Abstract
Background
The pandemic caused by the coronavirus SARS-CoV-2 and the countermeasures taken to protect the public are having a substantial effect on the health of the population. In Germany, nationwide protective measures to halt the spread of the virus were implemented in mid-March for 6 weeks.
Methods
In May, the impact of the pandemic was assessed in the German National Cohort (NAKO). A total of 113 928 men and women aged 20 to 74 years at the time of the baseline examination conducted 1 to 5 years earlier (53%) answered, within a 30-day period, a follow-up questionnaire on SARS-CoV-2 test status, COVID-19-associated symptoms, and self-perceived health status.
Results
The self-reported SARS-CoV-2 test frequency among the probands was 4.6%, and 344 participants (0.3%) reported a positive test result. Depressive and anxiety-related symptoms increased relative to baseline only in participants under 60 years of age, particularly in young women. The rate of moderate to severe depressive symptoms increased from 6.4% to 8.8%. Perceived stress increased in all age groups and both sexes, especially in the young. The scores for mental state and self-rated health worsened in participants tested for SARS-CoV-2 compared with those who were not tested. In 32% of the participants, however, self-rated health improved.
Conclusion
The COVID-19 pandemic and the protective measures during the first wave had effects on mental health and on self-rated general health.
The very first case of COVID-19 in Germany was detected on 27 January 2020 (1). The German health authorities isolated the first cases and traced and tested their contacts, but by mid-March 2020 community spread had become apparent in many regions. Testing capacities and dedicated medical care structures were set up to limit the spread and safeguard care of the general population. Within 2 weeks, nationwide countermeasures were introduced for a 6-week period. The goal was to contain the short- and long-term health impact of infection. However, concerns were raised regarding potential health consequences due to social isolation, increased stress and negative socioeconomic effects.
Large population-based cohort studies offer the opportunity to study emerging new diseases and their effects on health. Thus, they are ideal for measuring the spread of COVID-19 in the general population (2) and to evaluate the health impacts of protective measures (3). In the study presented here we analyzed data on more than 100 000 individuals from the German National Cohort (NAKO) (4). The following parameters were considered:
Regional differences in COVID-19 occurrence among NAKO participants in comparison with the official statistics in spring 2020
The frequency of COVID-19-associated symptoms
Changes in mental health and self-rated general health status compared with a baseline assessment 1 to 5 years earlier.
Methods
Between 2014 and 2019, the NAKO recruited 205 219 randomly selected persons aged 20 to 74 years for the baseline examination at 18 study centers (4). Approval had been given by all study centers’ local ethics committees, and all participants had provided written consent for study participation and repeat contact. The first follow-up examination started in 2019, but had to be halted in mid-March 2020 because of the COVID-19 pandemic and the Germany-wide protective countermeasures. Within a short time a new COVID-NAKO questionnaire was developed to collect information on SARS-CoV-2 tests and COVID-19-related symptoms and psychosocial factors. Further details can be found in eBox 1. The findings reported here rest on data collected from questionnaires completed during in the first 30 days (30 April to 29 May) by 113 928 COVID-NAKO participants (table). Questionnaire participants had the same age as non-participants (mean 50 years) and a slim majority were women (52%, against 49% in non-participants). Participation varied among the study regions, from 34% in the northeast to 67% in the southwest (eTable 1 and eTable 2).
eBOX 1. Content and distribution of the COVID-NAKO questionnaire.
The questionnaire was designed by a committee of NAKO scientists. It comprises 42 questions that derive mainly from validated instruments. Self-rated health was assessed by means of the first question from the Short Form Health Survey (SF-12) (“In general, how would you rate your health?”). Mental health was assessed by means of modules from the Patient Health Questionnaire (depressive symptoms: PHQ-9; anxiety symptoms: GAD-7; stress:
PHQ-stress).
Participants were asked whether they had undergone at least one test for coronavirus at a physician’s office, test center, or hospital. The exact type of test was not elucidated. If the participants answered “yes”, they were asked whether at least one test result was positive, with possible answers “yes” and “no.” Furthermore, the reasons for testing were relevant (e.g., contact with a person tested positive, returning from a high-risk area, suspicious symptoms). Symptoms were presented as a list of 12 items (plus the option “other”), and participants were asked to check what applied. The 12 symptoms were tiredness, breathing problems, headache, nausea, fever, chills, pain in extremities, cough, runny nose, diarrhea, and impairments of the senses of smell and taste.
Participants who had given an an e-mail address received the questionnaire electronically through a web-based survey tool with specific links; otherwise, a paper questionnaire was sent through the mail. All questionnaires were sent between 30 April and 15 May 2020, and participants received one reminder. Overall, 199 001 persons were eligible; 6218 individuals had died, had left Germany for good, declined to be contacted with the COVID-NAKO questionnaire, or had not consented to be recontacted at all.
Table. Description of the study sample of the German National Cohort and the frequency of SARS-CoV-2 testing as self-reported in the COVID-NAKO questionnaire.
| Study region characteristics | Sample characteristics | SARS-CoV-2 infections | ||||||||
| Population. N*1 | COVID-19 cases per 100 000*2 | Baseline | COVID-19 follow-up | % women | Age in years. mean | (Number of) SARS-CoV-2 tests | (Number) observed to be positive | (Number) expected to be positive*3 | p-value*4 | |
| Age range 15–79 years | Age range 15–79 years | |||||||||
| All study centers | 5 741 000 | 227 | 205 219 | 113 928 | 51,8 | 50 | 5245 | 344 | 257 | < 0.001 |
*1 Sum of all study centers. The numbers of inhabitants in the respective districts were obtained from the official records of the Federal Statistical Office (www.genesis.destatis.de/genesis/online). Numbers are given for all inhabitants in the age range 15–79 years (as of 31 December 2019) (see eTable 4).
*2 Unweighted mean of all study centers. The numbers of COVID-19 cases per 100 000 inhabitants in the period 1–30 March 2020 were obtained from the official data of the Robert Koch Institute [https://npgeo-corona-npgeo-de.hub.arcgis.com/. 02.06.2020]. Cumulative incidences were calculated as weighted mean based on the age groups 15–34 years. 35–59 years and 60–79 years. weighted by the number of inhabitants in the respective age group in each district (see eTable 4).
*3 The number of expected SARS-CoV-2 infections was calculated. based on the number of participants and the cumulative COVID-19 incidence. as the weighted mean across the age groups 15–34 years. 35–59 years and 60–79 years. weighted by the number of participants in the respective age group in each district (see eTable 4).
*4 Comparison of numbers of expected vs observed SARS-CoV-2 infections. based on a goodness-of-fit chi-squared test for probabilities. with the probabilities given by the expected number of infections.
eTable 1. Age and sex distribution of COVID-NAKO questionnaire respondents by study region.
| Participants | Sex | Age group (years) | |||||||||||||||
| Male | Female | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70 + | ||||||||||
| N | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| All | 113 928 | 54 954 | 48,2 | 58 974 | 51,8 | 10 330 | 9.1 | 11 670 | 10,2 | 30 407 | 26,7 | 31 009 | 27,2 | 28 306 | 24,8 | 2206 | 1,9 |
| Neubrandenburg | 7433 | 3624 | 48,8 | 3809 | 51,2 | 624 | 8.4 | 923 | 12,4 | 1958 | 26,3 | 2084 | 28 | 1756 | 23,6 | 88 | 1,2 |
| Leipzig | 5968 | 2823 | 47,3 | 3145 | 52,7 | 508 | 8.5 | 572 | 9.6 | 1584 | 26,5 | 1628 | 27,3 | 1518 | 25,4 | 158 | 2,6 |
| Kiel | 5641 | 2700 | 47,9 | 2941 | 52,1 | 490 | 8.7 | 567 | 10,1 | 1441 | 25,5 | 1576 | 27,9 | 1503 | 26,6 | 64 | 1,1 |
| Halle | 5346 | 2482 | 46,4 | 2864 | 53,6 | 441 | 8.2 | 513 | 9.6 | 1460 | 27,3 | 1551 | 29 | 1289 | 24,1 | 92 | 1,7 |
| Essen | 5382 | 2613 | 48,6 | 2769 | 51,4 | 561 | 10,4 | 671 | 12,5 | 1443 | 26,8 | 1388 | 25,8 | 1244 | 23,1 | 75 | 1,4 |
| Augsburg | 11 740 | 5810 | 49,5 | 5930 | 50,5 | 1061 | 9.0 | 1175 | 10 | 3138 | 26,7 | 3166 | 27 | 2926 | 24,9 | 274 | 2,3 |
| Mannheim | 5521 | 2760 | 50 | 2761 | 50 | 536 | 9.7 | 635 | 11,5 | 1473 | 26,7 | 1523 | 27,6 | 1265 | 22,9 | 89 | 1,6 |
| Berlin | 19 650 | 9316 | 47,4 | 10 334 | 52,6 | 1787 | 9.1 | 1872 | 9.5 | 5392 | 27,4 | 5337 | 27,2 | 4750 | 24,2 | 512 | 2,6 |
| Hanover | 4686 | 2306 | 49,2 | 2380 | 50,8 | 434 | 9.3 | 432 | 9.2 | 1238 | 26,4 | 1233 | 26,3 | 1251 | 26,7 | 98 | 2,1 |
| Bremen | 6150 | 3010 | 48,9 | 3140 | 51,1 | 565 | 9.2 | 648 | 10,5 | 1588 | 25,8 | 1669 | 27,1 | 1577 | 25,6 | 103 | 1,7 |
| Münster | 6514 | 3121 | 47,9 | 3393 | 52,1 | 542 | 8.3 | 577 | 8.9 | 1696 | 26 | 1792 | 27,5 | 1841 | 28,3 | 66 | 1 |
| Düsseldorf | 5253 | 2458 | 46,8 | 2795 | 53,2 | 541 | 10,3 | 561 | 10,7 | 1337 | 25,5 | 1381 | 26,3 | 1337 | 25,5 | 96 | 1,8 |
| Hamburg | 5300 | 2525 | 47,6 | 2775 | 52,4 | 541 | 10,2 | 613 | 11,6 | 1477 | 27,9 | 1393 | 26,3 | 1168 | 22 | 108 | 2 |
| Saarbrücken | 5992 | 2857 | 47,7 | 3135 | 52,3 | 472 | 7.9 | 657 | 11 | 1583 | 26,4 | 1588 | 26,5 | 1638 | 27,3 | 54 | 0,9 |
| Regensburg | 6545 | 3276 | 50,1 | 3269 | 49,9 | 592 | 9.0 | 592 | 9.0 | 1758 | 26,9 | 1818 | 27,8 | 1583 | 24,2 | 202 | 3,1 |
| Freiburg | 6807 | 3273 | 48,1 | 3534 | 51,9 | 635 | 9.3 | 662 | 9.7 | 1841 | 27 | 1882 | 27,6 | 1660 | 24,4 | 127 | 1,9 |
eTable 2. Description of the 16 study areas of the German National Cohort and the frequency of SARS-CoV-2 testing as reported in the COVID-NAKO questionnaire.
| Study region characteristics | Sample characteristics | SARS-CoV-2 infections | |||||||||||||
| # counties | Inhabitants in 1000 *1 | urban | rural | COVID-19 cases per 100 000 inhabitants *2 | Baseline | COVID-19 Follow-up | % women | Age. years mean | SARS-CoV-2 Tested | # observed positive | # expected*3 | p-value*4 | |||
| all | Age range 15–79y | all | Age range 15–79y | ||||||||||||
| All study centers | 205 219 | 113 928 | 51,8 | 50 | 5245 | 344 | 257 | < 0.001 | |||||||
| Neubrandenburg | 1 | 259 | 190 | x | 47 | 57 | 22 006 | 7433 | 51,2 | 49,5 | 277 | 9 | 4 | 0,02 | |
| Leipzig | 2 | 846 | 627 | x | x | 88 | 117 | 10 874 | 5968 | 52,7 | 50,5 | 277 | 13 | 7 | 0,02 |
| Kiel | 3 | 649 | 488 | x | x | 100 | 120 | 9504 | 5641 | 52,1 | 50,4 | 253 | 9 | 7 | 0,46 |
| Halle | 2 | 424 | 310 | x | x | 109 | 124 | 10 139 | 5346 | 53,6 | 50,2 | 362 | 12 | 7 | 0,05 |
| Essen | 1 | 583 | 435 | x | 145 | 163 | 10 623 | 5382 | 51,4 | 48,8 | 155 | 15 | 9 | 0,05 | |
| Augsburg | 3 | 680 | 517 | x | x | 170 | 178 | 20 621 | 11 740 | 50,5 | 50,2 | 400 | 40 | 22 | < 0.001 |
| Mannheim | 1 | 309 | 240 | x | 156 | 186 | 10 287 | 5521 | 50 | 49,2 | 321 | 17 | 10 | 0,03 | |
| Berlin *5 | 3 | 4040 | 3064 | x | x | 186 | 215 | 31 202 | 19 650 | 52,6 | 50,1 | 916 | 53 | 42 | 0,08 |
| Hanover | 1 | 1158 | 863 | x | x | 211 | 242 | 10 049 | 4686 | 50,8 | 50,4 | 177 | 12 | 11 | 0,83 |
| Bremen | 1 | 569 | 427 | x | 227 | 259 | 10 486 | 6150 | 51,1 | 50 | 266 | 13 | 15 | 0,66 | |
| Münster | 1 | 314 | 246 | x | 226 | 268 | 10 031 | 6514 | 52,1 | 50,7 | 309 | 23 | 18 | 0,24 | |
| Düsseldorf | 1 | 619 | 471 | x | 231 | 272 | 9146 | 5253 | 53,2 | 49,7 | 250 | 21 | 14 | 0,06 | |
| Hamburg | 1 | 1841 | 1399 | x | 277 | 315 | 10 083 | 5300 | 52,4 | 49,1 | 256 | 11 | 17 | 0,15 | |
| Saarbrücken | 2 | 472 | 355 | x | x | 294 | 345 | 10 038 | 5992 | 52,3 | 50,4 | 345 | 26 | 21 | 0,29 |
| Regensburg | 2 | 346 | 268 | x | x | 329 | 373 | 10 030 | 6545 | 49,9 | 50,3 | 302 | 34 | 24 | 0,04 |
| Freiburg | 2 | 396 | 304 | x | x | 367 | 399 | 10 100 | 6807 | 51,9 | 50 | 379 | 36 | 29 | 0,18 |
*1 The numbers of inhabitants in the respective districts were obtained from the official records of the Federal Statistical Office (www.genesis.destatis.de/genesis/online). Numbers are given for all inhabitants (as of 31 December 2018). as well as for all inhabitants in the age range of 15–79 years (as of 31 December 2019) (see eTable 4).
*2 The numbers of COVID-19 cases per 100 000 inhabitants in the period 1–30 March 2020 were obtained from the official data of the Robert Koch Institute [https://npgeo-corona-npgeo-de.hub.arcgis.com/. 02.06.2020]. The cumulative incidences were calculated as arithmetic mean of all age groups. as well as averaged based on the age groups 15–34 years. 35–59 years and 60–79 years. weighted by the number of inhabitants in the respective age group in the counties (see eTable 4).
*3 The number of expected positive tests/SARS-CoV-2 infections was calculated as weighted mean. based on the number of participants and the cumulative COVID-19 incidence. for the total population and for the age groups 15–34 years. 35–59 years and 60–79 years. weighted by the number of participants in the respective age group in each district (see eTable 4).
*4 Comparison of numbers of expected vs observed SARS-CoV-2 infections. based on a goodness-of-fit chi-squared test for probabilities. with the probabilities given by the expected number of infections. Since the absolute numbers of cases are sometimes very small. the resulting p-values have to be interpreted with caution. *5 Three NAKO study centers jointly cover the Berlin NAKO study region.
Numbers of expected COVID-19 cases were calculated on the basis of official records from the Robert Koch Institute and the Federal Statistical Office, as described in eBox 2. The frequencies of COVID-19-related symptoms and their co-occurrence were graphically evaluated by means of bar plots and Euler diagrams.
eBOX 2. Calculation of observed and expected COVID-19 cases.
Official data on COVID-19 cases reported by local health authorities were obtained from the website “National Platform for Geographic Data (NPGEO) Corona Hub 2020” of the Robert Koch Institute (www.npgeo-corona-npgeo-de.hub.arcgis.com/; last accessed on 2 June 2020). The populations of the respective districts were obtained from the official records of the Federal Statistical Office (www.genesis.destatis.de/genesis/online). Numbers were retrieved for all inhabitants (as of 31 December 2018) and for inhabitants in the age range of 15–79 years (as of 31 December 2019) (see eTable 4). Cumulative incidences were calculated as weighted mean, based on the overall population and on the age groups 15–34 years, 35–59 years, and 60–79 years, weighted by the number of inhabitants in the respective age group in each district (see eTable 4). The number of expected positive tests/SARS-CoV-2 infections was calculated, based on the number of participants and the cumulative COVID-19 incidence, for the whole population and as weighted mean across the age groups 15–34 years, 35–59 years and 60–79 years, weighted by number of participants in the respective age group for each district (see eTable 4). For comparison of the expected versus observed numbers of SARS-CoV-2 infections we used a chi-squared goodness-of-fit test for probabilities, with the probabilities given by the expected number of infections.
The NAKO baseline examination included (4):
Physical examinations
A standardized personal interview
Self-administered questionnaires and tests
Acquisition of biological samples.
Several modules from the German version of the Patient Health Questionnaire (PHQ) (5, 6) for the assessment of mental health were included in the questionnaires to assess the severity of depressive symptoms (PHQ-9), anxiety symptoms (GAD-7), and perceived psychosocial strains (PHQ-stress).
The assessment of mental health in the COVID-NAKO questionnaire comprised the same scales (PHQ-9, GAD-7 and PHQ-stress). Summary scores for all three mental health scales were calculated according to the PHQ manual. The respective minimum and maximum scores are 0 to 27 points for PHQ-9, 0 to 21 points for GAD-7 and 0 to 20 points for PHQ-stress. The test-retest reliability of PHQ-9 and GAD-7 is high (7, 8). Differences between the COVID-NAKO questionnaire and the baseline examination were analyzed for all participants with data available at both time points. Student´s t-test was used to assess differences in mental health scores according to study center, age group, and sex. Multivariable linear regression models were applied with the difference in score between the COVID-NAKO questionnaire and baseline for each scale as dependent variable and age at baseline, sex, and baseline score as independent variables.
Self-rated health was assessed using the first question from the Short Form Health Questionnaire (SF-12). Changes in subjective state of health between the baseline examination and the time of the COVID-NAKO questionnaire were evaluated graphically and by means of adjusted logistic regression models. The binary outcome was worsening of self-rated health compared with baseline.
Results
Cumulative incidence of SARS-CoV-2-positive test results
Overall, 4.6% of NAKO participants reported having been tested for SARS-CoV-2 since 1 February 2020. Of the 5245 tested participants, 344 (6.6%) were positive for SARS-CoV-2, yielding an overall cumulative incidence of 0.3%. The mean age of participants who were tested for SARS CoV-2 was higher than for than those who were not tested (50 years vs 47 years) and the proportion of women among the tested participants was slightly higher (57%). The number of cases tested positive in our study was 34% (p < 0.001) higher than was predicted on the basis of the official statistics. More than 80% of the cases that tested positive had been detected by mid-April (efigure 1a). A higher cumulative incidence was observed in the more strongly affected southern study centers (Freiburg, Saarbrücken, Regensburg) than in the north and east (Neubrandenburg, Leipzig, Kiel) (eFigure 1b, eTable 2).
eFigure 1a.
Numbers of SARS-CoV-2 infections and tests, as self-reported in COVID-NAKO questionnaires
eFigure 1b.
NAKO study regions and cumulative COVID-19 incidence as of 29 May 2020
Frequency and distribution of symptoms
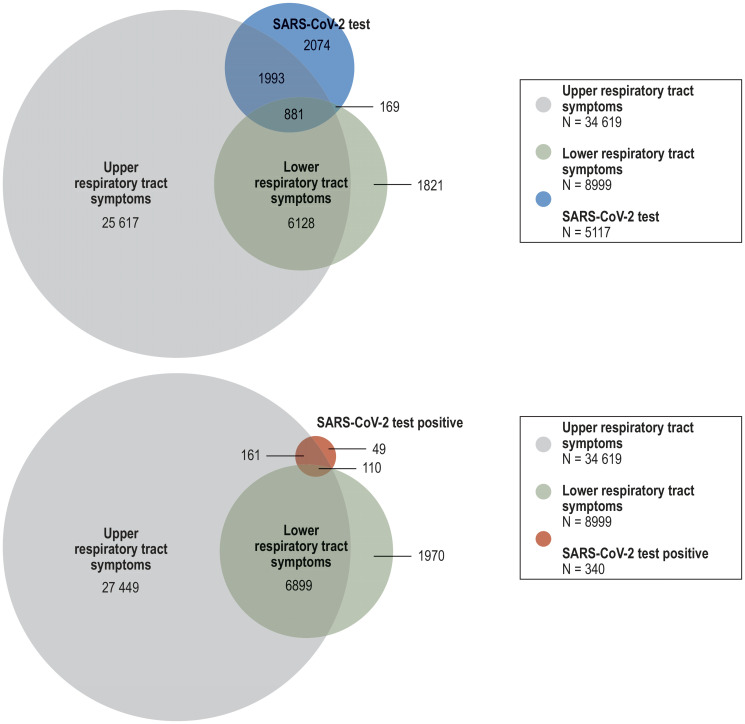
Across all regions, upper and lower respiratory tract symptoms were reported by 31% and 8% of the participants, respectively (etable 3). Of the 36 609 participants with either upper or lower respiratory tract symptoms in the preceding 4 months, 8.3% had been tested for SARS-CoV-2 infection. Among those tested, the rate of respiratory tract symptoms was much higher (59%). Specifically, 39% reported upper respiratory tract symptoms only, 3% lower respiratory tract symptoms only, and 17% reported symptoms in both segments (efigure 2). Persons who tested positive constituted only a minor fraction (0.93%) of all participants with respiratory tract symptoms (efigure 2). However, those with a positive test result reported on average more symptoms—such as fatigue, non-specific pain, loss of taste and smell—than those with a negative result (efigure 3). Thirty-six percent of participants with a positive test result reported no symptoms at all.
eTable 3. Distribution of respiratory tract symptoms and disease impact in the 16 NAKO study regions.
| Participants | Respiratory tract symptoms | Disease impact | |||||||||
| Upper respiratory tract | Lower respiratory tract | Bedridden | Sick leave | Hospital admission | |||||||
| N | n | % | n | % | n | % | n | % | n | % | |
| All study centers | 113 928 | 34 929 | 30,7 | 9242 | 8.1 | 15 627 | 13,7 | 16 141 | 14,2 | 617 | 0,5 |
| Neubrandenburg | 7433 | 1960 | 26,4 | 545 | 7.3 | 827 | 11,1 | 944 | 12,7 | 42 | 0,6 |
| Leipzig | 5968 | 1561 | 26,2 | 380 | 6.4 | 558 | 9.3 | 648 | 10,9 | 26 | 0,4 |
| Kiel | 5641 | 1805 | 32 | 458 | 8.1 | 810 | 14,4 | 827 | 14,7 | 26 | 0,5 |
| Halle | 5346 | 1372 | 25,7 | 317 | 5.9 | 516 | 9.7 | 643 | 12 | 25 | 0,5 |
| Essen | 5382 | 1664 | 30,9 | 448 | 8.3 | 779 | 14,5 | 784 | 14,6 | 26 | 0,5 |
| Augsburg | 11 740 | 3468 | 29,5 | 999 | 8.5 | 1640 | 14 | 1708 | 14,5 | 63 | 0,5 |
| Mannheim | 5521 | 1675 | 30,3 | 449 | 8.1 | 765 | 13,9 | 753 | 13,6 | 28 | 0,5 |
| Berlin | 19 650 | 6143 | 31,3 | 1482 | 7.5 | 2748 | 14 | 2877 | 14,6 | 120 | 0,6 |
| Hanover | 4686 | 1483 | 31,6 | 349 | 7.4 | 648 | 13,8 | 638 | 13,6 | 32 | 0,7 |
| Bremen | 6150 | 1957 | 31,8 | 456 | 7.4 | 891 | 14,5 | 921 | 15 | 19 | 0,3 |
| Münster | 6514 | 2067 | 31,7 | 573 | 8.8 | 961 | 14,8 | 961 | 14,8 | 35 | 0,5 |
| Düsseldorf | 5253 | 1630 | 31 | 429 | 8.2 | 749 | 14,3 | 706 | 13,4 | 27 | 0,5 |
| Hamburg | 5300 | 1719 | 32,4 | 425 | 8.0 | 775 | 14,6 | 764 | 14,4 | 20 | 0,4 |
| Saarbrücken | 5992 | 1973 | 32,9 | 618 | 10,3 | 887 | 14,8 | 914 | 15,3 | 38 | 0,6 |
| Regensburg | 6545 | 2135 | 32,6 | 623 | 9.5 | 932 | 14,2 | 914 | 14 | 49 | 0,7 |
| Freiburg | 6807 | 2317 | 34 | 691 | 10,2 | 1141 | 16,8 | 1139 | 16,7 | 41 | 0,6 |
eFigure 2.
Prevalence of symptoms of upper and lower respiratory tract infections in persons with available data on such symptoms, SARS-CoV-2 test, and result of SARS-CoV-2 test (N=111 582)
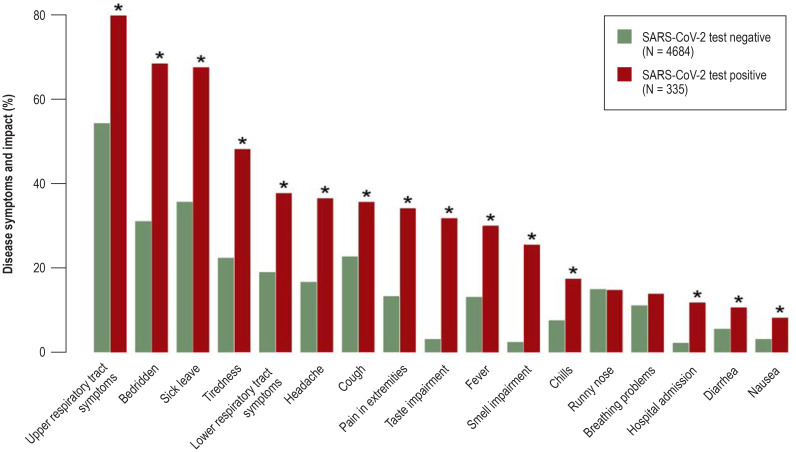
eFigure 3.
Prevalence of disease symptoms and consequences (bedridden, sick leave, hospital admission) in persons with positive SARS-CoV-2 test results and in those with negative SARS-CoV-2 test results. Statistically significant differences (p-value from chi-squared test < 0.0005) are indicated by *. The graph is based on the data of all persons who received a SARS-CoV-2 test and provided information on the result of the test and on all symptoms and measures of disease impact.
Changes in mental health
Figure 1 and eTable 5 illustrate the changes in mental health scores between the NAKO baseline examination and the time of the COVID-NAKO questionnaire. Figure 1 shows the mean increase in summary scores for self-perceived stress (1.14 ± 0.02) and for the severity of depressive (0.38 ± 0.02) and anxiety symptoms (0.36 ± 0.02), stratified by age and sex.
Figure 1.
Mean differences in mental health summary scores between the time of the COVID-NAKO questionnaire and the NAKO baseline examination, stratified by age group and sex
PHQ-stress; PHQ-9, depressive symptoms; GAD-7, anxiety symptoms; CI, confidence interval
eTable 5. Mental health summary scores at baseline and at the time of the COVID-NAKO questionnaire.
| PHQ-9 | GAD-7 | PHQ-stress | ||||
| Mean (SD) baseline | Mean (SD) COVID questionnaire | Mean (SD) baseline | Mean (SD) COVID questionnaire | Mean (SD) baseline | Mean (SD) COVID questionnaire | |
| All study centers | 3.68 (3.50) | 4.05 (3.91) | 3.01 (3.06) | 3.36 (3.42) | 3.36 (2.91) | 4.49 (3.48) |
| Neubrandenburg | 3.47 (3.44) | 3.73 (3.82) | 2.90 (3.03) | 3.16 (3.35) | 3.31 (2.96) | 4.50 (3.59) |
| Leipzig | 3.61 (3.40) | 3.70 (3.75) | 2.97 (3.05) | 3.07 (3.35) | 3.34 (2.85) | 4.29 (3.41) |
| Kiel | 3.59 (3.54) | 3.97 (3.88) | 2.85 (3.02) | 3.25 (3.37) | 3.24 (2.87) | 4.32 (3.44) |
| Halle | 3.50 (3.39) | 3.81 (3.78) | 2.91 (2.99) | 3.19 (3.33) | 3.34 (2.85) | 4.45 (3.41) |
| Essen | 3.94 (3.79) | 4.31 (4.11) | 3.15 (3.20) | 3.46 (3.52) | 3.41 (2.93) | 4.58 (3.51) |
| Augsburg | 3.53 (3.34) | 3.85 (3.84) | 2.99 (2.97) | 3.23 (3.40) | 3.35 (2.92) | 4.42 (3.52) |
| Mannheim | 3.78 (3.55) | 4.15 (3.98) | 3.00 (3.10) | 3.38 (3.47) | 3.35 (2.96) | 4.48 (3.46) |
| Berlin | 3.72 (3.50) | 4.24 (3.99) | 3.04 (3.07) | 3.45 (3.45) | 3.39 (2.92) | 4.60 (3.47) |
| Hanover | 3.58 (3.43) | 4.10 (3.83) | 2.86 (2.90) | 3.36 (3.38) | 3.24 (2.78) | 4.35 (3.36) |
| Bremen | 3.78 (3.49) | 4.19 (3.95) | 3.06 (3.08) | 3.42 (3.39) | 3.40 (2.93) | 4.46 (3.42) |
| Münster | 3.42 (3.29) | 3.90 (3.72) | 2.78 (2.88) | 3.32 (3.29) | 3.12 (2.74) | 4.29 (3.37) |
| Düsseldorf | 3.84 (3.63) | 4.27 (4.06) | 3.09 (3.15) | 3.53 (3.61) | 3.39 (2.94) | 4.53 (3.51) |
| Hamburg | 3.87 (3.69) | 4.39 (4.00) | 3.08 (3.17) | 3.55 (3.42) | 3.33 (2.88) | 4.69 (3.52) |
| Saarbrücken | 3.90 (3.65) | 4.15 (4.04) | 3.28 (3.26) | 3.56 (3.62) | 3.59 (3.06) | 4.67 (3.61) |
| Regensburg | 3.76 (3.56) | 4.00 (3.90) | 3.14 (3.10) | 3.41 (3.45) | 3.39 (2.97) | 4.52 (3.58) |
| Freiburg | 3.65 (3.39) | 4.06 (3.81) | 2.98 (2.91) | 3.48 (3.37) | 3.38 (2.87) | 4.61 (3.46) |
PHQ-stress; PHQ-9, depressive symptoms; GAD-7, anxiety symptoms; SD, standard deviation
Increases in perceived stress were observed across all age groups, while increases in depressive symptoms and anxiety symptoms were limited to those below the age of 60 years. The most pronounced increases on all three scales were seen in the younger age groups. Women showed much higher increases than men, e.g., a rise of 1.94 points on the stress scale (minimum 0, maximum 20) in the age group 30–39 years.
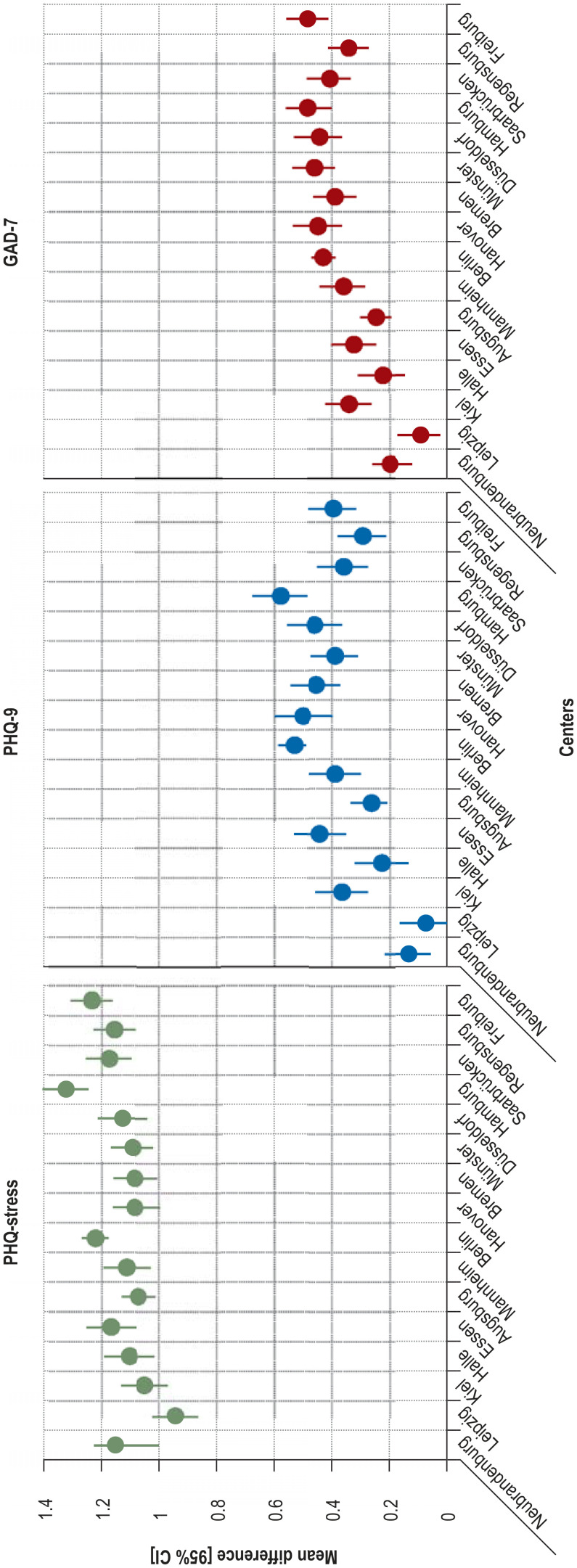
On all three scales for mental health, the differences from baseline were somewhat less pronounced in the NAKO regions with a low cumulative incidence than in the regions with intermediate or high incidence (efigure 4). This pattern was apparent for all scales regardless of the absolute increase in score.
eFigure 4.
Differences in mean summary scores for mental health, stratified by study center (in increasing order of background infection rate) and adjusted for sex, age at baseline, and summary score at baseline. PHQ-stress; PHQ-9, depressive symptoms; GAD-7, anxiety symptoms; CI, confidence interval
Participants who reported having been tested for SARS-CoV-2, regardless of whether the test result was positive or negative, had higher scores on all scales for mental health than those who had not been tested (efigure 5). The increase in mean severity of both depressive symptoms and anxiety symptoms raised the proportion of those who were above the cut-off points on these two scales (≥10 points): from 6.4% to 8.8% (depression) and from 4.3% to 5.7% (anxiety). The cut-off value shows symptoms of depression or anxiety with clinical relevance (9).
eFigure 5.
Increase in summary scores for mental health, stratified by SARS-CoV-2 test status. Mean differences in summary scores, with 95% confidence interval, between the NAKO baseline examination and the COVID-NAKO questionnaire, adjusted for sex, age at baseline, and summary score at baseline
PHQ-stress; PHQ-9, depressive symptoms; GAD-7, anxiety symptoms
Changes in self-rated health status
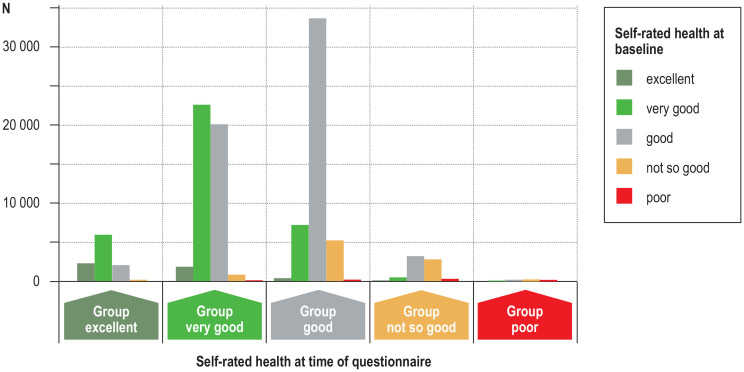
Thirty-two percent of the participants stated an improvement in self-rated state of health since baseline (figure 2), while 12% reported deterioration. Worsening was reported predominantly by persons who had been tested (odds ratio for those tested negative: 1.68, 95% confidence interval [1.54; 1.82], odds ratio for those tested positive: 2.38 [1.83; 3.10]), after adjustment for age, sex, study center, and self-rated health at baseline (etable 6). Furthermore, there was a relationship between deterioration in self-rated health and worsening of mental health (efigure 6).
Figure 2.
Self-rated health during the pandemic (x-axis) compared with the NAKO baseline examination (color coding). Y-axis: Number of participants. Overall, 56% of participants reported their health status as unchanged, 32% rated it as better during the pandemic, and 12% stated that it was worse during the pandemic than at the time of the NAKO baseline examination.
eTable 6. Associations of baseline characteristics. study center and SARS-CoV-2 testing status with deterioration in self-rated health from baseline to time of COVID-NAKO questionnaire*1, 2.
| OR | [95% CI] | p-value | |
| Characteristics at baseline examination | |||
| Age. per 5 years | 1.11 | [1.10; 1.11] | < 0.001 |
| Women | 1,3 | [1.25; 1.35] | < 0.001 |
| Self-rated health. continuous | 0,22 | [0.21; 0.23] | < 0.001 |
| Study center: reference Neubrandenburg | |||
| Leipzig | 0,93 | [0.83; 1.05] | 0.25 |
| Kiel | 0,99 | [0.88; 1.11] | 0.84 |
| Halle | 0,99 | [0.88; 1.11] | 0.82 |
| Essen | 1 | [0.89; 1.13] | 0.97 |
| Augsburg | 0,91 | [0.82; 1.00] | 0.05 |
| Mannheim | 0,91 | [0.81; 1.02] | 0.10 |
| Berlin | 1,03 | [0.95; 1.13] | 0.46 |
| Hanover | 1,05 | [0.93; 1.19] | 0.39 |
| Bremen | 0,99 | [0.89; 1.11] | 0.90 |
| Münster | 0,99 | [0.89; 1.11] | 0.93 |
| Düsseldorf | 0,8 | [0.70; 0.90] | < 0.001 |
| Hamburg | 0,84 | [0.75; 0.95] | < 0.001 |
| Saarbrücken | 1,03 | [0.92; 1.16] | 0.58 |
| Regensburg | 0,95 | [0.85; 1.07] | 0.41 |
| Freiburg | 0,87 | [0.78; 0.97] | 0.01 |
| SARS-CoV-2 test result: reference “not tested” | |||
| Negative test result | 1,68 | [1.54; 1.82] | < 0.001 |
| Positive test result | 2,38 | [1.83; 3.10] | < 0.001 |
*1 The parameters investigated were age. sex. self-rated health status at baseline examination. study center. and SARS-CoV-2 test status.
*2 Results from a logistic regression model with the dichotomous outcome: “self-rated health status at the time of the COVID-NAKO questionnaire is worse than at the baseline examination”. Self-rated health at the baseline examination was regarded in the model as continuous. with 1 meaning “excellent” and _5 signifying “poor”.
OR. Odds ratio; CI. confidence interval
eFigure 6.
Description of change in self-rated health status between baseline examination and COVID-NAKO questionnaire with changes in summary scores for mental health
X-axis: Change in self-rated health between baseline examination and questionnaire, categorized into unchanged/better/worse
Y-axis: Difference in mean summary scores between time of questionnaire and baseline.
PHQ-stress; PHQ-9, depressive symptoms; GAD-7, anxiety symptoms
Discussion
Consistently with official figures from local health offices, the results of this large, population-based cohort study indicate that up to the end of May 2020, a low proportion of infections with SARS-CoV-2 were self-reported (0.3%). Nevertheless, the NAKO data revealed 34% more positive test results than predicted on the basis of official reporting statistics. Selection bias may be at work here, as persons who tested positive may have been more likely to participate in the survey. The data cover the time from the start of the pandemic until it reached its peak in Europe (10). Early on, the epidemic was driven mainly by people coming back from abroad. This group tended to be of higher socio-economic status, a stratum which is also overrepresented in the NAKO. Furthermore, the higher cumulative incidence could also be due to increased health consciousness on the part of the NAKO participants. The implementation of a test-based case identification strategy along with countermeasures such as social distancing could have contributed to the decline in new SARS-CoV-2 infections in the NAKO study regions observed in our sample (11– 13).
Most of the persons with positive test results described their symptoms as mild, with 36% reporting no symptoms and 12% requiring hospitalization. Our data confirm that loss of smell and taste is associated with a higher likelihood of a positive SARS-CoV-2 test (14, 15).
Participants reported more perceived stress and more symptoms of depression and anxiety during the pandemic than at the time of the baseline examination, conducted 1 to 5 years earlier. While various factors may have contributed to this change over time, the fact that NAKO participants living in regions of low SARS-CoV-2 incidence reported fewer mental problems than those from regions of higher incidence supports a relation with the pandemic. Greater severity of depressive and anxiety symptoms was restricted to those younger than 60, with a focus on young adults between the ages of 20 and 39 years. Similar findings have recently been reported in the UK (16) and in a small follow-up survey conducted in April 2020 at Johns Hopkins University. The latter found a clear increase in severe psychological distress compared with a prior assessment in 2018, particularly in young adults aged 18 to 29 years (17).
A study with Dutch students showed that the lockdown in March 2020 negatively affected the students’ ability to stabilize their mood through familiar activities (18). Young and middle-aged adults were under particular pressure, having to manage various tasks in a situation of limited services and multiple challenges associated with the advice to stay at home. This included, for example, the coordination of working from home or other changes in working conditions with home schooling, childcare, or care for the elderly.
Very recent commentaries and recommendations (3, 19) emphasize the urgent necessity to collect high-quality data on the mental health effects of the COVID-19 pandemic across the whole population and in vulnerable groups (3) and point to the fact that the pandemic may have considerable implications for individual and collective health as well as for emotional and social functioning (19). They also address the need to provide mental health services that target patients’ health needs and reduce (social) disparities (20).
Self-rated health deteriorated in participants who underwent testing, especially in those with a positive test result. However, self-rated health also improved in a considerable number of participants. Given that this is self-perceived health, subjective changes in health consciousness rather than objective improvements may be responsible for this observation. While contact restrictions were in place, beginning in mid-March, essential shopping, access to the workplace (in the absence of reduced hours or working from home), and outdoor exercising were allowed at all times in the NAKO study regions. The national government and the individual federal states implemented a wide range of support programs to lessen the socioeconomic burden.
While working conditions became much worse for some employees, such as those in the health care sector, other population groups gained additional leisure time and experienced a slower pace of life, increased health consciousness, and neighborhood support. The results were not adjusted for individual socioeconomic factors; future analyses should specifically examine their potential role as modifiers.
A major strength of the results presented here is that they are derived from a large, population-based cohort with a defined sampling frame from 16 geographic regions of Germany. The baseline data supply a detailed characterization of the health status of the participants before the outbreak of the COVID-19 pandemic. The COVID-NAKO questionnaire offered a timely longitudinal follow-up and included several questions and scales previously employed in the baseline assessment. This provided the opportunity to analyze changes in health scores over time. Limitations arise from the fact that all responses are based on self-reports. Changes in the scores for mental health could be attributable to the pandemic, to the countermeasures, or to other unrelated factors. The mental health scores were analyzed on the dimensional scale only; in other words, no (subtype) diagnoses, e.g., major depressive disorder, were applied. The reported SARS-CoV-2 test results are only a snapshot, reflecting the situation at the time of filling in the questionnaire.
The worsening of results regarding mental health was stronger in regions with a higher background cumulative incidence. Moreover, it was slightly more pronounced among participants who had undergone the baseline examination only 1 or 2 years previously. This speaks for an association between worsening of mental health and the pandemic. Because the population was confronted with constantly changing regulations concerning health and general behavior, the results need to be discussed within the context of the dynamics of the pandemic. Repeated assessments are required to determine whether the consequences of the countermeasures will persist for a longer period.
Conclusion
Although the cumulative incidence of detected SARS-CoV-2 infections was low on the population level in Germany in spring 2020, we observed a deterioration in mental health scores during the nationwide 6-week period of protective measures in the entire NAKO cohort, irrespective of test or infection status. Our results indicate health consequences at population level that go substantially beyond the direct health impact of COVID-19.
BOX – AUTHORS. Complete list of authors and affiliations.
Annette Peters1,2,3; Susanne Rospleszcz1,2; Karin H. Greiser4; Marco Dallavalle1; André Karch5; Rafael Mikolajczyk6; Gérard Krause7; Stefanie Castell7; Alexandra Nieters8; Daniel Kraft9; Robert Wolff10; Gunthard Stübs10; Olga Lang1; Leo Panreck11; Marcella Rietschel12; Dan Rujescu13; Nico Dragano14; Börge Schmidt15; Heiko Becher16; Hermann Brenner17; Antje Damms-Machado4; Beate Fischer18; Claus-Werner Franzke19; Sylvia Gastell20; Kathrin Günther21; Anne Hermes22; Bernd Holleczek23,24; Lina Jaeschke25; Karl-Heinz Jöckel15; Rudolf Kaaks4; Thomas Keil26,27,28; Yvonne Kemmling29; Alexander Kluttig6; Oliver Kuß30; Nicole Legath5; Michael Leitzmann18; Wolfgang Lieb22; Markus Loeffler31, 32; Claudia Meinke-Franze10; Karin B. Michels19; Nadia Obi16; Tobias Pischon25; Tamara Schikowski33; Matthias B. Schulze34,35; Andreas Stang15; Sigrid Thierry1, 36; Henry Völzke10; Stefan N. Willlich37; Kerstin Wirkner31,32; Kathrin Wolf1; Hajo Zeeb38, 39; Klaus Berger5
1 Institute of Epidemiology, Helmholtz Center Munich, Germany
2 Department of Epidemiology, Institute for Medical Information Processing, Biometry and Epidemiology, Medical Faculty, Ludwig-Maximilians-Universität München, Munich, Germany
3 Department of Environmental Health, Harvard T. H. Chan School of Public Health, Boston, MA, USA
4 Department of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
5 Institute of Epidemiology and Social Medicine, University of Münster, Germany
6 Institute of Medical Epidemiology, Biometrics and Informatics, University of Halle-Wittenberg, Halle (Saale), Germany
7 Department of Epidemiology, Helmholtz Center for Infection Research, Braunschweig, Germany
8 Center for Chronic Immunodeficiency (CCI), University of Freiburg Medical Center, Germany
9 Medical Informatics for Translational Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany
10 Institute of Community Medicine, University of Greifswald, Germany
11 NAKO e.V., Heidelberg, Germany
12 Department of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health), Mannheim, Germany
13 Department of Psychiatry, Psychotherapy, and Psychosomatics, University of Halle-Wittenberg, Halle (Saale), Germany
14 Institute of Medical Sociology, University Hospital of Düsseldorf, Germany
15 Institute for Medical Informatics, Biometry and Epidemiology, University Hospital of Essen, Germany
16 Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
17 Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany
18 Department of Epidemiology and Preventive Medicine, Regensburg University Medical Center, Germany
19 Institute for Prevention and Cancer Epidemiology, Faculty of Medicine, University of Freiburg Medical Center, Germany
20 NAKO Study Center South Berlin/Brandenburg, German Institute for Nutritional Research Potsdam-Rehbruecke, Nuthetal, Germany
21 Leibniz Institute for Prevention Research and Epidemiology— BIPS, Bremen, Germany
22 Institute of Epidemiology, Kiel University, Germany
23 Saarland Cancer Registry, Saarbrücken, Germany
24 Division of Clinical Epidemiology and Aging Research, German Cancer Research Center (DKFZ), Heidelberg, Germany
25 Molecular Epidemiology Research Group, Max Delbrück Center for Molecular Medicine (MDC), Berlin, Germany
26 Institute for Social Medicine, Epidemiology, and Health Economics, Charité—University Medical Center Berlin, Germany
27 Institute for Clinical Epidemiology and Biometry, University of Wuerzburg, 97080 Würzburg, Germany
28 Bavarian State Office of Health, Health and Food Safety, Bad Kissingen, Germany
29 Department of Epidemiology, Helmholtz Center for Infection Research, Braunschweig, Germany
30 Institute for Biometrics and Epidemiology, German Diabetes Center, Leibniz Center for Diabetes Research, University of Düsseldorf, Germany
31 Institute for Medical Informatics, Statistics and Epidemiology (IMISE), University of Leipzig, Germany
32 Leipzig Research Center for Civilization Diseases (LIFE), University of Leipzig, Germany
33 IUF—Leibniz Institute for Environmental Medicine, Düsseldorf, Germany
34 Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbruecke, Nuthetal, Germany
35 German Institute for Nutritional Research Potsdam-Rehbruecke, University of Potsdam, Germany
36 NAKO Study Center, University Hospital Augsburg, Germany
37 Institute for Social Medicine, Epidemiology, and Health Economics, Charité – University Medical Center Berlin, Germany
38 Leibniz Institute for Prevention Research and Epidemiology— BIPS, Bremen, Germany
39 Human and Health Sciences, University of Bremen, Germany
eTable 4. Age group-specific cumulative incidences* and observed and expected case numbers.
| Age group (years) | Population | COVID-19 cases per 100 000 | Participants | SARS-CoV-2 positive | ||
| Observed (n) | Expected (n) | |||||
| All study centers | 15–34 | 3 435 142 | 230 | 16 322 | 48 | 36 |
| 35–59 | 4 672 133 | 235 | 67 094 | 252 | 155 | |
| 60–79 | 2 095 850 | 218 | 30 512 | 44 | 66 | |
| Neubrandenburg | 15–34 | 43 430 | 74 | 1045 | 0 | 1 |
| 35–59 | 90 073 | 59 | 4544 | 8 | 3 | |
| 60–79 | 56 742 | 41 | 1844 | 1 | 1 | |
| Leipzig | 15–34 | 211 238 | 116 | 774 | 1 | 1 |
| 35–59 | 281 408 | 128 | 3518 | 12 | 4 | |
| 60–79 | 134 047 | 98 | 1676 | 0 | 2 | |
| Kiel | 15–34 | 154 987 | 99 | 756 | 1 | 1 |
| 35–59 | 222 087 | 115 | 3318 | 5 | 4 | |
| 60–79 | 110 632 | 157 | 1567 | 3 | 2 | |
| Halle | 15–34 | 91 664 | 108 | 695 | 1 | 1 |
| 35–59 | 139 541 | 140 | 3270 | 10 | 5 | |
| 60–79 | 79 202 | 115 | 1381 | 1 | 2 | |
| Essen | 15–34 | 143 102 | 138 | 925 | 4 | 1 |
| 35–59 | 194 927 | 178 | 3138 | 10 | 6 | |
| 60–79 | 96 833 | 170 | 1319 | 1 | 2 | |
| Augsburg | 15–34 | 169 685 | 148 | 1674 | 3 | 2 |
| 35–59 | 239 483 | 191 | 6866 | 31 | 13 | |
| 60–79 | 107 730 | 196 | 3200 | 6 | 6 | |
| Mannheim | 15–34 | 90 495 | 188 | 875 | 3 | 2 |
| 35–59 | 105 056 | 200 | 3292 | 12 | 7 | |
| 60–79 | 44 576 | 148 | 1354 | 2 | 2 | |
| Berlin | 15–34 | 1 032 897 | 224 | 2727 | 9 | 6 |
| 35–59 | 1 425 486 | 215 | 11 661 | 37 | 25 | |
| 60–79 | 605 183 | 200 | 5262 | 7 | 11 | |
| Hanover | 15–34 | 279 960 | 241 | 693 | 0 | 2 |
| 35–59 | 398 418 | 259 | 2644 | 11 | 7 | |
| 60–79 | 184 410 | 205 | 1349 | 1 | 3 | |
| Bremen | 15–34 | 147 443 | 327 | 923 | 2 | 3 |
| 35–59 | 189 949 | 243 | 3547 | 10 | 9 | |
| 60–79 | 89 629 | 182 | 1680 | 1 | 3 | |
| Münster | 15–34 | 102 805 | 249 | 855 | 3 | 2 |
| 35–59 | 100 386 | 291 | 3752 | 18 | 11 | |
| 60–79 | 42 803 | 259 | 1907 | 2 | 5 | |
| Düsseldorf | 15–34 | 160 799 | 281 | 861 | 5 | 2 |
| 35–59 | 220 553 | 277 | 2959 | 11 | 8 | |
| 60–79 | 89 857 | 240 | 1433 | 5 | 3 | |
| Hamburg | 15–34 | 494 366 | 301 | 854 | 1 | 3 |
| 35–59 | 654 050 | 315 | 3170 | 9 | 10 | |
| 60–79 | 250 439 | 341 | 1276 | 1 | 4 | |
| Saarbrücken | 15–34 | 107 896 | 309 | 774 | 3 | 2 |
| 35–59 | 156 771 | 355 | 3526 | 22 | 13 | |
| 60–79 | 90 160 | 370 | 1692 | 1 | 6 | |
| Regensburg | 15–34 | 92 180 | 400 | 911 | 6 | 4 |
| 35–59 | 121 691 | 378 | 3849 | 20 | 15 | |
| 60–79 | 53 922 | 313 | 1785 | 8 | 6 | |
| Freiburg | 15–34 | 112 195 | 330 | 980 | 6 | 3 |
| 35–59 | 132 254 | 431 | 4040 | 26 | 17 | |
| 60–79 | 59 685 | 457 | 1787 | 4 | 8 | |
*Obtained from the data of the Robert Koch Institute (https://npgeo-corona-npgeo-de.hub.arcgis.com/. 02.06.2020) n. Number
Acknowledgments
We thank all staff at the study centers, the NAKO data management center, and the NAKO head office who made this study possible. Furthermore, we thank Maren Albrecht and Dr. Barbara Bohn for their committed work and their valuable contributions to the implementation of the questionnaire, as well as Dr. Susanne Göttlicher for her assistance in finalizing the manuscript.
Footnotes
Conflict of interest statement
Prof. Lieb owns shares in Biontech. The remaining authors declare that no conflict of interest exists.
Funding and support
The German National Cohort (NAKO) (www.nako.de) is funded by the Federal Ministry of Education and Research (project numbers: 01ER1301A/B/C and 01ER1511D), the federal states, and the Helmholtz Association, with additional financial support from the participating universities and the participating institutes of the Leibniz Association and the Helmholtz Association.
References
- 1.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 3.Holmes EA, O‘Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.German National Cohort Consortium. The German National Cohort: aims, study design and organization. Eur J Epidemiol. 2014;29:371–382. doi: 10.1007/s10654-014-9890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study Primary care evaluation of mental disorders Patient health questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 6.Lowe B, Grafe K, Zipfel S, et al. Detecting panic disorder in medical and psychosomatic outpatients: comparative validation of the hospital anxiety and depression scale, the patient health questionnaire, a screening question, and physicians‘ diagnosis. J Psychosom Res. 2003;55:515–519. doi: 10.1016/s0022-3999(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 7.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 9.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 11.Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324:859–870. doi: 10.1001/jama.2020.14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. The Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce M, Hope H, Ford T, et al. Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiatry. 2020;7:883–892. doi: 10.1016/S2215-0366(20)30308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGinty EE, Presskreischer R, Han H, Barry CL. Psychological distress and loneliness reported by US adults in 2018 and April 2020. JAMA. 2020;324:93–94. doi: 10.1001/jama.2020.9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taquet M, Quoidbach J, Fried EI, Goodwin GM. Mood homeostasis before and during the coronavirus disease 2019 (COVID-19) lockdown among students in the Netherlands. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.2389. e202389. doi: 10.1001/jamapsychiatry.2020.2389. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferbaum B, North CS. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383:510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 20.Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7:813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]