Abstract
Sexual selection often leads to evolution of conspicuous signals, raising the chances of attracting not only potential mates, but also predators. In lacertid lizards, ultraviolet (UV)–blue spots on flanks and shoulders represent such a trait. Some level of correlation between male and female ornamentation is also known to exist. Therefore, the phenotype of females may change in the absence of sexual selection. We tested this hypothesis on a complex of parthenogenetic and bisexual lizards of the genus Darevskia. We evaluated area, counts, and chromatic properties (UV opponency, saturation) of UV–blue spots and compared the values between the clones and their bisexual progenitor species. We found a fair heterogeneity between the parthenogenetic species, but no general tendency toward higher crypsis or conspicuousness. Values of the parthenogens were not significantly different from the values of sexual females. A possible explanation is that the changes in selective forces associated with parthenogenetic reproduction are too small to affect the resulting pattern of selective pressures on the studied traits, or that the phenotypes of the parthenogens result from the unique combination of parental genomes and are conserved by clonal reproduction.
Keywords: clonal reproduction, coloration, reptiles, sexual selection, visual modeling
Sexual selection frequently leads to the evolution of sexually dimorphic traits, with males being the more conspicuous sex (Darwin 1871; Andersson 1994). These traits may indicate good health condition or social dominance and thus increase the male’s mating success (Hamilton and Zuk 1982; Møller 1988; Milinski and Bakker 1990; Kodric-Brown 1993). In reptiles, and lizards, in particular, a whole range of colorful ornaments evolved, many of them having a role in intraspecific/social signaling (Sinervo and Lively 1996; Nicholson et al. 2007; Pérez I de Lanuza et al. 2014). As the visual system of diurnal reptiles (but also birds, for instance) is tetrachromatic with ultraviolet-sensitive (UVS) cones, it is natural that UV coloration takes part in their signaling too (Fleishman et al. 1993; Loew et al. 2002; Stevens and Cuthill 2007; Mullen and Pohland 2008; Pérez I de Lanuza et al. 2013; Marshall and Stevens 2014; Pérez I de Lanuza and Font 2014). UV ornamentation is frequently involved in sexual selection in lizards (Whiting et al. 2006; Pérez I de Lanuza et al. 2013; Lisboa et al. 2017). A number of lacertid species have UV–blue spots on their throats, shoulders, flanks, or bellies, which are more prominent in males than females (e.g., Gallotia galloti (Molina-Borja et al. 2006), Timon lepidus (Font et al. 2009), Podarcis sp. (Pérez I de Lanuza and Font 2010; Pérez I de Lanuza et al. 2014; Names et al. 2019)). The UV coloration in males also positively correlates with their fighting ability or mating success, as was reported, for example, in the European green lizard, Lacerta viridis (Bajer et al. 2011; Molnár et al. 2012), sand lizard, L. agilis (Olsson et al. 2011), Schreiber’s green lizard, L. schreiberi (Martín and López 2009), or common wall lizard, Podarcis muralis (Pérez I de Lanuza et al. 2014; MacGregor et al. 2017).
In our study, we focused on UV–blue spots on shoulders and outer ventral scales (henceforth OVS) in Caucasian rock lizards of the genus Darevskia (Lacertidae) (Arribas 2012; Abramjan et al. 2015). The genus is remarkable for comprising 7 obligatory parthenogenetic diploid species, which arose from interspecific hybridization of at least 4 bisexual species, 2 being paternal, and 2 being maternal ancestors (Moritz et al. 1992; Freitas et al. 2016, 2019; Tarkhnishvili et al. 2017). Darevskia lizards cluster into 3 main groups, with paternal species belonging to 1 group and maternal species to another (Murphy et al. 2000). As the parthenogens lack males, we decided to explore whether the absence of sexual selection affects their UV–blue coloration. First, the conspicuous traits may positively correlate between the sexes due to shared genetic basis (Lande 1980; Potti and Canal 2011). Furthermore, in mutual mate choice, not only females, but also males actively select more attractive partners (Jones and Hunter 1993; Amundsen 2000). Therefore, we may presume that in the absence of males, other evolutionary pressures can take precedence, which might subsequently affect females’ coloration. Various scenarios can be imagined. Predation can select for better crypsis (Stuart-Fox et al. 2003; Husak et al. 2006; Marshall et al. 2015). By contrast, competition over resources may enhance female ornamentation (Jenssen et al. 2000; LeBas 2006; Pryke 2007; Hegyi et al. 2008). This may be possible in the parthenogenetic Darevskia lizards, as they occur in high densities and their social interactions are more frequent than in sexual species (Darevsky 1967). Yet, their interactions are usually peaceful and their home ranges overlap more than in females of bisexual species (Galoyan 2013a). On the contrary, increased aggressiveness among the clonal females was reported from hybrid zones, where they mate with males of related bisexual species and compete for their attention (Danielyan et al. 2008; Galoyan 2013a, 2013b; Spangenberg et al. 2017).
Male appearance is rather costly and can reduce fitness due to physiological and behavioral causes (Swierk and Langkilde 2013). Higher conspicuousness also leads to better detectability. In many lacertids, including Darevskia sp., males copulate with multiple females, selecting the largest ones, likely in order to increase paternity chances (Carretero et al. 2018). During the mating, the male holds the female for some time, biting her inguinal region or thigh and leaving noticeable jaw marks on her body (Darevsky 1967). Thus, being easily detected and frequently mated can induce a considerable reproductive cost on females, for example, by increasing risk of predation due to reduced mobility, risk of injuries, or sexual transmission of pathogens (Le Galliard et al. 2005; White et al. 2011). Parthenogenetic females would be spared sexual conflict, so there would be no need for decreased conspicuousness. In spite of this, copulation between parthenogenetic Darevskia armeniaca or D. unisexualis and bisexual D. valentini is a common phenomenon in mixed communities. Such hybridizations usually result in sterile triploid offspring (Darevsky 1966), but may sporadically lead to the return to bisexuality via higher level of ploidy in the process of reticulate evolution (Danielyan et al. 2008; Carretero et al. 2018).
To sum up, there are multiple evolutionary pressures which can potentially affect the ornamentation in parthenogenetic females. In order to find out whether the conspicuousness of UV–blue coloration differs between sexual and parthenogenetic species, we examined the quantity and color properties of UV–blue spots in 4 parthenogens: (D. armeniaca, D. dahli, D. rostombekowi, and D. unisexualis) and their respective paternal (D. portschinskii, D. valentini) and maternal (D. raddei raddei/D. raddei nairensis, D. mixta) and 1 unrelated (D. caucasica) species.
Materials and Methods
The material comprised 326 lizards of 9 species. One species, D. caucasica, is unrelated to the parthenogens, but belongs to the same phylogenetic group as the maternal species D. raddei and D. mixta (Murphy et al. 2000). The lizards were captured in the wild in Armenia during their breeding season from May to July (2010–2019). Lizards from Georgia, also captured in the wild during the same period, were accessed at a private breeder. Shortly after acquiring the lizards, we recorded their snout-vent length (SVL) using a digital caliper with 0.01 mm precision and took digital images for further color analyses. Sample sizes are given in Table 1. For populations, see Appendix 1. For better precision, we decided to discriminate between D. raddei nairensis and D. raddei raddei in this study. Although conspecific, each of the subspecies contributed to formation of a distinct parthenogen; D. unisexualis is related to D. r. nairensis (Freitas et al. 2016), whereas D. rostombekowi is probably related to D. r. raddei (Fu et al. 2000). Unfortunately, no live D. mixta, the maternal ancestor of D. armeniaca and D. dahli, was available to us. We could examine only ethanol-fixed museum specimens, whose coloration was partially faded. We excluded them from the statistical models, but we present the data in the results.
Table 1.
Sample sizes used in this study
| Species | Males | Females |
|---|---|---|
| Bisexual | ||
| Darevskia caucasica | 14 | 17 |
| Darevskia mixta a | 14 | 24 |
| Darevskia raddei nairensis | 16 | 12 |
| Darevskia raddei raddei | 20 | 10 |
| Darevskia portschinskii | 21 | 21 |
| Darevskia valentini | 12 | 15 |
| Parthenogenetic | ||
| Darevskia armeniaca | 76 | |
| Darevskia dahli | 24 | |
| Darevskia rostombekowi | 15 | |
| Darevskia unisexualis | 15 |
Ethanol-fixed specimens.
Image acquisition and processing
We focused on both quantitative and chromatic characteristics of UV–blue spots in 2 body regions—the row of OVS and the shoulder. In OVS, we assessed the quantitative parameters (n = 326): area of the UV–blue coloration and number of UV–blue spots. We scanned each lizard together with a millimeter scale with an Epson GT-S640 scanner at 600 dpi resolution. We cut out both left and right rows of OVS in Adobe Photoshop CS6 and calculated the area of the blue color (corresponding to UV–blue) for each row with Barvocuc software (Rádlová et al. 2016) (Figure 1). The hue angle for blue was set to 175°–275° and the upper threshold for gray (nonsaturated colors) to 8%. Blue areas from left and right flank were averaged. Absolute values in square millimeter were used for statistical modeling. In the graphs, we present percentage of UV–blue per row of OVS, as it is more intuitive. The number of UV–blue spots was counted manually for each side of a lizard. Outputs from Barvocuc were used for counting and the original scans as a control. Counts from both sides were summed for each lizard.
Figure 1.
Examples of OVS rows used for the analysis of quantitative parameters of the UV–blue spots. Each pair shows the original scan (left) and the output from Barvocuc software (right). In D. mixta, the middle row shows the digitally reconstructed pattern; (a) D. caucasica male, (b) D. caucasica female, (c) D. mixta male, (d) D. mixta female, (e) D. r. nairensis male, (f) D. r. nairensis female, (g) D. portschinskii male, (h) D. portschinskii female, (i) D. r. raddei male, (j) D. r. raddei female, (k) D. valentini male, (l) D. valentini female, (m) D. armeniaca, (n) D. dahli, (o) D. rostombekowi, (p) D. unisexualis. (a–l) Bisexual species, (m–o) parthenogenetic species. Scale not preserved.
The museum specimens of D. mixta were photographed on a millimeter paper with a Nikon E4500 digital camera. Because their UV–blue spots were partially faded, they had to be manually selected and highlighted in Adobe Photoshop, to make them “visible” to Barvocuc software. The measurements were compared with data retrieved from photographs of live D. mixta, kindly provided by D. Tarkhnishvili. In order to make the estimation as objective as possible, the procedure was repeated twice by two of the authors (A.A. and D.F.). Unfortunately, the same result could not be obtained repeatedly—the difference between live and fixed specimens was significant in 2 out of 4 tests. The discrepancy was likely caused by high variance in coloration between live and fixed individuals and by varying angles and framings in each set of photographs. Therefore, we excluded these data from the statistical models, but we present the best estimates we could achieve in the results.
Chromatic parameters were measured for both OVS and shoulder spots and included 1) the UV opponency, which we use as a measure of dominance of the UV color and 2) saturation (chroma). For this purpose, we used UV photography and Multispectral Image Calibration and Analysis (MICA) Toolbox v2 (van den Berg et al. 2020), a freely available plug-in operating on ImageJ platform (Schneider et al. 2012). Our multispectral photo equipment was available only during one part of our study, therefore a limited number of lizards could be examined photographically. Besides, some of them had only one or no UV–blue spots on their flanks or shoulders. Therefore, only smaller subsets of individuals could be used for saturation and UV opponency measurements (OVS subset n = 91; shoulder spot subset n = 70; see Appendix 2 for details).
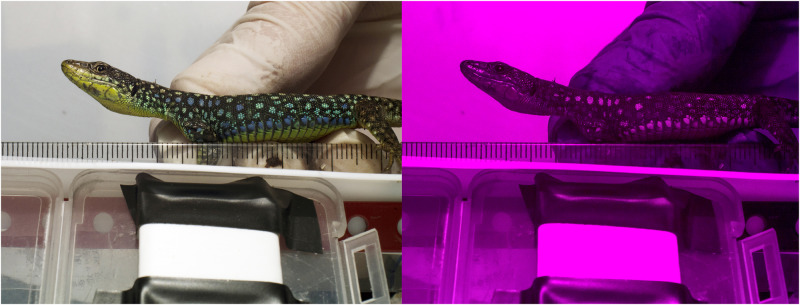
Animals with at least 3 UV–blue spots on OVS or 1 UV–blue spot on shoulder, respectively, were selected. Each lizard was photographed from its lateral side through UV/IR cut filter, transmitting visible light 400–700 nm, and then UV pass Baader U-Venus filter, transmitting 300–400 nm (Figure 2). We used a Samsung NX1000 camera, adapted for UV photography according to the instructions by J. Troscianko available at https://www.jolyon.co.uk/2014/07/full-spectrum-nx1000/, and a 35 mm Novoflex Noflexar lens. Lizards were placed 16 cm away from the lens and illuminated by Iwasaki ColorEyeArc bulb with its UV blocking coating removed. The lamp was set 20 cm above the lens. One-millimeter thick white PTFE (Teflon) plates, obtained from a local manufacturer (Techseal, www.techseal.cz), were put around the lizard to eliminate undesirable shadows. Photographs were calibrated against a white PTFE tape having flat 99% reflectance from 300 to 700 nm, which was checked by spectrophotometer against white WS-1 diffuse reflectance standard. The photographs were taken at a setting ISO400, F/16, and further processed with the MICA toolbox according to the methodology of Troscianko and Stevens (2015) and van den Berg et al. (2020).
Figure 2.
Example of photos taken in the visible and UV spectra, used for multispectral image analysis of saturation and UV opponency. The photo shows a male D. r. nairensis.
The 3 largest UV–blue spots were selected on each lizard’s OVS and treated together as 1 region of interest. As for the shoulder spots, we always selected the largest. For visual modeling, we used the photoreceptor data for P. muralis, cone abundance ratio UVS:SWS:MWS:LWS 1:2:5:9, Weber fraction 0.05 (Martin et al. 2015). Each multispectral stack was then converted to receptor noise limited (RNL) XYZ chromaticity system. XYZ stands for the 3 axes corresponding to opponent channels in a tetrachromat’s vision. X, Y, and Z axes represent “red-green” (LW:MW), “yellow-blue” ([LW + MW]:SW), and (LW + MW + SW):UV opponency, respectively. Each color is therefore described by 3 coordinates in the RNL XYZ chromaticity color space and the distance between any 2 points is in units of “just noticeable distances” (jnds) (van den Berg et al. 2020). UV opponency is expressed in Z-axis values with UV dominance increasing in the positive direction. Saturation of the spot is expressed as the mean of Euclidean distances of each pixel’s color to the achromatic origin of the color space.
Statistical analysis
We checked the data for normality and applied log transformation where needed (UV–blue area, spot saturation). In statistical models involving log-transformed response variables, we also used log-transformed SVL to allow interpretation in terms of allometry. A dataset comprising 83 males, 75 sexual, and 130 parthenogenetic females was used for statistical models analyzing UV–blue area and spot count (both for OVS). UV opponency and saturation were analyzed on smaller subsets (Appendix 2).
Calculations were performed with R v3.6.2 (“nlme” package) (R Core Team 2018) and Statistica 8 software (StatSoft 2007). First, we tested for differences between males and females in bisexual species. We ran linear models (LMs) for the following response variables: UV–blue area (n = 158), OVS UV opponency (n = 46), OVS saturation (n = 46), shoulder UV opponency (n = 33), and shoulder saturation (n = 33). Sex, species, SVL, and their interactions (sex × species, sex × SVL, species × SVL) were set as predictors. We also checked for the effect of outliers using Cook’s distances and reran the models without the selected observations. The results were unaffected. The full model was further reduced according to AIC (command “step”). In the case of spot count (n = 158), we used generalized linear model (GLM) with quasipoisson distribution and chi-square test. The order of the predictors was the same as in the LM.
Next, we compared differences between all 3 “sex categories” (males, sexual females, and parthenogenetic females) in quantitative as well as chromatic parameters. We used generalized least squares (GLSs) method for analyzing UV–blue area (n = 288), OVS UV opponency (n = 91), OVS saturation (n = 91), shoulder UV opponency (n = 70), and shoulder saturation (n = 70). This method enables analyzing correlated data, in our case treating species as independent observations and individual measurements within each species as pseudoreplications (see, e.g., Pekár and Brabec 2016). We ran a marginal model with “sex category,” SVL, and their interaction as predictors, affiliation to species and sex as a grouping variable, and we chose a “compound symmetry” correlation structure. Correction for the heterogeneity of variance was also applied. For analysis of the spot count (n = 288), we used a generalized estimated equation model (GEEGLM), again with “sex category,” SVL, and their interaction as predictors. Poisson distribution was assumed, affiliation to species and sex was set as the grouping variable and ANOVA chi-square test executed. For traits where the sex category was a significant factor, we checked differences between the parthenogens and their ancestral species with planned Tukey’s Unequal N HSD post hoc test.
We examined the correlation between SVL and UV–blue area for each species and sex with linear regression. The same SVLs may have different interpretations in males and females (Kratochvíl et al. 2003), therefore, we used relative SVL instead for the purpose of plotting, as it better expressed the “ontogenetic time.” It is calculated as a proportion of maximum SVL for each species and sex. Data for SVLmax were obtained from the literature (Darevsky 1967; Arakelyan et al. 2011) or from our measurements when our record was higher.
Results
Our results confirmed that UV–blue coloration is sexually dimorphic in Darevskia lizards. Proportion of UV–blue area was about 1.4–3.5 times larger in males than in females, depending on the species (Figure 3, Appendix 2). Males also tended to have higher number of spots, higher UV opponency, and higher saturation on both OVS and shoulder (Figure 4, Appendix 2). LMs (GLM in the case of spot count) revealed that sex was a significant factor for all traits (P ≤ 0.024) along with species (P ≤ 0.005) and SVL (P ≤ 0.016) (Table 2). Sex × species interaction was significant for OVS UV–blue area (P = 0.003), UV opponency (P = 0.013), and saturation (P = 0.008). Species × SVL interaction was significant for OVS UV opponency (P = 0.003), shoulder UV opponency (P = 0.034), and shoulder saturation (P = 0.001). Sex × SVL interaction was significant for UV–blue area (P = 0.001) (Table 2). Values of the parthenogens fell within the range of female values of the related bisexual species.
Figure 3.
Quantitative parameters of OVS spots by species and sex. Dark gray = males, light gray = females, white = parthenogens. Box plots: middle line = median, box = 1st–3rd quartile, whiskers = nonoutlier range, dots = outliers. *Values of D. mixta are based on measurements of ethanol-fixed individuals.
Figure 4.
Chromatic parameters of OVS and shoulder spots by species and sex. Middle line = median, box = 1st–3rd quartile, whiskers = nonoutlier range, dots = raw data. Y-axis in the UV opponency graph represents the (LW + MW + SW):UV color opponency. Both Y-axes are in units of “just noticeable differences” (jnds).
Table 2.
ANOVA/ANODEV results for the quantitative and chromatic parameters of the UV–blue spots
| OVS: UV–blue area |
OVS: Spot count |
|||||||
|---|---|---|---|---|---|---|---|---|
| LM |
GLM |
|||||||
| df | F | P | df | Deviance | Resid. df | Desid. dev | P | |
| Residuals | 142 | 157 | 1296.64 | |||||
| Sex | 1 | 43.261 | <0.001 | 1 | 94.155 | 156 | 1202.49 | <0.001 |
| Species | 4 | 22.910 | <0.001 | 4 | 265.594 | 152 | 936.89 | <0.001 |
| SVL | 1 | 47.748 | <0.001 | 1 | 66.606 | 151 | 870.29 | <0.001 |
| Sex × species | 4 | 4.129 | 0.003 | 4 | 63.008 | 147 | 807.28 | 0.006 |
| Sex × SVL | 1 | 11.519 | 0.001 | 1 | 6.95 | 146 | 800.33 | 0.206 |
| Species × SVL | 4 | 2.374 | 0.055 | 4 | 24.346 | 142 | 775.98 | 0.232 |
| OVS: UV opponency |
OVS: saturation |
Shoulder: UV opponency |
Shoulder: saturation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LM |
||||||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | |
| Residuals | 37 | 39 | 21 | 26 | ||||||||
| Sex | 1 | 16.082 | <0.001 | 1 | 26.890 | <0.001 | 1 | 17.239 | <0.001 | 1 | 5.729 | 0.024 |
| Species | 2 | 9.677 | <0.001 | 2 | 26.710 | <0.001 | 2 | 3.931 | 0.035 | 2 | 6.649 | 0.005 |
| SVL | 1 | 9.284 | 0.004 | 1 | 10.706 | 0.002 | 1 | 0.205 | 0.656 | 1 | 6.702 | 0.016 |
| Sex × species | 2 | 4.911 | 0.013 | 2 | 5.544 | 0.008 | 2 | 2.298 | 0.125 | — | — | |
| Sex × SVL | — | — | — | — | 1 | 0.068 | 0.797 | — | — | |||
| Species × SVL | 2 | 6.869 | 0.003 | — | — | 2 | 3.987 | 0.034 | 2 | 9.516 | 0.001 | |
Significant values are marked in boldface.
SVL, snout–vent length; df, degrees of freedom.
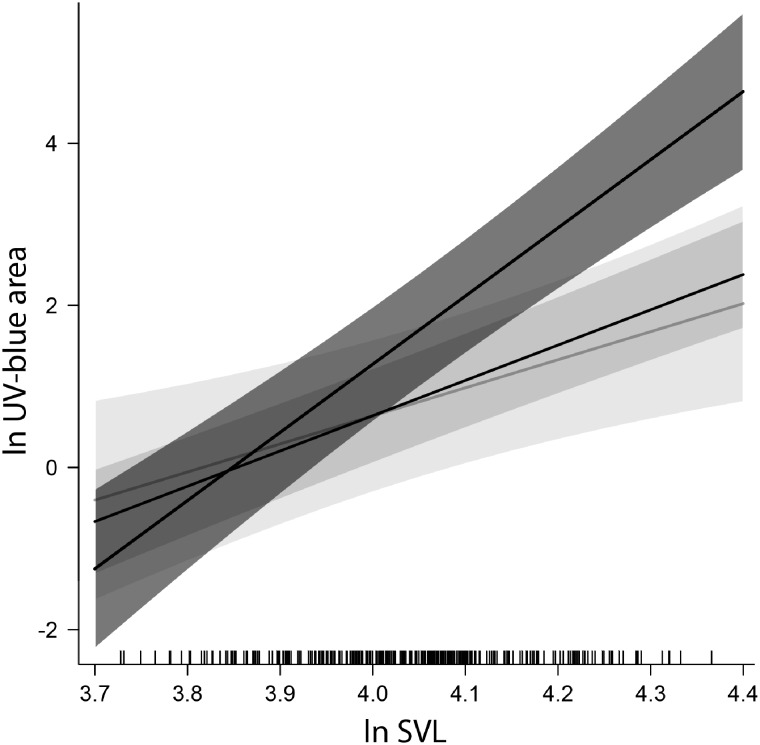
GLS comparisons of males, sexual females, and parthenogens revealed a significant effect of SVL in UV–blue area (F1,282 = 165.226, P < 0.001), OVS UV opponency (F1,85 = 49.466, P < 0.001), OVS saturation (F1,85 = 67.611, P < 0.001), and shoulder spot saturation (F1,64 = 7.057, P = 0.01). SVL was also significant in the spot count according to GEEGLM (χ2 = 12.534, P < 0.001). Sex category was significant only in OVS UV opponency (F2,85 = 12.457, P < 0.001) and OVS saturation (F2,85 = 11.396, P < 0.001). SVL × sex category interaction was significant in UV–blue area (F2,282 = 7.845, P < 0.001), which was caused by the effect of males (t = 3.259, P = 0.001; Table 4). No significant effect of parthenogens was detected in any of the traits, either in sex category alone or in the interaction with SVL (Tables 3 and 4). Effects of each sex category on UV–blue area, as predicted by the GLS model, are plotted in Figure 5.
Table 4.
Coefficients of the GLS/GEEGLM models comparing the sex categories
|
OVS: spot counta |
|||||||
|---|---|---|---|---|---|---|---|---|
| Value | SE | t | P | Estimate | SE | Wald | P | |
| (Intercept) | −13.221 | 4.696 | −2.816 | 0.005 | 0.960 | 0.405 | 5.613 | 0.018 |
| Sex category M | −19.161 | 6.187 | −3.097 | 0.002 | 1.047 | 0.675 | 2.410 | 0.121 |
| Sex category P | −3.566 | 5.023 | −0.710 | 0.478 | −0.364 | 1.068 | 0.116 | 0.733 |
| SVL | 3.465 | 1.152 | 3.008 | 0.003 | 0.031 | 0.006 | 26.973 | <0.001 |
| Sex cat. M × SVL | 4.949 | 1.519 | 3.259 | 0.001 | −0.012 | 0.011 | 1.212 | 0.271 |
| Sex cat. P × SVL | 0.891 | 1.232 | 0.724 | 0.470 | 0.006 | 0.016 | 0.161 | 0.689 |
| OVS: UV opponency Rho = −0.006 |
OVS: saturation Rho = 0.120 |
Shoulder: UV opponency Rho = 0.383 |
Shoulder: saturation Rho = 0.244 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | SE | t | P | Value | SE | t | P | Value | SE | t | P | Value | SE | t | P | |
| (Intercept) | −123.435 | 69.403 | −1.779 | 0.079 | −9.721 | 3.679 | −2.642 | 0.010 | −38.057 | 128.755 | −0.296 | 0.769 | −0.902 | 2.216 | −0.407 | 0.685 |
| Sex category M | 8.821 | 76.778 | 0.115 | 0.909 | 4.005 | 4.143 | 0.967 | 0.337 | 6.667 | 134.058 | 0.050 | 0.961 | −0.847 | 3.049 | −0.278 | 0.782 |
| Sex category P | 38.287 | 71.105 | 0.538 | 0.592 | 2.586 | 3.969 | 0.652 | 0.517 | 30.011 | 131.597 | 0.228 | 0.820 | 1.503 | 2.586 | 0.581 | 0.563 |
| SVL | 31.813 | 17.419 | 1.826 | 0.071 | 2.996 | 0.920 | 3.257 | 0.002 | 9.879 | 31.989 | 0.309 | 0.758 | 0.825 | 0.551 | 1.497 | 0.139 |
| Sex cat. M × SVL | −1.031 | 19.230 | −0.054 | 0.957 | −0.934 | 1.034 | −0.904 | 0.369 | −0.064 | 33.286 | −0.002 | 0.999 | 0.234 | 0.755 | 0.310 | 0.758 |
| Sex cat. P × SVL | −9.847 | 17.833 | −0.552 | 0.582 | −0.710 | 0.991 | −0.717 | 0.476 | −7.190 | 32.682 | −0.220 | 0.827 | −0.389 | 0.641 | −0.606 | 0.546 |
GEEGLM model.
M, males; P, parthenogens; SVL, snout–vent length.
Table 3.
Outputs of GLS/GEEGLM models comparing the sex categories
| OVS: UV–blue area |
OVS: spot count |
OVS: UV opponency |
OVS: saturation |
Shoulder: UV opponency |
Shoulder: saturation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLS model |
GEEGLM |
GLS model |
||||||||||||||
| All species | df | F | P | Chi2 | P | F | P | F | P | F | P | F | P | |||
| (Intercept) | 1 | 28.564 | <0.001 | 132.719 | <0.001 | 2819.614 | <0.001 | 15.288 | <0.001 | 4205.164 | <0.001 | |||||
| Sex category | 2 | 2.393 | 0.093 | 4.326 | 0.115 | 12.457 | <0.001 | 11.396 | <0.001 | 2.609 | 0.081 | 1.153 | 0.322 | |||
| SVL | 1 | 165.226 | <0.001 | 12.534 | <0.001 | 49.466 | <0.001 | 67.611 | <0.001 | 0.981 | 0.326 | 7.057 | 0.010 | |||
| SVL × sex category | 2 | 7.845 | 0.001 | 0.645 | 0.724 | 0.588 | 0.558 | 0.410 | 0.665 | 0.207 | 0.814 | 0.575 | 0.565 | |||
Figure 5.
GLS model prediction of the effects of sex categories on the UV–blue area. Dark gray = males, light gray = sexual females, mid gray = parthenogens. Bands show the 95% confidence interval.
Examining each species separately, we found positive correlation between SVL and OVS UV–blue area mainly in the males (D. r. raddei: r = 0.498, P = 0.025; D. r. nairensis: r = 0.791, P < 0.001, D. caucasica: r = 0.552, P = 0.041, D. mixta: r = 0.625, P = 0.016), but also in females D. r. raddei (r = 0.819, P = 0.004) and in all parthenogens except D. rostombekowi (D. armeniaca: r = 0.442, D. dahli: r = 0.606, D. unisexualis: r = 0.767; all P ≤ 0.001; Figure 6).
Figure 6.
Correlation between relative body size (proportion of maximum SVL recorded for each species and sex) and OVS UV–blue area in bisexual and parthenogenetic lizards. Open dots + dashed lines: males, D. armeniaca, D. unisexualis; full dots + solid lines: sexual females, D. dahli, D. rostombekowi. *Values of D. mixta are based on measurements of ethanol-fixed individuals.
Planned post hoc tests found no significant difference in the UV–blue area between the parthenogenetic species and females of their respective ancestral bisexual species. The only exception was D. dahli, which had significantly smaller UV–blue area compared with females of its paternal ancestor D. portschinskii (P = 0.011). When taking into account the data for the ethanol-fixed D. mixta, a significant difference between D. armeniaca and females of D. mixta also appeared, with D. armeniaca having larger UV–blue area (P = 0.001).
Discussion
We have confirmed sexual dimorphism in both quantitative and chromatic parameters of the UV–blue traits in Darevskia lizards. Our results are consistent with other studies on lacertids showing that the blue/UV–blue lateral spots occupy larger area and/or are more numerous in males than in females, for example, Psammodromus algirus (Carretero 2002), G. galloti (Molina-Borja et al. 2006), T. lepidus (Font et al. 2009), P. muralis (Names et al. 2019). Significant sex × species interactions in the LMs confirm that sexual dimorphism in OVS (UV–blue area, spot count, and chromatic parameters) varies considerably across the species (Figure 6). The same was not proven for the shoulder spots, however, this could have been caused by small sample sizes in this particular subset of data. Despite the high interspecific variability, the GLS model predicts a general tendency of OVS UV–blue area to grow with body size in males, rather than females (Figure 5), which is also consistent with observations in Podarcis lizards (Names et al. 2019).
The GLS model/GEEGLM did not reveal any systematic difference between the parthenogens and sexual females. The most noticeable distinction between these 2 groups appears when considering each parthenogenetic species separately. Three out of 4 species (D. armeniaca, D. unisexualis, and D. dahli) show a positive correlation between SVL and UV–blue spot area, which is a feature typical of males, but not sexual females. In general, the slope of the parthenogens corresponds to the slope of sexual females according to the GLS model prediction (Figure 5), but correlation coefficients are higher in the parthenogens (Figure 6). Therefore, we cannot rule out that the correlation can be present in sexual females as well, but was not detected due to their higher variance and lower sample sizes.
Our results did not confirm the hypothesis that UV–blue ornamentation in the parthenogenetic females would become either less or more conspicuous than in sexual females due to the absence of males. Based on our data, the only candidate for a notably duller parthenogen is D. dahli. It has significantly smaller UV–blue area than females of its paternal ancestor D. portschinskii, but whether the same applies to the difference from its maternal ancestor D. mixta is a matter of question. Our test did not detect a significant difference. Nonetheless, our samples of D. mixta were ethanol-fixed specimens, so the areas of their UV–blue spots could have been underestimated and a false negative result obtained. Darevskia dahli, however, is just 1 of 7 known parthenogenetic species of the genus Darevskia, each of them having a fairly distinct phenotype. We find larger and “UV-bluer” (e.g., D. armeniaca, D. unisexualis) as well as smaller and duller parthenogenetic species (e.g., D. dahli, D. rostombekowi) within the genus, which applies to both quantitative and chromatic qualities of the UV–blue ornaments (Figures 3 and 4). The reason why the parthenogens showed no systematic tendency toward lower (or higher) conspicuousness may be just a consequence of this diversity, which apparently arose from their hybrid origin. Each parthenogenetic species has a unique combination of parental genomes (Moritz et al. 1992; Freitas et al. 2019), which leads to different expression of UV–blue ornaments in each of the hybrids. These phenotypes could have been established at the genesis of the clones and then conserved by clonal reproduction (Abramjan et al. 2019).
The result that the parthenogens do not significantly differ from sexual females is much harder to interpret in terms of various selective pressures, as their effect is not so obvious in such a case. Considering predation, for instance, it can be stated that it does not reduce the UV ornaments in the parthenogens, because they are not very costly. This can be either due to signal partitioning (the spots restricted to OVS are exposed to the conspecifics, but hidden from the sight of aerial predators; Marshall and Stevens 2014), or the ornaments had been already selected for a “safe amount of conspicuousness” and do not need any further reduction. Shoulder spots, however, may respond differently. Unlike OVS, they cannot be concealed from predators. Our results suggest their chromatic qualities are comparable between sexual and parthenogenetic females, but we did not examine their sizes and counts. Hence, we cannot rule out that these may vary between the 2 groups. Furthermore, some species like D. valentini or D. mixta were not available to us for chromatic measurements and their inclusion in further studies would help to draw a more accurate image.
Our study was aimed mainly on the UV–blue coloration itself. Besides, chromatic properties only were assessed in the shoulder spots. A complex coverage of various species and evaluation of their overall pattern would be desirable in further research, especially focusing on the coloration of the back, belly, and the lateral flanks, and taking into account the natural background and variation between populations of the same species.
Acknowledgments
We are grateful to Valentina F. Orlova for kindly providing the material from the Zoological Museum in Moscow, David Tarkhnishvili for sharing his photographs of live D. mixta, Miroslav Švátora, Pavel Němec, Petra Frýdlová, Jitka Jančúchová-Lásková, and Petra Hnidová (Suchomelová) for assistance with collecting and processing the material, Marcel Honza for auxiliary spectrophotometric measurements, Michal Šulc for checking the white reflectance standards with a spectrophotometer, Valentina Azaryan for helping us with administrative and field issues, Jaroslav Staněk, Jaromír Antoch, Jakub Kreisinger, Kristina Kverková, Petr Tureček, Iveta Štolhoferová and Stano Pekár for statistical advice, and Petr Salaba for technical support. This article is dedicated to the memory of prof. Eduard G. Yavruyan, to whom we pay our gratitude for making our long-term scientific collaboration and field expeditions possible.
Finalization of this article was made possible thanks to Erasmus+ mobility program.
Appendix 1: List of Material Used in This Study
| Species | Population | Country | Males | Females |
|---|---|---|---|---|
| Bisexual | ||||
| Darevskia caucasica | Kazbek | Georgia | 9 | 12 |
| Darevskia caucasica | Tusheti | Georgia | 5 | 5 |
| Darevskia mixtaa | Bakuriani | Georgia | 7 | 18 |
| Darevskia mixtaa | SW of Tbilisi | Georgia | 7 | 6 |
| Darevskia raddei nairensis | Hayrivank | Armenia | 9 | 8 |
| Darevskia raddei nairensis | Yerevan | Armenia | 7 | 4 |
| Darevskia raddei raddei | Geghard | Armenia | 8 | 3 |
| Darevskia raddei raddei | Tatev | Armenia | 12 | 6 |
| Darevskia raddei raddei | Gosh | Armenia | 1 | |
| Darevskia portschinskii | Kojori | Georgia | 12 | 15 |
| Darevskia portschinskii | Gori | Georgia | 5 | 5 |
| Darevskia portschinskii | Gosh | Armenia | 4 | 1 |
| Darevskia valentini | Lchashen | Armenia | 8 | 7 |
| Darevskia valentini | Kuchak | Armenia | 1 | 1 |
| Darevskia valentini | Sepasar | Armenia | 3 | 7 |
| Parthenogenetic | ||||
| Darevskia armeniaca | Dilijan | Armenia | 60 | |
| Darevskia armeniaca | Lchashen | Armenia | 3 | |
| Darevskia armeniaca | Hankavan | Armenia | 13 | |
| Darevskia dahli | Kojori | Georgia | 12 | |
| Darevskia dahli | Dilijan | Armenia | 13 | |
| Darevskia rostombekowi | Dilijan | Armenia | 15 | |
| Darevskia unisexualis | Sevan | Armenia | 15 | |
Material from the collections of the Zoological Museum in Moscow.
Appendix 2: Mean, Minimum, and Maximum Values for Quantitative and Chromatic Parameters of UV–Blue Spots
| Quantitative parameters | SVL (mm) |
OVS: UV–blue area (%) per row |
OVS: UV–blue spots count per row |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| species | Sex | N | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max |
| Bisexual | |||||||||||
| Darevskia caucasica | F | 17 | 55.92 | 43.2 | 63.1 | 5.0 | 0.2 | 11.3 | 7.0 | 0 | 17 |
| Darevskia caucasica | M | 14 | 52.84 | 43.2 | 59.8 | 8.9 | 1.8 | 28.3 | 8.8 | 0 | 19 |
| Darevskia mixtaa | F | 24 | 55.59 | 45.7 | 60.7 | 2.9 | 0.0 | 10.0 | 4.0 | 0 | 9 |
| Darevskia mixtaa | M | 14 | 54.14 | 43.0 | 63.0 | 12.2 | 4.3 | 21.6 | 8.3 | 6 | 13 |
| Darevskia raddei nairensis | F | 12 | 59.59 | 55.8 | 65.2 | 16.4 | 4.7 | 34.6 | 11.4 | 2 | 15 |
| Darevskia raddei nairensis | M | 16 | 61.98 | 53.5 | 68.1 | 22.9 | 0.4 | 47.2 | 11.9 | 1 | 21 |
| Darevskia raddei raddei | F | 10 | 56.51 | 51.7 | 61.9 | 3.3 | 0.3 | 7.4 | 3.6 | 0 | 18 |
| Darevskia raddei raddei | M | 20 | 58.04 | 44.8 | 63.2 | 10.1 | 0.2 | 25.3 | 7.3 | 3 | 15 |
| Darevskia portschinskii | F | 21 | 51.89 | 43.9 | 57.8 | 5.2 | 0.4 | 13.7 | 7.2 | 0 | 20 |
| Darevskia portschinskii | M | 21 | 50.49 | 41.6 | 56.6 | 18.4 | 2.2 | 43.4 | 14.9 | 7 | 20 |
| Darevskia valentini | F | 15 | 67.45 | 46.8 | 78.7 | 8.3 | 0.8 | 22.4 | 11.6 | 3 | 21 |
| Darevskia valentini | M | 12 | 67.57 | 59.6 | 75.2 | 28.1 | 10.0 | 71.3 | 16.6 | 12 | 23 |
| Parthenogenetic | |||||||||||
| Darevskia armeniaca | F | 76 | 58.39 | 44.9 | 70.6 | 11.7 | 4.4 | 25.6 | 9.8 | 3 | 15 |
| Darevskia unisexualis | F | 15 | 62.82 | 47.1 | 73.0 | 9.9 | 3.0 | 17.0 | 9.4 | 0 | 13 |
| Darevskia dahli | F | 24 | 54.54 | 45.4 | 60.0 | 1.8 | 0.0 | 5.2 | 3.9 | 0 | 9 |
| Darevskia rostombekowi | F | 15 | 51.00 | 41.7 | 58.3 | 4.1 | 1.9 | 7.4 | 5.1 | 0 | 10 |
| Chromatic parameters | OVS: UV opponency |
OVS: saturation |
Shoulder: UV opponency |
Shoulder: saturation |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Sex | N | Mean | Min | Max | Mean | Min | Max | N | Mean | Min | Max | Mean | Min | Max |
| Bisexual | |||||||||||||||
| Darevskia raddei nairensis | F | 4 | 12.70 | 6.49 | 17.10 | 17.40 | 11.60 | 22.78 | 4 | 6.21 | 5.49 | 7.81 | 12.10 | 11.68 | 13.24 |
| Darevskia raddei nairensis | M | 10 | 12.34 | −5.32 | 19.14 | 16.81 | 9.02 | 22.60 | 10 | 9.16 | 3.14 | 14.40 | 13.04 | 9.45 | 17.82 |
| Darevskia raddei raddei | F | 3 | −2.27 | −10.81 | 7.22 | 10.33 | 9.24 | 11.43 | 3 | −0.97 | −7.56 | 5.83 | 9.35 | 8.37 | 9.87 |
| Darevskia raddei raddei | M | 8 | 9.38 | −5.47 | 19.65 | 13.98 | 6.56 | 22.32 | 7 | 4.59 | −2.46 | 8.40 | 11.08 | 9.23 | 13.92 |
| Darevskia portschinskii | F | 11 | 1.45 | −3.35 | 8.04 | 6.45 | 4.64 | 10.99 | 4 | −0.35 | −12.00 | 9.54 | 12.59 | 11.15 | 14.56 |
| Darevskia portschinskii | M | 10 | 6.41 | 3.92 | 9.79 | 10.08 | 6.81 | 13.74 | 5 | 11.06 | 2.79 | 15.69 | 13.86 | 6.65 | 19.20 |
| Darevskia valentinib | M | 1 | 8.83 | — | — | 11.69 | — | — | — | — | — | — | — | — | — |
| Parthenogenetic | |||||||||||||||
| Darevskia armeniaca | F | 14 | 5.68 | −1.37 | 9.93 | 9.80 | 5.28 | 14.22 | 14 | 6.57 | −0.67 | 11.71 | 11.35 | 6.13 | 15.79 |
| Darevskia unisexualis | F | 7 | 5.37 | 1.62 | 9.90 | 11.43 | 6.84 | 17.01 | 8 | 6.16 | 3.92 | 9.46 | 12.64 | 9.21 | 16.53 |
| Darevskia dahli | F | 14 | 2.02 | −6.03 | 6.44 | 6.69 | 4.05 | 9.22 | 11 | −2.00 | −7.86 | 4.49 | 9.15 | 7.42 | 12.30 |
| Darevskia rostombekowi | F | 10 | 2.12 | −2.40 | 7.27 | 6.83 | 4.99 | 10.35 | 4 | 0.30 | −7.43 | 4.36 | 9.85 | 8.62 | 10.75 |
Values of D. mixta are based on measurements of ethanol-fixed individuals.
An individual of D. valentini not included in the analyses of chromatic parameters.
References
- Abramjan A, Bauerová A, Somerová B, Frynta D, 2015. Why is the tongue of blue-tongued skinks blue? Reflectance of lingual surface and its consequences for visual perception by conspecifics and predators. Sci Nat 102:1–12. [DOI] [PubMed] [Google Scholar]
- Abramjan A, Frýdlová P, Jančúchová-Lásková J, Suchomelová P, Landová E. et al. , 2019. Comparing developmental stability in unisexual and bisexual rock lizards of the genus Darevskia. Evol Dev 21:175. [DOI] [PubMed] [Google Scholar]
- Amundsen T, 2000. Why are female birds ornamented?. Trends Ecol Evol 15:149–155. [DOI] [PubMed] [Google Scholar]
- Andersson MB, 1994. Sexual Selection. Princeton: Princeton University Press. [Google Scholar]
- Arakelyan MS, Danielyan FD, Corti C, Sindaco R, Leviton AE, 2011. Herpetofauna of Armenia and Nagorno Karabakh. Salt Lake City: Society for the Study of Amphibians and Reptiles. [Google Scholar]
- Arribas O, 2012. The ultraviolet photography of nature: techniques, material and (especially) Lacertini results. Butlletí la Soc Catalana Herpetol 20:72–114. [Google Scholar]
- Bajer K, Molnar O, Torok J, Herczeg G, 2011. Ultraviolet nuptial colour determines fight success in male European green lizards (Lacerta viridis). Biol Lett 7:866–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero MA, 2002. Sources of colour pattern variation in Mediterranean Psammodromus algirus. Netherlands J Zool 52:43–60. [Google Scholar]
- Carretero MA, García-Muñoz E, Argaña E, Freitas S, Corti C. et al. , 2018. Parthenogenetic Darevskia lizards mate frequently if they have the chance: a quantitative analysis of copulation marks in a sympatric zone. J Nat Hist 52:405–413. [Google Scholar]
- Danielyan F, Arakelyan M, Stepanyan I, 2008. Hybrids of Darevskia valentini, D. armeniaca and D. unisexualis from a sympatric population in Armenia. Amphibia Reptilia 29:487–504. [Google Scholar]
- Darevsky IS, 1966. Natural parthenogenesis in a polymorphic group of Caucasian rock lizards related to Lacerta saxicola Eversmann. J Ohio Herpetol Soc 5:115. [Google Scholar]
- Darevsky IS, 1967. Rock Lizards of the Caucasus: Systematics, Ecology and Phylogenesis of the Polymorphic Groups of Caucasian Rock Lizards of the Subgenus Archaeolacerta [in Russian]. Leningrad: Nauka, Academy of Sciences of USSR. [Google Scholar]
- Darwin C, 1871. The Descent of Man and Selection in Relation to Sex. London: Murray. [Google Scholar]
- Fleishman LJ, Loew ER, Leal M, 1993. Ultraviolet vision in lizards. Nature 365:397–397. [Google Scholar]
- Font E, Pérez I de Lanuza G, Sampedro C, 2009. Ultraviolet reflectance and cryptic sexual dichromatism in the ocellated lizard, Lacerta (Timon) lepida (Squamata: Lacertidae). Biol J Linn Soc 97:766–780. [Google Scholar]
- Freitas S, Rocha S, Campos J, Ahmadzadeh F, Corti C. et al. , 2016. Parthenogenesis through the ice ages: a biogeographic analysis of Caucasian rock lizards (genus Darevskia). Mol Phylogenet Evol 102:117–127. [DOI] [PubMed] [Google Scholar]
- Freitas SN, Harris DJ, Sillero N, Arakelyan M, Butlin RK. et al. , 2019. The role of hybridisation in the origin and evolutionary persistence of vertebrate parthenogens: a case study of Darevskia lizards. Heredity (Edinb) 123:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JZ, Murphy RW, Darevsky IS, 2000. Divergence of the cytochrome b gene in the Lacerta raddei complex and its parthenogenetic daughter species: evidence for recent multiple origins. Copeia 2000:432–440. [Google Scholar]
- Le Galliard JF, Fitze PS, Ferrière R, Clobert J, 2005. Sex ratio bias, male aggression, and population collapse in lizards. Proc Natl Acad Sci USA 102:18231–18236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galoyan E, 2013. a. Joint space use in a parthenogenetic Armenian rock lizard Darevskia armeniaca suggests weak competition among monoclonal females. J Herpetol 47:97–104. [Google Scholar]
- Galoyan EA, 2013. b. Intersexual relations within and among species of rock lizards of Darevskia genus. In: Modern Herpetology: Problems and Ways of Their Solutions. 25–27 November 2013. Saint Petersburg: Russian Academy of Sciences. 61–65. [Google Scholar]
- Hamilton WD, Zuk M, 1982. Heritable true fitness and bright birds: a role for parasites? Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Hegyi G, Garamszegi LZ, Eens M, Török J, 2008. Female ornamentation and territorial conflicts in collared flycatchers Ficedula albicollis. Naturwissenschaften 95:993–996. [DOI] [PubMed] [Google Scholar]
- Husak JF, Macedonia JM, Fox SF, Sauceda RC, 2006. Predation cost of conspicuous male coloration in collared lizards Crotaphytus collaris: an experimental test using clay-covered model lizards. Ethology 112:572–580. [Google Scholar]
- Jenssen TA, Orrell KS, Lovern MB, 2000. Sexual dimorphisms in aggressive signal structure and use by a polygynous lizard Anolis carolinensis. Copeia 2000:140–149. [Google Scholar]
- Jones IL, Hunter FM, 1993. Mutual sexual selection in a monogamous seabird. Nature 362:238–239. [Google Scholar]
- Kodric-Brown A, 1993. Female choice of multiple male criteria in guppies: interacting effects of dominance, coloration and courtship. Behav Ecol Sociobiol 32:415–420. [Google Scholar]
- Kratochvíl L, Fokt M, Rehák I, Frynta D, 2003. Misinterpretation of character scaling: a tale of sexual dimorphism in body shape of common lizards. Can J Zool 81:1112–1117. [Google Scholar]
- Lande R, 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305. [DOI] [PubMed] [Google Scholar]
- LeBas NR, 2006. Female finery is not for males. Trends Ecol Evol 21:170–173. [DOI] [PubMed] [Google Scholar]
- Lisboa C, Bajer K, Pessoa DMA, Huber MAA, Costa GC, 2017. Female Brazilian whiptail lizards Cnemidophorus ocellifer prefer males with high ultraviolet ornament reflectance. Behav Process 142:33–39. [DOI] [PubMed] [Google Scholar]
- Loew ER, Fleishman LJ, Foster RG, Provencio I, 2002. Visual pigments and oil droplets in diurnal lizards: a comparative study of Caribbean anoles. J Exp Biol 205:927–938. [DOI] [PubMed] [Google Scholar]
- MacGregor HEA, While GM, Barrett J, Pérez I de Lanuza G, Carazo P. et al. , 2017. Experimental contact zones reveal causes and targets of sexual selection in hybridizing lizards. Funct Ecol 31:742–752. [Google Scholar]
- Marshall KLA, Philpot KE, Stevens M, 2015. Conspicuous male coloration impairs survival against avian predators in Aegean wall lizards, Podarcis erhardii. Ecol Evol 5:4115–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall KLA, Stevens M, 2014. Wall lizards display conspicuous signals to conspecifics and reduce detection by avian predators. Behav Ecol 25:1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín J, López P, 2009. Multiple color signals may reveal multiple messages in male Schreiber’s green lizards Lacerta schreiberi. Behav Ecol Sociobiol 63:1743–1755. [Google Scholar]
- Martin M, Le Galliard J-F, Meylan S, Loew ER, 2015. The importance of ultraviolet and near-infrared sensitivity for visual discrimination in two species of lacertid lizards. J Exp Biol 218:458–465. [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker TCM, 1990. Female sticklebacks use male colouration as a basis for mate choice and hence avoid parasitised males. Nature 344:330–333. [Google Scholar]
- Molina-Borja M, Font E, Mesa Avila G, 2006. Sex and population variation in ultraviolet reflectance of colour patches in Gallotia galloti (Fam. Lacertidae) from Tenerife (Canary Islands.). J Zool 268:193–206. [Google Scholar]
- Møller AP, 1988. Badge size in the house sparrow Passer domesticus: effects of intra- and intersexual selection. Behav Ecol Sociobiol 22:373–378. [Google Scholar]
- Molnár O, Bajer K, Török J, Herczeg G, 2012. Individual quality and nuptial throat colour in male European green lizards. J Zool 287:233–239. [Google Scholar]
- Moritz C, Uzzell T, Spolsky C, Hotz H, Darevsky I. et al. , 1992. The maternal ancestry and approximate age of parthenogenetic species of Caucasian rock lizards (Lacerta: Lacertidae). Genetica 87:53–62. [Google Scholar]
- Mullen P, Pohland G, 2008. Studies on UV reflection in feathers of some 1000 bird species: are UV peaks in feathers correlated with violet-sensitive and ultraviolet-sensitive cones? Ibis 150:59–68. [Google Scholar]
- Murphy RW, Fu J, MacCulloch RD, Darevsky IS, Kupriyanova LA, 2000. A fine line between sex and unisexuality: the phylogenetic constraints on parthenogenesis in lacertid lizards. Zool J Linn Soc 130:527–549. [Google Scholar]
- Names G, Martin M, Badiane A, Galliard JL, 2019. The relative importance of body size and UV coloration in influencing male-male competition in a lacertid lizard. Behav Ecol Sociobiol 73:98. [Google Scholar]
- Nicholson KE, Harmon LJ, Losos JB, 2007. Evolution of Anolis lizard dewlap diversity. PLoS ONE 2:e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Andersson S, Wapstra E, 2011. UV-deprived coloration reduces success in mate acquisition in male sand lizards Lacerta agilis. PLoS ONE 6:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekár S, Brabec M, 2016. Marginal models via GLS: A convenient yet neglected tool for the analysis of correlated data in the behavioural sciences. Ethology 122:621–631. [Google Scholar]
- Pérez I de Lanuza G, Carazo P, Font E, 2014. Colours of quality: structural (but not pigment) coloration informs about male quality in a polychromatic lizard. Anim Behav 90:73–81. [Google Scholar]
- Pérez I de Lanuza G, Font E, 2010. Lizard blues: blue body colouration and ultraviolet polychromatism in lacertids. Rev Española Herpetol 24:67–84. [Google Scholar]
- Pérez I de Lanuza G, Font E, 2014. Ultraviolet vision in lacertid lizards: evidence from retinal structure, eye transmittance, SWS1 visual pigment genes and behaviour. J Exp Biol 217:2899–2909. [DOI] [PubMed] [Google Scholar]
- Pérez I de Lanuza G, Font E, Monterde JL, 2013. Using visual modelling to study the evolution of lizard coloration: sexual selection drives the evolution of sexual dichromatism in lacertids. J Evol Biol 26:1826–1835. [DOI] [PubMed] [Google Scholar]
- Potti J, Canal D, 2011. Heritability and genetic correlation between the sexes in a songbird sexual ornament. Heredity 106:945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke SR, 2007. Fiery red heads: female dominance among head color morphs in the Gouldian finch. Behav Ecol 18:621–627. [Google Scholar]
- R Core Team. 2018. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Rádlová S, Viktorin P, Frynta D, 2016. Barvocuc 2.0. Prague: Software for Color Image Analysis.
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinervo B, Lively CM, 1996. The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380:240–243. [Google Scholar]
- Spangenberg V, Arakelyan M, Galoyan E, Matveevsky S, Petrosyan R. et al. , 2017. Reticulate evolution of the rock lizards: meiotic chromosome dynamics and spermatogenesis in diploid and triploid males of the genus Darevskia. Genes 8:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StatSoft, 2007. STATISTICA (data analysis software system), version 8.0. Tulsa, OK.
- Stevens M, Cuthill IC, 2007. Hidden messages: are ultraviolet signals a special channel in avian communication? Bioscience 57:501–507. [Google Scholar]
- Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF, 2003. Conspicuous males suffer higher predation risk: visual modeling and experimental evidence from lizards. Anim Behav 66:541–550. [Google Scholar]
- Swierk L, Langkilde T, 2013. Bearded ladies: females suffer fitness consequences when bearing male traits. Biol Lett 9:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkhnishvili D, Murtskhvaladze M, Anderson CL, 2017. Coincidence of genotypes at two loci in two parthenogenetic rock lizards: how backcrosses might trigger adaptive speciation. Biol J Linn Soc 121:1–14. [Google Scholar]
- Troscianko J, Stevens M, 2015. Image calibration and analysis toolbox: a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol Evol 6:1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CP, Troscianko J, Endler JA, Marshall NJ, Cheney KL, 2020. Quantitative colour pattern analysis (QCPA): a comprehensive framework for the analysis of colour patterns in nature. Methods Ecol Evol 11:316–336. [Google Scholar]
- White J, Richard M, Massot M, Meylan S, 2011. Cloacal bacterial diversity increases with multiple mates: evidence of sexual transmission in female common lizards. PLoS ONE 6:e22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting MJ, Stuart-Fox DM, O’Connor D, Firth D, Bennett NC. et al. , 2006. Ultraviolet signals ultra-aggression in a lizard. Anim Behav 72:353–363. [Google Scholar]