Abstract
Reinforcement can occur when maladaptive hybridization in sympatry favors the evolution of conspecific preferences and target traits that promote behavioral isolation (BI). In many systems, enhanced BI is due to increased female preference for conspecifics. In others, BI is driven by male preference, and in other systems both sexes exert preferences. Some of these patterns can be attributed to classic sex-specific costs and benefits of preference. Alternatively, sex differences in conspecific preference can emerge due to asymmetric postzygotic isolation (e.g., hybrid offspring from female A × male B have lower fitness than hybrid offspring from female B × male A), which can lead to asymmetric BI (e.g., female A and male B are less likely to mate than female B and male A). Understanding reinforcement requires understanding how conspecific preferences evolve in sympatry. Yet, estimating conspecific preferences can be difficult when both sexes are choosy. In this study, we use Lucania killifish to test the hypothesis that patterns of reinforcement are driven by asymmetric postzygotic isolation between species. If true, we predicted that sympatric female Lucania goodei and sympatric male L. parva should have lower levels of BI compared with their sympatric counterparts, as they produce hybrid offspring with the highest fitness. To address the problem of measuring BI when both sexes are choosy, we inferred the contribution to BI of each partner using assays where one sex in the mating pair comes from an allopatric population with potentially low preference, whereas the other comes from a sympatric population with high preference. For one hybrid cross direction, we found that both female L. parva and male L. goodei have high contributions to BI in sympatry. In the other hybrid cross direction, we found that only female L. goodei contribute to BI. Sympatric male L. parva readily engaged in hybrid spawnings with allopatric L. goodei females. These results indicate that both asymmetric postzygotic isolation and the traditional sex-specific costs to preference likely affect the nature of selection on conspecific preferences and target traits.
Keywords: reinforcement, behavioral isolation, female mate choice, male mate choice, asymmetric costs to hybridization
Understanding how and why reproductive isolation forms between groups is an outstanding question in evolutionary biology. The initial stages of divergence often occur in allopatry (Mayr 1942; Coyne and Orr 2004; Ridley 2004), but the critical question is what happens to these groups upon secondary contact. Four different outcomes are possible (Coyne and Orr 2004). First, the levels of reproductive isolation between groups may be sufficient to maintain species boundaries (i.e., good species). Second, levels of reproductive isolation between groups may be insufficient, and groups may collapse into a hybrid swarm. Third, one group may simply outcompete the other, causing one group to become locally extinct. Finally, groups in secondary contact may hybridize at low levels, and produce maladaptive hybrids. In this scenario, selection against hybrids may increase conspecific preferences within groups, and complete the speciation process in sympatry (Butlin 1987; Noor 1999; Servedio and Noor 2003). This phenomenon, known as reinforcement, was initially met with skepticism, but theoretical and empirical work has since found support for reinforcement.
Early theoretical work considered systems where females acted as choosers and males acted as courters (Liou and Price 1994; Kelly and Noor 1996), and many empirical studies have shown that, indeed, behavioral isolation (BI) among taxa is often due to female mating preferences (Butlin and Ritchie 1991; Rundle and Schluter 1998; Servedio 2007; Dyer et al. 2014). However, other systems have provided good evidence for BI due to male mating preferences (Coyne et al. 1994; Peterson et al. 2005; Servedio 2007; Espinedo et al. 2010; Moran et al. 2017; Moran and Fuller 2018), and others have shown a mix where both females and males have preferences for conspecifics (Gregorio et al. 2012; West and Kodric-Brown 2015; but see Kozak et al. 2009). The question of what determines which sex exerts conspecific preferences and creates BI is unresolved. One possibility is that the classic sex-specific patterns of costs and benefits of choice at the within-species level determine the levels of conspecific preference in males and females. One sex, typically females, often invest more in a given reproductive event and have fewer overall mating attempts than males (Andersson 1994; Shuster and Wade 2003; Clutton-Brock 2007, 2009; Servedio 2007; Kozak et al. 2009). Here, the cost of hybridization may be greater for females than males, leading to high levels of female conspecific preference. Of course, sex ratios, densities of prospective mates, and predation risk can all affect the costs/benefits of choosing and courting (Clutton-Brock and Parker 1992; Kvarnemo and Ahnesjo 1996; Shuster and Wade 2003; Jennions and Petrie 2007).
In addition to the effects of classic sexual selection/mating system biology, the nature of postzygotic isolation can also create costs and benefits of choosing and courting that are unique to hybridization between species (Coyne and Orr 2004). Asymmetric postzygotic isolation is common and occurs when hybrids in one direction (e.g., female A × male B) have lower fitness than hybrids in another direction (e.g., female B × male A), and empirical examples of these types of costs have been documented in insects (Shapiro 2006; Hochkirch and Lemke 2011; Sánchez-Guillén et al. 2012; Yukilevich 2012), fish (Crow et al. 2007; Van Der Sluijs et al. 2008; Martin and Mendelson 2013), mammals (Smadja and Ganem 2005; Beysard et al. 2015; Shipley et al. 2016), amphibians (Pfennig and Simovich 2002; Arntzen et al. 2009), and plants (Tiffin et al. 2001; Ramsey et al. 2003; Coughlan and Willis 2018). In these scenarios, the expectation is that BI will be concordant with the direction of postzygotic isolation (i.e., females of species A and males of species B will be less likely to engage in hybrid matings than females of species B and males of species A). Theoretically, this variation in hybridization costs may affect the patterns of reproductive character displacement and, ultimately, the outcomes of reinforcing selection (Veen et al. 2001; Pfennig and Simovich 2002; Clutton-Brock 2007; Yukilevich 2012).
The Lucania system is excellent for investigating how variation in hybridization costs may affect patterns of reproductive character displacement, and reinforcement, for several reasons: The Lucania system contains 2 closely-related sister species (Duggins et al. 1983; Whitehead 2010)—the bluefin killifish (L. goodei) and the rainwater killifish (L. parva). L. goodei and L. parva can be found in sympatry and allopatry across Florida, with additional populations of allopatric L. parva across the Atlantic coast and the Gulf of Mexico. Previous studies suggest that these sister species diverged in allopatry and, in some populations, came back into secondary contact approximately 2 million years ago (Ghedotti and Davis 2017). Behavioral and genetic data also suggest that conspecific mate preference is stronger in sympatry compared with allopatry for both L. goodei and L. parva, a pattern consistent with reproductive character displacement and reinforcement (Fuller et al. 2007; Berdan and Fuller 2012; Gregorio et al. 2012; Kozak et al. 2015). Therefore, there is good support for reinforcement and a pattern of reproductive character displacement in this system.
Second, there is variation in hybridization costs due to sex and species identity in the Lucania system that may affect the outcome of reinforcement. Previous studies have documented asymmetric fitness costs to hybridization due to species identity and cross direction, where male F1 hybrids, produced from male L. goodei and female L. parva parents, suffer a significant reduction in fitness compared with hybrids formed from female L. goodei and male L. parva (Fuller 2008). Backcrosses into L. goodei also suffer reduced fitness whereas backcrosses into L. parva do not. Reinforcement via this process predicts that male L. goodei and female L. parva should have increased levels of conspecific mate preference compared with female L. goodei and male L. parva in sympatry. On the contrary, the Lucania system follows traditional sex roles and females energetically invest more into reproductive events than males, suggesting that females of both species may have increased levels of conspecific preference in sympatry compared with their male counterparts. Evidence supporting these predictions is mixed (Berdan and Fuller 2012; Kozak et al. 2015; St. John and Fuller 2019), and no clear connection between variation in hybridization costs and reinforcement has been documented in this system.
In this study, we used no-choice assays to investigate the roles of sex and cross direction on the patterns of conspecific mate preference in the Lucania system (hereafter referred to as BI). We hypothesize that hybridization costs associated with the asymmetry in the genetic incompatibility (i.e., cross direction) may cause concordant patterns of asymmetry in BI, and predict that L. goodei females and L. parva males from sympatric populations should have lower levels of conspecific preference than L. parva females and L. goodei males from the same populations. Alternatively, the sex-specific costs of reproduction and hybridization predict that conspecific preference may be higher for females of both species in sympatry compared with their male counterparts. We found that male L. parva from sympatry contributed less to BI than all other sympatric groups, supporting the hypothesis that the asymmetry in the genetic incompatibility alters the nature of reproductive character displacement. However, we found that female L. goodei—who we expected to follow the same pattern as male L. parva—had consistently high levels of BI. We conclude that the patterns of BI in the killifish system cannot solely be explained by the asymmetric costs to hybridization that genetic incompatibilities and cross direction produce. Instead, we suggest alternative factors that may be affecting the patterns of BI observed in this system.
Materials and Methods
Collection and care
During the summers of 2015 and 2016, we collected 4 types of populations for this study: 1) a sympatric population of L. goodei from Salt Springs (Marion County, FL; St. John’s river drainage), 2) sympatric populations of L. parva from California Creek (Dixie County, FL; Suwannee river drainage) and Salt Springs (Marion County, FL; St. John’s river drainage), 3) an allopatric population of L. goodei from Blue Springs (Gilchrist County, FL; Santa Fe river drainage), and 4) an allopatric population of L. parva from Lake Pontchartrain (St. Tammany County, LA) (for a complete list of sympatric and allopatric populations of Lucania across Florida, see Fuller and Noa 2008). We used dip nets and seines to collect males and females from each of these populations. Using coolers, we transported the fish back to the University of Illinois Urbana-Champaign where they resided for the duration of the study period. At the University of Illinois Urbana-Champaign, fish were kept in large cattle tanks in an outdoor greenhouse. Fish were exposed to natural light cycles and were fed a diet of brine shrimp and blood worms daily.
Egg production as a metric of male and female preference
We used the total number of eggs produced by mate pairs in a no-choice assay as a proxy for mate preference for each of the 4 population types. Typically, male killifish hold small territories around vegetation, where females visit them. During these visits, a female may assess a male and vice versa. If a male wish to mate with a female, the male will begin courting her by shaking his head and swimming around her. During this time, a female may: 1) continue to assess the male, 2) decide that she is willing to mate with him, or 3) decide that she is uninterested and swim away. If a female decides to mate with a male, they swim side by side and deposit eggs on vegetation in the male’s territory. Killifish only deposit 1–2 eggs per spawning bout—and females do not deposit eggs in isolation or without a male spawning partner—indicating that number of eggs produced from a spawning pair is a good proxy for the number of spawning bouts, and thus a good measure of preference for both individuals.
Number or frequency of spawning/mating bouts is a common metric of preference across many different systems (Hoikkala and Aspi 1993; Coyne et al. 2005; Schöfl et al. 2011; Dougherty and Shuker 2014), and our previous work has explicitly investigated the ability of egg production—along with other behavioral metrics—to detect conspecific preference in Lucania. Although measurement of association time and the number of courting bouts reliably detected preference for male L. goodei, they were not good measures of preference for female L. goodei. Instead, we found that number of eggs produced in no choice assays could reliably detect conspecific mate preference for both male and female L. goodei, which is why we use this measurement here (St. John and Fuller 2019).
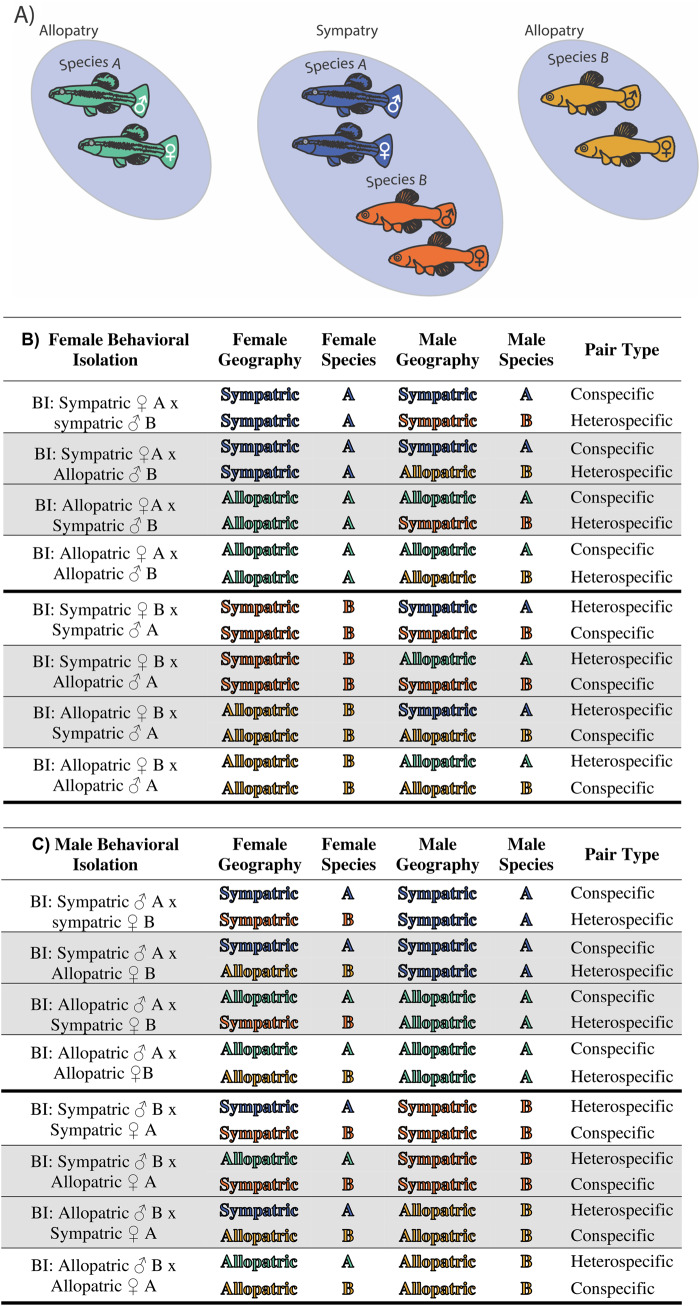
One challenging aspect of using no-choice assays and egg production as a proxy of mate preference is that it can be difficult to parse out the relative contributions of the female and male to BI. Previous studies investigating reinforcement have traditionally compared estimates of BI using conspecific and heterospecific pairs from sympatry and allopatry (Figure 1). These population pairs are obviously required to compare the levels of BI for sympatric versus allopatric populations. However, the use of traditional sympatric and allopatric population pairs does not inform as to the contributions of each species and sex to BI. For example, a pairing between a female of species A and a male of species B may fail to mate. The traditional assumption is that a failure to mate is a property of female mating preference and male traits. However, in systems where both sexes choose, both sexes can act as choosers and both sexes possess target traits that the other sex can assess. To address this, we repeatedly estimated preferences for each individual by producing conspecific and heterospecific pairs with individuals from both sympatry and allopatry (4 mates per individual). Repeated measures of an individual’s mate preference typically provide a more precise estimate of said preference (Wagner 1998; Dougherty and Shuker 2015). Furthermore, for our sympatric individuals, measuring their mate preference using mates from allopatry—who presumably have weaker or no conspecific mate preference—allows us to parse out the contributions of a given individual to a mating pair.
Figure 1.
(A) Species A and B and their geographic relationships. (B) Crosses used to measure BI for females as a function of sympatry and allopatry. (C) Crosses used to measure BI for males as a function of sympatry and allopatry. Traditional crosses used to diagnose RCD in white. Nontraditional crosses used to diagnose the roles of species and sex are shown in gray fill.
Experimental design
In total, we measured mate preference for 20 allopatric L. goodei individuals (10 males and 10 females from Blue Springs), 20 sympatric L. goodei individuals (10 males and 10 females from Salt Springs), 14 allopatric L. parva individuals (6 males and 8 females from Lake Pontchartrain, LA), and 26 sympatric L. parva individuals (11 males and 9 females from California Creek; 3 males and 3 females from Salt Springs). To measure preference for all 80 individuals, we set up 40 aquaria comprised 10 blocks of 4 tanks. Four males (a sympatric L. goodei male, an allopatric L. goodei male, an allopatric L. parva male, and a sympatric L. parva male) and 4 females (a sympatric L. goodei female, an allopatric L. goodei female, an allopatric L. parva female, and a sympatric L. parva female) were randomly assigned to each block. Within each block, we paired each male and female fish over the course of 5 weeks in July and August 2016. At the end of the study period, each block produced data for 16 unique male–female pairings (see Figure 2 for all pair types). In total, 10 replicates produced data for 160 unique pairings.
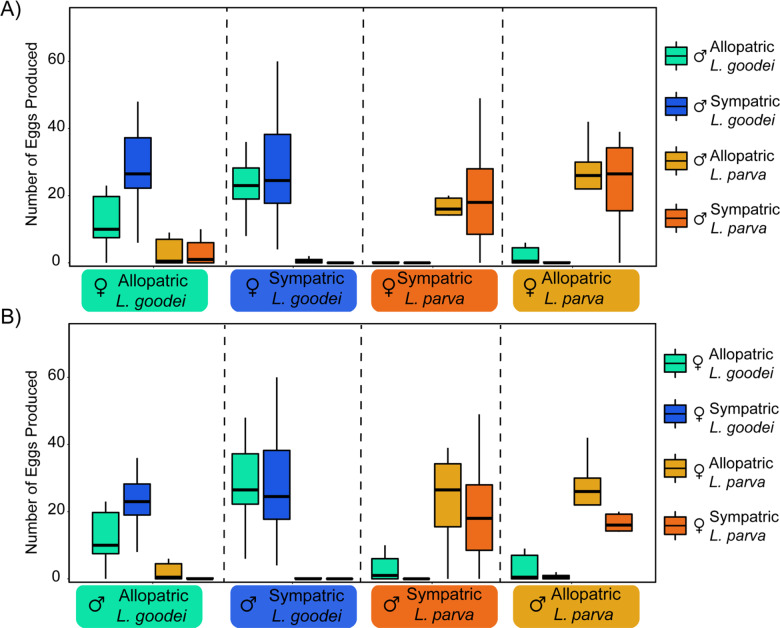
Figure 2.
Number of eggs produced by 16 unique mate pair types. (A) The 16 pairs from the female perspective. (B) The 16 pairs from the male perspective. The x-axis shows species and geographic designations, whereas boxplot colors indicate species and geographic designations of the mate.
Administration of assays
Immediately preceding the start of the study, we randomly assigned females to 1 of the 4 38-L tanks in their block. Females remained in their individual tanks for the entirety of the study, whereas males were moved between female tanks (but stayed in their assigned block). Each individual experienced 4 no-choice assays, which each lasted for 10 days. Assays began when a male was placed into a female tank along with 2 top mops (yarn attached to a Styrofoam ball) and 2 bottom mops (yarn attached to PVC pipe) that served as spawning substrate. The first 3 days of the no-choice assay were used as acclimation time for the mating pair. Any eggs collected during this time were disregarded. During the remaining 7 days, we collected and counted eggs from each mating pair and used the total number of eggs produced during this time as a proxy for preference. After egg collection on the 7th day, we removed males from their assigned tanks, randomly paired them with another female in their block, and repeated the process. We also followed this procedure for Weeks 3 and 4 so that all males were paired for 1 week (not including the acclimation time) with all females.
One caveat for this design is that we have a smaller sample size of L. parva from Lake Pontchartrain (6 males, 8 females). We had also hoped to use sympatric L. goodei and L. parva from the same location, but only had 3 males and 3 females of Salt Springs L. parva. To bolster the sample size and ensure that all animals experienced the same number of mates across replicate groups, we supplemented sympatric L. parva from California Creek (a separate sympatric site) wherever there was a missing L. parva. For blocks missing a sympatric L. parva from the Salt Springs population, we simply used animals from California Creek. For blocks missing an allopatric L. parva from Lake Pontchartrain, we used L. parva from California Creek as a “filler animal.” This ensured that all of the animals in the block could spawn for the same number of days. Hence, all blocks had male and female L. parva from a sympatric population, but 2 blocks lacked an allopatric L. parva female and 4 lacked an allopatric L. parva male. Additionally, one individual expired mid-assay and was thus removed from the dataset.
Statistics
Generalized linear mixed models
We used a GLMM to determine if the different combination of species and geography affected the total number of eggs a pair produced. First, we investigated whether block or week should be included in our overall model. We used a GLM with a negative binomial distribution to determine if either of these factors affected the total number of eggs produced by pairs. We found no effect of week (χ2 = 4.95, df = 3, P = 0.18) or block (χ2 = 2.94, df = 9, P = 0.97) on the total number of eggs produced by pairs, and therefore did not include them in our final model. As part of our experimental design, we purposefully randomized the order in which females were exposed to males in an effort to reduce order effects. We used a GLM with a negative binomial distribution to investigate whether the order of exposure to conspecific males or the order of exposure to native conspecific males affected the total number of eggs produced by subsequent pairs, but found no effect of either factor (conspecific exposure: LR χ2 = 0.14, df = 1, P = 0.71; native conspecific exposure: LR χ2 = 0.10, df = 1, P = 0.75). Next, we used t-tests to investigate whether the supplemental individuals added to blocks with missing mates were statistically different from their original groups. We found that neither the additional “filler” females (t = −0.053, df = 8.50, P = 0.96) or “filler” males (t = −0.78, df = 17.57, P = 0.44) were statistically different from their original groups and therefore included them with their appropriate groups for the overall model. For our final model, we used a GLMM with a negative binomial distribution and included the total number of eggs produced by a pair as the response variable in the model. Female species, male species, female geography, male geography, and the interactions between these variables were all included as fixed effects. We also included female and male ID as separate random effects (Table 1).
Table 1.
Results of GLMM to determine how species designation, geography, and their interaction affect the total number of eggs produced by a pair
| Response | Predictors | χ2 | df | P-value |
|---|---|---|---|---|
| Number of eggs produced by a pair | ♀ Species | 4.736 | 1 | 0.03 |
| ♂ Species | 1.463 | 1 | 0.226 | |
| ♀Geography | 1.565 | 1 | 0.211 | |
| ♂Geography | 1.856 | 1 | 0.173 | |
| ♀Species: ♂Species | 7.85 | 1 | 0.005 | |
| ♀Species: ♀Geography | 13 | 1 | 0.0003 | |
| ♂Species: ♀Geography | 10.239 | 1 | 0.001 | |
| ♀Species: ♂Geography | 11.428 | 1 | 0.001 | |
| ♂Species: ♂Geography | 1.315 | 1 | 0.252 | |
| ♀Geography: ♂Geography | 0.779 | 1 | 0.377 | |
| ♀Species: ♂Species: ♀Geography | 14.117 | 1 | 0.0002 | |
| ♀Species: ♂Species: ♂Geography | 8.012 | 1 | 0.005 | |
| ♀Species: ♀Geography: ♂Geography | 4.753 | 1 | 0.029 | |
| ♂Species: ♀Geography: ♂Geography | 0.004 | 1 | 0.95 | |
| ♀Species: ♂Species: ♀Geography: ♂Geography | 1.378 | 1 | 0.24 |
Significant predictors are indicated in bold.
Measuring BI
To make direct comparisons between groups, we used a standardized formula to quantify BI. We used Stalker’s isolation index (1942) with total number of eggs produced with a mate as a proxy for mate preference:
Stalker’s isolation index ranges from −1 to +1, with negative values representing heterospecific preference, positive values representing conspecific preference, and these values represent a linear relationship between mate preference and behavioral/reproductive isolation (Stalker 1942; Sobel and Chen 2014). Using this formula, we measured BI in 2 different ways: First, by comparing the number of eggs a group (i.e., male sympatric L. goodei, female allopatric L. parva, etc.) produced with conspecific mates (either L. goodei or L. parva) from their home population versus the number of eggs a group produced with heterospecific mates from a population of the same geography (i.e., sympatry or allopatry). For example, we calculated BI for sympatric L. goodei females by considering the number of eggs females laid with sympatric L. goodei males and sympatric L. parva males. For allopatric L. goodei females, we considered the number of eggs produced when paired with allopatric L. goodei males and allopatric L. parva males. This allowed us to calculate BI for each sex of each species in both ♀sympatric: ♂ sympatric crosses and ♀ allopatric: ♂ allopatric crosses (8 measures total). We refer to this as the traditional pairings (Figure 1; Table 2).
Table 2.
Estimated levels of BI for traditional and nontraditional crosses for L. goodei ♀ × L. parva ♂ and L. parva ♀ × L. goodei ♂
| L. goodei ♀ × L. parva ♂ | Female BI (CL) | Male BI (CL) | |
|---|---|---|---|
| Sympatric L. goodei ♀ × sympatric L. parva ♂ | 0.98 (0.93,1) | 0.97 (0.9,1) | |
| Sympatric L. goodei ♀ × allopatric L. parva ♂ | 0.95 (0.89,1) | 0.95 (0.82, 1) | |
| Allopatric L. goodei ♀ × sympatric L. parva ♂ | 0.41 (−0.4, 0.86) | 0.52 (0.18, 0.89) | |
| Allopatric L. goodei ♀ × allopatric L. parva ♂ | 0.33 (−0.24,1) | 0.54 (−0.12,1) | |
|
L. parva ♀ × L. goodei ♂ |
Female BI (CL) |
Male BI (CL) |
|
| Sympatric L. parva ♀ × sympatric L. goodei ♂ | 0.97 (0.9, 1) | 0.98 (0.93, 1) | |
| Sympatric L. parva ♀ × allopatric L. goodei ♂ | 0.95 (0.88, 1) | 0.94 (0.83, 1) | |
| Allopatric L. parva ♀ × sympatric L. goodei ♂ | 0.97 (0.88, 1) | 0.97 (0.92,1) | |
| Allopatric L. parva ♀ × allopatric L. goodei ♂ | 0.68 (0.15, 0.99) | 0.52 (−0.00063, 0.97) |
BI estimates were calculated using stalker’s isolation index, and confidence intervals were calculated using bootstrapping methods (10,000 iterations). Traditional crosses are shown in white. Nontraditional crosses are shown in gray.
Second, we compared the number of eggs a group produced with conspecific mates from their home population versus the number of eggs they produced with heterospecific mates from a population of the opposite geography (e.g., if the focal individual is from sympatry, we paired them with a heterospecific from allopatry). For example, we calculated BI for sympatric L. goodei females by considering the number of eggs females laid with sympatric L. goodei males and allopatric L. parva males. For allopatric L. goodei females, we considered the number of eggs produced when paired with allopatric L. goodei males and sympatric L. parva males. This allowed us to calculate BI for each sex of each species in both ♀ sympatric: ♂ allopatric crosses and ♀ allopatric: ♂ sympatric crosses (8 measures total). We refer to these additional pairings as nontraditional pairings (Figure 1; Table 2).
Although all 16 types of pairs are represented in the dataset, they were not present in equal numbers. The unequal numbers prevented us from calculating BI values for each individual. Instead, we used a bootstrap resampling method to calculate BI and 95% confidence intervals. We calculated BI for each group for 10,000 replicates. BI values were considered significant if 95% confidence intervals did not overlap with zero (Table 2). All analyses were performed in R (version 3.5.1).
Results
Traditional measures of conspecific preference reveal a pattern of reproductive character displacement in the Lucania system
Figure 2 shows the number of eggs produced for each cross-type from 1) the female perspective (Figure 2A), and 2) the male perspective (Figure 2B). Table 2 shows the patterns of BI that emerge from these crosses. There is a clear pattern of reproductive character displacement that is consistent with reinforcement when considering traditional measures of conspecific preference. Conspecific crosses produced many more eggs than did heterospecific crosses (post hoc pairwise Wilcoxon rank-sum test with a Bonferroni correction; P = 7.4 × 10−11) and this pattern was heightened as a function of sympatry versus allopatry. Heterospecific crosses involving animals from sympatric populations produced few (if any) eggs in comparison to heterospecific crosses from allopatric populations (Figure 2, post hoc pairwise Wilcoxon rank-sum test with a Bonferroni correction; P = 0.0055). Estimates of BI from traditional assays show that sympatric groups have strong, significant, conspecific preferences because their estimates are large positive values, that do not overlap with zero (which represents no mate preference; Table 2). Conversely, allopatric groups exhibited much lower estimates of BI that did overlap with zero indicating that, in general, they had weaker or nonexistent conspecific mate preferences (Table 2). However, there is a trend for slightly higher BI for crosses between male L. goodei and female L. parva in comparison to crosses between male L. parva and female L. goodei.
Nontraditional crosses show that male L. parva contribute less to BI than all other sympatric groups
Nontraditional crosses, involving a combination of animals from sympatric and allopatric populations, allow us to determine which partner has larger effects on BI. We first concentrate on crosses between L. parva females and L. goodei males, which produce offspring with reduced hybrid fitness and are predicted to have high BI. The inclusion of either a sympatric L. parva female or a sympatric L. goodei male creates high BI in this cross direction, and both nontraditional cross types produce high BI (Table 2). The implication is that both L. goodei males and L. parva females differ in preference/target traits between allopatric and sympatric populations such that both sexes contribute to BI.
Crosses in the opposite direction, L. goodei females crossed with L. parva males, produce hybrids with higher fitness and are predicted to have lower BI in comparison to the reciprocal hybrid cross. Here, estimates of BI for crosses involving allopatric L. goodei females do not differ from zero, regardless of the population of origin of L. parva, but estimates of BI for crosses involving sympatric L. goodei females are always significantly different from zero, indicating strong conspecific preference (Table 2). Conversely, L. parva males from sympatric populations appear to be willing to mate with L. goodei females. Specifically, male sympatric L. parva produced significantly more eggs when their female L. goodei mate was from an allopatric population compared with a sympatric population (post hoc pairwise Wilcoxon rank-sum tests with a Bonferroni correction; P = 0.0072), and BI estimates fell from 0.97 (CI 0.90–1) to 0.52 (CI 0.18–0.89) when L. goodei females were from allopatry compared with sympatry. The interpretation is that sympatric L. parva males will more readily engage in hybrid mating events than will L. goodei males.
Finally, our GLMM shows that the interaction between female species, male species, and female geography (χ 2 = 14.117, df = 1, P = 0.0002) and the interaction between female species, male species, and male geography (χ2 = 8.012, df = 1, P = 0.005) were both significant predictors for the number of eggs produced by a pair. This result not only suggests that there is variation in the total number of eggs produced by conspecific versus heterospecific pairings (as predicted by reinforcement), but that the number of eggs produced from a conspecific or heterospecific pairing may also depend on whether a mate is from sympatry or allopatry (i.e., whether their preference was measured in a traditional or nontraditional assay).
Discussion
In this study, we aimed to test whether the costs of hybridization associated with cross direction and sex affect the pattern of conspecific mate preference in the Lucania system. We made 2 predictions: First, the hypothesis that asymmetric genetic incompatibilities lead to asymmetric BI predicts that L. goodei females and L. parva males from sympatric populations should have lower levels of conspecific preference than L. parva females and L. goodei males from the same populations. Second, the hypothesis that traditional costs/benefits of mate preference in traditional mating systems also affect the costs/benefits of conspecific preference predicts that females of both species should have high BI relative to males in sympatry. Ultimately, we found that sympatric male and female L. goodei, and sympatric female L. parva had high contributions to BI. Regardless of the geographic identity of their conspecific or heterospecific mate partners, sympatric male and female L. goodei, and sympatric female L. parva did not engage in hybrid matings at high levels (Table 2; Figure 2). However, we found that sympatric male L. parva produced more eggs with heterospecific partners when said partner was from an allopatric population (Table 2; Figure 2)—suggesting that they have weaker conspecific mate preferences and lower contributions to BI than all other sympatric groups. Taken together, these results suggest that neither hypothesis alone fully explains the pattern of BI observed in the Lucania system. Instead, we suggest that costs associated with both sex and cross direction may be acting together to influence the species- and sex-specific patterns in reproductive character displacement.
Asymmetric postzygotic isolation does not solely explain the pattern of BI in Lucania
The asymmetric postzygotic isolation between Lucania species is well documented (Fuller 2008) and predicts that male L. goodei and female L. parva from sympatric populations should have high conspecific preference. We found strong evidence supporting this prediction. Both male L. goodei and female L. parva from sympatric populations abstained from hybrid matings regardless of whether they were paired with sympatric or allopatric heterospecifics (Table 2). Likewise, the asymmetry in postzygotic isolation also predicts that female L. goodei and male L. parva from sympatric populations should have lower levels of BI. However, we found that this prediction was not completely supported. We found that male L. parva from sympatric populations readily engaged in hybrid matings when they were paired with allopatric female L. goodei in our nontraditional assays (Table 2). This finding supports the predictions of reinforcement and indicates that previously strong estimates of conspecific preference for sympatric male L. parva (Fuller et al. 2007; Gregorio et al. 2012; Kozak et al. 2015) were at least partially due to the mate preferences or traits of the heterospecific mate. However, we also found that female L. goodei from sympatric populations exhibited high levels of conspecific preference regardless of whether they were paired with sympatric or allopatric heterospecific males—a finding that does not support our predictions.
Alternative explanations for patterns of BI
If female L. goodei produce F1 hybrids with relatively high fitness, then why do they continually exhibit high levels of conspecific preference? One possibility is that selection has favored increased conspecific preference in female L. goodei due to the low fitness of their F2 backcrosses. Fuller (2008) found that F1 hybrids with an L. goodei mother exhibited no difference in fitness compared with purebred crosses, but F2 generations had extremely low viability when backcrossed into L. goodei. This was not the case for L. parva hybrids. Instead, F1 hybrids with L. parva mothers exhibit significantly lower fitness than purebred F1s, but when backcrossed into L. parva the F2 generation exhibited fitness levels on par with purebreds (Fuller 2008). Hence, even though crosses between L. goodei females and L. parva males create F1 offspring with high viability and high fertility, crosses between L. goodei females and F1 hybrid males reduces offspring survival. One caveat for this explanation is that this hypothesis needs a theoretical model to determine whether selection against backcrossed offspring could alter levels of conspecific preference—especially considering that these F1 animals are rare in nature (Hubbs 1955), which would diminish the strength of selection on conspecific preference.
A second possibility is that female L. goodei incur additional costs to hybridization that are not experienced by male L. parva. For example, females generally invest more in reproduction (i.e., production of eggs, fewer reproductive events) than their male counterparts (Clutton-Brock and Parker 1992; Hayward and Gillooly 2011; Lipshutz 2018). Previous studies have shown that female L. goodei have stronger conspecific mate preference than their male counterparts and even exhibit preferences consistent with cascade reinforcement (St. John and Fuller 2019). Additional studies using Drosophila also indicate that rapid evolution of female mate preference via reinforcement may even curtail the evolution of male preference (Yukilevich and Peterson 2019). It is possible that reinforcement acted to increase conspecific mate preference for male L. goodei and female L. parva due to poor hybrid fitness, and also acted to increase conspecific mate preference for female L. goodei due to the costs associated with egg production. The data here support both scenarios.
It is also possible that the patterns of BI and conspecific preference observed in the Lucania system are not the result of reinforcement or selection against hybrids. Instead, these patterns could be 1) the incidental by-product of differences in selection or gene flow between populations (Coyne and Orr 2004; Cooley 2007), 2) the result of differences in sexual selection across populations (Langerhans and Riesch 2013), or 3) due to selection on a magic trait (Servedio et al. 2011). There is some support for these possibilities in our data. For example, Figure 2A shows that allopatric L. goodei females produced more eggs with sympatric L. goodei males than with L. goodei males from their home population. This could suggest that sexual selection in sympatry has shifted male L. goodei target traits, subsequently making them more attractive. However, both traditional and nontraditional estimates of BI measurements indicate that L. goodei females from allopatry ultimately exhibit nonsignificant conspecific preferences, suggesting that future work is needed to confirm or rule out this possibility.
Differences in natural selection across populations may have also incidentally shifted mating traits and preferences in the Lucania system. For example, previous studies have documented differences in lighting environment across killifish populations in Florida and have connected this variation to differences in male coloration (Fuller 2002; Fuller et al. 2010). It could be that the differences in conspecific preference and BI observed in this study are due to variation in male traits because of natural selection. However, the role of female mate choice in establishing the population patterns in lighting environment and male coloration is unclear (Fuller and Noa 2010; Mitchem et al. 2018). Finally, the effects of variation in hybridization costs on BI may vary with time since initial secondary contact. The process of reinforcement is expected to increase BI between groups over time, however, once groups approach complete isolation reinforcing selection becomes weaker. Furthermore, as time passes other forces, such as drift or natural selection may erase the patterns of BI produced through variation in hybridization costs.
Experimental design can affect measures of BI
Our novel experimental design also shows that high levels of BI between groups can be due to the behavior/target traits of one or both sexes. We found low levels of BI when using animals from allopatric populations. This pattern is consistent with the predictions of reinforcement and was wholly expected. However, for most crosses, BI increased dramatically when allopatric animals were paired with sympatric heterospecifics (Table 2). The increase in BI can be attributed to the preference/target traits of the sympatric animal. Reproductive character displacement (i.e., increased BI in sympatry) can be diagnosed by comparing BI for sympatric and allopatric animals. However, assessing the relative effect of each sex of each species requires measuring BI in all combinations of species, sex, and geography (i.e., allopatry vs. sympatry).
One caveat for our chosen design is that we used no-choice assays, which have some clear pros and cons with regard to documenting mate preference (Wagner 1998; Dougherty and Shuker 2015; Ryan and Taylor 2015). The advantage of no-choice assay is that they directly measure mating, which is the ultimate behavior of interest. However, there are clear disadvantages with regard to identifying the precise traits that create BI. In the case of the Lucania system, there are differences in male color patterns between spring and swamp populations in L. goodei, and there are differences in anal fin size between sympatric and allopatric populations of L. parva (Kozak et al. 2015). Hence, it is unclear whether preference per se or if a combination of preference and target traits differ between sympatric and allopatric populations in both species. Previous research on Drosophila subquinaria revealed that sympatric and allopatric populations differ in their cuticular hydrocarbon (CHC) phenotypes and that females from sympatry prefer sympatric CHC phenotypes (Dyer et al. 2014; Rundle and Dyer 2015). However, some literature does suggest that preference might diverge more than signaling traits in regard to reinforcement (Sullivan-Beckers and Cocroft 2010; Debelle et al. 2014; Wheatcroft and Qvarnström 2017). For example, work in birds suggests that reinforcement via discrimination is likely very common and does not require any further diversification of traits (Hudson and Price 2014). This claim is further supported by selection experiments that have successfully altered discrimination windows without noticeably diversifying traits (Kovach 1990). Still, the problem of identifying the relative importance of divergence in preference versus divergence in signaling traits requires direct measurements of these precise traits, and the need to do so is greater when both sexes choose.
Conclusion
In conclusion, we investigated whether patterns of BI in the Lucania system matched the predictions of reinforcement when asymmetric postzygotic isolation is present between species. We used a novel experimental design to separately measure mate preference and estimate the contribution to BI for males and females of both species. We found that reinforcement solely due to asymmetric postzygotic isolation does not explain the patterns of BI that we detected. Instead, reinforcement may be acting to increase mate preferences in some groups due to the dramatically reduced fitness of backcrosses or due to the increased costs of hybridization that females incur. Finally, we also highlighted the importance of experimental design when measuring mate preferences and urge future studies to consider the geographic identity of stimulus mates when investigating mate preferences and reinforcement.
Acknowledgments
L.D. Mitchem, C.-H. Chang, R. Moran, A. Bell, and S. Berlocher provided comments that improved the manuscript. Experiments were approved by Illinois Institutional Animal Care and Use Committee (#15246).
Funding
Work was funded by the NSF (DEB 0953716), the UI Department of Animal Biology Odum-Kendeigh Fund, and the UI School of Integrative Biology Harley and Francis Clark Fund. M.E. St. John was supported by an NIH SEPA Award (R25 OD020203) to B. Hug and R.C.F. L.D. Mitchem, J. Knox, and Z. Osterholz helped collect fish.
Authors’ Contributions
M.E.S.J. and R.C.F. conceptualized the project and reviewed and edited drafts; M.E.S.J. collected and analyzed data and wrote original draft; R.C.F. provided funding.
Conflict of Interest statement
The authors declare no conflicts of interest.
References
- Andersson MB, 1994. Sexual Selection. Princeton (NJ: ): Princeton University Press.[TQ1][TQ2] [Google Scholar]
- Arntzen JW, Jehle R, Bardakci F, Burke T, Wallis GP, 2009. Asymmetric viability of reciprocal-cross hybrids between crested and marbled newts (Triturus cristatus and T. marmoratus). Evolution 63:1191–1202. [DOI] [PubMed] [Google Scholar]
- Berdan EL, Fuller RC, 2012. A test for environmental effects on behavioral isolation in two species of killifish. Evolution 66:3224–3237. [DOI] [PubMed] [Google Scholar]
- Beysard M, Krebs-Wheaton R, Heckel G, 2015. Tracing reinforcement through asymmetrical partner preference in the European common vole Microtus arvalis. BMC Evol Biol 15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin R, 1987. Speciation by reinforcement. Trends Ecol Evol 2:8–13. [DOI] [PubMed] [Google Scholar]
- Butlin RK, Ritchie MG, 1991. Variation in female mate preference across a grasshopper hybrid zone. J Evol Biol 4:227–240. [Google Scholar]
- Clutton-Brock T, 2007. Sexual selection in males and females. Science 318:1882–1885. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, 2009. Sexual selection in females. Anim Behav 77:3–11. [Google Scholar]
- Clutton-Brock TH, Parker GA, 1992. Potential reproductive rates and the operation of sexual selection. Q Rev Biol 67:437–456. [Google Scholar]
- Cooley JR, 2007. Decoding asymmetries in reproductive character displacement. Proc Acad Nat Sci Philadelphia 156:89–96. [Google Scholar]
- Coughlan JM, Willis JH, 2018. Parallel patterns of development between independent cases of hybrid seed inviability in Mimulus. bioRxiv 458752.
- Coyne J, Crittenden A, Mah K, 1994. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265:1461–1464. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Elwyn S, Rolán-Alvarez E, 2005. Impact of experimental design on drosophila sexual isolation studies: direct effects and comparison to field hybridization data. Evolution 59:2588–2601. [PubMed] [Google Scholar]
- Coyne JA, Orr HA, 2004. Speciation. Sunderland (MA: ): Sinauer Associates. [Google Scholar]
- Crow KD, Munehara H, Kanamoto Z, Balanov A, Antonenko D. et al. , 2007. Maintenance of species boundaries despite rampant hybridization between three species of reef fishes (Hexagrammidae): implications for the role of selection. Biol J Linn Soc 91:135–147. [Google Scholar]
- Debelle A, Ritchie MG, Snook RR, 2014. Evolution of divergent female mating preference in response to experimental sexual selection. Evolution 68:2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Shuker DM, 2014. Precopulatory sexual selection in the seed bug Lygaeus equestris: a comparison of choice and no-choice paradigms. Anim Behav 89:207–214. [Google Scholar]
- Dougherty LR, Shuker DM, 2015. The effect of experimental design on the measurement of mate choice: a meta-analysis. Behav Ecol 26:311–319. [Google Scholar]
- Duggins CF, Karlin AA, Relyea KG, 1983. Electrophoretic variation in the killifish genus Lucania. Copeia 1983:564. [Google Scholar]
- Dyer KA, White BE, Sztepanacz JL, Bewick ER, Rundle HD, 2014. Reproductive character displacement of epicuticular compounds and their contribution to mate choice in Drosophila subquinaria and Drosophila recens. Evolution 68:1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinedo CM, Gabor CR, Aspbury AS, 2010. Males, but not females, contribute to sexual isolation between two sympatric species of Gambusia. Evol Ecol 24:865–878. [Google Scholar]
- Fuller RC, 2002. Lighting environment predicts the relative abundance of male colour morphs in bluefin killifish (Lucania goodei) populations. Proc R Soc B Biol Sci 269:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RC, 2008. Genetic incompatibilities in killifish and the role of environment. Evolution 62:3056–3068. [DOI] [PubMed] [Google Scholar]
- Fuller RC, McGhee KE, Schrader M, 2007. Speciation in killifish and the role of salt tolerance. J Evol Biol 20:1962–1975. [DOI] [PubMed] [Google Scholar]
- Fuller RC, Noa LA, 2008. Distribution and stability of sympatric populations of Lucania goodei and L. parva across Florida. Copeia 2008:699–707. [Google Scholar]
- Fuller RC, Noa LA, 2010. Female mating preferences, lighting environment, and a test of the sensory bias hypothesis in the bluefin killifish. Anim Behav 80:23–35. [Google Scholar]
- Fuller RC, , Noa LA, , Strellner RS, 2010. Teasing apart the many effects of lighting environment on opsin expression and foraging preference in bluefin killifish. Am Nat 176:1–13. [DOI] [PubMed] [Google Scholar]
- Ghedotti MJ, Davis MP, 2017. The taxonomic placement of three fossil Fundulus species and the timing of divergence within the North American topminnows (Teleostei: Fundulidae). Zootaxa 4250:577–586. [DOI] [PubMed] [Google Scholar]
- Gregorio O, Berdan EL, Kozak GM, Fuller RC, 2012. Reinforcement of male mate preferences in sympatric killifish species Lucania goodei and Lucania parva. Behav Ecol Sociobiol 66:1429–1436. [Google Scholar]
- Hayward A, Gillooly JF, 2011. The cost of sex: quantifying energetic investment in gamete production by males and females. PLoS ONE 6:e16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochkirch A, Lemke I, 2011. Asymmetric mate choice, hybridization, and hybrid fitness in two sympatric grasshopper species. Behav Ecol Sociobiol 65:1637–1645. [Google Scholar]
- Hoikkala A, Aspi J, 1993. Criteria of female mate choice in Drosophila littoralis, D. montana, and D. ezoana. Evolution 47:768–777. [DOI] [PubMed] [Google Scholar]
- Hubbs CL, 1955. Hybridization between fish species in nature. Syst Biol 4:1–20. [Google Scholar]
- Hudson EJ, Price TD, 2014. Pervasive reinforcement and the role of sexual selection in biological speciation. J Hered 105:821–833. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M, 2007. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev 72:283–327. [DOI] [PubMed] [Google Scholar]
- Kelly JK, Noor MA, 1996. Speciation by reinforcement: a model derived from studies of Drosophila. Genetics 143:1485–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J, 1990. Nonspecific imprintability of quail to colors: response to artificial selection. Behav Genet 20:91–96. [DOI] [PubMed] [Google Scholar]
- Kozak GM, Reisland M, Boughmann JW, 2009. Sex differences in mate recognition and conspecific preference in species with mutual mate choice. Evolution 63:353–365. [DOI] [PubMed] [Google Scholar]
- Kozak GM, Roland G, Rankhorn C, Falater A, Berdan EL. et al. , 2015. Behavioral isolation due to cascade reinforcement in Lucania killifish. Am Nat 185:491–506. [DOI] [PubMed] [Google Scholar]
- Kvarnemo C, Ahnesjo I, 1996. The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408. [DOI] [PubMed] [Google Scholar]
- Langerhans RB, Riesch R, 2013. Speciation by selection: a framework for understanding ecology’s role in speciation. Curr Zool 59:31–52. [Google Scholar]
- Liou LW, Price TD, 1994. Speciation by reinforcement of premating isolation. Evolution 48:1451. [DOI] [PubMed] [Google Scholar]
- Lipshutz SE, 2018. Interspecific competition, hybridization, and reproductive isolation in secondary contact: missing perspectives on males and females. Curr Zool 64:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MD, Mendelson TC, 2013. Incomplete behavioural isolation and asymmetric female preference in darter sister species (Percidae: Etheostoma). J Fish Biol 83:1371–1380. [DOI] [PubMed] [Google Scholar]
- Mayr E, 1942. Systematics and the Origin of Species: From the Viewpoint of a Zoologist. Cambridge (MA: ): Harvard University Press. [Google Scholar]
- Mitchem LD, Stanis S, Sutton NM, Turner Z, Fuller RC, 2018. The pervasive effects of lighting environments on sensory drive in bluefin killifish: an investigation into male/male competition, female choice, and predation. Curr Zool 64:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RL, Fuller RC, 2018. Male-driven reproductive and agonistic character displacement in darters and its implications for speciation in allopatry. Curr Zool 64:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran RL, Zhou M, Catchen JM, Fuller RC, 2017. Male and female contributions to behavioral isolation in darters as a function of genetic distance and color distance. Evolution 71:2428–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor MAF, 1999. Reinforcement and other consequences of sympatry. Heredity 83:503–508. [DOI] [PubMed] [Google Scholar]
- Peterson MA, Honchak BM, Locke SE, Beeman TE, Mendoza J. et al. , 2005. Relative abundance and the species-specific reinforcement of male mating preference in the Chrysochus (Coleoptera: Chrysomelidae) hybrid zone. Evolution 59:2639–2655. [PubMed] [Google Scholar]
- Pfennig KS, Simovich MA, 2002. Differential selection to avoid hybridization in two toad species. Evolution 56:1840–1848. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Bradshaw HD, Schemske DW, 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57:1520–1534. [DOI] [PubMed] [Google Scholar]
- Ridley M, 2004. Evolution. Oxford: Blackwell Publishing. [Google Scholar]
- Rundle HD, Dyer KA, 2015. Reproductive character displacement of female mate preferences for male cuticular hydrocarbons in Drosophila subquinaria. Evolution 69:2625–2637. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Schluter D, 1998. Reinforcement of stickleback mate preferences: sympatry breeds contempt. Evolution 52:200–208. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Taylor RC, 2015. Measures of mate choice: a comment on Dougherty & Shuker. Behav Ecol 26:323–324. [Google Scholar]
- Sánchez-Guillén RA, Wullenreuther M, Cordero Rivera A, 2012. Strong asymmetry in the relative strengths of prezygotic and postzygotic barriers between two damselfly sister species. Evolution 66:690–707. [DOI] [PubMed] [Google Scholar]
- Schöfl G, Dill A, Groot AT, 2011. Allochronic separation versus mate choice: nonrandom patterns of mating between fall armywormhost strains. Am Nat 177:470–485. [DOI] [PubMed] [Google Scholar]
- Servedio MR, 2007. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution 61:2772–2789. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Noor MAF, 2003. The role of reinforcement in speciation: theory and data. Annu Rev Ecol Evol Syst 34:339–364. [Google Scholar]
- Servedio MR, Van Doorn GS, Kopp M, Frame AM, Nosil P, 2011. Magic traits in speciation: “magic” but not rare? Trends Ecol Evol 26:389–397. [DOI] [PubMed] [Google Scholar]
- Shapiro LH, 2006. Asymmetric assortative mating between two hybridizing Orchelimum katydids (Orthoptera: Tettigoniidae). Am Midl Nat 145:423–427. [Google Scholar]
- Shipley RJ, Campbell P, Searle JB, Pasch B, 2016. Asymmetric energetic costs in reciprocal-cross hybrids between carnivorous mice (Onychomys). J Exp Biol 219:3803–3809. [DOI] [PubMed] [Google Scholar]
- Shuster SM, Wade MJ, 2003. Mating Systems and Strategies. Vol. 61. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Smadja C, Ganem G, 2005. Asymmetrical reproductive character displacement in the house mouse. J Evol Biol 18:1485–1493. [DOI] [PubMed] [Google Scholar]
- Sobel JM, Chen GF, 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68:1511–1522. [DOI] [PubMed] [Google Scholar]
- St. John ME, Fuller RC, 2019. The effects of experimental design on mating preferences and reproductive isolation in killifish. Behav Ecol 30:92–100. [Google Scholar]
- Stalker HD, 1942. Sexual isolation studies in the species complex Drosophila virilis. Genetics 27:238–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Beckers L, Cocroft RB, 2010. The importance of female choice, male-male competition, and signal transmission as causes of selection on male mating signals. Evolution 64:3158–3171. [DOI] [PubMed] [Google Scholar]
- Tiffin P, Olson MS, Moyle LC, 2001. Asymmetrical crossing barriers in angiosperms. Proc Biol Sci 268:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluijs I, Van Dooren TJM, Seehausen O, Van Alphen JJM, 2008. A test of fitness consequences of hybridization in sibling species of Lake Victoria cichlid fish. J Evol Biol 21:480–491. [DOI] [PubMed] [Google Scholar]
- Veen T, Borge T, Griffith SC, Saetre GP, Bures S. et al. , 2001. Hybridization and adaptive mate choice in flycatchers. Nature 411:45–50. [DOI] [PubMed] [Google Scholar]
- Wagner WE, 1998. Measuring female mating preferences. Anim Behav 55:1029–1042. [DOI] [PubMed] [Google Scholar]
- West RJD, Kodric-Brown A, 2015. Mate choice by both sexes maintains reproductive isolation in a species flock of pupfish (Cyprinodon spp) in the Bahamas. Ethology 121:793–800. [Google Scholar]
- Wheatcroft D, Qvarnström A, 2017. Reproductive character displacement of female, but not male song discrimination in an avian hybrid zone. Evolution 71:1776–1786. [DOI] [PubMed] [Google Scholar]
- Whitehead A, 2010. The evolutionary radiation of diverse osmotolerant physiologies in killifish (Fundulus sp.). Evolution 64:2070–2085. [DOI] [PubMed] [Google Scholar]
- Yukilevich R, 2012. Asymmetrical patterns of speciation uniquely support reinforcement in Drosophila. Evolution 66:1430–1446. [DOI] [PubMed] [Google Scholar]
- Yukilevich R, Peterson EK, 2019. The evolution of male and female mating preferences in Drosophila speciation. Evolution 73:1759–1773. [DOI] [PubMed] [Google Scholar]