Abstract
Aims:
The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network initiated a second observational cohort study - the Symptom Patterns Study (SPS) - to further investigate the underlying pathophysiology of Urologic Chronic Pelvic Pain Syndrome (UCPPS) and to discover factors associated with longitudinal symptom changes and responses to treatments.
Methods:
This multi-site cohort study of males and females with UCPPS features a run-in period of four weekly web-based symptom assessments prior to a baseline visit, followed by quarterly assessments up to 36-months. Controls were also recruited and assessed at baseline and 6-months. Extensive clinical data assessing urological symptoms, non-urological pain, chronic overlapping pain syndromes, and psychosocial factors were collected. Diverse biospecimens for biomarker and microbiome studies, quantitative sensory testing (QST) data under multiple stimuli, and structural and functional neuroimaging scans were obtained under a standardized protocol.
Results:
Recruitment was initiated (July 2015) and completed (February 2019) at 6 Discovery Sites. A total of 620 males and females with UCPPS and 73 Controls were enrolled, including 83 UCPPS participants who re-enrolled from the first MAPP Network cohort study (2009–2012). Baseline neuroimaging scans, QST measures and biospecimens were obtained on 578 UCPPS participants. Longitudinal follow-up of the cohort is ongoing.
Conclusions:
This comprehensive characterization of a large UCPPS cohort with extended follow-up greatly expands upon earlier MAPP Network studies and provides unprecedented opportunities to increase our understanding of UCPPS pathophysiology, factors associated with symptom change, clinically relevant patient phenotypes, and novel targets for future interventions.
Introduction
Urologic chronic pelvic pain syndrome (UCPPS) encompasses two highly prevalent, non-malignant urologic disorders, interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), primarily characterized by chronic and often debilitating pain in the pelvic region and/or genitalia, and typically a spectrum of bladder and lower urinary tract symptoms.1,2 As with many chronic pain disorders, UCPPS is poorly understood, and treatment is mostly empirical and unsatisfactory. To better understand the etiology and treated natural history of UCPPS, and to identify clinical and biological factors and research measurements that define clinically relevant patient sub-groups and sex-based differences, the NIDDK established the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network3 (http://www.mappnetwork.org/). During MAPP I (2008–2014), a prospective cohort study, the Trans-MAPP Epidemiology/ Phenotyping Study (EPS)4, enrolled 1,039 males and females, including participants with UCPPS (n=424); participants with chronic overlapping pain conditions (COPCs), including fibromyalgia, irritable bowel syndrome, and/or chronic fatigue syndrome (n=200); and healthy controls (n=415). All participants were extensively characterized at baseline, and UCPPS participants were followed for 48 weeks using a web-based data capture system to obtain questionnaire responses on a bi-weekly schedule. Biospecimens were collected on all participants at baseline and follow-up visits to identify plasma and urine biomarkers and potential infectious agents. In addition, baseline neuroimaging and quantitative sensory testing (QST) studies were performed in a subset of participants.
Key MAPP I findings include observations that: 1) urologic pain and urinary symptoms co-vary, with only moderate correlation (r=0.5), and thus should be evaluated separately5; 2) UCPPS participants who report pain beyond the pelvis have more severe UCPPS symptoms and more symptom flares than those with pelvic pain only6,7; 3) UCPPS participants report more psychosocial difficulties than pain-free controls, and poor psychosocial functioning is associated with a lower likelihood of longitudinal symptom improvement8–10; 4) UCPPS is associated with changes in structure and function of pain-related neural networks, as well as brain-level sensorimotor systems regulating urine storage11–13; 5) UCPPS symptom profiles can be distinguished by biological correlates (e.g., immune factors)14; 6) QST assessment reveals that UCPPS participants have significantly increased pressure pain sensitivity compared to healthy controls, and higher sensitivity was associated with less likelihood of UCPPS symptom improvement.15 These primary findings were generally consistent in both males and females, although some sex-specific differences were observed16.
For MAPP II (2014–2019), the Trans-MAPP Symptom Patterns Study (SPS) enrolled 620 males and females with UCPPS into the second MAPP longitudinal cohort study that included a significantly longer follow-up (36 months) and a highly integrated and more comprehensive cross-modality assessment of clinical, biological, brain imaging and pain sensitivity measures to further investigate factors associated with longitudinal symptom change profiles. Detailed information about targeted usual-care therapy and treatment response is also being collected to identify UCPPS participants with potential to preferentially respond to different therapies.
These SPS phenotyping studies were motivated by the following Primary Aims:
To characterize longitudinal urological and non-urological symptom patterns in a cohort of UCPPS participants enriched for pain restricted to the pelvis (i.e., regional pain) at baseline.
To identify associations between baseline clinical factors and urinary and non-urinary symptom progression in a cohort of UCPPS participants enriched for pain restricted to the pelvis (i.e., regional pain) at study entry (baseline).
To characterize transitions between regional pain (pelvic pain only) and systemic pain (pelvic pain and beyond) for UCPPS over time, and the corresponding association with symptom improvement or worsening and selected chronic overlapping pain conditions (e.g., fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome).
To identify associations between baseline objective quantitative pain sensitivity measures and symptom progression in a cohort of UCPPS participants enriched for pain restricted to the pelvis at baseline.
To create accurate, non-invasive diagnostic and prognostic tests, based on specific, definable and validated levels of biomarker(s) that will objectively identify and stratify UCPPS participants and guide the diagnosis, treatment, and long-term management of patients with UCPPS.
To identify brain signatures that remain stable, while other brain signatures are correlated with symptom change over time.
To compare the relative effectiveness of UCPPS therapies administered to MAPP participants with peripheral vs. central patient phenotypes.
Materials and Methods/Design
MAPP Network Organization
The MAPP II Research Network consists of six recruiting Discovery Sites (Los Angeles, CA; Chicago, IL; St. Louis, MO; Iowa City, IA, Seattle, WA, and Ann Arbor, MI); three research project-based Discovery Sites (Palo Alto, CA; Boston MA; Ontario, CN); a Data Coordinating Core (DCC; Philadelphia, PA); a Tissue Analysis and Technology Core (TATC; Aurora, CO); and a Neuro-imaging scan repository and Reading Center (UCLA/USC; Los Angeles, CA) (Appendix 1). Additional integrated work is supported via ancillary studies and sub-contracts. An External Experts Panel (EEP) appointed by the NIDDK provides comments/recommendations on study design and progress.
Overview of the MAPP II Symptom Patterns Study (SPS)
As illustrated (Figure 1), the SPS design includes in-clinic screening and enrollment of eligible UCPPS participants (week 0) who continue weekly follow-up via web-based data capture (weeks 1,2,3). These four repeated measures provide a unique opportunity to quantify the variability and propensity of symptom classifiers prior to the “deep-phenotyping” of baseline symptoms (week 4) and to adjust for “regression-to-the-mean” phenomena17.
Figure 1. MAPP II Symptom Patterns Study (SPS) and Embedded ATLAS Module.
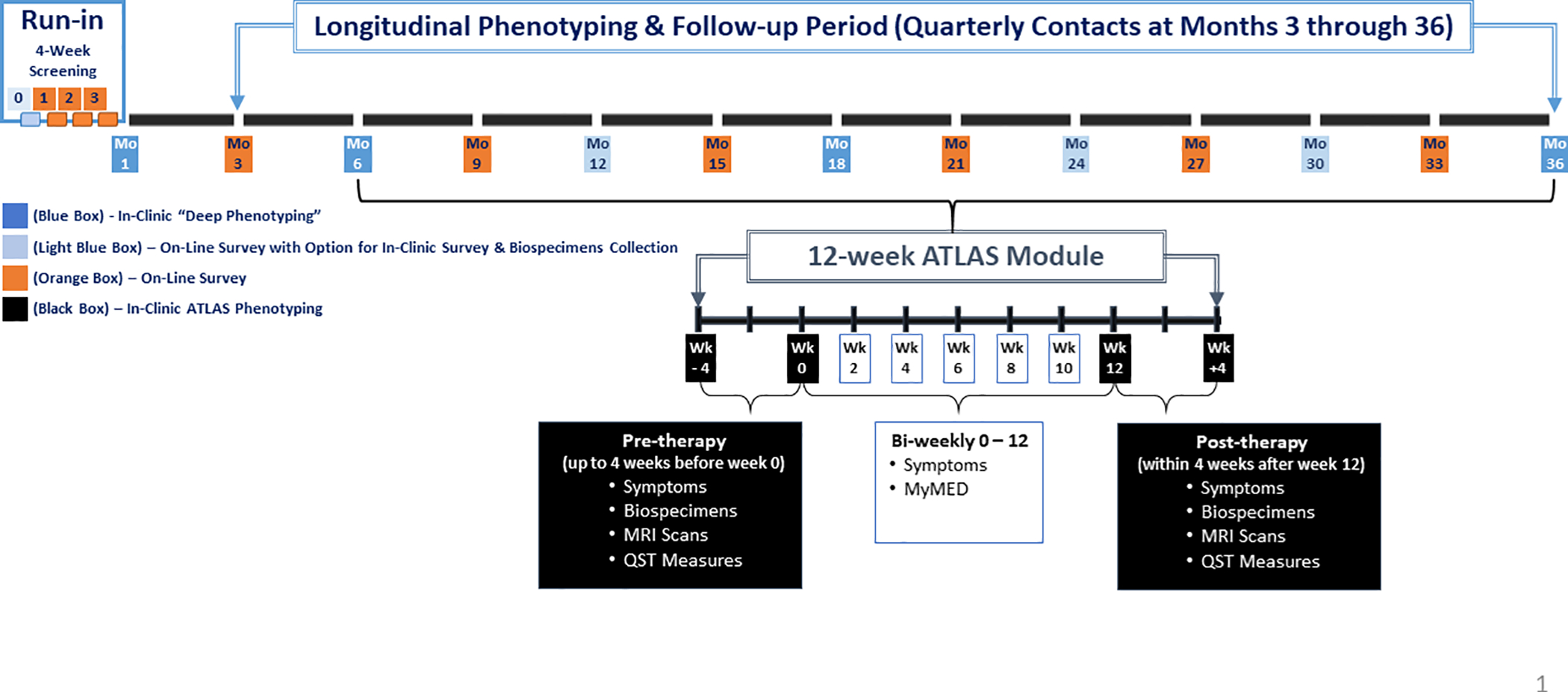
Following baseline assessments (week 4), longitudinal follow-up includes web-based monthly medication updates, quarterly symptom assessments, and in-clinic “deep phenotyping” assessments (6, 18, 36 months). Enrolled UCPPS participants were also invited to optional “in-clinic” semi-annual visits for additional biospecimen collections. The overall target sample size for enrollment was 600 UCPPS participants, aiming for a 1:1 ratio of females:males and 50% reporting pain in the pelvic region only (using body map assessments). In addition, 72 Controls without UCPPS were recruited and completed baseline and 6-month in-clinic “deep phenotyping” assessments.
Eligibility Criteria
Eligibility criteria for the SPS protocol are nearly identical to those for the MAPP I EPS4. Inclusion criteria for UCPPS participants include: 1) UCPPS symptoms present for a majority of the time during most recent 3 months; 2) age ≥18 years; and 3) response ≥1 on the bladder/prostate or pelvic pain/pressure/discomfort scale during past 2 weeks. However, re-enrolling MAPP I participants with a pain/pressure/discomfort score of 0 were eligible, in order to provide insight into factors influencing UCPPS symptom resolution. Exclusion criteria include symptomatic urethral stricture, neurological disease or disorder affecting the bladder, bladder fistula, a history of cystitis caused by tuberculosis, radiation therapy or chemotherapy, prior augmentation cystoplasty or cystectomy, active autoimmune or infectious disorder, history of pelvic cancer, current major psychiatric disorder, severe cardiac, pulmonary, renal, or hepatic disease, unilateral orchalgia (without pelvic symptoms), and prior prostate procedures (transurethral microwave thermotherapy (TUMT), transurethral needle ablation (TUNA), balloon dilation, prostate cryo-surgery, or laser procedure).
Risk Factors and Outcome Measures
Extensive risk factor and outcome data were collected during the screening/run-in period (weeks 0,1,2,3), as well as ‘deep phenotyping’ in-clinic visits (week 4; months 6,18,36), and web-based quarterly and semi-annual follow-up contacts (Table 1). These data include demographics, comprehensive measures of urologic symptoms, non-urologic symptoms, and psychosocial variables (https://www.mappnetwork.org/assets/documents/MAPP-Phase_I_&_II_CRF_Lists_20150326.pdf).
Table 1.
Phenotyping Domains, Case Report Forms (CRFs) and Timetable for Data Capture and Biological Samples Collection
| Screening/Run-in Period: Weeks 0, 1, 2, 3 | In-Clinic Deep Phenotyping Visits: Week 4; Months 6, 18, 36/ATLAS Visits | On-Line Quarterly Contacts: Months 3, 9, 15, 21, 27, 33 | On-Line with In-Clinic Option Contacts: Months 12, 24, 30 | |
|---|---|---|---|---|
| DEMO | Consent & Eligibility Confirmation† | |||
| UROLOGIC, NEUROSCAN, QST | Urine Dipstick/Culture | Urine Dipstick/Culture | Urine Dipstick/Culture | |
| SYM-Qs/Flare Status | SYM-Qs/Flare Status | SYM-Qs/Flare Status | SYM-Qs/Flare Status | |
| GRA (Run-In only) | GRA | GRA | GRA | |
| RICE; GUPIs; IC-SI/PI | RICE; GUPIs; IC-SI/PI, AUASI | RICE; GUPIs; IC-SI/PI | RICE; GUPIs; IC-SI/PI | |
| Pelvic Exam/ CystoHx† | Pelvic Exam | |||
| History: Med., Inf, Fam, Antibiotics† | Hx Antibiotics | Hx Antibiotics | ||
| Medications/PTHX† | Medications/PTHX | Medications (monthly) | Medications/PTHX | |
| Bladder Impact; SEAR, FSFI, IIEF, EFS | ||||
| Neuroimaging Scans; QST Measures | ||||
| BIO-SPECIMENS | Microbiome Urine (VB1, VB2, VB3) ¥ | Blood Collection: plasma, STIM tubes# | Blood Collection: plasma | |
| Vaginal and Rectal Swabsƛ | Biomarker Urine: spot urine | Biomarker Urine: spot urine | ||
| Microbiome Urine (VB1, VB2, VB3)¥ | Microbiome Urine (VB2)ŧ | |||
| Saliva (cortisol)€
Vaginal and Rectal Swabsƛ |
||||
| NON-UROLOGIC/PSYCHOCOCIAL | BPI/CHOIR Body Map | BPI/CHOIR Body Map | BPI/CHOIR Body Map | BPI/CHOIR Body Map |
| PainDetect | PainDetect | PainDetect | PainDetect | |
| McGill Pain; Gracely Box Scales | ||||
| CMSI checklist & Fibromyalgia module | CMSI checklist (Incl. CMSI modules) | CMSI checklist & Fibromyalgia module | CMSI checklist & Fibromyalgia module | |
| WHO-DAS | WHO-DAS, SF12 | WHO-DAS | WHO-DAS, SF12 | |
| IPAQ, WPAI, PANAS | IPAQ, WPAI, PANAS | |||
| HADS | HADS | HADS | HADS | |
| PROMIS: Fatigue, Sleep | PROMIS: Fatigue, Sleep | PROMIS: Fatigue, Sleep | PROMIS: Fatigue, Sleep | |
| MASQ | MASQ | |||
| Perceived Stress | Perceived Stress | Perceived Stress | Perceived Stress | |
| Catastrophizing; Personality: TIPI§ | ||||
| Trauma: CTES†; RTES†Ŧ |
Demographic/Urological: Optional In-Clinic visit@12,24,30 months.
W0
Follow-up
W4
STIM tubes: LPS (TLR4 agonist), FSL (TLR-2 agonist), and NULL (control) stimulated plasma collected at W4, M6, M18
Microbiome urine full set (VB1, VB2, VB3) collected only at W0, M18 & any In-Clinic visit if participant is experiencing a flare; Only VB2 collection at M6, M36
Microbiome (VB1, VB2, VB3) is collected at optional visits M12, 24, 30 if participant is experiencing a flare
Salivette 3-day (am+pm) collected at W4; Salivette 7-day (am+pm) collected at M6, M18, M36
Vaginal and Rectal swabs only collected at W0, M18.
Sociodemographics, Pelvic Examination, Medical and Family History
Demographics, physical measures, a standardized pelvic examination (including assessment of pelvic floor musculature tenderness) and a detailed personal and family medical history were obtained. A monthly, web-based module (“MyMeds”) was used to capture prescription, over-the-counter and non-medication therapies.
UCPPS Symptom Measures
Multiple urologic symptom measures, previously included in the MAPP I EPS protocol4, were retained in the SPS (Table 1). From these items, the two primary UCPPS severity scales (urologic pain, urinary frequency/urgency) were derived18. Additional urologic measures, as well as self-esteem/relationship, and sexual function were assessed during “deep phenotyping” visits.
Non-urologic Pain and Psychosocial Measures
An extensive array of non-urologic pain measures, previously included in the MAPP I EPS4, were also included in the SPS (Table 1), including a modified CHOIR body map19, together with a pain severity level for each checked site http://choir.stanford.edu. In addition, ‘fibromyalgia-ness’ is assessed using the fibromyalgia diagnostic criteria20 and a widespreadness pain index derived from the CHOIR body map; pain severity and interference is assessed using the Body Pain Index (BPI)21; and neuropathic-like pain symptoms are assessed by the PAIN Detect questionnaire22.
Assessment of Flares
“Flares” of UCPPS symptoms, defined as “symptoms much worse than usual”, were assessed at each quarterly contact. At the Screening visit, each participant provided the details of a flare management plan, if they had one. If a participant reported a current flare at any in-clinic visit, an additional “flare” urine sample was requested.
ATLAS Module
The SPS includes an embedded module, termed “Analysis of Therapies during the Longitudinal Assessment of Symptoms (ATLAS)” (Figure 1), designed to capture “deep-phenotyping” measures at the initiation of a select therapy and following 12 weeks of therapy, in order to correlate therapy response with specific phenotypic profiles. Eligibility for an ATLAS module began immediately following the 6-month visit, triggered by a UCPPS participant’s treating physician recommending initiation of one of seven targeted therapeutic interventions.
The seven ATLAS treatments selected for study were categorized as “central(C)’ or “peripheral(P)”, based on mechanism of action, as follows: i) oral opioids (C), ii) tricyclic antidepressants (C), iii) pelvic floor physical therapy (P), iv) cystoscopy with hydrodistention (P), v) alpha-adrenergic antagonists (men only) (P), vi) pentosan polysulfate (P), and vii) neuropathic pain agents (gabapentin, pregabalin) (C).
Daily Assessments via Mobile Phone App
To capture daily symptom fluctuations, minimizing recall bias in reporting, a novel mobile phone app (the “(M)APP”) was developed to monitor urologic and non-urologic pain symptoms, sleep, mood and activities 4 times per day for 14 consecutive days. These data will be evaluated for feasibility, validity, and utility of the (M)APP as a new clinical tool for patient assessment.
Biomarker and Microbiome Assessments
A comprehensive set of biological specimens (Table 1) was collected at screening/baseline and during longitudinal in-clinic and ATLAS visits. The collection of blood, urine, and saliva (cortisol) specimens, as well as vaginal and rectal swabs, together with their derivatives (genomic, bacterial, and fungal DNA) at the same time points allows for integrated comparison of biological and microbial profiles with clinical factors cross-sectionally and longitudinally. Biomarker and microbiome studies conducted in the MAPP II SPS were designed to validate and extend ongoing findings from MAPP I.
Neuroimaging
The SPS neuroimaging assessment includes two resting-state (RS) functional MRI scans, one following standardized bladder filling and another following voiding, as well as a T1-weighted structural MRI (T1), and a diffusion tensor imaging (DTI) scan. Assessments of pain and urgency are made throughout the scanning session. In contrast with MAPP I, in which scanning was only collected at baseline and without any provocation, SPS neuroimaging data are collected at multiple time-points to examine longitudinal changes in brain function and include a bladder filling procedure designed to identify brain networks mediating the sensation of bladder filling.
Quantitative Sensory Testing (QST)
The SPS QST study includes four testing methods that assess generalized and segmental pain sensitivity and endogenous pain modulation. As in MAPP I, Generalized (global) Mechanical Sensitivity is evaluated using the computer-controlled MAST system23,15 As in MAPP I, generalized pain sensitivity is measured using the MAST system which delivers a series of increasing discrete pressures to the thumbnail bed15,23, and is terminated when participants reach their maximum tolerable pain level.
Additional MAPP II tests include Segmental Mechanical Sensitivity using a flat rubber probe to deliver quantifiable pressure stimuli to the suprapubic area and to a control site on the forearm24. Temporal Summation (increased perception of pain to repetitive painful stimuli), is evaluated by applying a series of pinprick stimuli to the skin in the suprapubic area and to a control site on the forearm25–27.
Additional MAPP II tests include pressure algometry at the suprapubic area and the forearm and trapezius (control sites) to assess segmental pain sensitivity24. Temporal summation (increased perception of pain to repetitive painful stimuli) and pain aftersensations are evaluated by applying a series of pinprick stimuli (256 mN) to the skin at the suprapubic area and at a control site on the forearm25,26. Conditioned pain modulation assesses responses to a painful test stimulus during painful conditioning stimulation vs. neutral (non-painful) conditioning stimulation. Moderately painful pressure stimuli delivered via the MAST system to the dominant thumbnail serves as test stimulus28. Immersion of the contralateral foot into a circulating water bath with either body temperature water (32.0–33.0°C) or moderately painful hot water (44.0–46.5°C)29 serves as a neutral and painful conditioning stimulus, respectively.
Statistical Considerations and Operations
Run-in Period Measures
In order to incorporate week-to-week variability of symptoms into estimates of baseline associations and predictions of longitudinal change, alternate measures of symptom classifiers are being compared, including the propensity to be reported present during each week prior to baseline (week 4) (Figure 1).
Study Outcomes and Approach to Data Analysis
The SPS provides a rich array of biological and clinical outcomes within urologic, non-urologic and psychosocial data domains, including quality of life, healthcare seeking, and detailed factors reported at the time of urologic symptom flares. In particular, the severity scales (urologic pain, urinary symptoms) provide standard benchmarks to compare SPS and EPS participants.18
A variety of statistical approaches will be used to test hypotheses specific to each modality of data (e.g. neuroimaging, biomarkers, etc.) and to test associations across modalities and across time. Unsupervised consensus clustering (CC) methods30–34 will be utilized to derive reproducible clinical subgroups using baseline symptom data. Modeling multivariate symptom patterns will also be conducted to correlate baseline clinical phenotyping data, neuroimaging, QST, and biomarker data with longitudinal symptom patterns. Examples include investigating imaging-based cluster membership for potential mediation of reported treatment and symptom change, adjusted for cross-modality clinical phenotypes. Patient-specific variability measures derived from the run-in period will be evaluated as predictors of symptom change, and responsiveness to therapy change will be investigated to gain further insights into UCPPS subtypes.
Results
Recruitment, Screening and Enrollment
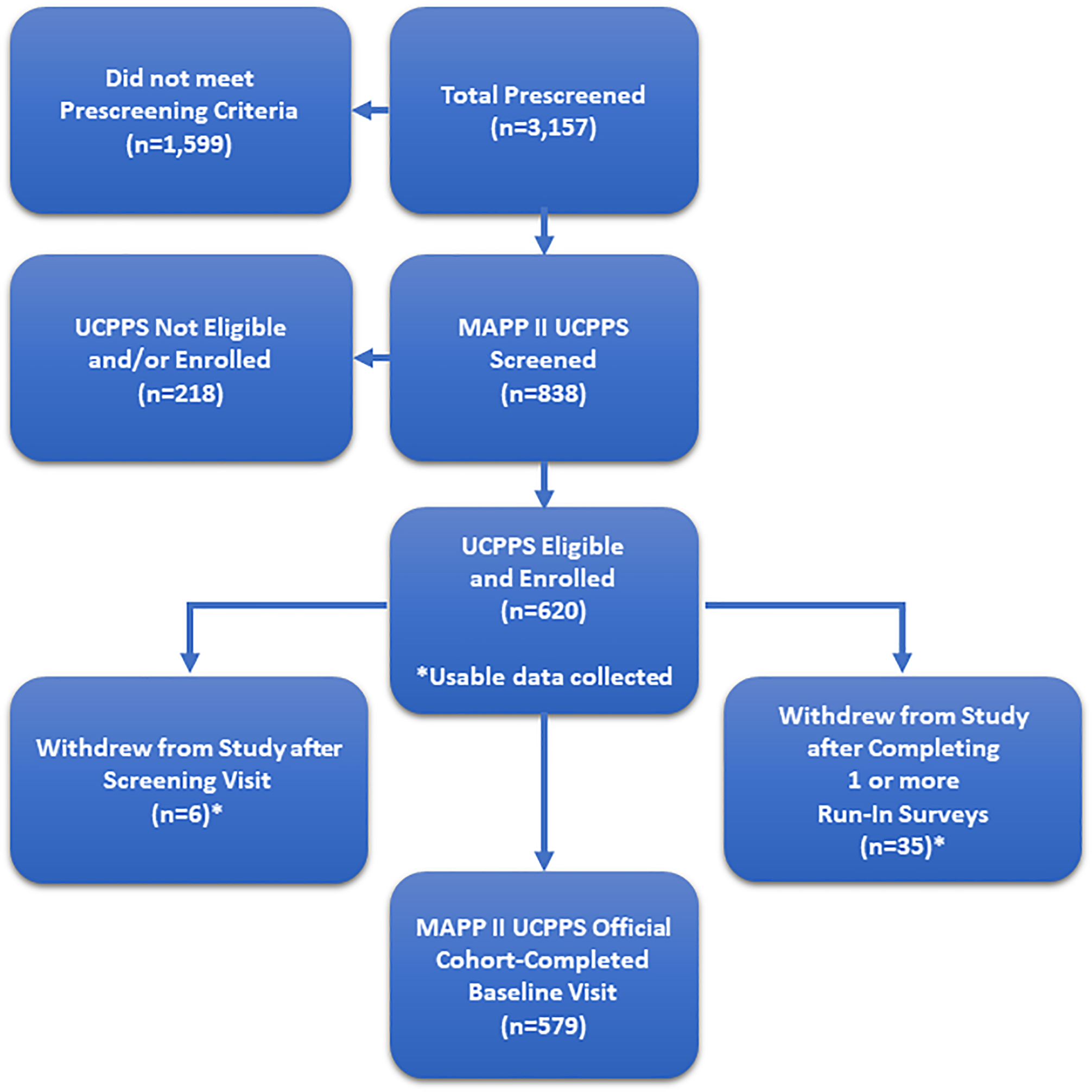
Participant recruitment was conducted at 6 Discovery Sites from July 1, 2015 through February 28, 2019. As summarized in the CONSORT Diagram (Figure 2), prescreening of 3,157 potentially eligible participants yielded 620 males and females meeting the UCPPS eligibility criteria at the screening visit (week 0), with 579 individuals progressing through the run-in period to complete the baseline visit (week 4). Of these 620 UCPPS participants, 83 were re-enrollees from the MAPP I EPS. In addition, 73 Control participants were enrolled into the SPS and followed through the 6-month visit.
Figure 2. MAPP II Symptom Patterns Study (SPS) CONSORT Diagram.
Collection of Study Data
The same screening/baseline measures were assessed for UCPPS and Control participants. Additionally, UCPPS participants reported weekly on a subset of measures during a run-in period. During the ‘deep phenotyping’ visits (week 4, months 6, 18, 36), neuroimaging scans, QST measures, and biomarker and microbiome specimens were obtained. Data from these 3 domains were obtained from 578 UCPPS participants, with complete overlap of results available for 374 (Figure 3, left panel). Furthermore, for 498 UCPPS participants, data from all 3 domains were obtained at both baseline (week 4) and 6-months, with a unique opportunity for paired comparisons (1 vs 6 months) of results from all 3 domains for 235 UCPPS participants (Figure 3, right panel).
Figure 3. VENN Diagrams.
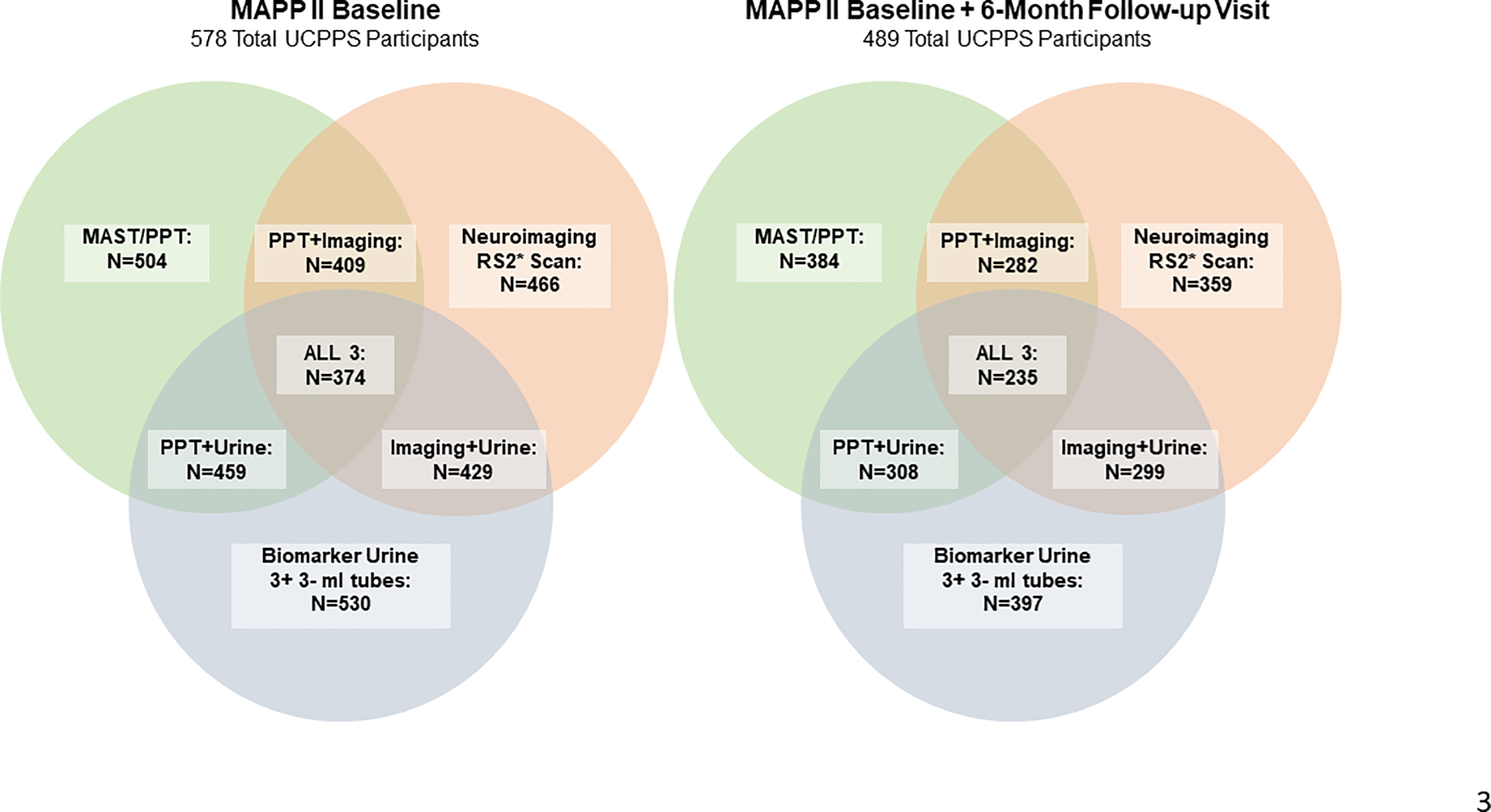
QST quantitative sensory testing] Measures of generalized and segmental pain sensitivity and endogenous pain modulation
Neuroimaging: RS2 fMRI scan results with a QC rating of “Pass” or “Pass (Issues)”
Biomarker Urine: At least 3 3-ml tubes available
*RS2 Scan – Empty Bladder Scan
UCPPS Participant Composition
The UCPPS participants enrolled into the MAPP II SPS (n=620) were compared to those in the MAPP I EPS (n=424) (Table 2). On average, MAPP II SPS participants were 1-year older (p<0.0001) with 3-years longer UCPPS symptom duration (p<0.0001), in large part attributable to the 83 participants from MAPP I EPS who re-enrolled into the MAPP II SPS (Table 2). Fewer males were recruited into the MAPP II SPS (34%) than into the MAPP I EPS (45%), but otherwise the SPS and EPS were not significantly different (p > 0.01) for any of the other demographic variables. For each of the primary urological symptoms, the SPS and EPS participants were not significantly different (p > 0.01), except for urological pain severity (SYM-Q1), reflecting the relaxed inclusion criteria for re-enrollment of MAPP I EPS participants into SPS. Otherwise, UCPPS participants in the MAPP I EPS and MAPP II SPS were not significantly different (p > 0.01) across a wide range of non-urological pain, psychosocial or quality of life measures.
Table 2.
Composition of MAPP I EPS, MAPP II SPS and MAPP II SPS/EPS Participants
| Domain | Measurement | MAPP I EPS | MAPP II SPS | p-value (MAPP I vs. II) | MAPP II (Re-enrolled from MAPP I) | ||
|---|---|---|---|---|---|---|---|
| Demographics | Number of Participants | N | 424 | 620 | 83 | ||
| Age (years) | Mean (StdDev) | 43.4 (15.1) | 44.4 (15.7) | <0.0001 | 48.8 (14.5) | ||
| Sex=Male | N (%) | 191 (45.1%) | 210 (33.9%) | 0.001 | 45 (54.2) | ||
| Race=White | N (%) | 374 (88.8%) | 543 (87.7%) | 0.61 | 81 (97.6) | ||
| Employed | N (%) | 278 (65.7%) | 394 (63.6%) | 0.29 | 53 (63.9) | ||
| Income | $50,000 or less | N (%) | 143 (37.1%) | 201 (36.4%) | 0.70 | 26 (34.2) | |
| $50,001 to $100,000 | 122 (31.7%) | 196 (35.4%) | 21 (27.6) | ||||
| More than $100,000 | 120 (31.2%) | 156 (28.2%) | 29 (38.2) | ||||
| Duration of Symptoms (years) | Mean (StdDev) | 8.5 (10.6) | 11.9 (11.5) | <0.0001 | 13.0 (9.3) | ||
| Urological Symptoms | Pain (0–10) (SYMQ-1) | Mean (StdDev) | 5.1 (2.2) | 4.8 (2.0) | 0.002 | 4.0 (2.1) | |
| Overall Pelvic/Urologic Symptoms (0–10) (SYMQ-5) | Mean (StdDev) | 5.2 (2.3) | 5.1 (2.2) | 0.24 | 4.2 (2.2) | ||
| GUPI Total Score (0–45) | Mean (StdDev) | 25.5 (8.7) | 25.7 (8.5) | 0.21 | 22.0 (8.9) | ||
| GUPI Pain Subscale (0–23) | Mean (StdDev) | 12.5 (4.5) | 12.8 (4.2) | 0.98 | 11.4 (4.3) | ||
| IC Symptom Index (0–20) | Mean (StdDev) | 9.7 (4.7) | 10.0 (4.5) | 0.61 | 9.6 (4.3) | ||
| IC Problem Index (0–16) | Mean (StdDev) | 8.5 (4.4) | 8.7 (4.0) | 0.87 | 7.6 (4.1) | ||
| AUA Symptom Index (0–35)* | Mean (StdDev) | 15.5 (8.5) | 14.8 (8.2) | 0.09 | 14.5 (8.2) | ||
| UCPPS Primary Severity Scales | Urologic Pain Severity Scale (0–28) | Mean (StdDev) | 14.9 (5.6) | 15.5 (5.2) | 0.52 | 13.8 (5.4) | |
| Urinary Severity Scale (0–25) | Mean (StdDev) | 12.6 (6.2) | 12.5 (6.0) | 0.36 | 11.7 (5.8) | ||
| Non-Urological Symptoms | Overall NON-Pelvic/NON-Urologic Symptoms (0–10) (SYMQ-6) | Mean (StdDev) | 3.3 (2.7) | 3.5 (2.7) | 0.04 | 3.5 (2.6) | |
| Pain-Detect Total (−1–38) | Mean (StdDev) | 10.8 (6.2) | 9.3 (6.5) | ||||
| Fibromyalgia Total Score (0–31) | Mean (StdDev) | 8.4 (5.4) | 8.5 (5.2) | 0.90 | 7.9 (6.0) | ||
| FM-WPI Score (0–19) | Mean (StdDev) | 3.3 (3.5) | 2.9 (3.3) | 0.76 | 2.9 (3.9) | ||
| FM-SS Score (0–12) | Mean (StdDev) | 5.1 (2.8) | 5.5 (3.0) | 0.11 | 4.9 (3.1) | ||
| HADS Anxiety (0–21) | Mean (StdDev) | 7.7 (4.5) | 8.0 (4.7) | 0.95 | 6.6 (4.3) | ||
| HADS Depression (0–21) | Mean (StdDev) | 5.4 (4.2) | 5.4 (4.0) | 0.40 | 4.5 (3.6) | ||
| Perceived Stress Score (0–40) | Mean (StdDev) | 16.4 (7.9) | 16.5 (7.9) | 0.13 | 13.8 (7.3) | ||
| PROMIS Fatigue (T-Score) | T-Score | 54.8 (8.8) | 55.8 (9.4) | 0.50 | 53.9 (9.1) | ||
| PROMIS Sleep (T-Score) | T-Score | 54.4 (9.3) | 55.3 (8.9) | 0.40 | 54.3 (9.0) | ||
| WHO-DAS (0–48) | Mean (StdDev) | 11.1 (9.0) | 8.8 (8.6) | ||||
| SF12* | PCS* (Z-Score) | Mean (StdDev) | 47.4 (10.3) | 45.6 (10.0) | 0.02 | 47.7 (10.2) | |
| MCS* (Z-Score) | Mean (StdDev) | 43.8 (10.5) | 43.3 (10.8) | 0.93 | 45.1 (9.2) | ||
MAPP II AUASI and SF12 data from baseline (visit 5) rather than screening (visit 1) since it was not collected at screening
Discussion
The MAPP SPS protocol represents a unique and highly integrated study design permitting a comprehensive evaluation of UCPPS biological and clinical characteristics over 3 years. The SPS incorporates diverse urologic and non-urologic symptom measures, as well as evaluations of the relationship between UCPPS and commonly associated COPCs and sex-related differences. This design with broad inclusion criteria affords an opportunity to define clinically relevant UCPPS sub-groups and correlate those phenotypes with differing symptom patterns and associated risk factors. The SPS incorporates many of the most promising methods in pain research (e.g., functional and structural neuroimaging, quantitative sensory testing, correlations with biological measures) to characterize pelvic and systemic pain in UCPPS. Furthermore, the novel 4-week run-in period captures week-to-week symptom variability to help establish the degree of stability of baseline symptoms. The extended follow-up period offers unprecedented opportunities to characterize UCPPS symptom change over 36 months. Furthermore, this longitudinal phenotyping allows for the discovery of factors that may underly or predict symptom improving or worsening, as well as test hypotheses on transition of UCPPS manifestations over extended time (e.g., potential changes from peripheral to centralized disease). The unique approach to collection of ATLAS treatment data permits correlations of UCPPS phenotypic characteristics with response to selected therapies, thus offering important insights for future clinical trial designs.
Conclusions
The MAPP Research Network has successfully designed and implemented a novel longitudinal observational study of UCPPS with a primary focus on discovery of factors that may define pathophysiology and clinical phenotypes and correlate with symptom changes over time. This unique study design extends the MAPP I EPS cohort study, including expanded longitudinal measures, a run-in period, and a 36-month longitudinal follow-up period. The MAPP Network continues to serve as a model for the integrated and comprehensive characterization of syndromic disorders of unknown etiology. The MAPP II SPS is expected to further the MAPP Network’s central goals of informing future clinical studies and ultimately improving clinical management of UCPPS.
Supplementary Material
Acknowledgments:
The MAPP Research Network acknowledges support through NIH grants. The NIDDK and MAPP Network investigators wish to thank the Interstitial Cystitis Association and the Prostatitis Foundation for their assistance in study participant recruitment and other network efforts, as well as all the participants who enrolled in these important research studies.
FUNDING.
Research funders must be listed at the end of the document. Funding for any publication should be clearly stated, and the role of the research funder as well as all parties contributing to all aspects of the research and its subsequent publication, must be made clear.
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) [DK082315 (Andriole, G; Lai, H), DK082316 (Landis, J), DK082325 (Buchwald, D), DK082333 (Lucia, MS), DK082342 (Klumpp, D; Schaeffer A), DK082344 (Kreder, K), DK082345 (Clauw, D; Clemens, JQ), DK082370 (Mayer, E; Rodriguez L), DK103227 (Moses, M), DK103260 (Anger, J; Freeman, M), DK103271 (Nickel, J)].
Reference List
- 1.Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol 2007;177(2):450–456. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JQ, Markossian TW, Meenan RT, O’Keeffe Rosetti MC, Calhoun EA. Overlap of voiding symptoms, storage symptoms and pain in men and women. J Urol 2007;178(4 Pt 1):1354–1358; discussion 1358. [DOI] [PubMed] [Google Scholar]
- 3.Clemens JQ, Mullins C, Kusek JW, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith JW, Stephens-Shields AJ, Hou X, et al. Pain and urinary symptoms should not be combined into a single score: psychometric findings from the MAPP Research Network. Journal of Urology. 2016;195(4P1):949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai HH, Jemielita T, Sutcliffe S, et al. Characterization of whole body pain in urological chronic pelvic pain syndrome at baseline: A MAPP research network study. Journal of Urology. 2017;198(3):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutcliffe S, Gallop R, Henry Lai HH, et al. A longitudinal analysis of urological chronic pelvic pain syndrome flares in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU Int 2019;124(3):522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naliboff BD, Stephens AJ, Afari N, et al. Widespread Psychosocial Difficulties in Men and Women With Urologic Chronic Pelvic Pain Syndromes: Case-control Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015;85(6):1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naliboff BD, Stephens AJ, Lai HH, et al. Clinical and Psychosocial Predictors of Urological Chronic Pelvic Pain Symptom Change in 1 Year: A Prospective Study from the MAPP Research Network. Journal of Urology. 2017;198(4):848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger JN, Stephens AJ, Landis JR, et al. Relationship between chronic nonurological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. Journal of Urology. 2015;193(4):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodworth D, Mayer E, Leu K, et al. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS One. 2015;10(10):e0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutch JJ, Labus JS, Harris RE, et al. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 2017;158(6):1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutch JJ, Ichesco E, Hampson JP, et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158(10):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrepf A, Bradley CS, O’Donnell M, et al. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain, behavior, and immunity. 2015;49(Oct):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harte SE, Schrepf A, Gallop R, et al. Quantitative assessment of nonpelvic pressure pain sensitivity in urologic chronic pelvic pain syndrome: a MAPP Research Network study. Pain. 2019;160(6):1270–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens JQ, Mullins C, Ackerman AL, et al. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol 2019;16(3):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens-Shields AJ, Clemens JQ, Jemielita T, et al. Symptom Variability and Early Symptom Regression in the MAPP Study: A Prospective Study of Urological Chronic Pelvic Pain Syndrome. Journal of Urology. 2016;196(5):1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith JW, Stephens-Shields AJ, Hou X, et al. Pain and urinary symptoms should not be combined into a single score: psychometric findings from the MAPP Research Network. J Urol 2016;195(4P1):949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StanfordPain. Home - Collaborative Health Outcomes Information Registry. [Google Scholar]
- 20.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. The Journal of rheumatology. 2011;38(6):1113–1122. [DOI] [PubMed] [Google Scholar]
- 21.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 22.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. [DOI] [PubMed] [Google Scholar]
- 23.Harte SE, Mitra M, Ichesco EA, et al. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Med Biol Eng Comput 2013;51(6):633–644. [DOI] [PubMed] [Google Scholar]
- 24.Lai HH, Gardner V, Ness TJ, Gereau RWt. Segmental hyperalgesia to mechanical stimulus in interstitial cystitis/bladder pain syndrome: evidence of central sensitization. J Urol 2014;191(5):1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12(11 Suppl):T61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155(7):1272–1279. [DOI] [PubMed] [Google Scholar]
- 28.Schoen CJ, Ablin JN, Ichesco E, et al. A novel paradigm to evaluate conditioned pain modulation in fibromyalgia. J Pain Res 2016;9:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granot M, Weissman-Fogel I, Crispel Y, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136(1–2):142–149. [DOI] [PubMed] [Google Scholar]
- 30.Moore A. K-Means and Hierarchical Clustering. Pittsburgh, PA. K-means and Hierarchical Clustering Tutorial Slides by Andrew Moore. Web site. https://www.cs.cmu.edu/~./awm/tutorials/kmeans.html. Published 2016. Accessed. [Google Scholar]
- 31.Monti S, Tamayo P, Mesirov J, Golub T. Consensus clustering: A resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52(1–2):91–118. [Google Scholar]
- 32.Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senbabaoglu Y, Michailidis G, Li JZ. Critical limitations of consensus clustering in class discovery. Sci Rep 2014;4:6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.gplots: Various R Programming Tools for Plotting Data. R package v 3.0.1; 2016. https://CRAN.R-project.org/package=gplots. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


