pg/ml.
The initial weights of all chow-fed mice were comparable (Figure 1B). The weight gain on chow-diet was greater in WT than ALR-H-HET or ALR-H-KO mice that gained least weight (Figure 1B). All HF/HC-fed mice started gaining more weight than those on chow diet between 6 and 8 weeks, the increase being highest for ALR-H-HET and lowest for ALR-H-KO mice. Thus, the net weight gain was 26% and 66% respectively for WT and ALR-H-HET mice on HF/HC- over those on chow-diet at the end of 16 weeks; weight gain of HF/HC-fed ALR-H-KO mice was only 13% over those on chow-diet (Figure 1B; Figure S1A). The differential weight gain was evident from much larger visceral and interscapular adipose tissue in HF/HC-fed ALR-H-HET compared to the WT mice, and relatively small increase in the ALR-H-KO mice after HF/HC feeding (Figure S1B–1E). Furthermore, increase in adipocytes size was greater in HF/HC-fed ALR-H-HET and lower in ALR-H-KO than the WT mice (Figure S1F). Fecal lipid content of ALR-H-KO mice on HF/HC diet was greater than ALR-H-HET or WT mice (Figure 1C).
The livers of chow-fed ALR-H-KO mice were larger than WT or ALR-H-HET mice. However, liver weight gain was greater in HF/HC-fed ALR-H-HET than WT mice, but no gain occurred in ALR-H-KO mice (Figure 1D). Consequently, increase in the liver/body weight ratio was greater in HF/HC-fed ALR-H-HET than WT mice, and no change occurred in ALR-H-KO mice (Figure 1E).
Differential reduction in hepatic ALR and increase in serum ALR in HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice
The basal hepatic ALR mRNA (Figure 1F) and protein (Figure 1G; Figure S2) expressions were lower in chow-fed ALR-H-HET and ALR-H-KO mice than in WT mice; ALR expression decreased significantly at the end of HF/HC feeding in all groups. Stressed/injured hepatocytes release greater amounts of ALR.20 Thus although basal serum ALR was lower in ALR-H-HET and ALR-H-KO than in WT mice, ALR increased in all HF/HC-fed mice but its levels were still lower in ALR-H-HET and ALR-H-KO mice than WT mice (Figure 1H). In regard to ALR expression in ALR-H-KO mice, almost complete depletion of ALR from hepatocytes between 1 and 2 weeks post-birth was found to cause excessive cell death. This stimulated biliary compartment as a compensatory mechanism to restore hepatocyte mass. These cells began expressing ALR (albeit at lower level than control)7.
Liver injury and differential steatosis in HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice
Livers of HF/HC-fed ALR-H-HET mice were paler than of WT mice while those of ALR-H-KO mice were coarse and granular (Figure 2A). The liver injury was mild in HF/HC-fed WT and slightly greater in ALR-H-HET mice as determined by serum ALT and AST levels (Figure 2B). ALT and AST levels were significantly elevated in chow diet-fed ALR-H-KO mice and did not change significantly by HF/HC feeding (Figure 2B). Interestingly, serum ALP, which were also much higher in chow-fed ALR-H-KO mice than in WT or ALR-H-HET mice, decreased after HF/HC feeding (Figure 2B). Decrease in ALP is reported in HF-fed mice21. It will be of interest to determine whether HF/HC alters biliary component in this model.
Figure 2: Liver injury and hepatic and serum lipids in HF/HC-fed WT and ALR-deficient mice.
Mice were fed chow or HF/HC diet for 16 weeks. (A) Liver of HF/HC-fed ALR-H-HET mouse looks paler than the WT mouse, while that of ALR-H-KO mouse is coarse. (B) Serum ALT, AST and ALP levels HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice. (C) H/E-stained (10X magnification) liver sections showing much greater steatosis in HF/HC-fed ALR-H-HET compared to WT mice and very low steatosis in ALR-H-KO mice. (D) Hepatic triglycerides are strongly increased in HF/HC-fed WT and ALR-H-HET mice, and mildly in ALR-H-KO mice. (E) Hepatic cholesterol is significantly increased in HF/HC-fed ALR-H-HET but not WT or ALR-H-KO mice. (F) Serum triglycerides are elevated in HF/HC-fed WT and ALR-H-HET mice. (G) Serum cholesterol is mildly but significantly elevated in all groups of mice fed HF/HC diet.
Histologically hepatic steatosis was greater in HF/HC-fed ALR-H-HET mice than in WT mice; in contrast, relatively low level lipid deposition occurred in ALR-H-KO mice (Figure 2C; Figure S3A). Hepatocellular ballooning and inflammatory infiltration were much prominent in HF/HC-fed ALR-H-HET than in WT mice (Figure 2C; Figure S3B). Inflammation, which was already present in ALR-H-KO mice increased after HF/HC feeding; however, necrosis was similar to that in chow-fed mice (Fig. 2C; Fig. S3B). The average NAS scores for chow-fed WT, ALR-H-HET and ALR-H-KO mice were 0, 0.67 and 2.5 and for HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice, 3.7, 4.8 and 4.7, respectively. Biochemical determinations showed greater increase in hepatic triglycerides and cholesterol in HF/HC-fed ALR-H-HET than in WT mice (Figure 2D, 2E). Increase in hepatic triglycerides was much less in ALR-H-KO mice and there was no change in cholesterol.
Serum triglycerides and cholesterol concentrations were higher in ALR-H-HET and ALR-H-KO mice than in WT mice. While serum triglycerides increased significantly in HF/HC-fed WT and ALR-H-HET mice, cholesterol increased significantly in all HF/HC-fed mice (Figure 2F, 2G).
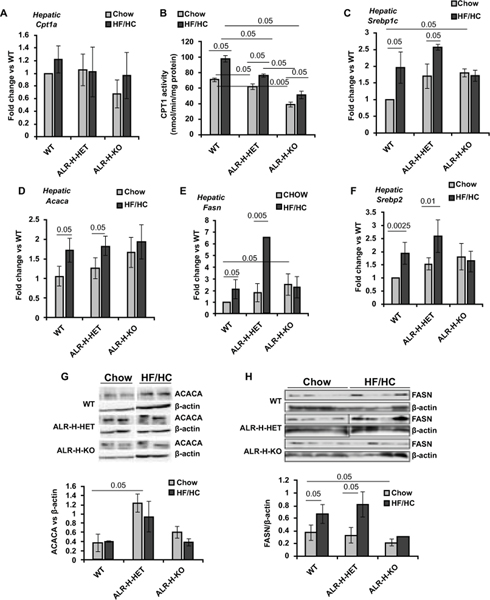
Although Hepatic CPT1a expression did not change significantly in HF/HC-fed WT, ALR-H-HET or ALR-H-KO mice (Figure 3A), CPT enzymatic activity increased significantly in all HF/HC-fed mice, the increase being greater in the WT mice (Figure 3B). Note that basal CPT activity was lower in chow-fed ALR-H-HET mice and even further reduced in ALR-H-KO mice. The expressions of SREBP1c, ACACA and FASN all increased in HF/HC-fed WT and ALR-H-HET but not ALR-H-KO mice (Figure 3C–3E). The increase in FASN expression was much greater in HF/HC-fed ALR-H-HET mice than the WT mice. SREBP2 expression increased similarly in HF/HC diet-fed WT and ALR-H-HET mice but remained unaltered in ALR-H-KO mice (Figure 3F). Western analysis showed no significant increase in ACACA protein in HF/HC-fed mice, but levels were already greater in chow-fed ALR-H-HET than in WT mice (Figure 3G). FASN protein increased in HF/HC-fed WT and ALR-H-HET mice but not in ALR-H-KO mice (Figure 3H). Together, these results indicate that hepatic lipid metabolism is compromised in HF/HC-fed mice and reflect both increased synthesis and decreased lipolysis, the magnitude being greater in ALR-H-HET than WT mice. In contrast, ALR-H-KO mice are resistant to alterations in the factors involved in lipid homeostasis.
Figure 3: Hepatic expression of enzymes of lipid homeostasis in HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice.
Mice were fed HF/HC diet for 16 weeks. (A, C, D, E, F) mRNA expression of indicated factors was measured by qRT-PCR. (B) Hepatic CPT enzymatic activity is lower in chow-fed ALR-H-HET and ALR-H-KO mice compared to WT mice. The activity increased in all HF/HC-fed groups but was still lower in ALR-H-HET and ALR-H-KO mice than in WT mice. (G) Hepatic protein expression of ACACA is greater in ALR-H-HET mice than WT mice. ACACA did not change after HF/HC feeding. (H) FASN expression increased in HF/HC-fed WT and ALR-H-HET mice but not in ALR-H-KO mice.
Insulin resistance and oxidative stress in HF/HC-fed ALR-H-HET and ALR-H-KO mice
Insulin resistance, a major feature of metabolic liver disease, contributes to steatosis and other characteristics of NAFLD11. We found similar serum insulin levels in chow-fed WT, ALR-H-HET and ALR-H-KO mice, which increased after HF/HC-feeding; the magnitude of increase was lower in ALR-H-KO mice (Figure 4A). The magnitude of insulin resistance (HOMA-IR) was highest in ALR-H-HET mice and lowest in ALR-H-KO mice (Figure 4B).
Figure 4: Glucose and pyruvate tolerance and oxidative stress in HF/HC-fed WT and ALR-deficient mice.
Mice were fed the HF/HC diet for 16 weeks. During the sixteenth week glucose or pyruvate tolerance test was performed. At the time of sacrifice, blood was drawn for insulin and glucose determinations. (A,B) Blood insulin levels and HOMA-IR. (C,D) Blood glucose at indicated times after glucose or pyruvate challenge. (E) DHE-stained liver sections (×20 magnification) and (F) ImageJ quantification demonstrating increased oxidative stress in chow-fed ALR-H-HET and ALR-H-KO mice, which increases greatly after HF/HC feeding. (G) 4HNE-stained liver sections (20× magnification) and (H) ImageJ quantification demonstrating much increased oxidative stress in chow-fed ALR-H-KO mice; 4HNE staining increased in all mice after HF/HC feeding, the magnitude being greater in ALR-H-HET and ALR-H-KO mice. (I) Malondialdehyde quantification showing already increased peroxidation in chow-fed ALR-H-HET mice compared to WT mice, which increased further after HF/HC feeding. Basal hepatic lipid peroxidation is much greater in ALR-H-KO mice and does not change after HF/HC feeding. (J) Hepatic GSH concentration in chow-fed or HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice. Abbreviations: FU, fluorescence unit; GSH, glutathione; HPF, high-power field; MDH, malondialdehyde.
Fasting blood glucose was similar in all chow-fed mice and increased modestly in WT and ALR-H-HET mice after HF/HC feeding (Figure 4C). Upon glucose challenge, blood glucose peaked at 30 min in all chow-fed mice and declined to the basal level only in WT mice at 120 min. The 30 min peak increase of blood glucose was greater in all HF/HC-fed than chow-fed mice, magnitude being higher in ALR-H-HET and ALR-H-KO mice. Glucose levels then declined in all mice, the rate being much slower in ALR-H-HET mice than the WT mice. Interestingly, glucose consumption by HF/HC-fed ALR-H-KO mice was much rapid, and blood glucose returned to basal level at 120 min.
Following pyruvate challenge, the gluconeogenic response was modestly greater in HF/HC-fed compared to chow-fed WT and ALR-H-HET mice, and blood glucose remained elevated even at 120 min; the rate of glucose clearance was somewhat slower in ALR-H-HET mice (Figure 4D). Interestingly, the initial increase in glucose in HF/HC-fed ALR-H-KO mice was of greater magnitude than in HF/HC-fed WT or ALR-H-HET mice, followed by much rapid clearance indicating high rate of glucose consumption.
Mild oxidative stress was apparent in hepatocytes of chow-fed ALR-H-HET mice as shown by increased dihydroethidium (DHE)- or 4-hydroxynonenal (4HNE) staining of the liver sections as well as malondealdehyde concentration (Figure 4E–I). High oxidative stress was already apparent in ALR-H-KO mice. HF/HC feeding strongly increased oxidative stress in ALR-H-HET mice, and while DHE staining increased robustly in ALR-H-KO mice, increases in 4HNE and malondealdehyde were modest (Figure 4E–I). Furthermore, GSH levels decreased in all HF/HC-fed mice; levels were already lower in chow-fed ALR-H-HET and ALR-H-KO mice (Figure 4J) suggesting that they are preconditioned to oxidative injury.
Increased inflammation and immune cell infiltration in HF/HC-fed ALR-deficient mice
Whereas a majority of subjects on high fat diet show steatosis, a subset progresses to steatohepatitis characterized by hepatic inflammation, and some develop fibrosis and cirrhosis. Hepatic frequency of TNFα- and IL6-expressing cells was higher in chow-fed ALR-H-HET and further increased in ALR-H-KO mice compared to WT mice (Figure 5A, 5B; Figure S4A, S4B). Their frequency increased following HF/HC feeding in WT mice and further in the ALR-H-HET mice; the increase in ALR-H-KO mice was not significant (Figure 5A, 5B; Figure S4A, 4B). CD4+IL17+ cell frequency was already higher in ALR-H-KO than WT and ALR-H-HET mice; CD4+IL17+ cells increased similarly in the WT and ALR-H-HET mice after HF/HC feeding, but no significant change occurred in ALR-H-KO mice (Figure 5C; Figure S4C). Conversely, immunosuppressive CD4+CD25+FoxP3+ regulatory T cell (Treg) frequency was lower in ALR-H-HET mice and further reduced in ALR-H-KO mice; their number decreased in all groups after HF/HC feeding but statistical significance reached only for WT mice (Figure 5D; Figure S4D). Furthermore, CD45+ leukocytes were found in the livers of chow-fed ALR-H-HET mice and even greater number in ALR-H-KO mice; following HF/HC feeding, CD45+ cell-infiltration increased in all HF/HC-fed mice, the effect being very high in ALR-H-KO and lowest in WT mice (Figure 5E; Figure S4E). Interestingly, the number of F4/80+ macrophages was greater in the ALR-H-HET and ALR-H-KO mice; F4/80+ cells increased upon HF/HC feeding in WT and ALR-H-HET but not in the ALR-H-KO mice (Figure 5F; Figure S4F). However, microgranulomas increased strongly in HF/HC-fed ALR-H-KO mice (Figure S4F). These data indicate that immune-inflammation already exists in ALR-deficient mice and HF/HC induces further increase in such inflammation.
Figure 5: Immune and inflammatory cells and inflammatory cytokines in the livers of HF/HC-fed WT and ALR-deficient mice.
Mice were fed HF/HC diet for 16 weeks. Nonparenchymal cells were isolated and following incubation with relevant antibodies were subjected to flow cytometry to identify specific cell types. Percent change in cells expressing (A) TNFα or (B) IL6, and (C) CD4+IL17+ and (D) CD4+CD25+FoxP3+ regulatory T cells after HF/HC feeding is shown. Quantification of CD45+ (E) and F4/80+ (F) cells in chow- or HF/HC-fed mouse liver. Representative histograms of (A-D) and stained sections (E and F) are shown in Supplemental Figure 4. Hepatic mRNA expression of (G) TNFα, (H) IL6, (I) IL17 and (J) IL10 was quantified by qRT-PCR in HF/HC-fed WT and ALR-deficient mice.
Consistent with the above findings, the basal TNFα expression was greater in ALR-H-HET mice, which increased further upon HF/HC feeding (Figure 5G). TNFα expression was also much greater in chow-fed ALR-H-KO mice, and increased strongly upon HF/HC feeding. IL6 expression was also greater in ALR-H-HET and ALR-H-KO mice than WT mice, and although increased in all groups after HF/HC feeding, statistical significance reached in WT mice (Figure 5H). IL17 expression increased similarly but significantly in all HF/HC-fed mice (Figure 5I). Expression of anti-inflammatory cytokine IL10 increased in HF/HC-fed WT and ALR-H-KO and showed reduction in ALR-H-HET mice (Figure 5J).
ALR-deficiency cause fibrosis/cirrhosis in ALR-deficient mice
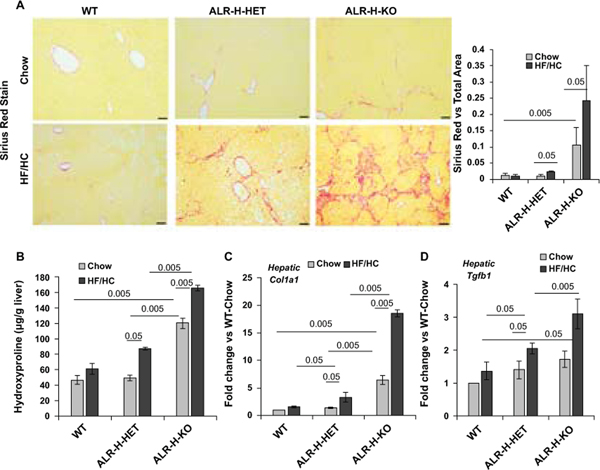
Sirius red staining showed minimal or no fibrosis in HF/HC-fed WT mice, modest fibrosis in ALR-H-HET mice and robust fibrosis or cirrhosis in ALR-H-KO mice (Figure 6A; Figure S5). Thus, fibrosis scores, based on NASH-CRN criteria19, for HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice were 0.3±0.29 (0 for chow-fed), 0.8±0.3 (0 for chow-fed) and 3.4±1.2 (0.8±0.29 for chow-fed) respectively (Table S3). Sirius red staining in HF/HC-fed ALR-H-HET and ALR-H-KO mice was consistent with increased number of hepatic stellate cells (HSCs) (Figure S6). Hydroxyproline increased in HF/HC-fed ALR-H-HET but not WT mice; basal hydroxyproline concentration was greater in ALR-H-KO mice and increased further upon HF/HC feeding (Figure 6B). Consistently, collagen 1 mRNA expression increased significantly in HF/HC-fed ALR-H-HET mice and robustly in ALR-H-KO mice (Figure 6C). The histopathological findings were supported by significantly increased hepatic expression of the potent fibrogenic cytokine TGFβ1 in HF/HC-fed ALR-H-HET and ALR-H-KO mice (Figure 6D).
Figure 6: Fibrosis in HF/HC-fed ALR-H-HET and ALR-H-KO mice.
Mice were fed HF/HC diet for 16 weeks. (A) Sirius Red staining (10X magnification) shows bridging fibrosis in ALR-H-KO mice fed chow-diet. After HF/HC feeding, ALR-H-HET mice showed moderate fibrosis whereas ALR-H-KO mice progressed to cirrhosis. No fibrosis was observed in the WT mice fed HF/HC diet. See Figure S5 for more images of Sirius Red-stained liver sections of HF/HC-fed ALR-H-HET and ALR-H-KO mice. Bar graph shows Image J quantification of Sirius Red-stained areas. (B) Hepatic TGFβ expression was already higher in chow-fed ALR-H-HET and ALR-H-KO mice. TGFβ expression did not increase in HF/HC-fed WT mice, but increased in ALR-H-HET and to even greater magnitude in the ALR-H-KO mice after HF/HC feeding. (C and D) Hepatic hydroxyproline content (major amino acid in collagens) and collagen 1 mRNA expression increased in HF/HC-fed ALR-H-HET and ALR-H-KO mice.
Lipid accumulation and inflammation in the adipose tissue of HF/HC-fed ALR-H-HET and ALR-H-KO mice
Upon examining biochemically the strong differences in the WAT of HF/HC-fed WT, ALR-H-HET and ALR-H-KO mice (Figure S1), we found increased expression of ACACA and FASN in HF/HC-fed WT and further increase in ALR-H-HET mice (Figure 7A, 7B). Note that the expressions of these enzymes were somewhat higher in chow-fed ALR-deficient compared to WT mice; expression of FASN was, however, lower in ALR-H-KO mice. HF/HC did not affect ACACA or FASN expression in ALR-H-KO mice. Expression of SREBP1c and SREBP2 was somewhat higher in chow-fed ALR-H-HET and ALR-H-KO mice (Figure 7C, 7D); while expression of SREBP1c did not change significantly after HF/HC-feeding, SREBP2 increased in WT and ALR-H-HET but not ALR-H-KO mice.
Figure 7: HF/HC-induced lipid accumulation and inflammation in WAT of ALR-H-HET and ALR-H-KO mice.
After 16 weeks of chow- or HF/HC-feeding, white adipose tissue was isolated. (A-D) mRNA expression of the enzymes of lipid homeostasis (ACACA, FAS, SREBP1c and SREBP2). (E) CD45-stained WAT sections (20X magnification) showing already greater number of leukocytes in ALR-H-HET and ALR-H-KO mice that increased further after HF/HC feeding. (F-G) Expression of TNFα but not IL6 increased in HF/HC-fed WT mice; both cytokines increased in HF/HC-fed ALR-H-HET and ALR-H-KO mice, the change being much greater in the latter. (H) Anti-inflammatory IL10 increased in all groups after HF/HC feeding but the magnitude of increase was much greater in the WT mice. (I) Leptin expression increased strongly in HF/HC-fed WT and ALR-HET mice and to a lower magnitude in ALR-H-KO mice.
Visceral adipose tissue inflammation is considered critical to the development of NAFLD/NASH11. Consistently, markedly higher number of CD45+ cells was found in the WAT of HF/HC-fed ALR-H-HET mice compared to the WT mice (Figure 7E). WAT of ALR-H-KO mice already had increased number of CD45+ cells that increased further after HF/HC feeding (Figure 7E). WAT-expression of TNFα and IL6 increased strongly in HF/HC-fed ALR-H-HET and ALR-H-KO mice, the magnitude being greater in ALR-H-KO mice (Figure 7F, 7G). In contrast, greater increase in the expression anti-inflammatory IL10 occurred in HF/HC-fed WT mice compared to ALR-H-HET and ALR-H-KO mice (Figure 7H). Leptin expression increased in all HF/HC-fed mice, the increase being much higher in WT and ALR-H-HET compared to ALR-H-KO mice (Figure 7I), which is consistent with the notion that fat-engorged adipocytes express increased leptin22.
Liver-WAT cross-signaling in HF/HC-fed ALR-deficient mice
Cross-communication between the liver and adipose tissue is critically important in pathogenesis of NAFLD. While TNFα and IL6 (produced in both organs) have been implicated in insulin resistance, evidence indicates possible roles of lipolytic fibroblast growth factor-21 (FGF21), and pro-inflammatory fetuin A (encoded by alpha 2-HS glycoprotein [AHSG]) and angiopoietin-like protein 3 (ANGPTL3). ANGPTL3 also has lipoprotein lipase inhibitory activity23. We found strongly increased hepatic FGF21 expression in HF/HC-fed WT and ALR-H-HET mice and modest increase in ALR-H-KO mice (Figure S7A). In contrast, WAT-expression of FGF21 increased strongly in ALR-H-KO and modestly in WT and ALR-H-HET mice (Figure S7B). Hepatic AHSG expression increased modestly in HF/HC-fed WT and ALR-H-HET mice but not ALR-H-KO mice; AHSG expression increased strongly in the WAT of WT and ALR-H-HET mice, and modestly ALR-H-KO mice (Figure S7C, S7D). Interestingly, hepatic ANGPTL3 expression, which was greater in chow-fed ALR-H-HET mice, decreased after HF/HC feeding; no change occurred in WT or ALR-H-KO mice (Figure S7E). In contrast, WAT ANGPTL3 expression was strongly up-regulated in HF/HC-fed WT and ALR-H-HET mice but reduced in ALR-H-KO mice (Figure S7F).
HF/HC feeding-induced NAFLD in ALR-deficient male mice
Since WT male mice are more susceptible to develop NAFLD than the female mice17, it was of interest to know whether hepatocyte-ALR deficiency predisposes female mice to aggressive NASH. Indeed, it was found to be such as demonstrated in Figures 1–7. When subjected to HF/HC diet for 16 weeks, similar (some at greater magnitude) pathological changes occurred in male ALR-H-HET and ALR-H-KO mice as in females (Figures S8 and S9).
ALR is reduced in human NASH and NASH-ESLD
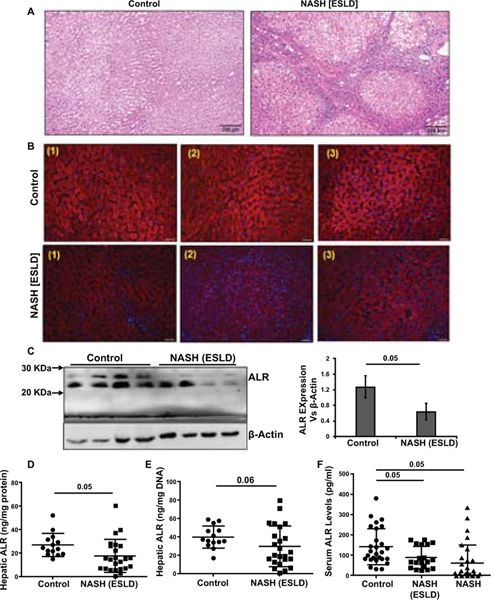
To determine whether the mouse data are relevant to human NAFLD, we analyzed ALR in the liver and serum of patients with NASH-induced ESLD (Table S4) and serum of NASH patients (Table S5). Histopathology demonstrated sporadic and often no lipid accumulation in the livers of NASH-ESLD patients (Figure 8A). IHC showed very low ALR protein expression in most areas although in some regions the intensity of ALR staining was quite high (Figure 8B; Figure S10). This observation was consistent with significantly lower hepatic ALR as determined by Western analysis and ELISA in NASH-ESLD than in control subjects (Figure 8C–E). The low levels of hepatic ALR in NASH-ESLD patients translated into lower serum ALR (Figure 8F). Interestingly, serum ALR was also significantly lower in NASH patients (Figure 8F) (details including serum-ALR in individual patients are shown in Tables S4 and S5) suggesting that ALR deficiency may be predisposing factor for progression of NAFLD to NASH and NASH-ESLD. Three NASH-ESLD patients had high (380, 272 and 288 pg/ml) serum ALR levels and these values are not shown, but high serum ALR seen in NASH patients are shown in Figure 8F.
Figure 8: ALR in human NASH-ESLD and NASH.
(A) The liver tissue of a liver transplant patient shows cirrhosis due to NASH. Note only sporadic lipid deposition. (B) So-called normal portions of resected liver tissue from 3 subjects with focal tumor show abundant ALR as determined by IHC. The NASH-cirrhotic liver, in contrast has much lower ALR expression in various portions of the tissue. Additional images are shown in Figure S10. (C) Western blot confirms low ALR expression in NASH-cirrhosis. (D and E) ELISA data showing tissue ALR concentration per mg protein (D) or mg DNA. (F) Serum ALR in normal control, NASH-ESLD (cirrhosis) and NASH subjects. We note that 3 NASH-ESLD patients had much higher hepatic ALR levels (380, 272 and 288 pg/ml) and these values are excluded from the graph.
DISCUSSION
Our investigation demonstrates for the first time that hepatic deficiency of ALR plays a major role in driving NAFLD progression from simple steatosis to nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis. The otherwise normal ALR-deficient ALR-H-HET mice gained more weight than WT mice during HF/HC feeding. HF/HC-fed ALR-H-KO mice with ALR levels similar to the ALR-H-HET mice gained minimal weight. Since HF/HC diet is enriched with carbohydrate (both solid pellets and drinking water) more than fat, de novo lipogenesis (DNL) appears to be equally if not more important in the weight gain for WT and ALR-H-HET mice24,25. Underlying pathology and inability of ALR-H-KO mice to increase enzymes of DNL therefore may be the plausible reasons for much less lipid accumulation both in the liver and adipose tissue. Higher weight gain by HF/HC-fed ALR-H-HET mice was associated with greater hepatic steatosis than WT mice. ALR-H-KO mice developed minimal steatosis but progressed to cirrhosis, which is similar to human NASH-induced cirrhosis with minimal or no steatosis26,27 (Figure 8). Of note, ALR-H-KO mouse develops spontaneous and excessive steatosis within 2 weeks of life, which regresses with time with appearance and expansion of ALR-expressing hepatocytes, but the mouse shows modest inflammation and fibrosis throughout its life7. Such undiagnosed underlying pathology may drive silent progression of the fatty liver disease to cirrhosis in humans. Even HF/HC-fed ALR-H-HET mice with apparently normal function and health, despite lower than normal ALR levels since early life, demonstrated NASH-like fibrosis. These data suggest that ALR deficiency may be a critical predisposing factor for progressive NASH. Indeed, hepatic and serum ALR levels are significantly lower in NASH-ESLD and serum of NASH patients. Importantly all HF/HC-fed mice showed similar mobility as chow-fed mice throughout the experimental period. Thus, although sedentary lifestyle is considered a major contributor for NAFLD/NASH in humans, development of aggressive disease in HF/HC-fed ALR-deficient mice exemplifies importance of ALR in resisting NAFLD progression.
Hepatic ALR decreased significantly in HF/HC-fed WT mice, and the decrease was even more pronounced in ALR-H-HET and ALR-H-KO mice, which already had lower basal ALR than WT mice. We previously observed reduced ALR expression in steatotic liver (e.g., in alcohol-fed or ob/ob mice) and in hepatocytes exposed to free fatty acids in vitro7,28. Serum ALR was also similarly lower in ALR-H-HET and ALR-H-KO mice than WT mice, and although increased after HF/HC feeding, was still significantly lower than WT mice. Because the liver (hepatocytes) is the primary source of circulating ALR1,20,29, these data and lower than normal serum ALR in human NASH suggest that ALR can be a diagnostic marker for NASH or predisposition to NASH.
Although CPT1a regulates hepatic lipid content by its role in mitochondrial uptake and β-oxidation of fatty acids, increased DNL, as evident from increased triglyceride synthesis, accounts for steatosis in NAFLD30. SREBP-1c regulates the expression of lipogenic enzymes ACACA and FASN and consequently DNL31–33. Although no significant change occurred in CTP1a expression in HF/HC-fed mice, the enzymatic activity increased and was greater in WT than ALR-deficient mice, which is consistent with increased fatty acid oxidation in NAFLD34. Thus increased levels of SREBP1c, ACACA and FASN as well as SREBP2 (regulates cholesterol synthesis) all in HF/HC-fed ALR-H-HET compared to the WT mice indicate that increased lipogenesis and relatively lower lipolysis (CPT activity) are responsible for greater steatosis. The expression of these factors was somewhat higher in chow-fed ALR-H-KO mice, but it did not change significantly after HF/HC feeding. Thus, higher plasma concentration of lipids in chow-fed ALR-H-HET and ALR-H-KO mice might be due to increased lipogenic enzymes and DNL.
Although mitochondrial dysfunction, and consequent oxidative stress, has been suggested to be critical to NAFLD pathogenesis14,34,35, it is unclear whether it is causal or consequence of the disease progression. Our data suggest that the subnormal but physiologically adequate mitochondrial function of ALR-H-HET mice (lower ATP levels: data not shown) predisposes the liver to greater HF/HC-induced injury due to significantly higher oxidative stress and peroxidation than WT mice.
A significant number of patients with NAFLD develop insulin resistance causing abnormal hepatic and adipose tissue carbohydrate and lipid metabolism. Interestingly, insulin resistance increased similarly in HF/HC-fed WT and ALR-H-HET mice, and to lower magnitude in ALR-H-KO mice. However, HF/HC-fed ALR-H-HET mice were unable to clear glucose as efficiently as WT mice; in contrast, HF/HC-fed ALR-H-KO mice cleared glucose more rapidly. PTT also demonstrated somewhat reduced glucose clearance by HF/HC-fed ALR-H-HET mice and much enhanced clearance by ALR-H-KO mice. It is proposed that cross talk between macrophages and steatotic hepatocytes via inflammatory cytokines (TNFα and IL6) imparts insulin resistance and regulates glucose homeostasis as NAFLD progresses36. Intriguingly, ALR-H-HET mice demonstrated mild hepatic inflammation evidenced by increased number of TNFα- and IL6-expressing cells, as well as CD45+ cells and F4/80+ macrophages. Inflammation increased in ALR-H-HET mice after HF/HC-feeding with further rise in TNFα- and IL6-expressing cells as well as CD45+ cells and F4/80+ macrophages. Such heightened inflammation in the liver as well as WAT of HF/HC-fed ALR-H-HET mice might be a reason for greater resistance to clear exogenously administered and endogenously generated glucose (via gluconeogenesis). However, it is interesting that ALR-H-KO mice with greater inflammation both in the liver and WAT are able to clear glucose much efficiently. Perhaps the energy need for the ALR-H-KO mice is greater and because of inability to store lipids, they adapt to the pathological changes and utilize glucose more rapidly.
Previous studies have shown that male gender17 or thermosneutral environment37 favors HF/HC-induced liver fibrosis. Thus an important finding of our investigation is the development of liver fibrosis in HF/HC-fed ALR-H-HET female mice housed in standard hypothermic (22ºC) environment. It is interesting that ALR-H-HET mice do not show any obvious sign of injury and fibrosis when on chow-diet despite greater than normal expression of TNFα and IL6, increased population of leukocytes and macrophages, and mildly increased oxidative stress. HF/HC-fed WT mice showed mildly increased hepatocellular oxidative stress and inflammation, but no fibrosis. Thus a plausible explanation for the normal liver structure and function of ALR-H-HET mice is that they adapt to such changes resisting pathological development, but remain primed to respond to additional stress as evident from significantly higher HF/HC-induced oxidative stress and inflammation than WT mice. Such environment is amenable to activation of hepatic stellate cells, the main fibrogenic cells in the liver, and their response to cytokines such as TGFβ38,39. While the endogenously generated injury-induced inflammation plays an essential role in liver fibrogenesis, inflammatory cytokines from WAT promote such progression in NAFLD. Indeed, the magnitude of inflammation in WAT of HF/HC-fed ALR-H-HET mice was greater (increased number of CD45+ cells), and although TNFα expression was similar, IL6 and leptin expression was greater in HF/HC-fed ALR-H-HET than WT mice. Interestingly, hepatic and WAT expression of anti-inflammatory IL10 expression increased HF/HC-fed WT mice but not ALR-H-HET mice, and this may be a cause of reduced injury in WT mice but not in the ALR-deficient mice.
Previously, we found that only 4 weeks of alcohol (Lieber de Carli)-supplemented diet produced cirrhosis in ALR-H-KO mice28. Thus, it was not surprising that HF/HC-fed ALR-H-KO mice progressed to cirrhosis, with very strong increase in TNFα and TGFβ. The underlying inflammation, injury and fibrosis, that remain modestly elevated in ALR-H-KO mice until about 9 months (about 40 weeks)7, increased excessively upon HF/HC feeding from 8 weeks till 24 weeks along with hepatocellular necrosis, oxidative stress and leukocyte infiltration. Interestingly, F4/80+ macrophage population did not change but numerous microgranulomas (Kupffer cell aggregates) could be seen within the lobules (Figure S4F), which is consistent with influx/activation of macrophages as essential component of NAFLD pathogenesis36,40.
The mice used in this study were hepatocyte-specific ALR-deficient. Thus it is not surprising to see excessive pathology progression in the liver after HF/HC feeding. However, much higher lipid deposition in WAT of HF/HC-fed ALR-H-HET mice and increased inflammation suggest that signaling from the liver could be promoting these changes. In this regard, liver-derived FGF21, fetuin-A and ANGPTL3 have been implicated in WAT pathogenesis23. However, hepatic expression of lipolytic FGF21 increased similarly in HF/HC-fed WT and ALR-H-HET mice, and anti-lipolytic fetuin-A increased modestly. Increase in FGF21 expression was modest and fetuin-A expression did not change after HF/HC feeding in ALR-H-KO mice. Furthermore, hepatic expression of pro-inflammatory ANGPTL3 tended to decrease in all HF/HC-fed mice. Although this may rule out the role of these factors derived from liver in WAT pathogenesis, their local generation appears to be important. FGF21 increased modestly, but fetuin-A increased strongly in the WAT of HF/HC-fed WT and to somewhat greater extent in ALR-H-HET mice perhaps explaining greater lipid accumulation. This suggestion is supported by much greater increase in FGF21 and very modest increase in fetuin-A expression in the WAT of HF/HC-fed ALR-H-KO mice. Surprisingly, ANGPTL3 expression was unaltered in HF/HC-fed ALR-H-KO mice and increased strongly but similarly in WT and ALR-H-HET mice. It remains to be determined which liver-derived circulating mediator(s) promotes WAT pathology in ALR-deficient mice.
In conclusion, our pre-clinical data implicating ALR deficiency as a potentially major determinant of NASH progression is supported strongly by significantly decreased ALR in human NASH-ESLD. Our data suggest that, similar to ALR-H-HET and ALR-H-KO mice, ALR deficiency may be a major determinant of accelerated progression of NAFLD to end-stage liver disease in humans, and serum ALR (as indicated by its reduced levels in NASH) as a biomarker of such predisposition. However, lack of information about whether lower serum ALR is due to reduced hepatic synthesis of the protein, and if so is it due to transcriptional or translational defect(s) is a limitation of our study. It will be critical to know how ALR gene is regulated, whether defect(s) in ALR gene is responsible for the protein deficiency, and/or endogenously produced factors inhibit its synthesis. These and precise knowledge of the mechanisms by which ALR regulates lipid homeostasis will be necessary to understand its role in (preventing) NAFLD progression. The implications of very high serum ALR in some NASH and NASH-ESLD subjects in the disease pathophysiology also require further investigation.
Supplementary Material
Acknowledgments
Financial Support: W81XWH-14-PRMRP-IIRA and VA Merit Review Award 1IO1BX001174 to CRG; NIH R01DK100314 to RK; NIH P30 DK078392 to the Digestive Diseases Health Center at Cincinnati Children’s Hospital Medical Center.
Abbreviations:
- ACACA
acetyl CoA carboxylase
- AHSG
Alpha 2-HS glycoprotein
- ALR
augmenter of liver regeneration
- ALR-H-HET
hepatocyte-specific ALR-heterozygous
- ALR-H-KO
hepatocyte-specific ALR-knockout
- ANGPTL3
angiopoietin-like protein 3
- CPT
carnitine palmitoyltransferase
- DHE
dihydroethidium
- DNL
de novo lipogenesis
- ESLD
end-stage liver disease
- FASN
fatty acid synthase
- FGF21
fibroblast growth factor-21
- GFER
growth factor ERV1 homolog
- HF/HC
high fat and high carbohydrate diet
- HNE
hydroxynonenal
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- IL
interleukin
- KO
knockout
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- SREBP
Sterol regulatory element-binding protein
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- Trig
regulatory T cells
- WAT
white adipose tissue
- WT
wild type
Footnotes
Conflict of interest: None of the authors participated in this study have anything to disclose regarding conflict of interest.
REFERENCES
- 1.Gandhi CR, Kuddus R, Subbotin VM, Prelich J, Murase N, Rao AS, Nalesnik MA, et al. A fresh look at augmenter of liver regeneration in rats. Hepatology 1999;29:1435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi CR. Augmenter of liver regeneration. Fibrogenesis Tissue Repair 2012;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirunavukkarasu C, Wang LF, Harvey SA, Watkins SC, Chaillet JR, Prelich J, Starzl TE, et al. Augmenter of liver regeneration: an important intracellular survival factor for hepatocytes. J Hepatol 2008;48:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francavilla A, Hagiya M, Porter KA, Polimeno L, Ihara I, Starzl TE. Augmenter of liver regeneration: its place in the universe of hepatic growth factors. Hepatology 1994;20:747–57. [PubMed] [Google Scholar]
- 5.Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, et al. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA 1994;91:8142–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giorda R, Hagiya M, Seki T, Shimonishi M, Sakai H, Michaelson J, et al. Analysis of the structure and expression of the augmenter of liver regeneration (ALR) gene. Molecular Medicine 1996;2:97–108. [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi CR, Chaillet JR, Nalesnik MA, Kumar S, Dangi A, Demetris AJ, Ferrell R, et al. Liver-Specific Deletion of Augmenter of Liver Regeneration Accelerates Development of Steatohepatitis and Hepatocellular Carcinoma in Mice. Gastroenterology 2015;148:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P, et al. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005;129:113–21. [DOI] [PubMed] [Google Scholar]
- 9.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- 10.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95 [DOI] [PubMed] [Google Scholar]
- 11.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- 13.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology 2002;122:1649–57. [DOI] [PubMed] [Google Scholar]
- 14.Kohli R, Pan X, Malladi P, Wainwright MS, Whitington PF. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. J Biol Chem. 2007;282:21327–36. [DOI] [PubMed] [Google Scholar]
- 15.Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, Lill R. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Rep 2001;2:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell SR, Thorpe C. Augmenter of liver regeneration: a flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemistry 2005;44:1532–41. [DOI] [PubMed] [Google Scholar]
- 17.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010;52:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, Kohli R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014;34:427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic steatohepatitis: Pathology and pathogenesis. Annu Rev Pathol 2010;5:145–71. [DOI] [PubMed] [Google Scholar]
- 20.Vodovotz Y, Prelich J, Lagoa C, Barclay D, Zamora R, Murase N, Gandhi CR. Augmenter of Liver Regeneration (ALR) is a novel biomarker of hepatocellular stress/inflammation: In vitro, in vivo, and in silico studies. Mol Med 2013;18:1421–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arisqueta L, Navarro-Imaz H, Labiano I, Rueda Y, Fresnedo O. High-fat diet overfeeding promotes nondetrimental liver steatosis in female mice. Am J Physiol Gastrointest Liver Physiol. 2018;315:G772-G780 [DOI] [PubMed] [Google Scholar]
- 22.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395(6704):763–70. [DOI] [PubMed] [Google Scholar]
- 23.Scheja L, Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. J Hepatol. 2016;64:1176–86. [DOI] [PubMed] [Google Scholar]
- 24.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 2008;138:1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR et al. 2004. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72 [DOI] [PubMed] [Google Scholar]
- 26.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 27.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29;664–69. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Wang J, Rani R, Gandhi CR. Hepatic Deficiency of Augmenter of Liver Regeneration Exacerbates Alcohol-Induced Liver Injury and Promotes Fibrosis in Mice. PLoS One 2016;11:e0147864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi CR, Murase N, Subbotin VM, Uemura T, Nalesnik M, Demetris AJ, Fung JJ, et al. Portacaval shunt causes apoptosis and liver atrophy despite increases in endogenous levels of major hepatic growth factors. J Hepatology 2002; 37:340–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997;89:331–40. [DOI] [PubMed] [Google Scholar]
- 32.Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans. 2002;30(Pt 6):1091–5. [DOI] [PubMed] [Google Scholar]
- 33.Soyal SM, Nofziger C, Dossena S, Paulmichl M, Patsch W. Targeting SREBPs for treatment of the metabolic syndrome. Trends Pharmacol Sci 2015;36:406–16. [DOI] [PubMed] [Google Scholar]
- 34.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120:1183–92. [DOI] [PubMed] [Google Scholar]
- 35.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol 2005;42:928–40. [DOI] [PubMed] [Google Scholar]
- 36.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009;51:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 2017;23:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425–56. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa D, Wallace MC, Friedman SL. Stellate cells and hepatic fibrosis. In: Gandhi CR, Pinzani M, eds. Stellate Cells in Health and Disease. Elsevier; 2015:41–62. [Google Scholar]
- 40.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem 2012;287:40161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.