Dear Editor,
In a recent letter to the editor, Langenbach and colleagues [1] cited concerns about the repeated use of Ag/AgCl electrodes in 4x1 High Definition transcranial direct current stimulation (HD-tDCS). Briefly, the authors reported increased impedance (with sudden, occasional cessation of stimulation) and visual evidence of oxidation (blackening of the electrode surface) after about 8 sessions in which the same electrode was used as the anode (2mA) and four other “ring” electrodes served as cathodes for up to 20 minutes per session. Relevant additional methodological information obtained via personal communication with Dr. Langenbach (3/4/2020) includes the following: 1) stimulation used a neuroConn DC-Stimulator MC (software version 1.6.0), which allows for independent electrical current modulation of each electrode, 2) investigators used a standard EEG cap and placed gel directly into the holes (i.e., no specialized holders were used), 3) a variable amount of gel was used (both across electrodes and participants) but impedance was always <8kΩ, 4) total duration of use varied for each set of electrodes. Langenbach and colleagues discussed methods to reduce wear including electrode rotation as well as the need for increased transparency in reporting about electrode use/care.
Given the above noted concerns, we felt it important to share our experiences with HD-tDCS (or multi-electrode tDCS) using data from our ongoing randomized trial as an example (NCT02155946 (see also ref 3)). In this study, participants with mild cognitive impairment (MCI) receive 5 consecutive daily sessions of active (2mA for 20 minutes/session) or sham HD-tDCS. As previously noted [3], the center anode is positioned at F5, while the ring cathodes are positioned at Fp1, F1, C5, and F9. Upon starting this line of research, our initial review of the literature [2] and recommendations from representatives at Soterix Medical Inc. (from whom units were acquired) indicated that electrodes are reusable for about 10 sessions at an intensity of 2mA if proper electrode rotation is maintained, such that no one electrode acts as the “center” for more than two sessions. Such recommendations precede, but ultimately support, Langenbach et al.’s suggestion for electrode rotation. Figure 1 is an example of our standard rotation, where each electrode is assigned a letter and team members are required to position electrodes at the pre-designated locations for each session. In this study [3], each set of electrodes is used for two participants (participant 1 = sessions 1-5; participant 2 = sessions 6-10) so that each electrode serves as the center for only two sessions.
Figure 1.
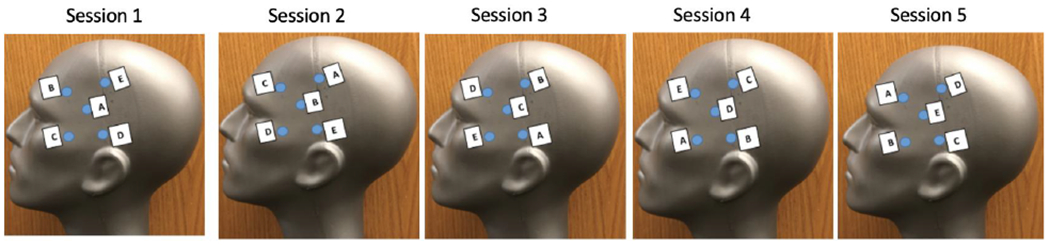
Electrode rotation across 5 sessions applied for NCT02155946 (note that locations are for demonstration purposes and are not meant to be precise)
Our trial uses a Soterix Medical Inc. 4x1 HD-tDCS unit, which provides full amplitude to the center channel/electrode but passively splits current to the ring electrodes, and is powered by a Soterix Medical Inc. Clinical Trials (CT) unit. We use electrode holders (available from Soterix Medical Inc.) that are affixed to the head using netting (Surgilast, manufactured by Derma Sciences) since, in our experience, this allows for greater accuracy and flexibility than standard EEG or comparable caps. Each electrode holder is filled with a standard 10ml of conductive gel, the electrode placed, additional gel added as necessary to ensure optimal conductivity, and then secured with a holder cap. We evaluate resistance/impedance values at multiple time points, with the first being immediately after all electrodes are placed (i.e., presaturation). This initial measurement identifies any atypically high values and allows team members to identify and correct any causal factors (e.g., removing air bubbles or hair). We then start a 10-minute phase in which the gel can “saturate” the skin (i.e., saturation phase), thereby reducing resistance/impedance (see below). We use this phase to provide instructions and/or collect various forms (e.g., pre-stimulation side effect questionnaires [4]). Impedance/resistance is measured after this 10-minute phase and any additional adjustments are made at that time (i.e., post-saturation). Our final measurement comes after completion of the 20 (or designated duration) -minutes of stimulation. We report values for each of these timepoints below noting that they are represented by a “quality unit” (QU), which is a ratio of impedance and voltage used by the Soterix Medical Inc. equipment. A QU of around 2 is considered acceptable for stimulation [5]. Side effect presence, severity, and location are evaluated following completion of study activities for each session [4]. Electrodes are gently washed with a steady stream of warm water and holders are cleaned with a toothbrush and liquid soap after each session.
Table 1 clearly demonstrates that QUs from 210 sessions decreased significantly across the 3 measurement points (pre-saturation > post-saturation > post-stimulation; Greenhouse-Geisser correction: F(1.251, 261.537)=933.523, p<.001)). As is also evident, QUs were lower during Sessions 1-5 relative to Sessions 6-10 regardless of the measurement period (all p<.05). However, QUs were stable across Sessions 1-5 and across Sessions 6-10 (all p>.283), as demonstrated in Figure 2. Thus, there seem to be some QU related changes (~30% increase in this sample) when using electrodes for multiple sessions on multiple participants. Regardless, the 10-minute saturation phase substantially reduced QUs; a critical finding since tDCS units inherently monitor resistance/impedance and adjust voltage in order to sustain the designated current amplitude (e.g., 2mA). Thus, higher resistance/impedance (or QU) values are problematic primarily to the extent that they affect tolerability. There were no unexpected or severe adverse events in any participant and Table 2 shows a comparable rate of known side effects. In fact, the reported rate of ‘burning sensation’ declined in Sessions 6-10 relative to Sessions 1-5. Thus, the relatively higher QU values found for the second participants came at no obvious cost.
Table I.
Descriptive Statistics for Set 1 Use (Sessions 1-5) and Set 2 Use (Sessions 6-10) of Electrodes
| Sessions 1-5 | Sessions 6-10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | Std. Deviation | N | Min | Max | Mean | Std. Deviation | |
| Pre-Saturation | 105 | 0.29 | 2.32 | 0.92 | 0.43 | 105 | 0.35 | 2.55 | 1.29 | 0.48 |
| Post-Saturation | 105 | 0.20 | 1.70 | 0.60 | 0.32 | 105 | 0.20 | 2.15 | 0.90 | 0.38 |
| Post-Stimulation | 105 | 0.06 | 0.93 | 0.26 | 0.19 | 105 | 0.06 | 1.76 | 0.44 | 0.31 |
Figure 2.
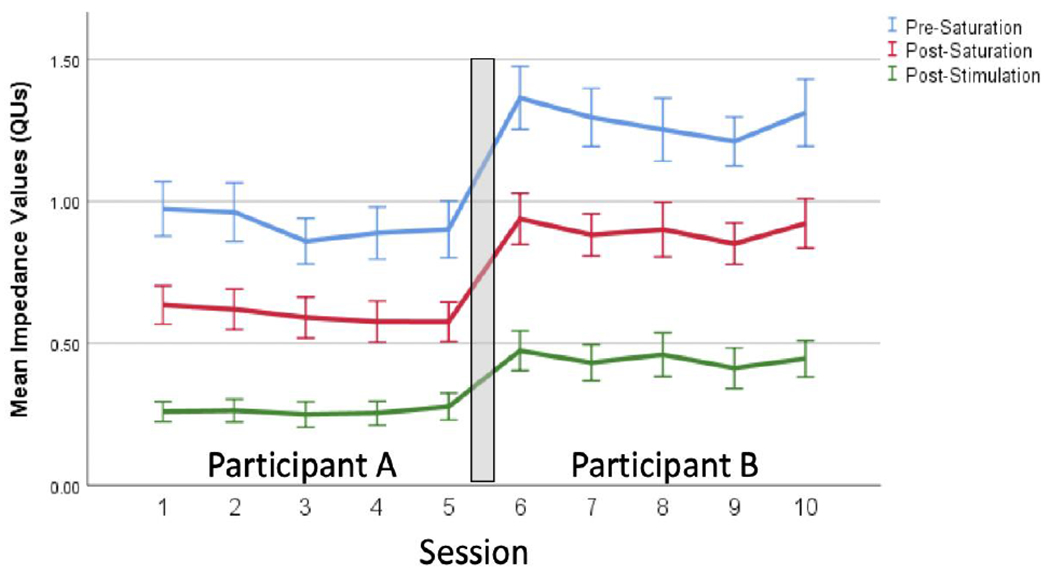
Mean quality units (QUs)values (SEM) across 10 sessions of electrode use. Note: electrodes were used for separate two participants.
Table 2.
Frequencies of reported side effects
| Sensation | Sessions 1-5 | Sessions 6-10 | Chi-square |
|---|---|---|---|
| Burning | 31.4% | 11.4% | 12.473, p<0.001 |
| Tingling | 41.9% | 33.3% | 1.644, p=0.2 |
| Itching | 18.1% | 15.2% | 0.309, p=.579 |
| Scalp Pain | 2.9% | 6.7% | 1.680, p=.195 |
| Trouble | 5.7% | 5.7% | 0, p=1.0 |
| Concentrating Sleepiness | 2.9% | 6.7% | 1.680, p=.195 |
| Headache | 1.0% | 1.9% | 0.338, p=.561 |
| Mood Change | 1.9% | 5.7% | 2.079, p=.149 |
| Neck Pain | 0% | 0% | n/a |
| Skin Redness | 0% | 2.9% | 3.043, p=.081 |
| Other | 0% | 1.9% | 2.019, p=.155 |
Other responses are not attributable to tDCS and include: rib pain from fall (n=1) and discomfort from chair (n=1). Data based on number of sessions in which a symptom of any severity (i.e., mild, moderate, severe) was reported.
Taken as a whole, there is no indication that using electrodes for up to 10 sessions is problematic when best practices (e.g., electrode rotation, consistent amount of conductive gel, saturation phase and impedance monitoring) are applied. We have occasionally encountered “bad” electrodes (e.g., severed wires) that occurred during manufacturing, shipping, or standard cleaning procedures, which have prevented the tES units from delivering stimulation. Thus, it is important to visually inspect the entire electrode prior to every use. Finally, we are aware that some units have been programmed so that their sham condition intermittently reports an impedance-related spike that “ends” the session; thereby mimicking changes that could occur during active stimulation. Users should therefore fully understand the parameters of their sham settings.
Acknowledgements
We wish to acknowledge funding from Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service (IRX001534) as well as the National Institute on Aging (R01 AG058724). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Langenbach BP, Savic B, Baumgartner T, & Knoch D (2020). Repeated anodal HD-tDCS stimulation might render silver chloride electrodes unreliable. Brain Stimulation, 13(3), 55–526, 10.1016/j.brs.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 2.DaSilva AF, Volz MS, Bikson M, & Fregni F (2011). Electrode positioning and montage in transcranial direct current stimulation. Journal of visualized experiment: JoVE, 51, 2744. doi: 10.3791/2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampstead BM, Sathian K, Bikson M, Stringer AY: Combined Mnemonic Strategy Training and High-Definition Transcranial Direct Current Stimulation for Memory Deficits in Mild Cognitive Impairment Alzheimer’s & Dementia: Translational Research & Clinical Interventions 3: 459–470, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reckow J, Rahman-Filipiak A, Garcia S, Schlaefflin S, Calhoun O, DaSilva AF, Bikson M, Hampstead BM: Tolerability and blinding of 4x1 High-Definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps Brain Stimulation 11: 991–997, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villamar MF, Volz MS, Bikson M, Datta A, DaSilva AF, & Fregni F (2013). Technique and Considerations in the Use of High-definition Transcranial Direct Current Stimulation (HD-tDCS). Journal of Visualized Experiments: JoVE, 77. 10.3791/50309 [DOI] [PMC free article] [PubMed] [Google Scholar]


