Abstract
Background
Nocardial brain abscesses are rare, and published literature describing brain abscesses due to Nocardia species is limited to individual case reports or small series. We report one of the largest contemporary retrospective studies describing risk factors, diagnostic evaluation, management, and outcomes of nocardial brain abscess.
Methods
Retrospective review of all adults with brain abscess due to culture-confirmed Nocardia species at our institution between January 1, 2009, and June 30, 2020.
Results
Overall, 24 patients had nocardial brain abscesses during the study period. The median age at presentation was 64 years, and 62.5% were immunocompromised. Pulmonary and cutaneous infections were the most common primary sites of nocardial infection. All 24 patients had magnetic resonance imaging performed, and the frontal lobe was the most commonly involved. The most common organism isolated was Nocardia farcinica, followed by Nocardia wallacei and Nocardia cyriacigeorgica. Thirteen patients were managed with antimicrobial therapy alone, while 11 had both medical and surgical management. In all patients, dual therapy was recommended for the initial 6 weeks of treatment, and 22 patients received at least 1 oral agent as part of their final antibiotic regimen, predominantly trimethoprim-sulfamethoxazole and linezolid. Fourteen patients achieved complete clinical and radiographic resolution of infection.
Conclusions
Nocardia is an important cause of brain abscess in the immunocompromised host. Early diagnostic and therapeutic aspiration may help health care providers confirm the diagnosis, choose an appropriate antimicrobial regimen, and achieve source control.
Keywords: brain abscess, management, Nocardia, risk factors
Nocardia is an important cause of brain abscess in the immunocompromised host. Early diagnostic and therapeutic aspiration may help confirm the diagnosis, choose an appropriate antimicrobial regimen, and achieve source control to attain a clinical and radiographic resolution of infection.
Brain abscesses are rare, with a worldwide estimated incidence ranging from 0.3 to 1.3 per 100 000 persons per year [1]. However, they tend to occur with considerably higher frequency in immunocompromised patients. Nocardial brain abscesses are exceedingly uncommon and comprise only 2% of all intracranial abscesses [2]. Nocardia has a unique tropism for the brain [3]. Infection is usually acquired through inhalation. Central nervous system (CNS) involvement may occur via hematogenous spread or direct extension from a contiguous cranial infection site following head trauma [4, 5]. The spectrum of CNS infection ranges from diffuse cerebral infiltration, meningitis, spinal cord infection, to brain abscess.
Over the last 30 years, the introduction of new antibiotics and diagnostic procedures has considerably changed the management of brain abscesses. However, even with the advancement of imaging technologies and antimicrobial therapy, mortality rates for nocardial brain abscesses remain high, up to 30%, compared with 10% for other bacterial causes [6].
Published data on the Nocardia spp. causing brain abscesses are dated or limited to case reports or small case series that often lack details about diagnostic and management interventions and clinical outcomes data. Understanding the interplay between patient factors and laboratory and and radiologic findings of nocardial brain abscesses may improve the identification of individuals at risk. Therefore, we aimed to describe the clinical presentation, treatment, and outcomes of patients with Nocardia brain abscess in a contemporary cohort at a large referral center.
METHODS
We retrospectively reviewed all adult patients (≥18 years old) with an International Classification of Disesases, Ninth Revision and Tenth Revision, Clinical Modification diagnosis of brain abscess at our institution from January 1, 2009, through June 30, 2020. A brain abscess was defined as a localized intracerebral collection of necrotic material surrounded by a well-vascularized capsule associated with at least 1 of the following 3 characteristics: (a) positive blood cultures for Nocardia spp., (b) positive cultures for Nocardia spp. in brain abscess aspirate, (c) presence of Nocardia organisms on histopathology of the excised brain material.
Clinical specimens from blood and brain aspirate were cultured on-site in BD Bactec mycobacteria growth indicator tube (MGIT) 960 broth in mycobacterial growth indicator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and on Middlebrook 7H11/7H11S agar biplates incubated at 35°C to 37°C for up to 6 weeks. Positive MGIT broth was subcultured to a Middlebrook 7H11 agar plate, and isolated colony growth was identified using Sanger sequencing of a 500-bp region of the 16S rRNA gene. A 100% match to the database entry was required for identification. From August 2014, matrix-assisted laser desorption ionization time-of-flight mass spectrophotometry (MALDI-TOF MS) was added to supplement species identification using Sanger sequencing [7, 8].
Brain abscess cases were further categorized as health care–associated infections (HAIs) if brain abscess developed ≥48 hours after admission, was not present at the time of admission, and the patient was admitted for a cause other than Nocardia [9]. Immunocompromised patients were defined as solid organ or bone marrow transplant recipients, patients with hematologic or solid malignancy, and those currently on antineoplastic chemotherapy, immunomodulators, or other immunosuppressive drugs, including corticosteroids (≥5 mg/d for >14 days). Final antibiotic therapy was defined as an antibiotic regimen used for >50% of the total duration of therapy. Species were labeled susceptible if >80% of isolates tested were susceptible. Relapsed was defined as the association of clinical and radiological signs of nocardiosis with the isolation of the same Nocardia species after the cessation of antimicrobial treatment for nocardiosis. Clinical, laboratory, and radiographic data were extracted from the electronic health record. The study was approved by the Mayo Clinic Institutional Review Board (IRB# 20-000488).
Categorical variables were reported as frequencies and proportions. Continuous variables were reported as median (interquartile range [IQR]). Five-year mortality was reported with a Kaplan-Meier curve. Statistical analysis was performed using JMP, version 14.1.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic Characteristics
A total of 247 adult patients with brain abscess were screened, and 24 (9.7%) patients met the study criteria. The median age at presentation (IQR) was 64 (58.5–71.2) years, and 75% were males. The most common underlying medical conditions were chronic kidney disease (45.8%), hypertension (33.3%), and diabetes mellitus (29.1%). Fifteen (62.5%) patients were immunocompromised, including 3 patients (20%) with head and neck malignancy. Other malignancies included prostate cancer (20%), lung adenocarcinoma (13.3%), and lymphoma (13.3%). Two patients (8.3%) had head and neck surgery. Nine patients (37.5%) were on prednisone, with a median dose (IQR) of 10 (8.7–23) mg for >2 weeks. Seven (29.2%) patients were on other immunomodulatory therapies as summarized in Table 1. The median time from solid organ transplant (SOT) to diagnosis of Nocardia spp. brain abscess (IQR) was 876 (261–1698) days. A total of 4 patients were on trimethoprim-sulfamethoxazole (TMP-SMX) for Pneumocystis pneumonia prophylaxis. The median Charlson Comorbidity Index (CCI) score (IQR) was 7 (4.25–10).
Table 1.
Clinical Characteristics of Patients With Nocardial Brain Abscess
| Variables | Nocardial Brain Abscess Cases (n = 24) |
|---|---|
| Demographics, No. (%) | |
| Female | 6 (25) |
| Male | 18 (75) |
| Age, median (IQR), y | 64 (58.5–71.2) |
| Race (%) | |
| White | 23 (95.8) |
| Black or African American | 1 (4.2) |
| Comorbidities, No. (%) | |
| Malignancy | 13 (54.1) |
| Head and neck | 3 (12.5) |
| Chronic kidney disease | 11 (45.8) |
| Hemodialysis | 4 (16.6) |
| Essential hypertension | 8 (33.3) |
| Diabetes mellitus | 7 (29.1) |
| Insulin-dependent | 3 (12.5) |
| Congestive heart failure | 6 (25) |
| Peripheral vascular disease | 5 (20.8) |
| Chronic obstructive pulmonary disease | 3 (12.5) |
| Immunocompromised, No. (%) | 15 (62.5) |
| Solid organ transplant | 7 (29.1) |
| Kidney | 4 (16.7) |
| Lung | 2 (8.3) |
| Liver | 1 (4.2) |
| Bone marrow transplant | 3 (12.5) |
| Prednisone (≥5 mg/d) | 9 (37.5) |
| Calcineurin inhibitors | 6 (25) |
| Antiproliferative agents | 6 (25) |
| mTOR inhibitors | 1 (4.2) |
| CCI, median (IQR) | 7 (4.5–10) |
Abbreviations: CCI, Charlson Comorbidity Index; IQR, interquartile range; mTOR, mammalian target of rapamycin.
Clinical and Radiologic Presentation
Lung and cutaneous infections were the most common primary sites of nocardial infection (37.5% and 12.5%, respectively). Lung infections were more frequent in immunocompromised than immunocompetent hosts (71% vs 29%). The immunocompetent host was more likely to have nocardiosis due to presumed direct inoculation secondary to trauma than the immunocompromised patients (62% vs 38%). Three (12.5%) cases were HAIs, and the infectious diseases (ID) team was consulted on all patients during their hospitalization.
All 24 patients had magnetic resonance imaging (MRI) performed, and 19 (79.1%) patients had a head computed tomography (CT). Of the patients who had a head CT, 13 (68.4%) had similar findings, but 6 (31.6%) had fewer lesions when compared with the MRI. Eight (33.3%) patients had >1 intracranial fluid collection. The most common location was the frontal lobe (41.6%), followed by parietal (37.5%), temporal (33.3%), and occipital (12.5%). Two patients had cerebellar involvement. The fluid collections’ median diameter size (IQR) was 14 (10–21) mm, and midline shift was observed in 8.4% of cases.
Microbiology
All 24 patients had a positive culture result from direct brain abscess sampling. The most common species isolated were Nocardia farcinica (n = 9; 37.5%), N. wallacei (n = 3; 12.5%), N. cyriacigeorgica (n = 3; 12.5%), N. abscessus (n = 1; 4.1%), N. otitidiscaviarum (n = 1; 4.1%), N. transvalensis (n = 1; 4.1%), and N. argoensis (n = 1; 4.1%). Similarly, all 24 patients had peripheral blood cultures performed, but only 3 patients had a positive blood culture for Nocardia spp. (Table 2).
Table 2.
Clinical Presentation and Management of Patients With Nocardial Brain Abscess
| Cases | Age, Gender | Immunocompromised Host | Primary Source | Brain Abscess Location | Diameter, mma | Positive Blood Cultures | Nocardia Spp. on Direct Brain Abscess Sampling | Final Antibiotic Therapy | Final Antibiotic Route | Duration of Therapy, d | Type of Surgical Intervention | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80, M | Yes | Pulmonary | Frontal | 12 | No | N. wallacei | Moxifloxacin, amikacin | Oral/IV | 84 | None | Diedb |
| 2 | 72, M | Yes | Skin | Frontal, parietal and midbrain | 17 | Yes | N. farcinica | TMP-SMX | IV | 180 | Open aspiration | Relapsed and died |
| 3 | 74, M | No | Unknown | Parietal, brain stem and cerebellum | 13 | No | N. farcinica | TMP-SMX | Oral | 336 | Open aspiration | Permanent neurologic deficit |
| 4 | 63, F | No | Pulmonary | Frontal, occipital and temporal | 21 | No | N. cyriacigeorgica | Ceftriaxone | IV | 168 | None | Cured |
| 5 | 77, F | No | CNS trauma | Temporal | 24 | No | Nocardia spp. | TMP-SMX, doxycycline | IV/oral | 168 | Open aspiration | Cured |
| 6 | 50, M | Yes | Unknown | Temporal | 41 | No | N. farcinica | Linezolid, amoxicillin-clavulanate | Oral | 224 | Open aspiration | Relapse and died |
| 7 | 94, M | Yes | Pulmonary | Temporal and parietal | 15 | No | N. farcinica | Linezolid, imipenem | Oral/IV | 14 | Stereotactic | Diedb |
| 8 | 73, F | No | Unknown | Parietal | 8 | No | N. wallacei | Linezolid, TMP-SMX, minocycline | Oral | 308 | None | Relapse and diedb |
| 9 | 65, M | Yes | Pulmonary | Cerebellum and forebrain | 10 | No | N. farcinica | TMP-SMX, moxifloxacin, doxycycline | Oral | 280 | None | Cured |
| 10 | 69, M | No | Pulmonary | Frontal | 10 | No | N. cyriacigeorgica | TMP-SMX | Oral | 365 | None | Required delayed surgical intervention |
| 11 | 50, M | Yes | Unknown | Parietal | 5 | No | N. otitidiscaviarum | TMP-SMX, amikacin | Oral/IV | 260 | None | Cured |
| 12 | 66, M | Yes | Skin | Brain stem | 4 | No | Nocardia spp. | TMP-SMX, amoxicillin-clavulanate | Oral | 365 | None | Cured |
| 13 | 49, M | No | Pulmonary | Parietal | 6.6 | Yes | N. farcinica | TMP-SMX, moxifloxacin | Oral | 365 | None | Cured |
| 14 | 61, M | Yes | Skin | Frontal | 4 | No | Nocardia spp. | TMP-SMX | Oral | 365 | None | Cured |
| 15 | 64, M | Yes | Unknown | Midbrain | 21 | No | Nocardia spp. | TMP-SMX | Oral | 365 | None | Permanent neurologic deficit |
| 16 | 60, F | Yes | Unknown | Temporal and parietal | 10 | No | N. argoensis | TMP-SMX, moxifloxacin | Oral | 365 | Open aspiration | Cured |
| 17 | 34, M | Yes | Pulmonary | Temporal | 26 | No | Nocardia spp. | TMP-SMX | Oral | 365 | Open aspiration | Cured |
| 18 | 64, M | Yes | Unknown | Occipital and temporal | 17 | No | N. cyriacigeorgica | Linezolid, TMP-SMX | Oral | 504 | Open aspiration | Cured |
| 19 | 50, M | Yes | CNS trauma | Frontal, occipital, temporal, and parietal | 13 | No | N. farcinica | TMP-SMX, ceftriaxone | Oral/IV | 196 | None | Died |
| 20 | 61, F | No | Unknown | Frontal | 10 | No | N. farcinica | Linezolid, ceftriaxone | Oral/IV | 182 | None | Cured |
| 21 | 69, M | No | Unknown | Parietal | 22 | No | N. farcinica | TMP-SMX | Oral | 365 | Stereotactic | Cured |
| 22 | 69, F | Yes | Pulmonary | Frontal | 20 | No | N. transvalensis | TMP-SMX, doxycycline | Oral | 365 | None | Cured |
| 23 | 58, M | No | Unknown | Frontal | 20 | No | N. abscessus | TMP-SMX, amoxicillin-clavulanate | Oral | 365 | Open aspiration | Cured |
| 24 | 63, M | Yes | Pulmonary | Frontal | 25 | Yes | N. wallacei | TMP-SMX, imipenem | Oral/IV | 20 | Stereotactic | Died |
Abbreviations: CNS, central nervous system; F, female; IV, intravenous; M, male; TMP-SMX, trimethoprim-sulfamethoxazole.
aDiameter of the largest lesion in cases when more than 1 lesion was present.
bDeath related to Nocardia infection.
Management and Outcomes
Thirteen (54.2%) patients were managed solely by medical management, while 11 (45.8%) had both medical and surgical management (Table 2). The median time from nocardial brain abscess diagnosis to surgical therapeutic intervention (IQR) was 3 (0.2–6) days.
Corticosteroids were used as part of the medical treatment in 10 (41.6%) patients, mostly due to midline shift and neurologic deficits. Dexamethasone was most commonly used (8; 33.3%), with a median dose (IQR) of 6 (4–12) mg per day and a median duration (IQR) of 14 (5–71.5) days.
The most common empiric antibiotic therapy used alone and/or in combination was TMP-SMX in 41.6% of cases, followed by vancomycin (37.5%), linezolid (33.3%), metronidazole (33.3%), and meropenem (21.1%). A total of 20 patients (83.3%) had at least 1 active antibiotic started empirically. The most common definitive antibiotic regimen based on susceptibilities is summarized in Table 2. Susceptibility testing varied among different species. All isolates were susceptible to TMP-SMX. Additional trends included N. farcinica susceptible to linezolid, moxifloxacin, and imipenem; N. wallacei was susceptible to minocycline and linezolid; N. cyriacigeorgica was susceptible to ceftriaxone and linezolid. In all patients, dual therapy was administered for the initial 6 weeks of treatment. A total of 8 (33.3%) patients received monotherapy, with 7 receiving TMP-SMX monotherapy as part of the final regimen after 6 weeks of induction therapy. The median duration of parenteral antibiotic therapy was 21 days. Twenty-two (91.7%) patients received at least 1 oral agent as part of their final antibiotic regimen, mainly TMP-SMX and linezolid. The final median antibiotic duration (IQR) was 322 (180.5–365) days. Thirty-six percent of patients receiving immunosuppressive therapy had reductions in immunosuppression as part of their management.
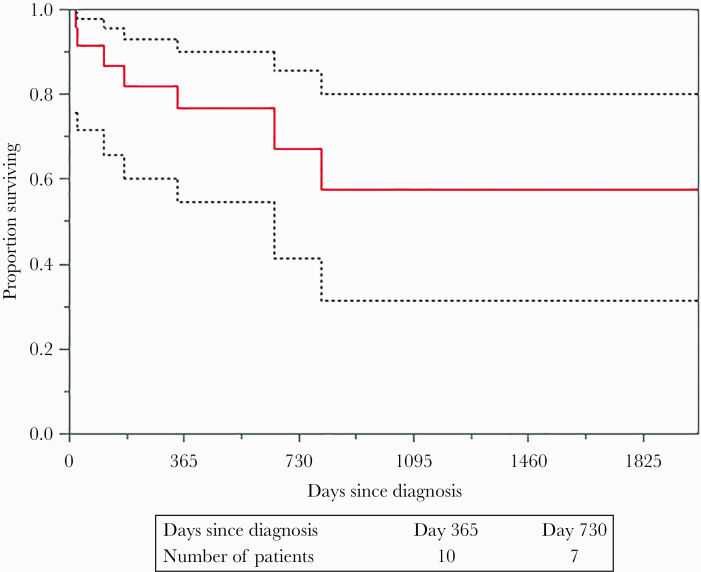
Fourteen (58.3%) patients achieved complete resolution of the clinical manifestations and radiographic resolution of brain fluid collection. Two (8.3%) had permanent neurological deficits (left hemiparesis and seizure, respectively), 3 (12.5%) patients relapsed, and 1 (4.1%) patient progressed despite medical therapy requiring surgical intervention at a later time during his hospital course. A total of 7 (29.1%) patients died, with a median time from diagnosis to death of 169 days. Four of these (16.6%) deaths were related to underlying chronic conditions including malignancy and cardiovascular disease. The average follow-up time for this cohort was 19 months. Figure 1 shows that ~60% of patients with nocardial brain abscess survived at 5 years.
Figure 1.
Survival curve is patients with Nocardia spp. brain abscess.
DISCUSSION
The current study is one of the largest contemporary cohorts to describe the risk factors, clinical and radiographic features, management interventions, and outcomes of nocardial brain abscess. Nocardia brain abscess occurs mostly in the fifth to sixth decades of life [10, 11], with the peak occurrence between ages 43 and 75 years. Purported risk factors for developing nocardial brain abscess include age, gender, and immunocompromised status [12, 13]. In our cohort, patients who presented with a nocardial brain abscess were older and predominantly Caucasian males, consistent with prior reports [14–16]. Higher incidence of nocardial brain abscesses in older age may be due to immunosenescence, a physiological part of aging linked to a higher risk of infection in part due to the excessive production of pro-inflammatory cytokines by macrophages and fibroblasts that may impact the innate and adaptive immune systems, which are crucial in the development of nocardial brain abscess [17, 18].
Systemic infection with Nocardia spp. most often occurs in immunosuppressed patients and rarely in immunocompetent individuals [4, 19, 20]. In our cohort, the percentage of immunocompromised individuals, including 10 transplant patients and those receiving corticosteroids, was higher than in other published studies [21–26]. Cell-mediated immune deficiencies, in particular, seem to be a major predisposing factor for Nocardia infections [20, 27]. As seen in our series, steroids or other immunosuppressive medications such as calcineurin inhibitors, antiproliferative agents, and mTOR inhibitors can suppress cell-mediated immunity, likely contributing to the high prevalence of Nocardia infections [20, 27, 28]. As the overall population ages and more patients receive various immunosuppressive therapies due to expanding indications, we may see more nocardial abscesses in the future.
Chronic corticosteroid therapy, especially at higher doses and with prolonged durations, has been associated with increased risk of opportunistic infections such as Pneumocystis pneumonia—thus the recommendation for TMP-SMX prophylaxis [5, 29]. Daily TMP-SMX prophylaxis also may prevent nocardiosis and account for the reduced prevalence of this organism in patients with AIDS and SOT recipients [30, 31]. However, breakthrough nocardial infections may occur in the context of low-dose or intermittent TMP-SMX prophylaxis [32–34]. Interestingly, in our series, 4 patients were on 1 TMP-SMX double-strength tablet daily for prophylaxis and still developed nocardial brain abscess. Therefore, if the suspicion for Nocardia infection is high, use of TMP-SMX prophylaxis should not dissuade clinicians from considering nocardial abscesses in differential diagnosis.
Primary lung infection, likely due to inhalation, was the most frequent mechanism of infection acquisition in our cohort, similar to earlier observations [26, 35]. Hematologic spread with a high propensity to the skin and subcutaneous tissue or CNS has also been described [5, 19, 36]. In our series, Nocardia spp. grew in the blood cultures of only 3 patients. Detailed skin examination and CT of the chest can be helpful to identify the primary site of infection. Interestingly, although ID was consulted in all cases in our series and a thorough evaluation was performed, the primary infection source could not be identified in 41.7% of the cases. We hypothesize that infection may have started as a direct inoculum from trauma or inhalation but infection at the primary site was subclinical and therefore no primary source was identified.
Nocardial brain abscesses may present as a hyperenhancing multiloculated ring lesions. As the infection usually occurs through inhalation and from direct spread from the sinuses [37], the frontal lobe is commonly involved. However, location, size, and appearance of nocardial brain abscesses alone cannot be used to differentiate these from other causes of bacterial abscesses. Sometimes it is difficult to differentiate nocardial abscesses from intracranial metastatic malignancy on CT or MRI [38]. Therefore, while MRI continues to be the preferred radiologic method for imaging of suspected nocardial abscesses [39], diagnostic aspiration is necessary to confirm the diagnosis and for selection of appropriate antimicrobial therapy.
The importance of isolating and culturing the Nocardia spp. is due to concern for resistance to specific antimicrobial agents [40]. However, Nocardia spp. have relatively slow growth, and they can be difficult to culture in the laboratory, making the 16S rRNA gene sequencing method a reliable alternative for identification [19]. Kiska et al. [41] concluded that no single method could accurately identify all Nocardia spp. associated with human infections. A combination of the antimicrobial susceptibility pattern, colony pigment, biochemical tests, and molecular techniques could potentially identify all isolates to the species level. To date, no specific brain tropism has been identified to a particular species. The present study was concordant with previous reports [3, 42–45] where N. farcinica and N. abscessus were the most commonly encountered species.
In general, the most active agents against Nocardia species include TMP-SMX, amikacin, minocycline, and imipenem [46, 47]. However, no randomized trials have been performed to compare the efficacy of different antibiotic regimens for nocardiosis. A study by Brown-Elliott et al. involving 522 clinical isolates reported that only 2% of the isolates demonstrated in vitro resistance to TMP-SMX [40]. Hamdi et al. reported similar results [46]. Sulfonamides and trimethoprim are small lipophilic antibiotics. At high doses, the penetration into the cerebrospinal fluid, in both the absence and the presence of meningeal inflammation, is considered sufficient for the treatment of CNS infections with susceptible bacteria [48]. Based on these observations, TMP-SMX is considered the mainstay of therapy [26, 49]. Acknowledging the high morbidity and mortality associated with nocardial brain abscesses, TMP-SMX should be used in combination with another highly bioavailable antimicrobial with good CNS penetration for induction therapy for nocardial brain abscess. Given the small number of patients in our study and earlier publications, no definitive recommendations can be made, and larger, multicenter studies are needed to determine the optimal treatment regimen.
Despite the limited literature, surgical excision is considered necessary in most cases [2, 50]. Lee et al. reported aspiration alone in 90.9% of their patients with no reported deaths [51]. In a study by Hall et al. [52], surgical aspirations alone were considered appropriate as the initial management of brain abscess. Others have proposed that craniotomy and excision of the entire abscess and wall are more effective than aspiration and drainage [2]. In our patient population, 54.2% of the cases had no surgical management, likely due to the smaller median brain abscess diameter size in our cohort (14 mm). In general, surgical aspiration is recommended and preferred in lesions larger than 2.5 cm in diameter [2].
Patients with nocardial brain abscess may have residual motor deficits and hearing impairment even with successful treatment for underlying infection [2]. In our cohort, hemiparesis and seizure were encountered only in few cases. The majority (62.5%) of our patients had a good outcome. Poor outcomes, including death, were mainly due to patients’ underlying comorbidities. This is further supported by a high median CCI score in our cohort. Due to the small size, we were unable to conclude if medical management vs a combination of medical and surgical management impacts patient outcomes. However, it stands to reason that early diagnosis and surgical management may be associated with reduced morbidity and mortality due to early and effective source control.
Limitations
The retrospective nature of the study with a case determination that was based on the claims data set is the primary limitation. Decisions regarding diagnostic testing and therapeutic interventions were left to the discretion of treating physicians and were not based on any standardized protocol. Due to the small size of our cohort, we were unable to assess the statistical significance of our observation. Finally, even though the Mayo Clinic in Minnesota receives patients from other cities and rural areas, the high prevalence of immunocompromised patients and those presenting from the Midwest limits our ability to generalize our findings.
CONCLUSIONS
Older, immunocompromised, and high-morbidity populations are at increased risk for nocardial brain abscesses. Early diagnostic and therapeutic aspiration may help health care providers confirm diagnosis, choose an appropriate antimicrobial regimen, and reduce morbidity and mortality due to early and adequate source control.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. None for all authors.
Potential conflicts of interest. M.R.S.: honoraria/consulting fee: Medtronic Inc., Philips, and Aziyo Biologics, Inc. (all <US$10K); research grant: Medtronic. J.C.O. has provided consulting services for Elsevier, Inc., and Bates College. All other authors: none. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The design of the work was approved by the local institutional review board (IRB# 20-000488). The study was considered minimal risk, and patient consent requirements were waived.
Author contributions. Cristina Corsini Campioli, MD: conception or design of the work, data collection, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published; Natalia E. Castillo Almeida, MD: drafting the article, critical revision of the article, final approval of the version to be published; John C. O’Horo, MD: conception or design of the work, critical revision of the article, final approval of the version to be published; Douglas Challener, MD: data analysis and interpretation, critical revision of the article; John Raymond Go, MD: critical revision of the article; Daniel C. DeSimone, MD: critical revision of the article; M. Rizwan Sohail, MD: conception or design of the work, drafting the article, critical revision of the article, final approval of the version to be published.
References
- 1. Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 2014; 82:806–13. [DOI] [PubMed] [Google Scholar]
- 2. Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery 1994; 35:622–31. [DOI] [PubMed] [Google Scholar]
- 3. Al Tawfiq JA, Mayman T, Memish ZA. Nocardia abscessus brain abscess in an immunocompetent host. J Infect Public Health 2013; 6:158–61. [DOI] [PubMed] [Google Scholar]
- 4. Alijani N, Mahmoudzadeh S, Hedayat Yaghoobi M, et al. Multiple brain abscesses due to Nocardia in an immunocompetent patient. Arch Iran Med 2013; 16:192–4. [PubMed] [Google Scholar]
- 5. Cattaneo C, Antoniazzi F, Caira M, et al. Nocardia spp. infections among hematological patients: results of a retrospective multicenter study. Int J Infect Dis 2013; 17:e610–4. [DOI] [PubMed] [Google Scholar]
- 6. Cassir N, Million M, Noudel R, et al. Sulfonamide resistance in a disseminated infection caused by Nocardia wallacei: a case report. J Med Case Rep 2013; 7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem 2015; 61:100–11. [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter SP, Olson SL, Connelly BJ, et al. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium species, Nocardia species, and other aerobic Actinomycetes. J Clin Microbiol 2016; 54:376–84. Available at: https://www.cdc.gov/hai/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Healthcare-associated infections.
- 10. Mcclelland CJ, Craig BF, Crockard HA. Brain abscesses in Northern-Ireland - 30-year community review. J Neurol Neurosur Psychiatry 1978; 41:1043–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicolosi A, Hauser WA, Musicco M, Kurland LT. Incidence and prognosis of brain abscess in a defined population: Olmsted County, Minnesota, 1935-1981. Neuroepidemiology 1991; 10:122–31. [DOI] [PubMed] [Google Scholar]
- 12. Zhang C, Hu L, Wu X, et al. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect Dis 2014; 14:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muzumdar D, Jhawar S, Goel A. Brain abscess: an overview. Int J Surg 2011; 9:136–44. [DOI] [PubMed] [Google Scholar]
- 14. Manzar N, Manzar B, Kumar R, Bari ME. The study of etiologic and demographic characteristics of intracranial brain abscess: a consecutive case series study from Pakistan. World Neurosurg 2011; 76:195–200; discussion 79–83. [DOI] [PubMed] [Google Scholar]
- 15. Menon S, Bharadwaj R, Chowdhary A, et al. Current epidemiology of intracranial abscesses: a prospective 5 year study. J Med Microbiol 2008; 57:1259–68. [DOI] [PubMed] [Google Scholar]
- 16. Ong CT, Tsai CF, Wong YS, Chen SC. Epidemiology of brain abscess in Taiwan: a 14-year population-based cohort study. PLoS One 2017; 12:e0176705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 2005; 579:2035–9. [DOI] [PubMed] [Google Scholar]
- 19. Chung TT, Lin JC, Hsieh CT, et al. Nocardia farcinica brain abscess in an immunocompetent patient treated with antibiotics and two surgical techniques. J Clin Neurosci 2009; 16:1675–7. [DOI] [PubMed] [Google Scholar]
- 20. Al-Tawfiq JA, Al-Khatti AA. Disseminated systemic Nocardia farcinica infection complicating alefacept and infliximab therapy in a patient with severe psoriasis. Int J Infect Dis 2010; 14:e153–7. [DOI] [PubMed] [Google Scholar]
- 21. Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol 2006; 65:557–62; discussion 562. [DOI] [PubMed] [Google Scholar]
- 22. Xiao F, Tseng MY, Teng LJ, et al. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol 2005; 63:442–9; discussion 449–50. [DOI] [PubMed] [Google Scholar]
- 23. Landriel F, Ajler P, Hem S, et al. Supratentorial and infratentorial brain abscesses: surgical treatment, complications and outcomes-a 10-year single-center study. Acta Neurochir 2012; 154:903–11. [DOI] [PubMed] [Google Scholar]
- 24. Frank M, Woschnagg H, Mölzer G, Finsterer J. Cerebellar nocardiosis and myopathy from long-term corticosteroids for idiopathic thrombocytopenia. Yonsei Med J 2010; 51:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YK, Sung H, Jung J, et al. Impact of immune status on the clinical characteristics and treatment outcomes of nocardiosis. Diagn Microbiol Infect Dis 2016; 85:482–7. [DOI] [PubMed] [Google Scholar]
- 26. Yang M, Xu M, Wei W, et al. Clinical findings of 40 patients with nocardiosis: a retrospective analysis in a tertiary hospital. Exp Ther Med 2014; 8:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adjamian N, Kikam A, Wessell KR, et al. Nocardia brain abscess and CD4(+) lymphocytopenia in a previously healthy individual. Case Reports Immunol 2015; 2015:374956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc 2012; 87:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martínez Tomás R, Menéndez Villanueva R, Reyes Calzada S, et al. Pulmonary nocardiosis: risk factors and outcomes. Respirology 2007; 12:394–400. [DOI] [PubMed] [Google Scholar]
- 30. Peterson PK, Ferguson R, Fryd DS, et al. Infectious diseases in hospitalized renal transplant recipients: a prospective study of a complex and evolving problem. Medicine (Baltimore) 1982; 61:360–72. [DOI] [PubMed] [Google Scholar]
- 31. Goodlet KJ, Tokman S, Nasar A, Cherrier L, Walia R, Nailor MD. Nocardia prophylaxis, treatment, and outcomes of infection in lung transplant recipients: A matched case-control study. Transpl Infect Dis 2020:e13478. doi: 10.1111/tid.13478. [DOI] [PubMed] [Google Scholar]
- 32. Chouciño C, Goodman SA, Greer JP, et al. Nocardial infections in bone marrow transplant recipients. Clin Infect Dis 1996; 23:1012–9. [DOI] [PubMed] [Google Scholar]
- 33. Coussement J, Lebeaux D, van Delden C, et al. ; European Study Group for Nocardia in Solid Organ Transplantation. Nocardia infection in solid organ transplant recipients: a multicenter European case-control study. Clin Infect Dis 2016; 63:338–45. [DOI] [PubMed] [Google Scholar]
- 34. Lebeaux D, Freund R, van Delden C, et al. Outcome and treatment of nocardiosis after solid organ transplantation: new insights from a European study. Clin Infect Dis 2017; 64:1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matulionyte R, Rohner P, Uçkay I, et al. Secular trends of Nocardia infection over 15 years in a tertiary care hospital. J Clin Pathol 2004; 57:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev 1994; 7:357–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lange N, Berndt M, Jörger AK, et al. Clinical characteristics and course of primary brain abscess. Acta Neurochir (Wien) 2018; 160:2055–62. [DOI] [PubMed] [Google Scholar]
- 38. Lin YJ, Yang KY, Ho JT, et al. Nocardial brain abscess. J Clin Neurosci 2010; 17:250–3. [DOI] [PubMed] [Google Scholar]
- 39. Sartoretti E, Sartoretti T, Gutzwiller A, et al. Advanced multimodality MR imaging of a cerebral nocardiosis abscess in an immunocompetent patient with a focus on amide proton transfer weighted imaging. BJR Case Rep 2020; 6:20190122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown-Elliott BA, Biehle J, Conville PS, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol 2012; 50:670–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kiska DL, Hicks K, Pettit DJ. Identification of medically relevant Nocardia species with an abbreviated battery of tests. J Clin Microbiol 2002; 40:1346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farran Y, Antony S. Nocardia abscessus-related intracranial aneurysm of the internal carotid artery with associated brain abscess: a case report and review of the literature. J Infect Public Health 2016; 9:358–61. [DOI] [PubMed] [Google Scholar]
- 43. Galacho-Harriero A, Delgado-López PD, Ortega-Lafont MP, et al. Nocardia farcinica brain abscess: report of 3 cases. World Neurosurg 2017; 106:1053.e15–24. [DOI] [PubMed] [Google Scholar]
- 44. Benek HB, Akcay E, Yilmaz H, Yis R, Yurt A. Nocardia cyriacigeorgica brain abscess with Pemphigus vulgaris: first report. Br J Neurosurg. In press. [DOI] [PubMed] [Google Scholar]
- 45. Kumar VA, Augustine D, Panikar D, et al. Nocardia farcinica brain abscess: epidemiology, pathophysiology, and literature review. Surg Infect (Larchmt) 2014; 15:640–6. [DOI] [PubMed] [Google Scholar]
- 46. Hamdi AM, Fida M, Deml SM, Abu Saleh OM, Wengenack NL. Retrospective analysis of antimicrobial susceptibility profiles of Nocardia species from a tertiary hospital and reference laboratory, 2011 to 2017. Antimicrob Agents Chemother. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mosel D, Harris L, Fisher E, et al. Disseminated Nocardia infection presenting as hemorrhagic pustules and ecthyma in a woman with systemic lupus erythematosus and antiphospholipid antibody syndrome. J Dermatol Case Rep 2013; 7:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang EE, Prober CG. Ventricular cerebrospinal fluid concentrations of trimethoprim-sulphamethoxazole. J Antimicrob Chemother 1983; 11:385–9. [DOI] [PubMed] [Google Scholar]
- 49. Rosman Y, Grossman E, Keller N, et al. Nocardiosis: a 15-year experience in a tertiary medical center in Israel. Eur J Intern Med 2013; 24:552–7. [DOI] [PubMed] [Google Scholar]
- 50. Cooper CJ, Said S, Popp M, et al. A complicated case of an immunocompetent patient with disseminated nocardiosis. Infect Dis Rep 2014; 6:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee GY, Daniel RT, Brophy BP, Reilly PL. Surgical treatment of nocardial brain abscesses. Neurosurgery 2002; 51:668–71; discussion 671–2. [PubMed] [Google Scholar]
- 52. Hall WA, Martinez AJ, Dummer JS, Lunsford LD. Nocardial brain abscess: diagnostic and therapeutic use of stereotactic aspiration. Surg Neurol 1987; 28:114–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.