Abstract
In domestic dogs Canis familiaris, vocal traits have been investigated for barks and growls, and the relationship between individual body size and vocal traits investigated for growls, with less corresponding information for whines. In this study, we examined the frequency and temporal traits of whines of 20 adult companion dogs (9 males, 11 females), ranging in body mass from 3.5 to 70.0 kg and belonging to 16 breeds. Dog whines (26–71 per individual, 824 in total) were recorded in conditioned begging contexts modeled by dog owners. Whines had 3 independent fundamental frequencies: the low, the high and the ultra-high that occurred singly as monophonic calls or simultaneously as 2-voice biphonic or 3-voice polyphonic calls. From the smallest to largest dog, the upper frequency limit varied from 0.24 to 2.13 kHz for the low fundamental frequency, from 2.95 to 10.46 kHz for the high fundamental frequency and from 9.99 to 23.26 kHz for the ultra-high fundamental frequency. Within individuals, the low fundamental frequency was lower in monophonic than in biphonic whines, whereas the high fundamental frequency did not differ between those whine types. All frequency variables of the low, high, and ultra-high fundamental frequencies correlated negatively with dog body mass. For duration, no correlation with body mass was found. We discuss potential production mechanisms and sound sources for each fundamental frequency; point to the acoustic similarity between high-frequency dog whines and rodent ultrasonic calls and hypothesize that ultra-high fundamental frequencies function to allow private, “tete-a-tete” communication between members of social groups.
Keywords: acoustic communication, Canis familiaris, companion dogs, high-frequency calls, nonlinear phenomena, vocalization
Studying the vocalizations of domestic dogs Canis familiaris has considerable scientific value, and is of interest to the general public (Faragó et al. 2014; Taylor et al. 2014; Amiot et al. 2016). Vocal attributes prove useful in estimating dog emotional valence, discomfort, and arousal in veterinary (Riede et al. 2001; Kim et al. 2005; Curi and Talamoni 2006; Gilbert-Gregory et al. 2016), and biomedical research (Box and Spielmann 2005; Dellarco et al. 2009; Hasiwa et al. 2011; Rowell et al. 2011; Gilmore and Greer 2015). In addition, studying acoustic diversity of canine whines is requisite to the further development of automated methods for classifying context and emotional content of dog vocalizations (Molnár et al. 2008; Espinosa et al. 2015).
Domestic dogs produce 4 main call types: barks, growls, howls, and whines (Cohen and Fox 1976; Tembrock 1976; Volodina et al. 2006a; Yeon 2007; Faragó et al. 2014; Taylor et al. 2014; Grobman et al. 2019). Dog barks and growls have been studied in some detail (barks: Riede et al. 2001, 2005; Yin and McCowan 2004; Chulkina et al. 2006; Lord et al. 2009; Larranaga et al. 2015; growls: Riede and Fitch 1999; Taylor et al. 2008), whereas dog howls and whines are poorly studied (Riede et al. 2000; Volodina et al. 2006a). For communication with humans, dogs primarily use barks and whines (Pongrácz et al. 2010; Faragó et al. 2014; Taylor et al. 2014; Westgarth et al. 2016; Parsons et al. 2019). With whines, dogs can protest at separation (Mariti et al. 2013; Huber et al. 2017) and manipulate their owners (Volodina et al. 2006a) in a manner similar to the excessive manipulative meowing of domestic cats Felis catus (Nicastro 2004; McComb et al. 2009).
Humans commonly perceive canine whines as sad calls (Filippi et al. 2017; Parsons et al. 2019). Many dog owners complain about the uncontrolled vocalization of their companion dogs (Beaver 1994; Pongrácz et al. 2010; Westgarth et al. 2016; Parsons et al. 2019). However, some dog owners voluntarily or involuntarily promote whining by their dogs via a conditioned response mechanism (Volodina et al. 2006a; Faragó et al. 2014). This kind of communication between dogs and owners develops via classical or operant conditioning with positive reinforcement, either deliberate or not (e.g., Guerra and Silva 2010). So, in some dog–owner pairs, dog whining represents a part of everyday routine communication (Volodina et al. 2006a; Faragó et al. 2014). Whines, recorded in conditioned response contexts (e.g., of begging for food), can be used to study acoustic diversity in domestic dogs (Volodina et al. 2006a). These whines represent an “actor’s play” for manipulating their owners rather than expressing the dog’s submission or frustration.
In terrestrial mammals, there is an inverse relationship between vocalization fundamental frequency (f0) and body size, the larger an animal (and therefore the size of the vocal folds within larynx), the lower the fundamental frequency (f0) it can produce (Fletcher 2004; Taylor et al. 2008; Baotic et al. 2015; Charlton and Reby 2016; Martin et al. 2017). The domestic dog provides a convenient model for studying vocal cues to body size across breeds (Riede and Fitch 1999; Taylor et al. 2008), because over 400 breeds of domestic dogs are currently recognized (Borge et al. 2011; Shearman and Wilton 2011), which differ considerably in body size (Yordy et al. 2020).
In domestic dogs, the relationship between body size and acoustic variables has been studied only for growls (Riede and Fitch 1999; Taylor et al. 2008). Wideband growls encode information about dog size (Riede and Fitch 1999; Taylor et al. 2008) via call formant frequencies, representing the resonances of the vocal tract (Riede and Fitch 1999; Taylor and Reby 2010), and by call f0, reflecting the rate of vibration of the vocal folds in the larynx (Taylor et al. 2010a, 2010b). The larger a dog’s size and vocal tract length, the lower are the formant frequencies of the growls (Riede and Fitch 1999; Taylor et al. 2008). Information about body size, encoded in dog growls, is perceived by other dogs (Faragó et al. 2010; Taylor et al. 2010a, 2011) and by humans (Taylor et al. 2008, 2010b).
In contrast to the wideband growls, in which formants are clearly visible (Riede and Fitch 1999; Taylor et al. 2008, 2010a), the narrowband dog whines do not reveal their underlying formants (Volodin et al. 2005, 2006a; Taylor and Reby 2010). Therefore, in whines, potential variables encoding body size in domestic dogs include call duration and frequencies of the low (f0) and the high (g0) fundamental frequencies (Volodina et al. 2006a). Aside from domestic dogs, these f0 and g0 frequencies were also reported in other species of dog-like canids: African wild dogs Lycaon pictus (Wilden 1997, 1998), dholes (Volodin et al. 2001; Volodin and Volodina 2002; Volodina et al. 2006b; Frey et al. 2016), timber wolves Canis lupus (Schassburger 1987; Coscia et al. 1991), dingos C. lupus dingo (Tembrock 1976; Déaux and Clarke 2013) and red wolves Canis rufus (Schneider and Anderson 2011).
Two main hypotheses have been proposed for mechanisms underlying the production of the high fundamental frequency in canids (Frey et al. 2016). The first one is the hole-tone whistle mechanism (Roberts 1975) for producing an independent tone in the narrowings of the nasal vocal tract (Frey et al. 2016). The second one is the edge-tone whistle mechanism (Riede et al. 2017) for producing the independent tone as a result of interaction between a glottal exit jet and the edge of the lateral laryngeal ventricle (Frey et al. 2016). These whistle mechanisms of vocal production in canids were not yet considered in domestic dogs (Taylor et al. 2014), although Solomon et al. (1995) considered it unlikely that the vocal folds produced fundamental frequencies from 2.95 to 3.75 kHz in dog whines and proposed that these calls were produced by the whistle mechanism. High-speed cineradiography of vocalizing domestic dogs revealed that high-frequency whines were emitted through the nose, whereas low-frequency barks were emitted through the mouth (Fitch 2000). It has been shown earlier that the domestic dogs emitted whines, consisting only of the high fundamental frequency g0, with a closed mouth, whereas the appearance of the low fundamental frequency f0 (resulting the in appearance of the biphonic whine) was accompanied by mouth opening (Volodina et al. 2006a, see also Supplementary material, Movies S1 and S2). The morphological study of the vocal apparatus of the dhole, the canid species that produce calls similar to those of dogs, with 2 fundamental frequencies (Volodin and Volodina 2002; Volodina et al. 2006b), revealed a few potential sites and morphological structures which can serve as potential sources for the production of high-frequency whistling calls (Frey et al. 2016). However, these hypotheses have not yet received empirical support.
Whines of domestic dogs contain all known vocal nonlinear phenomena: subharmonics, deterministic chaos, biphonation, and frequency jumps (Wilden et al. 1998; Fitch et al. 2002; Volodin et al. 2005). The occurrence of vocal nonlinear phenomena (primarily deterministic chaos and subharmonics) was investigated in domestic dog barks (Riede et al. 2001, 2005; Tokuda et al. 2002) and howls (Tooze et al. 1990; Riede et al. 2000). The occurrence of different kinds of nonlinear phenomena in domestic dog whines was investigated only for a small sample of 9 dogs (Volodin et al. 2005; Volodina et al. 2006a). Temporal and frequency characteristics of domestic dog whines have yet to be investigated.
In domestic dog whines, the f0 and g0 can occur either alone as separate calls or both within the same call, one-by-one as a frequency jump or simultaneously as biphonation (Volodina et al. 2006a; Frey et al. 2016). Biphonation may be recognized from call spectrograms by: (1) frequency bands that are not integer multiples of a fundamental frequency; (2) differences in frequency modulation between some of the frequency bands; and (3) appearance of additional frequency bands, representing linear combinations of original frequencies. The additional frequency bands can be calculated by the formula n*f0 + m*g0, where n and m are integer multiples of f0 and g0 (Wilden et al. 1998; Volodin and Volodina 2002; Frey et al. 2016).
Preliminary analyses indicated that some whines of domestic dogs and dholes contained a third independent frequency h0, which was visible on a spectrogram but was too high (>15 kHz) to be heard by most adult humans (Volodina and Volodin 2018). For domestic dogs, production of whines with the high and ultra-high fundamental frequencies, extending into the ultrasonic range, is not surprising, as the high-frequency cut-off hearing range in the domestic dog is 41–47 kHz (Heffner 1983). It is interesting that, in spite of the differences in body size and in the respective areas of the tympanic membrane, the high-frequency cut-off value did not depend on dog body size, being similar in Chihuahua and Saint Bernard dogs (Heffner 1983). The peak hearing sensitivity for the dogs of different breeds is similar at 8 kHz (Heffner 1983) and corresponds well to the mean g0 values of 5.26–6.32 kHz in our study.
The aim of this study was to investigate the 3-voice complexity in the begging whines of companion domestic dogs and to test for any relationship between acoustic variables of whines and dog body size. We describe (1) the structural variation of the low-frequency (f0 alone), high-frequency (g0 alone) and the biphonic (f0 and g0) whines on a representative sample of dogs of different breeds and body sizes. (2) We investigate the relationship between frequency-temporal variables of whines (f0, g0, and f0&g0) with log body mass of the dogs. (3) We compare the f0 and g0 values between the monophonic and the biphonic whines with the expectation that both f0 and g0 frequencies will be different between the monophonic and biphonic whines within individuals. (4) For the first time, we describe the occurrence and acoustic variables for the third independent ultra-high (>15 kHz) fundamental frequency h0 of dog whines.
For f0, we predicted a lower f0 in bigger dogs. This prediction was based on the common rule for mammalian f0, that the frequency of the sound produced by the vocal folds is inversely related to an animal's body size (Fletcher 2004; Charlton and Reby 2016; Martin et al. 2017). For g0, we also predicted a lower g0 in bigger dogs. This prediction was based on data for human whistling, reporting that the whistle fundamental frequency is inversely related to the size of the attached resonator, the oral cavity (Azola et al. 2018). For h0, recently reported for both the domestic dog and in the dhole (Volodina and Volodin 2018) we had no a priori prediction.
Materials and Methods
Animals
Study animals were 20 adult (older than 1 year) individual companion domestic dogs C. familiaris (9 males, 11 females), belonging to different breeds, ranging in body mass from 3.5 to 70 kg (Table 1). All dogs were intact to avoid potential effects of castration or ovariectomy on vocalization (Kim et al. 2005). Body-mass score was provided by dog owners accurate to 0.1 kg for the smallest breeds and about 2 kg for the largest breeds (Table 1). The inclusion rule for dogs in this study were reports of the owners that their dogs regularly produce whines during some routine everyday dog-owner communication, e.g., when begging for snacks, for opening the door, for toys, and so on. The inclusion of dogs in this study also required apparent owner interest in the study and their informed consent to participate and help with recordings.
Table 1.
Domestic companion dogs (n = 20 individuals) provided whines for this study
| ID-number | Name | Breed | Sex | Mass(kg) | n recording sessions |
n analyzed whines |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| n f-whines | n g-whines | n f&g-whines | n h-whines | Total | ||||||
| 1 | Chris | Toy dachshund | m | 4 | 1 | 10 | 14 | 12 | – | 36 |
| 2 | Chrom | Poodle mix | m | 6 | 1 | 17 | 12 | 11 | – | 40 |
| 3 | Hilda | Dachshund | f | 7 | 2 | 11 | 11 | 10 | – | 32 |
| 4 | Reed | Collie | m | 20 | 1 | 13 | 13 | 10 | – | 36 |
| 5 | Grach | Giant schnauzer | m | 50 | 1 | 4 | 12 | 12 | – | 28 |
| 6 | Darjyal | Caucasian shepherd | m | 70 | 2 | 11 | 13 | 12 | – | 36 |
| 7 | Laska | Toy dachshund | f | 3.5 | 10 | 13 | 13 | 12 | 12 | 50 |
| 8 | Rick | Toy terrier | f | 3.7 | 1 | 5 | 12 | 4 | 11 | 32 |
| 9 | Knopa | Toy terrier and pincher | f | 6 | 2 | 4 | 13 | 24 | 41 | |
| 10 | Chloe | Dachshund | f | 7 | 5 | 12 | 12 | 11 | 11 | 46 |
| 11 | Krosha | Dachshund | f | 7 | 13 | 12 | 20 | 15 | 13 | 60 |
| 12 | Lisa | Fox terrier | f | 7 | 6 | 22 | 16 | 5 | 43 | |
| 13 | Vyusha | Dachshund | f | 9 | 1 | 11 | 12 | 8 | 9 | 40 |
| 14 | Dina | Spaniel mix | f | 15 | 1 | 20 | 28 | 12 | 11 | 71 |
| 15 | Lyalya | Greyhound hortaya | f | 20 | 1 | 13 | 14 | 13 | 13 | 53 |
| 16 | Mikhai | Husky | m | 25 | 1 | 10 | 10 | 12 | – | 32 |
| 17 | Martin | Husky | m | 25 | 2 | 11 | 12 | 11 | – | 34 |
| 18 | Gracie | Weimar hound | f | 25 | 1 | 13 | 13 | 15 | 10 | 51 |
| 19 | Kurt | Kurtshaar | m | 30 | 1 | 11 | 4 | 11 | – | 26 |
| 20 | Fedor | East European shepherd | m | 55 | 1 | 13 | 13 | 11 | – | 37 |
| Total | – | – | – | – | – | 236 | 267 | 231 | 90 | 824 |
ID-number, name, breed, sex, mass, the number of recording sessions included in analysis (n recording sessions) and the number of whines included in spectrographic and statistical analysis (n analyzed whines) are indicated. Designations: n f-whines—number of f-whines (with the low fundamental frequency f0 alone); n g-whines—number of g-whines (with the high fundamental frequency g0 alone); n f&g-whines—number of f&g-whines (with both f0 and g0); n h-whines—number of h-whines (with the ultra-high fundamental frequency h0 emitted alone or together with f0, g0, or both).
Call recording
Whines of 20 study dogs were recorded in 2000–2016. Call recordings were made from unrestrained dogs in an environment familiar to the dog, either inside or outside the home. Dogs produced whines in a conditioned begging context, designed by their owners based on everyday routine communication with their dogs. The recording situations varied depending on individual dog: dogs could vocalize spontaneously; when begging for snacks; when begging for opening a door to another room; before a walk; when begging for a toy out of the dog’s access, and so on (see Supplementary material, Movies S1 and S2 for examples of dog vocal begging). Nevertheless, the general context for acoustic recording was uniform for all dogs: by producing the whines, the dog manipulated the owner, who could provide the dog with a reinforcing object or context.
The call collector (this could be the author, dog owner, or student helper) recorded the whines in vicinity (within 5 m) of the unrestrained focal dog and its owner, who modeled the situation provoking the whining. In some cases, the focal dog was in the room together with 1–2 other dogs of the same owner; in this case, the call collector commented on whines belonging to the focal dog by voice. The owner could be in the same room, in the neighboring room or nearby, but outside. All study dogs were tolerant toward researchers and the recording procedure. Distance from the microphone to the animal was 0.5–5 m; orientation of the dog caller could change during the recordings. Recording sessions lasted 1–20 min; the number of recording sessions per dog was 1–13. Time spans between separate recordings of the same individual were irregular and could vary from1through a few days apart, up to 1 year.
Due to the long (16 years) overall data collection period in this study, the equipment applied for acoustic recording was different between the recording periods of 2000–2005 (for dogs #1–6, Table 1) and 2006–2016 (for Dogs #7–20, Table 1). From 2000 to 2005, for acoustic recordings (frequency range 40–12, 500 Hz, after-recording digitizing at 48,000 Hz, 16 bit), we used an analog SONY WM-D6C cassette tape recorder (Sony Corp., Tokyo, Japan) with Type II chrome audiocassettes EMTEC-CS II (EMTEC Consumer Media, Ludwigshafen, Germany) and a Tesla-AMD-411N (Tesla VÚST, Prague, Czech Republic) cardioid dynamic microphone. All cassette recordings were digitized within a month after recording.
In 2006–2016, for acoustic recordings (frequency range 40–24,000 Hz, sampling frequency 48,000 Hz), we used solid state recorders Marantz-PMD660 (DandM Professional, Kanagawa, Japan) or Zoom-H1 and Zoom-H4 digital recorders (Zoom Corp., Tokyo, Japan) and cardioid electret condenser microphones Sennheiser K6-ME64 (Sennheiser electronic, Wedemark, Germany) or AKG-C1000S (AKG-Acoustics Gmbh, Vienna, Austria). To capture the possible ultrasonic frequencies in dog whines, for the smallest dog (#7, Table 1), some acoustic recordings (frequency range 100–48,000 Hz, sampling frequency 96,000 Hz) were also made by using a hand-held Pettersson D1000X recorder with built-in microphone (Pettersson Electronik AB, Uppsala, Sweden) at distance 0.5–1 m. During all acoustic recordings, the overloading (clipping) effects due to too high a level of recording were avoided by manual control of the recording level.
Call samples
Audio files were analyzed for the presence of high-quality whines appropriate for spectrographic analyses using Avisoft SASLab Pro software (Avisoft Bioacoustics, Berlin, Germany). Following Gogoleva et al. (2008), we considered sound utterances as separate calls if they were separated with a silent interval longer than 30 ms. Following Volodina et al. (2006a), we scored each whine for the presence of the low (f0) and the high (g0) fundamental frequencies. In addition, we scored files for the presence of the ultra-high (h0) fundamental frequency. We considered that the f0, g0, or h0 was present in the call, if their duration was 30 ms or longer.
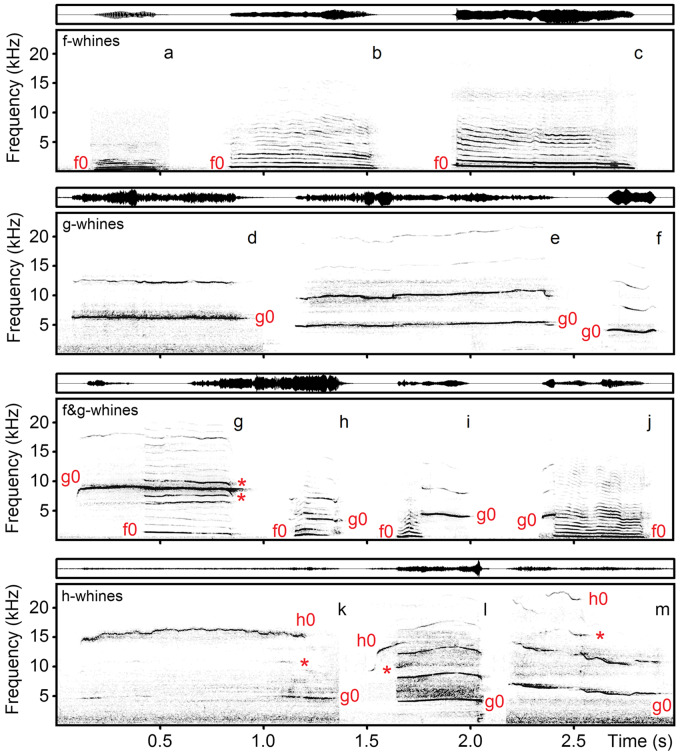
One author (O.V.S.), blind to data collection, classified each call visually to 1 of 4 structural types (Figure 1), and then classifying accuracy was additionally confirmed by another author (I.A.V.). We classified whines with f0 alone and its harmonics as f-whines. We classified whines with g0 alone and its harmonics as g-whines. We classified the whines with both f0 and g0, their harmonics and combinatory frequency bands resulting from interaction between f0 and g0 as f&g-whines (Figure 1). We classified any whine containing the ultra-high (h0) fundamental frequency as h-whine because the h0 rarely occurred in dog whines and could combine with either f0 or g0 or with both f0 and g0 frequencies (Figure 1).
Figure 1.
Spectrograms (below) and waveforms (above) of different whines of domestic dogs: f-whines—the whines with the low fundamental frequency f0 alone; g-whines—the whines with the high fundamental frequency g0 alone; f&g-whines—the whines with both low f0 and high g0 fundamental frequencies, combinatory frequency bands between f0 and g0 are labeled with asterisk; h-whines—the whines containing the ultra-high (h0) fundamental frequency, combinatory frequency bands between g0 and h0 are labeled with asterisk. (a) f-whine of Caucasian shepherd #6; (b) f-whine of spaniel mix #14; (c) f-whine of toy dachshund #7; (d) g-whine of spaniel mix #14; (e) g-whine of toy terrier and pincher #9; (f) g-whine of husky #16; (g) f&g-whine of dachshund #11; (h) f&g-whine of East European shepherd #20; (i) f&g-whine of husky #16; (j) f&g-whine of Weimar hound #18; (k) h-whine of dachshund #10; (l) h-whine of greyhound hortaya #15; (m) h-whine of toy terrier #8. The spectrograms were created with Hamming window, 48 kHz sampling rate, FFT 1024 points, frame 50% and overlap 93.75%. The audio file with these calls is provided in Supplementary material Audio S3.
For analyses, we selected all whines with high signal-to-noise ratio, non-overlapped with noises, human voices, calls of nonfocal dogs, or excessive wind noise (Table 1). We excluded from analyses all calls recorded with too high a level of recording that resulted in clipping. When possible, we avoided using structurally similar whines that immediately followed each other.
For the 20 study dogs, we included in the analysis from 4 to 22 f-whines per individual (236 f-whines in total); from 4 to 28 g-whines per individual (267 g-whines in total) and from 4 to 24 f&g-whines per individual (231 f&g-whines in total). For the total of 8 study dogs in which we found h-whines, we included in the analysis from 9 to 13 h-whines per individual (90 h-whines in total). The total sample of analyzed whines (f-whines, g-whines, f&g-whines, and h-whines) was 824 whines (Table 1).
Call analysis
Spectrographic analysis of f-whines, g-whines, and f&g-whines was conducted by using Avisoft SASLab Pro software with a 22.05 kHz sampling frequency, the Hamming window, Fast Fourier transform (FFT) length of 1024 points, frame 50% and overlap 93.75%. Spectrographic analysis of h-whines was conducted using either a 48 or 96 kHz sampling frequency, the Hamming window, FFT length of 1024 points, frame 50% and overlap 93.75%.
We measured 4 (2 frequency, 2 temporal) acoustic variables per f-whine, g-whine, or h-whine, and 7 (4 frequency, 3 temporal) acoustic variables per f&g-whine (Table 2). On the screen in the spectrogram window, we measured the duration with the standard marker cursor and the f0, g0, and h0 variables with a free reticule cursor (Figure 2). All measurements were exported automatically to Microsoft Excel (Microsoft Corp., Redmond, WA, USA). In either f-whines and g-whines, the duration of the measured f0 or g0 frequency band was equal to the total duration of a call (Figure 2). In h-whines, the duration of the h0 frequency band could be less than or equal to the total duration of a call. For calls with noncontinuous f0, g0, or h0 bands, the total duration of the respective frequency band was calculated as the sum of its parts (Figure 2).
Table 2.
Measured acoustic variables in different whines of domestic dog and their corresponding abbreviations
| Acoustic variable | Abbreviation | Different categories of whines |
|||
|---|---|---|---|---|---|
| f-whine | g-whine | f&g-whine | h-whine | ||
| Maximum low fundamental frequency (kHz) | f0max | + | + | ||
| Minimum low fundamental frequency (kHz) | f0min | + | + | ||
| Maximum high fundamental frequency (kHz) | g0max | + | + | ||
| Minimum high fundamental frequency (kHz) | g0min | + | + | ||
| Maximum ultra-high fundamental frequency (kHz) | h0max | + | |||
| Minimum ultra-high fundamental frequency (kHz) | h0min | + | |||
| Duration of low-frequency call part (ms) | f-dur | + | + | ||
| Duration of high-frequency call part (ms) | g-dur | + | + | ||
| Duration of ultra-high-frequency call part (ms) | h-dur | + | |||
| Total duration of a call (ms) | call-dur | + | + | + | + |
Designations: f-whine—the whine with the low fundamental frequency f0 alone, g-whine—the whine with the high fundamental frequency g0 alone, f&g-whine—the whine with both f0 and g0, h-whine—any whine containing the ultra-high (h0) fundamental frequency. Shadowed cells labeled with “+” mean that the given variable was measured in whines of the given category.
Figure 2.
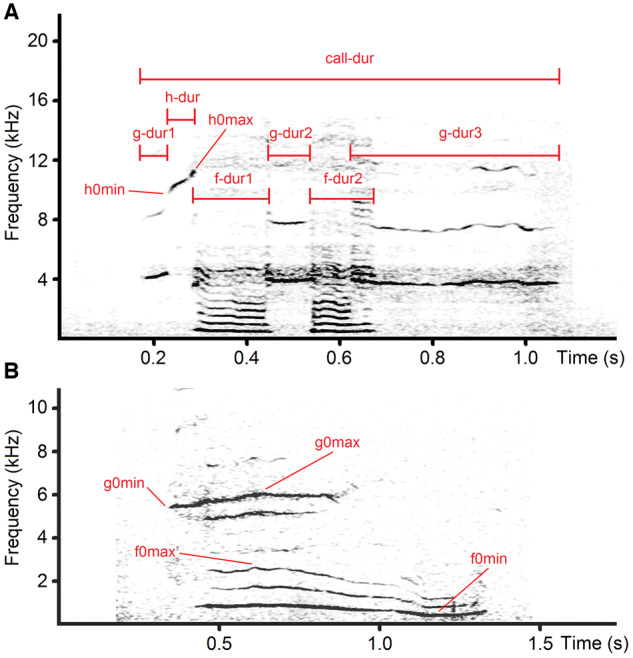
Measured acoustic variables in domestic dog whines. (A) h-whine of Weimar hound #18; (B) f&g-whine of dachshund #3. Designations: call-dur—total duration of a call; f-dur=f-dur1+f-dur2—duration of low-frequency call part; g-dur=g-dur1+g-dur2+g-dur3—duration of high-frequency call part; h-dur—duration of ultra-high-frequency call part; h0max—maximum ultra-high fundamental frequency; h0min—minimum ultra-high fundamental frequency; g0max—maximum high fundamental frequency; g0min—minimum high fundamental frequency; f0max—maximum low fundamental frequency; f0min—minimum low fundamental frequency. The spectrograms were created with Hamming window, 48 kHz (A) and 22.05 kHz (B) sampling rate, FFT 1024 points, frame 50%, and overlap 96.87%.
Statistical analyses
Statistical analyses were made with STATISTICA, version 8.0 (StatSoft, Tulsa, OK, USA). Means are presented as mean ± standard deviation (SD) , all tests were 2-tailed and differences were considered significant whenever P < 0.05. For the statistical analysis, we used the averaged per individual values of the acoustic variables for f-whines, g-whines, and f&g-whines for each of the 20 study dogs and for h-whines for each of the 8 study dogs which provided h-whines. All distributions of measured parameter values did not depart from normality (Kolmogorov–Smirnov test, P > 0.05), so we could use the parametric tests. We used linear regression analysis and the Pearson correlation with Bonferroni corrections for multiple testing to estimate the relationship between the acoustic variables of f-whines, g-whines, and f&g-whines and body size (via log body mass) for 20 individual dogs. We used a repeated measured Analysis of variance (ANOVA) to compare the variables of the low, and high or ultra-high frequencies within and between f-whines, g-whines, and f&g-whines.
Ethical note
This research involved non-invasive experiments. The situations in which dog whines were recorded, representing routine dog-owner communication (begging for snacks, for opening the door, etc.), were designed by dog owners informed about the aims and circumstances of the investigation, and were conducted in environments familiar to the dogs. Dog owners were equally interested in both the scientific output and in the welfare of their pets; they could stop the recording trial at their will. The authors adhered to the “Guidelines for the treatment of animals in behavioral research and teaching” (Anim. Behav., 2020, 159, I–XI) and the legal requirements of Russia pertaining to the protection of animal welfare. The experimental procedure was approved by the Committee of Bio-ethics of Lomonosov Moscow State University, research protocol # 2011-36.
Results
All 20 study dogs produced f-whines, g-whines, and the biphonic f&g-whines (see Supplementary Table S4 for mean ± SD values for all acoustic variables). Duration of f-whines (0.59 ± 0.39 ms) did not differ significantly from durations of either g-whines (0.52 ± 0.36 ms, r-m ANOVA, F1,19 = 0.82, P = 0.38) or f&g-whines (0.66 ± 0.35, r-m ANOVA, F1,19 = 1.76, P = 0.20), whereas the duration of g-whines was shorter than the duration of f&g-whines (r-m ANOVA, F1,19 = 7.55, P = 0.01). The duration of the low-frequency call component (0.41 ± 0.19 ms) did not differ significantly from the duration of the high-frequency call component (0.45 ± 0.27 ms) in the biphonic f&g-whines of the same individual (r-m ANOVA, F1,19 = 1.49, P = 0.24).
In f-whines, f0max varied from 0.59 to 1.17 kHz and f0min varied from 0.24 to 0.89 kHz between different dogs (Supplementary Table S4). In the biphonic f&g-whines, f0max varied from 0.74 to 2.13 kHz and f0min varied from 0.38 to 1.49 kHz among different dogs. The f0max was lower in the monophonic f-whines (0.80 ± 0.20 kHz) than in the biphonic f&g-whines (1.07 ± 0.34 kHz) of the same individual (r-m ANOVA, F1,19 = 34.5, P < 0.001) (Figure 3). The f0min was lower in the monophonic f-whines (0.46 ± 0.16 kHz) than in the biphonic f&g-whines (0.69 ± 0.27 kHz) of the same individual (r-m ANOVA, F1,19 = 37.4, P < 0.001) (Figure 3). The duration of the monophonic f-whines (0.59 ± 0.39 ms) was significantly longer than the duration of the low-frequency call component (0.41 ± 0.19 ms) of the biphonic f&g-whines in the same individual (r-m ANOVA, F1,19 = 8.19, P = 0.01).
Figure 3.
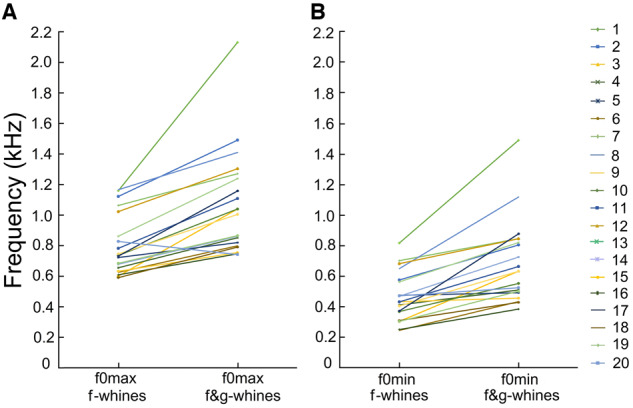
Plots illustrating the differences in (A) f0max between the monophonic f-whines and the biphonic f&g-whines; (B) f0min between the monophonic f-whines and the biphonic f&g-whines.
In g-whines, g0max varied from 3.59 to 10.43 kHz and g0min varied from 2.95 to 9.46 kHz between different dogs (Supplementary Table S4). In the biphonic f&g-whines, g0max varied from 3.43 to 10.46 kHz and g0min varied from 3.01 to 9.51 kHz between different dogs. The g0max did not differ between the monophonic g-whines (6.32 ± 2.12 kHz) and the biphonic f&g-whines (6.17 ± 1.98 kHz) of the same individual (r-m ANOVA, F1,19 = 2.84, P = 0.11) (Figure 4). The g0min did not differ between the monophonic g-whines (5.35 ± 1.90 kHz) and the biphonic f&g-whines (5.26 ± 1.79 kHz) of the same individual (r-m ANOVA, F1,19 = 1.29, P = 0.27) (Figure 4). Duration of the monophonic g-whines (0.52 ± 0.36 ms) did not differ significantly from the duration of the high-frequency call part (0.45 ± 0.27 ms) in the biphonic f&g-whines of the same individual (r-m ANOVA, F1,19 = 1.96, P = 0.18).
Figure 4.
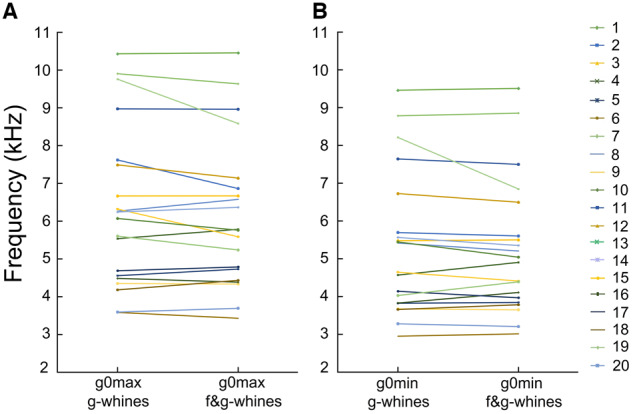
Plots illustrating the differences in (A) g0max between the monophonic g-whines and the biphonic f&g-whines; (B) g0min between the monophonic g-whines and the biphonic f&g-whines.
Frequency ranges of f0 and g0 did not overlap either within dog or in the total sample of the f-, g-, and f&g-whines of all 20 study dogs (Supplementary Table S4). In the monophonic whines, the highest f0max of f-whines was lower than the lowest g0min of g-whines (r-m ANOVA, F1,19 = 130.0, P < 0.001) (Figure 5). In the biphonic f&g-whines, the highest f0max was lower than the lowest g0min (r-m ANOVA, F1,19 = 147.0, P < 0.001). Therefore, even in the smallest dog, toy dachshund #7 (3.5 kg), the f0max was always lower-frequency than g0min of the biggest dog, Caucasian shepherd #6 (70 kg) (Figure 5).
Figure 5.
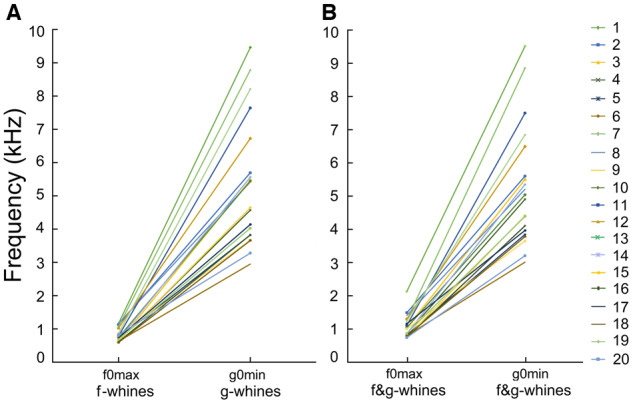
Plots illustrating the differences in (A) f0max and g0min of the monophonic f- and g-whines; (B) f0max and g0min of the biphonic f&g-whines.
Only 8 of the 20 study dogs had h-whines, that is, the whines containing the ultra-high fundamental frequency h0 (Supplementary Table S4). The duration of h-whines (0.91 ± 0.23 ms) did not differ from the duration of f&g-whines (0.79 ± 0.23 ms, F1,7 = 2.17, P = 0.18) in the 8 study dogs. The duration of the ultra-high-frequency call component (0.38 ± 0.26 ms) comprised on average 40.3 ± 20.3% of the entire call duration of h-whines and varied from 18.2% to 73.2% among individuals.
In the h-whines, the h0max (16.54 ± 4.21 kHz) varied from 11.08 to 23.26 kHz and h0min (15.26 ± 4.19 kHz) varied from 9.99 to 22.16 kHz among different dogs (Supplementary Table S4). Frequency ranges of g0 and h0 did not overlap, either within dog or in the total sample of the g-, f&g-, and h-whines of the 8 study dogs (Supplementary Table S4). The lowest h0min of h-whines was higher than the highest g0max of either the monophonic g-whines (r-m ANOVA, F1,7 = 52.7, P < 0.001) or the biphonic f&g-whines (r-m ANOVA, F1,7 = 56.6, P < 0.001) (Figure 6). Even in the smallest dog, a toy dachshund #7, the g0max (9.90 kHz) was slightly lower in frequency than h0min (9.99 kHz) in the larger-sized Weimar hound #18 (Figure 6A).
Figure 6.
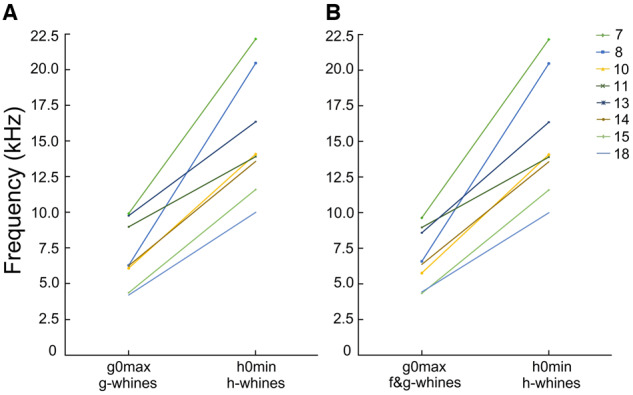
Plots illustrating differences between (A) g0max in monophonic g-whines and h0min in h-whines; (B) g0max in biphonic f&g-whines and h0min in h-whines.
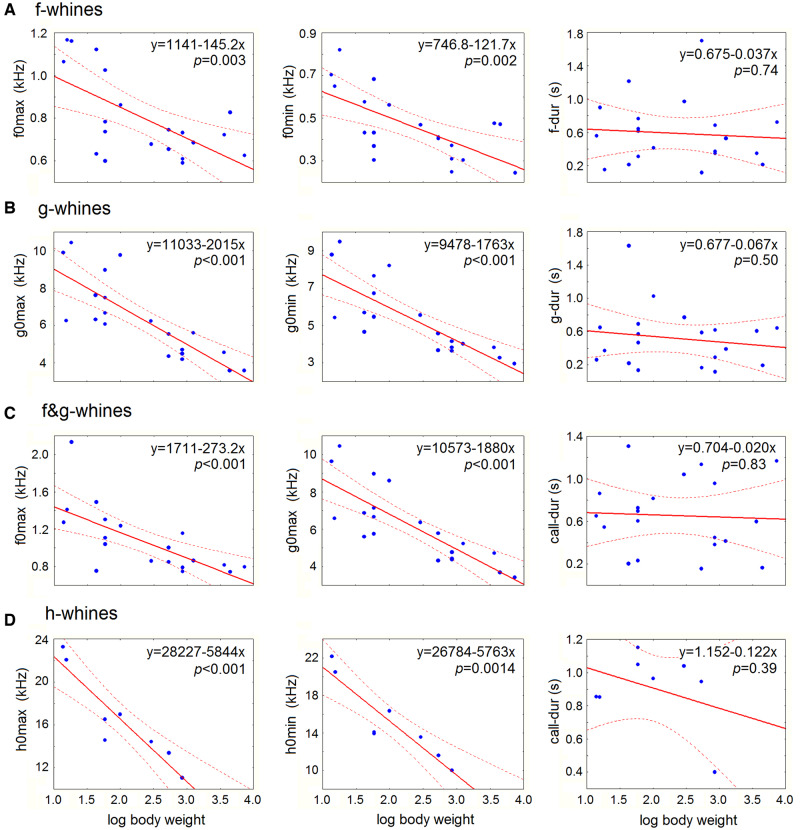
In all categories of whines (f-, g-, f&g-, and h-whines), the variables of the low (f0max, f0min), the high (g0max, g0min) and the ultra-high (h0max, h0min) fundamental frequencies correlated negatively with dog log body mass serving as a proxy for body size (Figure 7 and Table 3). In contrast, in all categories of whines (f-, g-, f&g-, and h-whines), all variables of duration (f-dur, g-dur, h-dur, and call-dur) did not correlate with log body mass (Figure 7 and Table 3).
Figure 7.
Scatterplots illustrating the relationships between acoustic variables of different categories of whines and log body mass of the study dogs. (A) f-whines; (B) g-whines; (C) f&g-whines; (D) h-whines. For decoding the abbreviations of measured acoustic variables, see Table 2. Linear regression lines with 95% confidence intervals are shown. P-values indicate the Pearson correlation results.
Table 3.
Pearson correlation between measured acoustic variables of domestic dog whines and log body mass
| Different categories of whines | Acoustic variable | n | Pearson correlation results |
|||
|---|---|---|---|---|---|---|
| r | R 2 | t | P-value | |||
| f-whine | f-dur | 20 | −0.08 | 0.006 | −0.34 | 0.74 |
| f0max | 20 | −0.63 | 0.39 | −3.43 | 0.003 | |
| f0min | 20 | −0.65 | 0.42 | −3.64 | 0.002 | |
| g-whine | g-dur | 20 | −0.16 | 0.03 | −0.69 | 0.50 |
| g0max | 20 | −0.81 | 0.66 | −5.85 | <0.001 | |
| g0min | 20 | −0.79 | 0.62 | −5.45 | <0.001 | |
| f&g-whine | call-dur | 20 | −0.05 | 0.002 | −0.21 | 0.83 |
| f-dur | 20 | 0.05 | 0.002 | 0.21 | 0.84 | |
| f0max | 20 | −0.68 | 0.47 | −3.96 | <0.001 | |
| f0min | 20 | −0.63 | 0.40 | −3.48 | 0.0027 | |
| g-dur | 20 | 0.08 | 0.001 | 0.33 | 0.74 | |
| g0max | 20 | −0.81 | 0.65 | −5.83 | <0.001 | |
| g0min | 20 | −0.78 | 0.61 | −5.26 | <0.001 | |
| h-whine | call-dur | 8 | −0.36 | 0.13 | −0.94 | 0.39 |
| h-dur | 8 | −0.002 | 0 | −0.005 | 0.99 | |
| h0max | 8 | −0.92 | 0.86 | −5.96 | <0.001 | |
| h0min | 8 | −0.92 | 0.84 | −5.57 | 0.0014 | |
Designations: n—number of dogs; f-whine—the whine with the low fundamental frequency f0 alone, g-whine—the whine with the high fundamental frequency g0 alone, f&g-whines—the whine with both f0 and g0, h-whine—any whine containing the ultra-high (h0) fundamental frequency. For decoding the abbreviations of measured acoustic variables, see Table 2. P estimates <0.0038 (after Bonferroni correction for f-, g-, and f&g-whines) and <0.0125 (after Bonferroni correction for h-whines) are shown in bold.
Discussion
This study showed that the 3 independent fundamental frequencies, the low f0, the high g0, and the ultra-high h0, found in the begging whines of companion domestic dogs, provide vocal cues to dog body size. Dog body size was only encoded in frequency variables, correlating negatively with individual body weight.
Temporal (duration) variables did not correlate with dog body mass. The present data are not consistent with comparative data for primates without air sacs, which reports a significant positive relationship between body mass and call duration (Hewitt et al. 2002).
The ranges of the low f0, the high g0, and the ultra-high h0 fundamental frequencies did not overlap, neither within individuals nor across dog breeds, and their simultaneous production indicates 3 different sound sources for their production by the dog vocal apparatus. There is experimental evidence that the low fundamental frequency f0 of dog whines is produced with the vibration of the vocal folds in the larynx, coming from experimental research studying sound production in relation to the activity of laryngeal muscles and subglottal pressure, carried out on anesthetized domestic dogs (Solomon et al. 1995; Berry et al. 1996), and excised dog larynges (Alipour et al. 1997, 2007; Finnegan and Alipour 2009). Values for f0, obtained in these experiments: from 0.09 to 0.11 kHz (Finnegan and Alipour 2009), from 0.1 to 0.2 kHz (Berry et al. 1996), from 0.12 to 0.24 kHz (Alipour et al. 1997), from 0.14 to 0.28 kHz (Alipour et al. 2007), and from 0.48 to 1.01 kHz (Solomon et al. 1995) coincide well with the ranges of f0 values in domestic dog whines obtained in our study: from 0.24 to 2.13 kHz for mean values per individual, and from 0.12 kHz (in Dogs #10 and #16) to 3.42 kHz (Dog #1) with accounting for individuality (Supplementary Table S4).
Frequency jumps and biphonations occurring in domestic dog whines between the high g0 and the ultra-high h0 fundamental frequencies are surprisingly reminiscent of the structural patterns of rodent ultrasonic calls. Further similarity between the high-frequency whines of domestic dogs and the ultrasonic calls of rodents is in the non-overlapping ranges between fundamental frequencies produced with vocal folds (f0) and those produced by the whistling mechanism (Riede 2011; Brudzynski 2014; Pasch et al. 2017). Experiments showed that a few rodent species use the whistling mechanism only for producing the ultrasonic calls (Riede 2011, 2013, 2018; Pasch et al. 2017; Riede et al. 2017), whereas the audible calls are produced by vibration of the vocal folds (Riede et al. 2011; Pasch et al. 2017). In laboratory rats Rattus norvegicus, domestic mice Mus musculus, fat-tailed gerbils Pachyuromys duprasi, and yellow steppe lemmings Eolagurus luteus, ultrasonic calls are produced in 2 different frequency ranges, so call fundamental frequency either jumps between these ranges or 2 fundamental frequencies are emitted in the different ranges simultaneously, which results in the biphonic call (Scattoni et al. 2008; Grimsley et al. 2011; Riede 2011, 2013; Zaytseva et al. 2019; Yurlova et al. 2020). Further experimental study in a helium–oxygen atmosphere (Riede 2011; Pasch et al. 2017) is necessary to confirm the potential whistling nature of domestic dog high-frequency whines.
In the biphonic f&g-whines of 19 of the 20 study dogs, the low fundamental frequency f0 was higher-frequency than f0 of the monophonic f-whines of the same individuals (Figure 3). Similar data were reported for the monophonic and biphonic contact calls of the dhole (Frey et al. 2016). Probably, g0 produced along with f0 within a call could slightly affect the vocal fold vibration via the so-called vortex-induced vibrations mechanism (Herzel and Reuter 1997; Mergell and Herzel 1997).
We found that all variables of the low f0, the high g0 and the ultra-high h0 frequencies were inversely related to dog body size. The value of the low fundamental frequency f0 depends on the size of the vocal folds: the larger the vocal folds, the lower f0 (Baotic et al. 2015), so the larger animal will produce lower-frequency calls (Fletcher 2004; Charlton and Reby 2016; Martin et al. 2017; but see Riede and Brown 2013 regarding variation in f0 that is independent of body size and vocal fold length). This agrees well with the negative correlation between the low fundamental frequency f0 of the whines and dog body mass found in this study. Previously, only a single study demonstrated a negative relationship between dog body size and the f0 of their growls (Taylor et al. 2008), whereas results of many playback studies suggest a possibility of call-based recognition of dog body size by recipient dogs and by humans (Taylor et al. 2008, 2010a, 2010b, 2011; Faragó et al. 2010,).
This study also provides the first evidence for the inverse relationship of the “whistling” g0 and h0 fundamental frequencies in dog whines with body size. The relationship between the fundamental frequency of the whistling calls and body size is poorly studied. There is a single study on humans, showing an inverse relationship between the fundamental frequency of the whistle and the volume of the attached Helmholtz resonator, modeling a part of the oral cavity (Azola et al. 2018). We propose that larger-sized dogs have respectively larger lateral laryngeal ventricles, playing the role of the Helmholtz resonator for the edge-tone mechanism of whistle production in canids (Frey et al. 2016). We also propose that larger-sized dogs have respectively larger-volume air cavities in the vocal tract in vicinity of the sources for producing the high and ultra-high fundamental frequencies, located in the narrowings of the nasal vocal tract (Frey et al. 2016). At the same time, for the whistling ultrasonic calls of rodents, the available data about the relationship between the fundamental frequency and body size are contradictory. For example, with body growth, the fundamental frequency of the ultrasonic calls decreases in the yellow steppe lemming (Yurlova et al. 2020) but increases in the fat-tailed gerbil (Zaytseva et al. 2019).
Thus, in whines of domestic dogs, all 3 fundamental frequencies, f0, g0, and h0, provide indicators of dog body size. The relationship between fundamental and formant frequencies was reported previously for dog growls (Riede and Fitch 1999; Taylor et al. 2008). As with the earlier studies, our study was conducted with dogs of different breeds, and thus considerable variation in body size. It remains unclear, whether the acoustic cues to body size are also presented in whines of dogs of similar size within breeds.
The communicative role of whines in domestic dogs and their ancestor timber wolves is still poorly understood (Schassburger 1987; Faragó et al. 2014; Taylor et al. 2014). However, we can propose potential communicative functions of whines based on data on other social canids. In the dhole, the biphonic f&g yap-squeaks better discriminate individuals compared with either f-yaps or g-squeaks (Volodina et al. 2006b), and better encode the position of a caller relative to a listener owing to different propagation of the low f0 and high g0 fundamental frequencies (Volodin et al. 2006c). This allows easy and quick recognition of vocalizing individuals relative to the direction of their approach and may therefore serve in the context of managing the social relationships in pack-living canids, thereby diffusing intra-pack aggression (Wilden 1997; Ludwig and Ludwig 2000; Frey et al. 2016).
The strong structural variability of whines may also be used by domestic dogs for attracting the attention of their owners in situation where the dog cannot cope with a problem. Whereas the repeatedly produced monotonous vocal sequences suppress responding in listeners (Hauser 1993; Hare 1998; Fitch and Kelly 2000), the use of calls with a few fundamental frequencies can function as a mechanism diminishing the probability of ignoring, or habituating to a call (Fitch et al. 2002).
Because high frequencies have greater directionality, greater attenuation, greater scattering, and decreased localizability than low frequencies (Musolf and Penn 2012), the ultra-high frequency (h0) can only be heard at close range. Dogs may be able to use the ultra-high frequency for private communication “tete-a-tete” with preferred pack members, just as Wilson and Hare (2004, 2006) suggested for ultrasonic alarm calls of Richardson’s ground squirrels limiting receivers to close kin. Whereas pack members in close vicinity, would hear the ultra-high-whines, dogs at greater distances or behind the caller would hear nothing. In addition to domestic dogs, the ultra-high fundamental frequency was also found in the contact calls of the highly social canid, the dhole (Volodina and Volodin 2018). Other social canids, e.g., the African wild dogs, timber wolves, dingos, and red wolves, may also be capable of producing the ultra-high fundamental frequency. However, such research would require acoustic recording at close distance followed by analysis of calls within the high-frequency range.
Supplementary Material
Acknowledgments
We thank dog owners: A. Blehman, D. Galas, O. Ilchenko O. Lifanova, E. Olehnovich, O. Rozdina, M. Rutovskaya, N. Soldatova, and A. Volodin for their cooperation. We thank all students and researchers who helped us with data collection: O. Filatova, D. Galas, V. Matrosova, M. Monakhova, A. Pavlyukov, and D. Smirnova. We thank Daniel Blumstein, Susan Lingle, and an anonymous reviewer for their useful comments on the manuscript. During our work, we adhered to the “Guidelines for the treatment of animals in behavioral research and teaching” (Anim. Behav., 2020, 159, I–XI) and requirements of the Russian Federation, pertaining to the protection of animal welfare.
Funding
This study was supported by the Russian Science Foundation grant 19-14-00037.
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Alipour F, Scherer RC, Finnegan EM, 1997. Pressure-flow relationship during phonation as a function of adduction. J Voice 11:187–194. [DOI] [PubMed] [Google Scholar]
- Alipour F, Jaiswal S, Finnegan EM, 2007. Aerodynamic and acoustic effects of false folds and epiglottis in excised larynx models. Ann Otol Rhinol Laryngol 116:135–144. [DOI] [PubMed] [Google Scholar]
- Amiot C, Bastian B, Martens P, 2016. People and companion animals: it takes two to tango. BioScience 66:552–560. [Google Scholar]
- Azola A, Palmer J, Mulheren R, Hofer R, Fischmeister F. et al. 2018. The physiology of oral whistling: a combined radiographic and MRI analysis. J Appl Physiol 124:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baotic A, Sicks F, Stoeger AS, 2015. Nocturnal “humming” vocalizations: adding a piece to the puzzle of giraffe vocal communication. BMC Res Notes 8:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver BV, 1994. Owner complaints about canine behavior. J Am Vet Med Assoc 204:1953–1955. [PubMed] [Google Scholar]
- Berry DA, Herzel H, Titze IR, Story BH, 1996. Bifurcations in excised larynx experiments. J Voice 10:129–138. [DOI] [PubMed] [Google Scholar]
- Borge KS, Tønnessen R, Nødtvedt A, Indrebø A, 2011. Litter size at birth in purebred dogs-A retrospective study of 224 breeds. Theriogenology 75:911–919. [DOI] [PubMed] [Google Scholar]
- Box RJ, Spielmann H, 2005. Use of the dog as non-rodent test species in the safety testing schedule associated with the registration of crop and plant protection products (pesticides): present status. Arch Toxicol 79:615–626. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, 2014. Social origin of vocal communication in rodents. In: Witzany G, editor. Biocommunication of Animals. Dordrecht, the Netherlands: Springer, 63–79. [Google Scholar]
- Charlton BD, Reby D, 2016. The evolution of acoustic size exaggeration in terrestrial mammals. Nat Commun 7:12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulkina MM, Volodin IA, Volodina EV, 2006. Individual, intersexual and interbreed variability of barks in dog Canis familiaris (Carnivora, Canidae). Zool Zhur 85: 544–555 [Google Scholar]
- Cohen JA, Fox MW, 1976. Vocalizations in wild canids and possible effects of domestication. Behav Proc 1:77–92. [DOI] [PubMed] [Google Scholar]
- Coscia EM, Phillips DP, Fentress JC, 1991. Spectral analysis of neonatal wolf Canis lupus vocalizations. Bioacoustics 3:275–293. [Google Scholar]
- Curi NHA, Talamoni SA, 2006. Trapping, restraint and clinical-morphological traits of wild canids (Carnivora, Mammalia) from the Brazilian Cerrado. Rev Brasil Zool 23:1148–1152. [Google Scholar]
- Déaux EC, Clarke JA, 2013. Dingo Canis lupus dingo acoustic repertoire: form and contexts. Behaviour 150:75–101. [Google Scholar]
- Dellarco VL, Rowland J, May B, 2009. A retrospective analysis of toxicity studies in dogs and impact on the chronic reference dose for conventional pesticide chemicals. Critic Rev Toxicol 40:16–23. [DOI] [PubMed] [Google Scholar]
- Espinosa HP, Pérez-Martínez JM, Durán-Reynoso JÁ, Reyes-Meza V, 2015. Automatic classification of context in induced barking. Res Comput Sci 100:63–74. [Google Scholar]
- Faragó T, Pongrácz P, Miklósi A, Huber L, Virányi Z. et al. 2010. Dogs’ expectation about signalers’ body size by virtue of their growls. PLoS One 5:e15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragó T, Townsend S, Range F, 2014. The information content of wolf (and dog) social communication. In: Witzany G, editor. Biocommunication of Animals. Dordrecht, the Netherlands: Springer. 41–62. [Google Scholar]
- Filippi P, Gogoleva SS, Volodina EV, Volodin IA, De Boer B, 2017. Humans identify negative (but not positive) arousal in silver fox vocalizations: implications for the adaptive value of interspecific eavesdropping. Curr Zool 63:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan EM, Alipour F, 2009. Phonatory effects of supraglottic structures in excised canine larynges. J Voice 23:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT, 2000. The phonetic potential of nonhuman vocal tracts: comparative cineradiographic observations of vocalizing animals. Phonetica 57:205–218. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Kelly JP, 2000. Perception of vocal tract resonances by whooping cranes Grus americana. Ethology 106:559–574. [Google Scholar]
- Fitch WT, Neubauer J, Herzel H, 2002. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim Behav 63:407–418. [Google Scholar]
- Fletcher NH, 2004. A simple frequency-scaling rule for animal communication. J Acoust Soc Am 115:2334–2338. [DOI] [PubMed] [Google Scholar]
- Frey R, Volodin IA, Fritsch G, Volodina EV, 2016. Potential sources of high frequency and biphonic vocalization in the dhole Cuon alpinus. PLoS One 11:e0146330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Gregory SE, Stull JW, Rice MR, Herron ME, 2016. Effects of trazodone on behavioral signs of stress in hospitalized dogs. J Am Vet Med Assoc 249:1281–1291. [DOI] [PubMed] [Google Scholar]
- Gilmore KM, Greer KA, 2015. Why is the dog an ideal model for aging research? Exp Gerontol 71:14–20. [DOI] [PubMed] [Google Scholar]
- Gogoleva SS, Volodin IA, Volodina EV, Trut LN, 2008. To bark or not to bark: vocalization in red foxes selected for tameness or aggressiveness toward humans. Bioacoustics 18:99–132. [Google Scholar]
- Grimsley JMS, Monaghan JJM, Wenstrup JJ, 2011. Development of social vocalizations in mice. PLoS One 6:e17460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobman M, Lever T, Reinero CR, 2019. Discrimination between respiratory and non-respiratory sound waveforms in dogs using acoustic wave recordings: an objective metric of cough. Vet J 253:105380. [DOI] [PubMed] [Google Scholar]
- Guerra LGGC, Silva MTA, 2010. Learning processes and the neural analysis of conditioning. Psychol Neurosci 3:195–208. [Google Scholar]
- Hare JF, 1998. Juvenile Richardson’s ground squirrels Spermophilus richardsonii discriminate among individual alarm callers. Anim Behav 55:451–460. [DOI] [PubMed] [Google Scholar]
- Hasiwa N, Bailey J, Clausing P, Daneshian M, Eileraas M. et al. , 2011. Critical evaluation of the use of dogs in biomedical research and testing in Europe. ALTEX 28:326–340. [DOI] [PubMed] [Google Scholar]
- Hauser MD, 1993. Do vervet monkey infants cry wolf? Anim Behav 45:1242–1244. [Google Scholar]
- Heffner HE, 1983. Hearing in large and small dogs: absolute thresholds and size of the tympanic membrane. Behav Neurosci 97:310–318. [Google Scholar]
- Herzel H, Reuter R, 1997. Whistle register and biphonation in a child’s voice. Folia Phoniatr Logo 49:216–224. [DOI] [PubMed] [Google Scholar]
- Hewitt G, MacLarnon A, Jones KE, 2002. The functions of laryngeal air sacs in primates: a new hypothesis. Folia Primatol 73:70–94. [DOI] [PubMed] [Google Scholar]
- Huber A, Barber ALA, Faragó T, Müller CA, Huber L, 2017. Investigating emotional contagion in dogs Canis familiaris to emotional sounds of humans and conspecifics. Anim Cognition 20:703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Yeon SC, Houpt KA, Lee HC, Chang HH. et al. , 2005. Acoustic feature of barks of ovariohysterectomized and intact German Shepherd bitches. J Vet Med Sci 67:281–285. [DOI] [PubMed] [Google Scholar]
- Larrañaga A, Bielza C, Pongrácz P, Faragó T, Bálint A. et al. 2015. Comparing supervised learning methods for classifying sex, age, context and individual Mudi dogs from barking. Anim Cognition 18:405–421. [DOI] [PubMed] [Google Scholar]
- Lord K, Feinstein M, Coppinger R, 2009. Barking and mobbing. Behav Proc 81:358–368. [DOI] [PubMed] [Google Scholar]
- Ludwig W, Ludwig C, 2000. Beobachtungen zur sozialen organisation eines Rudels Rothunde Cuon alpinus im Zoo Dresden. Der Zool Garten N F 70:39–59. [Google Scholar]
- Mariti C, Ricci E, Zilocchi M, Gazzano A, 2013. Owners as a secure base for their dogs. Behaviour 150:1275–1294. [Google Scholar]
- Martin K, Tucker MA, Rogers TL, 2017. Does size matter? Examining the drivers of mammalian vocalizations. Evolution 71:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb K, Taylor AM, Wilson C, Charlton BD, 2009. The cry embedded within the purr. Curr Biol 19:R507–R508. [DOI] [PubMed] [Google Scholar]
- Mergell P, Herzel H, 1997. Modelling biphonation: the role of the vocal tract. Speech Commun 22:141–154. [Google Scholar]
- Molnár C, Kaplan F, Roy P, Pachet F, Pongrácz P. et al. 2008. Classification of dog barks: a machine learning approach. Anim Cognition 11:389–400. [DOI] [PubMed] [Google Scholar]
- Musolf K, Penn DJ, 2012. Ultrasonic vocalizations in house mice: a cryptic mode of acoustic communication. In: Macholan M, Baird SJE, Munclinger P, Pialek J, editors. Evolution of the Housemouse. Cambridge: Cambridge University Press, 253–277. [Google Scholar]
- Nicastro N, 2004. Perceptual and acoustic evidence for species-level differences in meow vocalizations by domestic cats Felis catus and African wild cats Felis silvestris lybica. J Compar Psychol 118:287–296. [DOI] [PubMed] [Google Scholar]
- Parsons CE, LeBeau RT, Kringelbach ML, Young KS, 2019. Pawsitively sad: pet-owners are more sensitive to negative emotion in animal distress vocalizations. Royal Soc Open Sci 6:181555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasch B, Tokuda IT, Riede T, 2017. Grasshopper mice employ distinct vocal production mechanisms in different social contexts. Proc Royal Soc B 284:20171158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongrácz P, Molnár C, Miklósi A, 2010. Barking in family dogs: an ethological approach. Vet J 183:141–147. [DOI] [PubMed] [Google Scholar]
- Riede T, 2011. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol 106:2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, 2013. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. J Exp Zool A 319:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, 2018. Peripheral vocal motor dynamics and combinatory call complexity of ultrasonic vocal production in rats. In: Brudzynski SM, editor. Handbook of Ultrasonic Vocalization. V. 25. A Window into the Emotional Brain. Amsterdam, Netherlands: Elsevier, Academic Press, 45–60. [Google Scholar]
- Riede T, Brown C, 2013. Body size, vocal fold length, and fundamental frequency: implications for mammal vocal communication. Nova Acta Leopoldina NF b295–314. [Google Scholar]
- Riede T, Fitch WT, 1999. Vocal tract length and acoustics of vocalization in the domestic dog Canis familiaris. J Exp Biol 202:2859–2867. [DOI] [PubMed] [Google Scholar]
- Riede T, Herzel H, Mehwald D, Seidner W, Trumler E. et al. , 2000. Nonlinear phenomena in the natural howling of a dog-wolf mix. J Acoust Soc Am 108:1435–1442. [DOI] [PubMed] [Google Scholar]
- Riede T, Herzel H, Hammerschmidt K, Brunnberg L, Tembrock G, 2001. The harmonic-to-noise ratio applied to dog barks. J Acoust Soc Am 110:2191–2197. [DOI] [PubMed] [Google Scholar]
- Riede T, Mitchell BR, Tokuda I, Owren MJ, 2005. Characterizing noise in nonhuman vocalizations: acoustic analysis and human perception of barks by coyotes and dogs. J Acoust Soc Am 118:514–522. [DOI] [PubMed] [Google Scholar]
- Riede T, York A, Furst S, Müller R, Seelecke S, 2011. Elasticity and stress relaxation of a very small vocal fold. J Biomech 44:1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Borgard HL, Pasch B, 2017. Laryngeal airway reconstruction indicates that rodent ultrasonic vocalizations are produced by an edge-tone mechanism. Royal Soc Open Sci 4:170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LH, 1975. The rodent ultrasound production mechanism. Ultrasonics 13:83–88. [DOI] [PubMed] [Google Scholar]
- Rowell JL, McCarthy DO, Alvarez CE, 2011. Dog models of naturally occurring cancer. Trends Mol Med 17:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN, 2008. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One 3:e3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schassburger RM, 1987. Wolf vocalization: an integrated model of structure, motivation and ontogeny. In: Frank H, editor. Man and Wolf. Dordrecht, Netherland: Dr W. Junk Publishers, 313–346. [Google Scholar]
- Schneider JN, Anderson RE, 2011. Tonal vocalizations in the red wolf Canis rufus: potential functions of nonlinear sound production. J Acoust Soc Am 130:2275–2284. [DOI] [PubMed] [Google Scholar]
- Shearman JR, Wilton AN, 2011. Origins of the domestic dog and the rich potential for gene mapping. Genet Res Int 2011:579308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NP, Luschei E, Kang L, 1995. Fundamental frequency and tracheal pressure during three types of vocalizations elicited from anaesthetized dogs. J Voice 9:403–412. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Reby D, 2010. The contribution of source-filter theory to mammal vocal communication research. J Zool 280:221–236. [Google Scholar]
- Taylor A, Reby D, McComb K, 2008. Human listeners attend to size information in domestic dog growls. J Acoust Soc Am 123:2903–2909. [DOI] [PubMed] [Google Scholar]
- Taylor A, Reby D, McComb K, 2010. a. Size communication in domestic dog Canis familiaris growls. Anim Behav 79:205–210. [Google Scholar]
- Taylor A, Reby D, McComb K, 2010. b. Why do large dogs sound more aggressive to human listeners: acoustic bases of motivational misattributions. Ethology 116:1–8.21132114 [Google Scholar]
- Taylor A, Reby D, McComb K, 2011. Cross modal perception of body size in domestic dogs Canis familiaris. PLoS One 6:e17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Ratcliffe VF, McComb K, Reby D, 2014. Auditory communication in domestic dogs: vocal signalling in the extended social environment of a companion animal. In: Kaminski J, Marshall-Pescini S, editors. The Social Dog: Behavior and Cognition. Amsterdam, Netherlands: Elsevier, Academic Press, 131–163. [Google Scholar]
- Tembrock G, 1976. Canid vocalizations. Behav Proc 1:57–75. [DOI] [PubMed] [Google Scholar]
- Tokuda I, Riede T, Neubauer J, Owren MJ, Herzel H, 2002. Nonlinear analysis of irregular animal vocalizations. J Acoust Soc Am 111:2908–2919. [DOI] [PubMed] [Google Scholar]
- Tooze ZJ, Harrington FH, Fentress JC, 1990. Individually distinct vocalizations in timber wolves, Canis lupus. Anim Behav 40:723–730. [Google Scholar]
- Volodin IA, Volodina EV, 2002. Biphonation as a prominent feature of the dhole Cuon alpinus sounds. Bioacoustics 13:105–120. [Google Scholar]
- Volodin IA, Volodina EV, Isaeva IV, 2001. Vocal repertoire in the Dhole Cuon alpinus (Carnivora, Canidae) in captivity. Entomol Rev 81:S346–S361. [Google Scholar]
- Volodin IA, Volodina EV, Filatova OA, 2005. Structural peculiarities, occurrence and functional significance of nonlinear phenomena in calls of terrestrial mammals. J Gen Biol 66:346–362. [PubMed] [Google Scholar]
- Volodin IA, Volodina EV, Nagaylik MM, 2006. c. Cues to orientation of a caller to a listener in biphonic and non-biphonic close range contact calls in the dhole Cuon alpinus. Adv Bioacous Dissert SASA Hist Nat 47:245–255. [Google Scholar]
- Volodina EV, Volodin IA, 2018. Dogs Canis familiaris and dholes Cuon alpinus squeak close to ultrasound. In: Gridley T, editor. 1st African Bioacoustics Community Conference. Cape Town. South Africa: University of Cape Town, 50. [Google Scholar]
- Volodina EV, Volodin IA, Filatova OA, 2006. a. The occurrence of nonlinear vocal phenomena in frustration whines of the domestic dog Canis familiaris. Adv Bioacoust Dissert SASA Hist Nat 47:257–270. [Google Scholar]
- Volodina EV, Volodin IA, Isaeva IV, Unck C, 2006. b. Biphonation may function to enhance individual recognition in the dhole Cuon alpinus. Ethology 112:815–825. [Google Scholar]
- Westgarth C, Knuiman M, Christian HE, 2016. Understanding how dogs encourage and motivate walking: cross-sectional findings from RESIDE. BMC Public Health 16:1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden I, 1997. Phonetische Variabilitat in Der Lautgebung Afrikanischer Wildhunde (Lycaon Pictus) Und Deren Fruhe Ontogenese. Aachen, Germany: Snaker Verlag. [Google Scholar]
- Wilden I, Herzel H, Peters G, Tembrock G, 1998. Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics 9:171–196. [Google Scholar]
- Wilson DR, Hare JF, 2004. Ground squirrel uses ultrasonic alarms. Nature 430:523. [DOI] [PubMed] [Google Scholar]
- Wilson DR, Hare JF, 2006. The adaptive utility of Richardson’s ground squirrel Spermophilus richardsonii short-range ultrasonic alarm signals. Can J Zool 84:1322–1330. [Google Scholar]
- Yeon SC, 2007. The vocal communication of canines. J Vet Behav 2:141–144. [Google Scholar]
- Yin S, McCowan B, 2004. Barking in domestic dogs: context specificity and individual identification. Anim Behav 68:343–355. [Google Scholar]
- Yordy J, Kraus C, Hayward JJ, White ME, Shannon LM. et al. 2020. Body size, inbreeding, and lifespan in domestic dogs. Conservat Genet 21:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurlova DD, Volodin IA, Ilchenko OG, Volodina EV, 2020. Rapid development of mature vocal patterns of ultrasonic calls in a fast-growing rodent, the yellow steppe lemming Eolagurus luteus. PLoS One 15:e0228892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaytseva AS, Volodin IA, Ilchenko OG, Volodina EV, 2019. Ultrasonic vocalization of pup and adult fat-tailed gerbils Pachyuromys duprasi. PLoS One 14:e0219749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.