Abstract
In sexually dimorphic species characterized by exaggerated male ornamentation, behavioral isolation is often attributed to female preferences for conspecific male signals. Yet, in a number of sexually dimorphic species, male mate choice also results in behavioral isolation. In many of these cases, the female traits that mediate species boundaries are unclear. Females in sexually dimorphic species typically lack many of the elaborate traits that are present in males and that are often used for taxonomic classification of species. In a diverse and largely sexually dimorphic group of fishes called darters (Percidae: Etheostoma), male mate choice contributes to behavioral isolation between a number of species; however, studies addressing which female traits males prefer are lacking. In this study, we identified the dominant female pattern for two sympatric species, Etheostoma zonale and Etheostoma barrenense, using pattern energy analysis, and we used discriminate function analysis to identify which aspects of female patterning can reliably classify species. We then tested the role of female features in male mate choice for E. zonale, by measuring male preference for computer animations displaying the identified (species-specific) conspecific features. We found that the region above the lateral line is important in mediating male mate preferences, with males spending a significantly greater proportion of time with animations exhibiting conspecific female patterning in this region than with animations exhibiting heterospecific female patterning. Our results suggest that the aspects of female phenotypes that are the target of male mate choice are different from the conspicuous male phenotypes that traditionally characterize species.
Keywords: darters, discriminant function analysis, Etheostoma, female phenotype, male mate choice, 3D animations
Behavioral isolation is the reduction of gene flow between populations or species due to differences in courtship signals and preferences and is often considered as one of the most important barriers to gene flow between closely related animal species (Coyne and Orr 2004). In species characterized by extreme sexual dimorphism, behavioral isolation is most often attributed to female preferences for elaborate conspecific male traits (Kraaijeveld et al. 2011), as female preferences are thought to be primarily responsible for male trait elaboration (Andersson 1994). However, male mate choice has received increasing attention as more studies demonstrate that males, even of sexually dimorphic species with conventional sex roles, are choosy (Amundsen et al. 1997; Amundsen and Forsgren 2001; Pizzari et al. 2003; Werner and Lotem 2003; Reading and Backwell 2007), suggesting that male preferences also contribute to behavioral isolation in sexually dimorphic species. Identifying the courtship signals whose differences contribute to behavioral isolation for both males and females is therefore fundamental to understanding the dynamics of many species boundaries.
Elaboration of male traits via female mate choice may promote behavioral isolation if divergence in traits and preferences reduce interbreeding between species (Ryan and Rand 1993; Coyne and Orr 2004). In these cases, traits that promote behavioral isolation would be species-specific, conspicuous male traits that are preferred by conspecific females (e.g., coloration in haplochromine cichlids, Seehausen and van Alphen 1998; pulse rate of acoustic signals in Gryllus and Laupala crickets, Gray and Cade 2000; Mendelson and Shaw 2002; visual and vocal signals in Passerina spp., Baker and Baker 1990). In contrast, female ornaments are predicted to be less conspicuous in lineages with traditional sex roles because of potential tradeoffs between investment in reproduction and investment in trait elaboration (Bonduriansky 2001; Servedio and Lande 2006). Furthermore, if females are unlikely to remain unmated (e.g., because of an abundance of sexually available males relative to females), then the benefits of elaborated female traits may be small (Fitzpatrick et al. 1995; LeBas 2006) or outweighed by their costs (Long et al. 2009). However, females of many sexually dimorphic species do possess reduced forms of male elaborations, such as the subdued carotenoid-based coloration present in some female house finches, Carpodacus mexicanus (Hill 1993). There are also examples of female-specific elaborations, such as the yellow-orange coloration of eggs visible through the female’s abdomen in the two-spotted goby, Gobiusculus flavescens (Amundsen and Forsgren 2001). These examples raise the possibility that behavioral isolation via male mate choice could be mediated by signals shared between the sexes or by female-specific elaborations.
Darters (Percidae: Etheosotma) are a diverse group of freshwater fish, largely characterized by elaborate, male-limited coloration (Kuehne and Barbour 1983). Male mate choice has been found to contribute to behavioral isolation across many darter species (Ciccotto et al. 2013; Martin and Mendelson 2016) and, in some cases, male preferences appear to contribute as much as, or more than, female preferences to isolation (Moran et al. 2017; Roberts and Mendelson 2017; Mendelson et al. 2018). Male choice may be prevalent in this group due to a relatively balanced operational sex ratio because many species of darters provide no parental care (Page et al. 1985), and/or because males generally initiate courtship, which may select for male choice if the costs of female rejection are high (von Schilcher and Dow 1977; Edward and Chapman 2011). Behavioral isolation is the strongest isolating barrier measured between the sympatric species pair Etheostoma zonale and Etheostoma barrenense (Williams and Mendelson 2014). Both males and females in these species strongly prefer conspecific mates, and those preferences appear to be mediated based largely on visual cues (Williams and Mendelson 2010; Roberts and Mendelson 2017). These findings suggest that females possess species-specific visual features, even if they are phenotypically less distinct than males, and that males prefer species-specific female phenotypes.
Etheostoma zonale is the focal species of this study. During the breeding season, typically March through May, male E. zonale have alternating green and yellow bars along the body, whereas males of the sympatric congener E. barrenense display primarily red-orange coloration with black blotches fused along the lateral line to form a stripe (Figure 1A, B). Females of both species display the patterning of conspecific males but only muted coloration, if any at all (Figure 1C, D). In addition, females are easily distinguished from males during the breeding season by swollen white bellies that dampen (reduce the contrast intensity of) or eliminate the patterning below the lateral line. In a previous study aimed to identify the visual cues that explain female preference for conspecific males in this species pair, Williams and Mendelson (2011) used hand-painted motorized models and showed that females of both species prefer conspecific male color (green versus red models) and pattern (bars versus stripe). In contrast, the female traits that drive male preference for conspecific females remain unclear.
Figure 1.
Representative photos taken during the breeding season of male E. zonale (A), E. barrenense (B), female E. zonale (C), and E. barrenense (D).
In this study, we aimed to determine which aspects of female visual signals explain preference in male E. zonale for conspecific over heterospecific E. barrenense females. We first used pattern energy analysis to identify the dominant female breeding pattern in both species (Stoddard and Stevens 2010; Troscianko and Stevens 2015). Pattern energy analysis analyzes the contribution of different marking sizes to a given pattern and has been used to quantify various aspects of pattern attributes (e.g., marking size, coverage) in studies of host–parasite egg matching (Stoddard and Stevens 2010). We then used discriminant function analysis (DFA) to determine which features of the dominant female region identified via pattern energy analysis can reliably classify species. Female pattern features that could reliably classify species were then tested in behavioral assays to measure their effect on the behavior of male E. zonale. We also tested male preference for female barred patterning, because that pattern element (vertical bars) is shared between the sexes in E. zonale and is preferred by females (Williams and Mendelson 2011). We utilized 3D digital animations in behavioral assays, allowing us to test whether addition of conspecific features to heterospecific animations would increase male preference.
Materials and Methods
Quantifying focal regions: pattern energy analysis
RAW photographs of female (N = 38) and male (N = 27) E. zonale and female (N = 29) and male (N = 12) E. barrenense were taken with a macrophotography setup using a Canon Mark IV camera with a Canon EF 100 mm f/2.8 L macro lens (Canon USA, Inc., Lake Success, NY, USA). Fishes were positioned laterally and pinned with all fins extended (with the exception of the pectoral fin which was removed in order to provide an unobstructed view of body color and pattern) in 10% formalin for ∼10 min. The camera was mounted on a Cognisys Stackshot Extended Macro Rail (Cognisys Inc., Traverse City, MI, USA) and set to focus the camera on the highest focal plane of each fish. Photographs were then taken at 0.5 mm intervals until the lowest focal plane of the fish was in focus, generating a series of images at different focal planes. These images were then stacked using Zerene Stacker (Zerene Systems, Richland, WA, USA) to create a single high-resolution image for each fish, and we calculated the predicted photoreceptor response of the darter visual system for each pixel in the image using the Image Analysis and Calibration Toolbox plugin in ImageJ (Troscianko and Stevens 2015; Roberts et al. 2017).
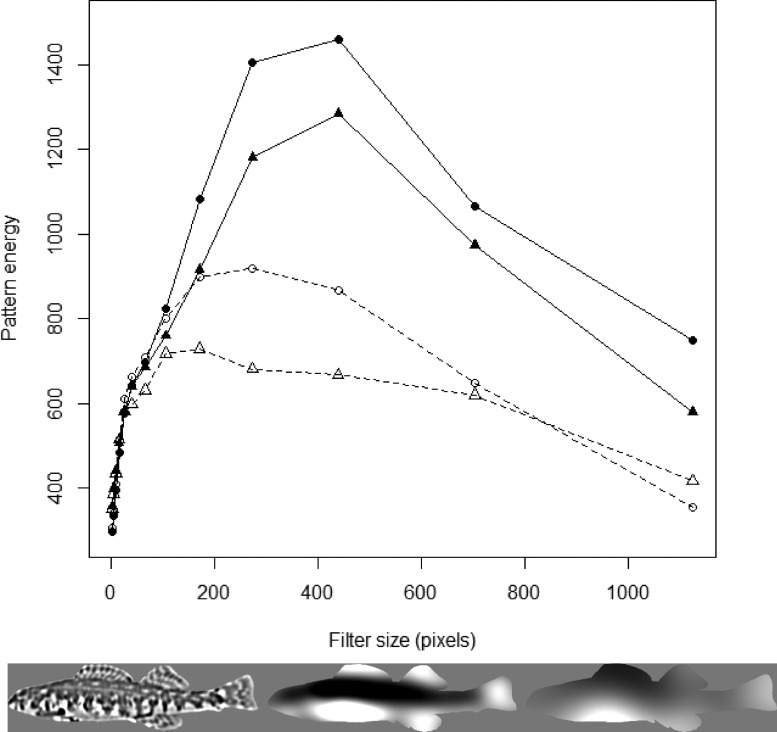
To identify the dominant body pattern, we used the pattern energy analysis function in the ImageJ plugin (Troscianko and Stevens 2015). Pattern energy analysis filters an original image into a series of other images, each representing a different spatial scale (Barbosa et al. 2008; Chiao et al. 2009; Stoddard and Stevens 2010). This process is similar to a sieve, in which different filter sizes allow different aspects of an image to be retained, with the sum of all filtered images closely approximating the original image. Pattern energy is then calculated as the standard deviation of all pixel values for each filtered image, with the filtered image that has the highest pattern energy (i.e., highest standard deviation of pixel values) containing the pattern elements that make up an individual’s dominant marking (Stoddard and Stevens 2010; Troscianko and Stevens 2015).
We filtered images using a 1.6 step multiplier, starting at a 4-pixel scale to generate 13 filtered images of each individual. We calculated pattern energy for each of the 13 filtered images and then averaged pattern energy at the sex and species level to identify the species-specific dominant marking for male and female E. zonale and E. barrenense (Figure 2). In the filtered image containing the dominant marking, we used the threshold tool in ImageJ to select the lowest pixel values (i.e., darker areas) until 25% of total pixel values were selected. We term this 25% selection the “focal region.”
Figure 2.
Average pattern energy spectra for E. zonale (females: closed triangles; males: open triangles) and E. barrenense (females: closed circles; males: open circles) across 13 filter sizes. Image series below spectra shows an example photograph of a female E. zonale processed at different filter sizes (from left to right: 67, 440, and 1,126 pixels). The center image (440 pixels) is the filter size with the highest pattern energy, indicating this is the dominant marking size.
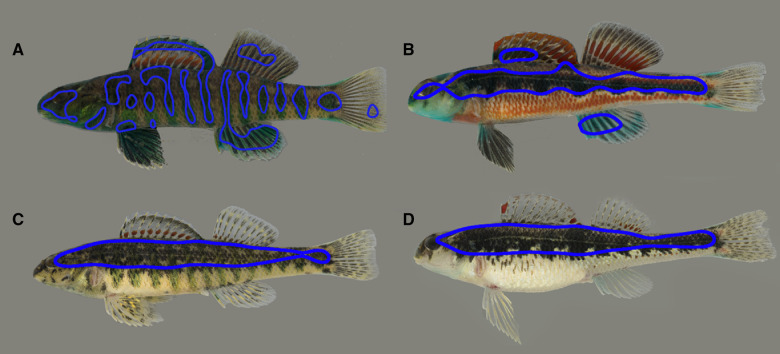
The 25% cutoff value was somewhat arbitrarily chosen; ideally, we would test a range of cutoff values in behavioral assays, but the short (3 months) breeding season of darters is prohibitive. However, using the 25% cutoff value for images of males captured the barred and stripe patterning, respectively, known to be preferred by females (Figure 3A, B; Williams and Mendelson 2011, 2013), suggesting that the 25% cutoff value can identify behaviorally relevant body patterns. Using the 25% cutoff value for images of females captured nearly the entire region above the lateral line (Figure 3C, D). Thus, through pattern energy analysis and using a 25% threshold to select the dominant marking, we selected the female focal region as the flank above the lateral line for both E. zonale and E. barrenense (Figure 3C, D).
Figure 3.
Representative images of focal regions (outlined in blue) for male E. zonale (A), E. barrenense (B), female E. zonale (C), and E. barrenense (D) obtained by selecting 25% of the total body area representing the darker portion of the dominant pattern indicated by pattern energy analysis.
DFA of female focal regions
We quantified the color, luminance, size, and shape of the female focal region in each female E. zonale. We then tested the effect of focal region color, luminance, size, and shape, both individually and collectively, on species classification (using discriminate function analysis) and male mate choice (using behavioral assays).
To calculate focal region color and luminance, we estimated quantal catch from the darter medium wavelength-sensitive (MWS) and long wavelength-sensitive (LWS) cone types using the ImageJ plugin (Gumm et al. 2012; Roberts et al. 2017; Troscianko and Stevens 2015). Color was defined as the difference in quantal catch between the LWS and MWS cone types (Williams and Mendelson 2013), and luminance was calculated as quantal catch from LWS types (Marshall et al. 2003). Focal region size was quantified as the total number of pixels contained within the focal region. Focal region shape was quantified using elliptical Fourier analysis (Kuhl and Giardina 1982; Rohlf and Archie 1984; McLellan and Endler 1998) which breaks down a shape outline into mathematically defined ellipses (i.e., harmonics) of different sizes, orientations, and eccentricities to describe increasingly finer shape details. This method has been used to classify complex biological shapes such as corals (Pseudopterogorgia spp., Carlo et al. 2011) and face patterns in guenon monkeys (tribe: Cercopithecini; Allen and Higham 2015). Using the program Shape (version 1.3.; Iwata and Ukai 2002), we extracted 20 harmonics, which achieved a close visual match to segmented shape, and then performed PCA to reduce shape descriptors.
To determine whether or which of the features of the female focal region (i.e., color, luminance, size, and shape) could accurately predict species identity, we used linear DFA in R (version 3.5.0; R Core Development Team 2015) using the package MASS and code written by R. Mundry (MPA for Evolutionary Anthropology, Leipzig, Germany). DFA attempts to classify groups on the basis of a set of predictor variables. We evaluated classification performance by evaluating whether our DFA model was able to correctly classify species significantly better than when compared with a null model. The null model is created by randomly assigning observations to either group (i.e., E. zonale or E. barrenense), irrespective of their actual group membership, and then performing DFA on the randomized dataset. Thus, for the null model, there should be no discriminability between groups. The classification accuracy of the observed data can then be compared with the null model to assess whether classification performance is significantly better than the null model.
In addition to performing DFA on each measured focal region feature individually, we also performed DFA on a “full” model that included all focal region measurements, that is, focal region color, luminance, size, and shape. The full model considers the additive effect of all measured focal region features on species discrimination.
Behavioral assays
Behavioral assays were conducted in 2018 (Year 1) and 2019 (Year 2). Experimental protocols were changed between years based on results obtained in Year 1 and to improve overall testing procedures. Methods for creating animations and for behavioral assays that are consistent between Years 1 and 2 assays are described first. Methodological details specific to Years 1 and 2 are described in their respective sections.
To test the effect of female body pattern elements on male mate preference, we created 3D animations to be presented to males in behavioral assays, following methods in Roberts et al. (2019). 3D digital models of E. zonale and E. barrenense were purchased from Turbosquid (product ID female E. zonale: 928023; female E. barrenense: 957636) and imported into the animation software program Autodesk Maya (Autodesk Inc, San Rafael, CA, USA). To create a path for models to follow, we imported video of live darters into Maya and matched the model’s location and body position to stills from the video footage. All animations in a given year followed the same path, that is, displayed the same behavioral sequence. “Skins” were then applied over 3D models in Maya to provide conspecific or heterospecific features to models. Skins are 2D images that are wrapped around a 3D model so that the 2D image covers the 3D space of the model. After applying a path and skin to 3D models, animations were exported as a video file to be used in behavioral assays. Etheostoma zonale responds to video and computer animations in a comparable manner to live stimuli (Roberts et al. 2017, 2019) and therefore was used as the focal species for behavioral assays.
Experimental setup for the behavioral assays followed methods in Roberts et al. (2019), with a 37.9-L central test tank (50 L × 25 W × 30 H cm) flanked on either short side by two computer monitors with two 10-cm “association zones” marked on each side of the tank closest to the computer monitors. Male E. zonale were placed in the test tank while the monitors displayed a gray screen for 10 min to allow for acclimation to the testing environment. One monitor then began playing an animation of a conspecific female (or conspecific female feature), whereas the other monitor played an animation of a heterospecific female (or feature). Once the focal male had visited both 10-cm association zones and subsequently returned to the neutral zone (i.e., was not in either association zone), we began recording the amount of time a male spent in the conspecific and heterospecific association zones over a 10-min period (“trial”) using JWatcher (Blumstein et al. 2000).
Year 1
In Year 1, we used videos of live male E. zonale and E. barrenense as a reference to create a movement path for animations, which was a composite of 15 s E. zonale behavior and 15 s E. barrenense behavior. To create conspecific skins, we randomly selected RAW photographs of female E. zonale from Line Creek and Middle Fork Red River (N = 3 from each site). Heterospecific skins were created using RAW photographs of female E. barrenense from the East Fork Barren River (N = 6).
Based on the DFA results, we tested the effect of focal feature (1) color, (2) shape, and (3) the full focal region on male preferences. We created 5 different types of animation stimulus pairs (treatments) for Year 1 assays: baseline, color-only, shape-only, unaltered focal region, and random conspecific area (Figure 4A–D, F). RAW photographs were manipulated in Adobe Photoshop (Adobe Inc., San Jose, CA, USA) to generate the skins.
Figure 4.
Examples of images used as skins to make 3D animated models for behavioral trials. Unaltered conspecific female E. zonale and unaltered heterospecific female E. barrenense used in baseline trials (A). Color-only animations were created using the normalized average focal region color for conspecific and heterospecific coloration applied to 3D models (B). Shape-only animations used conspecific and heterospecific focal region shape in black on a white background applied to the female E. barrenense 3D model (C). Full focal region animations were created by applying a conspecific E. zonale focal region over a heterospecific E. barrenense female image (D). Barred animations were created by applying a conspecific barred patterning over a heterospecific female image (E). Random area control animations were created by randomly selecting 100 × 100 pixel squares of conspecific E. zonale and applying these over a heterospecific female image (F). Nonfocal area control animations were created by selecting a contiguous patch representing 25% of conspecific E. zonale area excluding any area of the focal region and applying it over a heterospecific female image (G). Stimuli consisting of a heterospecific base model and image with conspecific elements applied over the top (D–G) were paired in behavioral trials with the unaltered heterospecific female.
Baseline animations were created using unaltered photographs of female E. zonale and E. barrenense (Figure 4A). Male E. zonale were predicted to show strong preference for conspecific female animations in baseline trails, based on previous studies using both live (Roberts and Mendelson 2017) and animated (Roberts et al. 2019) females. Thus, male preference in baseline treatments acted as a positive control.
To create color-only animations, we measured the average RGB pixel value for female E. zonale and E. barrenense focal regions. The focal region RGB values were normalized so the sum of each independent color channel was equal between all photographs, allowing us to test the effect of focal region color independent of differences in overall brightness. The normalized average RGB values of conspecific and heterospecific focal regions were applied as the skins for animations. This resulted in animations that displayed either conspecific or heterospecific focal region color over the entire 3D model (Figure 4B).
We created the shape-only animations by filling in the focal region area with pure black and applying pure white fill to the remainder of the skin (Figure 4C). This was done for both conspecific and heterospecific focal region shape to generate conspecific shape-only animations and heterospecific shape-only animations. The shape-only skin was then applied to animations, allowing us to test the effect of focal region shape without any effect of color.
Because the full DFA model including all focal region features also significantly predicted species identity, we created unaltered focal region animations to test the effect of focal region features collectively on male mate choice. To create the unaltered focal region animation, we took a photograph of a heterospecific female and pasted a focal region from E. zonale over the original image (Figure 4D). This skin was added to the models, resulting in an animation of a heterospecific female displaying a conspecific female focal region. The focal region animation allowed us to test whether the addition of the conspecific focal region to a heterospecific female increases male preference for an otherwise heterospecific animation. Focal region animations were paired with unaltered heterospecific animations in the focal region treatment.
The random conspecific area animations were created by randomly selecting 100 × 100 pixel squares of female E. zonale patterning representing 25% of the total female body and applying these over an image of a female E. barrenense (Figure 4F). Randomized selection of 100 × 100 pixel squares was achieved by overlaying a numbered grid over female E. zonale images in Adobe Photoshop and selecting numbered squares to be included in the final skin using a random number generator. Random area animations were a type of negative control, allowing us to control for the skin editing process in general and potentially confounding effects of pasting conspecific elements over a heterospecific female. The random area control also indicated whether conspecific pattern elements outside of those being directly tested in the focal region treatment influence male preferences. Random area animations were paired with unaltered heterospecific animations in the random control treatment.
During Year 1, we tested N = 12 males with all five treatments: baseline, color, shape, full focal region, and random conspecific area animations. Each male was first shown a baseline treatment, followed by the remaining treatment types in random order. All skins used to create animations were applied to the female E. barrenense 3D model, with the exception of the baseline animations. The side of the tank that the conspecific feature was presented on was switched between treatments for each individual.
Year 2
In Year 2, we used videos of live female E. zonale as a reference to create a path for animations, which was 47 s of nose jabbing (typically performed prior to spawning) and swimming behavior. To create conspecific skins, we randomly selected RAW photographs of female E. zonale from Line Creek (N = 5). Heterospecific skins were created using RAW photographs of E. barrenense from the East Fork Barren River (N = 5).
The only treatments that resulted in a significant preference in Year 1 were the baseline and focal region treatments. We therefore decided to replicate these treatments in Year 2. In addition, we created two new types of animations for Year 2 treatments: barred pattern, and a new negative control featuring a nonfocal contiguous region (Figure 4E, G).
Baseline and focal region animations were created in the same manner as in Year 1. A barred animation treatment was added to Year 2 because the vertical bars characteristic of E. zonale are shared between the sexes (Figure 1A, C) and have been shown to be preferred by female E. zonale (Williams and Mendelson 2011). We therefore created and tested barred animations in Year 2 to determine if the barred patterning is as effective in eliciting male preference as the female focal region we identified in the pattern energy analysis. We created the barred animation by manually selecting female E. zonale bars using the polygon selection tool in Adobe Photoshop and adding the barred patterning over a heterospecific female skin. This skin was applied to animations, resulting in animations of female E. barrenense (heterospecific) displaying female E. zonale (conspecific) bars (Figure 4E). Barred animations were paired with unaltered heterospecific animations in the barred treatment.
We also replaced the random conspecific area control animations with nonfocal, contiguous conspecific control animations. The nonfocal area control was created by selecting a contiguous body area of a female E. zonale, excluding any area that was within the focal region, and applying this over a female E. barrenense skin. This resulted in animations that had 25% nonfocal conspecific phenotype over an otherwise heterospecific female (Figure 4G). Because focal regions were largely contiguous areas of female patterning, contiguous nonfocal area animations allowed for a more comparable control of the editing process than the random area controls in Year 1. The nonfocal area control tests whether male preferences in the focal region treatment are simply due to receiving sufficient (i.e., 25%) conspecific signal, regardless of its location on the body. Nonfocal contiguous controls were paired with unaltered heterospecific animations in the nonfocal contiguous control treatment.
In Year 2, males were subjected to (1) two trials of the baseline treatment, followed by one trial each for the (2) focal region, (3) barred, and (4) nonfocal contiguous control treatments, in randomized order. The first baseline trial presented males with an unaltered conspecific and an unaltered heterospecific female skin. The second baseline trial showed the same unaltered skins but with the conspecific side of the tank reversed. These animations displayed the full skins of conspecific and heterospecific females, but unlike Year 1, both animations were the E. barrenense 3D model (body shape). This helped ensure that males preferred the patterning of conspecific females independent of body shape, and also that males were consistent in their preference across two trials. Applying an unaltered conspecific skin to the 3D E. barrenense model also provided a better comparison to male preferences for manipulated animations, which were created using the female E. barrenense 3D model. We tested N = 25 males with each of the four treatment types. The side of the tank that the conspecific feature was presented on was switched between treatments for each individual.
Analysis
For both years, we calculated the proportion of time a male spent in the conspecific and heterospecific association zone relative to the entire 10-min trial time. We tested whether males spent a larger proportion of trial time with the conspecific animation relative to the heterospecific animation for all treatments as a proxy of male preference. We used association time as a proxy for mating preference in this study because association time has been shown to reliably predict mating outcomes in other species of fishes (Aspbury and Basolo 2002; Gonçalves and Oliveira 2003; Lehtonen and Lindström 2008; Jeswiet and Godin 2011) and association times in dichotomous choice assays are consistent with mating preferences in unrestricted stream trials for darters (Williams and Mendelson 2010; Martin and Mendelson 2013).
To determine whether parametric or nonparametric tests should be used in data analysis, we assessed normality of the data residuals using Shapiro–Wilks tests. Parametric t-tests or Wilxocon signed rank tests (nonparametric) were used where appropriate. We also computed effect sizes (d) for comparisons between proportion of time spent in the conspecific and heterospecific association zone for all treatments using the “effsize” package in R. Effect sizes provide a description of the magnitude of the effect of an observed treatment independent of sample size and are valuable for comparing treatments within and between studies (Fritz et al. 2012). Benchmarks for interpreting effect sizes described by Cohen (1988) suggest d values of 0.8 are large, 0.5 are intermediate, and 0.2 are small, although the exact interpretation of effect size depends on the particular area of study.
We also used a generalized linear mixed-effects model to test whether relative percent time spent in the conspecific and heterospecific association zone differed between treatments. Because the response variable was a proportional response (i.e., percent time associating with the conspecific animation relative to percent time associating with the heterospecific animation), we specified a binomial distribution in the model parameters (Crawley 2007). The model included both Year 1 and Year 2 data in the same analysis, consequently year was included as a random effect in the model, as was individual ID, with treatment type as a fixed effect. Model validation results indicated no violations of the assumptions of homogeneity and normality of the final model. The effect of treatment on association time was assessed by comparing the above model with a null model that did not include any fixed effects (i.e., treatment) using analysis of variance (ANOVA). Post hoc analyses to compare differences between treatment types were conducted with a Tukey correction for multiple comparisons.
To test whether a male exhibited side bias, we calculated the proportion of time spent in the left-hand and right-hand side association zones, relative to the total amount of time spent in both association zones across all treatments. One male from Year 1 and three males from Year 2 spent >75% of their total association time on one side of the tank across all treatments and were removed from analysis for potential side bias, resulting in a final sample of N = 11 for Year 1 and N = 22 for Year 2. After removing males that displayed side bias, there was no significant difference in the amount of time each male spent on the left- and right-hand side of the tank (Wilcoxon signed-rank test: Z = 0.54, P = 0.59). All statistical analyses were conducted in R (version 3.5.0).
Ethical note
Fish used for pattern energy analysis were collected in 2017 between March and April. Etheostoma zonale was collected from the Middle Fork Red River, Powell Co., KY, USA (37.815002, −83.71877) and Line Creek, Monroe Co., KY, USA (36.651835, −85.820182). Etheostoma barrenense was collected from the East Fork Barren River, Monroe Co., KY, USA (36.745964, −85.696728). Males used for behavioral assays were collected from Line Creek in Clay Co., TN, USA (36.606639, −85.745970) and Monroe Co., KY, USA (36.651835, −85.820182) on 22 March 2018 and from the same site in KY the following year on 23 March 2019. All fishes were transported to the University of Maryland Baltimore County and housed in a recirculating aquarium system (Aquatic Habitats, Inc., Apopka, FL, USA) until euthanized for photography or used in behavioral assays. Water temperature, conductivity, and pH for fish housing approximated conditions in the natural habitat. Fishes were maintained on a diet of live black worms provided once daily. Permission to collect fish was granted by the Kentucky Department of Fish and Wildlife Resources (2017: #SC1711121, 2018: #SC1811149, 2019: #SC1911185) and the Tennessee Wildlife Resource Agency (Scientific Collection Permit #1424). Authority to work with live animals was granted by the IACUC (OLAW Assurance Number D16-00462, Protocol TM011061821).
Results
DFA
DFA showed that for females, focal region color (78.9% correctly identified, P < 0.05) and shape (89.5% correctly identified, P < 0.01) reliably classified species identity. The full model, including all of the focal region features, also performed significantly better than the null model, with 91.5% of all cases being correctly classified (P < 0.05).
Quantifying focal regions: pattern energy analysis
Female E. zonale and E. barrenense had the highest pattern energy at the same filter size (440 pixels) and the shape of the pattern spectra was qualitatively similar (Figure 2), indicating similarities between species in the spatial statistics of female pattern. Pattern energy was higher for female E. barrenense than for female E. zonale at the dominant (440 pixel) filter size (t-test: t = 3.67, P = 0.0006; d = 0.94; Figure 2), indicating higher contrast for E. barrenense females in their dominant marking. Males of both species exhibited lower pattern energy overall than females (Figure 2) and also differed from females in their dominant marking size: the filter size with maximum energy for male E. zonale was 172 pixels and for male E. barrenense was 275 pixels. Our analysis thus indicates that pattern is sex-specific, both in dominant marking size and in overall contrast, within each species (Figure 3).
Year 1: behavioral assays
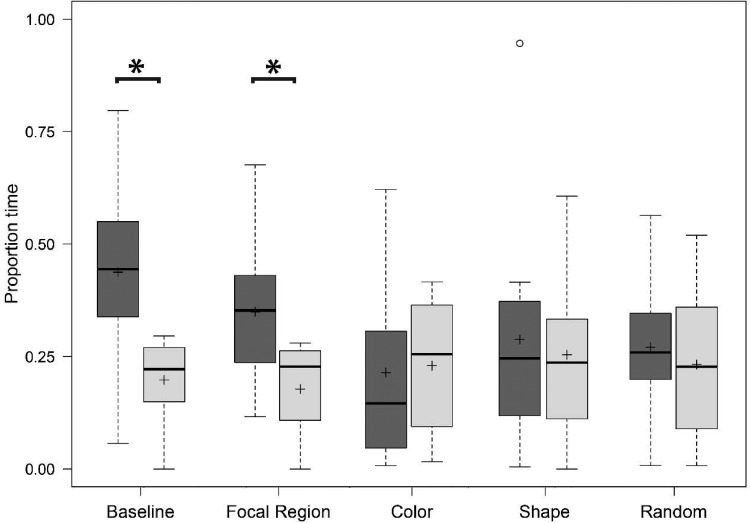
Males spent a significantly larger proportion of time in the conspecific association zone than the heterospecific association zone for baseline animations (paired t-test: t = 2.73, P = 0.021; Figure 5), spending a mean ± SE of 43.77 ± 7.09% of total trial time in the conspecific association zone compared with 19.79 ± 2.86% of total trial time in the heterospecific association zone. Males also spent a significantly larger proportion of time in the conspecific association zone in the focal region treatment (paired t-test: t = 2.90, P = 0.016; Figure 5), spending 34.94 ± 4.91% of total trial time in the conspecific “focal region” association zone compared with 17.76 ± 3.14% of total trial time in the heterospecific association zone for this treatment. There were large effects of treatment type on the proportion of time spent with each stimulus for both the baseline (d = 0.82) and focal region (d = 0.87) treatments. There were no significant differences in the proportion of time spent in either association zone for the color-only (paired t-test: t = −0.17, P = 0.87; Figure 5), shape-only (Wilcoxon signed-rank test: Z = 0.18, P = 0.89; Figure 5), or the random area control treatments (paired t-test: t = 0.46, P = 0.65; Figure 5) and effect sizes for these three treatments were small to negligible (d = 0.05, 0.08, and 0.14, respectively).
Figure 5.
Proportion of trial time spent in the conspecific (dark grey) and heterospecific (light grey) association zones in Year 1. Bars represent medians, boxes indicate upper and lower quartiles, whiskers show sample minima and maxima, open circles show outliers, and crosses indicate sample means. Asterisks indicate a significant difference between conspecific and heterospecific association times within a treatment.
Year 2: behavioral assays
Because males in Year 2 were subjected to two baseline trials prior to experimental treatments, we compared male preference in the first and second baseline trials to determine whether male preference was consistent. To compare male preference across the two baseline trials, we used a standardized metric of strength of preference (SOP), calculated as the difference in time spent with conspecific relative to heterospecific stimuli over total time spent with both stimuli (Stalker 1942). We found no significant difference in SOP between the first and second baseline trials (paired t-test: t = 0.63, P = 0.53, d = 0.13); therefore, we used the average of time spent in the conspecific and heterospecific association zones across the 2 baseline trials to calculate the proportion of time spent in each zone for each individual for the baseline treatment.
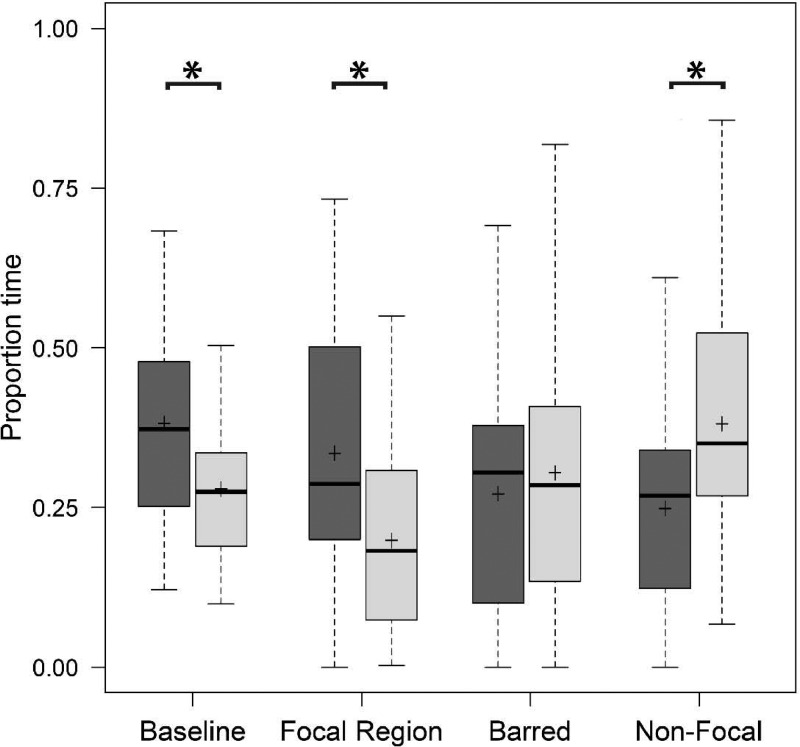
Males spent a significantly larger proportion of time in the conspecific association zone than the heterospecific association zone for the baseline treatment (paired t-test: t = 2.16, P = 0.043; Figure 6) and the focal region treatment (paired t-test: t = 2.11, P = 0.047; Figure 6), as predicted by Year 1 results. In the baseline treatment, males spent 38.16 ± 3.26% of total trial time in the conspecific association zone and 27.91 ± 2.33% of total trial time in the heterospecific association zone. In the focal region treatment, males spent 33.49 ± 4.42% of total trial time in the conspecific “focal region” zone and 19.81 ± 3.29% of total trial time in the heterospecific zone. Effect sizes approached values indicative of an intermediate effect for baseline (d = 0.46) and focal region (d = 0.45) treatments. We also found a significant difference in the proportion of time spent in the conspecific zone relative to the heterospecific zone in the contiguous nonfocal control treatment (paired t-test: t = −2.13, P = 0.045; Figure 6) along with an intermediate effect size (d = 0.50); however, in this case, males spent a larger proportion of time associating with the heterospecific animation (38.11 ± 4.17% of total trial time) than with the conspecific animation (24.85 ± 3.49% of total trial time). There was no significant difference in the proportion of time spent in the conspecific or heterospecific association zones for the barred treatment (paired t-test: t = −0.45, P = 0.67; Figure 6 with a negligible effect size (d = 0.02).
Figure 6.
Proportion of trial time spent in the conspecific (dark grey) and heterospecific (light grey) association zones in Year 2. Bars represent medians, boxes indicate upper and lower quartiles, whiskers show sample minima and maxima, and crosses indicate sample means. Asterisks indicate a significant difference between conspecific and heterospecific association times within a treatment.
Comparison of treatment types
Results of the model suggested a significant effect of treatment type on the relative percentage of time spent with the conspecific and heterospecific stimuli, with the model including treatment performing significantly better than the null model that did not include treatment (ΔAIC (Akaike information criterion) = 210.5; P < 0.0001). Post hoc analyses revealed a significant difference in preference between the baseline treatment and all treatment types except the focal region treatment (Table 1), indicating that preference for the conspecific focal region was not statistically distinguishable from preference for the baseline animations.
Table 1.
Post hoc analysis with Tukey correction of the model predicting the effect of treatment type on the percent of time spent with the conspecific and heterospecific stimuli
| Comparison to baseline | Estimate | SD | z | P-value |
|---|---|---|---|---|
| Barred | −0.47 | 0.08 | −6.00 | <0.001 |
| Color-only | −0.80 | 0.12 | −6.81 | <0.001 |
| Focal region | 0.04 | 0.07 | 0.58 | 0.997 |
| Nonfocal region | −0.80 | 0.08 | −10.57 | <0.001 |
| Random area | −0.54 | 0.11 | −4.80 | <0.001 |
| Shape | −0.56 | 0.11 | −5.16 | <0.001 |
Discussion
Our results suggest that male preference for conspecific females in E. zonale is mediated, at least in part, by a patterning element that we identified as the female focal region, a patch of the body immediately dorsal to the lateral line. Males spent significantly more time with a heterospecific female animation that displayed the conspecific female focal region than with the same animation without the conspecific focal region (Figures 5 and 6). Our data therefore suggest that patterning in a patch immediately dorsal to the lateral line is an important female signal that affects male mate choice in E. zonale.
In species with sexual dimorphism, the palette of potential traits that could mediate behavioral isolation is different for males and females. Moreover, female traits mediating behavioral isolation via male choice are potentially more challenging to identify in sexually dimorphic species, like darters, with “drab” females. Previous studies of female choice in E. zonale indicate that male color mediates behavioral isolation via female choice. Models painted with conspecific (green) versus heterospecific (red) dominant color alone elicited preference for conspecific color in female E. zonale (Williams and Mendelson, 2011, 2013). However, males in our study did not prefer color-only or shape-only conspecific animations (Figure 5), despite results of the DFA showing that both the color and shape of the female focal region alone were able to significantly predict species identity. It is possible that color replication by the computer monitors was not a good representation of actual female color, although a previous study in E. zonale found that computer monitors replicate the majority of darter body coloration similarly to live fish (Roberts et al. 2017). It may also be that males are not the intended receiver of female color or shape, and therefore would not be expected to discriminate between females based on those signals alone. For example, both male and female turquoise-brown motmots (Eumomota superciliosa) exhibit elaborate tail ornaments, which appear to be a sexual signal in males, but there is no apparent function of elaborate female tails in male mate choice (Murphy, 2007a, 2008). Rather, elaborate female tails appear to function as a deterrent against attack by predators (Murphy, 2006, 2007b). Thus, the color and/or shape of the focal region in E. zonale and E. barrenense could function in other interactions, for example with predators, in female–female interactions, or may be preferred by males when tested with species other than the one tested in this study. Alternatively, these features may not be biologically relevant in isolation. For example, in the wolf spider Schizocosa ocreata, females were significantly less receptive to vibrational and visual cues when presented in isolation than when these cues were presented together (Uetz et al. 2009). In the butterfly Heliconius erato, males preferred models displaying homotypic color and pattern more strongly than models displaying homotypic pattern or color in isolation (Finkbeiner et al. 2014). Our result, whether an artifact of experimental design or true biological relevance, suggests that males do not prefer the color or shape of the female focal region alone.
We also found that the barred patterning characteristic of E. zonale was not preferred by males when superimposed on a heterospecific female background. The pattern of E. zonale consists of vertical bars of contrasting intensity that span the entire dorsoventral axis in males (Figures 1 and 3). Our pattern energy analysis confirmed that, for male E. zonale during the breeding season, the barred pattern has the highest energy, that is, represents the dominant pattern (Figure 3). Previous work showed that the barred pattern is attractive to conspecific females, independent of color, and likely contributes to behavioral isolation between E. zonale and E. barrenense (Williams and Mendelson 2011). Females also exhibit barred patterning; however, during the breeding season, their bright, distended abdomens appear to shift the dominant pattern toward countershading, with the highest contrast for females occurring between the pigmentation above the lateral line and the abdomen below (Figure 3). The barred pattern superimposed on a heterospecific female animation may have obscured the dominant countershading pattern and bright, monochromatic abdomen that characterize females.
Our results therefore suggest that the traits that mediate behavioral isolation between E. zonale and E. barrenense are different for males and females. Females show strong preference for conspecific male color (green) and conspecific male pattern (bars) (Williams and Mendelson 2011, 2013), whereas these two elements were not sufficient to elicit preference in males in this study. A difference between males and females in the traits that mediate behavioral isolation is also observed in Drosophila, in which male preference for conspecific females is often driven by pheromonal responses (Cobb and Jallon 1990; Gleason et al. 2005), whereas females tend to prefer conspecific male song (Doi et al. 2001; Gleason 2005). Similarly, Uy et al. (2018) identified several examples of bird species in which traits that mediate behavioral isolation are not the same for males and females, though notably they cite many more examples for which the traits do not differ between males and females.
The two control regions (random area and nonfocal contiguous area) also failed to elicit a preference for the conspecific animation, suggesting there is not some minimum threshold of conspecific area that must be present to elicit a male response. However, one unexpected result was that males preferred the unaltered heterospecific animations over the nonfocal controls in the nonfocal contiguous control treatment in Year 2. The nonfocal control animations displayed a contiguous patch of conspecific pattern from an area of the body outside the focal region (Figure 4), meaning that nonfocal control and focal region animations differed primarily in the location of conspecific signal being displayed. Preference for heterospecific animations in the nonfocal control treatment suggests that males could discriminate between the two stimulus types, yet shifted their preference away from the model displaying some element of conspecific pattern. One explanation is that the nonfocal control represented an abnormal stimulus, which generally has aversive properties in choice assays (Yamazaki et al. 2004). Avoidance of sick or aberrant individuals has been noted across a variety of species (Oaten et al. 2011). For instance, killifish (Fundulus diaphanus) prefer not to associate with conspecific individuals that have been injected with black ink, mimicking the visual phenotype of parasitized individuals (Krause and Godin 1996). Alternatively, the nonfocal conspecific patch may have obstructed or altered the abdomen in some of the nonfocal controls, such that the heterospecific animation, with an unobstructed white abdomen, may have been more attractive.
The region we identified as critical to male mate choice is in close proximity to the abdomen. Female darters with ripe eggs display a visibly distended, mostly monochromatic white abdomen, and there is evidence that males prefer females with larger abdomens in other species of fish (Rowland 1982; Nuttall and Keenleyside 1993). The abdomen in female darters therefore likely conveys information about female fecundity to males. The abdomen is generally similar between species, however, with the majority of species-specific patterning located above the lateral line or within the caudal peduncle in females (Figure 1). Thus, one hypothesis generated by our results is that the focal region, a species-specific phenotype that may indicate compatibility, and the abdomen, a fecundity indicator, together form a composite phenotype that elicit a preferred mating response from male E. zonale. That hypothesis is consistent with a lack of male preference for bars, which may have obscured the abdomen, and with a preference for heterospecific female animations if the nonfocal control region also obscured the abdomen.
In summary, we identified a region of female body patterning that appears to mediate behavioral isolation via male mate choice between sympatric species of darters. The female trait we identified here differs from the dominant pattern in male E. zonale (i.e., vertical bars that span the entire dorsoventral axis) and also differs from the traits that contribute to behavioral isolation via female choice. Male traits that affect female mate choice tend to be those that are easily identified by a human observer in sexually dimorphic species. Identifying female traits that are biologically relevant in male mate choice is a critical first step in understanding female trait evolution and speciation.
Acknowledgments
We would like to thank S. Hulse for assistance in the field and with photography, T. Bhardvay and S. Parsa for their assistance in behavioral assays, and T. Cronin, R. Fuller, J. Leips, and K. Omland for their feedback on manuscript preparation.
Funding
This research was funded in part by an Animal Behavior Society Student Research Grant provided to NSR.
Author contributions
N.S.R. and T.C.M. conceived and designed the experiment and collected field collections. N.S.R. collected data and performed statistical analyses. The manuscript was written by N.S.R. with input from T.C.M. Both authors gave approval of the final manuscript prior to submission.
Conflict of Interest statement
The authors declare that there is no conflict of interest.
References
- Allen WL, Higham JP, 2015. Assessing the potential information content of multicomponent visual signals: a machine learning approach. Proc R Soc B 282:20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, 2001. Male mate choice selects for female coloration in a fish. Proc Natl Acad Sci USA 98:13155–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen T, Forsgren E, Hansen LT, 1997. On the function of female ornaments: male bluethroats prefer colourful females. Proc Royal Soc B 264:1579–1586. [Google Scholar]
- Andersson MB, 1994. Sexual Selection. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Aspbury AS, Basolo AL, 2002. Repeatable female preferences, mating order and mating success in the poeciliid fish Heterandria formosa. Behav Ecol Sociobiol 51:238–244. [Google Scholar]
- Baker MC, Baker AEM, 1990. Reproductive behavior of female buntings: isolating mechanisms in a hybridizing pair of species. Evolution 44:332–338. [DOI] [PubMed] [Google Scholar]
- Barbosa A, Mäthger LM, Buresch KC, Kelly J, Chubb C. et al. , 2008. Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vision Res 48:1242–1253. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Evans CS, Daniel JC, 2000. JWatcherTM 1.0. Available from: http://www.jwatcher.ucla.edu/ (accessed April 2016).
- Bonduriansky R, 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339. [DOI] [PubMed] [Google Scholar]
- Carlo JM, Barbeitos MS, Lasker HR, 2011. Quantifying complex shapes: elliptical Fourier analysis of octocoral sclerites. Biol Bull 220:224–237. [DOI] [PubMed] [Google Scholar]
- Chiao CC, Chubb C, Buresch K, Siemann L, Hanlon RT, 2009. The scaling effects of substrate texture on camouflage patterning in cuttlefish. Vision Res 49:1647–1656. [DOI] [PubMed] [Google Scholar]
- Ciccotto PJ, Gumm JM, Mendelson TC, 2013. Male association preference for conspecifics in the redband darter Etheostoma luteovinctum (Teleostei: Percidae) based on visual cues. Copeia 2013:154–159. [Google Scholar]
- Cobb M, Jallon JM, 1990. Pheromones, mate recognition and courtship stimulation in the Drosophila melanogaster species sub-group. Anim Behav 39:1058–1067. [Google Scholar]
- Coyne JA, Orr H, 2004. Speciation. Sunderland (MA: ): Sinauer. [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillside (NJ: ): Erlbaum. [Google Scholar]
- Crawley MJ, 2007. The R Book. Hoboken (NJ: ): John Wiley & Sons. [Google Scholar]
- Doi M, Matsuda M, Tomaru M, Matsubayashi H, Oguma Y, 2001. A locus for female discrimination behavior causing sexual isolation in Drosophila. Proc Natl Acad Sci USA 98:6714–6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DA, Chapman T, 2011. The evolution and significance of male mate choice. Trends Ecol Evol 26:647–654. [DOI] [PubMed] [Google Scholar]
- Finkbeiner SD, Briscoe AD, Reed RD, 2014. Warning signals are seductive: relative contributions of color and pattern to predator avoidance and mate attraction in Heliconius butterflies. Evolution 68:3410–3420. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S, Berglund A, Rosenqvis G, 1995. Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol J Linn Soc Lond 55:251–260. [Google Scholar]
- Fritz CO, Morris PE, Richler JJ, 2012. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141:2–18. [DOI] [PubMed] [Google Scholar]
- Gleason JM, 2005. Mutations and natural genetic variation in the courtship song of Drosophila. Behav Genet 35:265–277. [DOI] [PubMed] [Google Scholar]
- Gleason JM, Jallon JM, Rouault JD, Ritchie MG, 2005. Quantitative trait loci for cuticular hydrocarbons associated with sexual isolation between Drosophila simulans and D. sechellia. Genetics 171:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves DM, Oliveira RF, 2003. Time spent close to a sexual partner as a measure of female mate preference in a sex-role-reversed population of the blenny Salaria pavo (Risso) (Pisces: Blenniidae). Acta Ethol 6:1–5. [Google Scholar]
- Gray DA, Cade WH, 2000. Sexual selection and speciation in field crickets. Proc Natl Acad Sci 97:14449–14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumm JM, Loew ER, Mendelson TC, 2012. Differences in spectral sensitivity within and among species of darters (genus Etheostoma). Vision Res 55:19–23. [DOI] [PubMed] [Google Scholar]
- Hill GE, 1993. Male mate choice and the evolution of female plumage coloration in the house finch. Evolution 47:1515–1525. [DOI] [PubMed] [Google Scholar]
- Iwata H, Ukai Y, 2002. SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J Hered 93:384–385. [DOI] [PubMed] [Google Scholar]
- Jeswiet SB, Godin JGJ, 2011. Validation of a method for quantifying male mating preferences in the guppy Poecilia reticulata. Ethology 117:422–429. [Google Scholar]
- Kraaijeveld K, Kraaijeveld-Smit FJ, Maan ME, 2011. Sexual selection and speciation: the comparative evidence revisited. Biol Rev 86:367–377. [DOI] [PubMed] [Google Scholar]
- Krause J, Godin JGJ, 1996. Influence of parasitism on shoal choice in the banded killifish (Fundulus diaphanus, Teleostei, Cyprinodontidae). Ethology 102:40–49. [Google Scholar]
- Kuehne RA, Barbour RW,. 1983. The American Darters. Lexington (KY: ): University Press of Kentucky. [Google Scholar]
- Kuhl FP, Giardina CR, 1982. Elliptic Fourier features of a closed contour. Comput Vision Graph 18:236–258. [Google Scholar]
- LeBas NR, 2006. Female finery is not for males. Trends Ecol Evol 21:170–173. [DOI] [PubMed] [Google Scholar]
- Lehtonen TK, Lindström K, 2008. Repeatability of mating preferences in the sand goby. Anim Behav 75:55–61. [Google Scholar]
- Long TA, Pischedda A, Stewart AD, Rice WR, 2009. A cost of sexual attractiveness to high-fitness females. PLoS Biol 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NJ, Jennings K, McFarland WN, Loew ER, Losey GS, 2003. Visual biology of Hawaiian coral reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia 2003:467–480. [Google Scholar]
- Martin MD, Mendelson TC, 2013. Incomplete behavioural isolation and asymmetric female preference in darter sister species (Percidae: Etheostoma). J Fish Biol 83:1371–1380. [DOI] [PubMed] [Google Scholar]
- Martin MD, Mendelson TC, 2016. Male behaviour predicts trait divergence and the evolution of reproductive isolation in darters (Percidae: Etheostoma). Anim Behav 112:179–186. [Google Scholar]
- McLellan T, Endler JA, 1998. The relative success of some methods for measuring and describing the shape of complex objects. Syst Biol 47:264–281. [Google Scholar]
- Mendelson TC, Gumm JM, Martin MD, Ciccotto PJ, 2018. Preference for conspecifics evolves earlier in males than females in a sexually dimorphic radiation of fishes. Evolution 72:337–347. [DOI] [PubMed] [Google Scholar]
- Mendelson TC, Shaw Kl. 2002. Genetic and behavioral components of the cryptic species boundary between Laupala cerasina and L. kohalensis (Orthoptera: Gryllidae).Genetics of Mate Choice: From Sexual Selection to Sexual Isolation. Dordrecht, the Netherlands: Springer. 301–310 [PubMed] [Google Scholar]
- Moran RL, Zhou M, Catchen JM, Fuller RC, 2017. Male and female contributions to behavioral isolation in darters as a function of genetic distance and color distance. Evolution 71:2428–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TG, 2006. Predator-elicited visual signal: why the turquoise-browed motmot wag-displays its racketed tail. Behav Ecol 17:547–553. [Google Scholar]
- Murphy TG, 2007. a. Racketed tail of the male and female turquoise-browed motmot: male but not female tail length correlates with pairing success, performance, and reproductive success. Behav Ecol Sociobiol 61:911–918. [Google Scholar]
- Murphy TG, 2007. b. Dishonest ‘preemptive’ pursuit-deterrent signal? Why the turquoise-browed motmot wags its tail before feeding nestlings. Anim Behav 73:965–970. [Google Scholar]
- Murphy TG, 2008. Lack of assortative mating for tail, body size, or condition in the elaborate monomorphic Turquoise-browed Motmot Eumomota superciliosa. Auk 125:11–19. [Google Scholar]
- Nuttall DB, Keenleyside MH, 1993. Mate choice by the male convict cichlid (Cichlasoma nigrofasciatum; Pisces, Cichlidae). Ethology 95:247–256. [Google Scholar]
- Oaten M, Stevenson RJ, Case TI, 2011. Disease avoidance as a functional basis for stigmatization. Philos T R Soc B 366:3433–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LM, Smith P, Burr B, Mayden R, 1985. Evolution of reproductive behaviors in percid fishes. Ill Nat His Sur Bull 33:275–295. [Google Scholar]
- Pizzari T, Cornwallis CK, Løvlie H, Jakobsson S, Birkhead TR, 2003. Sophisticated sperm allocation in male fowl. Nature 426:70–74. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. 2015. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reading K, Backwell PR, 2007. Can beggars be choosers? Male mate choice in a fiddler crab. Anim Behav 74:867–872. [Google Scholar]
- Roberts NS, Gumm JM, Mendelson TC, 2017. Darter (Percidae: Etheostoma) species differ in their response to video stimuli. Anim Behav 131:107–114. [Google Scholar]
- Roberts NS, McCaulley C, Mendelson TC, 2019. Validating the use of computer animations in male Etheostoma zonale: a comparison of individual response to live and artificial stimuli. Curr Zool 65:725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NS, Mendelson TC, 2017. Male mate choice contributes to behavioural isolation in sexually dimorphic fish with traditional sex roles. Anim Behav 130:1–7. [Google Scholar]
- Rohlf FJ, Archie JW, 1984. A comparison of Fourier methods for the description of wing shape in mosquitoes (Diptera: Culicidae). Syst Zool 33:302–317. [Google Scholar]
- Rowland WJ, 1982. Mate choice by male sticklebacks Gasterosteus aculeatus. Anim Behav 30:1093–1098. [Google Scholar]
- Ryan MJ, Rand AS, 1993. Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47:647–657. [DOI] [PubMed] [Google Scholar]
- Seehausen O, van Alphen JJ, 1998. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav Ecol Sociobiol 42:1–8. [Google Scholar]
- Servedio MR, Lande R, 2006. Population genetic models of male and mutual mate choice. Evolution 60:674–685. [PubMed] [Google Scholar]
- Stalker HD, 1942. Sexual isolation studies in the species complex Drosophila virilis. Genetics 27: 238–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard MC, Stevens M, 2010. Pattern mimicry of host eggs by the common cuckoo, as seen through a bird's eye. Roy Soc B Biol Sci 277:1387–1393. P [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troscianko J, Stevens M, 2015. Image calibration and analysis toolbox: a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol Evol 6:1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz GW, Roberts JA, Taylor PW, 2009. Multimodal communication and mate choice in wolf spiders: female response to multimodal versus unimodal signals. Anim Behav 78:299–305. [Google Scholar]
- Uy JAC, Irwin DE, Webster MS, 2018. Behavioral isolation and incipient speciation in birds. Annu Rev Ecol Evol Syst 49:1–24. [Google Scholar]
- von Schilcher F, Dow M, 1977. Courtship behaviour in Drosophila: sexual isolation or sexual selection?. Z Tierpsychol 43304–310. [Google Scholar]
- Werner NY, Lotem A, 2003. Choosy males in a haplochromine cichlid: first experimental evidence for male mate choice in a lekking species. Anim Behav 66:293–298. [Google Scholar]
- Williams TH, Mendelson TC, 2010. Behavioral isolation based on visual signals in a sympatric pair of darter species. Ethology 116:1038–1049. [Google Scholar]
- Williams TH, Mendelson TC, 2011. Female preference for male coloration may explain behavioural isolation in sympatric darters. Anim Behav 82:683–689. [Google Scholar]
- Williams TH, Mendelson TC, 2013. Male and female responses to species-specific coloration in darters (Percidae: etheostoma). Anim Behav 85:1251–1259. [Google Scholar]
- Williams TH, Mendelson TC, 2014. Quantifying reproductive barriers in a sympatric pair of darter species. Evol Biol 41:212–220. [Google Scholar]
- Yamazaki Y, Shinohara N, Watanabe S, 2004. Visual discrimination of normal and drug induced behavior in quails (Coturnix coturnix japonica). Anim Cogn 7:128–132. [DOI] [PubMed] [Google Scholar]