Abstract
Individuals which have invaded urbanized environments are reported to engage in riskier behaviors, possibly influenced by the scarcity of predators in urbanized areas. Here, we studied the risk-taking behavior of birds which had invaded a new natural environment, rather than an artificial urban environment, using recently established populations of the bull-headed shrike Lanius bucephalus, which naturally colonized three subtropical islands in Japan. We compared flight initiation distance (FID), the distance at which an individual approached by a human initiates flight, between the islands and the temperate mainland. FID was longer for the insular shrikes compared with the mainland shrikes after controlling for other factors, indicating that the individuals which had invaded a new natural environment had a lower propensity for risk-taking. A possible explanation for these results is that low risk-taking behavior might be adaptive on the islands due to predation by the black rat Rattus rattus, an unfamiliar predator not found in shrike habitats on the temperate mainland. Further studies are needed to examine the nest predation rate, predator species, and nest site selection of these insular shrike populations.
Keywords: flight initiation distance, island population, Lanius bucephalus, natural introduction, predation
Risk-taking behavior is related to predator avoidance (Quinn and Cresswell 2005; Réale et al. 2007; Herborn et al. 2010). Risk-taking individuals are prone to suffer predation (Møller et al. 2008; Møller 2014), and risk-taking behavior changes in response to factors associated with predation risk, such as the existence of predators (Møller et al. 2017; Lapiedra et al. 2018) and the availability of hiding places (Martín and López 2000; Walther and Gosler 2001). Individuals which have invaded urbanized environments are reported to engage in riskier behaviors. In the common myna Acridotheres tristis, for example, individuals in highly urbanized environments return to feeding spots sooner than individuals in suburbs after encountering novel objects (Sol et al. 2011). In 44 species of European birds, individuals that had invaded urban environments less readily flee from a human than do conspecific individuals in rural habitats (Møller 2008). A meta-analysis of 180 bird species has also shown greater tolerance to human approach in urbanized birds than in rural or suburban populations (Samia et al. 2015). These studies suggest that pioneers in urban environments tend to take greater risks. Given that risk-taking behavior is related to vulnerability to predation, the risk-taking behavior of the birds in urbanized areas might have been influenced by the scarcity of predators in those areas (Shochat et al. 2006; Møller 2008). Thus, risk-taking behavior is probably related to the establishment of new populations in new environments. However, there has been little study of the risk-taking behaviors of animals which invade new natural environments rather than artificial urban environments.
The bull-headed shrike Lanius bucephalus has recently spread to some Japanese islands, making this species a suitable target for studying the risk-taking behavior of individuals which have invaded new natural environments and established new populations. The bull-headed shrike is, with rare exceptions, not distributed in the Ryukyu Archipelago (Nansei Islands) in southern Japan but has naturally colonized three small islands between the 1970s and 2000s (see Materials and Methods section for details). Using these recently established populations, we investigated risk-taking behavior. Flight initiation distance (FID), the distance at which an individual approached by a human initiates flight, is commonly used as a comparative metric of risk-taking behavior (Blumstein 2003, 2006). FID is known to increase with increasing predation risk (Cooper and Frederick 2007; Cooper 2009) and is correlated with susceptibility to predation even when measured for an approaching researcher rather than an actual predator (Díaz et al. 2013; see also Møller et al. 2010). Thus, FID is a good indicator of risk-taking behavior.
Here, we analyze the risk-taking behavior of bird populations that recently naturally colonized islands in comparison with mainland populations. We hypothesize that bull-headed shrikes on the islands take greater risks than those on the mainland, and we predict that FID is shorter for these insular shrikes than for the mainland shrikes.
Materials and Methods
Study Sites and Species
The bull-headed shrike is a medium-sized (34–52 g), open-nesting passerine, which breeds in subarctic and temperate zones in East Asia (Yosef 2008). Its habitat consists of open grounds (e.g., farmland, suburban parks) accompanied by scattered shrubs and forest edges (Yosef 2008). Bull-headed shrikes prey on arthropods and small vertebrates wandering on the ground, and use bushes and trees for nesting sites (Nakamura and Nakamura 1995). Most shrike populations are resident, but shrikes in northern Japan, in northern China, and on plateaus migrate to wintering grounds in temperate and subtropical zones (Nakamura and Nakamura 1995; Yosef 2008).
In the Ryukyu Archipelago (Nansei Islands), an approximately 1,000-km chain of islands in southern Japan, bull-headed shrikes are generally winter visitors and are not breeding (The Ornithological Society of Japan 2012), although there are exceptions. They have naturally settled and have been breeding on Minami- and Kita-daitojima in the Daito Islands since the 1970s (Takagi 2009). On Kikaijima in the Amami Islands and Nakanoshima in the Tokara Islands, breeding attempts of the shrikes were first confirmed in 2012 (Ijichi et al. 2013) and 1989 (Morioka 1990), respectively. We studied these recently established breeding populations on Minami-daitojima, Kikaijima, and Nakanoshima.
Kikaijima (28°19′N, 130°00′E; 56.8 km2) is located in the Central Ryukyus, 297 km south of Kyushu, which is one of the four main islands of Japan. Minami-daitojima (25°50′N, 131°14′E, 30.6 km2) is located 290 km southeast of Kikaijima. A large proportion of these islands is occupied by agricultural fields, which are mainly used to grow sugarcane (Saccharum spp.). Nakanoshima (29°50′N, 129°52′E; 34.5 km2) is located 160 km north of Kikaijima. Evergreen forest covers a large part of the island, and there is some farmland and pastureland, which are the habitat of the shrikes.
Potential predators of shrikes on these islands are rats Rattus spp. and the Japanese weasel Mustela itatsi (Tobai 1994; Sakagami et al. 2011; Matsui and Takagi 2012), both of which were artificially introduced. The feral cat Felis catus and the large-billed crow Corvus macrorhynchos are also among the potential predators, but the crows are extinct on Minami-daitojima (Takagi 2009). No snakes inhabit these islands.
To compare risk-taking behaviors on these islands with those on the mainland, we also studied bull-headed shrikes on three of the main islands of Japan, namely Kyushu, Honshu, and Hokkaido. The mainland study sites were Kanoya and surrounding areas (31°18′–31°30′N, 130°54′–131°00′E) in Kagoshima Prefecture, southern Kyushu; Tsukuba and Tsuchiura (36°03′–36°10′N, 140°06′–140°09′E) in Ibaraki Prefecture, central Honshu; and Teshikaga and surrounding areas (43°28′–43°56′N, 144°15′–144°39′E) in the Kushiro and Nemuro areas, eastern Hokkaido. These areas have open farmland, grasslands, and forests with scattered houses. Snakes (e.g., the Japanese rat snake Elaphe climacophora), Japanese weasels, and feral cats are known predators of shrikes on Honshu (Yamagishi 1981). In addition, Takagi and Abe (1996) assumed that the red fox Vulper vulpes, weasels Mustela spp., and rats are potential nest predators in Hokkaido.
Flight Initiation Distance
We used FID to compare the risk-taking behavior of bull-headed shrikes between the islands and the mainland. FID is defined as the distance at which an individual approached by a human initiates flight and was measured using the following standard technique (Blumstein 2006; Møller 2008; Garamszegi et al. 2009). When we found a bull-headed shrike resting or searching for food from a perch (e.g., the tops of tree, pole sprinklers, and utility wires), one observer walked toward the individual slowly (ca. 0.5 m/s). The horizontal distance at which the bird took flight was measured using a laser range finder (TruPulse 200, Laser Technology, Centennial, CO, USA) with accuracy of 0.1 m. We also recorded the distance from which we started walking toward the bird (starting distance). Sex of the individual was also recorded because previous studies have suggested that it may influence FID (Guay et al. 2013). Height above ground was recorded in 0.5-m intervals (measured by eye). FID was calculated as the square root of the sum of the squared horizontal distance and the squared height (Blumstein 2006; Møller 2008).
Data were collected from 56 individuals on the islands (25 on Kikaijima, 25 on Minami-daitojima, and 6 on Nakanoshima) and 66 individuals on the mainland (25 in Kyusyu, 31 in Honshu, and 10 in Hokkaido). Field studies were performed during 3 or 4 days at each site between 25 March and 27 June 2019 under sunny or cloudy conditions, but not rainy or windy conditions. The breeding season of the shrikes starts in February and continues until July (Nakamura and Nakamura 1995; Takagi 2006). The periods of field work at each study site corresponded to the mid- to late breeding season. Therefore, wintering visitors were not sampled in this study. Some shrikes were observed carrying food items to nestlings and fledglings, but we did not use these birds for the measurements to avoid the effects of their defense behavior on FID. We used measurements for adults only. To avoid duplicate sampling of the same individuals, we recorded one individual at a given site. The minimum distance between sampling sites was 200 m, which is enough to encounter new individuals because the territory size (length) of this species is approximately 200 m on the main islands of Japan (Yamagishi 1981; Takagi 2003). Exceptions were that we used two individuals <200 m apart when one was male and one was female and when two birds were observed at the same time.
Two male observers (Nakanoshima: H.H.; other study sites: S.H.) of similar stature and age collected the data after thorough consultation. In a previous study, two observers collected measurement data in a similar way (Fernández-Juricic et al. 2002), since FID can be assessed with high consistency by different observers (Møller et al. 2008).
Statistical analyses
First, we simply investigated the Pearson’s correlation between FID and starting distance in the insular and mainland shrikes, as previous studies have reported that starting distance has an effect on FID (Blumstein 2003, 2006; Rodriguez-Prieto et al. 2009). Next, to assess the influence of location (island/mainland) on FID, we constructed generalized linear mixed models (GLMMs) because factors other than the location were expected to affect FID. We applied an identity link function and Gaussian error distribution and used study site (Kikaijima, Minami-daitojima, Nakanoshima, Kyushu, Honshu, and Hokkaido) as a random factor. The following explanatory variables were included in the models: location, human population density, sex, starting distance, and height above the ground. Since starting distance affected FID (see Results section), and the interaction between starting distance and other factors can have an effect on FID (Blumstein 2003; Dumont et al. 2012), we included an interaction term between starting distance and location in the models. Human population density (per square kilometer) was calculated from the population and area of the municipality in which each study site was located (Government of Japan 2015). We used a model selection approach based on Akaike’s information criteria corrected for small sample size (AICc) to evaluate all possible models. The model with the lowest AICc value is the best model, and models with ΔAICc <2 are considered equally good as the model with the lowest AICc (Burnham and Anderson 2002). After model selection according to this procedure, we used likelihood ratio tests to assess the significance of the explanatory variables included in the models. We conducted the GLMM analysis using R software (version 3.6.1; R Core Team 2019). In all statistical tests, a P<0.05 was considered statistically significant.
Results
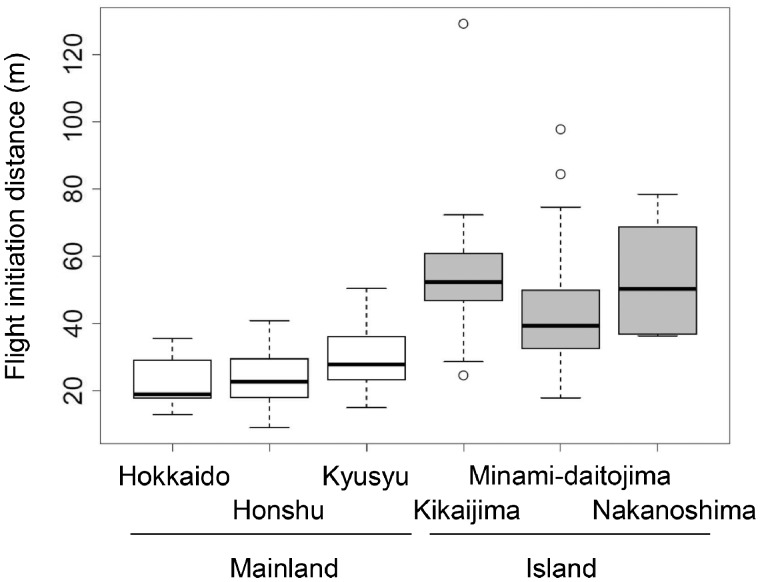
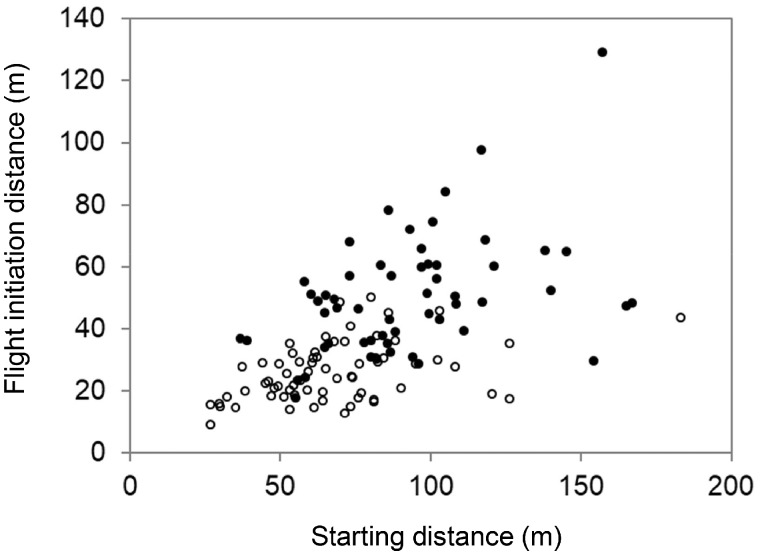
In the field studies, we observed 56 individuals on the islands and 66 individuals on the mainland. The proportion of males was not significantly different between the locations (island: 13/56; mainland: 8/66; Fisher’s exact test, P = 0.15). Height above the ground was also not significantly different between the locations (island: mean [range] = 5.9 m [2–12 m]; mainland: 5.4 m [1–15 m]; Welch’s t-test, t117.4 = 0.78, P = 0.44). Starting distance was longer on the islands (mean [range] = 93.0 m [36.7–167.0 m]) than on the mainland (67.8 m [26.7–183.0 m]) (t111.5 = 4.85, P <0.001). FID was also longer on the islands (mean [range] = 50.6 m [17.9–129.0 m]) than on the mainland (26.1 m [9.1–50.4 m]) (t76.7 = 8.69, P < 0.001; Figure 1). In both the insular and mainland populations, FID was positively correlated with starting distance (insular: Pearson correlation r54 = 0.42, P <0.01; mainland: r64 = 0.42, P < 0.001; Figure 2).
Figure 1.
Flight initiation distances of bull-headed shrikes on islands and mainland. Box plots show the 25–75th percentiles (boxes), medians (thick lines within boxes), and ±1.5 interquartile ranges (whiskers). Each open circle indicates an outlier.
Figure 2.
Relationship between flight initiation distance and starting distance of bull-headed shrikes. Closed and open circles indicate data obtained from insular and mainland shrikes, respectively.
In model selection, location (island/mainland) was included as an explanatory variable in all of the top 10 GLMMs (Table 1). Location, sex, and starting distance were predictors of FID in the best model. The Akaike weight of this model was high (wi = 0.404). The second-best model included the interaction between location and starting distance in addition to the explanatory variables in the best model. The difference in AICc between the two models was less than 2 (ΔAICc = 1.87), which indicates a high relative likelihood of these models. In the second-best models, location, sex, and starting distance had significant effects on FID. The effect of the interaction between location and starting distance was not significant (Table 2).
Table 1.
Predictors of flight initiation distance of the bull-headed shrike according to generalized linear mixed models
| Location | Density | Sex | SD | Height | Location: SD | AICc | ΔAICc | wi |
|---|---|---|---|---|---|---|---|---|
| + | + | 0.223 | 982.46 | 0.00 | 0.404 | |||
| + | + | 0.301 | + | 984.33 | 1.87 | 0.158 | ||
| + | 0.221 | 984.60 | 2.14 | 0.139 | ||||
| + | + | 0.222 | 0.054 | 984.78 | 2.33 | 0.126 | ||
| + | 0.229 | + | 986.45 | 3.99 | 0.055 | |||
| + | + | 0.301 | 0.024 | + | 986.72 | 4.27 | 0.048 | |
| + | 0.221 | 0.049 | 986.90 | 4.44 | 0.044 | |||
| + | 0.299 | 0.018 | + | 988.82 | 6.36 | 0.017 | ||
| + | 0.004 | + | 0.227 | 992.25 | 9.80 | 0.003 | ||
| + | 0.002 | + | 0.306 | + | 994.05 | 11.59 | 0.001 |
Explanatory variables were location, human population density, sex, starting distance (SD), and height from the ground. Interaction between location and SD was included. Difference in AICc between the best model and the top 10 models (ΔAICc) and Akaike weight (wi) are shown. Location = island or mainland.
Table 2.
Estimates, standard errors, and results of likelihood-ratio tests of factors in the well-fitted generalized linear mixed model for explaining variation in flight initiation distance in the bull-headed shrike
| Factor | Estimate | SE | χ21 | P-value |
|---|---|---|---|---|
| Intercept | 25.081 | 6.723 | ||
| Location (mainland) | −7.465 | 8.577 | 14.04 | < 0.001 |
| Sex (male) | −1.370 | 3.262 | 4.35 | 0.037 |
| SD | 0.301 | 0.060 | 18.14 | < 0.001 |
| Location: SD | −0.166 | 0.089 | 0.38 | 0.54 |
Location: estimate relative to “island”; sex: estimate relative to “female”. SD: starting distance.
Discussion
Contrary to our prediction, bull-headed shrikes had longer FID in the island populations compared with the mainland populations. Although there are reported cases where the interaction between starting distance and other factors affects FID (Blumstein 2003; Dumont et al. 2012), our results showed that the interaction between starting distance and location (island/mainland) did not have significant effect on FID. Therefore, we conclude that FID of the shrikes was longer on the island than on the mainland after controlling for other factors.
The result of model selection showed that human population density was not an effective predictor of FID. Some previous studies have shown that birds in areas with a high human population density become less sensitive to human approach because they learn that humans are not a great threat (Eason et al. 2006; Clucas and Marzluff 2012). However, we found that human population density did not affect FID, suggesting that the shorter FID of the mainland shrikes was not influenced by habituation to humans.
It is unclear what factors account for the difference in FID between the islands and the mainland. A possible explanation is that low risk-taking behavior is adaptive on the islands due to a local predator, the artificially introduced black rat Rattus rattus. On the subtropical Nansei Islands, black rats inhabit and often nest in bushes, which are the nesting site of the shrikes (Matsui et al. 2010; Sakagami et al. 2011). In contrast, in temperate mainland Japan, the rats live only near human settlements, not in shrubs (Hamao et al. 2009). On the Nansei Islands, some of the predation of shrike nests was suspected to be by rats (Matsui and Takagi 2012). As risk-taking behavior is related to predator avoidance (Quinn and Cresswell 2005; Réale et al. 2007; Herborn et al. 2010), nest predation by rats might explain the lower propensity of the insular shrikes for risk-taking.
A previous study by Møller et al. (2015) has shown that bird species which successfully established new populations on islands tend to have a higher breeding density in urban habitats than in rural habitats in their ancestral range. Because urbanized birds generally show high risk-taking behavior (Møller 2008; Samia et al. 2015), their results suggest that individuals taking higher risk might be more successful in settling on islands. In contrast, our study found lower risk-taking behavior on islands than on the mainland. A possible explanation for this difference is that Møller et al. (2015) focused on species that humans had intentionally introduced, which would likely make the ability to tolerate proximity to humans an important factor in successful settlement. In the naturally colonized populations of shrikes on the Nansei Islands, on the other hand, such an ability may not be related to settlement on the islands, and high risk-taking seems to confer no advantage in the invasion. Cooper et al. (2014) reported that escape distance (FID) is shorter in insular lizards than in mainland lizards, which is not consistent with our finding. They suggested that the shorter FID of insular lizards was attributable to the scarcity or absence of predators on the islands. On islands where predators are scarce or absent, less risk-sensitive behavior and shorter FID can be expected. However, if predation is more intense on islands than on the mainland, insular animals would come to take lower risk and have longer FID compared with those on the mainland. Our finding might demonstrate this possibility.
This study leads to the understanding that an uneven geographical pattern of risk-taking behavior is generated in the distribution range of the bull-headed shrike. Although individual animals exhibit behavioral plasticity in response to their environment (Piersma and Drent 2003; Thomson et al. 2012), behavioral traits are at least partly heritable (Fidler et al. 2007; Dochtermann et al. 2015). In particular, FID can be affected by factors such as starting distance (Blumstein 2003, 2006; Rodriguez-Prieto et al. 2009; present study) and availability of refuge from predators (Engelhardt and Weladji 2011; Guay et al. 2013), suggesting that the escape behavior of individuals changes in response to the situation. On the other hand, a substantial proportion of variation in FID can be explained by heritability (Møller 2014; Carrete et al. 2016; Sprau and Dingemanse 2017). Therefore, the low propensity for risk-taking of the insular shrikes is expected to be heritable to some extent. If the risk-taking behavior of the shrikes has a genetic basis, then the geographical pattern of the behavior would be sustained by the uneven distribution of genetic diversity (Canestrelli et al. 2016).
A local unfamiliar predator was a possible factor contributing to the low risk-taking behavior of the insular shrikes. In this case, the behavioral traits of the invading populations would be shaped by selection in the new environment. Behavioral traits, such as boldness/shyness, exploration, and aggressiveness, affect the success of introduction into a new environment across various stages of the process (Chapple et al. 2012). In the initial stage, individuals with boldness and exploratory propensity, which are related to risk-taking propensity, are more likely to become founders of the new population (Canestrelli et al. 2016) because they tend to disperse over long distances (Cote et al. 2013). In the shrikes on the Nansei Islands, low risk-taking individuals were assumed to respond adaptively to an unfamiliar predator. If so, this would be an example of selection acting through survival and reproduction in the later establishment stage in introduction process, leading to the uneven geographic pattern of behavioral traits.
To confirm this hypothesis, further studies are needed to examine the nest predation rates, predator species and nest site selection of insular shrike populations. Based on the hypothesis, black rats are expected to be an important factor of shrikes’ nesting failure, and risk-sensitive shrikes would prefer to safer nest sites. It would be also useful to identify the source population of the insular shrikes using molecular analysis to confirm the effects of dispersal.
Acknowledgments
We thank Sachiko Endo for her advice and help in the field and Masaoki Takagi, Shin Matsui, and Mutsuyuki Ueta for providing information about the shrikes in the Ryukyu Archipelago and Kyushu. We are also indebted to Toshitaka Suzuki and Craig Barnett for their comments on an earlier version of the manuscript and to John Zepernick for language editing. Thanks are also due to the editors and two anonymous reviewers for their constructive comments. Kikai Town Office provided facilities for the study. All data collection in this study complied with the current laws of Japan. Permission was not required as we neither captured the birds nor conducted invasive sampling.
Funding
This study was supported by the National Museum of Nature and Science, Tokyo (Nos 20183001 and 20193001 to S.H.).
Authors’ contributions
S.H., Y.Y., H.T., and T.I. designed the study. S.H. and H.T. collected the data. S.H. conducted statistical analyses. All contributed to writing the manuscript and have given final approval for publication.
Conflict of interest Statement
The authors declare that they have no conflict of interest.
References
- Blumstein DT, 2003. Flight-initiation distance in birds is dependent on intruder starting distance. J Wildl Manage 67:852–857. [Google Scholar]
- Blumstein DT, 2006. Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399. [Google Scholar]
- Burnham KP, Anderson DR, 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd edn. New York: Springer. [Google Scholar]
- Canestrelli D, Bisconti R, Carere C, 2016. Bolder takes all? The behavioral dimension of biogeography. Trends Ecol Evol 31:35–43. [DOI] [PubMed] [Google Scholar]
- Carrete M, Martínez-Padilla J, Rodríguez-Martínez S, Rebolo-Ifrán N, Palma A. et al. , 2016. Heritability of fear of humans in urban and rural populations of a bird species. Sci Rep 6:31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple DG, Simmonds SM, Wong BBM, 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64. [DOI] [PubMed] [Google Scholar]
- Clucas B, Marzluff JM, 2012. Attitudes and actions toward birds in urban areas: human cultural differences influence bird behavior. Auk 129:8–16. [Google Scholar]
- Cooper WE Jr, 2009. Optimal escape theory predicts escape behaviors beyond flight initiation distance: risk assessment and escape by striped plateau lizards Sceloporus virgatus. Curr Zool 55:123–131. [Google Scholar]
- Cooper WE Jr, Frederick WG, 2007. Optimal flight initiation distance. J Theor Biol 244:59–67. [DOI] [PubMed] [Google Scholar]
- Cooper WE Jr, Pyron RA, Garland T Jr , 2014. Island tameness: living on islands reduces flight initiation distance. Proc R Soc B 281:20133019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Fogarty S, Tymen B, Sih A, Brodin T, 2013. Personality-dependent dispersal cancelled under predation risk. Proc R Soc B 280:20132349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M, Møller AP, Flensted-Jensen E, Grim T, Ibáñez-Álamo JD. et al. , 2013. The geography of fear: a latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE 8:e64634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dochtermann NA, Schwab T, Sih A, 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc R Soc B 282:20142201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F, Pasquaretta C, Réale D, Bogliani G, Hardenberg A, 2012. Flight initiation distance and starting distance: biological effect or mathematical artefact? Ethology 118:1–12. [Google Scholar]
- Eason PK, Sherman PT, Rankin O, Coleman B, 2006. Affecting flight initiation distance in American robins. J Wildl Manage 70:1796–1800. [Google Scholar]
- Engelhardt SC, Weladji RB, 2011. Effects of levels of human exposure on flight initiation distance and distance to refuge in foraging eastern gray squirrels Sciurus carolinensis. Can J Zool 89:823–830. [Google Scholar]
- Fernández-Juricic E, Jimenez MD, Lucas E, 2002. Factors affecting intra- and inter-specific variations in the difference between alert distances and flight distances of birds in forested habitats. Can J Zool 80:1212–1220. [Google Scholar]
- Fidler AE, van Oers K, Drent PJ, Kuhn S, Meuller JC. et al. , 2007. Drd4 gene polymorphisms are associated with personality variation in a passerine bird. Proc R Soc B 274:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi LZ, Eens M, Török J, 2009. Behavioural syndromes and trappability in free-living collared flycatchers, Ficedula hypoleuca. Anim Behav 77:803–812. [Google Scholar]
- Government of Japan, 2015. Population census in 2015. e-Stat, Portal site of official statistics of Japan. Ministry of Internal Affairs and Communications, Government of Japan. <seurld>https://www.e-stat.go.jp/</seurld> (accessed 2019 Dec 10 ).
- Guay P-J, Lorenz RDA, Robinson RW, Symonds MRE, Weston MA, 2013. Distance from water, sex and approach direction influence flight distances among habituated black swans. Ethology 119:552–558. [Google Scholar]
- Hamao S, Nishimatsu K, Kamito T, 2009. Predation of bird nests by introduced Japanese weasel Mustela itatsi on an island. Ornithol Sci 8:139–146. [Google Scholar]
- Herborn KA, Macleod R, Miles WTS, Schofield ANB, Alexander L. et al. , 2010. Personality in captivity reflects personality in the wild. Anim Behav 79:835–843. [Google Scholar]
- Ijichi T, Torikai H, Hamao S, 2013. A breeding record of the Bull-headed Shrike on Kikai-jima Island in the Amami Islands group. Jpn J Ornithol 62:68–71. [Google Scholar]
- Lapiedra O, Schoener TW, Leal M, Losos JB, Kolbe JJ, 2018. Predator-driven natural selection on risk-taking behavior in anole lizards. Science 360:1017–1020. [DOI] [PubMed] [Google Scholar]
- Martín J, López P, 2000. Costs of refuge use affect escape decisions of Iberian rock lizards Lacerta monticola. Ethology 106:483–492. [Google Scholar]
- Matsui S, Takagi M, 2012. Predation risk of eggs and nestlings relative to nest-site characteristics of the Bull-headed Shrike Lanius bucephalus. Ibis 154:621–625. [Google Scholar]
- Matsui S, Hisaka M, Takagi M, 2010. Arboreal nesting and utilization of open-cup bird nests by introduced Ship Rats Rattus rattus on an oceanic island. Bird Conserv Int 20:34–42. [Google Scholar]
- Møller AP, 2008. Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol 63:63–75. [Google Scholar]
- Møller AP, 2014. Life history, predation and flight initiation distance in a migratory bird. J Evol Biol 27:1105–1113. [DOI] [PubMed] [Google Scholar]
- Møller AP, Díaz M, Flensted-Jensen E, Grim T, Ibáñez-Álamo JD. et al. , 2015. Urbanized birds have superior establishment success in novel environments. Oecologia 178:943–950. [DOI] [PubMed] [Google Scholar]
- Møller AP, Erritzøe J, Nielsen JT, 2010. Causes of interspecific variation in susceptibility to cat predation on birds. Chin Birds 1:1–15. [Google Scholar]
- Møller AP, Kwiecinski Z, Tryjanowski P, 2017. Prey reduce risk-taking and abundance in the proximity of predators. Curr Zool 63:591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Nielsen JT, Garamszegi LZ, 2008. Risk taking by singing males. Behav Ecol 19:41–53. [Google Scholar]
- Morioka H, 1990. Breeding birds of the Tokara Islands and their origin. Mem Natl Mus Nat Sci 23:151–166. [Google Scholar]
- Nakamura T, Nakamura M, 1995. Bird’s Life in Japan With Color Pictures: Birds of Mountain, Woodland and Field. Hoikusya, Osaka.
- Piersma T, Drent J, 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233. [Google Scholar]
- Quinn JL, Cresswell W, 2005. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour 142:1377–1402. [Google Scholar]
- R Core Team, 2019. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ, 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prieto I, Fernández-Juricic E, Martín J, Regis Y, 2009. Antipredator behavior in blackbirds: habituation complements risk allocation. Behav Ecol 20:371–377. [Google Scholar]
- Sakagami M, Hamao S, Mori Y, 2011. Predation of bird nests on Kikai Island, in the Amami Island group: differences in predation rates among nesting habitats and the identity of predators. Jpn J Ornithol 60:88–95. [Google Scholar]
- Samia D, Nakagawa S, Nomura F, Rangel TF, Blumstein DT, 2015. Increased tolerance to humans among disturbed wildlife. Nat Commun 6:8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat E, Warren PS, Faeth SH, Mclntyre NE, Hope D, 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. [DOI] [PubMed] [Google Scholar]
- Sol D, Griffin AS, Bartomeus I, Boyce H, 2011. Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS ONE 6:e19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprau P, Dingemanse NJ, 2017. An approach to distinguish between plasticity and non-random distributions of behavioral types along urban gradients in a wild passerine bird. Front Ecol Evol 5. doi: 10.3389/fevo.2017.00092. [Google Scholar]
- Takagi M, 2003. Philopatry and habitat selection in Bull-headed and Brown shrikes. J Field Ornithol 74:45–52. [Google Scholar]
- Takagi M, 2006. The Bull-headed Shrike. In: NPO Bird Research editor. Ecology of Japanese Birds. Tokyo: NPO Bird Research, 166–167. [Google Scholar]
- Takagi M, 2009. Avifauna of the Nansei Shoto (Southwest Islands), Japan, in relation to distances among islands. Jpn J Ornithol 58:1–17. [Google Scholar]
- Takagi M, Abe S, 1996. Seasonal change in nest site and nest success of Bull-headed Shrikes. Jpn J Ornithol 45:167–174. [Google Scholar]
- The Ornithological Society of Japan, 2012. Check-List of Japanese Birds. 7th Revised Edn. Sanda: The Ornithological Society of Japan. [Google Scholar]
- Thomson JS, Watts PC, Pottinger TG, Sneddon LU, 2012. Plasticity of boldness in rainbow trout Oncorhynchus mykiss: do hunger and predation influence risk-taking behaviour? Horm Behav 61:750–757. [DOI] [PubMed] [Google Scholar]
- Tobai S, 1994. The Tokara Islands, WWFNature Series 1. Tokyo: WWFJ. [Google Scholar]
- Walther BA, Gosler AG, 2001. The effects of food availability and distance to protective cover on the winter foraging behaviour of tits (Aves: Parus). Oecologia 129:312–320. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, 1981. The Bride of Bull-Headed Shrikes. Tokyo: Dainippon-tosho. [Google Scholar]
- Yosef R, 2008. Family Laniidae (Shrikes). In: del Hoyo J, Elliott A, Christie D, editors. Handbook of the Birds of the World. Vol. 13 Penduline-Tits to Shrikes. Barcelona: Lynx Edicions, 732–796. [Google Scholar]