Abstract
Background:
Traumatic brain injury (TBI) in older adults leads to considerable morbidity and mortality. Outcomes among older adults with TBI are disparately worse than in younger adults. Differences in immunologic response to injury may account for at least some of this disparity. Understanding how aging differentially affects the immune response to TBI and how older age and these immunologic changes affect the natural history of recovery following TBI are the goals of this study.
Design/Methods:
A prospective multiple cohort design is being used to assess the effects of aging and TBI on immune makers and to test predictors of impairment and disability in older adults following mild TBI. Older adults (≥ 55 years) with mild TBI are enrolled with three comparison groups: younger adults (21–54 years) with mild TBI, non-injured older adults (≥ 55 years) and non-injured young adults (21–54 years). For the primary analysis, we will assess the association between immune markers and Glasgow Outcome Scale-Extended at 6 months, using logistic regression. Predictors of interest will be inflammatory biomarkers. Multivariate linear regression will be used to evaluate associations between biomarkers and other outcomes (symptoms, function and quality of life) at 3 and 6 months. Exploratory analyses will investigate the utility of biomarkers to predict outcome using ROC curves.
Discussion:
A better understanding of the recovery trajectory and biological rationale for disparate outcomes following TBI in older adults could allow for development of specific interventions aimed at reducing or eliminating symptoms. Such interventions could reduce impairment and health care costs.
Keywords: brain injury, older adult, cohort study, disability, biomarkers, immune function
INTRODUCTION
Traumatic brain injury (TBI) in older adults is common, accounting for over 25% of brain injuries in the US each year and results in considerable mortality, long-term impairment and disability.1 The vast majority (>75%) of these injuries are considered mild.2 Outcomes among older adults with TBI are worse when compared to their younger counterparts with similar injuries. Clinical efforts to treat TBI in older adults have not yielded the expected improvements in outcome.3 These poor outcomes may be due, in part, to the inability to identify those older adults most vulnerable to adverse outcomes early so that interventions, such as rehabilitation, can occur in a timely manner. A critical barrier to the management of TBI is the limited knowledge about potential differences that results from aging in the response to TBI, and how older age may affect pathology and recovery following TBI.
To date, relatively little is known about the trajectory of symptoms experienced by older adults following mild TBI. Recent studies demonstrate that being older and female is associated with an increased likelihood of developing symptoms as well as the number and type of symptoms.4–8 It is unclear if age or other factors (gender, social support) contributed to the resulting impairment as these factors have not been evaluated. Information on the functional status of older adults following mild TBI is limited. In studies that have examined impairment or disability following TBI in adults, there is evidence to suggest that older adult survivors of TBI have increased dependence as measured by global outcome instruments such as the Glasgow Outcome Scale (GOS)9–11compared to younger TBI patients. Adults older than 50 years of age had the highest disability at 1 to 2 years following TBI12, further emphasizing the long-term functional implications of this disease. Our study will provide critical knowledge regarding the natural history of recovery following TBI in older adults.
Inflammation is one of the primary immune responses of the brain to TBI. Both chemokines and cytokines are released both locally at the site of injury and systemically in response to injury.13,14 Increases in pro-inflammatory chemokines and cytokines have been reported following experimental and clinical TBI and may be causally related to sequelae of injury.15–21 “Sickness behavior” is a normal homeostatic syndrome that occurs in response to pro-inflammatory cytokines in the brain22,23and is not attenuated with steroid administration.24 Sickness behaviors include decreased activity and socialization, and reduced appetite. Therefore, there is a known physiologic and causal mechanism for symptom development that is associated with cytokine release following experimental TBI, making it an appropriate area for investigation and potential intervention. Studies indicate that the concentrations of inflammatory cytokines following TBI vary according to the time post-injury25, but little information is available regarding inflammatory cytokines in the post-acute phase. Furthermore, the natural history of cytokine expression is not well characterized in large clinical samples of adults/older adults.26 No study has identified if symptoms that are present following injury could be correlated to changes in inflammatory cytokine concentrations or impaired cellular immune response. Blood biomarkers show promise for prediction of outcome from TBI27, but have not been evaluated for prognostic purposes in older adults.
Aging itself has been described as state of immunosenescence, characterized by changes in innate immunity and a chronic low-level proinflammatory status.28,29 These changes have been associated with higher morbidity and mortality.28–31 Cognitive dysfunction and fatigue are also linked to immune changes induced by traumatic injury, but the relationship of these symptoms to immune and inflammatory markers in an older population remains unexplored. In this study, we will test a model of impairment and disability following mild TBI in older adults based on the Institute of Medicine’s Disability Framework32,33 in which aging is a biological factor that contributes to the pathological response following TBI by modulating the immune response. This increase in inflammation, in turn, is hypothesized to correlate with the increased symptom burden, increased functional limitation and disability, and poorer quality of life reported in older adults following TBI.
A better understanding of the trajectory of recovery is important in the prediction of outcomes and the development of interventions for management of symptoms and reduction of disability following mild TBI in older adults. Biomarkers could provide important tools for screening and estimating impairment severity and determining the need for further resources or treatment.
METHODS
Study design
A single-center prospective multiple cohort design is being used to assess the effects of aging and TBI on immunological biomarkers and to test predictors of impairment and disability in older adults following mild TBI. TBI study participants (Groups 1 and 2) are being recruited within 24 hours of injury from Harborview Medical Center, Seattle, WA, USA, an urban level I regional trauma center. Age- and gender-matched non-injured controls (Groups 3 and 4) are recruited from the community. Study findings will be reported in accordance with the STROBE statement.34 Approval for the study was provided by the Human Subjects Division of the University of Washington IRB #41718.
Study site and recruitment
Older adults (≥ 55 years of age) with mild traumatic brain injury (Group 1) are enrolled with three comparison groups: younger adults (21–54 years of age) with mild TBI (Group 2), non-injured older adults (≥ 55 years of age) (Group 3) and non-injured young adults (21–54 years of age) (Group 4). The decision to use 55 years of age as the cut point for younger and older adults was based upon the trauma literature since it appears to be an inflection point for increased mortality after trauma.35,36 In order to achieve the most representative sample of patients who present to the emergency department (ED) for treatment for mild TBI, we are enrolling subjects prospectively in a consecutive manner. Patients seeking treatment for traumatic injury are screened for initial eligibility by trained research staff working in the ED. Written informed consent is obtained from all participants or their legally authorized representative at time of enrollment.
To be eligible, older adult mild TBI subjects (Group 1) must have a clinical diagnosis of mild TBI from blunt trauma within the past 24 hours based on CDC criteria (see Table 1)1); be ≥ 55 years of age; speak and read English and have a documented home address within western Washington State. Younger mild TBI subjects (Group 2) will meet the same criteria as Group 1, but must be 21–54 years of age.
Table 1.
Clinical diagnosis of mild TBI. 1
| Signs and symptoms consistent with a diagnosis of mild TBI (require one or more of the following): 1) confusion or disorientation 2) amnesia near the time of the injury and Glasgow Coma Scale (GCS) of 15 by 24 hours 3) a loss of consciousness up to 30 minutes 4) neurological or neuropsychological problems, and/or 5) an initial score of 13 or higher on the GCS |
Inclusion and exclusion criteria were developed to maximize generalizability. In order to increase the likelihood of subjects from all groups completing the protocol, we exclude those persons 1) planning to leave the area within 6 months; or 2) who have a documented estimated life expectancy < 6 months. Persons taking drugs that might alter the inflammatory response, 3) oral or injectable steroids within the last 30 days, NSAIDS more than 3 days per week, COX-2 Inhibitors, biologic inhibitors of cytokines, and other immune modulating agents will also be excluded. As the following conditions significantly alter the recovery pattern from mild TBI, we exclude persons who have: 4) Cervical spine trauma at time of injury; 5) Previous TBI or stroke in the past year; 6) Previous history of dementia. Given the known differences in outcomes between blunt and penetrating injury we exclude individuals with 7) penetrating head injury as a mechanism. 8) In order to better distinguish the effects of TBI from non-head injury, we exclude 8) persons with injuries to other body regions that are classified as greater than a moderate injury (Abbreviated Injury Scale (AIS) >2).37
In order to achieve a control sample that most closely represents the injury populations related to transitional factors (Figure 1), non-injured older adults (Group 3) and non-injured younger adults (Group 4) are recruited by having injured individuals identify a non-injured friend of similar age to participate. In instances where an individual is unwilling or unable to identify a friend to be approached for study participation, the remainder of the control sample are recruited from the local community. In order to be eligible, non-injured older adult subjects (Group 3) must : 1) be ≥ 55 years of age; 2) independently perform all activities of daily living38; 3) speak and read English; and 4) have a documented home address and telephone number within western Washington State. Eligibility for non-injured younger adult subjects (Group 4) is the same as group 3, but 21–54 years of age. As we are seeking healthy non-injured controls, persons with: 1) previous TBI or stroke by self-report in the past year; 2) hospitalization within the past 6 months; or 3) history of dementia are excluded from Groups 3 and 4. Finally, participants in the control groups are excluded if they are taking immune modulating medications (the same as in Groups 1 and 2) at the time of screening.
Figure 1. Conceptual Model for development of impairment and disability in older adults following TBI.
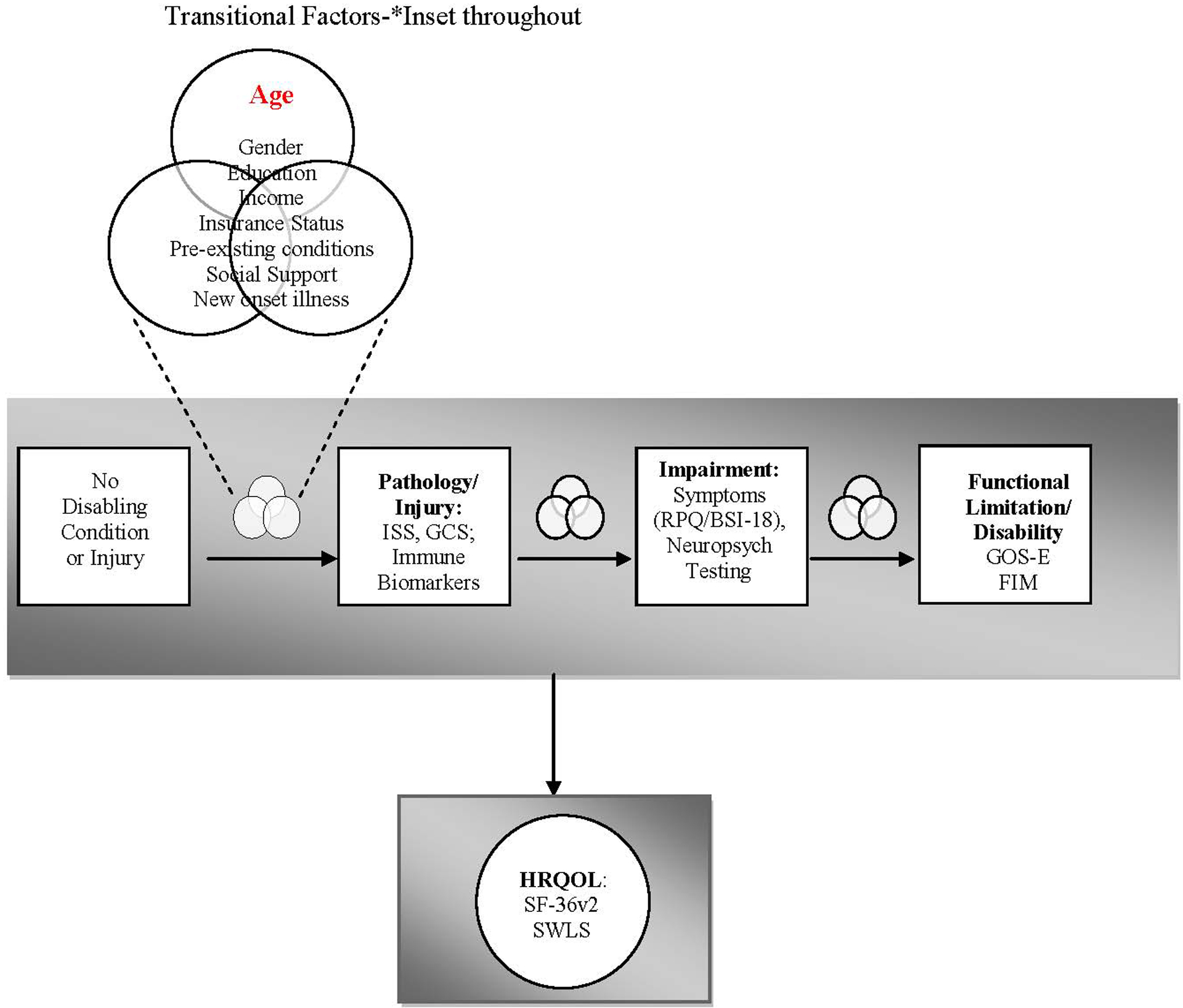
Aging is a biological factor that may contribute to the pathological response to TBI by modulating the immune response. These immune changes are, in turn, associated with the increased symptom burden (impairment), increased functional limitation and disability, and poorer quality of life reported in older patients following TBI. Transitional factors are those variables that may either promote or prevent and individual from moving through the stages of acquiring disability.
PROCEDURES
Traumatic Brain Injury Subjects (Groups 1 and 2).
Day 0, 3, 7:
Blood samples are collected at time 0 in the ED or hospital for immune biomarkers. The timing for the initial day 0 sample is as close as possible to time of admission to the emergency department, within 24 hours of injury. Data on demographics, injury data (type, location, mechanism, and severity) are extracted from the medical record. Questionnaires are administered for assessment of pre-injury functional limitations and health-related quality of life as indicated by the protocol (See Tables 2 and 3). Data regarding new medications, emergency department visits or rehospitalizations is also collected.
Table 2.
Research Protocol.
| Groups 1 and 2 Study Protocol-All in Person Visits | |||||
|---|---|---|---|---|---|
| Enrollment: Day 0 | Day 3 | Day 7 | 1 month | 3 month | 6 month |
| DEMOGRAPHIC & INJURY DATA Blood Sample |
Blood Sample Questionnaires to assess pre-injury status: Social support Functional limitations HRQOL |
Blood Sample Neuropsychological Testing Questionnaires: Symptoms Functional Limitations/Disability HRQOL |
Blood Sample Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
Blood Sample Neuropsychological Testing Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
Blood Sample Neuropsychological Testing Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
| Groups 3 and 4 Study Protocol-Visit in Person unless noted | |||||
| Enrollment: Day 0 | 1 month | 3 month (Phone) | 6 month | ||
| DEMOGRAPHIC & HEALtd DATA Blood Sample Neuropsychological Testing Questionnaires: Social Support Symptoms Functional Limitations/Disability HRQOL |
Blood Sample Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
Blood Sample Neuropsychological Testing Questionnaires: Symptoms Functional Limitations/Disability HRQOL New onset illness or injury |
||
Table 3.
Summary of Measures
| Measure | Source (Instrument) | Instrument Psychometrics/ Measurement properties |
Frequency | Groups | |
|---|---|---|---|---|---|
| Transitional Factors | Age Insurance Status Gender |
Medical Record Review (Patient Enrollment Form) | Day 0 | 1, 2, 3, 4 | |
| Education Income Pre-existing conditions |
Self/Family Report (Patient Enrollment Form, Elixhauser) | Day 0 Day 3 (or as early as able to obtain information reliably) |
3, 4 1, 2 |
||
| New onset illness or injury | Self/Family Report on Patient Data Form-Follow up visit | Day 7 1, 3, 6 Month |
1, 2 1, 2, 3, 4 |
||
| Social Support | MOS Social Support Survey43 | α >0.91 | Day 0 Day 3 (or when able to obtain reliable information) |
3, 4 1, 2 |
|
| Pathology: Injury | Injury Type Location Mechanism Severity |
Medical record (GCS, Injury severity score (ISS)44, Injury Mechanism E-code45, Head CT findings) Confirm with Trauma Registry (Patient Enrollment Form) |
Day 0 | 1,2 | |
| Inflammatory Markers Interleukin (IL)-1 beta IL-2, IL-4, IL-5, IL-6, Ll-7,, IL-10, IL-12, IL-13, GM-CSF, TNF-alpha, leptin, fractalkine |
Plasma (Multiplex Bead based assay) | Day 0, 3, 7 1, 3 and 6 Months (Day 3, 7 and 3 month for TBI groups only*) |
1, 2, 3, 4 | ||
| Cellular Immune Response (IFN-γ secretion in response to PHA or LPS) | PBMCs (ELISpot assay) | Inter-rater reliability in pilot >.92; test retest reliability>.90 | Day 0, 3, 7 1, 3 and 6 Months (Day 3, 7 and 3 month for TBI groups only*) |
1, 2, 3, 4 | |
| Impairment: Symptoms | Symptom Presence and Burden | Rivermead Post-concussion Symptom Questionnaire (RPQ)46 | Rho=.67 at 3 months post-injury; .56 at 6 months. Test-retest reliability 0.8946 | Day 0 Day 7 1, 3, 6 month |
3, 4 1,2 1, 2, 3, 4 |
| Brief Symptom Inventory-18 (BSI-18)47 | Test-retest in TBI patients .57–.67 on the subscales; Internal consistency reported to be α=.75–.9147 | ||||
| Neuropsychological Impairment | Rey Auditory Verbal Learning Test (RAVLT)48 | Internal reliability high with reported α>.9; test retest good.48–50 | Day 0 Day 7 3, 6 month (3 month for TBI groups only*) |
3, 4 1, 2 1, 2, 3, 4 |
|
| Trail Making Test (TMT)48 | Interrater reliability is reported as .94 for Part A and .90 for Part B51 | ||||
| Processing Speed Index from Wechsler Adult Intelligence Scale52 | High internal consistency (.8–.89). Good test-retest reliability48 | ||||
| Functional Limitation Disability | Functional Change Post-Injury Disability | Functional Independence Measure53 | Construct and predictive validity demonstrated in TBI patients. Inter-rater reliability of the FIM is reported to be > .90; >.90 test-retest reliability53 | Day 0 Day 7 1, 3, 6 month |
3, 4 1, 2 1, 2, 3, 4 |
| Glasgow Outcome Scale-Extended (GOS-E)54 | Inter-rater reliability of the GOS-E is reported to be .78 with a weighted kappa of.8554 | 3, 6 month | 1, 2, 3, 4 | ||
| Quality of Life | Health-related Quality of Life | Self-Report (MOS Short Form-36v2 Acute Recall)55 | Reliability of the SF-36 reported to be .92 and .88 for the subscales55,56. In mild TBI patients, α .83–.91 reported57 | Day 0 Day 7 1, 3, 6 month |
3, 4 1, 2 1, 2, 3, 4 |
| Satisfaction with Life Scale (SWLS)58 | Internal consistency exceeds 0.80; Test-retest reliability 0.82–0.8959 | Day 0 Day 7 1, 3, 6 month |
3, 4 1, 2 1, 2, 3, 4 |
Legend: Group 1: Older mild TBI; Group 2: Younger mild TBI; Group 3: Older non-inured Control; Group 4: Younger non-injured Control
GCS: Glasgow Coma Scale; ISS: Injury Severity Score;
For non-injured control no 3 day visit, phone visit only at 3 month visit to reduce burden and increase subject retention based upon pilot work.
1, 3, and 6-month post-injury visits:
Questionnaires are administered for assessment of impairment, functional limitations/disability, and HRQOL as indicated by the protocol (See Tables 2 and 3). Blood is sampled at each visit. Data regarding changes in health status (new medications, conditions, ED visits, surgery or hospitalization is also collected.
Blood sampling procedures:
12 ml of blood will be collected from each subject and samples are transported for processing within 4 hours of collection. Two EDTA-containing (Becton-Dickinson) tubes are used to collect blood samples for plasma and PMBC isolation. Following collection, tubes are then centrifuged at 1,000 x g for 10 minutes at room temperature in order to separate plasma from the buffy coat interface. Plasma is stored at −70°C until batch evaluation. Ficoll gradient technique is used to isolate PBMCs from the buffy coat layer. Once the cell pellet is obtained, it is washed and cell viability and counts are obtained. The cells are adjusted to a concentration of 107 cells per ml with complete Freezing Media (Fetal Calf serum containing 10% DMSO) in storage vials (1 mL per vial). These tubes are then put into a room temperature Slo-Cooler box and placed in a −70°C degree freezer overnight. The next day, these tubes are transferred into liquid nitrogen for long-term storage until batch analysis.
Non-injured Controls (Groups 3 and 4):
Following initial screening via telephone, the subject meets with research staff to obtain informed consent, answer questionnaires and have neuropsychological testing (Tables 2 and 3). A blood sample is drawn for immune markers at this visit (Day 0). 1, 3 and 6-month visits occur as per Table 2.
Measures
Demographic characteristics.
All subjects will be asked about their age, gender, race/ethnicity, formal educational achievement, income level, insurance status and pre-existing conditions. In studies of persons with severe TBI, their pre-injury level of functioning was found to be more predictive of outcome than the actual injury severity39 and therefore both of these variables will thus be considered as covariates in our analysis. Further, age, lack of or public insurance (Medicaid), lower income and lower levels of social support have been related to lower HRQOL at 12-months post-TBI40,41 and will therefore also be considered as transitional factors influencing the development of disability and changes in HRQOL.
Inflammatory Cytokine/Chemokine measurements.
Multiplex cytokine assay using a fluorescent microsphere suspension array, will be used to detect and quantify protein concentrations of selected inflammatory markers.
Cellular Immune Response.
ELISpot will be performed on bulk PBMCs. In order to determine the number of PBMCs producing interferon (IFN)-γ in response to stimulus, cells will be plated in 96-well plates (Millipore) previously coated with primary antibody along with 10uL of stimulant (lipopolysaccharide [LPS] or phytohemagglutinin [PHA]) or control (media alone) following the protocol of Hagiwara and colleagues.42 All experiments will be performed in triplicate. Cells secreting INF-ʏ when cultured produce a colored spot in response to the immune assay within the well. Spots will counted by two independent investigators blinded to group aided by a semi-automated software system.
Impairment, Disability and Health-Related Quality of Life.
We consulted the National Institutes of Health/National Institute of Neurologic Disorders and Stroke (NINDS) common data elements (CDEs) for TBI to select outcome measures, and include all recommended core measures within the domains of impairment, function/disability, and HRQOL (See Table 3).
ANALYSIS
Measurements of inflammatory cytokines will be log-transformed in order to obtain normality of distribution within groups. Means, standard deviations and distributions will be used to describe cellular immune and inflammatory biomarkers, impairment, functional limitation/disability and HRQOL at each time point. Data will be examined to determine if baseline characteristics of those persons lost-to-follow up differ from those participants who complete the protocol. Multiple imputation techniques60 will be used to manage missing data. Sensitivity analyses, e.g. counting those lost-to-follow up as all having a poor outcome or all having a good outcome will indicate the degree to which lost-to-follow up may sway outcome in the analyses.
Transitional factors such as gender, income, insurance type, education, pre-injury morbidity (preexisting conditions and function), social support (MOS SSS) and insurance status may be confounders; if they are related to group (p<.10) they will be treated as covariates in all analyses addressing impairment, functional limitations/disability and HRQOL. To reduce the effects of sample heterogeneity, in all analyses of TBI subjects we will adjust for additional covariates to include: pupil reactivity and presence of a significant extracranial injury (ISS) as per the basic recommendations of the MRC CRASH trial collaborators.61 To adjust for multiple comparisons, we will use the Benjimini-Hochberg testing procedure.62 We will collect data at each visit from the medical record and from subject reports of any new onset or change in illness that could be potential confounders (e.g. new medications, surgeries, infections, acute hospitalizations) and determine if any additional adjustment is required between groups.
For the primary analysis, we will assess the association between biomarker concentrations and GOS-E at 6 months, using ordinal logistic regression per the IMPACT study group recommendations (proportional odds methodology). The predictors of interest will be a) log-transformed inflammatory biomarker concentrations and b) cell counts. Included in the model will be the aforementioned covariates. Multivariate linear regression will be used to evaluate associations between biomarkers and other outcomes (symptom scores, neuropsychological function, FIM and HRQOL) at 3 and 6 months. We will use error-in-variables regression to account for measurement error in the predictor variable (biomarker) if indicated. Exploratory analyses will investigate the prognostic utility of biomarkers to predict poor outcomes following TBI using receiver-operating characteristic (ROC) curves.
Linear mixed models for longitudinal data63 will be used to estimate 1) geometric mean number of cells and 2) inflammatory biomarker concentrations, and mean impairment, disability and HRQOL across time for the 4 groups adjusted for covariates (vide supra). Time will be modeled as a categorical variable to detect non-linear effects over time. This model will allow for estimating the response to injury relative to a non-injured control for both younger and older adults. The interaction of age group and injury group will indicate whether there is a more extreme injury effect in older adults. To compare immune function and outcomes among older and younger TBI subjects, a second set of linear mixed models will be used to model immune biomarkers (Th1 response or cytokine concentrations) and outcomes, restricted to groups 1 and 2, which will have more observations per individual. These models will include transitional and injury covariates.
Sample Size
Based on pilot data for the biomarkers and assuming a conservative α of 0.0125, we will have 90% power to detect a difference of ±0.6 in log-transformed cytokine concentrations between older and younger adults with TBI with 75 participants in each of the four groups (N=300). This corresponds to one group having 4 times the cytokine concentration of the other. We will also have will 90% power to detect correlation coefficients greater than .42 between biomarkers and functional outcomes with 75 participants in each group. Based on standard deviation estimates for markers and outcomes obtained from our pilot data, we will be able to detect a 40% increase in odds of a worse outcome on the GOS-E given a 2-fold difference in a biomarker.
DISCUSSION
This study will lead to an increased understanding of the natural history of the recovery and disability trajectory following mild TBI in older adults, and hopefully aid in prediction of outcome as well as clinical care. This study will potentially identify biological predictors of poor outcome in older adults with mild TBI that are worthy of follow up in subsequent studies. The proposed study will begin to examine mechanisms underlying symptoms and functional impairment experienced by older adult patients following TBI to determine if they are associated with changes in cellular immune function and cytokine concentrations. Biomarkers could provide an important tool for delineating the degree of impairment and determining the need for further resources or treatment. As important, biomarkers could provide indirect measures of treatment response for use in clinical trials and treatment.
Acknowledgements.
We would like to thank Drs. John Amory, Patrick Heagerty and Jeffery Probstfield for critical input on the study design as it evolved through the ITHS KL2 program.
Funding.
This work was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R01NS077913. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests. None.
Ethics Approval. University of Washington Institutional Review Board.
Contributor Information
Hilaire J. Thompson, Biobehavioral Nursing and Health Informatics, University of Washington, Harborview Injury Prevention and Research Center, Box 357266, Seattle, WA, 98195-7266 USA
Frederick P. Rivara, Department of Pediatrics, School of Medicine, University of Washington, Department of Epidemiology, School of Public Health, University of Washington, Harborview Injury Prevention and Research Center, Seattle, WA, USA
Kyra J. Becker, Departments of Neurology and Neurological Surgery, School of Medicine, University of Washington, Seattle, WA, USA
Ronald V. Maier, Department of Surgery, School of Medicine, University of Washington, Surgeon-in-Chief, Harborview Medical Center, Harborview Injury Prevention and Research Center, Seattle, WA, USA
Nancy Temkin, Department of Neurosurgery, School of Medicine, University of Washington, Department of Biostatistics, School of Public Health, University of Washington, Seattle, WA, USA.
References
- 1.National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention, 2003. [Google Scholar]
- 2.Centers for Disease Control. Traumatic Brain Injury in the United States: A Report to Congress. Atlanta, GA: CDC, 2001. [Google Scholar]
- 3.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med 2006;354(4):366–78. [DOI] [PubMed] [Google Scholar]
- 4.Hibbard MR, Uysal S, Sliwinski M, et al. Undiagnosed health issues in individuals with traumatic brain injury living in the community. J Head Trauma Rehabil 1998;13(4):47–57. [DOI] [PubMed] [Google Scholar]
- 5.Breed ST, Flanagan SR, Watson KR. The relationship between age and the self-report of health symptoms in persons with traumatic brain injury. Arch Phys Med Rehabil 2004;85(4 Suppl 2):S61–7. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein FC, Levin HS. Cognitive outcome after mild and moderate traumatic brain injury in older adults. J Clin Exp Neuropsychol 2001;23(6):739–53. [DOI] [PubMed] [Google Scholar]
- 7.Bazarian JJ, Blyth B, Mookerjee S, et al. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma 2009;27(3):527–39. doi: 10.1089/neu.2009.1068 [doi] [published Online First: 2009/11/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dikmen S, Machamer J, Fann JR, et al. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc 2010;16(3):401–11. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, Pentland B. Head injuries in elderly patients. Neurosurg Rev 1989;12 Suppl 1:441–5. [DOI] [PubMed] [Google Scholar]
- 10.Pentland B, Jones PA, Roy CW, et al. Head injury in the elderly. Age Ageing 1986;15(4):193–202. [DOI] [PubMed] [Google Scholar]
- 11.Mosenthal AC, Lavery RF, Addis M, et al. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma 2002;52(5):907–11. [DOI] [PubMed] [Google Scholar]
- 12.Testa JA, Malec JF, Moessner AM, et al. Outcome after traumatic brain injury: effects of aging on recovery. Arch Phys Med Rehabil 2005;86(9):1815–23. [DOI] [PubMed] [Google Scholar]
- 13.Gosain A, Gamelli RL. A primer in cytokines. J Burn Care & Rehabilitation 2005;26:7–12. [DOI] [PubMed] [Google Scholar]
- 14.Utagawa A, Truettner JS, Dietrich WD, et al. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Experimental Neurology 2008;211:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson HJ, Hoover RC, Tkacs NC, et al. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J Cereb Blood Flow Metab 2005;25(2):163–76. [DOI] [PubMed] [Google Scholar]
- 16.Bermpohl D, You Z, Lo EH, et al. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab 2007 [DOI] [PubMed] [Google Scholar]
- 17.Morganti-Kossmann MC, Rancan M, Stahel PF, et al. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care 2002;8(2):101–5. [DOI] [PubMed] [Google Scholar]
- 18.Frati A, Salvati M, Mainiero F, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg 2004;100(1):24–32. [DOI] [PubMed] [Google Scholar]
- 19.Mussack T, Biberthaler P, Kanz KG, et al. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med 2002;30(12):2669–74. [DOI] [PubMed] [Google Scholar]
- 20.Arand M, Melzner H, Kinzl L, et al. Early inflammatory mediator response following isolated traumatic brain injury and other major trauma in humans. Langenbecks Arch Surg 2001;386(4):241–8. [DOI] [PubMed] [Google Scholar]
- 21.Kyrkanides S, O’Banion MK, Whiteley PE, et al. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol 2001;119(2):269–77. [DOI] [PubMed] [Google Scholar]
- 22.Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol 2010. doi: S0014–4886(10)00415–2 [pii] 10.1016/j.expneurol.2010.11.014 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 2007;21(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman KJ, Goss CW, Simkins RM, et al. Sickness behaviors following medial frontal cortical contusions in male rats. J Neurotrauma 2001;18(10):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy A, Carpenter KL, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab 2010;31(2):658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JC, Reinhart K. Biomarkers of sepsis. Crit Care Med 2009;37(7):2290–8. [DOI] [PubMed] [Google Scholar]
- 27.Dash PK, Zhao J, Hergenroeder G, et al. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics 2010;7(1):100–14. doi: S1933–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol 2010;104(3):183–90; quiz 90–2, 210. doi: S1081–1206(09)00010–6 [pii] 10.1016/j.anai.2009.11.009 [doi] [DOI] [PubMed] [Google Scholar]
- 29.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 30.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004;39(5):687–99. [DOI] [PubMed] [Google Scholar]
- 31.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003;23(1):15–39. [DOI] [PubMed] [Google Scholar]
- 32.Pope AM, Tarlov AR, editors. Disability in America: A National Agenda for Prevention. Washington, DC: National Academies Press, 1991. [Google Scholar]
- 33.Brandt EN, Pope AM, editors. Enabling America: Assessing the Role of Rehabilitation Science and Engineering. Washington, DC: National Academies Press, 1997. [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. International Journal of Surgery (London, England) 2014;12(12):1495–9. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 35.Thompson HJ, Voss JG, Rue T. Age and the inflammatory response to traumatic brain injury. Journal of Neurotrauma 2010;27(5):A–90. [Google Scholar]
- 36.Jacoby SF, Ackerson TH, Richmond TS. Outcome from serious injury in older adults. J Nurs Scholarsh 2006;38(2):133–40. [DOI] [PubMed] [Google Scholar]
- 37.Scaling CoI. The Abbreviated Injury Scale (2005)-Update 2008 Manual. Des Plaines, IL: Association for the Advancement of Automotive Medicine; 2008. [PMC free article] [PubMed] [Google Scholar]
- 38.Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist 1970;10(1):20–30. [DOI] [PubMed] [Google Scholar]
- 39.Novack TA, Bush BA, Meythaler JM, et al. Outcome after traumatic brain injury: pathway analysis of contributions from premorbid, injury severity, and recovery variables. Arch Phys Med Rehabil 2001;82(3):300–5. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy ML, MacKenzie EJ, Durbin DR, et al. Health-related quality of life during the first year after traumatic brain injury. Arch Pediatr Adolesc Med 2006;160(3):252–60. [DOI] [PubMed] [Google Scholar]
- 41.von Wild KR. Posttraumatic rehabilitation and one year outcome following acute traumatic brain injury (TBI): data from the well defined population based German Prospective Study 2000–2002. Acta Neurochir Suppl 2008;101:55–60. [DOI] [PubMed] [Google Scholar]
- 42.Hagiwara E, Abbasi F, Mor G, et al. Phenotype and frequency of cells secreting IL-2, IL-4, IL-6, IL-10, IFN and TNF-alpha in human peripheral blood. Cytokine 1995;7(8):815–22. doi: 10.1006/cyto.1995.0098 [DOI] [PubMed] [Google Scholar]
- 43.Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine (1982) 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 44.Baker SP, O’Neill B. The injury severity score: an update. J Trauma 1976;16(11):882–5. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Recommended framework of E-code groupings for presenting injury mortality and morbidity data Atlanta, GA: CDC; 2005. [updated 2/16/05. Available from: http://www.cdc.gov/ncipc/whatsnew/matrix2.htm accessed May 1, 2006 2006. [Google Scholar]
- 46.King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995;242(9):587–92. [DOI] [PubMed] [Google Scholar]
- 47.Meachen SJ, Hanks RA, Millis SR, et al. The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch Phys Med Rehabil 2008;89(5):958–65. [DOI] [PubMed] [Google Scholar]
- 48.Strauss EE, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 2006. [Google Scholar]
- 49.Mitrushina M, Satz P, Chervinsky A, et al. Performance of four age groups of normal elderly on the Rey Auditory-Verbal Learning Test. J Clin Psychol 1991;47(3):351–7. [DOI] [PubMed] [Google Scholar]
- 50.Mitrushina M, Satz P. Effect of repeated administration of a neuropsychological battery in the elderly. J Clin Psychol 1991;47(6):790–801. [DOI] [PubMed] [Google Scholar]
- 51.Giovagnoli AR, Del Pesce M, Mascheroni S, et al. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 1996;17(4):305–9. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D Wechsler Adult Intelligence Scale III. San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- 53.Hamilton BB, Laughlin JA, Fiedler RC, et al. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med 1994;26(3):115–9. [PubMed] [Google Scholar]
- 54.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 1998;15(8):573–85. [DOI] [PubMed] [Google Scholar]
- 55.Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincon, RI: QualityMetric, Inc; 2000. [Google Scholar]
- 56.Ware JE, Kosinski M, Keller SK. SF-36® Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 57.Findler M, Cantor J, Haddad L, et al. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Inj 2001;15(8):715–23. doi: 10.1080/02699050010013941 [doi] [published Online First: 2001/08/04] [DOI] [PubMed] [Google Scholar]
- 58.Diener E, Emmons RA, Larsen RJ, et al. The Satisfaction With Life Scale. J Pers Assess 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 59.Pavot W, Diener E. Review of the satisfaction with life scale. Journal of Personality Assessment 1993;5:164–72. [DOI] [PubMed] [Google Scholar]
- 60.Rue T, Thompson HJ, Rivara FP, et al. Managing the common problem of missing data in trauma studies. J Nurs Scholarsh 2008;40(4):373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 2008;336(7641):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289. [Google Scholar]
- 63.Diggle P, Heagerty P, Liang K, et al. Analysis of Longitudinal Data. Oxford: Oxford University Press; 2002. [Google Scholar]


