Abstract
Cerebral palsy (CP) is a non-progressive motor dysfunction leading to multiple morbidities, including spasticity, which can be managed with botulinum toxin injection (BTI). This literature review aims to examine published studies on the efficacy and safety of different interventions used to reduce pain and anxiety associated with BTI in children with CP. A literature review of all published evidence in English language, or with an English translation between 1999 and 2019, using PubMed, EBSCO host, and Medline databases was carried out. All identified papers were screened for inclusion criteria. Data from included papers were entered and analyzed on an Excel database. Twenty-one studies conducted in multiple clinical settings identified 10 different analgesia and sedation modalities including intravenous ketamine, midazolam, inhaled nitrous oxide, general anesthesia, and Eutectic Mixture of Local Anesthetics (EMLA®) cream. Most of the studies were descriptive with the exception of two clinical trials and one qualitative study. All interventions had some adverse effects, but they were generally mild and no long-term sequelae were reported. The combination of inhaled nitrous oxide with EMLA® cream showed promising primary results. However, ketamine and midazolam combination could be a safe alternative. Currently, there is no sufficient data to draw on the superiority of any modality. Further high-quality studies are warranted.
Keywords: Analgesia, Botulinum toxin injection, Cerebral palsy, General anesthesia, Spasticity
INTRODUCTION
Cerebral palsy (CP) refers to a spectrum of conditions that affect the developing brain leading to central nervous system (CNS) permanent, non-progressive motor dysfunction that affects muscle tone, posture, and movement [1]. The main associated morbidity is motor impairment leading to limitations in function and activity with a wide spectrum of clinical presentations that change as the CNS matures [1]. Approximately 80% of the children with CP will have spasticity as the main motor abnormality [1], but they could also have intellectual disability, communication, behavioral difficulties, and seizure disorders [2].
Botulinum toxin injection (BTI) has been used as the primary method of treatment for focal spasticity in children [2]. Other anti-spasticity interventions include medications such as baclofen, benzodiazepines, dantrolene, and tizanidine, as well as denervation via chemical agents, such as phenol and alcohol, for segmental and localized spasticity [3,4]. The primary indications for spasticity treatment whether focal or generalized are to reduce pain and muscle spasms, minimize contractures and deformity, enabling brace use, improve posture, aid mobility, and improve patient’s ease of self-care [3].
The efficacy and safety of treatment with BTI in the management of spasticity has been established in children with CP [5,6], with several studies demonstrating the effectiveness and improvements in spasticity, deformity, and gait [7-9]. However, pain and anxiety related to intramuscular injections are significant concerns to a relatively safe procedure [4]. Hence, in the effort to reduce pain and anxiety in children, the selection of an appropriate procedural sedation and analgesia (PSA) agents is essential [10]. Currently, there are no established standards of care for PSA for children undergoing BTI and there is a great variability among clinicians for pain prevention protocols ranging from no intervention to topical anesthesia, oral sedation, inhaled nitrous oxide to general anesthesia (GA) [11]. Alternative approaches including the use of medical clowns or play therapists have also been reported [9-11].
In this study, we conducted a literature review to identify the different PSAs used for BTI sessions in children with CP to assess their feasibility and adverse effects.
Materials and Methods
Search strategy
A comprehensive literature search through PubMed, EBSCO, and Medline was carried out using the following terms: [“botulinum toxins” (MeSH Terms) AND “injection” (MeSH Terms) AND sedation (All Fields)] AND (“CP”[MeSH Terms]) AND (“child” [MeSH Terms). The inclusion criteria for the studies were all studies conducted between 1999 and 2019, studies using modalities of PSA in children with CP undergoing BTI for spasticity, aged 2-21 years, and all publications in English or with an English translation available.
Study selection
All articles identified during initial stages were reviewed by two reviewers. Articles that clearly did not meet the eligibility criteria were rejected at initial review. Articles marked for potential inclusion were then obtained electronically or in a paper copy and reassessed for inclusion. Studies that met the inclusion criteria were appraised by two independent authors. In case of any disagreements regarding inclusion of studies, a third author was consulted. Whenever applicable, when assessments of patients had been reported at different times, only the latest and most comprehensive assessments were included.
Data analysis
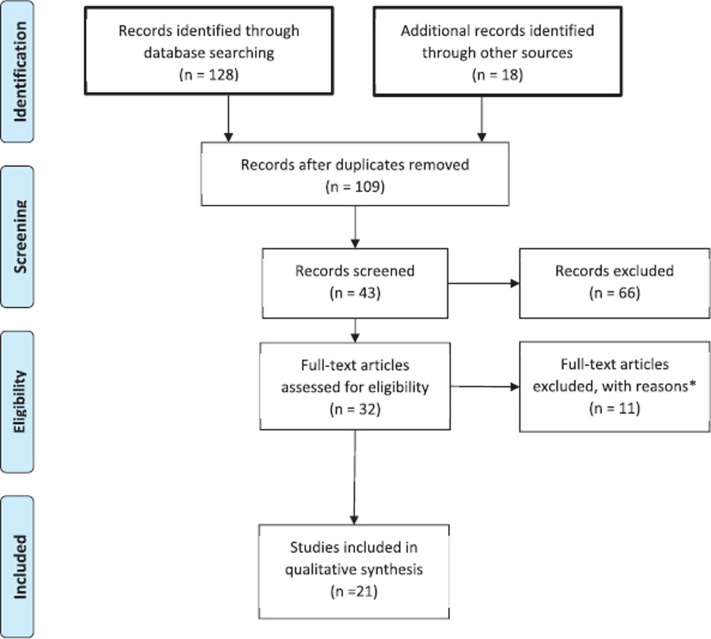
Data were extracted from selected studies and entered into an excel database. Variables included in the database were number of patients per study, age of the patients recruited from each study, type of PSA used (if any), reported adverse effects (if any), study limitation, and study outcome. The PRISMA checklist guidelines [9] were used for reporting our review findings (Figure 1). The study was approved by Mohammed Bin Rashid University of Medicine and Health Sciences Research Ethics Committee.
Figure 1.
Study selection flowchart. *Due to language of publication (one in Spanish and one in Portuguese), nine were excluded because they had no clear indication as to the sedation method used.
RESULTS AND DISCUSSION
A total of 109 studies were screened for eligibility, of which 32 were selected for full-text reviewing. Eleven studies were excluded after initial assessment based on the following reasons: two studies were excluded due to language of publication (Spanish and Portuguese) and nine were excluded because they had no clear indication as to the sedation method used. The remaining studies were included in this review (Figure 1). Of the 21 studies, nine (42.85%) were retrospective studies, six (28.57%) were prospective analysis studies, three (14.28%) were randomized control studies (one cohort, one case control, and one qualitative study) (each representing 4.76%). The main outcomes were adverse effects, safety, feasibility, and parents’ satisfaction.
The most frequent PSA methods used for BTI were inhaled nitric oxide (iN2O), EMLA® topical cream, and midazolam given via different routes. Using iN2O as the sole mode of PSA was reported in only one study, which concluded that iN2O was efficacious and effective in terms of cost and was a suitable alternative to conscious sedation or GA [12]. In six studies, iN2O was used with either EMLA® cream or midazolam [12-17]. Inhaled nitric oxide (iN2O) was associated with lower pain scores and was considered more cost-effective and efficacious when compared with GA, but nausea, vomiting, and headache were the most reported adverse effects [18]. Brochard et al. [19], compared iN2O with midazolam and concluded that iN2O was more effective in terms of post-procedural sedation. The authors also reported no difference in ease of procedure between the groups [19].
Midazolam use was reported in six studies and in different preparations (oral, rectal, intramuscular, and intravenous) [12,15-17,20,21]. The drug had an overall good feasibility, but it was associated with adverse effects including pain, nausea, and sleep disturbance. Although midazolam might provide adequate sedation and amnesia for the procedure, its usefulness was tempered by its lack of an analgesic effect [17].
Topical EMLA® cream was used in five studies: three in combination with iN2O [14,16,17], one with paracetamol [13], and one with midazolam [22]. Combining iN2O with local EMLA® cream application showed that around 50% of the patients did not receive sufficient analgesia. Henceforth, the need for extra sedation during the BTI procedure was required [19].
Ketamine is one of the commonly used sedation methods that have been shown to have a good safety profile in addition to its efficacy. Commonly reported adverse effects include rash, nausea, vomiting, sleep disturbance, and mild headaches [14]. The combination of intravenous ketamine with either intravenous or rectal midazolam was reported to be easy and resulted in better pain tolerance [14,21]. Nonetheless, the main adverse effects included nausea and vomiting, limb tremors, and headaches.
GA, using remifentanil and propofol, was linked to a safer adverse effects profile and parental perception of less pain. Moreover, BTI under GA was thought to lead to a more accurate injection site choice [23,24].
The use of distraction techniques, such as clowns, combined with iN2O was preferred by parents but had no effect on pain or anxiety; nonetheless, a recent controlled crossover study reported lower pain that continued after the crossover [25,26].
The literature review showed that over the last 20 years, different sedation modalities have been used as PSA in children with CP but with no clear-cut evidence preferring one intervention over the other. Ketamine and midazolam have been shown to be effective in managing BTI-associated pain and anxiety when either one is used alone. However, there is individual response variation to these medications. Moreover, high doses are associated with serious adverse effects, such as dysphoric reactions, with ketamine and respiratory depression with midazolam [27]. A combination of both medications at smaller doses might offer the desired pharmacological effect [28]. On the contrary, iN2O provides analgesia in addition to procedural sedation and amnesia. iN2O produced higher levels of procedural sedation compared to enteral midazolam; however, those who received iN2O were less sedated at the end of the clinic encounter. iN2O was observed to be more effective in reducing pain than midazolam. Furthermore, iN2O was viewed as a better choice during BTI sessions by parents, in comparison to prior experience with midazolam. It also achieved a higher level of sedation than midazolam [17]. iN2O has proven to be advantageous as it seems to offer an almost pain- and anxiety-free procedure. Besides, it requires neither an intravenous line nor post-procedure monitoring given its short half-life. Another benefit is that it blunts any unpleasant memories [22]. On the downside, the need for high concentrations of iN2O as a general anesthetic is linked to the suppression of reflexes and is associated with the risk of airway compromise. When used in outpatient or day care facilities, iN2O can be self-administered. Although this can be beneficial for the children able to cooperate, it can, unfortunately, limit its use in the group of children who may have a degree of cognitive impairment or in those who are too young.
The use of propofol alone can be effective but higher doses may be required to provide optimal muscle relaxation during such painful procedures. Sedative and systemic analgesic agents are generally required to provide amnesia and analgesia. Patient selection undergoing this procedure should be well thought out, as post-procedure monitoring is required, as well as the presence of a trained anesthetist, which might pose a burden on the healthcare system, as it requires hospital admission and use of resources [23,24]. In comparison to GA, conscious sedation requires less staff and resources with a consequent lowering of cost and time. On the other hand, the use of GA was reported to result in a more accurate choice of injection sites [23]. Both approaches had similar carer’s satisfaction rates and minimal adverse events [24]. Louer et al. [19], used a combination of propofol and ketamine for PSA during BTI sessions. The authors reported adverse events in 10% during their study timeline. Major adverse effects included hypoxemia, transient apnea, and both hypoxemia and apnea which was reported in less than 1% of the cases with adverse events [29].
The combination of iN2O and EMLA® cream was reported to provide sufficient pain control, with or without the use of benzodiazepines, such as midazolam, and alleviate the procedure-related anxiety. This is consistent with the collective experience of the participating clinicians and carers [14,17]. On the contrary, a study by Brochard et al. [19], found that 38% of children infiltrated with iN2O and EMLA®, required more analgesia to carry out the procedure [30]. The authors concluded that the clinical characteristics seem to not strongly correlate with the success or failure of the 50% N2O/oxygen-EMLA® protocol [30].
Inhaled N2O analgesia is a cost-effective and efficacious alternative to conscious sedation or GA for minor pediatric surgical procedures when administered by an experienced practitioner [17]. iN2O can be safely applied on a patient with a full stomach and it has the advantage of rapid onset of action. Furthermore, there is minimal preparation, and utilization of resources with no requirement for an intravenous line or post-procedure monitoring [22]. Nausea and vomiting seem to be more when iN2O is administered on an empty stomach. These adverse effects could be prevented by feeding the child immediately before beginning the procedure [22].
CONCLUSION
To our knowledge, no previous extensive reviews were conducted to assess the use of PSA in BTI in children with CP, henceforth the need for such a paper. BTI-associated pain and anxiety is a significant concern to a relatively safe procedure but could be alleviated with the right choice of medications.
Inhaled nitrous oxide is one of the promising options to consider either alone or in combination with EMLA® topical analgesic. Ketamine and midazolam in intravenous formulation combination could also be a safe alternative to iN2O/EMLA® mixture for patients who do not tolerate inhalational analgesia [20,31,32]. Both of these methods have no serious adverse effects when regular precautions are taken into account.
One of the limitations of this review is language bias, as only manuscripts in English or with English translation were included.
RECOMMENDATIONS
The lack of an overall consensus and a unified guideline calls for more studies to be conducted. Future research should also keep in mind the fact that children with CP, especially those with the severe spastic quadriplegic form, are at a higher risk of airway-related problems during PSA, as they have increased respiratory secretions, which may present as a disadvantage to the use of iN2O or GA. Future studies should also include standardized and validated scales for pain and anxiety in verbal and non-verbal children with CP.
CONFLICT OF INTEREST
The author declares that there are no competing interests.
FUNDING
None.
ETHICAL APPROVAL
The study was approved by Mohammed Bin Rashid University of Medicine and Health Sciences Research Ethics Committee.
REFERENCES
- 1.Agarwal A, Verma I. Cerebral palsy in children: an overview. J Clin Orthop Trauma. 2012;3(2):77–81. doi: 10.1016/j.jcot.2012.09.001. https://doi.org/10.1016/j.jcot.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortellazzi P, Lamperti M, Minati L, Falcone C, Pantaleoni C, Caldiroli D. Sedation of neurologically impaired children undergoing MRI: a sequential approach. Pediatr Anesth. 2007;17(7):630–36. doi: 10.1111/j.1460-9592.2006.02178.x. https://doi.org/10.1111/j.1460-9592.2006.02178.x. [DOI] [PubMed] [Google Scholar]
- 3.Kannikeswaran N, Mahajan P, Sethuraman U, Groebe A, Chen X. Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Pediatr Anesth. 2009;19(3):250–56. doi: 10.1111/j.1460-9592.2008.02900.x. https://doi.org/10.1111/j.1460-9592.2008.02900.x. [DOI] [PubMed] [Google Scholar]
- 4.Rabach I, Peri F, Minute M, Aru E, Lucafò M, Di Mascio A, et al. Sedation and analgesia in children with cerebral palsy: a narrative review. World J Pediatr. 2019;15(5):432–40. doi: 10.1007/s12519-019-00264-0. https://doi.org/10.1007/s12519-019-00264-0. [DOI] [PubMed] [Google Scholar]
- 5.Fehlings D, Novak I, Berweck S, Hoare B, Stott N, Russo R. Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: international consensus statement. Eur J Neurol. 2010;17:38–56. doi: 10.1111/j.1468-1331.2010.03127.x. https://doi.org/10.1111/j.1468-1331.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 6.Love S, Novak I, Kentish M, Desloovere K, Heinen F, Molenaers G, et al. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. 2010;17:9–37. doi: 10.1111/j.1468-1331.2010.03126.x. https://doi.org/10.1111/j.1468-1331.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Sung I, Yoo J, Park E, Park S. Effects of different dilutions of botulinum toxin type a treatment for children with cerebral palsy with spastic ankle plantarflexor: a randomized controlled trial. J Rehabil Med. 2009;41(9):740–45. doi: 10.2340/16501977-0418. https://doi.org/10.2340/16501977-0418. [DOI] [PubMed] [Google Scholar]
- 8.Molenaers G, Fagard K, Van Campenhout A, Desloovere K. Botulinum toxin a treatment of the lower extremities in children with cerebral palsy. J Child Orthop. 2013;7(5):383–87. doi: 10.1007/s11832-013-0511-x. https://doi.org/10.1007/s11832-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copeland L, Edwards P, Thorley M, Donaghey S, Gascoigne-Pees L, Kentish M, et al. Botulinum toxin a for nonambulatory children with cerebral palsy: a bouble blind randomized controlled trial. J Pediatr. 2014;165(1):140–46.e4. doi: 10.1016/j.jpeds.2014.01.050. https://doi.org/10.1016/j.jpeds.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Cantador-Hornero M, Jiménez-Espuch P, de Torres-Garcia I, Contreras-Jiménez M, Martínez-Mezo G, Morales de los Santos J, et al. Sedation-analgesia protocol for the injection of botulinum toxin a in cerebral palsy. An Pediatr (Engl Ed) 2019;91(5):317–27. doi: 10.1016/j.anpedi.2018.12.018. https://doi.org/10.1016/j.anpede.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Bakheit A. Botulinum toxin in the management of childhood muscle spasticity: comparison of clinical practice of 17 treatment centres. Eur J Neurol. 2003;10(4):415–19. doi: 10.1046/j.1468-1331.2003.00619.x. https://doi.org/10.1046/j.1468-1331.2003.00619.x. [DOI] [PubMed] [Google Scholar]
- 12.Zier J, Rivard P, Krach L, Wendorf H. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin a injections in children. Dev Med Child Neurol. 2008;50(11):854–58. doi: 10.1111/j.1469-8749.2008.03069.x. https://doi.org/10.1111/j.1469-8749.2008.03069.x. [DOI] [PubMed] [Google Scholar]
- 13.Löwing K, Thews K, Haglund-Åkerlind Y, Gutierrez-Farewik E. Effects of botulinum toxin-a and goal-directed physiotherapy in children with cerebral palsy GMFCS levels I & II. physical & occupational therapy in pediatrics. 2016;37(3):268–82. doi: 10.3109/01942638.2016.1150384. https://doi.org/10.3109/01942638.2016.1150384. [DOI] [PubMed] [Google Scholar]
- 14.Lorin K, Forsberg A. Treatment with botulinum toxin in children with cerebral palsy: a qualitative study of parents’ experiences. Child Care Health Dev. 2016;42(4):494–503. doi: 10.1111/cch.12350. https://doi.org/10.1111/cch.12350. [DOI] [PubMed] [Google Scholar]
- 15.Lee Z, Cho D, Choi W, Park D, Byun S. Effect of botulinum toxin type a on morphology of salivary glands in patients with cerebral palsy. Ann Rehabil Med. 2011;35(5):636–40. doi: 10.5535/arm.2011.35.5.636. https://doi.org/10.5535/arm.2011.35.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unlu E, Cevikol A, Bal B, Gonen E, Celik O, Kose G. Multilevel botulinum toxin type a as a treatment for spasticity in children with cerebral palsy: a retrospective study. Clinics (Sao Paulo) 2010;65(6):613–13. doi: 10.1590/S1807-59322010000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paget S, Swinney C, Burton K, Bau K, O’Flaherty S. Systemic adverse events after botulinum neurotoxin a injections in children with cerebral palsy. Dev Med Child Neurol. 2018;60(11):1172–77. doi: 10.1111/dmcn.13995. https://doi.org/10.1111/dmcn.13995. [DOI] [PubMed] [Google Scholar]
- 18.Burnweit C, Diana-Zerpa J, Nahmad M, Lankau C, Weinberger M, Malvezzi L, et al. Nitrous oxide analgesia for minor pediatric surgical procedures: an effective alternative to conscious sedation? J Pediatr Surg. 2004;39(3):495–99. doi: 10.1016/j.jpedsurg.2003.11.037. https://doi.org/10.1016/j.jpedsurg.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Brochard S, Blajan V, Lempereur M, Garlantezec R, Houx L, Le Moine P, et al. Determining the technical and clinical factors associated with pain for children undergoing botulinum toxin injections under nitrous oxide and anesthetic cream. Eur J Paediatr Neurol. 2011;15(4):310–15. doi: 10.1016/j.ejpn.2010.12.006. https://doi.org/10.1016/j.ejpn.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Chow C, Choong C. Ketamine-based procedural sedation and analgesia for botulinum toxin a injections in children with cerebral palsy. Eur J Paediatr Neurol. 2016;20(2):319–22. doi: 10.1016/j.ejpn.2015.11.009. https://doi.org/10.1016/j.ejpn.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson S, Brunsson I, Askljung B, Påhlman M, Himmelmann K. A rectally administered combination of midazolam and ketamine was easy, effective and feasible for procedural pain in children with cerebral palsy. Acta Paediatr. 2017;106(3):458–62. doi: 10.1111/apa.13710. https://doi.org/10.1111/apa.13710. [DOI] [PubMed] [Google Scholar]
- 22.Papavasiliou A, Nikaina I, Foska K, Bouros P, Mitsou G, Filiopoulos C. Safety of botulinum toxin a in children and adolescents with cerebral palsy in a pragmatic setting. Toxins. 2013;5(3):524–36. doi: 10.3390/toxins5030524. https://doi.org/10.3390/toxins5030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Lee E, Park S. Validity of bispectral index monitoring during deep sedation in children with spastic cerebral palsy undergoing injection of botulinum toxin. Korean J Anesthesiol. 2019;72(6):592–98. doi: 10.4097/kja.19129. https://doi.org/10.4097/kja.19129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soudant D, Staal H, Witlox A, Vles J. Conscious sedation or general anaesthetic for intramuscular botulinum toxin injections in children - a two centre cross-sectional prospective audit. Eur J Paediatr Neurol. 2013;17(2):219–20. doi: 10.1016/j.ejpn.2012.06.012. https://doi.org/10.1016/j.ejpn.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Pazi H, Cohen A, Kroyzer N, Lotem-Ophir R, Shvili Y, Winter G, et al. Clown-care reduces pain in children with cerebral palsy undergoing recurrent botulinum toxin injections- A quasi-randomized controlled crossover study. PLoS One. 2017;12(4):e0175028. doi: 10.1371/journal.pone.0175028. https://doi.org/10.1371/journal.pone.0175028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houx L, Dubois A, Brochard S, Pons C. Do clowns attenuate pain and anxiety undergoing botulinum toxin injections in children? Ann Phys Rehabil Med. 2019;19:1877–84. doi: 10.1016/j.rehab.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Richards A, Griffiths M, Scully C. Wide variation in patient response to midazolam sedation for outpatient oral surgery. Oral Surg Oral Med Oral Pathol. 1993;76(4):408–11. doi: 10.1016/0030-4220(93)90004-n. https://doi.org/10.1016/0030-4220(93)90004-N. [DOI] [PubMed] [Google Scholar]
- 28.Parker R, Mahan R, Giugliano D, Parker M. Efficacy and safety of intravenous midazolam and ketamine as sedation for therapeutic and diagnostic procedures in children. Pediatrics. 1997;99(3):427–31. doi: 10.1542/peds.99.3.427. https://doi.org/10.1542/peds.99.3.427. [DOI] [PubMed] [Google Scholar]
- 29.Louer R, McKinney R, Abu-Sultaneh S, Lutfi R, Abulebda K. Safety and efficacy of a propofol and ketamine based procedural sedation protocol in children with cerebral palsy undergoing botulinum toxin a injections. PM&R. 2019;11(12):1320–25. doi: 10.1002/pmrj.12146. https://doi.org/10.1002/pmrj.12146. [DOI] [PubMed] [Google Scholar]
- 30.Brochard S, Blajan V, Lempereur M, Le Moine P, Peudenier S, Lefranc J. et al. Effectiveness of nitrous oxide and analgesic cream (lidocaine and prilocaine) for prevention of pain during intramuscular botulinum toxin injections in children. Ann Phys Rehabil Med. 2009;52(10):704–16. doi: 10.1016/j.rehab.2009.09.001. https://doi.org/10.1016/j.rehab.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Nugud A, Nugud S, Nugud A, Nugud A, Kathamuthu R, Jalal M. Perinatal risk factors for development of retinopathy of prematurity in a tertiary neonatal intensive care unit. J Taibah Univ Med Sci. 2019;14(3):306–11. doi: 10.1016/j.jtumed.2019.05.001. https://doi.org/10.1016/j.jtumed.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alwahab A, Kharsa A, Nugud A, Nugud S. Occipital meningoencephalocele case report and review of current literature. Chin Neurosurg J. 2017;3(1):50–4. https://doi.org/10.1186/s41016-017-0104-5. [Google Scholar]