SUMMARY
Tissue-resident memory (Trm) CD8+ T cells mediate protective immunity in barrier tissues, but the cues promoting Trm cell generation are poorly understood. Sensing of extracellular adenosine triphosphate (eATP) by the purinergic receptor P2RX7 is needed for recirculating CD8+ T cell memory, but its role for Trm cells is unclear. Here we showed that P2RX7 supported Trm cell generation by enhancing CD8+ T cell sensing of TGF-β, which was necessary for tissue residency. P2RX7-deficient Trm cells progressively decayed in non-lymphoid tissues and expressed dysregulated Trm-specific markers. P2RX7 was required for efficient re-expression of the receptor TGF-βRII through calcineurin signaling. Forced Tgfbr2 expression rescued P2RX7-deficient Trm cell generation, and TGF-β sensitivity was dictated by P2RX7 agonists and antagonists. Forced Tgfbr2 also rescued P2RX7-deficient Trm cell mitochondrial function. Sustained P2RX7 signaling was required for long-term Trm cell maintenance, indicating that P2RX7 signaling drives induction and CD8+ T cell durability in barrier sites.
In Brief
Borges da Silva et al. report that the extracellular ATP receptor P2RX7 promotes generation of virus-specific tissue-resident memory (Trm) CD8+T cells through induction of the TGF-β signaling pathway. Furthermore, long-term maintenance of Trm cells relies on continuous P2RX7 signaling.
Graphical Abstract

INTRODUCTION
Immunological memory is a hallmark of the adaptive immune system, where distinct memory cell types contribute to mediate enhanced specific antigen recognition (Farber et al., 2016). Among these types, memory CD8+ T cells are crucial to mediate long-term protection against intracellular pathogens. Memory CD8+ T cells can be divided by their migration pattern. Central memory T (Tcm) cells circulate through lymphoid tissues and are long-lived, providing surveillance in these sites. In contrast, tissue-resident memory (Trm) cells are a first line of defense against pathogens in non-lymphoid tissues (NLTs) (Jameson and Masopust, 2018; Schenkel and Masopust, 2014). The intracellular pathways promoting establishment of memory CD8+ T cells are subject of intense research (Araki et al., 2009; Henning et al., 2018; Milner and Goldrath, 2018; Zhang et al., 1998), paving the way for strategies to boost the generation of long-lived memory CD8+ T cells upon immunization or infection. Extracellular signals also play a critical role in memory CD8+ T cell homeostasis, such as cytokine (Zhang et al., 1998) and chemokine (Alanio et al., 2018; Jung et al., 2016) sensing. However, many other signals are present in the extracellular milieu, and less is known about how these affect memory CD8+ T cell generation and maintenance. In particular, the extracellular cues that drive generation of Trm cells are unclear.
Among the established connections between tissue-derived signals and tissue residency, the role of transforming growth factor β (TGF-β) is widely known. TGF-β is crucial for formation of CD8+ Trm cells in the small intestine intraepithelial lymphocyte (SI IEL) population, skin epidermis and kidney (Casey et al., 2012; Mackay et al., 2013; Zhang and Bevan, 2013; Ma et al., 2017), as well as of tissue-resident CD8αα+ cells that arise naturally in the SI-IEL pool (Konkel et al., 2011). In T cells, TGF-β induces a Trm cell transcriptional program in tissue-infiltrating CD8+ T cells (Nath et al., 2019) and promotes CD103-expressing Trm cells in epithelial sites (Mackay et al., 2013; Sheridan et al., 2014; Zhang and Bevan, 2013). CD103, αE-integrin, which pairs with β7-integrin to bind E-cadherin, is important for retention of some CD8+ and CD4+ Trm cells (Iijima and Iwasaki, 2015; Schenkel and Masopust, 2014), but TGF-β plays additional roles in promoting Trm cells, including negative regulation of T-bet and Eomes (Mackay et al., 2015) and the ability to downregulate expression of KLF2, a transcription factor essential for T cell trafficking (Skon et al., 2013). TGF-β receptors are expressed by naive T cells, and T cell receptor (TCR) stimulation provokes their transient downregulation (Tu et al., 2018); however, the cues that promote re-expression of TGF-β receptors in T cells are poorly defined, leaving it unclear how activated T cells regain TGF-β sensitivity during generation of the Trm cell pool.
The purinergic receptor P2RX7 is crucial for establishment of long-lived memory CD8+ T cells in a cell-intrinsic way (Borges da Silva et al., 2018), affecting recirculating and resident memory populations. P2RX7 is an ATP-gated ion channel and, in the presence of micromolar extracellular ATP (eATP) concentrations, induces cytosolic calcium (Ca2+) influx and potassium (K+) efflux (Khadra et al., 2013). In contrast, high eATP concentrations or sustained eATP stimulation leads to opening of non-specific cytolytic pores and subsequent cell death (Ferrari et al., 1996; Rissiek et al., 2015). Although P2RX7 promotes mitochondrial homeostasis in Tcm cells, its precise role in Trm cells is not understood. Indeed, there is controversy whether P2RX7 is beneficial or detrimental to CD8+ Trm cell maintenance (Borges da Silva et al., 2018, 2019; Stark et al., 2018). Because Trm cells in at least some NLTs may be exposed to sustained high concentrations of eATP (Proietti et al., 2019), P2RX7 signaling could shape not only the induction but also the durability of the Trm cell pool.
Here we show that P2RX7 is critical for differentiation and maintenance of CD8+ Trm cells primarily by inducing re-expression of TGF-β receptors in effector CD8+ T cells. We report that P2RX7 is necessary for tissue-infiltrating CD8+ T cells to fully up-regulate CD103 and establish residency but also required for long-term maintenance of established Trm cells. Forced Tgfbr2 expression restored the ability of P2rx7−/− CD8+ T cells to establish residency in some NLTs and also enhanced mitochondrial function and homeostasis of virus-specific CD8+ T cells, suggesting an unanticipated link between P2RX7, TGF-β signaling, and mitochondrial function in CD8+ T cells.
RESULTS
CD8+ Trm cells require P2RX7 expression upon activation to establish residency in NLTs
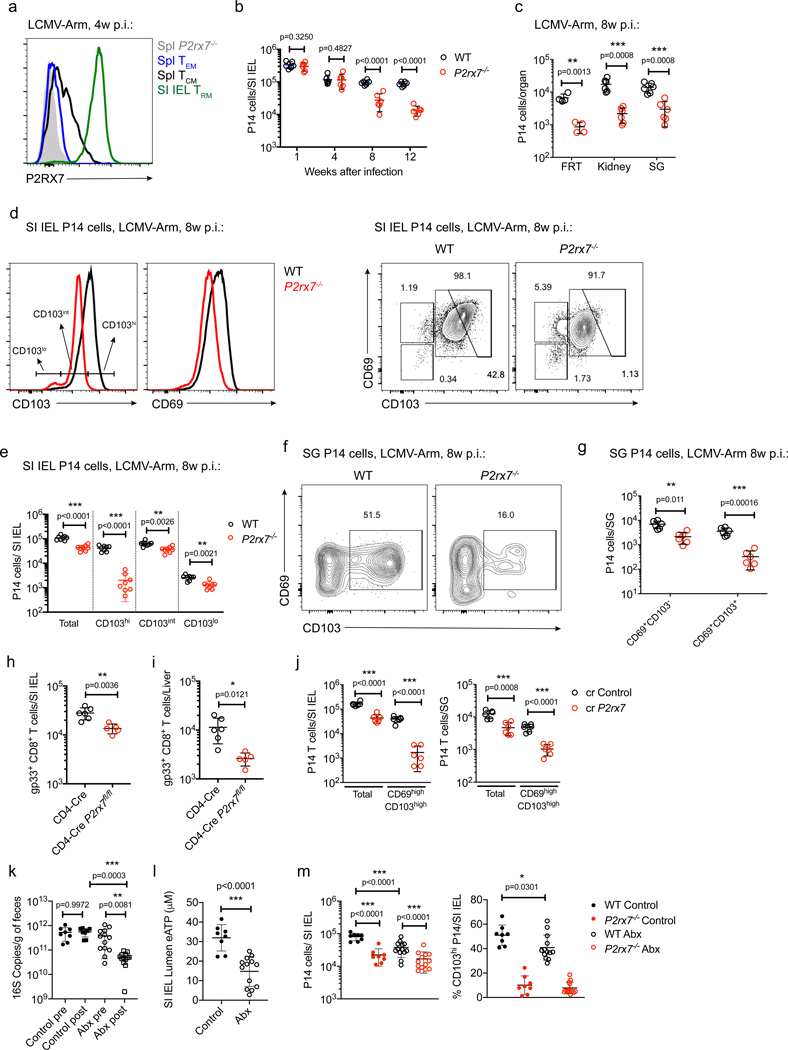
We initially examined P2RX7 expression and function in adoptively transferred P14 CD8+ T cells primed by infection with the lymphocytic choriomeningitis virus (LCMV) Armstrong strain (LCMV). As reported previously (Borges da Silva et al., 2018, 2019), P2RX7 was highly expressed by Trm cells, especially those in mucosal tissues, but also detectable on Tcm cells under suitable staining conditions (Figures 1A, S1A, and S1B). Confirming our previous findings, germline P2RX7-deficient (P2rx7−/− ) P14 TCR transgenic CD8+ T cells failed to establish long-lived Trm cells in several NLTs compared with wild-type (WT) P14 cells: in the SI IEL population (Figure 1B), female reproductive tract (FRT), kidney and salivary glands (SGs) (Figure 1C), and liver (Figure S2A). In the SI IEL population at 8 weeks, we observed a marked defect in the numbers of P2rx7−/− P14 cells with high cell-surface expression of CD69 and CD103 (Figures 1D and 1E; Borges da Silva et al., 2018), markers often used to identify Trm cells (Mackay et al., 2013), although other phenotypic subsets were also reduced significantly. Similarly, CD69hiCD103hi populations were markedly reduced among P2rx7−/− P14 cells in the SGs (Figures 1F and 1G) and kidneys (Figure S2B) following LCMV infection, and skin P2rx7−/− OT-I TCR transgenic CD8+ T cells (OT-I) following infection with the vesicular stomatitis virus Indiana strain expressing ovalbumin (VSV-OVA) (Figure S2C) although, again, some reduction in the numbers of P2rx7−/− CD69+CD103− cells was also observed in all sites.
Figure 1. CD8+ Trm Cells Require P2RX7 to Establish in NLTs.
(A–G) WT and P2rx7−/− P14 cells (distinguished by congenic markers) were transferred into recipient mice, which were infected with LCMV.
(A) Representative histogram plots showing P2RX7 expression on different WT memory P14 subsets. Naive P2rx7−/− P14 cells provided a staining control. (B and C) Numbers of WT and P2rx7−/− P14 cells at the indicated times after infection in the small intestine intraepithelial lymphocyte population (SI IEL; B) or in the female reproductive tract (FRT), kidneys, and salivary glands (SGs) 8 weeks after infection (C).
(D–G) 8 weeks after LCMV infection, WT and P2rx7−/− P14 cells were analyzed for CD69 and CD103 expression in the SI IEL (D) and SGs (F) and for the numbers of subpopulations distinguished by CD103 expression in the SI IEL (E) and SGs (G).
(H and I) CD4-Cre P2rx7+/+ (CD4-Cre) and CD4-Cre P2rx7fl/fl mice were infected with LCMV, and more than 4 weeks later, the numbers of gp33/Db tetramer-binding (gp33+) CD8+ T cells were quantified in the SI IEL (H) and liver (I).
(J) Congenically distinct P14 cells were activated in vitro and electroporated with Cas9 plus single guide RNAs for P2rx7 (sgP2rx7) or Cd19 as a control (sgControl) and then co-transferred into recipient mice that were infected with LCMV. After 5 weeks, the numbers of SI IEL (left) and SG (right) P14 cells were quantified. (K–M) WT and P2rx7−/− P14 cells were transferred into mice infected with LCMV; the mice were treated between days −7 and +28 (relative to LCMV infection) with PBS (control) or antibiotics (Abx).
(K) 16S bacterial RNA counts at days −7 (pre) and +28 (post) relative to LCMV infection.
(L) eATP concentration in the SI IEL lumen at day 28 after LCMV infection.
(M) Numbers (left) and percentages of CD103hi (right) SI IEL P14 cells at day 28 after LCMV infection.
Data are from 2–4 independent experiments; n = 4–13 per experimental group. Graphical data (B, C, E–M) is shown as means with error bars indicating SD; p values from two-tailed t-test are indicated: *, p <0.05; **, p<0.01; ***, p<0.001. See also Figures S1–S3.
Trm cells can also form in secondary lymphoid organs (SLOs) (Schenkel et al., 2014), a population that also highly expressed P2RX7 (Figure S2D) and was under-represented among P2rx7−/− CD8+ T cells (Figures S2E and S2F), demonstrating a requirement for P2RX7 for Trm cells even in lymphoid sites.
Because CD8+ T cells might be affected by other P2rx7−/− cells in germline knockout mice, we extended these studies. Defects in circulating and resident P2rx7−/− CD8+ T cell memory populations were observed in T cell-specific conditional P2rx7−/− mice (CD4-Cre P2rx7fl/fl) (Figures 1H, 1I, S2G, and S3A–S3D) and in P2rx7−/− cells from mixed bone marrow chimeras (Figures S2H and S2I). To confirm that our studies reflect P2RX7 function following T cell activation, we ablated P2rx7 via CRISPR-Cas9 of in-vitro-activated WT P14 cells prior to adoptive transfer and LCMV infection (Figure S3E). P14 cells ablated for P2RX7 (sgP2rx7) failed to establish long-lived circulating and resident memory compared with controls (Figures 1J and S3F–S3H). These results indicate that cell-intrinsic P2RX7 expression following T cell activation is crucial for long-lived CD8+ Trm cell establishment.
The in vivo source of eATP that promotes P2RX7 stimulation is unclear. In the gut, local microbiota contribute to luminal eATP concentrations (Perruzza et al., 2017; Proietti et al., 2019), and bacterial depletion using antibiotics (Abx) has been reported to reduce these concentrations (Proietti et al., 2019), which we confirmed (Figures 1K and 1L). Abx treatment caused a partial decrease in WT SI IEL P14 Trm cell numbers and reduced CD69hiCD103hi pool frequency (Figure 1M). This effect was specific for WT intestinal P14 Trm cells because no changes in SI IEL P2rx7−/− P14 cells or P14 in the SGs or SLOs (Figures S2J and S2K) was detected. These results suggest that microbiota-derived eATP plays a partial but important role in gut Trm cell establishment.
P2RX7 Promotes the Trm Cell Phenotype of CD8+ T Cells Recruited to NLTs and Their Attachment to the Intestinal Epithelium
Upregulation of CD103 and CD69 in effector CD8+ T cells occurs soon after entry into the SI IEL pool (Masopust et al., 2006) and is correlated with high P2RX7 expression (Figure 2A). Hence, we investigated whether P2RX7 regulates the efficiency or phenotype of CD8+ T cells recently recruited to NLTs. P2rx7−/− P14 cells initially entered the SI IEL pool as efficiently as WT cells overall, but we noted a severe underrepresentation of P2rx7−/− CD69+CD103+ P14 cells and a milder but significant decrease in CD69loCD103+ (Figures 2B–2D). This was also observed in other sites (such as the SGs; data not shown), and the effect was magnified for CD69highCD103high cells 7 days after LCMV infection (Figures 2E and 2F). Overall, these results suggest that P2RX7 expression does not limit NLTs recruitment but does influence initial upregulation of CD69 and CD103.
Figure 2. P2RX7 Is Crucial for Initial Upregulation of CD103 and CD69 in NLTs.
(A–F) P14 cells (WT or P2rx7−/− ) were adoptively transferred, and recipient mice were infected with LCMV for 5 (A–D) or 7 (E and F) days.
(A) Representative flow cytometry plots showing the relationship between CD69 and CD103 expression (left) and P2RX7 protein expression (center, right) among SI IEL WT P14 cells.
(B–F) Co-transferred WT and P2rx7−/− P14 cells were analyzed at day 5 (B–D) or 7 (E and F) after infection and evaluated for CD103 and CD69 expression (B and E; representative data), and the ratio (C) or absolute number (D and F) of WT and P2rx7−/− P14 cells was determined for the indicated phenotypic subsets in the indicated tissues.
Data are from 2–4 independent experiments; n = 5–10 per experimental group. Graphical data is shown as means with error bars indicating SD (A, C, D) or SEM (F). P-values from One-way ANOVA (A) or two-tailed t-test (C-D, F) are indicated: *, p <0.05; ***, p<0.001.
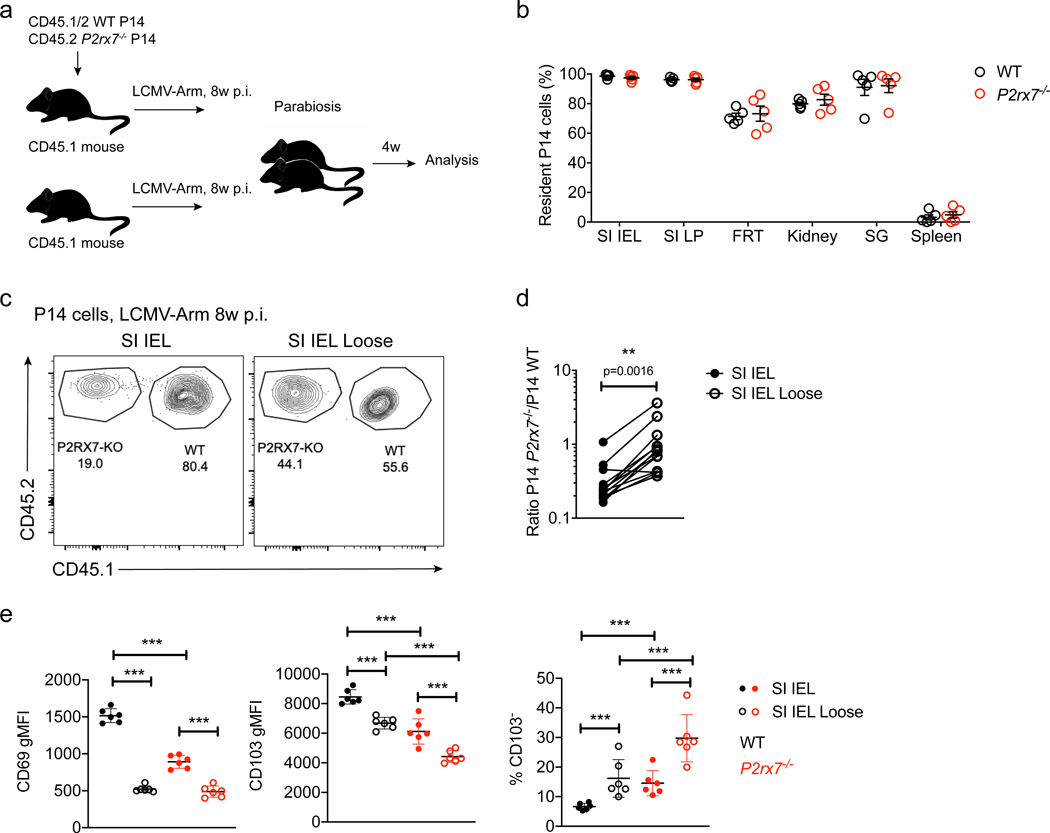
Because CD103 is critical for SI IEL CD8+ Trm cells (Casey et al., 2012), it was possible that P2rx7−/− memory CD8+ T cells in this and other NLTs were not truly tissue-resident at all but, rather, a population of recirculating effector memory T (Tem) cells. To address this, we performed parabiosis studies, conjoining mice that had received co-adoptive transfer of WT and P2rx7−/− P14 cells and LCMV infection (>30 days earlier) with non-transferred, infection-matched controls (Figure 3A). P2rx7−/− P14 cells were numerically disadvantaged in comparison with WT P14 in immune parabionts, highlighting their deficiency in forming Trm cells (Figure S3I). However, within these populations, WT and P2rx7−/− P14 cells exhibited similar residency profiles in diverse NLTs (Figure 3B), showing that the rare P2rx7−/− P14 cells in NLTs are indeed Trm cells. However, the progressive loss of P2rx7−/− Trm cells (Borges da Silva et al., 2018; Figure 1B) suggested that this population is poorly retained in the long term. Indeed, when we used a modified small intestine T cell purification protocol (Zhang and Bevan, 2013), which distinguishes between resident CD8+ T cells that are “loosely” attached to the SI IEL (“SI IEL Loose”) from total SI IEL P14 cells, we observed that P2rx7−/− P14 cells are more prominent in the SI IEL Loose compartment, suggesting that these cells are not as effectively anchored in this tissue (Figures 3C–3E). Furthermore, SI IEL P2rx7−/− P14 cells exhibited increased percentages of cell death at late (but not early) memory time points (Figure S3J), correlating with their decay over time.
Figure 3. P2rx7–/– CD8+ T Cells in NLTs Are Resident but Less Firmly Attached to the Intestinal Epithelium.
(A and B) Eight weeks after co-transfer of WT and P2rx7−/− P14 cells and LCMV infection, mice were surgically conjoined to non-transferred, infection-matched mice, and parabiosis was maintained for 4 weeks (A) before sacrifice and analysis to determine the percentage of tissue-resident WT and P2rx7−/− P14 cells in the indicated tissues (B; 100% indicates fully resident, and 0% indicates equilibration between the parabionts).
(C–E) Samples of small intestine were recovered from mice co-transferred with WT and P2rx7−/− P14 cells and infected with LCMV 8 weeks earlier and subjected to normal processing (SI IEL) or mock enzymatic digestion before mechanical disruption (SI IEL Loose).
(C and D) The relative frequencies of WT and P2rx7−/− P14 cells in the SI IEL and SI IEL Loose fractions was determined (C shows representative flow cytometry data). Protein expression of CD69 and CD103 (left, center; average gMFI) and the percentages of CD103lo cells were determined for each group (E).
Data are from 2–4 independent experiments; n = 5–13 per experimental group. Graphical data is shown as means with error bars indicating SD (E) or SEM (B). p values from two-tailed t-test are indicated: “ns” (not significant), p>0.05; **, p<0.01; ***, p<0.001.
P2RX7-Mediated eATP Sensing Primes CD8+ T Cells to Respond to TGF-β
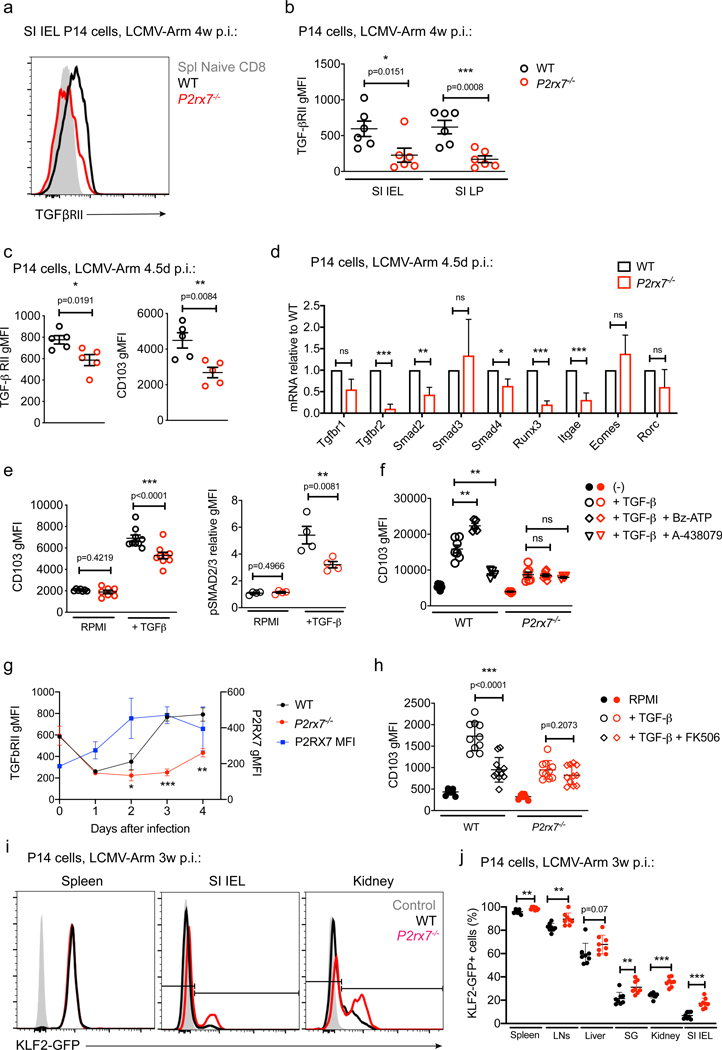
Expression of TGF-β receptors is required for generation of CD8+ Trm cells in some NLTs (Zhang and Bevan, 2013). TGF-β stimulation promotes expression of CD103 (Casey et al., 2012; Mackay et al., 2013) and can induce downregulation of KLF2 and, subsequently, S1PR1 (Skon et al., 2013), which indirectly leads to elevated CD69 cell surface expression (Jameson and Masopust, 2018). Because P2RX7 was required for optimal CD69 and CD103 expression, we considered whether P2RX7 signaling impinges on TGF-β sensitivity. As shown in Figures 4A and 4B, expression of TGF-β receptor II (TGF-βRII) was reduced significantly on P2rx7−/− P14 SI IEL and SI Lamina Propria (LP) Trm cells. We next determined whether defective TGF-β sensitivity would extend to early effector phase populations in lymphoid sites prior to “seeding” NLTs (Mackay et al., 2013; Masopust et al., 2010; Milner et al., 2017). Expression of TGF-β RII and CD103 was reduced in the day 4.5 splenic P2rx7−/− population (Figure 4C), and gene expression analysis showed reductions in numerous components of the TGF-β sensing and signaling pathway (Figure 4D). These defects were transient because gene expression for these molecules was similar in WT and P2rx7−/− splenic P14 cells 8 days post-LCMV infection (Figure S4C; Borges da Silva et al., 2018). In addition to inducing CD103, TGF-β inhibits expression of the transcription factors T-bet and Eomes in CD8+ Trm cells (Mackay et al., 2015); although no difference in Eomes expression was observed, T-bet protein expression geometric mean fluorescence intensity (gMFI) was elevated in P2rx7−/− Trm cells (Figures S4A and S4B), consistent with reduced TGF-β stimulation.
Figure 4. P2RX7-Dependent eATP Sensing Potentiates the Ability of CD8+ T Cells to Respond to TGF-β.
(A–G) Mice co-transferred with WT and P2rx7−/− P14 cells were sacrificed at 4 weeks (A and B) or 4.5 days (C–F) or multiple time points (G) after LCMV infection. Donor cells in the spleen, SI IEL and SI LP were assessed for TGF-βRII expression gMFI (A and B; A shows representative flow cytometry data). Alternatively, spleens from isolated 4.5 days post-LCMV infection, donor cells sorted cell surface expression of TGF-βRII and CD103 (C), and mRNA prepared to assess expression of the listed genes (D).
(E and F) Splenocytes were cultured for 40 h with the indicated stimuli, and WT and P2rx7−/− P14 donor cells were assessed for expression of CD103 (E, left panel, and F) and phosphorylated SMAD2/3 (pSMAD2/3; E, right panel) (data are presented as median fluorescent intensity). In (F), some cells were incubated with a P2RX7 agonist (Bz-ATP) or antagonist (A438079).
(G) Mice were sacrificed on days 1–4 post-infection, and donor cells were assessed for expression of P2RX7 (WT, blue line) and TGF-βRII (WT, black line; P2rx7−/−, red line) (data are presented as geometric mean fluorescence intensity [gMFI]).
(F) Naive WT and P2rx7−/− P14 cells were in-vitro-activated with αCD3, αCD28, and interleukin-2 (IL-2) for 72 h and then assessed for CD103 expression after exposure to the indicated factors for a further 40 h (FK506, when used, was introduced 6 h prior to hTGF-β addition).
(I and J) In-vitro-activated KLF2-GFP P14 cells were electroporated with Cas9 and sgRNAs for P2rx7 (sgP2rx7) or Cd19 (sgCd19) and then co-transferred into recipient mice that were infected with LCMV for 3 weeks, at which point average KLF2-GFP MFI was assessed in the indicated tissues (I shows representative data).
Data are from 2–4 independent experiments; n = 4–11 per experimental group. Graphical data is shown as means with error bars indicating SD (G, H, J) or SEM (B–F). p values from two-tailed t-test are indicated: “ns” (not significant), p>0.05; *, p <0.05; **, p<0.01; ***, p<0.001 See also Figures S4 and S5.
These results would predict that P2RX7 is required for normal TGF-β sensitivity. To test this, we stimulated effector P14 cells with TGF-β in vitro (Casey et al., 2012) and read out responsiveness by CD103 upregulation and phosphorylation of the TGF-βRII targets Smad2 and Smad3. All of these responses were reduced in P2rx7−/− compared with WT P14 effector cells (Figure 4E). This system also let us evaluate whether TGF-β sensitivity was influenced by pharmacological manipulation of P2RX7 using the P2RX7 agonist 2’(3’)-O-(4-benzoylbenzoyl)-ATP (Bz-ATP) or antagonist A-438079. Bz-ATP treatment led to increased upregulation of CD103, whereas A-438079 had the opposite effect (Figure 4F); both responses were P2RX7 dependent because no effect was observed on P2rx7−/− P14 cells. Moreover, short-term incubation of in vitro-activated CD8+ T cells with Bz-ATP induced elevated Itgae and Tgfbr2 mRNA expression in a P2RX7-dependent way (Figure S5A). These data suggest that P2RX7 signaling primes effector CD8+ T cells to respond to TGF-β. Indeed, upregulation of P2RX7 in activated P14 cells procedeed and was required for re-expression of TGF-βRII in activated P14 cells in vivo (Figure 4G) and in vitro (Figure S5B) – which, as described previously (Tu et al., 2018), is initially downregulated by TCR stimulation. We extended these studies and showed that pre-treatment with a P2RX7 agonist or antagonist affected the subsequent response to TGF-β stimulation (Figures S5C and S5D). Together, these results suggest that P2RX7 activation primes effector CD8+ T cell sensitivity to TGF-β. Because P2RX7 stimulation induces calcium influx, we explored whether these effects might involve Calcineurin activity (Lewis, 2001). Calcineurin blockade using low-dose FK506 reduced TGF-β-induced CD103 expression in WT (but not P2rx7−/− ) effector P14 cells (Figure 4H), in keeping with a role of Ca2+ and Calcineurin involvement in P2RX7-mediated TGF-β sensitivity.
A putative function of TGF-β is to aid in downregulation of the transcription factor KLF2, which is required for CD8+ T cells to establish tissue residency (Skon et al., 2013). To assess how P2RX7 deficiency influences KLF2 downregulation, we used CRISPR-Cas9 to ablate P2rx7 in KLF2-GFP reporter P14 cells. Loss of P2rx7 led to partially increased percentages of KLF2-GFP+ cells in distinct organs (Figures 4I and 4J), detected in the SI IEL as early as 5 days post-infection (p.i.; Figure S5E); KLF2 downregulation was inversely correlated with P2RX7 in NLTs (Figure S5F). These results suggest that P2RX7 contributes to KLF2 downregulation in developing Trm cells.
Forced Tgfbr2 Expression Rescues CD69+CD103+ Trm Cell Generation in P2rx7–/– CD8+ T Cells
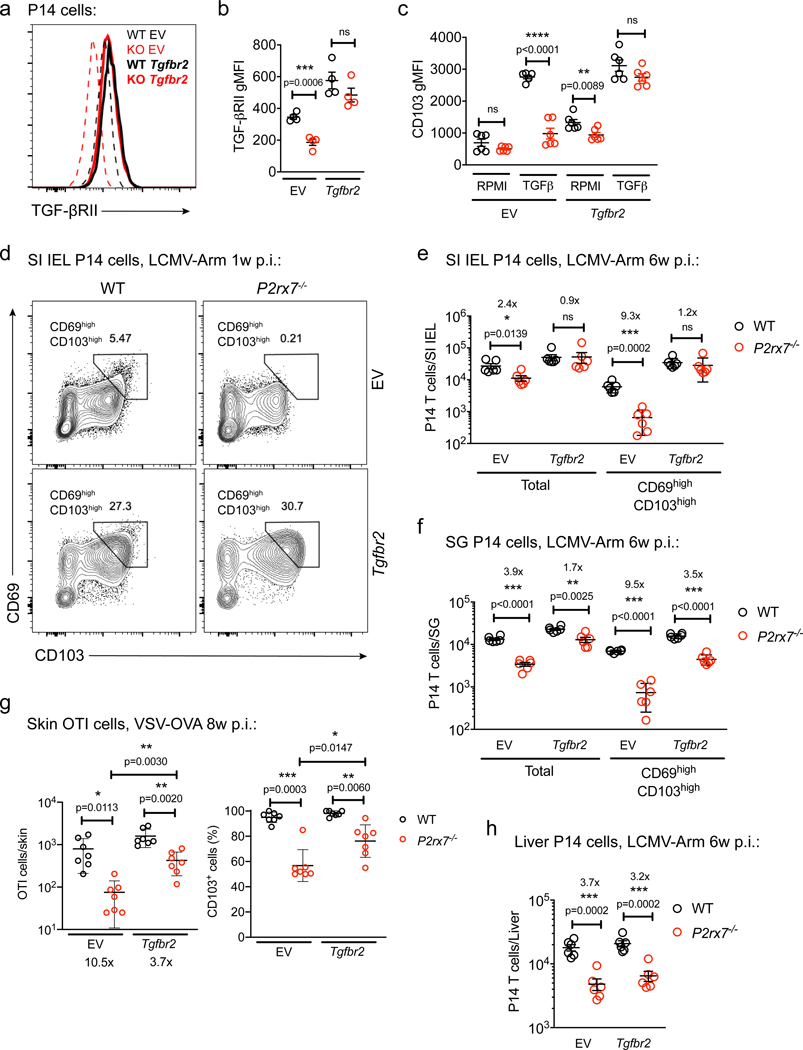
Because our studies suggested that P2RX7 is required for normal TGF-β sensitivity by activated CD8+ T cells, we assessed whether forced expression of TGF-βRII could compensate for P2RX7 deficiency. Retroviral transduction of activated P14 CD8+ T cells with a vector encoding the mouse Tgfbr2 gene led to similar TGF-βRII mRNA (Figure S6A) and protein expression (Figures 5A and 5B) in WT and P2rx7−/− P14 cells. Consequently, transduced WT and P2rx7−/− P14 cells showed equivalent TGF-β sensitivity in vitro (Figures 5C and S6B). This allowed us to test whether forced Tgfbr2 expression affected P2rx7−/− Trm cell generation in vivo. Although P2rx7−/− cells transduced with an empty vector (EV) showed defective generation and poor co-expression of CD69 and CD103 among SI IEL Trm cells, Tgfbr2 transduction largely compensated for these defects, minimizing the difference between WT and P2rx7−/− cells (Figures 5D and 5E). A similar “rescue” was observed for transduced Tgfbr2-P2rx7−/− P14 cells in the SGs (Figure 5F) and transduced P2rx7−/− OT-I cells in the skin following VSV-OVA infection (Figure 5G). On the other hand, liver P2rx7−/− P14 Trm cells were not rescued by forced Tgfbr2 expression (Figure 5H), indicating that P2RX7 promotes liver Trm cell generation independent of TGF-βRII expression.
Figure 5. Forced Expression of Tgfbr2 Restores Trm Cell Generation by P2rx7–/– CD8+ T Cells.
(A–H) WT or P2rx7−/− P14 (A–F and H) or OT-I CD8+ T cells (G) were activated in vitro and transduced with empty vector (EV) or Tgfbr2-encoding retroviruses. (A–C) Transduced cells were maintained in vitro and assessed for TGF-βRII protein expression (A and B, shown as gMFI; A shows representative data) or expression of CD103 after 40 h of culture with or without human TGF-β (hTGF-β).
(D–H) Retrovirally transduced WT and P2rx7−/− P14 (D–F and H) or OT-I (G) cells were co-transferred into recipient mice that were then infected with LCMV (D–F and H) or VSV-OVA (G) and analyzed 1 week (D), 6 weeks (E, F, and H), or 8 weeks (G) post-infection.
(D) Representative flow cytometry plots showing expression of CD69 and CD103 in the indicated P14 populations.
(E–H) Numbers of EV- and Tgfbr2-transduced WT and P2rx7−/− P14 cells were determined in the SI IEL (E), SGs (F), or liver (H). In (G), EV- and Tgfbr2-transduced WT and P2rx7−/− OT-I cells were isolated from the skin and assessed for absolute numbers and frequency of CD103+ cells.
Data are from 2–3 independent experiments; n = 4–7 per experimental group. Graphical data is shown as means with error bars indicating SD (G) or SEM (B, C, E, F, H). p values from two-tailed t-test are indicated: “ns” (not significant), p>0.05; *, p <0.05; **, p<0.01; ***, p<0.001. See also Figure S6.
Forced Tgfbr2 Expression Restores Mitochondrial Homeostasis of P2rx7–/– CD8+ T Cells
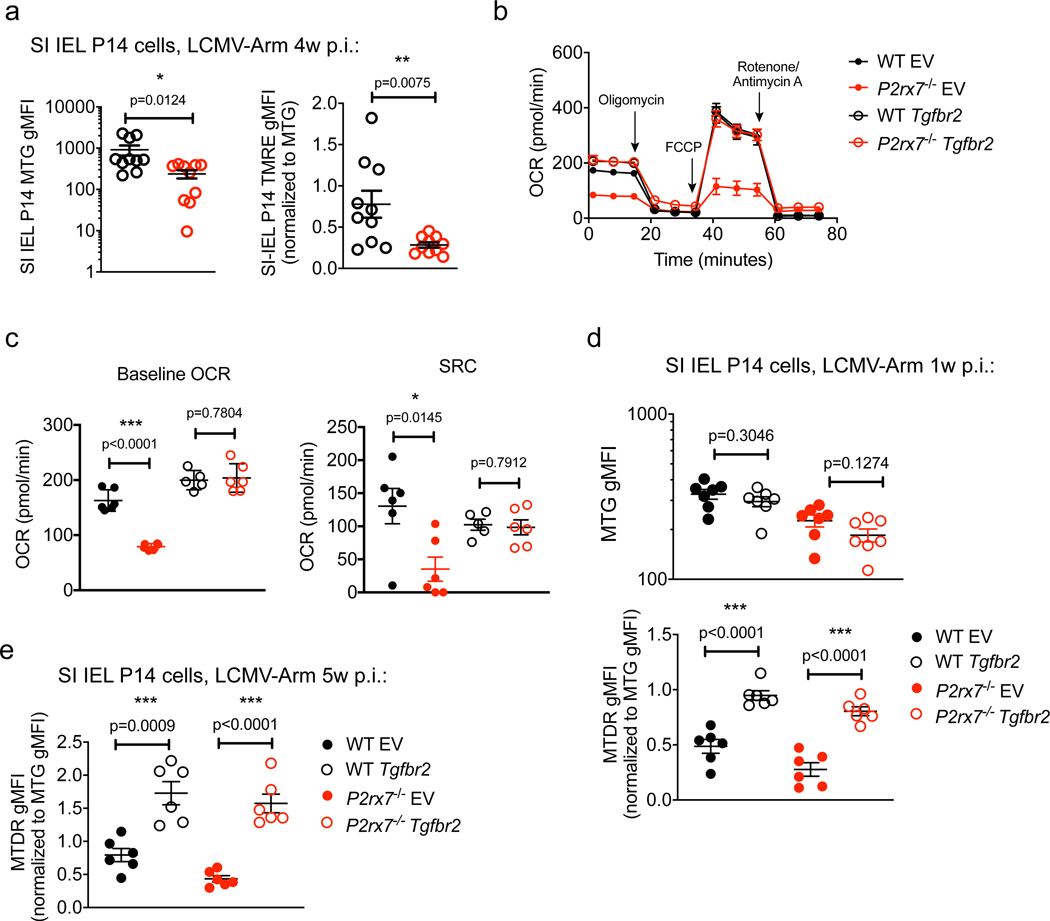
In addition to rescuing most Trm cells, forced expression of TGF-βRII partially restored generation of P2rx7−/− KLRG1loCD127hi (memory precursor - MP) cells, which contain precursors of circulating and resident memory populations (Mackay et al., 2013; Sarkar et al., 2008), and enhanced generation of P2rx7−/− Tcm cells in lymphoid sites (Figures S6C–S6F). Because these populations do not require CD103 or CD69 to form, it was unclear how TGF-βRII expression altered their differentiation. P2RX7 deficiency impairs mitochondrial maintenance in circulating memory CD8+ T cells (Borges da Silva et al., 2018), and we observed that mitochondrial mass and membrane potential were also reduced in P2rx7−/− SI IEL Trm cells (Figure 6A). We therefore tested whether P2RX7-dependent TGF-β sensitivity regulates mitochondrial metabolism in effector and memory CD8+ T cells. Upon incubation with TGF-β and IL-15 in vitro, P2rx7−/− P14 “memory-like” cells exhibited decreased mitochondrial respiration rates (oxygen consumption rate [OCR]) in comparison with WT P14 cells, and forced expression of TGF-βRII rescued the OCR of these in vitro memory-like P2rx7−/− cells (Figures 6B and 6C). In vivo, forced TGF-βRII expression restored mitochondrial memory potential (but not mitochondrial mass) in P2rx7−/− P14 CD8+ T cells from both the spleen and SI IEL (Figures 6D, 6E, and S6G). Overall, these results suggest that the role of P2RX7 in regulating CD8+ T cell metabolism may, to some extent, be mediated by enhanced TGF-β sensitivity.
Figure 6. Tgfbr2 Overexpression Restores Trm Cell Mitochondrial Homeostasis in the Absence of P2RX7.
(A) WT and P2rx7−/− P14 cells in the SI IEL 4 weeks after adoptive transfer and LCMV infection were stained for mitochondrial mass (Mitotracker green [MTG], left) and mitochondrial membrane potential (TMRE, right). Data for TMRE are normalized to MTG gMFI.
(B and C) WT or P2rx7−/− P14 cells were transduced with EV or Tgfbr2-encoding retroviral vectors during in vitro activation. Cells were then incubated for 40 h with hTGF-β and IL-15 and subjected to a mitochondrial stress test. Oxygen consumption rates (OCRs) were determined for the indicated groups after sequential addition of oligomycin, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP), and rotenone plus An-timycin A (B), and the baseline OCR concentrations (left) and spare respiratory capacity (SRC; the difference between maximal and baseline OCR concentrations; right) were determined (C). (D and E) Retrovirally transduced WT and P2rx7−/− P14 cells were co-transferred into recipient mice that were then infected with LCMV for 1 week (D) or 5 weeks (E), at which time donor cells in the SI IEL were assessed for mitochondrial mass (MTG; D, above) and mitochondrial membrane potential (Mitotracker Deep Red [MTDR] corrected for MTG staining; D, below, and E).
Data are from 3–4 independent experiments; n = 6–10 per experimental group. Graphical data (A, C–E) is shown as means with error bars indicating SEM. p values from two-tailed t-test are indicated: *, p <0.05; **, p<0.01; ***, p<0.001. See also Figure S6.
P2RX7 Is Required for Long-Term Trm Cell Maintenance
P2rx7 deficiency led to progressive loss of Trm and Tcm cells over time (Borges da Silva et al., 2018; Figure 1B), and although our studies suggest defective differentiation of P2rx7−/− memory precursors, there may also be a role of sustained P2RX7 activity following memory CD8+ T cell establishment. Indeed, pharmacological blockade of P2RX7 leads to decay of pre-formed Tcm cells (Borges da Silva et al., 2018). To address this more precisely, we utilized an inducible P2rx7 knockout approach for “late” P2rx7 ablation. P2rx7fl/fl Ert2-Cre+ P14 (P2rx7fl/fl) and P2rx7+/+ Ert2-Cre+ P14 (P2rx7+/+) cells were mixed 1:1 and co-transferred to host mice, followed by LCMV infection. At various times, the mice were treated with tamoxifen to induce Cre activity (detected via a floxed-stop-yellow fluorescent protein reporter). To confirm the validity of this approach, we applied tamoxifen on days 1–4 post-infection, which ablated P2rx7 during the effector phase (Figure 7A). This led to a 5- to 10-fold loss of memory P2rx7fl/fl P14 cells (relative to P2rx7+/+ counterparts) in circulating and Trm cell pools (Figures 7B, S7A, and S7B), similar to other approaches (Figures 1, S1, and S2). To test whether P2RX7 is required during Trm cell maintenance, we delayed tamoxifen treatment until 28–35 days post-infection (Figure 7C). Late tamoxifen treatment also led to profound loss of P2rx7fl/fl compared with P2rx7+/+ counterparts in circulating and tissue-resident P14 memory cells (Figure 7D). The effect of late P2rx7 ablation initially appeared to be selective for certain Trm cell populations (Figures S7C–S7E), although this was likely related to slow decay of cell-surface P2RX7 in populations that initially had high expression of the receptor (such as SI IEL) (Figures S7F–S7H). Together, these data suggest that sustained P2RX7 expression is required for long-term Trm (and Tcm) cell maintenance.
Figure 7. P2RX7 Is Required for Long-Term Trm Cell Maintenance.
(A–D) Congenically distinct P2rx7+/+ Ert2-Cre+ YFP and P2rx7fl/fl Ert2-Cre+ YFP P14 CD8+ T cells were co-transferred into recipient mice that were infected with LCMV. Recipients were treated with tamoxifen on days 1–4 (A and B) or 28–35 (C and D) post-infection and analyzed at least 30 days following the end of tamoxifen treatment (B and D). Representative flow cytometry plots (left) and ratios between P2rx7fl/fl Ert2-Cre+ YFP and P2rx7+/+ Ert2-Cre+ YFP P14 cells (right) for the indicated organs are shown for early (B) and late (D) tamoxifen treatment.
(E) P2rx7+/+ Ert2-Cre+ YFP and P2rx7fl/fl Ert2-Cre+ YFP P14 cells were activated in vitro, transduced with EV or Tgfbr2-encoding retroviral vectors, and then co-transferred into recipient mice subsequently infected with LCMV. Mice were treated with tamoxifen on days 1–4 or 28–35 post-infection. Data show the ratios between P2rx7fl/fl Ert2-Cre+ YFP and P2rx7+/+ Ert2-Cre+ YFP P14 cells in the indicated organs at day 63 post-infection. Data are from 2–3 independent experiments; n = 4–9 per experimental group. Graphical data is shown as means with error bars indicating SD (E) or SEM (B, D). p values from two-tailed t-test are indicated: *, p <0.05; **, p<0.01; ***, p<0.001 See also Figure S7.
Because forcing TGF-βRII expression in activated CD8+ T cells compensated for P2RX7 loss in Trm cell generation, we considered whether it would also compensate in Trm cell maintenance. As expected, induced P2rx7 deletion in cells transduced with the EV led to their loss in SLOs and NLTs after early or late tamoxifen treatment (Figures 7E, S7I, and S7J). Similar to our findings with germline KO cells, when P2rx7 was ablated early, forced Tgfbr2 expression largely rescued generation of P2rx7−/− P14 cells in SLOs and most NLTs (with the exception of the liver) (Figures 7E, S7I, and S7J). However, when P2rx7 knockout was induced late, the benefit of forced Tgfbr2 expression was lost (Figures 7E, S7I, and S7J). This correlated with the finding that endogenous TGF-βRII expression on memory P14 cells depended on early but not late P2RX7 expression (Figure S7K). Thus, the role of P2RX7 in sustained memory CD8+ T cell maintenance could not be substituted by correcting TGF-βRII expression during priming.
DISCUSSION
Trm cell establishment in barrier tissues is crucial to provide a frontline of defense against pathogens (Schenkel and Masopust, 2014), but the extracellular signals promoting Trm cell generation and long-term survival are not fully understood. Here we showed that sustained P2RX7 expression was required for establishment and long-term maintenance of CD8+ Trm cells in NLTs. Our findings are consistent with the persistent high expression of P2RX7 in Trm cells from diverse NLTs (Borges da Silva et al., 2018; Rissiek et al., 2018), which we confirmed here. In addition to a role of P2RX7 in regulating mitochondrial homeostasis in memory CD8+ T cells (Borges da Silva et al., 2018), our data suggest that P2RX7 supports TGF-β sensitivity in effector CD8+ T cells, which favors tissue residency by inducing expression of the tissue adhesion molecule CD103 and KLF2 downregulation. P2RX7-mediated KLF2 downregulation may also contribute to CD69 expression as an indirect effect of S1PR1 downregulation (Skon et al., 2013). Therefore, P2RX7 signaling promotes metabolic fitness and efficient Trm cell generation in virus-specific CD8+ T cells. Few P2rx7−/− CD8+ T cells establish long-lived Trm cells; our data suggest that these are truly resident but may not be firmly embedded in NLTs (perhaps because of reduced expression of tissue adhesion molecules) and are also more susceptible to cell death—two features that may be related.
Our data suggest that P2RX7 controls TGF-β sensitivity, at least in part, through control of TGF-βRII expression in activated CD8+ T cells. T cell activation leads to rapid loss of TGF-β receptor I (TGF-βRI) and TGF-βRII gene expression, but the factors leading to re-expression of these receptors in subsequent days are unclear (Tu et al., 2018). P2RX7 serves as an eATP-stimulated calcium ion channel in various cell types, including CD8+ T cells (Borges da Silva et al., 2018; Di Virgilio et al., 2017), and we found that Calcineurin blockade impaired TGF-β sensitivity of in-vitro-activated CD8+ T cells, suggesting that a calcium-sensitive signaling pathway may regulate TGF-βRII re-expression. It will be important to investigate candidate transcriptional regulators, including nuclear factor of activated T cells (NFAT) proteins, some of which can be activated downstream of P2RX7 stimulation (Ferrari et al., 1999). It is important to note that defective Tgfbr2 expression in splenic P2rx7−/− CD8+ T cells was observed early (day 4.5) but not later (day 8) in the response to LCMV, indicating that other pathways dictate TGF-β receptor expression as the effector response develops, at least in the spleen. Regardless, our findings suggest that eATP, signaling through P2RX7, promotes TGF-β sensitivity in CD8+ T cells, including the precursors of Trm cells, most of which are thought to seed NLTs during the effector phase (Masopust et al., 2010; Milner et al., 2017; Mueller and Mackay, 2016).
The importance of P2RX7-dependent TGF-β sensitivity, and its control of TGF-βRII expression specifically, was exemplified by the finding that forced TGF-βRII expression was sufficient to compensate for P2RX7 deficiency in generation of memory precursors as well circulating and resident memory cells (in many but not all NLTs). This was unexpected because gene expression of other components of the TGF-β signaling pathway (including TGF-βRI and various Smad proteins) was also reduced in P2rx7−/− day 4.5 splenocytes. This implies that TGF-βRII expression is the limiting factor in TGF-β sensitivity. Indeed, it is notable that forced expression of Tgfbr2 also enhanced generation of Trm cells among WT CD8+ T cells, suggesting that its physiological expression is limiting for this response. CD8+ T cell responsiveness to TGF-β occurs prior to T cell activation, and a recent report elegantly showed that TGF-β activation by dendritic cells pre-conditions naive CD8+ T cells to favor eventual Trm cell generation (Mani et al., 2019). Our studies using inducible P2RX7 knockout approaches indicate, however, that the critical phase of P2RX7-regulated TGF-β sensitivity occurs following CD8+ T cell activation. Our results suggest that formation of Trm cells and circulating memory CD8+ T cells relies on P2RX7-mediated TGF-βRII, suggesting that this pathway is important for formation of early memory precursors that can give rise to Trm cells or circulating memory CD8+ T cells, whose existence is postulated (Gaide et al., 2015). This implies that the P2RX7-TGF-βRII signaling pathway is engaged in SLOs during the effector phase, before migration to NLTs.
The finding that forced Tgfbr2 expression partially restored generation of P2rx7−/− Tcm cells in the circulation is consistent with reports showing that sustained signaling through TGF-βRII promotes a memory precursor phenotype in CD8+ T cells (Schwartzkopff et al., 2015) and establishment of circulating memory CD8+ T cells (Ma and Zhang, 2015). Those studies suggest that TGF-β stimulation may counteract inflammatory signals sensed by effector CD8+ T cells, promoting memory differentiation and maintenance. Our findings added another layer to this model, in which P2RX7, sensing a “danger signal” (eATP) promotes optimal expression of TGF-βRII, which, in turn, limits sensing of other inflammatory signals. Exposure to eATP does not automatically indicate danger, however; our studies indicate that eATP derived from commensal bacterial may provide the eATP that stimulates P2RX7 in developing SI IEL Trm cells. Furthermore, eATP is released by activated immune cells, such as antigen-presenting cells or CD8+ T cells themselves. Most immune cells express surface channels that mediate eATP export, such as Pannexin 1 (Dosch et al., 2018; Schenk et al., 2008), and Pannexin 1 and P2RX7 act together to mediate optimal mitochondrial metabolism of CD8+ T cells in vitro (Borges da Silva et al., 2018). Dissecting the role of this “autocrine” pathway for P2RX7 stimulation and memory T cell generation will be important in future studies (Wanhainen et al., 2019).
However, our results should not imply that P2RX7 promotes Trm cell establishment exclusively through TGF-βRII expression; forced Tgfbr2 was unable to rescue several P2rx7−/− defects, including generation of P2rx7−/− CD8+ Trm cells in the liver. This might indicate that elevated TGF-βRII expression alone was insufficient to restore suitable TGF-β sensitivity to cells in that site or that this population of Trm cell requires other P2RX7-regulated cues. Likewise, forced expression of TGF-βRII was unable to preserve established Trm cells in the face of late P2rx7 ablation.
P2RX7 regulates mitochondrial homeostasis in CD8+ Trm cells, as it does in circulating memory cells (Borges da Silva et al., 2018), and recent reports suggest that mitochondrial metabolism governs the long-term survival of skin (Pan et al., 2017) and lung Trm cells (Li et al., 2019). A role of TGF-β in regulating CD8+ T cell metabolism was unexpected but is consistent with a report showing that TGF-β signaling inhibits the mechanistic target of rapamycin (mTOR) pathway and promotes mitochondrial metabolism in regulatory T cells (Priyadharshini et al., 2018). However, forced TGF-βRII did not restore all mitochondrial defects in P2rx7−/− CD8+ T cells, and it will be important to dissect the TGF-β-dependent and -independent mechanisms by which P2RX7 controls T cell metabolism.
Our conclusion that P2RX7 promotes differentiation and survival of CD8+ Trm cells contrasts with some previous reports suggesting that P2RX7 stimulation mediates T cell death. Strong activation of P2RX7 can induce cell death following membrane pore formation in a variety of cells, and some T cell populations are especially vulnerable to this process. For example, survival and function of CD4+ T follicular helper (Tfh) cells in Peyer’s patches are enhanced in P2rx7−/− mice and in situations where eATP generated by commensal microbes is degraded (Proietti et al., 2014, 2019). Relevant to our studies however, is that Stark et al. (2018) have contradictory data regarding the role of P2RX7 in CD8+ SI IEL Trm cell homeostasis. That study shows that irreversible activation of P2RX7 (through nicotinamide adenine dinucleotide - NAD+ - stimulation of the ectoenzyme ARTC2.2, which ADP-ribosylates P2RX7 and activates its pore function) leads to CD8+ Trm cell death. It is not clear whether this pathway is engaged during normal homeostasis, and we deliberately blocked ARTC2.2 just prior to mouse sacrifice to minimize cell death induced by tissue disruption (Borges da Silva et al., 2019; Rissiek et al., 2018). Nevertheless, it will be important to assess how this NAD+-ARTC2.2-P2RX7 pathway may be induced in situations of sterile or infection-related tissue damage.
More concerning, Stark et al. (2018) also report that P2RX7 deficiency has negligible effects on generation of circulating or resident memory CD8+ T cell populations in studies of mixed bone marrow chimeras similar to ours. The basis of these discrepancies is unclear, and differences in mouse substrains and/or commensal microbes cannot be excluded. On the other hand, our current study entailed multiple gene ablation models (germline, conditional, inducible knockouts, and CRISPR-Cas9, targeting two different P2rx7 exons), tracked responses by TCR transgenic and polyclonal CD8+ T cells (including mixed bone marrow chimeras), and tested the effects of P2RX7 pharmacological blockade in vivo, all of which led to concordant results. Hence, although the basis of the distinct findings of Stark et al. (2018) is unclear, our use of diverse approaches reinforces the model that P2RX7 promotes generation of and is important for maintenance of CD8+ Tcm and Trm cells.
Signaling through eATP plays diverse roles in T cells, often inducing enhanced homeostasis (Adinolfi et al., 2005; Di Virgilio et al., 2017). Our findings highlight a fundamental role of eATP sensing through P2RX7 to induce long-lived Trm cells following viral infection, in part because of P2RX7 promoting TGF-β sensitivity required for tissue residency programs. Furthermore, our data indicate that continuous P2RX7 engagement is required to sustain and shape adaptive immunological memory at circulation and barrier sites.
Limitations of Study
This study did not include an in-depth analysis of how P2RX7 stimulation regulates transcription of Tgfbr2 and other components in the TGF-β signaling pathway, beyond a proposed role for calcium influx and calcineurin activation. Also, the basis of how increased expression of TGF-βRII mediates elevated mitochondrial function in memory CD8+ T cells is unclear, and further work is needed to better understand the effect of P2RX7 on mitochondrial homeostasis overall. Our data measuring mitochondrial mass and membrane potential (Figure 6A) argue that P2RX7 supports optimal mitochondrial homeostasis in Trm cells, but those analyses provide an incomplete view of mitochondrial function; detailed studies of mitochondrial turnover and activity will allow a better understanding of this aspect of memory CD8+ T cell biology. The finding that forced expression of TGF-βRII largely rescues CD8+ T cell memory differentiation by cells that were induced to lose P2rx7 early but not late suggests a complex relationship between these two factors that will benefit from future study. Finally, studies focusing on human samples will be necessary to understand to what extent P2RX7 promotes Trm cells in human NLTs. Further assessment of the expression pattern of P2RX7 in human Trm cells and its correlation with P2RX7 gene variants (Sorge et al., 2012) may offer fundamental insights into how eATP sensing influences the generation of long-term adaptive immunity in the human population.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be addressed to and will be fulfilled by the Lead Contact Stephen C. Jameson (james024@umn.edu).
Resource Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Female Six- to 8-week old adult C57BL/6 (B6) and B6.SJL (expressing the CD45.1 allele) mice were purchased from Charles River (via the National Cancer Institute) and were allowed to acclimate to the housing facility for at least one week. P2rx7−/−, CD4-Cre, Ert2-Cre+ and R26-EYFP (YFP) mice were obtained from Jackson Laboratories; P2rx7fl/fl mice were obtained from Drs. Gyorgy Hasko (Rutgers University) and Matyas Sandor (U-Wisconsin). OVA-KbSIINFEKL-specific TCR transgenic OTI and LCMV-DbGP33-specific TCR transgenic P14 mice were fully backcrossed to B6 and P2rx7−/− mice, with introduction of Thy1.1, Thy1.2, CD45.1 and CD45.2 congenic markers for identification. Animals were maintained under specific-pathogen-free conditions at the University of Minnesota. In all experiments, mice were randomly assigned to experimental groups. All experimental procedures were approved by the institutional animal care and use committee at the University of Minnesota (IACUC 1709–35136A).
Viral strains
LCMV Armstrong strain was maintained at −80°C until infection and diluted to 2 ×106 PFU/ml in PBS. VSV-OVA strain was maintained at −80°C and diluted to 1 × 107 PFU/ml in PBS at the time of infection studies.
METHOD DETAILS
Infection studies
Wild-type, P2rx7−/−, or Ert2-Cre-YFP-P2rx7fl/fl P14 cells were adoptively transferred into naive wild-type mice, which were infected with LCMV Armstrong strain (2 × 105 PFU, intraperitoneally (i.p.)). In some experiments, wild-type or CD4-Cre P2rx7fl/fl mice were infected with LCMV Armstrong. In other experiments, wild-type or P2rx7−/− OTI cells were adoptively transferred into naive wild-type mice, which were infected with VSV-OVA strain (1 × 106 PFU, intravenously (i.v.)).
Primary cell cultures
Naive P14 cells were obtained from six- to 8-week old female mice with a C57BL/6 background. P14 cells were cultured in complete RPMI media: RPMI 1640 (Corning) supplemented with 10% Fetal Bovine Serum (Atlanta Biologicals), 100 U/ml penicillin/streptomycin (Thermo Fisher Scientific) and 2 mM L-glutamine (Corning). All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Administration of tamoxifen in mice
Tamoxifen (Sigma-Aldrich) (diluted in Sunflower Oil) was administered to mice i.p. daily during the indicated treatment periods at a daily dose of 1 mg (Milner et al., 2017). Two different treatment periods were used, in which Tamoxifen was given between days 1 and 4 or between days 28 and 35 relative to LCMV infection.
Administration of antibiotics in mice
A cocktail of Saccharin (Sweet’n’Low), Vancomycin (Chem-Impex), Ampicilin (Millipore Sigma), Metronidazole (Sigma-Aldrich) and Gentamicin (Invitrogen) was administered to mice in drinking water during the indicated treatment period as described (Chu et al., 2019). Briefly, a mix of 250 mg of Vancomycin, 500 mg of Ampicilin, 500 mg of Metronidazole and 500 mg of Gentamicin was diluted in drinking water. Saccharin (1 mg) was added to make the antibiotics cocktail more palatable. Following antibiotic treatment, mice exhibited no significant differences in weight gain.
Parabiosis experiments
Parabiosis surgeries were performed as previously described (Skon et al., 2013). Briefly, mice were anesthetized with ketamine, and the flank hair was shaved and further removed using Nair. After making lateral incisions, mice were joined with interrupted horizontal mattress sutures with 5–0 NOVAFIL. Additional sutures were placed through the olecranon and knee joints to secure the legs of the conjoined pairs. Parabiotic pairs were analyzed between 2 and 4 weeks after surgeries. Percentages of residency are calculated accordingly to Steinert et al. (2015), using the following formula: % resident = 100 × (1 – (# of P14 in non-transferred parabiont organ × 2/# of P14 in non-transferred + immune parabiont organs)). In this formula, a value of 100% means that, within the organ studied, all P14 cells are non-circulating.
Flow cytometry
Lymphocytes were isolated from tissues including spleen, inguinal lymph nodes, cervical lymph nodes, blood, small intestine epithelium (SI IEL), small intestine lamina propria (SI LP), female reproductive tract, kidney and salivary glands (SG) as previously described (Skon et al., 2013; Steinert et al., 2015). In summary, organs were removed and cut in small pieces into erlenmeyers containing 30 mL of 0.5 mg/ml Collagenase type I solution (kidney and SG), 0.5 mg/ml Collagenase type IV (FRT) or 0.15 mg/ml Dithioerythritol (SI IEL). Following this period, lymphocytes were isolated by 44/67% Percoll gradient isolation. During isolation of lymphocytes from non-lymphoid tissues, in all experiments, 50 μg of Treg-Protector (anti-ARTC2.2) nanobodies (BioLegend) were injected i.v. 30 minutes prior to mouse sacrifice (Borges da Silva et al., 2019). Direct ex vivo staining and intracellular staining were performed as described (Renkema et al., 2016; Skon et al., 2013). In some experiments, small intestines were incubated for 30 min in the absence of enzymatic digestion, to collect the cells loosely attached to the SI IEL compartment (“SI IEL Loose”) (Zhang and Bevan, 2013). To identify LCMV-specific CD8+ T cells, tetramers were prepared as described previously (Daniels and Jameson, 2000): monomers were tetra-merized by incubation with APC- or PE-Streptavidin for 1h at room temperature (RT) in the dark. For detection of vascular-associated lymphocytes in non-lymphoid organs, in vivo i.v. injection of PerCP-Cy5.5-conjugated CD8α antibody was performed (Anderson et al., 2014). Due to unclear division between bona-fide Trm and circulating memory using i.v. CD8α injection in the liver, we used total liver P14 numbers in all experiments where this organ is listed. Among LCMV-specific CD8+ T cells, the following markers were used to distinguish these respective populations: TCM (CD44+CD62L+), TEM (CD44+CD62L−CD127+), LLEC (CD44+ CD62L−CD127−KLRG1hi), Trm (i.v.CD8α−CD69+/−CD103hi/int/lo), SLO Trm (CD44+CD62L−CD69+), MPECs (CD127+KLRG1−), SLECs (CD127−KLRG1+). For mitochondrial mass and membrane potential measurements, cells were incubated with MTG (Thermo Fisher Scientific) and Mitotracker Deep Red (Thermo Fisher Scientific) or TMRE (Cell Signaling Technology) simultaneously for 15 min at 37°C prior to ex vivo staining. For detection of the intracellular factors Eomes and T-bet, surface-stained cells were permeabilized, fixed and stained by using the eBioscience Foxp3 staining kit, according to manufacturer instructions. For intracellular detection of pSmad2/3, cells were stained with surface markers then fixed with Paraformaldehyde 1%, permeabilized with 90% Methanol and stained with pSmad2/3 for 20 min at room temperature. Flow cytometric analysis was performed on LSR II or LSR Fortessa (BD Bio-sciences) and data was analyzed using FlowJo software (Treestar).
Cell sorting
Cell sorting was performed on a FACS Aria III device (BD Biosciences). Quantitative PCR analysis experiments were performed on KLRG1+CD127− (SLEC) and KLRG1−CD127+ (MPEC) CD8+ T cells sorted from mice 8 days post-LCMV infection, or with WT and P2rx7−/− CD8+ T cells 4.5 days post-LCMV infection. The population purity after cell sorting was > 95% in all experiments.
RNA expression analysis
P14 cells from d4.5 post-LCMV and P14 MPECs/SLECs from d8 post-LCMV were first homogenized using QIAshredder columns (QIAGEN) and RNA was extracted using RNeasy kit (QIAGEN) following manufacturer instructions. From the isolated RNA samples, cDNA was prepared with the SuperScript III First-Strand Synthesis SuperMix (ThermoFisher Scientific) following manufacturer instructions. Quantitative PCR analysis was done as described previously (Lee et al., 2015), using triplicates for each sample with an ABI 7700 sequence-detection system and amplification was detected with SYBR Green PCR Master Mix (Applied Biosystems). For analysis, we used the delta-delta Ct algorithm. Cycling threshold values for the control target gene (Hprt) were subtracted from cycle threshold values for the gene of interest. The list of primers used in this study can be found in Table S1.
Metabolic assays
In the indicated in vitro-activated CD8+ T cell populations, OCR and ECAR were assessed using a 96-well XF Extracellular flux analyzer, following manufacturer instructions (Seahorse Bioscience). Spare respiratory capacity (SRC) was defined as described previously (van der Windt et al., 2012), i.e., the subtraction between the maximum OCR values and the baseline OCR values.
In vitro culture experiments
P14 cells were isolated from naive mice with mouse CD8+ T cell isolation and stimulated up to 120 h with plate-bound αCD3 (10 μg/ml), αCD28 (20 μg/ml) and IL-2 (10 ng/ml). When indicated, Bz-ATP (300 μM, Sigma-Aldrich) or A-438079 (25 μM) was added to the cultures between 66–72 h post-activation. In some experiments, after 72 h of activation, P14 cells were incubated for an additional 60 h with recombinant human TGF-β (10 ng/ml; Peprotech) and/or IL-15 (10 ng/ml). In some experiments, TGF-β-cultured P14 cells were cultured in the presence of 300 μM Bz-ATP or 25 μM A-438079 for 60 h.
Retroviral transduction experiments
P14 cells isolated as described above were activated with plate-bound αCD3, αCD28 and IL-2. 24 h after activation, cells were spin-infected by retroviruses MiT-Tgfbr2 or control empty vector (MiT-EV) as described previously (Skon et al., 2013). At 24h after activation, P14 cells were incubated for 30 min at 37°C with 10 mg/ml polybrene and 20 ng/ml human IL-2. After centrifugation of the plates (at 411 g for 5 min at 21°C), the culture medium was removed and 1 mL of the supernatant of retroviral vector expressing MiT-Tgfbr2 or MiT-EV was added to each well, which also had 10 μg/ml polybrene and 20 ng/ml IL-2. Spin infection (1,141 g for 90 min at 21°C) was followed by incubation for 1h at 37°C to allow the cells to ‘rest’. At 1 day after transduction, a 1:1 mix of 1×105 P14 cells transduced with MiT-Tgfbr2 or MiT-EV were transferred into host mice that were infected with LCMV the next day. For assessment of transduction efficiency, a portion of transduced cells were stained for TGF-βRII and assessed by flow cytometry, together with the transduction markers Thy1.1 (MiT-EV) and mCherry (MiT-Tgfbr2).
CRISPR-Cas9 experiments
Cas9/RNP nucleofection of P14 cells was performed as described previously (Seki and Rutz, 2018). Briefly, P14 cells were isolated and activated as described above. While cells were maintained in culture, single guide RNAs for P2rx7 (sgP2rx7), Cd19 (sgCd19) or scrambled control (sgControl) and Cas9 protein were mixed by pipetting up and down and pre-complexed at room temperature for at least 10 min. After this period, 1–10 million activated P14 cells were re-suspended in 20 μL primary cell nucleofection solution (Lonza), then mixed and incubated with the crRNA/Cas9 mix for 2 min at room temperature. The P14 cell/Cas9/RNP mixes were transferred to Nucleofection cuvette strips (4D-Nucleofector X kit S; Lonza). Cells were electroporated using a 4D nucleofector (4D-Nucleofector Core Unit; Lonza). After nucleofection, prewarmed complete RPMI was used to transfer transfected P14 cells in 96-well plates. After 2 h, P14 cells were cultured in 24-well plates in complete RPMI for 48 h, before adoptive transfer into recipient mice.
QUANTIFICATION AND STATISTICAL ANALYSIS
Details on statistics used can be found in figure legends. Data were subjected to the Kolmogorov-Smirnov test to assess normality of samples. Statistical differences were calculated by using unpaired two-tailed Student’s t test (or one-way ANOVA with Tukey post-test, where indicated). All experiments were analyzed using Prism 7 (GraphPad Software). Graphical data was shown as mean values with error bars indicating the SD or SEM. P values of < 0.05 (*), < 0.01 (**) or < 0.001 (***) indicated significant differences between groups.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies and Dyes | ||
| Anti-mouse CD8a BUV395 | BD Biosciences | Clone 53–6.7, Cat no. 563786; RRID:AB_2732919 |
| Anti-mouse CD44 Af700 | Tonbo Biosciences | Clone IM7, Cat no. 80-0441-U100; RRID:AB_2621985 |
| Anti-mouse CD62L BV605 | BioLegend | Clone MEL-14, Cat no. 104438;RRID:AB_2563058 |
| Anti-mouse CD69 PE-Cy7 | Tonbo Biosciences | Clone H1.2F3, Cat no. 60-0691-U100; RRID:AB_2621856 |
| Anti-mouse KLRG1 PE-Cy7 | eBioscience/Thermo | Clone 2F1, Cat no. 25-5893-82; RRID:AB_1518768 |
| Anti-mouse IFN-g PE-Cy7 | Tonbo Biosciences | Clone XMG1.2, Cat no. 60-7311-U100; RRID:AB_2621871 |
| Anti-mouse CD45.2 PE-Cy7 | Tonbo Biosciences | Clone 104, Cat no. 60-0454-U100; RRID:AB_2621851 |
| Anti-mouse CD45.1 PE-Cy7 | Tonbo Biosciences | Clone A20, Cat no. 60-0453-U100; RRID:AB_2621850 |
| Anti-mouse CD62L BV786 | BD Biosciences | Clone MEL-14, Cat no. 564109; RRID:AB_2738598 |
| Anti-mouse CD44 BV786 | BD Biosciences | Clone IM7, Cat no. 563736; RRID:AB_2738395 |
| Anti-mouse CD8a BV786 | BD Biosciences | Clone 53–6.7, Cat no. 563332; RRID:AB_2721167 |
| Anti-mouse CD90.2 BV785 | BioLegend | Clone 30-H12, Cat no. 105331; RRID:AB_2562900 |
| Anti-mouse CD8a PerCP-Cy5.5 | Tonbo Biosciences | Clone 53–6.7, Cat no. 65-0081-U100; RRID:AB_2621882 |
| Anti-mouse CD45.2 PerCP-Cy5.5 | Tonbo Biosciences | Clone 104, Cat no. 65-0454-U100; RRID:AB_2621894 |
| Anti-Mouse EOMES PerCP-eFluor 710 | eBioscience/Thermo | Clone Dan11mag, Cat no. 46-4875-80; RRID:AB_10609215 |
| Annexin V FITC | BD Biosciences | Cat no. 65874X |
| Anti-mouse TNFa FITC | eBioscience/Thermo | Clone MP6-XT22, Cat no. 11-7321-82; RRID:AB_465418 |
| Ghost Dye Red 780 Viability Dye | Tonbo Biosciences | Cat no. 13-0865-T100 |
| Anti-mouse CD62L APC | BioLegend | Clone MEL-14, Cat no. 104412;RRID:AB_313099 |
| Anti-mouse CD127 APC | eBioscience/Thermo | Clone A7R34, Cat no. 17-1271-82; RRID:AB_469435 |
| Anti-mouse CD45.2 APC | BioLegend | Clone 104, Cat no. 109814; RRID:AB_389211 |
| Anti-mouse KLRG1 BV711 | BD Biosciences | Clone 2F1, Cat no. 564014; RRID:AB_2738542 |
| Anti-mouse/rat CD90.1 eFluor450 | eBioscience/Thermo | Clone HIS51, Cat no. 48-0900-82; RRID:AB_1272254 |
| Anti-mouse CD45.1 vf450 | Tonbo Biosciences | Clone A20, Cat no. 75-0453-U100; RRID:AB_2621949 |
| Anti-mouse CD45.2 vf450 | Tonbo Biosciences | Clone 104, Cat no. 75-0454-U100; RRID:AB_2621950 |
| Anti-mouse CD45.2 FITC | Tonbo Biosciences | Clone 104, Cat no. 35-0454-U500; RRID:AB_2621692 |
| Anti-mouse CD90.2 FITC | BioLegend | Clone 30-H12, Cat no. 105306; RRID:AB_313177 |
| Anti-mouse P2X7R APC | BioLegend | Clone 1F11, Cat no. 148706; RRID:AB_2650954 |
| Anti-mouse CD103 BV510 | BD Biosciences | Clone M290, Cat no. 563087; RRID:AB_2721775 |
| Anti-mouse P2X7 PE | BD Biosciences | Clone 1F11, Cat no. 565345; RRID:AB_2739198 |
| Anti-human/mouse T-bet PE-Cy7 | BioLegend | Clone 4B10, Cat no. 644824; RRID:AB_2561761 |
| Anti-mouse CD186 (CXCR6) PE | BioLegend | Clone SA051D1, Cat no. 151104; RRID:AB_2566546 |
| Anti-mouse CD127 PE | eBioscience/Thermo | Clone A7R34, Cat no. 12-1271-83; RRID:AB_465845 |
| Anti-mouse/rat CD90.1 PE | eBioscience/Thermo | Clone HIS51, Cat no. 12-0900-83; RRID:AB_465774 |
| Anti-mouse TGF-beta RII APC | R&D Systems | Cat no. FAB532A-025 |
| Anti-human Granzyme B PE | Invitrogen/Thermo | Clone GB11, Cat no. GRB04 |
| InVivoMAb anti-mouse CD3ε | BioXCell | Clone 145–2C11, Cat no. BE0001–1; RRID:AB_1107634 |
| InVivoMAb anti-mouse CD28 | BioXCell | Clone 37.51, Cat no. BE0015–1-A050mg; RRID:AB_1107624 |
| Anti-mouse pSMAD2/3 (pS465/pS467) PE | BD Biosciences | Clone 072–670, Cat no. 562586; RRID:AB_11151915 |
| MitoTracker® Green FM | Thermo | Cat no. M7514 |
| MitoTracker® Deep Red FM | Thermo | Cat no. M22426 |
| Mitochondrial Membrane Potential Assay Kit (II) (TMRE) | Cell Signaling Technologies | Cat no. 13296S |
| Treg-Protector (anti-ARTC2 Nanobody) [S+16a] | BioLegend | Clone S+16a, Cat no. 149802; RRID:AB_2565494 |
| Chemicals, Peptides and Recombinant Proteins | ||
| Collagenase, Type 1 | Worthington | Cat no. LS004197 |
| Collagenase, Type 3 | Worthington | Cat no. LS004182 |
| DnaseI | Sigma-Aldrich | Cat no. 10104159001 |
| Percoll | GE Healthcare | Cat no. 17–0891-09 |
| Dithioerythritol | EMD Millipore | Cat no. 233152–5GM |
| Tamoxifen | Sigma-Aldrich | Cat no. T5648 |
| Human recombinant TGF-ß | Peprotech | Cat no. 100–21-10ug |
| Mouse recombinant IL-2 | R&D Systems | Cat no. 402-ML-500/CF |
| TrueCut Cas9 Protein v2 | Thermo | Cat no. A36499 |
| 2'(3')-O-(4-Benzoylbenzoyl) adenosine 5'- triphosphate triethylammonium salt (Bz-ATP) | Sigma-Aldrich | Cat no. B6396–25MG |
| EMS 16% Paraformaldehyde Aqueous Solution, EM Grade | Electron Microscopy Sciences | Cat no. 15710 |
| Methanol | Sigma-Aldrich | Cat no. 322415 |
| VetaKet (Ketamine HCl) | Akorn Animal Health | Cat no. 59399–114-10 |
| Dimethyl Sulfoxide (DMSO) | Fisher Scientific | Cat no. BP231–1 |
| Sunflower seed oil from Helianthus annuus | Sigma-Aldrich | Cat no. S5007–250ML |
| RPMI 1640 | Corning | Cat no. 10–040-CV |
| HBSS 10x | Corning | Cat no. 20–021-CV |
| XF Base Medium Minimal DMEM | Agilent Technologies | Cat no. 102353–100 |
| Fetal Bovine Serum | Atlas Biologicals | Cat no. FS-0500-AD |
| L-Glutamine, 100x, Liquid | Corning | Cat no. 25–005-CI |
| Pennicilin/Streptomycin | Thermo | Cat no. 15070063 |
| Mouse recombinant IL-15 | Peprotech | Cat no. 210–15-50ug |
| AnaSed Injection (Xylazine) | Akorn Animal Health | Cat no. 59399–111 -50 |
| Vancomycin hydrochloride | Chem-Impex | Cat no. 00315 |
| Metronidazole,bioxtra | Sigma-Aldrich | Cat no. M1547–5G |
| Ampicillin | Millipore Sigma | Cat no. 10835242001 |
| Gentamicin | Invitrogen | Cat no. 15750060 |
| Critical Commercial Assays | ||
| Foxp3 / Transcription Factor Staining Buffer Kit | Tonbo Biosciences | Cat no. TNB-0607-KIT |
| CD8a+ T cell Isolation Kit, Mouse | Miltenyi Biotec | Cat no. 130–104-075 |
| Seahorse XF Cell Mito Stress Test Kit | Agilent Technologies | Cat no. 103015–100 |
| QiaShredder Columns | QIAGEN | Cat no. 79654 |
| RNeasy Plus Mini Kit | QIAGEN | Cat no. 74134 |
| SuperScript III First-Strand Synthesis SuperMix | Invitrogen | Cat no. 11752050 |
| P4 Primary Cell 4D-Nucleofector X Kit S | Lonza | V4XP-4032 |
| ATP Determination Kit | Thermo | A22066 |
| Experimental Models: Organisms/Strains | ||
| Mouse: B6.SJL-PtprcaPepcb/ BoyCrCrl (CD45.1) | NCI Charles River | Strain Code 564 |
| Mouse: C57BL/6NCrl (C57BL/6) | NCI Charles River | Strain Code 556 |
| Mouse: P14 | Dr. R. Ahmed, Emory University | N/A |
| Mouse: OTI | Dr. K. Hogquist, University of Minnesota | N/A |
| Mouse: B6.Cg-Ndor1Tg(UBC-cre/ERT2)1Ejb/1J (ERT2-Cre) | Jackson | Stock no. 007001 |
| Mouse: B6.129X1-Gt(ROSA) 26Sortm1(EYFP)Cos/J (LSL-YFP) | Jackson | Stock no. 006148 |
| Mouse: P2rx7fl/fl | Dr. Gyorgy Hasko, Rutgers University | N/A |
| Mouse: B6.129P2-P2rx7tm1Gab/J (P2rx7−/−) | Jackson | Stock no. 005576 |
| Mouse: B6.PL-Thy1a/CyJ (Thy1.1) | Jackson | Stock no. 000406 |
| Virus: Lymphocytic choriomeningitis virus (LCMV) Armstrong strain | Dr. R. Ahmed, Emory University | N/A |
| Virus: Vesicular stomatitis virus (VSV)- Indiana strain expressing ovalbumin (VSV-OVA) | Dr. L. Lefrancois, University of Connecticut | N/A |
| Oligonucleotides | ||
| PCR primers - see Table S1 | Synthego | N/A |
| P2rx7 single guide (sg) RNA1: UGAGCGAUAAGCUGUACCAG | Synthego | N/A |
|
P2rx7 sgRNA2: UAUCAGCUCCGUGCACACCA |
Synthego | N/A |
| Scrambled sgRNA1: GCACUACCAGAGCUAACUCA | Synthego | N/A |
| Scrambled sgRNA2: GUACGUCGGUAUAACUCCUC | Synthego | N/A |
| Cd19 sgRNA1: | Synthego | N/A |
| CCUGGCCUGGGAUUGCACGU | ||
| Cd19 sgRNA2: | Synthego | N/A |
| GAGAAGCUGGCUUGGUAUCG | ||
| Software and Algorithms | ||
| GraphPad Prism v8 | GraphPad Software | https://www.graphpad.com |
| FlowJo v10 | FlowJo | https://www.flowjo.com |
| FacsDIVA | BD Biosciences | N/A |
| Seahorse Wave Desktop Software | Agilent Technologies | https://www.agilent.com |
Highlights.
Generation of long-lived CD103+ CD69+ Trm cells requires P2RX7 expression
P2RX7 controls Trm cell generation through induction of the TGF-β signaling pathway
Trm cell long-term maintenance requires continuous expression of P2RX7
ACKNOWLEDGMENTS
We thank Wanjun Chen (NIDCR) for providing the MiT-Tgfbr2 overexpression vector. We also thank the members of the Jamequist lab for critically reviewing the manuscript. S.C.J. was funded by NIAID (R01 AI038903 and AI145147). H.B.d.S. was funded by NIAID (K99/R00 AI139381) and a Paul C. Shiverick/CRI Irvington fellowship.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.immuni.2020.06.010.
DECLARATIONS OF INTEREST
The authors declare no competing interests.
REFERENCES
- Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, and Di Virgilio F. (2005). Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol. Biol. Cell 16, 3260–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanio C, Barreira da Silva R, Michonneau D, Bousso P, Ingersoll MA, and Albert ML (2018). CXCR3/CXCL10 Axis Shapes Tissue Distribution of Memory Phenotype CD8+ T Cells in Nonimmunized Mice. J. Immunol 200, 139–146. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D. (2014). Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc 9, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, and Ahmed R. (2009). mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, et al. (2018). The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature 559, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Wang H, Qian LJ, Hogquist KA, and Jameson SC (2019). ARTC2.2/P2RX7 Signaling during Cell Isolation Distorts Function and Quantification of Tissue-Resident CD8+ T Cell and Invariant NKT Subsets. J. Immunol 202, 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. (2012). Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol 188, 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, et al. (2019). The microbiota regulate neuronal function and fear extinction learning. Nature 574, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, and Jameson SC (2000). Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multi-mers. J. Exp. Med 191, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, and Falzoni S. (2017). The P2X7 Receptor in Infection and Inflammation. Immunity 47, 15–31. [DOI] [PubMed] [Google Scholar]
- Dosch M, Gerber J, Jebbawi F, and Beldi G. (2018). Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci 19, 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DL, Netea MG, Radbruch A, Rajewsky K, and Zinkernagel RM (2016). Immunological memory: lessons from the past and a look to the future. Nat. Rev. Immunol 16, 124–128. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Stroh C, and Schulze-Osthoff K. (1999). P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J. Biol. Chem 274, 13205–13210. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, and Di Virgilio F. (1996). Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol 156, 1531–1539. [PubMed] [Google Scholar]
- Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, and Kupper TS (2015). Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med 21, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning AN, Roychoudhuri R, and Restifo NP (2018). Epigenetic control of CD8+ T cell differentiation. Nat. Rev. Immunol 18, 340–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, and Iwasaki A. (2015). Tissue instruction for migration and retention of TRM cells. Trends Immunol 36, 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, and Masopust D. (2018). Understanding Subset Diversity in T Cell Memory. Immunity 48, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Kim HG, Perry CJ, and Kaech SM (2016). CCR7 expression alters memory CD8 T-cell homeostasis by regulating occupancy in IL-7- and IL-15-dependent niches. Proc. Natl. Acad. Sci. USA 113, 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadra A, Tomić M, Yan Z, Zemkova H, Sherman A, and Stojilkovic SS (2013). Dual gating mechanism and function of P2X7 receptor channels. Biophys. J 104, 2612–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, and Chen W. (2011). Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol 12, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, and Jameson SC (2015). The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity 42, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS (2001). Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol 19, 497–521. [DOI] [PubMed] [Google Scholar]
- Li C, Zhu B, Son YM, Wang Z, Jiang L, Xiang M, Ye Z, Beckermann KE, Wu Y, Jenkins JW, et al. (2019). The Transcription Factor βhlhe40 Programs Mitochondrial Regulation of Resident CD8+ T Cell Fitness and Functionality. Immunity 51, 491–507.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Mishra S., Demel EL., Liu Y., and Zhang N. (2017). TGF-β Controls the Formation of Kidney-Resident T Cells via Promoting Effector T Cell Extravasation. J. Immunol 198, 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, and Zhang N. (2015). Transforming growth factor-β signaling is constantly shaping memory T-cell population. Proc. Natl. Acad. Sci. USA 112, 11013–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. (2013). The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol 14, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, and Carbone FR (2015). T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111. [DOI] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366, eaav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. (2010). Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med 207, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, and Ahmed R. (2006). Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol 176, 2079–2083. [DOI] [PubMed] [Google Scholar]
- Milner JJ, and Goldrath AW (2018). Transcriptional programming of tissue-resident memory CD8+ T cells. Curr. Opin. Immunol 51, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al. (2017). Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, and Mackay LK (2016). Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol 16, 79–89. [DOI] [PubMed] [Google Scholar]
- Nath AP, Braun A, Ritchie SC, Carbone FR, Mackay LK, Gebhardt T, and Inouye M. (2019). Comparative analysis reveals a role for TGF-β in shaping the residency-related transcriptional signature in tissue-resident memory CD8+ T cells. PLoS ONE 14, e0210495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. (2017). Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruzza L, Gargari G, Proietti M, Fosso B, D’Erchia AM, Faliti CE, Rezzonico-Jost T, Scribano D, Mauri L, Colombo D, et al. (2017). T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 18, 2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadharshini B, Loschi M, Newton RH, Zhang JW, Finn KK, Gerriets VA, Huynh A, Rathmell JC, Blazar BR, and Turka LA (2018). Cutting Edge: TGF-β and Phosphatidylinositol 3-Kinase Signals Modulate Distinct Metabolism of Regulatory T Cell Subsets. J. Immunol 201, 2215–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, et al. (2014). ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity 41, 789–801. [DOI] [PubMed] [Google Scholar]
- Proietti M, Perruzza L, Scribano D, Pellegrini G, D’Antuono R, Strati F, Raffaelli M, Gonzalez SF, Thelen M, Hardt WD, et al. (2019). ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat. Commun 10, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkema KR, Lee JY, Lee YJ, Hamilton SE, Hogquist KA, and Jameson SC (2016). IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J. Exp. Med 213, 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissiek B, Haag F, Boyer O, Koch-Nolte F, and Adriouch S. (2015). P2X7 on Mouse T Cells: One Channel, Many Functions. Front. Immunol 6, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissiek B, Lukowiak M, Raczkowski F, Magnus T, Mittrücker HW, and Koch-Nolte F. (2018). In Vivo Blockade of Murine ARTC2.2 During Cell Preparation Preserves the Vitality and Function of Liver Tissue-Resident Memory T Cells. Front. Immunol 9, 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, and Ahmed R. (2008). Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med 205, 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, and Grassi F. (2008). Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal 1, ra6. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, and Masopust D. (2014). Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol 192, 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, and Masopust D. (2014). Tissue-resident memory T cells. Immunity 41, 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkopff S, Woyciechowski S, Aichele U, Flecken T, Zhang N, Thimme R, and Pircher H. (2015). TGF-β downregulates KLRG1 expression in mouse and human CD8(+) T cells. Eur. J. Immunol 45, 2212–2217. [DOI] [PubMed] [Google Scholar]
- Seki A, and Rutz S. (2018). Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J. Exp. Med 215, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, and Lefranç ois L. (2014). Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40, 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, and Jameson SC (2013). Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol 14, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, et al. (2012). Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med 18, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, Wesselink TH, Behr FM, Kragten NAM, Arens R, Koch-Nolte F, van Gisbergen KPJM, and van Lier RAW (2018). TRM maintenance is regulated by tissue damage via P2RX7. Sci. Immunol 3, eaau1022. [DOI] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, and Masopust D. (2015). Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu E, Chia CPZ, Chen W, Zhang D, Park SA, Jin W, Wang D, Alegre ML, Zhang YE, Sun L, and Chen W. (2018). T Cell Receptor-Regulated TGF-β Type I Receptor Expression Determines T Cell Quiescence and Activation. Immunity 48, 745–759.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL (2012). Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanhainen KM, Jameson SC, and da Silva HB (2019). Self-Regulation of Memory CD8 T Cell Metabolism through Extracellular ATP Signaling. Immunometabolism 1, e190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, and Bevan MJ (2013). Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, and Sprent J. (1998). Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or code.