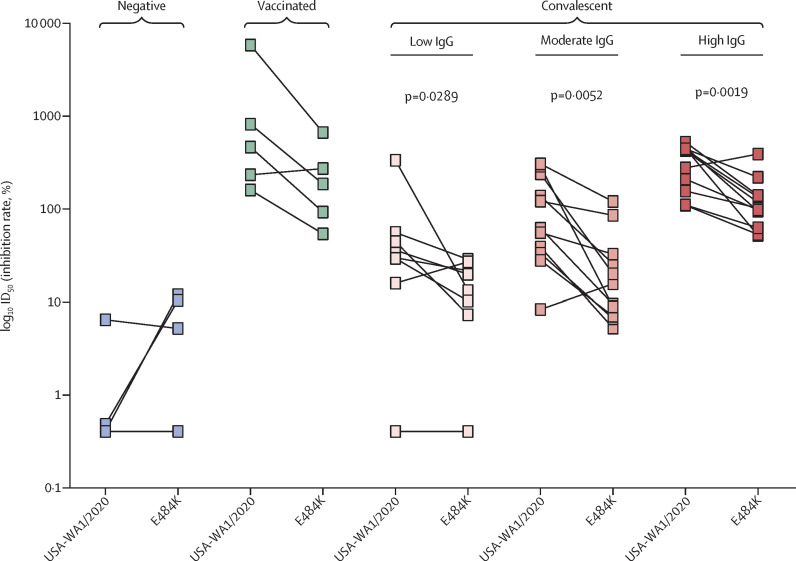
It is concerning that emerging variants of SARS-CoV-2 can evade neutralising antibodies induced by previous infection or vaccination through mutations in the spike protein, including the receptor-binding domain (RBD). The asparagine (N) to tyrosine (Y) substitution at position 501 (N501Y), present in variants of concern belonging to the B.1.1.7, B.1.351, and P.1 lineages, does not seem to affect in-vitro neutralisation of human convalescent or post-vaccination sera. However, additional substitutions, such as E484K present in B.1.351 and P.1 lineages, might allow evasion from neutralising antibodies.1, 2, 3, 4 We did in-vitro microneutralisation assays with the USA-WA1/2020 virus and a recombinant (r)SARS-CoV-2 virus, which is identical to USA-WA1/2020 except for the E484K mutation introduced in the spike RBD. A total of 34 sera were selected from study participants on the basis of their SARS-CoV-2 S ELISA antibody titre (negative [N=4] vs weak [N=8], moderate [N=11], or strong positive [N=11]; appendix p 1). Additionally, we included sera from five individuals who received two doses of the Pfizer–BioNTech SARS-CoV-2 vaccine BNT162b2 (V1-V5). The same sera have been tested for neutralisation studies with a N501Y SARS-CoV-2 variant in our recent report.5 Serum neutralisation efficiency was lower with E484K rSARS-CoV-2 versus USA-WA1/2020 for vaccinated (3·4-fold) and convalescent samples (2·4 fold for low IgG, 4·2 fold for moderate IgG, and 2·6 fold for high IgG, based on geometric means; figure ), and data were significantly different for the convalescent sera. These data suggest that the single E484K mutation in the RBD affects the binding of serum polyclonal neutralising antibodies. The decrease in neutralisation efficiency for sera with low or moderate IgG against SARS-CoV-2 spike protein could result in loss of neutralisation of the E484K recombinant virus. These data show the influence of a single mutation (E484K) present in SARS-CoV-2 B.1.351 and P.1 lineages on the neutralising activity of convalescent (infected with previous SARS-CoV-2 strains) and post-vaccination polyclonal sera. A limitation of our study is the small number of post-vaccination samples tested. However, sera with high neutralisation titres against the USA-WA1/2020 strain (convalescent and post-vaccination) were still able to neutralise E484K rSARS-CoV-2. Therefore, vaccinations should induce the highest neutralisation titres possible, to maximise protection against antigenically drifted SARS-CoV-2 strains. Most SARS-CoV-2 vaccines are recommended as a prime-boost regimen. Due to vaccine shortage, some public health authorities have recommended to postpone the booster vaccination to be able to provide more individuals with a primer vaccination. This approach will result in lower neutralising antibody titres. Our data suggest that lower neutralising antibody titres might be problematic in the context of newly emerging SARS-CoV-2 variants, considering that this approach could leave some vaccinees unprotected. Which neutralisation titre correlates with (full) protection, and to what extent immune mechanisms beyond direct virus neutralisation, such as antibody-dependent cellular cytotoxicity by non-neutralising antibodies or T-cell mediated immunity, contribute to protection is unclear. The worldwide vaccination effort should aim at fully vaccinating as many people as possible by use of vaccination strategies that result in high neutralising antibody titres.
Figure.
Human convalescent and post-vaccination sera neutralise E484K recombinant SARS-CoV-2 less efficiently than USA-WA1/2020 in an in-vitro microneutralisation assay
Convalescent sera are subdivided in low, moderate, and high IgG classes on the basis of anti-spike ELISA titres. Two-sided Mann Whitney-U tests were used to calculate statistical differences.
SJ and CY contributed equally. The Personalized Virology Initiative study group members and their affiliations, declarations of interests, and acknowledgments are listed in the appendix. This research was partly funded by the Center for Research for Influenza Pathogenesis, a Center of Excellence for Influenza Research and Surveillance supported by the National Institute of Allergy and Infectious Diseases (contract number HHSN272201400008C), by the National Cancer Institute grant U54CA260560, by the JPB Foundation and the Open Philanthropy Project (research grant 2020–215611 [5384], and by anonymous donors to AG-S. Work on SARS-CoV-2 in the Krammer and Simon laboratories was funded by the Collaborative Influenza Vaccine Innovation Centers' contract number 75N93019C00051. Research in the Martinez-Sobrido laboratory was partly funded by the New York Influenza Center of Excellence, a member of the National Institute of Allergy and Infectious Diseases, and was also partially funded by the National Institutes of Health, the Department of Health and Human Services, and the Centers of Excellence for Influenza Research and Surveillance (contract number HHSN272201400005C, New York Influenza Center of Excellence). Funding sources had no role in the study design, in the collection, analysis, and interpretation of the data, in the writing of the Correspondence, and in the decision to submit the Correspondence for publication. The García-Sastre laboratory has received research support from Pfizer, Senhwa Biosciences, and 7Hills Pharma. AG-S has consulting agreements for 7Hills Pharma, Avimex, and Esperovax involving cash, and Vivaldi Biosciences, Contrafect, Accurius, and Vaxalto involving stock. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and Newcastle disease virus-based SARS-CoV-2 vaccines which list FK as co-inventor. DS and VS are also listed on the serological assay patent application as co-inventors. FK has consulted for Merck and Pfizer (before 2020), and is currently consulting for Seqirus and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. All other authors declare no competing interests.
Contributor Information
Personalized Virology Initiative study group:
Hala Alshammary, Angela A. Amoako, Mahmoud H. Awawda, Katherine F Beach, Maria C. Bermúdez-González, Rachel L. Chernet, Lily Q. Eaker, Emily D. Ferreri, Daniel L. Floda, Charles R. Gleason, Giulio Kleiner, Denise Jurczyszak, Julia C. Matthews, Wanni A. Mendez, Lubbertus C.F. Mulder, Kayla T. Russo, Ashley-Beathrese T. Salimbangon, Miti Saksena, Amber S. Shin, Levy A. Sominsky, and Komal Srivastava
Supplementary Material
References
- 1.Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9 doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greaney AJ, Loes AN, Crawford KHD, et al. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Cell Host Microbe. 2021;29:463–476. doi: 10.1016/j.chom.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv. 2021 doi: 10.1101/2021.01.15.426911. published online Jan 30. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu K, Werner AP, Moliva JI, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021 doi: 10.1101/2021.01.25.427948. published online Jan 25. (preprint). [DOI] [Google Scholar]
- 5.Rathnasinghe R, Jangra S, Cupic A, et al. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera. medRxiv. 2021 doi: 10.1101/2021.01.19.21249592. published online Jan 20. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.