Abstract
Aim:
To identify the molecular mechanisms mediating the persistent defensive functions of the self-renewing junctional epithelium (JE).
Materials and methods:
Two strains of Wnt reporter mice, Axin2CreErt2/+;R26RmTmG/+ and Axin2LacZ/+, were employed, along with three clinically relevant, experimental scenarios where the function of the JE is disrupted: after tooth extraction, after a partial gingivectomy and after a complete circumferential gingivectomy.
Results:
Using transgenic Wnt reporter strains of mice, we established the JE is a Wnt-responsive epithelium beginning at the time of its formation, and that it maintains this status into adulthood. After tooth extraction, progeny of the initial Wnt-responsive JE population directly contributed to healing, and ultimately adopted an oral epithelium (OE) phenotype. In the traditional partial gingivectomy model, the JE completely regenerated and did so via progeny of the original Wnt-responsive population. However, following circumferential gingivectomy, the OE was incapable of reestablishing a functional JE.
Conclusions:
A Wnt-responsive niche at the interface between tooth and oral epithelia is required for a functional JE.
Keywords: gingiva, epithelial attachment, gingivectomy, Wnt-responsive, oral epithelium
Introduction
One of the primary functions of epithelia is protection: this layer of closely aggregated cells covers most body surfaces and lines most body cavities and thus serves as the first line of defense against pathogen invasion (Presland & Dale, 2000; Schroeder & Listgarten, 2003). When this barrier function is compromised, a host of diseases result. In the oral cavity, some of the epithelia are keratinized, some have specialized sensory organs and others produce mucus; all oral epithelia, however, share a barrier function.
Of all the oral epithelia one of the most interesting to us is the epithelium which forms an attachment to teeth. Multiple features distinguish this junctional epithelium (JE) from neighboring oral epithelia (OE): namely, its non-keratinized nature (Atsuta et al., 2005; Schroeder & Listgarten, 1997), the presence of large intercellular spaces its unique attachment to enamel and cementum (M. A. Listgarten, 1966), and its high mitotic activity (Skougaard, 1965). The formation of the JE occurs simultaneously with tooth eruption. Prior to penetration of the tooth into the oral cavity, the enamel epithelium flattens and transitions into a structure called the reduced enamel epithelium (REE). As the tooth breaches the OE, the REE fuses with it and transforms through as-yet-unknown mechanisms into a JE (reviewed in (Shimono et al., 2003)).
It is generally thought that the JE is gradually replaced by cells from the OE (Yajima-Himuro et al., 2014) and that following its surgical removal, the JE can rapidly reform from the OE (Kato et al., 2019; M. Listgarten, 1972; M. A. Listgarten, 1967; Masaoka et al., 2009; Wazen et al., 2015). The mechanisms by which OE cells transition into JE cells and adopt the adhesive functions and high mitotic index that characterize this unique barrier epithelium are largely unknown. If one understood this process in detail then theoretically, we could induce the formation of a new, functionally equivalent JE after diseases e.g., periodontitis or peri-implantitis.
In previous, we identified a Wnt-responsive stem cell population in the OE (Yuan et al., 2019). We wondered whether Wnt signaling is involved in the formation and/or homeostasis of the JE. We utilized Wnt reporter mice to identify Wnt responsive cells during JE formation, then follow the fate(s) of Wnt responsive cells after injury. We set to determine whether Wnt-responsive cells contribute to a newly forming JE. In doing so, we uncovered the unique characteristics of the JE that were neither replicated nor functionally replaced by the OE.
Method and Materials
Animals
All experimental protocols were approved by the Stanford Committee on Animal Research (protocol #13146). Axin2LacZ/+ mice express lacZ that is under control of the promoter for the Wnt target gene Axin2. Xgal staining faithfully reflects the Axin2 expression pattern, therefore used to monitor endogenous canonical Wnt signals (Al Alam et al., 2011; Lustig et al., 2002). Axin2CreERT2/+; R26RmTmG/+ mice were used for lineage tracing (Yuan et al., 2019). In this strain, Cre expression is under the control of the promoter of the Wnt target gene, Axin2. Exposure to tamoxifen triggers a recombination event that causes Axin2-positive cells to express GFP; hence these cells were referred to as Wnt-responsive cells. After tamoxifen is cleared from the body (~24h), any increase in the number of GFP positive (GFP+ve) cells reflects an expansion in the original Wnt- responsive population; hence these cells are referred to as progeny or descendants of Wnt-responsive cells. Tamoxifen (T5648, Sigma) was dissolved in ethanol and then diluted with sunflower seed oil to 10 mg/mL. A single dose of tamoxifen (5 mg/25 g body weight) was delivered intraperitoneally to Axin2CreERT2/+;R26RmTmG/+ mice to label Wnt-responsive cells.
Surgeries
Tooth extractions were performed on 2-month old mice as described (Yuan et al., 2018). Mice were anesthetized with Ketamine (80 mg/kg) and Xylazine (16 mg/kg), followed by subcutaneous injections of Buprenorphine (0.1 mg/kg). The mouth was rinsed using saline; bilateral removal of maxillary first molars (mxM1s) was achieved using fine forceps (Dumont #7, Fine Science Tools Inc., CA). Bleeding was controlled by local compression. Mice recovered in a controlled, heated environment. Healing was monitored for 2-week period - no signs of infection or prolonged inflammation were observed.
Unilateral partial gingivectomies were performed on 3-month old mice as described (Masaoka et al., 2009; Wazen et al., 2015). Following anesthesia, the palatal gingiva, extending from the maxillary first (mxM1) to third molars (mxM3), was excised using a scalpel blade (#11, Fine Science Tools Inc., CA). To fully eliminate JE, mxM2s on the left side were extracted from 2-month old mice. After healing for one week, the gingiva surrounding mxM1s was fully removed using a scalpel blade. Bleeding was controlled by local compression. Pain control was assured by delivery of Buprenorphine. Mice recovered in a controlled and heated environment. Gingival healing was monitored throughout a 2-week period, with no evidence of prolonged inflammation.
Detailed sample preparation, histology, immunohistochemistry, EdU injections and staining, and Xgal staining protocols can be found in the supporting information.
Results
The JE originates from Wnt-responsive cells and maintains the Wnt-responsiveness
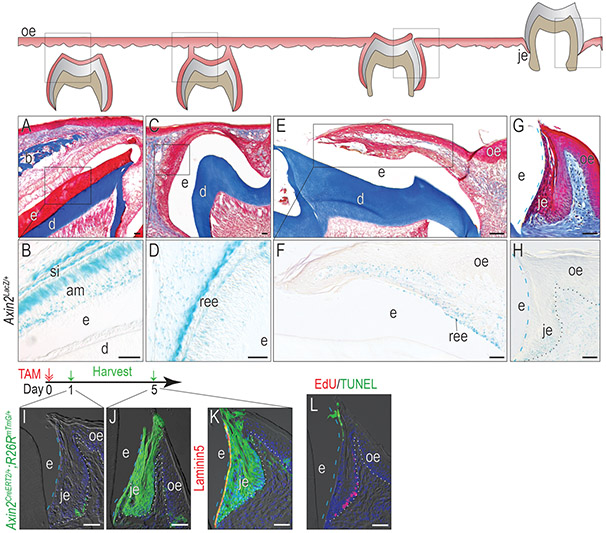
Molar tooth eruption begins around post-natal day 15 (P15) in mice, simultaneous with the formation of the JE around these teeth. We began analyses on tissues from post-natal day 9 (P9) Axin2LacZ/+ pups, where the Wnt-responsive status of ameloblasts and stratum intermedium (SI) was demonstrated by Xgal staining (Fig. 1A,B). By P14, reduced ameloblasts along with SI derived papillary layer formed the reduced enamel epithelium (REE, Fig. 1C); REE cells retained their Wnt-responsive status (Fig. 1D). By P15, when the tooth penetrated into the oral cavity, the flattened REE cells transitioned into a JE (Fig. 1E), which was also Wnt-responsive (Fig. 1F). Into adulthood e.g., 3 months of age, the fully formed JE maintained its Wnt-responsive state (Fig. 1G,H). Therefore, the Wnt-responsive status of the JE was established at its inception.
Figure 1. The JE originates from Wnt-responsive cells and maintains the Wnt-responsiveness.
(A) Masson’s trichrome staining showing the OE covering the unerupted maxillary first molar on P9. (B) In Axin2LacZ/+ mice, Xgal staining showing Xgal+ve Wnt-responsive cells in the JE on P9. (C) By P13, the enamel was completely formed, and the inner enamel epithelium reduced to a few layers of flat cuboidal cells called REE. At this stage, the REE started to fuse with OE. (D) Xgal staining showing Xgal+ve Wnt-responsive cells on P13. (E) On P14, the maxillary first molar penetrated to the oral cavity and the JE started to form. (F)Xgal staining showing Xgal+ve Wnt-responsive cells on P14. (G) Masson’s trichrome staining of the first maxillary molar in adult mice. (H) Xgal staining showing Wnt-responsive cells in adults. One dose of tamoxifen was given to adult Axin2CreERT2/+;R26RmTmG/+ mice and GFP+ve cells were analyzed (I) 1 day and (J) 5 days later. (K) Co-staining of 5-day chase GFP (green) with Laminin 5 (red) to show the attachment to the tooth surface. (L) TUNEL staining (green) showing apoptosis and EdU staining (red) showing cell proliferation in the JE.
Dashed blue lines indicate the shape of enamel. Dotted black and dotted white lines indicate the demarcation between the epithelium and the connective tissue. Abbreviations: e, enamel space; je, junctional epithelium; oe, oral epithelium; b, bone; d, dentin; ree, reduced enamel epithelium; am, ameloblasts. Blue dashed lines indicate the edge of the enamel and white dotted lines indicate the boundary between the epithelium and the connective tissue. Scale bars: 50μm.
The expression of β-galactosidase, and its detection by Xgal staining in Axin2LacZ/+ mice, establishes whether cells are responding to an endogenous Wnt signal. To follow the fate of these Wnt-responsive cells, we employed a lineage tracing strain Axin2CreERT2/+ ;R26RmTmG/+, in which GFP expression is under tamoxifen-inducible control of the Axin2 promoter. One day after tamoxifen injection, GFP+ve, Wnt-responsive cells were located at the base of the JE (Fig. 1I). After a 5-day chase, progeny of the initial Wnt-responsive cells populated the entire JE (Fig. 1J).
JE attaches to the enamel surface with hemidesmosomes mainly consisting of Laminin5 (Oksonen, Sorokin, Virtanen, & Hormia, 2001), and co-staining of Laminin5 with GFP showed that the progeny of Wnt-responsive cells maintained the attachment of the JE to the tooth (Fig. 1K). This dramatic expansion in the number of GFP+ve cells was in keeping with the high rate of cell proliferation revealed by EdU staining (Fig. 1L). TUNEL staining in the crestal-most cells suggested that cell turnover of the JE involved programmed cell death (Fig. 1L).
Collectively, these data demonstrate that from its inception, the JE originates from Wnt-responsive cells. Descendants of these Wnt-responsive cells contribute to the JE attachment to teeth and maintain the JE structure.
After tooth extraction, the Wnt-responsive JE disappears
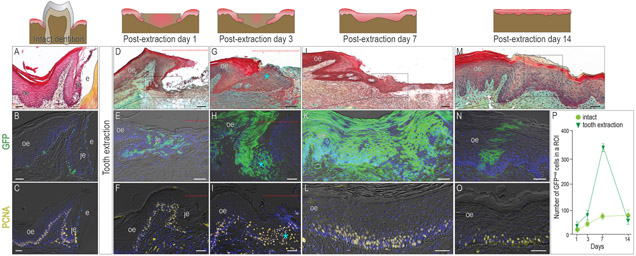
Since the JE forms with tooth eruption, we wondered whether the tooth was required to maintain its structure. Using morphologic and molecular features of an intact JE as a guide (Fig. 2A-C), the fate of the JE after tooth extraction was examined. Within 24h of tooth removal, remnants of the Wnt-responsive JE began to proliferate (Fig. 2D-F). By post-extraction day 3, the JE remnant was dramatically larger (asterisk, Fig. 2G) and comprised predominantly of proliferating progeny from the Wnt-responsive population (Fig. 2H,I).
Figure 2. After tooth extraction, the Wnt-responsive JE disappears.
In (A) an intact dentition, (B) one-day lineage tracing reveals a few Wnt-responsive cells in the JE and OE. (C) PCNA staining showing the proliferative cells in the OE and JE. One day after tooth extraction, (D) the JE remnant, (E) the progeny of Wnt-responsive cells, and (F) the cell proliferation were analyzed. By post-extraction day 3, (G) the enlarged JE, (H) burst of the progeny of Wnt-responsive cells, and (I) massive cell proliferation were observed. By post-extraction day 7, (J) the re-epithelization of the extraction socket, (K) the distribution of the Wnt-responsive progeny, and (L) the cell proliferation were analyzed. By post-extraction day 14, (M) the re-epithelization of the extraction socket, (N) the distribution of Wnt-responsive progeny, and (O) the cell proliferation were analyzed. (P) Quantification of Wnt-responsive cells (n=4). The data were expressed as mean ± SD.
Dotted Red lines indicate the extraction site. Dotted white lines indicate the demarcation between the epithelium and the connective tissue. Dashed blue lines indicate the shape of enamel. Abbreviations: je, junctional epithelium; oe, oral epithelium; e, enamel space. Scale bars: 50 μm.
By post-extraction day 7, re-epithelialization of the extraction site was nearly complete (Fig. 2J). This re-epithelialization was achieved by the robust proliferation of the initial Wnt-responsive population (Fig. 2K,L; quantified in P). By post-extraction day 14, the new epithelium had adopted all of the characteristics of an OE: its keratinization, multilaminar structure was now populated by individually Wnt-responsive clones which originated from the proliferating basal lamina (Fig. 2M-O; quantified in P).
Taken these together, the JE forms when the tooth penetrates the OE, disrupting the previously continuous epithelial tissue in the oral cavity. Following tooth extraction, the JE disappears and the continuity of epithelial tissue above the socket is restored.
After partial gingivectomy, a new JE is regenerated with an original Wnt-responsive population
The historic literature states that the JE can regenerate, and more recent studies suggest that it does so via contributions from the adjacent OE (Kato et al., 2019; Nakamura, 2018). The OE and JE, however, are histologically distinct since the former is keratinized and the latter is not (Fig. 3A). A gingivectomy was performed, in which the palatal JE and adjacent OE were surgically removed (Fig. 3B and supplementary Fig. 1A).
Figure 3. After a partial gingivectomy, a regenerated JE re-establishes its Wnt-responsiveness.
Pentachrome staining was used to analyze the morphology with the samples from (A) intact, post-GV day (B)1, (C) 3, (D) 5, (E) 7, and (F) 14. (G) One dose of tamoxifen was given to Axin2CreERT2/+;R26RmTmG/+ mice and GFP+ve cells were analyzed 5 days later. (H) EdU staining was performed to visualize proliferating cells. On post-GV day 1, (I) the progeny of Wnt-responsive cells, and (J) the cell proliferation were analyzed. On post-GV day 3, (K) the progeny of Wnt-responsive cells, and (L) the cell proliferation were analyzed. On post-GV day 5, (M) the progeny of Wnt-responsive cells, and (N) the cell proliferation were analyzed. On post-GV day7, (Q) the progeny of Wnt-responsive cells, (P) the cell proliferation, and (Q) Laminin 5 expression were analyzed. On post-GV day 14, (R) the progeny of Wnt-responsive cells and cell proliferation, and (S) Laminin 5 expression were analyzed. (T) An illustration of the healing process after a gingivectomy. (U) Pentachrome staining showing the two epithelial populations co-existed on post-GV day 3. The blue arrow points the non-keratinized epithelium tissue around the tooth. (V) lineage tracing shows two GFP+ve populations co-existed on post-GV day 3. The green star indicates the GFP+ve population around the tooth. (W) An illustration showing both JE and OE contribute to the regeneration after a gingivectomy.
Dashed blue lines indicate the shape of enamel. Dotted black and dotted white lines indicate the demarcation between the epithelium and the connective tissue. Abbreviations: GV, gingivectomy; M1, molar 1; je, junctional epithelium; oe, oral epithelium; e, enamel space. Scale bars: 50 μm.
On post-gingivectomy (GV) day 3, a dense cellular infiltrate occupied the injury site (Fig. 3C). On post-GV day 5, the cellular interface abutted the tooth surface (Fig. 3D). On post-GV day 7, evidence of a JE was detectable (Fig. 3E) and by post-GV day 14, the JE was fully regenerated (Fig. 3F).
Having established that the JE regenerated in a mouse model, equivalent to that reported in larger animals, we next examined whether the Wnt-responsive stem cell niche was also rebuilt along with the JE. Wnt-responsive cells existed in both the JE and OE (Fig. 2), but by delivering a single dose of tamoxifen to reporter mice, then evaluating the distribution of Wnt-responsive descendants after 5 days, it was clear that the baseline mitotic activity of Wnt-responsive cells was significantly higher in the JE than the OE. The entire JE was populated with GFP-positive cells whereas the OE had only a few isolated GFP-positive clones (Fig. 3G,H).
To determine whether a proliferating, Wnt-responsive population contributed to JE regeneration, mice were injected with a single dose of tamoxifen then one day later underwent gingivectomy. On post-GV day 1, essentially no GFP-positive or EdU-positive cells were detected around the tooth (Fig. 3I,J), confirming that the Wnt-responsive JE niche had been surgically removed. On post-GV day 3, GFP-positive cells in the adjacent OE had expanded dramatically, but a non-proliferative, non-GFP population was interposed between the OE and the tooth (Fig. 3K,L). By post-GV day 5, progeny of the Wnt-responsive population completely occupied the wound site (Fig. 3M) and the number of EdU-positive cells had decreased in the OE (Fig. 3N).
By post-GV day 7, the regenerated JE was comprised of progeny from the GFP-positive population (Fig. 3O). EdU labeling showed that proliferation had shifted from the OE to the JE (Fig. 3P). The expression pattern of Laminin 5, or lack thereof, on post-GV day 7 suggested the healing process was not finished and the JE attachment to the tooth surface was not fully re-established (Fig. 3Q).
Eventually, the newly regenerated JE was comprised entirely of Wnt-responsive progeny (Fig. 3R). The distribution of GFP-positive cells in the OE had returned to baseline levels (Fig. 3R). EdU labeling indicated that cell proliferation in the JE and OE had also returned to their respective baseline levels (Fig. 3R). Finally, the newly formed JE expressed Laminin 5 on the surface facing the tooth (Fig. 3S). Collectively, these data demonstrated a functional JE, along with its Wnt-responsive niche, had regenerated after gingivectomy.
A long-held point of view states that the OE contributes to the homeostasis of the JE (Shimono et al., 2003) and the gingivectomy model is one method by which this contribution can be observed (Fig. 3T). In tissue sections taken from the edge of the gingivectomy, however, we found evidence of a second population of cells that was distinct from the OE (Fig. 3U). One epithelial population was contiguous with the OE, but the other was separate from it, and positioned adjacent to the tooth surface (blue arrow, Fig. 3U and supplementary Fig. 1B). Filaggrin, a keratin bundling protein, is crucial for the barrier function and is exclusively expressed by the differentiated keratinocytes (Presland & Dale, 2000). Using it as a marker of differentiation/keratinization, we found the population contiguous with the OE was keratinized and, and another population was non-keratinized (Fig. 3U and supplementary Fig. 1C). Lineage tracing showed that both epithelial tissues contained progeny of an initial Wnt-responsive cell population but were separated by a blood clot (Fig. 3V). By post-GV day 5, the two populations appeared to coalesce (supplementary Fig. 1D), but one population remained keratinized while the other was not (supplementary Fig. 1D,E) and this maintained till the JE was fully regenerated by post-GV day 14 (supplementary Fig. 1F). These data suggested that descendants from another Wnt-responsive population were contributing to JE regeneration and the most obvious source for that population was the remaining JE (Fig. 3W).
A functional JE cannot be regenerated solely from the OE
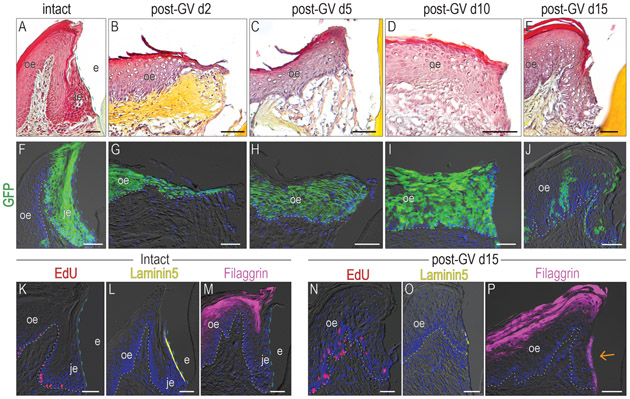
A standard gingivectomy model removes the JE from the palatal surface of the tooth but residual JE still exists on the mesial, distal and buccal surfaces. The presence of the remaining JE could be contributing to JE regeneration on the palatal tooth surface. Therefore, we modified a gingivectomy procedure to eliminate the entire JE. Maxillary M2 was extracted on the left side (supplementary Fig. 2A) and the gingiva surrounding M1 was excised circumferentially (supplementary Fig. 2B and C). Two days after the entire tooth attachment apparatus (Fig. 4A) was eliminated by the surgery, the palatal OE had thickened and a small projection of cells towards to the tooth suggested that the healing process had been initiated (Fig. 4B). On post-GV day 5, the keratinized OE had reached the tooth surface and reformed the missing keratinized epithelium (Fig. 4C). This keratinized interface persisted, without forming a JE (Fig. 4D,E). Thus, the OE served as a cell source for reepithelization, but did not appear to regenerate the JE structure on its own.
Figure 4. A functional JE cannot be solely regenerated from the OE.
Pentachrome staining was used to analyze the morphology with the samples from (A) intact group, post-GV day (B)2, (C) 5, (D) 10, and (E) 15. (F) One dose of tamoxifen was given to Axin2CreERT2/+;R26RmTmG/+ mice, GFP+ve cells were analyzed 5 days later. Lineage tracing showing the distribution of Wnt-responsive cells on post-GV day (G)2, (H) 5, (I) 10, (J) 15. In the intact samples, (K) EdU+ve cells, (L) Laminin 5 expression, and (M) Filaggrin expression pattern was examined. In the gingivectomy group, (N) EdU+ve cells, (O) Laminin 5 expression, and (P) Filaggrin expression pattern was examined on post-GV day 15. The orange arrow indicates the abnormal Filaggrin expression on the interface between the epithelium and tooth surface.
Dashed blue lines indicate the shape of enamel. Dotted white lines indicate the demarcation between the epithelium and the connective tissue. Abbreviations: GV, gingivectomy; je, junctional epithelium; oe, oral epithelium; e, enamel space. Scale bars: 50 μm.
The circumferential gingivectomy procedure specifically removed the Wnt-responsive JE population (Fig. 4F) while leaving behind Wnt-responsive OE populations. To gain insights into why the OE unable to regenerate the JE after its complete removal, we followed progeny of these Wnt-responsive OE cells. For example, on post-GV day 2, Wnt-responsive cells from in OE proliferated and migrated towards the tooth surface (Fig. 4G). On post-GV day 5, progeny of the Wnt-responsive OE cells had reached the tooth surface and reformed the missing keratinized epithelium (Fig. 4H). This GFP-positive keratinized epithelium persisted, without forming a JE (Fig. 4I). Once the repair process was complete on post-GV day 15, the number and distribution of progeny from the original Wnt-responsive colonies had returned to a baseline status (Fig. 4J).
Following circumferential gingivectomy, a keratinized epithelium had reformed around the tooth but was it a functional JE? Using EdU, Laminin5, and Filaggrin to monitor cell proliferation, cell attachment, and terminal epithelial cell differentiation, an intact JE (Fig. 4K-M) was compared with the structure that had formed on post-GV day 15 (Fig. 4N-P). Relative to the intact state, epithelial cells at the interface were less mitotically active (compare Fig. 4K,N) and were devoid of Laminin 5 expression (compare Fig. 4L,O; supplementary Fig. 3A-C). Instead, epithelial cells at the interface underwent terminal differentiation unlike in an intact JE (compare Fig. 4M,P; supplementary Fig. 3D,E). Collectively these data demonstrated that following complete JE removal, the OE contributed to re-epithelialization of the wound site but was incapable of regenerating a functional JE, contrasting to what we have observed in the partial gingivectomy model (supplementary Fig.4).
Discussion
The JE is formed from- and maintained by- two cell sources
Oral epithelial continuity is essential for preventing pathogen entry into the body, yet this continuity is disrupted by teeth. To compensate for this breach, a specialized JE forms in synchrony with the tooth eruption process, and this initial JE is derived from the REE (Nakamura, 2018; Shimono et al., 2003). Is the JE a separate entity, or is it an adaptation of the OE? Recent data argue that it is an adaptation because the OE eventually contributes cells to the JE (Kato et al., 2019). Our data support the first part of this model, in which the JE and its Wnt-responsive status are established at its origin from the REE (Fig. 1). In contrast, however, our data demonstrate that the OE cannot regenerate a functional JE by itself (Fig. 4), suggesting the JE is maintained by its own Wnt-responsive stem cell niche (Figs. 3,4). These findings argue that the JE is a separate entity that is not functionally replaced after its complete loss or destruction. If true, these data have potential clinical implications.
The OE may be necessary but is not sufficient for JE regeneration
The regeneration of a JE following gingivectomy was first observed decades ago (Engler, Ramfjord, & Hiniker, 1966; M. Listgarten, 1972). It is generally thought that the new JE derives from the OE (reviewed in (Bosshardt & Lang, 2005)) despite the fact that remnants of the JE clearly remained after gingivectomy (Masaoka et al., 2009; Nishio, Wazen, Kuroda, Moffatt, & Nanci, 2010). In alignment with historical data, we also showed that a JE structure regenerates following a partial, palatal gingivectomy, and that this new JE resembles an intact JE in terms of its cell cycle dynamics, its expression of cell adhesion molecules, and the presence of a Wnt-responsive niche (Fig. 3). When a complete, circumferential gingivectomy was performed, however, a JE was not regenerated, even after 15 days (Fig. 4).
The implications of these data have clinical relevance. After disease or injury, the reformation or regeneration of the JE is essential for gingival health. If the OE cannot undertake this regenerative task alone, then it is worthwhile considering why not, and how the process may be aided. Skin injury models are particularly informative in this context. For example, in adult animals, full thickness excisional wounds undergo re-epithelialization, but the healed site lacks sebaceous glands and hair follicles (Ito et al., 2007). Thus, skin wound healing in adults is deemed a reparative and not a regenerative process (reviewed in (Whyte, Smith, & Helms, 2012)). In our experiments here, excision of the complete JE also resulted in a re-epithelialized wound but one that lacked Wnt-responsive stem cell progeny, and a complete JE (Fig. 4). Our lineage-tracing data demonstrate that in a partial gingivectomy model, the JE regenerates because remnants of the pre-existing JE contribute Wnt-responsive stem cell progeny to contribute to its regeneration (Fig. 3). Taken together, these data suggest that inducing the reformation of a Wnt-responsive niche within interfacial tissue adjacent to teeth- or dental implants- may be sufficient to convert it into a functional JE. In skin wounding models, over-expression of Wnt7a in epithelia is sufficient to induce skin regeneration (Ito et al., 2007); perhaps a similar strategy would work in the OE.
Does a functional JE-like structure reform around a dental implant?
The JE is abolished after tooth extraction (Fig. 2). While JE remnants clearly contribute to re-epithelialization and wound closure, but lineage tracing data show that their numbers return to baseline levels within 2 weeks (Fig. 2). A similar “pruning event” follows the injury of the hard palate (Yuan et al., 2019) and the cell cycle kinetics of remaining Wnt-responsive cell populations are indistinguishable from intact OE (Fig. 2). Therefore, following tooth removal, the JE as well as its stem cell niche seems to completely disappear.
This statement prompts an obvious question: when an implant is placed in healed OE, does a functional JE-like structure reform? Clearly, a new peri-implant epithelium (PIE) forms around a dental implant, but we hypothesize that it is not functionally equivalent to the native JE. Published data (and also reviewed in (Atsuta et al., 2005; Ivanovski & Lee, 2018)) claim that the PIE shares structural, ultrastructural, and functional characteristics with the JE, but obvious differences such as the morphology (Heyman et al., 2018) and mitotic activity (Fujiseki, Matsuzaka, Yoshinari, Shimono, & Inoue, 2003) are demonstrated to exist between PIE and JE. Another difference is the pattern and onset of Laminin 5 expression following implant placement, which was both delayed and inconsistent within the PIE (Atsuta et al., 2005). We speculate that the PIE will bear a histological- but not functional- equivalency to a native JE, which may be attributable to the lack of a Wnt-responsive stem cell niche around an implant. Ongoing studies in our laboratory and others are seeking to clarify these points, which we believe will ultimately advance our understanding of peri-implant health and the etiologies of peri-implant diseases.
Limitations of the study
The lineage-tracing studies demonstrate that both the OE and JE contain Wnt-responsive cells and niches, that the properties of the stem cells in those niches e.g., mitotic activity, adhesion to the tooth) are dramatically different. Our data strongly suggest that JE cells can become OE cells (Fig. 2) but OE cells do not seem capable of converting into JE cells. The basis for this effect is not yet clear.
Conclusions
A functional JE contains a population of Wnt-responsive cells that contribute to OE repair once a tooth is extracted and that contributes to JE regeneration following partial gingivectomy. Following complete gingivectomy, however, the JE cannot regenerate despite the cellular contribution from the OE.
Supplementary Material
Clinical Relevance.
Scientific rationale for this study:
The junctional epithelium’s (JE) barrier function is critical for periodontal health. This study’s goal was to identify the molecular mechanisms mediating the persistent defensive functions of this self-renewing tissue.
Principal findings:
A Wnt-responsive cell population contributes to the initial JE derived from reduced enamel epithelium. Progeny from this Wnt-responsive population persist until tooth extraction, where they then contribute to oral epithelial repair. The JE can only regenerate after gingivectomy if remnants of this original Wnt-responsive population persist.
Practical implications:
Preserving Wnt-responsive cells in the JE may be a critical component of JE regeneration.
Acknowledgments
We thank Yunke Ren and Gladys Hernandez for their help in tissue processing and staining.
Sources of funding
The study was supported by a gift from the Bredt family to J.A.H. and K99DE028585-01 to X.Y.
Footnotes
Conflict of Interest Statement
All authors declare that no conflicts of interest exist.
References
- Al Alam D, Green M, Tabatabai Irani R, Parsa S, Danopoulos S, Sala FG, … Bellusci S (2011). Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS ONE, 6(8), e23139. doi: 10.1371/journal.pone.0023139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsuta I, Yamaza T, Yoshinari M, Mino S, Goto T, Kido MA, … Tanaka T (2005). Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials, 26(14), 1751–1760. doi: 10.1016/j.biomaterials.2004.05.033 [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, & Lang NP (2005). The junctional epithelium: from health to disease. J Dent Res, 84(1), 9–20. doi: 10.1177/154405910508400102 [DOI] [PubMed] [Google Scholar]
- Engler WO, Ramfjord SP, & Hiniker JJ (1966). Healing following simple gingivectomy. A tritiated thymidine radioautographic study. I. Epithelialization. J Periodontol, 37(4), 298–308. [DOI] [PubMed] [Google Scholar]
- Fujiseki M, Matsuzaka K, Yoshinari M, Shimono M, & Inoue T (2003). An experimental study on the features of peri-implant epithelium: immunohistochemical and electron-microscopic observations. Bull Tokyo Dent Coll, 44(4), 185–199. doi: 10.2209/tdcpublication.44.185 [DOI] [PubMed] [Google Scholar]
- Heyman O, Koren N, Mizraji G, Capucha T, Wald S, Nassar M, … Wilensky A (2018). Impaired Differentiation of Langerhans Cells in the Murine Oral Epithelium Adjacent to Titanium Dental Implants. Front Immunol, 9, 1712. doi: 10.3389/fimmu.2018.01712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, & Cotsarelis G (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature, 447(7142), 316–320. [DOI] [PubMed] [Google Scholar]
- Ivanovski S, & Lee R (2018). Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol 2000, 76(1), 116–130. doi: 10.1111/prd.12150 [DOI] [PubMed] [Google Scholar]
- Kato M, Tanaka J, Aizawa R, Yajima-Himuro S, Seki T, Tanaka K, … Yamamoto M (2019). Visualization of junctional epithelial cell replacement by oral gingival epithelial cells over a life time and after gingivectomy. Sci Rep, 9(1), 7640. doi: 10.1038/s41598-019-44065-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M (1972). Ultrastructure of the dento-gingival junction after gingivectomy. J Periodontal Res, 7(2), 151–160. doi: 10.1111/j.1600-0765.1972.tb00640.x [DOI] [PubMed] [Google Scholar]
- Listgarten MA (1966). Electron microscopic study of the gingivo-dental junction of man. Am J Anat, 119(1), 147–177. doi: 10.1002/aja.1001190109 [DOI] [PubMed] [Google Scholar]
- Listgarten MA (1967). Electron microscopic features of the newly formed epithelial attachment after gingival surgery. A preliminary report. J Periodontal Res, 2(1), 46–52. doi: 10.1111/j.1600-0765.1967.tb01995.x [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, … Behrens J (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol, 22(4), 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaoka T, Hashimoto S, Kinumatsu T, Muramatsu T, Jung HS, Yamada S, & Shimono M (2009). Immunolocalization of laminin and integrin in regenerating junctional epithelium of mice after gingivectomy. J Periodontal Res, 44(4), 489–495. doi: 10.1111/j.1600-0765.2008.01142.x [DOI] [PubMed] [Google Scholar]
- Nakamura M (2018). Histological and immunological characteristics of the junctional epithelium. Jpn Dent Sci Rev, 54(2), 59–65. doi: 10.1016/j.jdsr.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio C, Wazen R, Kuroda S, Moffatt P, & Nanci A (2010). P44-expression pattern of APIN and amelotin during formation and regeneration of the junctional epithelium. Bull Group Int Rech Sci Stomatol Odontol, 49(3), 111–112. [PubMed] [Google Scholar]
- Oksonen J, Sorokin LM, Virtanen, & Hormia M (2001). The junctional epithelium around murine teeth differs from gingival epithelium in its basement membrane composition. J Dent Res, 80(12), 2093–2097. doi: 10.1177/00220345010800121401 [DOI] [PubMed] [Google Scholar]
- Presland RB, & Dale BA (2000). Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med, 11(4), 383–408. doi: 10.1177/10454411000110040101 [DOI] [PubMed] [Google Scholar]
- Schroeder HE, & Listgarten MA (1997). The gingival tissues: the architecture of periodontal protection. Periodontol 2000, 13, 91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x [DOI] [PubMed] [Google Scholar]
- Schroeder HE, & Listgarten MA (2003). The junctional epithelium: from strength to defense. J Dent Res, 82(3), 158–161. doi: 10.1177/154405910308200302 [DOI] [PubMed] [Google Scholar]
- Shimono M, Ishikawa T, Ishikawa H, Matsuzaki H, Hashimoto S, Muramatsu T, … Inoue T (2003). Regulatory mechanisms of periodontal regeneration. Microsc Res Tech, 60(5), 491–502. doi: 10.1002/jemt.10290 [DOI] [PubMed] [Google Scholar]
- Skougaard M (1965). Turnover of the gingival epithelium in marmosets. Acta Odontol Scand, 23(6), 623–643. doi: 10.3109/00016356509041116 [DOI] [PubMed] [Google Scholar]
- Wazen RM, Moffatt P, Ponce KJ, Kuroda S, Nishio C, & Nanci A (2015). Inactivation of the Odontogenic ameloblast-associated gene affects the integrity of the junctional epithelium and gingival healing. Eur Cell Mater, 30, 187–199. doi: 10.22203/ecm.v030a13 [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, & Helms JA (2012). Wnt signaling and injury repair. Cold Spring Harb Perspect Biol, 4(8), a008078. doi: 10.1101/cshperspect.a008078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima-Himuro S, Oshima M, Yamamoto G, Ogawa M, Furuya M, Tanaka J, … Yamamoto M (2014). The junctional epithelium originates from the odontogenic epithelium of an erupted tooth. Sci Rep, 4, 4867. doi: 10.1038/srep04867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Pei X, Zhao Y, Tulu US, Liu B, & Helms JA (2018). A Wnt-Responsive PDL Population Effectuates Extraction Socket Healing. J Dent Res, 22034518755719. doi: 10.1177/0022034518755719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Xu Q, Zhang X, Van Brunt LA, Ticha P, & Helms JA (2019). Wnt-Responsive Stem Cell Fates in the Oral Mucosa. iScience, 21, 84–94. doi: 10.1016/j.isci.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.