Abstract
Multiple options are being tried for the management of 2019-nCoV infection since its pandemic started. Favipiravir (FPV) is one of drugs, which is also being tried for the management of 2019-nCoV infection. The present study aimed to evaluate the efficacy and safety of FPV in published literature. Comparative randomized or nonrandomized controlled clinical trials comparing FPV to the standard of care (SOC)/control or other antiviral agent/combinations were included. A total of 12 databases were searched and identify four studies which were further used for final analysis. The data analysis was done as pooled prevalence using a random effect model by “RevMan manager version 5.4.1 and “R” software. The point estimate, odds ratio (OR) with 95% confidence interval (CI) was calculated for dichotomous data. In the present study, the marginal beneficial effect was seen in the FPV group in overall clinical improvement comparison to SOC/control, i.e., (4 studies, log OR [95% CI] (−0.19 [−0.51, 0.13]). However, in all other outcomes, it was found to be comparable to the SOC/control arm namely “clinical improvement on day 7–10” (3 studies, OR [95% CI] 1.63 [1.07, 2.48]) while “clinical improvement on day 10–14” (3 studies, OR [95% CI] 1.37 [0.24, 7.82]) and viral negativity was seen (4 studies, OR [95% CI] 1.91 [0.91, 4.01]). No difference in efficacy was found between FPV versus lopinavir/ritonavir or arbidol groups. Regarding adverse effects, except for the occurrence of rash (higher in the FPV group), safety was comparable to SOC. In our study, there was a marginal difference between the FPV and the SOC arm in terms of “clinical improvement” on day 7–10 or 10–14, and “virological negativity” on day 10–14.” However, some benefit was observed in a few studies, but it was also comparable to the control drugs or SOC.
Keywords: COVID-19, favipiravir, meta-analysis, systematic review
Introduction
The outbreak of coronavirus-2 (SARS-CoV-2) has now invaded almost every country’s health and economical system. Scientists were working round-the-clock globally for its management and new drug evaluation. The nature and structure of COVID-19 make it more communicable and therefore need an urgent treatment. Data suggest that the cases are still rising and as of October 18, 2020, the total active cases was 8,954,631 in which 8,882,705 (99%) mild cases and 71,926 (1%) serious or critical ill cases.[1] As the COVID-19 pandemic escalates and subsequent consequences to global health and the economy, the scientific group is trying to figure out preparing effective therapeutic options.[2] Several drugs such as azithromycin,[3] hydroxychloroquine,[4] chloroquine phosphate,[5] arbidol,[6] lopinavir/ritonavir (LPV/RTV),[7] ribavirin,[8] remdesivir,[9] and tocilizumab[10] were tried into the 2019 nCoV infection. The use of favipiravir (FPV) is also being initiated in many countries as single-centered or multicentric clinical trials.[7,8] FPV, a purine nucleotide analog and viral RNA-dependent RNA polymerase inhibitor used as antiviral against the infection of the influenza-A virus. It is also being evaluated to combat several viral diseases including COVID-19.[6,7,11,12] There is a lack of evidence which states the effectiveness or safety of drugs being studying in the trials for COVID-19 management. COVID-19 is considered to be varying effects among the different populations, i.e., the elderly are more severe and then men, and with other chronic health conditions, such as diabetes, chronic respiratory disease, and cardiovascular diseases. Therefore, the present meta-analysis and systematic review have assessed the safety and efficacy of FPV in the COVID-19 management.
Materials and Methods
The “preferred reporting items for systematic reviews and meta-analysis statement (PRISMA)” and “Cochrane community” recommendations and guidelines[14] were followed for the data collection and analysis. The current meta-analysis and systematic review were performed to evaluate the efficacy and safety of FPV therapy in the management of COVID-19.
Clinical effectiveness of FPV was analyzed against SARS-CoV-2 as per defined outcome, i.e.,
Clinical improvements included symptomatic or radiological improvements
Virological cure (reverse transcription polymerase chain reaction [RT-PCR] negative COVID-19)
Clinical worsening/progression/or need for mechanical ventilation
Safety/tolerability of the studied drug.
Inclusion/exclusion criteria
The articles were screened based on clinical trials of randomized control trials (RCTs) or non-RCTs which investigated the efficacy or safety of FPV compared with other treatment (active) arm or control arm toward the treatment of patients, confirmed for 2019-nCoV infections. No restrictions were imposed concerning age, gender, ethnicity, blinding, or publication status. The research featured publications in English and other languages were included in the study. Patients with clinical outcome and viral negativity of the patient were the primary endpoint of the study. Additional outcomes were consisting of a need for mechanical ventilation and adverse events (outcomes). Case report, case series, and articles in English or other languages having no full text, or those full text is inaccessible were excluded from the study. Further, studies that consist of incomplete and inadequate data were not incorporated.
Database Search
The data were screened at 12 medical literature databases (CINAHL Plus, PubMed, Embase, Ovid, Scopus, Web of Science, Science Direct, Wiley Online Library, bioRxiv, medRxiv, CNKI, and Cochrane) were performed up to October 5, 2020 without any language restriction. Apart from these, the references of the included studies were also screened for possible inclusion criteria. The search was carried out using the keywords: favipiravir or Avigan and COVID-19 OR SARS-CoV-2 or new coronavirus OR 2019-nCoV OR 2019 novel coronavirus [Figure 1].
Figure 1.
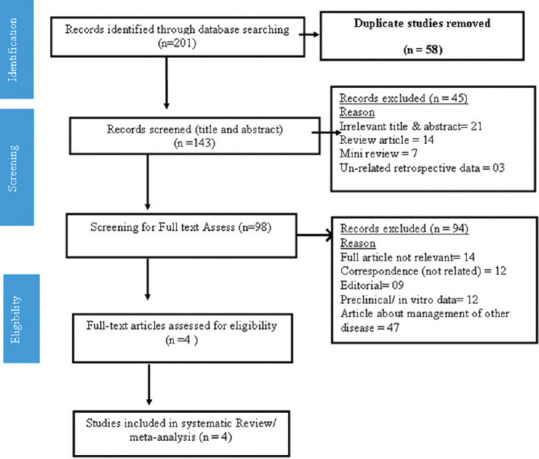
Preferred reporting items for systematic reviews and meta-analysis chart for the screening and included studies as per preferred reporting items for systematic reviews and meta-analysis statement
Screening of articles
After searching the databases with appropriate keywords, duplicates were removed and two reviewers (HS and AS) screened the studies using the title and abstract as predefined inclusion/exclusion criteria [Table 1]. Full-text of relevant studies was further evaluated for possible inclusion in the meta-analysis using the same inclusion/exclusion criteria. Any discrepancies among investigators were resolved by consulting with AP and BM.
Table 1.
Study characteristic of included studies
| Study, Author | Place | Study Design | Population | Intervention | Control or SOC | Virological cure (n) | Outcome |
|---|---|---|---|---|---|---|---|
| Cai Q, 2020[7] | China | Open control trial | RT-PCR confirmed COVID-19 (noncritically ill) | FPV (400/100 mg) BD (n=35) (day 1: 1600 mg twice daily; days 2-14: 600 mg twice daily | SOC (LPV/RTV: 1600 mg twice daily on day 1 and 600 mg twice daily on days 2-14) n=45 (days 1-14: 400 mg/100 mg twice daily) |
FPV: 32 LPV/RTV: 28 |
FPV showed better therapeutic responses on COVID-19 in terms of disease progression and viral clearance |
| Chen C, 2020[6] | China | RCT - multicenter trial | RT-PCR confirmed severe COVID-19 | FPV (n=116) (1600 mg*2/first day, followed by 600 mg*2/day) for 10 days | Arbidol (n=120) Conventional therapy plus umifenovir (arbidol) (200 mg*3/day |
FPV: 71 Arbidol: 62 |
FPV did not significantly improve the clinical recovery rate at day 7, compared to Arbidol |
| Lou Y, 2020[12] | China | Open control trial | RT-PCR confirmed severe COVID-19 | FPV (n=9) 1600 mg or 2200 mg orally, followed by 600 mg each time, 3 times a day, and the duration of administration was not>14 days |
Baloxavir marboxil group (n=10) and control group (n=10) (80 mg once a day orally on day 1 and day 4; for patients who are still positive in the virological test, they can be given again on day) | FPV: 7 Baloxavir: 7 Control: 10 |
Author did not found a significant difference as compared to control or SIOC in viral negativity on day 14 |
| Ivashchenko AA[12] | Russia | Multicenter, open-label, randomized (Phase II/III) | Mild to moderate COVID-19 | FPV (n=20): AVIFAVIR 1600 mg BID on day 1, followed by 600 mg BID on days 2–14 FPV (n=20): AVIFAVIR 1800 mg BID on day 1, followed by 800 mg BID on days 2-14 |
SOC (n=20): Russian guidelines for the treatment of COVID-19 | FPV: 37 SOC: 16 |
FPV demonstrated rapid antiviral response against SARS-CoV |
RT-PCR: Real-time polymerase chain reaction, RCT: Randomized controlled trial, FPV: Favipiravir, SOC: Standard of care, LPV/RTV: Lopinavir/ritonavir, SARS-CoV: Severe acute respiratory syndrome coronavirus
Data extraction
Data were extracted by two reviewers (HS and HK) independently extracted out from the included studies using a pretested data extraction form.
Risk of bias assessment
The randomized controlled clinical trials were evaluated as per Cochrane’s risk of bias (ROB) tool.[14] The methodological quality assessment of the clinical studies was performed employing the Newcastle-Ottawa Scale.[15]
Statistical analysis
The data are entered into Microsoft excel and summarized in Table 1. The data were analyzed for proportion analysis by Chi-squared test,[17] whereas “dichotomous data” point estimates were reported as odds ratio (OR) with 95% confidence interval (CI). Heterogeneity[17] was quantified by standard Chi-squared test and denoted by I2 for the level of irregularity. The statistically significant level was set at P < 0.05. Publication bias was studied by funnel plot[17] and distribution inclination plotted for assessment. Information was broke down by utilizing the “RevMan 5.4.1 (Cochrane Review)[16] and Metafor bundle in “R.”[17]
Results
We have screened a total of 201 studies and extracted 58 as duplicates. The remaining articles were assessed for the title and abstract, which given out the 98 relevant articles and excluded the rest 45 articles due to irrelevant title and abstract (n = 21), review articles (n = 14), mini-review (n = 07), and unrelated retrospective study (n = 03). Finally, we have reached to the four articles found suitable for the systematic review and meta-analysis and excluded 94 articles due to lack of full articles, editorials, case reports, or data not given properly to be qualified for inclusion [Figure 1]. The included study characteristics are represented in Table 1.
Overall safety and efficacy of favipiravir in SARS-CoV-2 (irrespective of disease severity): Overall patients recovery outcome
A total of the four studies (n = 405) included in the calculation of random effect size to evaluate the outcome, Figure 2a, a forest plot depicting the log OR – 0.19 (95% CI: −0.51, 0.13) with nonsignificant heterogeneity (P = 0.8560). Funnel plot of included studies assessed for the publication bias is shown in Figure 2b; symmetrical and all studies located under the curve. It suggests that exposure of the FPV lowers the odds of the outcome.
Figure 2.
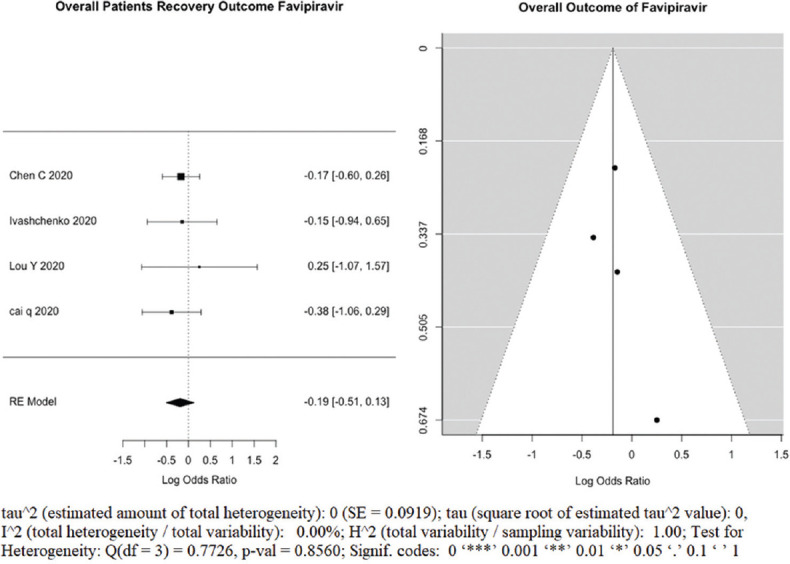
(a) Forest plot showing pooled overall recovery outcome of clinical improvement after favipiravir treatment of COVID-19 patients, logs Odd’s ratio. (b): Funnel plot showing there is no publication bias as it is symmetry and all studies under the curve, among studies evaluating overall clinical improvement after favipiravir treatment of COVID-19 patients
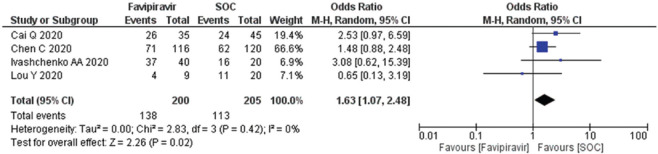
Clinical improvement after day 7–10 among COVID-19 patients
A total of four studies (n = 405) included for the calculation random effect size calculation, forest plot [Figure 3] showing that exposure of the FPV after 7–10 days, associated with higher odds of the outcome. The OR calculated as 1.63 (95% CI; 1.07, 2.48) in random effect size, which showed nonsignificant heterogeneity in the studies and there was no publication bias observed in funnel plot [Supplementary Figure 1 (196.7KB, tif) .
Figure 3.
Forest plot showing the pooled prevalence of clinical improvement after day 7–10 among COVID-19 patients. (Suppl. Figure: 1 (196.7KB, tif) - Funnel plot showing publication bias among studies evaluating clinical improvement on day 7-10 of COVID-19 patients.)
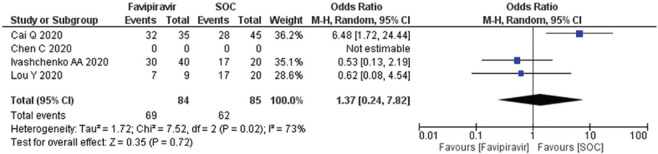
Clinical improvement after day 10–14 among COVID-19 patients
All four studies (n = 169) evaluated using random effect size model, forest plot [Figure 4], OR 1.37 (95% CI; 0.24, 7.82) suggested that exposure of the FPV for day 10–14 days associated with higher odds of the outcome. The funnel plot showed nonsignificant heterogeneity (I2) and no publication bias [Supplementary Figures 2 (195.8KB, tif) ].
Figure 4.
Forest plot showing the pooled prevalence of clinical improvement after day 10–14 among COVID-19 patients. (Suppl. Figure: 2 (195.8KB, tif) - Funnel plot showing publication bias among studies evaluating clinical improvement on day 14 of COVID-19 patients
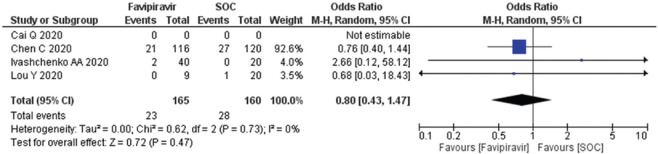
Need for mechanical ventilation
Three studies (n = 325) evaluated the need for mechanical ventilation. The OR reported as 0.80 (95% CI, 0.43, 1.47) suggested low odds and need for mechanical ventilation to the patients as compared to the SOC/control Figure 5. The data suggested that heterogeneity (I2) is 0% and there was no publication bias seen in the published literature [Supplementary Figure 3 (193.3KB, tif) ]. The number of patients needed for mechanical ventilation was less in all the arms [Supplementary Figures 4 (196.7KB, tif) ].
Figure 5.
Forest plot showing the pooled prevalence of requirement of mechanical ventilation among COVID-19 patients. (Suppl. Figure: 3 (193.3KB, tif) - Funnel plot showing publication bias among studies evaluating the requirement of mechanical ventilation of COVID-19 patients.)
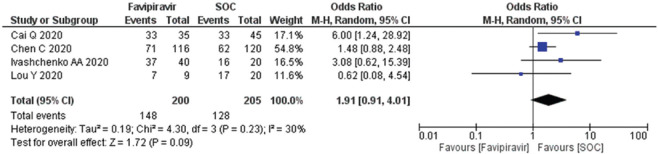
Viral negativity after day 10–14 among COVID-19 patients
Four studies (n = 405) were evaluated for the viral negativity on day 14 using a random effect size model and forest plot for publication bias [Figure 6]. The OR 1.91 (95% CI; 0.91, 4.01) suggested that FPV was associated with the lower odds as compared to the standard of care (SOC)/control arm. The data suggested that heterogeneity (I2) is 30% and there was no publication bias seen [Supplementary Figure 3 (193.3KB, tif) ].
Figure 6.
Forest plot showing the pooled prevalence of viral negativity among COVID-19 patients. (Suppl. Figure: 4 (196.7KB, tif) - Funnel plot showing publication bias among studies of viral negativity.)
Adverse drug reaction
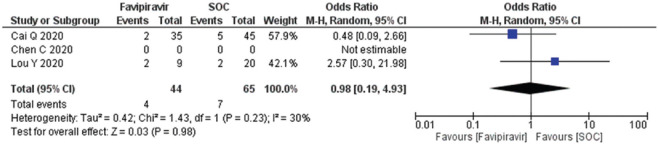
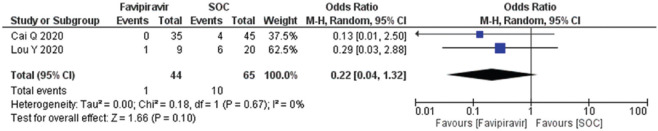
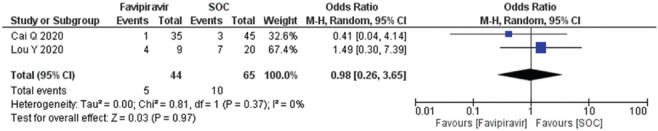
A total of two studies (n = 109) reported adverse drug events (diarrhea, nausea, rashes, and liver or kidney injury). The diarrhea OR calculated as 0.98 (95% CI; 0.19, 4.93) [Figure 8] and forest plot suggested that exposure of the FPV does not an adverse outcome and comparable to SOC/control. Heterogeneity (I2) in the studies reported was observed at 30%. The forest plots of nausea and vomiting and rashes estimated the OR 0.52 (95% CI; 0.00, 88.01) and 0.22 (95% CI; 0.04, 1.32), respectively, with heterogeneity (I2) 0% which was found significantly different to the standard operating care (P = 0.02) [Figure 9,10]. Besides, the occurrence of liver/kidney injury was calculated as OR 0.98 (95% CI; 0.26, 3.65), suggested that exposure of FPV does not affect odds of outcome [Figure 11] and there was no publication bias seen in the funnel plots in the supplementary material [Supplementary Figures 5 (194.8KB, tif) -8 (196.7KB, tif) ].
Figure 8.
Forest plot showing the pooled prevalence of diarrhea among COVID-19 patients after favipiravir treatment (Suppl. Figure: 5 (194.8KB, tif) - Funnel plot showing publication bias among studies evaluating diarrhea among COVID-19 patients after favipiravir treatment.)
Figure 9.
Forest plot showing the pooled prevalence of nausea and vomiting among COVID-19 patients after favipiravir treatment (Suppl. Figure: 6 (197.3KB, tif) - Funnel plot showing publication bias among studies evaluating nausea and vomiting among COVID-19 patients after favipiravir treatment.)
Figure 10.
Forest plot showing the pooled prevalence of rash among COVID-19 patients after favipiravir treatment (Suppl. Figure: 7 (197.9KB, tif) - Funnel plot showing publication bias among studies evaluating rash among COVID-19 patients after favipiravir treatment.)
Figure 11.
Forest plot showing the pooled prevalence of liver and kidney injury among COVID-19 patients after favipiravir treatment (Suppl. Figure: 8 (196.7KB, tif) - Funnel plot showing publication bias among studies evaluating liver and kidney injury among COVID-19 patients after favipiravir treatment.)
Risk of bias analysis
The ROB among the included studies is reported in Figure 7.
Figure 7.
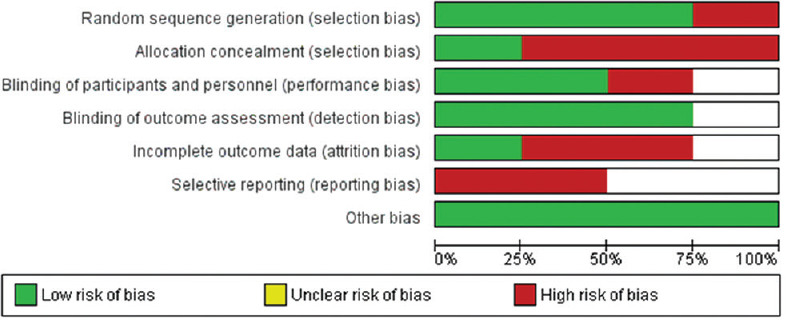
Risk of bias among the clinical studies included in the analysis among the favipiravir versus standard of care published/preprint publications
Discussion
No drugs were developed exclusively for the treatment of COVID-19. Favipiravir is being tried as a promising clinical intervention to combat SARS-CoV-2 infection with other drugs such as azithromycin,[3] hydroxychloroquine,[4] chloroquine,[5] LPV/RTV,[7] ribavirin,[8] remdesivir,[9] and tocilizumab.[10] All the drugs are being examined under the controlled trial as open or nonrandomized, cross-sectional, or randomized trials in single or multicentric trials. In all cases, issues regarding the study design or lack of control arm or other confounding variables may influence the study outcome. Cai et al., 2020,[7] showed that COVID-19 positive patients have shown faster viral clearance in comparison with the patients treated with LPV/RTV as compared to FPV.[7] The endpoint as viral clearance has been confirmed positive with computed tomography imaging, which has shown improvement in infected patients of COVID-19.[7] The study was open-labeled, nonrandomized, and received interferon- (5 million U daily) with FPV, and control may influence the study outcomes, whereas Wang et al., 2020, have shown an effective reduction in SARS-CoV-2 infection, tested in in vitro (EC50= 61.88/mol_L_1, CC50>400/mol_L_1, SI >6.46) by FPV;[9] in addition, Lou et al., 2020, have also demonstrated the effectiveness in in vitro study before proceeding for the exploratory single-center, open-label, randomized, controlled trial.[12]
Ivashchenko et al., 2020,[11] have demonstrated rapid antiviral response against COVID-19 and suggest that the proportion of patients who attained negative RT-PCR on day 5 on both dosing regimens of FPV was twice as high as in the reference group (P < 0.05). However, the author suggested that it is only a “proof of concept” study in moderately ill patients. But finding suggested promised good safety with mild to moderate adverse reactions and no toxicity results with the higher dosing of FPV[6,7,11] and clinical outcome in respect of symptomatic or radiological outcome is comparable with the SOCs.
Mechanical ventilation (noninvasive or invasive) is another important factor in the management of COVID-19. Therefore, drugs that reduced the use or improvement in oxygen consumption may be a good endpoint in the assessment of treating drugs. The study by Rattanaumpawan et al., 2020,[13] showed that FPV improves clinically in terms of oxygen status by 66.7% on day 7 and 92.5% in patients who did not require O2-supplementation. The study concluded that patients with the requirement of O2-supplementation improved 47.2% by day 7 of treatment with FPV. Finally, the rate of clinical improvement reaches 75% by day 14 and 83.7% by day 28. Furthermore, the study showed that without O2-supplementation requirement, patients improved 92.6% with FPV treatment[13] and clinical improvement on day 14 but found to be comparable to the SOC/control arm. The limitation of the Rattanaumpawan et al., 2020,[13] is that it was a retrospective observational study and does not have a control arm.
Chen et al., 2020,[6] conducted RCT which showed FPV clinical improvement to treat with the reduction in the symptoms such as pyrexia, cough, and difficulty in breathing in comparison to Arbidol. Clinical improvements by day 7 were reported at 71.4% with the improvement in the clinical symptoms such as fever and cough and reported that most frequent adverse event as raised serum uric acid.
Diarrhea was found to be more common and followed by rash, nausea, and vomiting. Liver and kidney impairment was also seen in a few patients.[6,7,13] However, several registered ongoing clinical trials are aiming on various antiviral agents against COVID-19 and the endpoints of studies have not conclusively united yet for a specific result to focus on a particular drug.
Limitation of the study
The present systematic review and meta-analysis were studied in a few samples. The extensive search of published literature and databases showed open level randomized trials and nonrandomized trials of single- or multicentric trials. As a result, we found one published article, whereas three other articles were in the preprint section, but found to be informative to be included in the study. The SOC arm included the other drugs such as (LPV/RTV), Arbidol, and Baloxavir. Therefore, for the translational value of effectiveness or safety of FPV, we need more published quality RCTs/non-RCTs or observation studies to conclude an outcome.
Conclusion
In the present study, no statistical difference was found in terms of “clinical improvement” and “virological cure on day 7–10” and 10–14. FPV therapy showed some help in respect of decreasing “time to virological clearance” as compared to LPV/RTV in T finding after day 14, and in addition, there was no difference observed of FPV compared to SOC (LPV/RTV, Arbidol, and Baloxavir).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Funnel plot showing publication bias among studies evaluating clinical improvement on day 7 of COVID-19 patients
Funnel plot showing publication bias among studies evaluating clinical improvement on day 14 of COVID-19 patients
Funnel plot showing publication bias among studies of viral negativity
Funnel plot showing publication bias among studies evaluating the requirement of mechanical ventilation of COVID-19 patients
Funnel plot showing publication bias among studies evaluating diarrhea among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating nausea and vomiting among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating rash among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating liver and kidney injury among COVID-19 patients after favipiravir treatment
References
- 1. [[Last assessed on 2020 Oct 18]]. Available from: https://www.worldometers.info/coronavirus/
- 2.Prajapat M, Sarma P, Shekhar N, Avti P, Sinha S, Kaur H, et al. Drug targets for corona virus: A systematic review. Indian J Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekhavati E, Jafari F, SeyedAlinaghi S, Jamalimoghadamsiahkali S, Sadr S, Tabarestani M, et al. Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial. Int J Antimicrob Agents. 2020;56:106143. doi: 10.1016/j.ijantimicag.2020.106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey JD, Johnson NJ, Semler MW, Collins SP, Aggarwal NR, Brower RG, et al. Rationale and design of ORCHID: A randomized placebo-controlled clinical trial of hydroxychloroquine for adults hospitalized with COVID-19. Ann Am Thorac Soc. 2020;17:1144–53. doi: 10.1513/AnnalsATS.202005-478SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Chen H, Shang Y, Zhu H, Chen G, Chen Y, et al. Efficacy of chloroquine versus lopinavir/ritonavir in mild/general COVID-19 infection: A prospective, open-label, multicenter, randomized controlled clinical study. Trials. 2020;21:622. doi: 10.1186/s13063-020-04478-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Zhang Y, Huang Y, Yin P, Cheng Z, Wu J, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv. 2020031720037432. [Google Scholar]
- 7.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020 Mar 18; doi: 10.1016/j.eng.2020.03.007. doi: 101016/jeng202003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–78. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92:814–8. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for treatment of patients with moderate COVID-19: Interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1176. ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: An exploratory randomized, Controlled Trial. Eur J Pharm Sci. 2020:105631. doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rattanaumpawan P, Jirajariyavej S, Lerdlamyong K, Palavutitotai N, Saiyarin J. Real-world experience with favipiravir for treatment of COVID-19 in Thailand: Results from a multi-center observational study. medRxiv. doi: 10.3390/antibiotics11060805. 2020062420133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottawa Hospital Research Institute. [[Last accessed on 2020 Apr 1]]. Available from: http://wwwohrica/programs/clinical_epidemiology/oxfordasp .
- 16.RevMan.5.41 Download. [[Last assessed on last assessed on 2020 Oct 11]]. Available from: /online learning/ core software cochrane reviews/revman/revman 5 download .
- 17.Homepage [The Metafor Package] [[Last accessed on 2020 Sep 27]]. Available from: http://wwwmetafor-projectorg/dokuphp .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot showing publication bias among studies evaluating clinical improvement on day 7 of COVID-19 patients
Funnel plot showing publication bias among studies evaluating clinical improvement on day 14 of COVID-19 patients
Funnel plot showing publication bias among studies of viral negativity
Funnel plot showing publication bias among studies evaluating the requirement of mechanical ventilation of COVID-19 patients
Funnel plot showing publication bias among studies evaluating diarrhea among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating nausea and vomiting among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating rash among COVID-19 patients after favipiravir treatment
Funnel plot showing publication bias among studies evaluating liver and kidney injury among COVID-19 patients after favipiravir treatment