Abstract
Background
Childhood cancer survivors experience significantly higher rates of hypertension, which potentiates cardiovascular disease, but the contribution and relationship of genetic and treatment factors to hypertension risk are unknown.
Objectives
This study sought to determine the contribution of a blood pressure polygenic risk score (PRS) from the general population and its interplay with cancer therapies to hypertension in childhood cancer survivors.
Methods
Using 895 established blood pressure loci from the general population, we calculated a PRS for 3,572 childhood cancer survivors of European ancestry from the Childhood Cancer Survivor Study (CCSS) original cohort, 1,889 from the CCSS expansion cohort, and 2,534 from the St. Jude Lifetime Cohort. Hypertension was assessed using National Cancer Institute criteria based on self-report of a physician diagnosis in CCSS and based on blood pressure measurement in the St. Jude Lifetime Cohort.
Results
In the combined sample of 7,995 survivors, those in the top decile of the PRS had an odds ratio (OR) of 2.66 (95% confidence interval [CI]: 2.03 to 3.48) for hypertension compared with survivors in the bottom decile. The PRS-hypertension association was modified by being overweight/obese (per standard deviation interaction OR: 1.13; 95% CI: 1.01 to 1.27) and exposure to hypothalamic-pituitary axis radiation (per standard deviation interaction OR: 1.18; 95% CI: 1.05 to 1.33). Attributable fractions for hypertension to the PRS and cancer therapies were 21.0% and 15.7%, respectively; they jointly accounted for 40.2% of hypertension among survivors.
Conclusions
A blood pressure PRS from the general population is significantly associated with hypertension among childhood cancer survivors and contributes to approximately one quarter of hypertension risk among survivors. These findings highlight the importance of screening for hypertension in all childhood cancer survivors and identifying higher-risk subgroups.
Key Words: cancer therapies, childhood cancer survivors, hypertension, polygenic risk score
Abbreviations and Acronyms: AF, attributable fraction; aOR, adjusted odds ratio; CCSS, Childhood Cancer Survivor Study; CI, confidence interval; COG, Children’s Oncology Group; HPA, hypothalamic-pituitary axis; IQRs, interquartile ranges; OR, odds ratio; PRS, polygenic risk score; SD, standard deviation; SJLIFE, The St. Jude Lifetime Cohort
Central Illustration
With improvements in survival rates of childhood cancer over the past decades, cardiovascular disease has emerged as a leading cause of noncancer morbidity and mortality in long-term survivors (1, 2, 3, 4, 5). Compared with siblings, survivors are at 5-fold increased risk of developing severe cardiac events (3) and at 8-fold increased risk of cardiovascular disease–related death (6). Although much of this risk can be attributed to exposure to cardiotoxic therapies including chest-directed radiation and/or anthracyclines, aging long-term survivors also develop traditional, modifiable risk factors that are primary contributors of cardiovascular disease in the general population, including hypertension, diabetes, dyslipidemia, and obesity (7). In survivors, hypertension was independently associated with up to a 19-fold increased risk of cardiovascular diseases and the combined effect of anthracycline and hypertension was significantly greater than the expected additive effects with an 86-fold increased risk of heart failure (7). These results suggest that hypertension can potentiate cancer treatment–related cardiotoxicity in a near-multiplicative manner (8,9).
The prevalence of hypertension in childhood cancer survivors increases sharply with age, exceeding 70% by age 50 years, and is substantially higher than the general population after accounting for age, sex, race/ethnicity, and body mass index (10). The Children’s Oncology Group (COG) long-term follow-up guidelines for survivors of childhood cancer currently designate chemotherapies (ifosfamide and heavy metals [cisplatin, carboplatin, and oxaliplatin]), abdominal, hypothalamic-pituitary axis (HPA), or total body radiation and nephrectomy as risk factors for hypertension, and recommend active surveillance of blood pressure in exposed survivors (11). However, hypertension could be attributed to these cancer treatments in only 9.3% of hypertensive survivors (12) and no other models of risk prediction for hypertension are currently available. As such, there is a need to enhance risk stratification to identify survivors at increased risk of hypertension who can be targeted for enhanced surveillance and lifestyle and pharmacological interventions that may minimize cardiovascular burden.
In the general population, both heritable and lifestyle risk factors contribute to the risk of hypertension. To date, 901 genetic variants are known to affect blood pressure levels in European populations and their cumulative effect is assessed in the form of a polygenic risk score (PRS) (13). This PRS is associated with a 3.34-fold increased risk of hypertension among individuals at the top decile of the PRS, compared with those at the bottom decile. The objective of this study was to evaluate this blood pressure PRS and its interplay with cancer therapies and being overweight/obese in association with hypertension, using 2 cohorts of childhood cancer survivors of European ancestry.
Methods
Study population
Participants were from 2 large cohort studies of childhood cancer survivors in North America, the Childhood Cancer Survivor Study (CCSS) (14,15) and the St. Jude Lifetime Cohort (SJLIFE) (16). The CCSS is a retrospective cohort study with a longitudinal follow-up of survivors of childhood cancer treated at 31 institutions in the United States and Canada. Study eligibility includes a diagnosis of cancer before 21 years of age and survival at 5 years after diagnosis of childhood cancer. Initial recruitment included survivors diagnosed between 1970 and 1986 (“CCSS original cohort”), which was subsequently expanded to include additional survivors diagnosed between 1987 and 1999 (“CCSS expansion cohort”). The CCSS study methodology and characteristics have been previously described (15).
The SJLIFE (16), initiated in 2007, is a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of 5-year survivors of childhood cancer treated at St. Jude Children’s Research Hospital since 1962. The Institutional Review Boards at St. Jude Children’s Research Hospital and each of the centers participating in the CCSS approved the study, and participants provided informed consent.
Considering the existing blood pressure PRS was derived from individuals of European ancestry via genome-wide association study, our analyses were restricted to survivors of European ancestry. In both CCSS and SJLIFE studies, genetic ancestry was determined by principal component analyses using the 1,000 European Genomes as the reference population.
Genetic data
Genotype data in the CCSS original cohort were obtained on Illumina HumanOmni5Exome array. After the quality control as described previously (17,18), genotypes were imputed up to the Haplotype Reference Consortium r1.1 (2016) haplotypes using Minimac3 (19) implemented in the Michigan Imputation Server. Paired-end whole-genome sequencing using the Illumina HiSeq X10 and/or NovaSeq sequencers was used to obtain genotype data in the CCSS expansion cohort and the SJLIFE. Details of the whole-genome sequencing, data processing, and quality control metrics are provided elsewhere (20, 21, 22). Principal components were generated based on the genotype data of an independent set of common variants using EIGENSTRAT in PLINK version 1.9 (23) and were used to control for population stratification.
Phenotypes
Participants in the CCSS completed a multi-item questionnaire at baseline and follow-up evaluations that included age at onset of hypertension based on self-report of medical diagnosis and pharmacological treatment. In SJLIFE, hypertension was ascertained through measured blood pressure and extent of lifestyle/medical intervention. Using modifications of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.03) (24), hypertension was graded and categorized as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening or disabling (grade 4), or fatal (grade 5). Survivors in both CCSS and SJLIFE are continuously assessed for hypertension based on survey and clinical ascertainment, respectively. In this study, survivors with Common Terminology Criteria for Adverse Events grade ≥2 at any of the hypertension assessments were considered hypertensive and those with grade <2 in all hypertension assessments were classified as hypertension-free.
Polygenic risk score
The largest genome-wide association study on blood pressure to date, including more than one million individuals of European ancestry, robustly implicated 901 loci (13). Using the reported beta estimates (on blood pressure) of these loci as their weights, a PRS was constructed as a weighted sum of the number of risk alleles carried by an individual. Of the 901 loci, 5 were identified in non-European populations and are monomorphic in Europeans, and 1 locus was absent in the CCSS original cohort, resulting in 895 loci for our PRS calculation. Risk alleles and weights of these loci are provided in Supplemental Table 1. The PRS was evaluated as a continuous variable or a categorical variable (1st decile, 2nd to 5th deciles, 6th to 8th deciles, 9th decile, and 10th decile, where the decile cutoffs were determined using the PRS distribution in the CCSS original cohort, the largest of the 3 cohorts).
Statistical analyses
For each PRS category, age-standardized prevalence ratio of hypertension was calculated as the ratio of the observed number of hypertensive survivors in each individual cohort to the expected number, which was calculated by applying the age-specific (10-year groups) prevalence of hypertension in the CCSS original cohort, the largest among the 3 cohorts, to the individual cohorts. The nonparametric bootstrap with 1,000 replicates was used to estimate the 95% confidence interval (CI).
Continuous variables are reported with their medians with interquartile ranges (IQRs) and categorical variables are presented with percentages. Multivariable analyses assessing association between PRS and risk of hypertension were performed in individual cohorts as well as in the combined sample of all survivors from the 3 cohorts. Association results were presented with estimated odds ratio (OR) and corresponding 95% CIs. The nongenetic baseline model was first fit among all survivors in the 3 cohorts with logistic regression for hypertension adjusting for the top 10 principal components and covariates, where the covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never), physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort, and SJLIFE), and the 6 COG risk factors (yes/no) including ifosfamide, heavy metals, abdominal radiation, HPA radiation, total body radiation, and nephrectomy. Of the 6 COG risk factors, multivariable analyses showed ifosfamide was not significantly associated with risk of hypertension (adjusted OR [aOR]: 1.18; p = 0.328), whereas a strong and statistically significant association was observed when all alkylating agents (bendamustine [treanda], busulfan, carmustine, chlorambucil [leukeran], cyclophosphamide, ifosfamide, lomustine, mechlorethamine [nitrogen mustard], melphalan, procarbazine, and thiotepa) were considered (aOR: 1.59; p < 0.001). Therefore, we replaced ifosfamide with alkylating agents (yes/no) in all the analyses. The PRS was then added in 2 ways: one as a continuous variable (z score normalized across all survivors) assessing adjusted prevalence of hypertension per standard deviation change in the PRS; and the other as a categorical variable. Stratified analyses based on body mass index (<18.5 kg/m2 [underweight], 18.5 to 24.9 kg/m2 [normal weight], or ≥25 kg/m2 [overweight/obese]) and each of the 6 COG risk factors already noted, except total body radiation due to insufficient numbers, were conducted, followed by analyses of their multiplicative interaction with the PRS.
In the combined sample, the attributable fraction (AF) for hypertension was calculated using multivariable logistic regression, adjusting for the same covariates of the regression model above and the top 10 principal components. Specifically, we first calculated the predicted prevalence of hypertension: 1) in presence of both cancer therapies (alkylating agents, heavy metals, abdominal radiation, HPA radiation, total body radiation, and nephrectomy) and the PRS (p11); 2) in absence of PRS by moving everyone to the bottom decile (the first decile) without changing the cancer therapies (p10); 3) in absence of the 6 cancer therapies without changing the PRS (p01); and 4) with elimination of both cancer therapies and the PRS (p00). The AF of hypertension due to cancer therapies and that due to the PRS were then calculated as (p11–p10)/p11 and (p11–p01)/p11, respectively. The AF of hypertension due to the joint effect of cancer therapies and the PRS, exceeding the individual effects of the 2 estimated separately above (25), was estimated as (p11–p10–p01+p00)/p11. The following stratified analyses were also conducted: 1) body mass index (<18.5 kg/m2 [underweight], 18.5 to 24.9 kg/m2 [normal weight], or ≥25 kg/m2 [overweight/obese]; 2) age (<35 years/≥35 years); and 3) the COG risk factor(s) showing significant interaction with the PRS. Statistical analyses were performed using R 3.4.0 and 2-sided p values <0.05 were considered statistically significant.
Results
After excluding survivors with missing phenotype and/or covariate data and of non-European ancestry, there were 7,995 survivors in both the CCSS and SJLIFE for the final analysis. Clinical, demographic, and treatment characteristics of all 7,995 study participants according to cohort are provided in Table 1. Participants in the CCSS original cohort were the oldest with median age at last contact of 41.5 years (IQR: 12.4 years), followed by those in the SJLIFE with median age at last contact of 35.5 years (IQR: 15.3 years) and in the CCSS expansion cohort with median age at last contact of 30.1 years (IQR: 8.5 years). The median body mass index in the CCSS original cohort, CCSS expansion cohort, and SJLIFE was 22.8 kg/m2 (IQR: 5.5 kg/m2), 24.8 kg/m2 (IQR: 6.9 kg/m2), and 26.4 kg/m2 (IQR: 8.9 kg/m2), respectively. Of the 7,995 survivors, 1,678 were hypertensive. The prevalence of hypertension was the highest in the clinically assessed SJLIFE cohort (28.7%), followed by the CCSS original cohort (22.1%) and expansion cohort (8.5%). The PRS distribution in the 3 cohorts was similar and followed approximately a normal distribution (Supplemental Figure 1).
Table 1.
Demographic and Treatment Characteristics of Eligible Participants in This Study
| CCSS Original Cohort (n = 3,572) | CCSS Expansion Cohort (n = 1,889) | SJLIFE (n = 2,534) | |
|---|---|---|---|
| Sex | |||
| Female | 1,876 (52.5) | 994 (52.6) | 1,198 (47.3) |
| Male | 1,696 (47.5) | 895 (47.4) | 1,336 (52.7) |
| Smoker | |||
| Never | 2,841 (79.5) | 1,368 (72.4) | 1,656 (65.4) |
| Current/past | 731 (20.5) | 521 (27.6) | 878 (34.6) |
| Hypertension | |||
| No | 2,782 (77.9) | 1,729 (91.5) | 1,806 (71.3) |
| Yes | 790 (22.1) | 160 (8.5) | 728 (28.7) |
| Abdominal radiation | |||
| No | 2,661 (74.5) | 1,570 (83.1) | 2,042 (80.6) |
| Yes | 911 (25.5) | 319 (16.9) | 492 (19.4) |
| Total body irradiation | |||
| No | 3,559 (99.6) | 1,875 (99.3) | 2,531 (99.9) |
| Yes | 13 (0.4) | 14 (0.7) | 3 (0.1) |
| Hypothalamic-pituitary axis radiation | |||
| No | 1,278 (35.8) | 1,151 (60.9) | 1,146 (45.2) |
| Yes | 2,294 (64.2) | 738 (39.1) | 1,388 (54.8) |
| Nephrectomy | |||
| No | 3,229 (90.4) | 1,736 (91.9) | 2,369 (93.5) |
| Yes | 343 (9.6) | 153 (8.1) | 165 (6.5) |
| Alkylating agents | |||
| No | 1,816 (50.8) | 849 (44.9) | 1,068 (42.1) |
| Yes | 1,756 (49.2) | 1,040 (55.1) | 1,466 (57.9) |
| Heavy metals | |||
| No | 3,406 (95.4) | 1,577 (83.5) | 2,231 (88.0) |
| Yes | 166 (4.6) | 312 (16.5) | 303 (12.0) |
| Age at cancer diagnosis, yrs | |||
| Median (IQR) | 7.7 (10.5) | 8.9 (9.9) | 7.7 (10.2) |
| Age at last contact, yrs | |||
| Median (IQR) | 41.5 (12.4) | 30.1 (8.5) | 35.5 (15.3) |
| Body mass index, kg/m2 | |||
| Median (IQR) | 22.8 (5.5) | 24.8 (6.9) | 26.4 (8.9) |
Values are n (%), unless otherwise indicated. All the survivors are of European ancestry.
CCSS = Childhood Cancer Survivor Study; IQR = interquartile range; SJLIFE = The St. Jude Lifetime Cohort.
Age-standardized prevalence ratio of hypertension
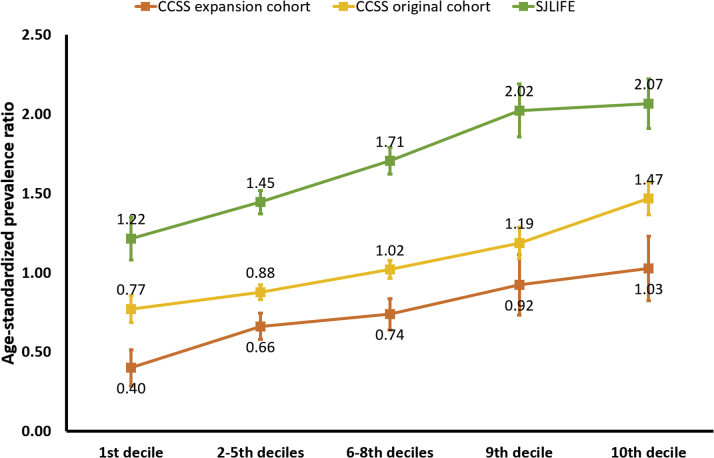
The PRS-decile-specific age-standardized prevalence ratios of hypertension in the CCSS original cohort, the CCSS expansion cohort, and the SJLIFE are provided in Figure 1. Among survivors with PRS in the top decile, the prevalence of hypertension was greater than expected in all 3 cohorts, with a prevalence ratio of 1.47 (CCSS original cohort), 1.03 (CCSS expansion cohort), and 2.07 (SJLIFE). Notably, in the clinically assessed SJLIFE, the prevalence ratio was >1 in all categories of the PRS.
Figure 1.
Age-Standardized Prevalence Ratios of Hypertension by PRS Deciles
For survivors in the CCSS original and expanded cohorts (self-report of hypertension) and the SJLIFE study (clinical assessment of hypertension), the ratio of the observed number of hypertensive survivors in each individual cohort to the expected number was calculated. The nonparametric bootstrap with 1,000 replicates was used to estimate the 95% confidence interval. Among survivors with the PRS in the top decile, the prevalence of hypertension was greater than expected in all 3 cohorts. CCSS = Childhood Cancer Survivor Study; PRS = polygenic risk score; SJLIFE = The St. Jude Lifetime Cohort.
Association of the PRS with hypertension
In the CCSS original cohort, the PRS was significantly associated with an increased prevalence of hypertension (aOR per 1-SD of the PRS: 1.31; 95% CI: 1.20 to 1.42; p < 0.001) (Table 2). Compared with survivors with the PRS in the lower decile, those in upper deciles had a greater prevalence of hypertension; for example, survivors in the top decile had a 2.63-fold higher odds (95% CI: 1.80 to 3.83; p < 0.001). Similar results were observed among survivors in the CCSS expansion cohort with a significantly increased prevalence of hypertension per SD change in the PRS (aOR: 1.33; 95% CI: 1.12 to 1.58; p < 0.001) and top versus bottom decile (aOR: 3.03; 95% CI: 1.39 to 6.60; p = 0.005). The adjusted ORs of clinically assessed hypertension in the SJLIFE were also comparable for both per SD change in the PRS (aOR: 1.39; 95% CI: 1.26 to 1.54; p < 0.001) and the top versus bottom decile (aOR: 2.60; 95% CI: 1.66 to 4.06; p < 0.001) than those in the CCSS cohorts with self-reported hypertension. In the combined sample of all 3 cohorts, the PRS remained consistently associated with hypertension (per SD change aOR: 1.34; 95% CI: 1.26 to 1.43; p < 0.001 and top vs. bottom decile aOR: 2.66; 95% CI: 2.03 to 3.48; p < 0.001). Survivors with the PRS in intermediate deciles also showed significantly increased prevalence of hypertension (aOR ≥1.24) compared with those with the PRS in the bottom decile (Table 2).
Table 2.
Multivariable Adjusted Associations of the Blood Pressure PRS From the General Population With Hypertension Among Childhood Cancer Survivors
| PRS | CCSS Original Cohort (790 Hypertensive Survivors; 2,782 Hypertension-Free Survivors) |
CCSS Expansion Cohort (160 Hypertensive Survivors; 1,729 Hypertension-Free Survivors) |
SJLIFE (728 Hypertensive Survivors; 1,806 Hypertension-Free Survivors) |
Combined Sample (1,678 Hypertensive Survivors; 6,317 Hypertension-Free Survivors) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | C-Index | OR (95% CI) | p Value | C-Index | OR (95% CI) | p Value | C-Index | OR (95% CI) | p Value | C-Index | |
| Per SD change | 1.31 (1.20–1.42) | <0.001 | 0.736 | 1.33 (1.12–1.58) | <0.001 | 0.747 | 1.39 (1.26–1.54) | <0.001 | 0.788 | 1.34 (1.26–1.43) | <0.001 | 0.785 |
| 1st decile | Ref. | |||||||||||
| 2th–5th deciles | 1.17 (0.85–1.62) | 0.329 | 0.736 | 1.83 (0.93–3.63) | 0.082 | 0.745 | 1.18 (0.81–1.73) | 0.385 | 0.787 | 1.24 (0.99–1.56) | 0.066 | 0.784 |
| 6th–8th deciles | 1.44 (1.04–2.00) | 0.030 | 1.89 (0.95–3.77) | 0.072 | 1.76 (1.20–2.58) | 0.004 | 1.61 (1.27–2.03) | <0.001 | ||||
| 9th decile | 1.89 (1.29–2.79) | 0.001 | 2.29 (1.03–5.10) | 0.042 | 2.32 (1.46–3.70) | <0.001 | 2.08 (1.58–2.75) | <0.001 | ||||
| 10th decile | 2.63 (1.80–3.83) | <0.001 | 3.03 (1.39–6.60) | 0.005 | 2.60 (1.66–4.06) | <0.001 | 2.66 (2.03–3.48) | <0.001 | ||||
Covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never), physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort, and SJLIFE), alkylating agents, heavy metals, abdominal radiation, hypothalamic-pituitary axis radiation, total body radiation, and nephrectomy.
The PRS showed greater effect in overweight/obese survivors (per SD change in PRS aOR: 1.39; top decile vs. bottom decile aOR: 3.51) than those with normal weight (per SD change in PRS aOR: 1.23; top vs. bottom decile aOR: 2.07) (Supplemental Table 2). These adjusted ORs were significantly different from each other (the interaction aOR per SD change in the PRS 1.13; p = 0.041; top decile vs. bottom decile aOR: 1.85; p = 0.001) (Table 3). We did not observe a significant modification of the effect of the PRS in underweight versus normal weight survivors (Supplemental Table 2). A greater PRS effect was also observed among survivors exposed to HPA radiation (per SD change in PRS aOR: 1.41; top decile vs. bottom decile aOR: 3.07) compared with unexposed survivors (per SD change in PRS aOR: 1.24; top vs. bottom decile aOR: 2.13; the interaction aOR per SD change in the PRS: 1.18; p = 0.005 and top vs. bottom decile aOR: 1.44; p = 0.195) (Supplemental Table 3). A slightly greater effect of the PRS on hypertension was observed among survivors with nephrectomy or exposed to abdominal radiation than unexposed survivors. However, no statistically significant interaction was found between the PRS and exposure to alkylating or platinum agents, abdominal radiation, or nephrectomy (Supplemental Table 3).
Table 3.
Interaction of Blood Pressure PRS From the General Population With Body Mass Index and Exposure to Hypothalamic-Pituitary Axis Radiation in Hypertension Risk Among Childhood Cancer Survivors
| PRS | PRS×Overweight/Obese |
PRS×Hypothalamic-Pituitary Axis Radiation |
||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Per SD change | 1.13 (1.01–1.27) | 0.041 | 1.18 (1.05–1.33) | 0.005 |
| 1st decile | Ref. (1.00) | |||
| 2th–5th deciles | 1.21 (0.95–1.55) | 0.120 | 1.02 (0.64–1.63) | 0.930 |
| 6th–8th deciles | 1.21 (0.94–1.55) | 0.140 | 1.23 (0.77–1.97) | 0.395 |
| 9th decile | 1.39 (0.93–2.06) | 0.104 | 1.45 (0.83–2.53) | 0.197 |
| 10th decile | 1.85 (1.27–2.69) | 0.001 | 1.44 (0.83–2.49) | 0.195 |
Covariates included age at childhood cancer diagnosis, age at last follow-up, sex, body mass index, smoking status (current or former vs. never), physical activity (vigorous intensity exercise in metabolic equivalents), cohort (CCSS original cohort, CCSS expansion cohort, and SJLIFE), alkylating agents, heavy metals, abdominal radiation, hypothalamic-pituitary axis radiation, total body radiation, and nephrectomy.
Attributable fraction of the PRS and cancer therapies for hypertension
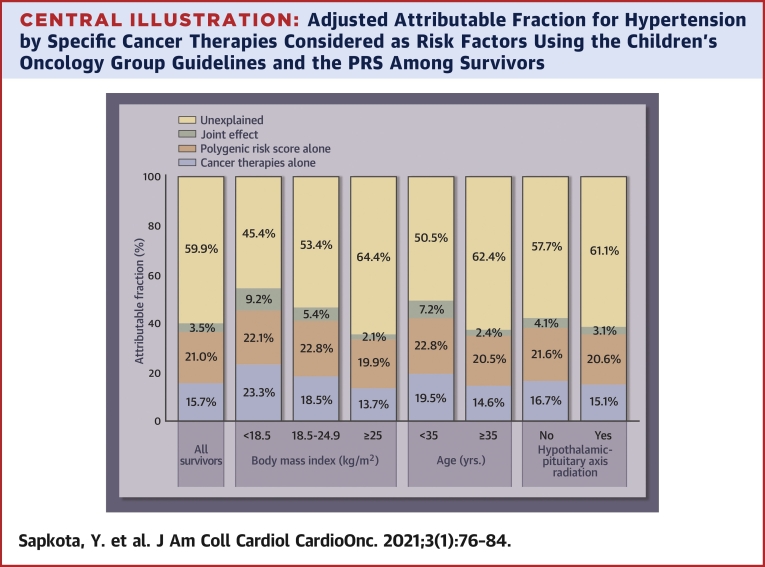
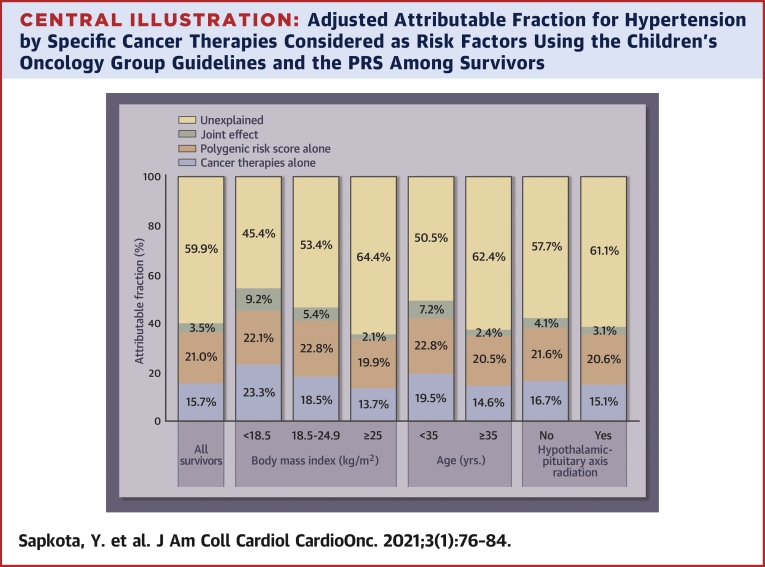
The adjusted AF for hypertension due to both the PRS and the specific cancer therapies considered as risk factors by the COG guidelines was 40.2% (Central Illustration). This total AF can be broken down into 3 components: 21.0% attributed to the PRS; 15.7% attributable to cancer therapies; and the remaining 3.5% due to the joint effect of both cancer therapies and the PRS together, exceeding the 2 individual effects. The AF for the PRS and cancer therapies was greater for survivors who were underweight (54.6%), had normal weight (46.7%), or were <35 years (49.5%), and these increases were mainly due to greater contribution of cancer therapies in these survivors. The contribution of the PRS remained consistent across the subgroups based on body mass index, age, and exposure to HPA radiation.
Central Illustration.
Adjusted Attributable Fraction for Hypertension by Specific Cancer Therapies Considered as Risk Factors Using the Children’s Oncology Group Guidelines and the PRS Among Survivors
The adjusted attributable fraction for hypertension due to both PRS and the specific cancer therapies was 40.2% (21.0% attributed to the PRS; 15.7% attributable to cancer therapies; and the remaining 3.5% due to the joint effect of both cancer therapies and the PRS together, exceeding the individual effects of the 2). The contribution of the PRS remained generally consistent across the subgroups based on body mass index, age, and exposure to hypothalamic-pituitary axis radiation. PRS = polygenic risk score.
Discussion
To our knowledge, this is the first study to apply and comprehensively evaluate the polygenic risk of blood pressure loci from the general population to childhood cancer survivors who are known to be at significantly greater risk of hypertension. Our results showed that survivors with the PRS in the top decile have a 2.7-fold increased odds of hypertension prevalence compared with those with the PRS in the bottom decile, and this risk was further exacerbated among overweight/obese survivors and those exposed to HPA radiation. Notably, our data indicate that the PRS and cancer therapies together accounted for 40.2% of hypertension among survivors, with the PRS alone contributing 21.0%.
We found a slightly attenuated PRS effect on hypertension among childhood cancer survivors compared with the 3.3-fold odds of hypertension prevalence among individuals with the PRS in the top decile in the general population (13). However, considering the absolute risk of hypertension in survivors is higher than those in the general population, the true effect of PRS among survivors of childhood cancer may be slightly greater than the observed 2.7-fold odds of hypertension prevalence. Thus, even a small increase in relative risk by the PRS can translate to substantial increase in absolute risk. Furthermore, the polygenic risk of hypertension was increased by being overweight/obese–a major late effect in childhood cancer survivors (26) and exposure to HPA radiation, which is a risk factor for obesity in survivors (27). However, the attributable fraction due to the PRS was slightly lower among overweight/obese survivors (19.9%) compared with those with normal weight (22.8%). These seemingly contradictory observations may be explained by the following: if the genetic contribution to the total risk is lower in presence of a strong nongenetic risk factor (overweight/obesity), the risk increase by the genetic factor is stronger because the baseline propensity for hypertension is elevated by the strong nongenetic risk factor.
An important finding in this study is that 40.2% of hypertension among survivors could be attributed to cancer therapies plus the PRS. About 16% of hypertension among survivors were attributed to cancer therapies and this contribution was higher (49% to 55%) among younger (<35 years) survivors or those with normal weight or those who are underweight. Approximately 21% of hypertension among survivors could be attributed to the polygenic risk, which remained generally consistent with respect to body mass index, age, and HPA radiation. Given that these estimates are independent of demographic, lifestyle, and treatment factors and that the polygenic risk is established at birth, survivors with a high blood pressure polygenic risk may benefit from earlier screening and intervention measures to reduce the risk of hypertension and comorbidities. Additionally, evaluating the polygenic risk requires a one-time genetic test (currently <$100), highlighting the feasibility of genetic risk assessment. Our results, therefore, suggest that consideration of blood pressure polygenic risk in combination with established cancer treatment–related risk factors can identify survivors at high risk of hypertension. Genetic testing for polygenic risk, in concert with assessment of clinical risk factors, could be used to risk stratify frequency of ongoing follow-up in normotensive survivors at high risk and therapeutic interventions (e.g., lifestyle, pharmacological) for those with elevated blood pressure or established hypertension.
Study limitations
Although the PRS and cancer therapies explained approximately 40% of cases of hypertension among survivors, further investigations are needed to understand the causes of the remaining 60% of cases of hypertension in survivors. In the CCSS survivors, hypertension was diagnosed based on self-report, which may be prone to misclassification and hence the reported blood pressure PRS effect on hypertension may be underestimated. Clinically assessed cohorts of childhood cancer survivors, such as the SJLIFE, provide a more accurate estimate of the PRS. Other such cohorts with both genetic and outcome data are not currently available, however. Kidney abnormalities are known predictors of hypertension; however, we were unable to assess their potential influence on the PRS-hypertension association because of limitations in self-reported data in the CCSS, which comprises approximately 68% of the study population. Nonetheless, the genotypes are independent of these potential confounders, and, if not, they would be in the pathway that the PRS, thus capturing their risk at least partially. The PRS could be calculated only for 5-year childhood cancer survivors who provided DNA samples in SJLIFE and CCSS. Thus, our findings may not be generalizable to patients with cancer who died before reaching the 5-year milestone. Our analyses were also restricted to survivors of European ancestry to be consistent with the populations used to derive the existing PRS. There is a need to assess the polygenic risk among survivors of non-European ancestry.
Conclusions
We found that the blood pressure PRS from the general population predicts hypertension among childhood cancer survivors in 3 independent cohorts. Survivors with higher polygenic risk were at significantly increased risk of hypertension, independent of demographic, lifestyle, and treatment risk factors, and this risk was further elevated among overweight/obese survivors and those exposed to HPA radiation. Approximately one quarter of the hypertensive survivors were attributed to PRS alone; together with the cancer therapies, this contribution increased to >40%. Our results, therefore, have strong implications for the personalized management of current survivors of childhood cancer and future patients with cancer. Although screening for hypertension in all childhood cancer survivors is important, enhanced awareness and prevention are warranted for those who are at higher risk due to PRS, clinical factors, and treatment exposures.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: The prevalence of hypertension in childhood cancer survivors is more than 2.5 times higher than in noncancer survivor controls. Importantly, survivors exposed to cardiotoxic cancer therapy have been shown to be at increased risk of cardiomyopathy, but those who also develop hypertension experience a near multiplicative increase in risk of cardiomyopathy. A blood pressure PRS established in the general population adds significantly to the prediction of high-risk subgroups for hypertension in childhood cancer survivors of European ancestry. Specifically, the attributable fraction of hypertension to PRS and cancer treatments together is >40%. Compared with survivors in the lower 10% of the PRS, survivors in all the other deciles of the PRS are at increased risk of hypertension, independent of demographic, lifestyle, and treatment risk factors. This PRS-associated risk was further elevated among survivors exposed to HPA radiation and those who were overweight/obese.
TRANSLATIONAL OUTLOOK: Although screening for hypertension in all childhood cancer survivors is important, enhanced awareness and prevention are warranted for those who are at higher risk due to PRS, clinical factors, and treatment exposures. Future research should be focused on the evaluation of genetic testing and application of polygenic risk scores, in concert with the assessment of clinical risk factors, to risk stratify frequency of ongoing follow-up in normotensive survivors at high risk and determine clinically appropriate therapeutic interventions (e.g., lifestyle, pharmacological) for those with elevated blood pressure or established hypertension.
Funding Support and Author Disclosures
The St. Jude Lifetime Cohort (U01 CA195547: to Drs. Hudson and Robinson, Principal Investigators) and the Childhood Cancer Survivor Study (CCSS; U24 CA55727; Dr. Armstrong Principal Investigator) are supported by the National Cancer Institute at the National Institutes of Health and the Cancer Center Support CORE grant (CA21765: C. Roberts, Principal Investigator). The CCSS original cohort genotyping was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. This work is also supported by R01 CA216354 (Drs. Zhang and Yasui, Principal Investigators) from the National Cancer Institute at the National Institutes of Health and the American Lebanese Syrian Associated Charities, Memphis, Tennessee. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Donna K. Arnett, PhD, MSPH, served as Guest Associate Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Armstrong G.T., Chen Y., Yasui Y. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulrooney D.A., Armstrong G.T., Huang S. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger K.C., Mertens A.C., Sklar C.A. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Bhakta N., Liu Q., Ness K.K. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390:2569–2582. doi: 10.1016/S0140-6736(17)31610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens A.C., Yasui Y., Neglia J.P. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong G.T., Oeffinger K.C., Chen Y. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian S.H., Xu L.F., Ky B. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow E.J., Baker K.S., Lee S.J. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson T.M., Li Z., Green D.M. Blood pressure status in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017;26:1705–1713. doi: 10.1158/1055-9965.EPI-17-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children's Oncology Group . Children's Oncology Group; Monrovia, CA: October 2018. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. [Google Scholar]
- 12.Hudson M.M., Ness K.K., Gurney J.G. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelou E., Warren H.R., Mosen-Ansorena D. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. doi: 10.1038/s41588-018-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robison L.L., Armstrong G.T., Boice J.D. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robison L.L., Mertens A.C., Boice J.D. Study design and cohort characteristics of the childhood cancer survivor study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 16.Hudson M.M., Ness K.K., Nolan V.G. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton L.M., Sampson J.N., Armstrong G.T. Genome-wide association study to identify susceptibility loci that modify radiation-related risk for breast cancer after childhood cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx058. djx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapkota Y., Turcotte L.M., Ehrhardt M.J. Genome-Wide association study in irradiated childhood cancer survivors identifies HTR2A for subsequent basal cell carcinoma. J Invest Dermatol. 2019;139:2042–2045e8. doi: 10.1016/j.jid.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S., Forer L., Schonherr S. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sapkota Y., Cheung Y.T., Moon W. Whole-genome sequencing of childhood cancer survivors treated with cranial radiation therapy identifies 5p15.33 locus for stroke: a report from the St. Jude Lifetime Cohort Study. Clin Cancer Res. 2019;25:6700–6708. doi: 10.1158/1078-0432.CCR-19-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapkota Y., Wilson C.L., Zaidi A.K. A novel locus predicts spermatogenic recovery among childhood cancer survivors exposed to alkylating agents. Cancer Res. 2020;80:3755–3764. doi: 10.1158/0008-5472.CAN-20-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z.M., Wilson C.L., Easton J. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36:2078–2087. doi: 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson M.M., Ehrhardt M.J., Bhakta N. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidem Biomar. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taguri M., Kuchiba A. Decomposition of the population attributable fraction for two exposures. Ann Epidemiol. 2018;28:331–334e1. doi: 10.1016/j.annepidem.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Wilson C.L., Liu W., Yang J.J. Genetic and clinical factors associated with obesity among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Cancer. 2015;121:2262–2270. doi: 10.1002/cncr.29153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig R.H., Hinds P.S., Ringwald-Smith K. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88:2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.