Abstract
Reverse total shoulder arthroplasty (RTSA) was originally developed because of unsatisfactory results with anatomic shoulder arthroplasty options for the majority of degenerative shoulder conditions and fractures.
After initial concerns about RTSA longevity, indications were extended to primary osteoarthritis with glenoid deficiency, massive cuff tears in younger patients, fracture, tumour and failed anatomic total shoulder replacement.
Traditional RTSA by Grammont has undergone a number of iterations such as glenoid lateralization, reduced neck-shaft angle, modular, stemless components and onlay systems.
The incidence of complications such as dislocation, notching and acromial fractures has also evolved.
Computer navigation, 3D planning and patient-specific implantation have been in use for several years and mixed-reality guided implantation is currently being trialled.
Controversies in RTSA include lateralization, stemless humeral components, subscapularis repair and treatment of acromial fractures.
Cite this article: EFORT Open Rev 2021;6:189-201. DOI: 10.1302/2058-5241.6.200085
Keywords: acromion fracture, arthroplasty, design, indications, reverse, shoulder, subscapularis repair
Introduction
The reverse total shoulder arthroplasty (RTSA) was developed in the 1980s as a treatment for rotator cuff tear arthropathy in the elderly.1 It has demonstrated excellent clinical outcomes and thus has become well-established as the treatment of choice for cuff tear arthropathy. National joint registries have reported 10-year survivorship for the diagnosis of rotator cuff arthropathy of 94.1%.2 Increasing surgeon experience with the reverse prosthesis has seen a decrease in complications and a change in the indications for surgery.3 An early expanded indication was primary osteoarthritis with loss of rotator cuff function.4 Massive irreparable rotator cuff tear without osteoarthritis has also been an accepted indication for a number of years, given numerous studies have reported good functional outcomes.5 Over the last 10 years the indications for RTSA have seen a huge expansion. The Australian National Joint Registry shows the proportion of primary total shoulder arthroplasty (TSA) cases that are reverses increased from 42.2% in 2009 to 77.9% in 2018.2
This review looks at some of the more recent evidence for the following indications: posterior glenoid deficiency with intact cuff, fracture, tumour, revision surgery and in the treatment of younger patients. It also looks at some of the evolutions in design from the classic Grammont prosthesis, new technologies for precise implantation, and some of the controversies including treatment of acromion fractures and repair of subscapularis.
Indications
Posterior glenoid deficiency/wear
There has been an increasing trend towards the use of RTSA in patients with osteoarthritis and an intact rotator cuff, in the presence of posterior glenoid wear and/or humeral head subluxation. Traditionally, posterior glenoid wear has been managed with anatomic TSA and asymmetric reaming, with posterior bone graft or a posteriorly augmented glenoid for larger corrections.6 Walch et al, however, reported high rates of glenoid loosening following anatomic TSA in a series of 92 patients with biconcave glenoids and primary osteoarthritis.7 At a mean follow-up of 77 months, glenoid loosening was observed in 20.6%, with a revision rate of 16.3%.
In anatomic TSA in the setting of posterior glenoid deficiency, it is likely the recurrence of humeral head subluxation that leads to early polyethylene wear and glenoid component loosening.8 This high rate of complications with anatomic TSA has resulted in surgeons considering the use of RTSA. The reverse’s semi-constrained design corrects the posterior head subluxation.
Mizuno et al reported excellent clinical outcomes in a series of 27 patients with primary osteoarthritis and biconcave glenoids treated with RTSA, with only one failure at a mean follow-up of 54 months.9 A retrospective review of 49 shoulders with primary osteoarthritis treated with RTSA similarly demonstrated satisfactory clinical outcomes, with only two failures (both due to infection, 4%), at a mean 7.7 years follow-up.10 Preoperative glenoid morphology had no influence on outcomes.
McFarland et al also reported on 42 consecutive RTSAs, implanted without bone-grafting using asymmetric reaming, to treat glenohumeral arthritis with severe glenoid bone loss (19 type A2, five B2, and 18 C glenoids) in cuff-intact shoulders.11 They demonstrated satisfactory clinical function with only one failure (due to baseplate loosening, 2%) at a mean of three years post surgery. Virk et al used posteriorly augmented glenoids for 67 RTSAs in patients with osteoarthritis and posterior glenoid wear (Walch B2, B3, or C glenoid).12 At a mean follow-up of 40 months (+/- 15.4 months), patients demonstrated satisfactory clinical outcomes with low complications (4.5%) and no aseptic loosening of the baseplate.
Glenoid bone loss resulting in a biconcave (B2) or severely retroverted and dysplastic (C) glenoid should be considerations for use of RTSA.
Fracture
RTSA is an option for treatment of three- and four-part proximal humeral fractures in elderly patients. Poor results following hemiarthroplasty for proximal humeral fractures are well documented and often relate to tuberosity migration with malunion or nonunion.13,14 A number of recent studies have demonstrated improved clinical outcomes with RTSA compared to hemiarthroplasty.
Meta-analyses comparing RTSA with hemiarthroplasty for the treatment of proximal humeral fractures have demonstrated improvements in clinical outcome scores,15–17 forward flexion,15–18 abduction16 and tuberosity healing.16,17 Some studies have shown increased complications with RTSA18 whilst others have shown decreased complications17 or no difference.15 A systematic review including 34 trials with 2165 patients, found that RTSA had the highest Constant score and lowest incidence of complications compared to both hemiarthroplasty and surgical fixation, in the treatment of displaced proximal humeral fractures in adults.19
One trial randomized 62 patients over the age of 70 years to either RTSA or hemiarthroplasty.20 They reported significantly higher mean University of California–Los Angeles (29.1 vs. 21.1) and Constant (56.1 vs. 40.0) scores, forward elevation (120.3° vs. 79.8°), and abduction (112.9° vs. 78.7°) in the RTSA group. Six patients in the hemiarthroplasty group were converted to RTSA because of severe pain and limited function. Interestingly, they still had poor function (Constant score mean, 21.8; range, 8–51) after conversion.
A recent randomized trial compared non-operative treatment with RTSA in 59 patients aged 80 years or older with three- and four-part proximal humerus fractures.21 They reported no difference in clinical outcomes at 12-months between the two groups. It should be noted that this was a population of low-demand patients with multiple comorbidities. Most surgeons would only operate on a patient aged over 80 years if they were high functioning with an elevated quality of life.
A sensible option is a trial of non-operative management with conversion to RTSA for those in whom non-operative treatment fails. A meta-analysis looked at acute versus delayed RTSA for the treatment of proximal humeral fractures in patients over 65 years.22 They found no differences in forward flexion, clinical outcome scores or all-cause reoperation between the two groups, suggesting that delaying the surgery does not affect the final outcome. However, a study by Mechlenburg et al investigated 837 shoulder arthroplasties for failed non-operative treatment of proximal humerus fracture and found a high revision rate of 7% for hemiarthroplasty and 11% for RTSA. They also found men receiving RTSA had a higher revision rate than men undergoing hemiarthroplasty (Hazard Ratio 6, 95% Confidence Interval 2–19).23
There are some inherent fracture patterns, however, that will lead to undesirable sequelae such as malunion, nonunion, avascular necrosis, chronic locked dislocations and post-traumatic arthritis. Martinez et al reported the results of 44 patients treated with RTSA for the sequelae of proximal humerus fractures.24 This included 16 valgus-impacted malunions, eight locked dislocations or fracture/dislocations with head collapse/necrosis, 14 surgical neck nonunions and six severe tuberosity malunions. The mean Constant score increased from 28 to 58, average anterior elevation increased from 40° to 100° and 86% (38 of 44 patients) were either very satisfied or satisfied. There was, however, a high prosthetic dislocation rate (13.6%).
Raiss et al similarly found a high dislocation rate (34%) in a study of 32 nonunions of the surgical neck treated with RTSA. They also reported similar improvements in Constant score and shoulder mobility.25 Increased risk of dislocation was associated with intraoperative resection of the tuberosities,25 so preservation of the tuberosities should be performed where possible. The mean age of patients was 68 years, with a range from 48 to 83 years.
These studies suggest that RTSA has good clinical outcomes as a treatment for three- or four-part proximal humeral fractures in an elderly population. Where possible in a younger patient, surgical fixation should be attempted. However, reverse TSA offers a satisfactory option for younger patients who present with the sequelae of fractures. The surgeon should be aware of the higher complication rate, particularly dislocations.
Revision
Numerous studies have reported on outcomes for revision of shoulder arthroplasty to RTSA.26–31 They report satisfactory functional outcomes, but with complication rates as high as 47%.29 The surgeon should be cautious when interpreting many of these studies as they often group together heterogenous indications for revision as well as different types of prosthetic revision (e.g. hemiarthroplasty, anatomic, reverse).
The most common reason for revision of anatomic TSA is cuff failure.32 A recent study by Shields and Wiater reported on outcomes of revision of anatomic TSA to RTSA exclusively for rotator cuff failure or component loosening.33 They matched 35 revision patients with 70 patients undergoing primary RTSA for cuff tear arthropathy. At a mean 50 months follow-up, pain and American Shoulder and Elbow Surgeons scores were similar (p = NS). The revision group had worse subjective shoulder value scores (63 vs. 79; p = 0.002), satisfaction (74% vs. 90%; p = 0.03), and more complications (31% vs. 13%; p = 0.02). They concluded that although function is comparable, one should expect more complications and lower satisfaction.
Recently, Hernandez et al reported on the results of RTSA revision surgery for instability in 62 anatomic TSAs, 13 hemiarthroplasties and 7 RTSAs.34 The survivorship free from dislocation at five years was 79%, with the hemiarthroplasty conversion group having the highest risk of instability. They also demonstrated decreased pain, improved functional outcome scores and range of motion following revision surgery. Complication rate was reasonably high, with 12 (18%) shoulders requiring repeat revision surgery.
Melis et al reported on 37 consecutive anatomical TSA revised to RTSA for aseptic glenoid loosening/failure.35 Glenoid bone grafting was performed in 29 cases (78%). The mean Constant score increased from 24 to 55 and active anterior elevation from 68° to 121°. A postoperative complication occurred after revision in 11 patients (30%). Eight patients (21%) needed a subsequent reoperation because of glenoid loosening (n = 3), prosthetic anterior instability (n = 3), and humeral subsidence (n = 2). Wagner et al noted a significantly higher rate of revision in RTSAs with concomitant bone grafting compared to those that did not require bone grafting (24% vs. 7% five-year revision rate) in a series of 143 revision RTSAs.26
Many shoulder arthroplasty systems now use a modular humeral stem that can be converted from a hemiarthroplasty or anatomic shoulder replacement to a reverse-compatible stem. Crosby et al, in a multicentre, retrospective analysis of 102 consecutive shoulder revisions, compared 73 shoulders that required exchange of the humeral stem with 29 that had retention of a convertible-platform humeral component.36 Patients with retention had significantly shorter operative time (mean and standard deviation, 130 ± 48 versus 195 ± 58 minutes) and lower estimated blood loss (292 ± 118 versus 492 ± 334 mL). The rate of intraoperative complications was significantly lower in the retention group (0% versus 15%). Patients with retention also had improved postoperative range of motion (active external rotation, 26° ± 23° versus 11° ± 23° [p = 0.006]; active forward elevation, 112° ± 37° versus 96° ± 33° [p = 0.055]).
These studies demonstrate that RTSA is a good option for the majority of shoulder arthroplasty revisions.
Tumour
Resection of the proximal humerus in the treatment of sarcomas or other neoplasms often includes the tuberosities and rotator cuff to achieve appropriate margins. RTSA is now an option for reconstruction that has demonstrated satisfactory functional outcomes. Some studies have demonstrated equivalent functional outcomes of RTSA for other indications.37
Grosel et al reported no early complications in 13 patients who underwent reconstruction with a reverse shoulder megaprosthesis.38 They recommend RTSA in patients with a life expectancy greater than six months, with good preoperative shoulder function and where preservation of the axillary nerve is planned. Maclean et al reported no revisions or complications at a mean follow-up of 49 months in eight patients who underwent RTSA following oncologic resection.39 Functional outcomes were not great (mean abduction 62°, Musculoskeletal Tumor Society (MTS) score 60%). De Wilde et al showed excellent functional outcomes (Constant score 76, mean abduction 157°) in 14 patients who underwent RTSA following oncologic resection at a mean 7.7 years follow-up.37 Three patients (21%) had major complications (two dislocations treated with closed reduction, one infection requiring revision and one aseptic loosening requiring revision). Kaa et al reported on 16 patients who underwent RTSA following oncologic resection at a mean 44 months follow-up.40 They reported acceptable functional outcomes (mean abduction 78°, MTS 77), but with a similarly high complication rate. Four patients (25%) underwent revision surgery (two aseptic loosening, one dislocation, one deep infection). Two patients (12.5%) had a perioperative pathological fracture.
The tumours in these studies were primary bone tumours including osteosarcoma, chondrosarcoma, giant cell tumour, and Ewing sarcoma or metastatic disease. Preservation of the axillary nerve and deltoid muscle function is essential for RTSA function and stability.41 In cases where the deltoid insertion is resected from the humerus, it may be re-attached to the prosthesis with non-absorbable sutures,40 which may be augmented with an ingrowth surface38 (e.g. trevira tube).39 These studies demonstrate that RTSA is a good option for restoration of satisfactory shoulder function following resection for neoplasia. Surgeons need to be cognisant of the high complication rates and need to consider the extent of bone and soft tissue resection, as well as patient function and life expectancy, before undertaking surgery.
Young patients
Greater numbers of RTSAs are being carried out in younger patients (under 60–65 years old). Surgeons have been reluctant to perform RTSAs in young patients due to concerns about longevity of the implant. Clinical results have been shown to deteriorate after six to 10 years.42,43 High rates of complications have been reported in younger patients.44 The Australian National Joint Registry (NJR) reports significantly increased revision rates in younger age groups (at seven years: > 75 2.7%; 65–74 3.6%; 55–64 5.7%).2 Higher rates of revision in younger patients, however, are seen in all types of arthroplasty not just RTSA. Wagner et al reviewed 5494 consecutive shoulder arthroplasties (anatomic, reverse and hemiarthroplasty) performed between 1970 and 2012.45 They reported a 3% decrease in the risk for revision surgery with every one-year increase in age. Subgroup analysis across the different types of prosthesis showed the same association with age for all.
More recent studies, however, have reported promising outcomes in younger age groups. Ernstbrunner et al reported the results of 20 patients (23 shoulders) with a mean age of 57 years (range, 47–59) at a mean of 11.7 years post surgery.46 Implant survivorship was satisfactory at 91%, and there was no drop-off in clinical function. A meta-analysis of patients aged < 65 years (mean age, 56; range, 21–65) who underwent RTSA for a failed previous arthroplasty or a cuff-deficient shoulder, included eight articles with a total of 417 patients.47 The overall complication rate was 17% (range, 7–38%), with the most common complications being instability (5%) and infection (4%). The reintervention rate was 10% at four years, with implant revision in 7% of cases. Clinical outcome measures were highly satisfactory and the authors concluded that RTSA was a reliable procedure in patients aged < 65 years. Goldenberg et al similarly concluded that RTSA is safe and effective in patients younger than 65 years in their recent meta-analysis.48 They found complication, reoperation, and revision rates were similar to those seen in older patient cohorts with significant improvements in clinical outcome scores up to a mean follow-up of 4.7 years (mean follow-up range, 3.0–7.8 years). Another meta-analysis of RTSA in patients < 60 years similarly demonstrated that early clinical and functional outcomes were favourable, with long-term implant survivorship comparable to older patients.49
All these studies confirm that RTSA is a viable option for patients under 60–65 years, and should no longer be reserved for elderly patients.
Design evolution
The medialization seen in the traditional Grammont design RTSA (see Fig. 1) leads to high rates of scapular notching (up to 96% in some series),50 which may lead to micro-motion of the baseplate and glenoid loosening.51 Numerous studies have demonstrated that increasing grade of notching is associated with worse clinical outcomes (lower Constant scores).52–55 Excessive medialization may also result in detensioning of any intact cuff, which may lead to instability and weakness in external rotation.
Fig. 1.
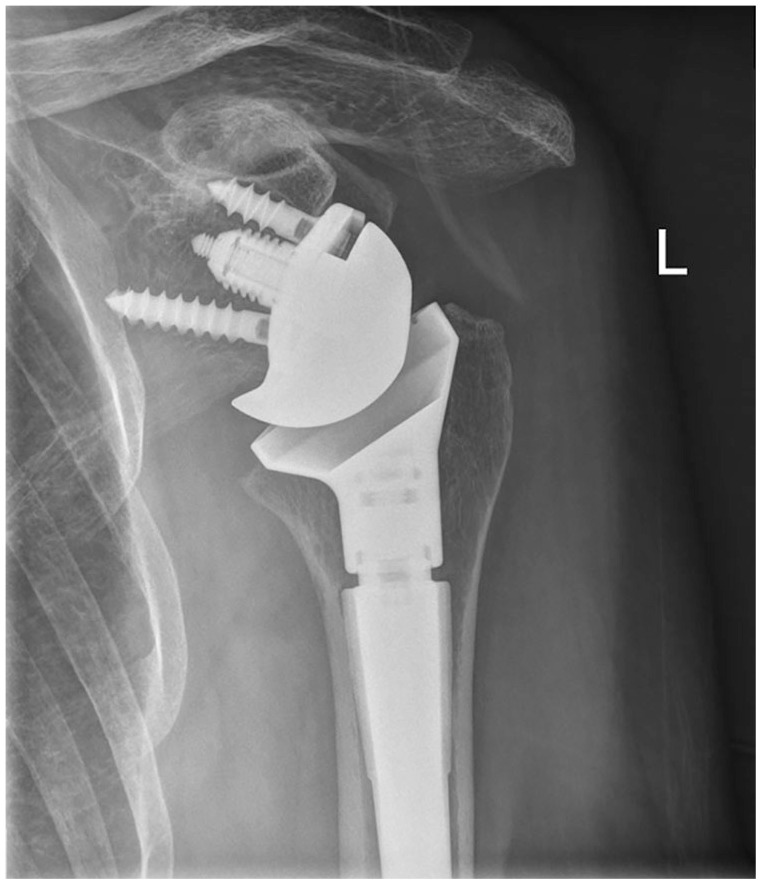
Traditional Grammont-style prosthesis with medialized glenoid and 155° neck-shaft angle and inlay humeral component. Eccentric glenosphere has been used to avoid notching.
Reverse prostheses have seen a number of evolutions to try to address some of the problems seen with the traditional design. These designs try to provide a more lateralized construct, which may be achieved at the glenoid, the humerus or both.56
Lateralization of the glenoid
Lateralization of the glenoid has been shown to decrease scapular notching. A systematic review, including 349 patients from 13 studies, found the incidence of scapular notching to be 5.4% in a lateralized glenoid group compared to 44.9% in a traditional, medialized glenoid group (p < 0.001).57 Furthermore, the lateralized group had increased mean active external rotation (46° vs. 24°, p < 0.001). However, the rate of clinically significant glenoid loosening was only 1.8% in the traditional, medialized group compared to 8.8% in the lateralized group (p = 0.003). The increase in glenoid loosening may be a result of increased shear forces at the baseplate interface. Biomechanical studies have demonstrated increased micro-motion with a lateralized design.58 Lateralization may be achieved using a prosthesis with a lateralized baseplate and glenosphere design or by bone grafting the glenoid (See Fig. 2).
Zumstein et al, similarly found an increased rate of glenoid loosening with a shoulder prosthesis using a lateralized centre of rotation compared to the traditional Grammont design (5.8% vs. 2.5%, p = 0.025).59 This study, however, only looked at one lateralized prosthesis design. A more recent systematic review of 6583 RTSAs from 103 studies, found only a slight difference in aseptic loosening rates between lateralized and medialized glenoid designs and this was not significant (1.15% medialized vs. 1.84% lateralized).60
In another systematic review comparing postoperative outcomes in patients treated with a medialized versus lateralized glenoid prostheses, Helmkamp et al demonstrated an increase in external rotation in the lateralized group (mean 21° vs. 7°).61 Otherwise, range of motion variables were similar. Lateralization also resulted in decreased scapular notching (4.3% vs. 49%). There were, however, no clear difference between groups in outcome scores.
There is no clear-cut answer on whether a lateralized glenoid is better than medialized. It is a trade-off between the different biomechanical effects. Medialization decreases forces across the glenoid component and creates compressive forces at the bone–implant interface, which may improve glenoid fixation. Lateralization avoids notching and may improve range of motion (particularly external rotation). This ambiguity is perhaps why the focus on lateralization has recently shifted to the humeral component.
Humeral component design
Lateralization on the humerus maintains the decreased torque of a medialized glenosphere/glenoid interface, with the biomechanical advantages that lateralization produces. Modifications to the humeral stem include onlay systems, curved short stems and changes to the neck-shaft angle.
The most widely used neck-shaft angle for RTSA has been 155° (see Fig. 1) for more than 10 years.4,54,62–66 However, a more horizontal humeral component is biomechanically more likely to impinge on the lateral pillar of the scapula. More recently, implants with neck-shaft angles of 135° and 145° have been developed in an attempt to reduce scapula notching. There are, however, concerns that a reduced neck-shaft angle may lead to a higher dislocation rate.1,67
Biomechanical studies have demonstrated increased range of motion with decreasing neck-shaft angle.68 A lower neck angle increases the range before scapula bone contact occurs and decreases contact area at the inferior scapular neck, indicating that decreased scapula notching would be expected. Decreasing the neck-shaft angle has also been coupled with a change to an onlay proximal interface in some systems (see Fig. 2). The original Grammont prosthesis had an inlay design to increase bony contact with the proximal component. This, however, requires increased reaming of the metaphyseal bone. An onlay proximal interface combined with a curved-stem design preserves proximal bone and the angled cut may be less likely to damage the greater tuberosity and any remaining cuff. It also allows for interchangeability between reverse prostheses with hemiarthroplasty and anatomic TSA. The onlay design results in more lateral displacement of the humerus,69 which produces increased tensioning of the anterior and posterior rotator cuff and lengthens the deltoid moment arm.70 A clinical trial comparing RTSAs performed using the onlay, curved-stem prosthesis demonstrated improved external rotation and decreased notching compared to a traditional design.71 There were, however, increased numbers of scapular fractures with the onlay stems. This increase in scapular fractures has been reported by other authors.72 Increased deltoid tension secondary to over-lengthening the arm has been proposed as a possible causative mechanism.73 Although prosthesis design has been demonstrated to affect deltoid load, we are unaware of any evidence directly linking deltoid tension to acromion and scapular fractures.74
Fig. 2.
Lateralized humeral component with 135° neck-shaft angle and onlay design. Lateralized glenoid component with metal augmentation (A) or bone augmentation (B).
A recent meta-analysis of 2222 shoulders undergoing RTSA (across 38 studies) compared the rate of scapula notching and dislocation between implants with neck-shaft angles of 155° and 135°.75 Of these, 1762 (79.3%) had implants with a neck-shaft angle of 155°, and 460 had implants with 135° with a lateralized glenosphere. Scapular notching was found to be more common in the 155° group at 16.80% compared to 2.83% in the 135° group (p < 0.0001). There was no statistical difference in dislocation rates between the two groups, at 2.33% for the 155° and 1.74% for the 135° group (p = ns). Further studies are needed to compare these neck-shaft angles with and without glenoid lateralization, as lateralization alone has been shown to decrease the rate of scapular notching with a 155° prosthesis.76 Another meta-analysis by the same group also demonstrated increased external rotation with a 135° humeral inclination compared to a 155° prosthesis (33° vs. 25°, p < 0.001).77
Modified humeral designs with lower neck-shaft angle may reduce scapula notching and increase external rotation without decreasing stability. Further clinical trials are required for this to be definitively proven as well as to investigate the effect on scapula fractures.
Stemless
Stemless total shoulder implants were first introduced to the market in 2004.78 Around seven different stemless designs (including anatomic and reverse) are available from six implant companies.79 The two main types of stemless implants are impaction and screw-in. The reported advantages of stemless prostheses include bone preservation, decreased surgical time, lower blood loss, less stress shielding distally, removing the diaphyseal stress riser and less lateralization.79–87 Disadvantages include dependence on proximal bone stock, increased cost, and reliance on subscapularis repair. There are also concerns regarding proximal bone response to a stemless implant and a lack of high-quality, long-term studies looking at their performance.88
Levy et al performed a prospective study of 102 consecutive patients who received cementless RTSA with the Verso implant (Innovative Design, London, UK) with a two- to seven-year follow-up.89 Seventy-eight patients were female, and the mean age was 74.4 years. Indications for surgery included rotator cuff arthropathy (65), fracture sequelae (12), rheumatoid arthritis (13), failed cuff repair (3), cuff deficiency with a loose prosthesis (3) and acute fracture (2). Patient satisfaction improved with an increase in Subjective Shoulder Value from 8/100 to 85/100. Constant scores improved with 14 to 59 (age and sex adjusted, 86; p < 0.0001). All range-of-motion parameters increased – from 47° to 129° in elevation, 10° to 51° in external rotation, and 21° to 65° in internal rotation. Radiographic analysis did not show any lucencies, subsidence or stress shielding, however, glenoid notching was noted in 21 patients.
Teissier et al prospectively analysed 105 RTSAs in patients receiving the Total Evolutive Shoulder System (TESS; Biomet, Warsaw, IN, USA).90 The mean age was 73 years and mean follow-up time 41 months. Ninety-six per cent of patients reported their satisfaction as good or excellent. Improvement was seen in the Constant score from 40 points preoperatively to 68 points at last follow-up (p < 0.001). Mean flexion was 143° (90–170°) and external rotation was 39° (20–70°). Scapular notching was noted in 17 patients.
Von Engelhardt evaluated 67 patients receiving the TESS implant (56 stemless, 11 stemmed) with a mean follow-up time of 17.5 months.91 A significant improvement in relative Constant (11.3% vs. 78.8%) and Disabilities of the Arm, Shoulder and Hand (DASH) scores (73.7 vs. 31.8) was noted without significant differences between stemless and stemmed. One stemless case was revised due to humeral component loosening and scapula notching was noted in nine cases.
There is limited evidence for stemless humeral components in RTSA, with the majority of studies reporting short- to medium- term results. Long-term survivorship needs to be determined. There are also complications specific to the design with periprosthetic fractures from impaction onto the lateral cortex being reported (see Fig. 3).
Fig. 3.
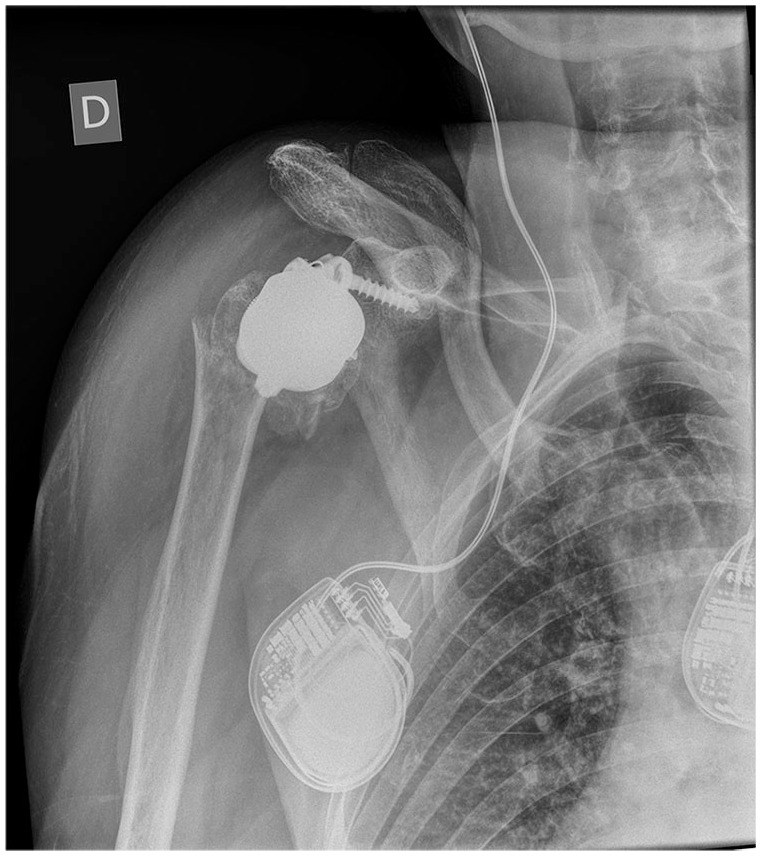
Stemless design reverse total shoulder arthroplasty (RTSA) with periprosthetic fracture.
Computer navigation, patient-specific instrumentation and mixed-reality guided implantation
Positioning of the glenoid is an important factor in the functional outcome and survival of RTSA.92 One of the most common postoperative complications of RTSA is failure of the glenoid component, and glenoid component malposition has been associated with humeral instability, increased stress at the bone–prosthesis interface, early failure and an inferior outcome.93–96 Poor visualization, complex and variable anatomy, limited bony landmarks as well as abnormal glenoid morphology are just some of the factors that make positioning of the glenoid component technically challenging.94,97 In recent years, computer navigation (NAV) and patient-specific instrumentation (PSI) have been developed to improve glenoid component positioning in shoulder arthroplasty.98
Throckmorton et al performed a study on 70 cadaver shoulders with radiologically confirmed arthritis that were randomized into PSI and standard instrumentation (SI) groups, with 36 anatomic TSAs and 34 RTSAs.99 The glenoid components in anatomic TSAs with PSI averaged 5° of deviation from the target version and 3° in inclination compared to 8° of version and 7° of inclination in the SI group. This finding was not replicated in the RTSA patients, with no statistical difference in degrees of variation in version or inclination. The number of outliers (defined as version or inclination errors greater than 10° or 4 mm offset error) was improved with statistical significance in the PSI group compared to SI (17% vs. 66%, p < 0.05). This finding was replicated by Hendel et al in their study of 31 anatomic TSAs, who also found a reduction in outliers in the PSI group (27% vs. 75%, p < 0.05).100
Kircher et al performed a prospective, randomized controlled trial of 20 patients with total shoulder arthroplasty for osteoarthritis, with or without glenoid navigation (Nano Station Praxim, Grenoble, France) with a passive optical tracking system for intraoperative navigation).101 The patients in the navigation group were found to have improved accuracy of glenoid version (measured on axial CT slices) with an average change of retroversion from 15.4° ± 5.8° to 3.7° ± 6.3°, compared to 14.4° ± 6.1° to 10.9° ± 6.8° in the SI group.101 However, it was noted that operative time was significantly longer in the navigated group at 169.5 ± 15.2 compared to 138 ± 18.4 minutes.101
A recent meta-analysis by Burns et al analysed 269 TSAs and RTSAs in 258 patients across nine studies (four controlled and five uncontrolled).102 They found no significant effect of navigation on version error or inclination error. However, there was a statistically significant improvement in PSI glenoid version error (mean difference –6.3°, 95% CI –0.7° to –11.8°, p = 0.03) and inclination error (mean difference –8.2°, 95% CI –2.0° to –14.4°, p < 0.01). Although these improvements are encouraging, it is unknown whether they correlate with clinical outcomes.
Another new innovative technology is mixed-reality guided implantation – a form of augmented reality that involves the use of a headset that projects a virtual 3D reconstruction of the scapula that can be manually positioned by the surgeon. Only one case report has been published for RTSA: Gregory et al used a Microsoft HoloLens to perform RTSA in an 80-year-old female with advanced arthritis and Walch A2-type glenoid.103 Although it is only in its infancy, this technology has immense potential for future surgeons as a practical and educational tool.
Challenges associated with navigation in shoulder arthroplasty are increased operative time, cost, increased labour and aborted use due to malfunction.98,101 PSI requires the ordering and manufacturing of a custom implant, which also creates a logistical challenge and cost. There are no studies directly comparing navigation with patient-specific instrumentation for shoulder arthroplasty, and the overall cost effectiveness of these technologies has not been researched. While some surgeons choose to use NAV or PSI routinely, there are no absolute indications for the use of either. In our experience, they are useful tools in patients with challenging anatomy, for example severe glenoid bone loss. A study of anatomic TSAs found that patients with greater than 16° of glenoid retroversion benefitted the most from PSI, with regard to accuracy of implantation.100
Controversies
Acromial fracture
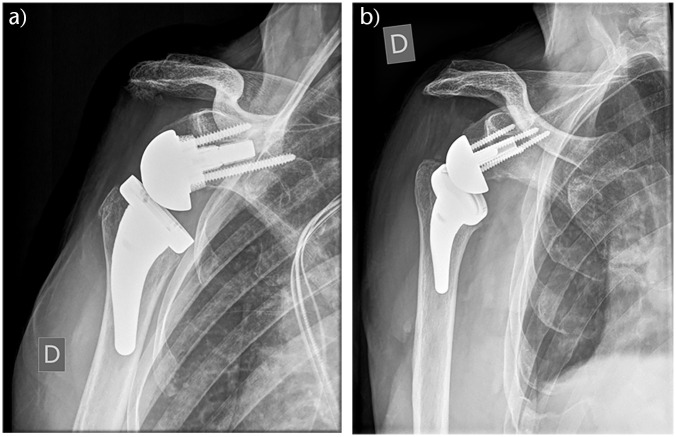
Acromial fracture after RTSA is posited to occur because the longer arm length and increased tension of the deltoid muscle transmits higher forces through the muscle origin (see Fig. 4). Additionally, acromial fractures have been associated with osteoporosis, prosthesis design, surgical approach, screw position and length in the glenoid, and technical factors such as deltoid tension.73,104–107
Fig. 4.
Radiograph of scapula spine fracture post reverse total shoulder arthroplasty (RTSA) (A). Three-dimensional surgical planning for plate fixation (B). Postoperative radiographs (C).
The majority of studies looking at this complication report low numbers of between 1 to 25 fractures.50,104,108–113 One of the largest studies was performed by Teusink et al (n = 1018 RTSAs), who retrospectively reviewed 25 (3.1%) non-operatively managed scapular fractures post RTSA with case controls. The patients were matched 1:4 to a control group, and a higher revision rate was found in the fracture group (8% vs. 2%); however, this was not statistically significant.113
Crosby et al reviewed the records of 400 RTSAs and identified 22 cases (5.5%) of acromial fracture, and described three patterns: type I, avulsion fractures of the anterior acromion; type II, fractures to the acromion posterior the acromioclavicular joint; and type III, scapular spine fractures.104 Eight (2.0%) type I fractures were successfully treated non-operatively. Of the 10 (2.5%) type II fractures, seven were managed operatively with improvement in their symptoms; four (1%) had type III fractures and were all successfully managed operatively.
Levy et al provided an alternative classification of acromial fractures post RTSA, with three types having varying involvement of the deltoid origin.114 Type I fractures involved a portion of the anterior and middle deltoid origin, type II at least the entire middle deltoid origin and type III the entire middle and posterior origin. In their study of 157 patients with RTSA, 18 acromial fractures were identified and were all managed non-operatively, however, they described a limitation in their functional outcomes.
There have been three recent published meta-analyses looking at acromial fracture post RTSA.115,116,117, Cho and colleagues reviewed 15 studies (n = 2857 shoulders) with a mean age of 72.9 years and found the incidence of acromial fracture to be 4.0%;115 87.7% of acromial fractures were managed non-operatively and 12.3% were managed with surgery, with a union rate of 43.8% and 87.5% respectively. The mean follow-up time was 34 months. Differences were found in the rate of acromial fracture of medial glenoid/medial humeral, lateral glenoid/medial humeral, and medial glenoid/lateral humeral prostheses with rates of 8.4%, 4.0%, and 2.8% respectively.
Patterson et al reviewed 32 studies (n = 3838 RTSAs) with 159 reported acromial fractures – an incidence rate of 4.14%.116 One hundred and thirty nine patients were treated non-operatively with a sling or brace, and 20 were treated with open reduction and internal fixation. All patients reported inferior functional outcome scores with average Constant and American Shoulder and Elbow Society (ASES) scores of 63 and 57, respectively when compared to their pre-fracture state. They also reported decreased forward flexion (95°) and abduction (76°) after acromial fracture.
King et al performed a systematic review of 90 articles (9048 RTSAs) and found the incidence of acromial and/or scapular spine fracture to be 2.8%.117 Risk factors for fracture included inflammatory arthritis (10.9%), massive rotator cuff tears (3.8%) and lateralized glenosphere designs (3.8%). The incidence was lowest in acute proximal humerus fractures (0%) and post-traumatic arthritis (2.1%).
These studies establish that acromial fracture has a marked detrimental effect on outcomes following RTSA. Further research is required to determine whether operative or non-operative treatment delivers improved results.
Subscapularis repair
Instability and dislocation are devastating complications following RTSA, and one hypothesized cause is a dysfunctional subscapularis tendon. However, management of the subscapularis tendon during RTSA is controversial, with conflicting studies reporting the outcomes after repair. A number of studies report a significant increase in dislocation rates when the subscapularis is not repaired.118–120 Edwards et al evaluated 138 consecutive RTSAs and found subscapularis was repairable in 62 and irreparable in 76. They found all seven dislocations occurred in the irreparable group.119 Trappey et al retrospectively analysed 284 patients undergoing RTSA (212 primary, 72 revision) and found that patients with an irreparable subscapularis tendon had a higher rate of instability (14 of 123 [11%]) compared to patients with a repairable tendon (1 of 161 [< 1%]).120 All three studies reported results of RTSA with medializing designs.
More recent studies have not shown any difference between repair and non-repair groups.4,121–123 Clark et al identified 65 patients who received RTSA with subscapularis repair and 55 without subscapularis repair.121 Dislocation was noted in two patients in the repair group and three in the non-repair group and was not statistically significant.121 Friedman et al analysed 340 RTSA patients with subscapularis repair and 251 without repair, and found that there was a dislocation rate of 0% in the repair group and 1.2% in the non-repair group, and this was not statistically significant.122 Vourazeris retrospectively studied 202 patients undergoing primary RTSA, and found no significant difference in the rate of dislocation in the subscapularis repair group (0% of 86) compared to the non-repair group (2.6% of 118).123 The study by Clark reflects results of RTSA with increased glenoid-sided lateralization built into the glenosphere, while the latter two studies report results of RTSA designs with humeral-sided lateralization. Modern lateralizing prosthesis designs are reported to decreased instability compared to classic Grammont design by increasing joint-reaction forces and the wrapping angle of the deltoid muscle.56,124
A recent meta-analysis by Matthewson and colleagues included 1306 patients post RTSA and found an overall lower dislocation rate when the subscapularis was repaired (OR 0.19, p < 0.001), but also found that in patients without repair, a lateralized centre of rotation (COR) resulted in a decreased dislocation rate compared to medialized COR (OR 0.24, p < 0.001).125 Other findings included largely equivalent clinical outcomes between subscapularis repair and non-repair groups after RTSA. However, there is a lack of high-quality studies looking at the effect of subscapularis repair.
It seems that repairing the subscapularis is less important for stability with modern lateralizing prosthesis designs. In non-lateralizing fracture prosthesis we recommend subscapularis and lesser tubercle repair. For elective RTSA, subscapularis repair is recommended as long as its tendon and muscle quality are appropriate, especially in cuff arthropathies. Repairing the subscapularis is reported to have an advantage for active internal rotation,126 but with a possible drawback of decreased passive external rotation.127 Subscapularis tendon release and repair should allow for intraoperative external rotation with the arm at side of at least 45°. With modern lateralizing prosthesis designs, we do not recommend tight repairs which have the potential to lead to stiffness and pain, influencing postoperative recovery.128
Conclusion
RTSA should be used in the management of posterior glenoid wear, glenoid retroversion and dysplasia, three- and four-part proximal humerus fracture, tumour reconstruction, failed anatomic TSA and is increasingly being used in younger patients. Modern RTSA designs with a lower neck-shaft may reduce scapula notching and increase external rotation without decreasing stability. Further clinical trials are required for this to be definitively proven as well as to investigate the effect on scapula fractures. Similarly, stemless RTSA has promising early results but will require larger, longer-term studies. NAV and PSI have been shown to improve the accuracy of glenoid implantation. Long-term clinical studies or registry results are required to determine whether this is translated to a proven clinical benefit. The cost-effectiveness should also be researched. Acromial fracture after RTSA is estimated to occur in 3–4% of cases and has a marked detrimental effect on outcomes following RTSA. Further research, ideally with randomized clinical trials, is required to determine whether operative or non-operative treatment delivers better results. We recommend subscapularis tendon repair in RTSA with Grammont design for fracture, which we believe to be an important factor in postoperative stability. The effect of subscapularis tendon repair on RTSA dislocation rates of current, lateralizing prosthesis designs should be investigated with high-quality registry data since large numbers are required for adequate statistical power.
Footnotes
ICMJE Conflict of interest statement: GW reports consulting fees, Support for travel to meetings for study or other purposes and royalties from Wright, related to the submitted work.
The other authors declare no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65–68. [DOI] [PubMed] [Google Scholar]
- 2. Australian Orthopaedic Association National Joint Replacement Registry. Hip, Knee & Shoulder Arthroplasty Annual Report 2019. Adelaide: AOA, 2019. [Google Scholar]
- 3. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg 2012;21:1470–1477. [DOI] [PubMed] [Google Scholar]
- 4. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476–1485. [DOI] [PubMed] [Google Scholar]
- 5. Sevivas N, Ferreira N, Andrade R, et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg 2017;26:e265–e277. [DOI] [PubMed] [Google Scholar]
- 6. Luedke C, Kissenberth MJ, Tolan SJ, Hawkins RJ, Tokish JM. Outcomes of anatomic total shoulder arthroplasty with B2 glenoids: a systematic review. JBJS Rev 2018;6:e7. [DOI] [PubMed] [Google Scholar]
- 7. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 2012;21:1526–1533. [DOI] [PubMed] [Google Scholar]
- 8. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am 2003;85:251–258. [DOI] [PubMed] [Google Scholar]
- 9. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am 2013;95:1297–1304. [DOI] [PubMed] [Google Scholar]
- 10. Collin P, Hervé A, Walch G, Boileau P, Muniandy M, Chelli M. Mid-term results of reverse shoulder arthroplasty for glenohumeral osteoarthritis with posterior glenoid deficiency and humeral subluxation. J Shoulder Elbow Surg 2019;28:2023–2030. [DOI] [PubMed] [Google Scholar]
- 11. McFarland EG, Huri G, Hyun YS, Petersen SA, Srikumaran U. Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J Bone Joint Surg Am 2016;98:1801–1807. [DOI] [PubMed] [Google Scholar]
- 12. Virk M, Yip M, Liuzza L, et al. Clinical and radiographic outcomes with a posteriorly augmented glenoid for Walch B2, B3, and C glenoids in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2020;29:e196–e204. [DOI] [PubMed] [Google Scholar]
- 13. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2002;11:401–412. [DOI] [PubMed] [Google Scholar]
- 14. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg 2003;12:569–577. [DOI] [PubMed] [Google Scholar]
- 15. Mata-Fink A, Meinke M, Jones C, Kim B, Bell J-E. Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: a systematic review. J Shoulder Elbow Surg 2013;22:1737–1748. [DOI] [PubMed] [Google Scholar]
- 16. Shukla DR, McAnany S, Kim J, Overley S, Parsons BO. Hemiarthroplasty versus reverse shoulder arthroplasty for treatment of proximal humeral fractures: a meta-analysis. J Shoulder Elbow Surg 2016;25:330–340. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Zhu Y, Zhang F, Chen W, Tian Y, Zhang Y. Meta-analysis suggests that reverse shoulder arthroplasty in proximal humerus fractures is a better option than hemiarthroplasty in the elderly. Int Orthop 2016;40:531–539. [DOI] [PubMed] [Google Scholar]
- 18. Ferrel JR, Trinh TQ, Fischer RA. Reverse total shoulder arthroplasty versus hemiarthroplasty for proximal humeral fractures: a systematic review. J Orthop Trauma 2015;29:60–68. [DOI] [PubMed] [Google Scholar]
- 19. Chen L, Xing F, Xiang Z. Effectiveness and safety of interventions for treating adults with displaced proximal humeral fracture: a network meta-analysis and systematic review. PLoS One 2016;11:e0166801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures: a blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg 2014;23:1419–1426. [DOI] [PubMed] [Google Scholar]
- 21. Lopiz Y, Alcobía-Díaz B, Galán-Olleros M, García-Fernández C, Picado AL, Marco F. Reverse shoulder arthroplasty versus nonoperative treatment for 3- or 4-part proximal humeral fractures in elderly patients: a prospective randomized controlled trial. J Shoulder Elbow Surg 2019;28:2259–2271. [DOI] [PubMed] [Google Scholar]
- 22. Torchia MT, Austin DC, Cozzolino N, Jacobowitz L, Bell J-E. Acute versus delayed reverse total shoulder arthroplasty for the treatment of proximal humeral fractures in the elderly population: a systematic review and meta-analysis. J Shoulder Elbow Surg 2019;28:765–773. [DOI] [PubMed] [Google Scholar]
- 23. Mechlenburg I, Rasmussen S, Unbehaun D, Amundsen A, Rasmussen JV. Patients undergoing shoulder arthroplasty for failed nonoperative treatment of proximal humerus fracture have low implant survival and low patient-reported outcomes: 837 cases from the Danish Shoulder Arthroplasty Registry. Acta Orthop 2020;91:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez AA, Calvo A, Bejarano C, Carbonel I, Herrera A. The use of the Lima reverse shoulder arthroplasty for the treatment of fracture sequelae of the proximal humerus. J Orthop Sci 2012;17:141–147. [DOI] [PubMed] [Google Scholar]
- 25. Raiss P, Edwards TB, da Silva MR, Bruckner T, Loew M, Walch G. Reverse shoulder arthroplasty for the treatment of nonunions of the surgical neck of the proximal part of the humerus (type-3 fracture sequelae). J Bone Joint Surg Am 2014;96:2070–2076. [DOI] [PubMed] [Google Scholar]
- 26. Wagner E, Houdek MT, Griffith T, et al. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 2015;97:1653–1660. [DOI] [PubMed] [Google Scholar]
- 27. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am 2007;89:292–300. [DOI] [PubMed] [Google Scholar]
- 28. Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br 2007;89:189–195. [DOI] [PubMed] [Google Scholar]
- 29. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006;15:527–540. [DOI] [PubMed] [Google Scholar]
- 30. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:1478–1483. [DOI] [PubMed] [Google Scholar]
- 31. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:514–522. [DOI] [PubMed] [Google Scholar]
- 32. Knowles NK, Columbus MP, Wegmann K, Ferreira LM, Athwal GS. Revision shoulder arthroplasty: a systematic review and comparison of North American vs. European outcomes and complications. J Shoulder Elbow Surg 2020;29:1071–1082. [DOI] [PubMed] [Google Scholar]
- 33. Shields E, Wiater JM. Patient outcomes after revision of anatomic total shoulder arthroplasty to reverse shoulder arthroplasty for rotator cuff failure or component loosening: a matched cohort study. J Am Acad Orthop Surg 2019;27:e193–e198. [DOI] [PubMed] [Google Scholar]
- 34. Hernandez NM, Chalmers BP, Wagner ER, Sperling JW, Cofield RH, Sanchez-Sotelo J. Revision to reverse total shoulder arthroplasty restores stability for patients with unstable shoulder prostheses. Clin Orthop Relat Res 2017;475:2716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg 2012;21:342–349. [DOI] [PubMed] [Google Scholar]
- 36. Crosby LA, Wright TW, Yu S, Zuckerman JD. Conversion to reverse total shoulder arthroplasty with and without humeral stem retention: the role of a convertible-platform stem. J Bone Joint Surg Am 2017;99:736–742. [DOI] [PubMed] [Google Scholar]
- 37. De Wilde L, Boileau P, Van der Bracht H. Does reverse shoulder arthroplasty for tumors of the proximal humerus reduce impairment? Clin Orthop Relat Res 2011;469:2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grosel TW, Plummer DR, Mayerson JL, Scharschmidt TJ, Barlow JD. Oncologic reconstruction of the proximal humerus with a reverse total shoulder arthroplasty megaprosthesis. J Surg Oncol 2018;118:867–872. [DOI] [PubMed] [Google Scholar]
- 39. Maclean S, Malik SS, Evans S, Gregory J, Jeys L. Reverse shoulder endoprosthesis for pathologic lesions of the proximal humerus: a minimum 3-year follow-up. J Shoulder Elbow Surg 2017;26:1990–1994. [DOI] [PubMed] [Google Scholar]
- 40. Kaa AKS, Jørgensen PH, Søjbjerg JO, Johannsen HV. Reverse shoulder replacement after resection of the proximal humerus for bone tumours. Bone Joint J 2013;95-B:1551–1555. [DOI] [PubMed] [Google Scholar]
- 41. De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res 2005;430:156–162. [DOI] [PubMed] [Google Scholar]
- 42. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006;88:1742–1747. [DOI] [PubMed] [Google Scholar]
- 43. Favard L, Levigne C, Nerot C, Gerber C, De Wilde L, Mole D. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res 2011;469:2469–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ek ETH, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013;22:1199–1208. [DOI] [PubMed] [Google Scholar]
- 45. Wagner ER, Houdek MT, Schleck CD, et al. The role age plays in the outcomes and complications of shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1573–1580. [DOI] [PubMed] [Google Scholar]
- 46. Ernstbrunner L, Suter A, Catanzaro S, Rahm S, Gerber C. Reverse total shoulder arthroplasty for massive, irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am 2017;99:1721–1729. [DOI] [PubMed] [Google Scholar]
- 47. Chelli M, Lo Cunsolo L, Gauci M-O, et al. Reverse shoulder arthroplasty in patients aged 65 years or younger: a systematic review of the literature. JSES Open Access 2019;3:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goldenberg BT, Samuelsen BT, Spratt JD, Dornan GJ, Millett PJ. Complications and implant survivorship following primary reverse total shoulder arthroplasty in patients younger than 65 years: a systematic review. J Shoulder Elbow Surg 2020;29:1703–1711. [DOI] [PubMed] [Google Scholar]
- 49. Bedeir YH, Gawish HM, Grawe BM. Outcomes of reverse total shoulder arthroplasty in patients 60 years of age or younger: a systematic review. J Hand Surg Am 2020;45:254.e1–254.e8. [DOI] [PubMed] [Google Scholar]
- 50. Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005;87:1476–1486. [DOI] [PubMed] [Google Scholar]
- 51. Roche CP, Stroud NJ, Martin BL, et al. The impact of scapular notching on reverse shoulder glenoid fixation. J Shoulder Elbow Surg 2013;22:963–970. [DOI] [PubMed] [Google Scholar]
- 52. Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017;26:1253–1261. [DOI] [PubMed] [Google Scholar]
- 53. Wellmann M, Struck M, Pastor MF, Gettmann A, Windhagen H, Smith T. Short and midterm results of reverse shoulder arthroplasty according to the preoperative etiology. Arch Orthop Trauma Surg 2013;133:463–471. [DOI] [PubMed] [Google Scholar]
- 54. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am 2007;89:588–600. [DOI] [PubMed] [Google Scholar]
- 55. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004;86:388–395. [DOI] [PubMed] [Google Scholar]
- 56. Werthel J-D, Walch G, Vegehan E, Deransart P, Sanchez-Sotelo J, Valenti P. Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop 2019;43:2349–2360. [DOI] [PubMed] [Google Scholar]
- 57. Lawrence C, Williams GR, Namdari S. Influence of glenosphere design on outcomes and complications of reverse arthroplasty: a systematic review. Clin Orthop Surg 2016;8:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 2005;14:162S–167S. [DOI] [PubMed] [Google Scholar]
- 59. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146–157. [DOI] [PubMed] [Google Scholar]
- 60. Rojas J, Choi K, Joseph J, Srikumaran U, McFarland EG. Aseptic glenoid baseplate loosening after reverse total shoulder arthroplasty: a systematic review and meta-analysis. JBJS Rev 2019;7:e7. [DOI] [PubMed] [Google Scholar]
- 61. Helmkamp JK, Bullock GS, Amilo NR, et al. The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2018;27:2099–2107. [DOI] [PubMed] [Google Scholar]
- 62. De Biase CF, Delcogliano M, Borroni M, Castagna A. Reverse total shoulder arthroplasty: radiological and clinical result using an eccentric glenosphere. Musculoskelet Surg 2012;96:S27–S34. [DOI] [PubMed] [Google Scholar]
- 63. Martinez AA, Bejarano C, Carbonel I, Iglesias D, Gil-Albarova J, Herrera A. The treatment of proximal humerus nonunions in older patients with reverse shoulder arthroplasty. Injury 2012;43:S3–S6. [DOI] [PubMed] [Google Scholar]
- 64. Randelli P, Randelli F, Arrigoni P, et al. Optimal glenoid component inclination in reverse shoulder arthroplasty: how to improve implant stability. Musculoskelet Surg 2014;98:15–18. [DOI] [PubMed] [Google Scholar]
- 65. Sayana MK, Kakarala G, Bandi S, Wynn-Jones C. Medium term results of reverse total shoulder replacement in patients with rotator cuff arthropathy. Ir J Med Sci 2009;178:147–150. [DOI] [PubMed] [Google Scholar]
- 66. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am 2011;93:1915–1923. [DOI] [PubMed] [Google Scholar]
- 67. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S–161S. [DOI] [PubMed] [Google Scholar]
- 68. Oh JH, Shin S-J, McGarry MH, Scott JH, Heckmann N, Lee TQ. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1091–1098. [DOI] [PubMed] [Google Scholar]
- 69. Beltrame A, Di Benedetto P, Cicuto C, Cainero V, Chisoni R, Causero A. Onlay versus inlay humeral steam in reverse shoulder arthroplasty (RSA): clinical and biomechanical study. Acta Biomed 2019;90:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roche CP, Diep P, Hamilton M, Crosby LA, Flurin P-H, Wright TW, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis 2013;71:284–293. [PubMed] [Google Scholar]
- 71. Merolla G, Walch G, Ascione F, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg 2018;27:701–710. [DOI] [PubMed] [Google Scholar]
- 72. Ascione F, Kilian CM, Laughlin MS, et al. Increased scapular spine fractures after reverse shoulder arthroplasty with a humeral onlay short stem: an analysis of 485 consecutive cases. J Shoulder Elbow Surg 2018;27:2183–2190. [DOI] [PubMed] [Google Scholar]
- 73. Mayne IP, Bell SN, Wright W, Coghlan JA. Acromial and scapular spine fractures after reverse total shoulder arthroplasty. Shoulder Elbow 2016;8:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Giles JW, Langohr GDG, Johnson JA, Athwal GS. Implant design variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res 2015;473:3615–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2015;24:988–993. [DOI] [PubMed] [Google Scholar]
- 76. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Erickson BJ, Harris JD, Romeo AA. The effect of humeral inclination on range of motion in reverse total shoulder arthroplasty: a systematic review. Am J Orthop (Belle Mead NJ) 2016;45:E174–E179. [PubMed] [Google Scholar]
- 78. Huguet D, DeClercq G, Rio B, Teissier J, Zipoli B; TESS Group. Results of a new stemless shoulder prosthesis: radiologic proof of maintained fixation and stability after a minimum of three years’ follow-up. J Shoulder Elbow Surg 2010;19:847–852. [DOI] [PubMed] [Google Scholar]
- 79. Churchill RS, Athwal GS. Stemless shoulder arthroplasty-current results and designs. Curr Rev Musculoskelet Med 2016;9:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kadum B, Wahlström P, Khoschnau S, Sjödén G, Sayed-Noor A. Association of lateral humeral offset with functional outcome and geometric restoration in stemless total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:e285–e294. [DOI] [PubMed] [Google Scholar]
- 81. Nyffeler RW, Sheikh R, Jacob HAC, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction: an in vitro study. J Bone Joint Surg Am 2004;86:575–580. [DOI] [PubMed] [Google Scholar]
- 82. Habermeyer P, Lichtenberg S, Tauber M, Magosch P. Midterm results of stemless shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg 2015;24:1463–1472. [DOI] [PubMed] [Google Scholar]
- 83. Berth A, März V, Wissel H, Awiszus F, Amthauer H, Lohmann CH. SPECT/CT demonstrates the osseointegrative response of a stemless shoulder prosthesis. J Shoulder Elbow Surg 2016;25:e96–e103. [DOI] [PubMed] [Google Scholar]
- 84. Bohsali KI, Wirth MA, Rockwood CA, Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006;88:2279–2292. [DOI] [PubMed] [Google Scholar]
- 85. Harmer L, Throckmorton T, Sperling JW. Total shoulder arthroplasty: are the humeral components getting shorter? Curr Rev Musculoskelet Med 2016;9:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Berth A, Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: a comparison of the functional outcome after a minimum of two years follow-up. J Orthop Traumatol 2013;14:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hawi N, Magosch P, Tauber M, Lichtenberg S, Habermeyer P. Nine-year outcome after anatomic stemless shoulder prosthesis: clinical and radiologic results. J Shoulder Elbow Surg 2017;26:1609–1615. [DOI] [PubMed] [Google Scholar]
- 88. Ew B, Ev F, Mt O, Ba P. Stemless humeral implants in total shoulder arthroplasty. J Am Acad Orthop Surg 2020;28:e277–e287. [DOI] [PubMed] [Google Scholar]
- 89. Levy O, Narvani A, Hous N, et al. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral implant without a stem: clinical and radiologic outcomes in prospective 2- to 7-year follow-up study. J Shoulder Elbow Surg 2016;25:1362–1370. [DOI] [PubMed] [Google Scholar]
- 90. Teissier P, Teissier J, Kouyoumdjian P, Asencio G. The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: clinical and radiographic results. J Shoulder Elbow Surg 2015;24:45–51. [DOI] [PubMed] [Google Scholar]
- 91. von Engelhardt LV, Manzke M, Filler TJ, Jerosch J. Short-term results of the reverse Total Evolutive Shoulder System (TESS) in cuff tear arthropathy and revision arthroplasty cases. Arch Orthop Trauma Surg 2015;135:897–904. [DOI] [PubMed] [Google Scholar]
- 92. Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524–528. [DOI] [PubMed] [Google Scholar]
- 93. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg 2005;14:111S–121S. [DOI] [PubMed] [Google Scholar]
- 94. Matsen FAI, III, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am 2008;90:885–896. [DOI] [PubMed] [Google Scholar]
- 95. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg 2002;11:130–135. [DOI] [PubMed] [Google Scholar]
- 96. Gregory TM, Sankey A, Augereau B, et al. Accuracy of glenoid component placement in total shoulder arthroplasty and its effect on clinical and radiological outcome in a retrospective, longitudinal, monocentric open study. PLoS One 2013;8:e75791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:48–55. [DOI] [PubMed] [Google Scholar]
- 98. Verborgt O, Vanhees M, Heylen S, Hardy P, Declercq G, Bicknell R. Computer navigation and patient-specific instrumentation in shoulder arthroplasty. Sports Med Arthrosc Rev 2014;22:e42–e49. [DOI] [PubMed] [Google Scholar]
- 99. Throckmorton TW, Gulotta LV, Bonnarens FO, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg 2015;24:965–971. [DOI] [PubMed] [Google Scholar]
- 100. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am 2012;94:2167–2175. [DOI] [PubMed] [Google Scholar]
- 101. Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: a prospective-randomized clinical study. J Shoulder Elbow Surg 2009;18:515–520. [DOI] [PubMed] [Google Scholar]
- 102. Burns DM, Frank T, Whyne CM, Henry PD. Glenoid component positioning and guidance techniques in anatomic and reverse total shoulder arthroplasty: a systematic review and meta-analysis. Shoulder Elbow 2019;11:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gregory TM, Gregory J, Sledge J, Allard R, Mir O. Surgery guided by mixed reality: presentation of a proof of concept. Acta Orthop 2018;89:480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hess F, Zettl R, Smolen D, Knoth C. Anatomical reconstruction to treat acromion fractures following reverse shoulder arthroplasty. Int Orthop 2018;42:875–881. [DOI] [PubMed] [Google Scholar]
- 106. Walch G, Mottier F, Wall B, Boileau P, Molé D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg 2009;18:495–502. [DOI] [PubMed] [Google Scholar]
- 107. Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg 2013;22:1514–1521. [DOI] [PubMed] [Google Scholar]
- 108. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 2005;87:1697–1705. [DOI] [PubMed] [Google Scholar]
- 109. Kurowicki J, Triplet JJ, Momoh E, Moor MA, Levy JC. Reverse shoulder prosthesis in the treatment of locked anterior shoulders: a comparison with classic reverse shoulder indications. J Shoulder Elbow Surg 2016;25:1954–1960. [DOI] [PubMed] [Google Scholar]
- 110. Mellano CR, Kupfer N, Thorsness R, et al. Functional results of bilateral reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:990–996. [DOI] [PubMed] [Google Scholar]
- 111. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJP. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res 2015;473:3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wahlquist TC, Hunt AF, Braman JP. Acromial base fractures after reverse total shoulder arthroplasty: report of five cases. J Shoulder Elbow Surg 2011;20:1178–1183. [DOI] [PubMed] [Google Scholar]
- 113. Teusink MJ, Otto RJ, Cottrell BJ, Frankle MA. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty? J Shoulder Elbow Surg 2014;23:782–790. [DOI] [PubMed] [Google Scholar]
- 114. Levy JC, Anderson C, Samson A. Classification of postoperative acromial fractures following reverse shoulder arthroplasty. J Bone Joint Surg Am 2013;95:e104. [DOI] [PubMed] [Google Scholar]
- 115. Cho C-H, Jung J-W, Na S-S, Bae K-C, Lee K-J, Kim D-H. Is acromial fracture after reverse total shoulder arthroplasty a negligible complication? A systematic review. Clin Orthop Surg 2019;11:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Patterson DC, Chi D, Parsons BO, Cagle PJ, Jr. Acromial spine fracture after reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2019;28:792–801. [DOI] [PubMed] [Google Scholar]
- 117. King JJ, Dalton SS, Gulotta LV, Wright TW, Schoch BS. How common are acromial and scapular spine fractures after reverse shoulder arthroplasty? A systematic review. Bone Joint J 2019;101-B:627–634. [DOI] [PubMed] [Google Scholar]
- 118. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:737–744. [DOI] [PubMed] [Google Scholar]
- 119. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:892–896. [DOI] [PubMed] [Google Scholar]
- 120. Trappey GJ, IV, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res 2011;469:2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg 2012;21:36–41. [DOI] [PubMed] [Google Scholar]
- 122. Friedman RJ, Flurin P-H, Wright TW, Zuckerman JD, Roche CP. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg 2017;26:662–668. [DOI] [PubMed] [Google Scholar]
- 123. Vourazeris JD, Wright TW, Struk AM, King JJ, Farmer KW. Primary reverse total shoulder arthroplasty outcomes in patients with subscapularis repair versus tenotomy. J Shoulder Elbow Surg 2017;26:450–457. [DOI] [PubMed] [Google Scholar]
- 124. Routman HD, Flurin P-H, Wright TW, Zuckerman JD, Hamilton MA, Roche CP. Reverse shoulder arthroplasty prosthesis design classification system. Bull Hosp Jt Dis 2015;73:S5–14. [PubMed] [Google Scholar]
- 125. Matthewson G, Kooner S, Kwapisz A, Leiter J, Old J, MacDonald P. The effect of subscapularis repair on dislocation rates in reverse shoulder arthroplasty: a meta-analysis and systematic review. J Shoulder Elbow Surg 2019;28:989–997. [DOI] [PubMed] [Google Scholar]
- 126. Dedy NJ, Gouk CJ, Taylor FJ, Thomas M, Tan SLE. Sonographic assessment of the subscapularis after reverse shoulder arthroplasty: impact of tendon integrity on shoulder function. J Shoulder Elbow Surg 2018;27:1051–1056. [DOI] [PubMed] [Google Scholar]
- 127. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics 2002;25:129–133. [DOI] [PubMed] [Google Scholar]
- 128. Werner BC, Wong AC, Mahony GT, et al. Clinical outcomes after reverse shoulder arthroplasty with and without subscapularis repair: the importance of considering glenosphere lateralization. J Am Acad Orthop Surg 2018;26:e114–e119. [DOI] [PubMed] [Google Scholar]