Abstract
Patients with lumbar spinal canal stenosis (LSS) have impaired activities of daily living because of pain or motor paralysis, but no effective preventive treatment is currently available. The number of patients with LSS is predicted to continually increase as the average age of the global population increases. To provide a conceptual framework for improving healthy life expectancy, the Japanese Orthopaedic Association introduced the concept of locomotive syndrome, to which LSS is related. Ours and other studies have shown that LSS exacerbates locomotive syndrome and that surgical treatment is one method for improving it. Furthermore, we propose that the two-step test, a locomotive syndrome risk test, is effective for assessing the risk for falls and severity of LSS. Meanwhile, lumbar spinal epidural lipomatosis (LSEL), which is a manifestation of LSS, has been shown to be related to metabolic syndrome. Previous studies have suggested that the whole LSS can be also associated with metabolic syndrome. Although locomotive syndrome is very different from metabolic syndrome, which involves lipid metabolism, these two syndromes overlap, such as in LSS. Conducting research on LSS from the perspectives of both locomotive syndrome and metabolic syndrome may lead to novel methods for prevention and treatment of LSS and, conversely, may yield clues for resolving symptoms of the two syndromes. This review provides an overview of LSS from the perspective of locomotive syndrome and metabolic syndrome, along with findings from our research group.
Keywords: lumbar spinal canal stenosis, locomotive syndrome, metabolic syndrome
1.Introduction
Patients with lumbar spinal canal stenosis (LSS) have impaired activities of daily living because of pain or motor paralysis, but no effective preventive treatment is currently available. Additionally, considering the cost of management therapy for patients with LSS, development of therapies for LSS is greatly needed to make treatment economical. With the continued aging of the global population, the number of patients with LSS is also expected to increase further.
Hypertrophy of the ligamentum flavum, degeneration of intervertebral discs and facet joints, and other factors lead to spinal canal stenosis in patients with LSS. This causes chronic compression of the cauda equina and nerve root, clinically resulting in neurologic symptoms, such as pain and numbness from the low back to the lower extremities, motor deficits, sensory disturbances, and intermittent claudication. Magnetic resonance imaging (MRI) and myelography are useful tools for the diagnosis of LSS1-3). First-line treatments for LSS include conservative treatments, such as medications, epidural blocks, and nerve root blocks4-8). Surgical treatment is indicated in patients for whom these treatments are ineffective and activities of daily living are markedly impaired. Therapeutic outcomes of surgical treatment are generally favorable9).
Although epidural fat is commonly found in the lumbar spinal canal, excessive accumulation of epidural fat occasionally compresses the cauda equina and nerve root, which potentially causes neurologic symptoms, such as low back and lower extremity pain. This state, known as lumbar spinal epidural lipomatosis (LSEL), is considered to be a manifestation of LSS. Although LSEL has been shown to be related to obesity and metabolic syndrome10-16), its pathology is largely unknown, and clear treatment guidelines are not available.
In order to provide a conceptual framework for improving healthy life expectancy, the Japanese Orthopaedic Association in 2007 introduced the concept of locomotive syndrome, to which LSS is related17-19). Locomotive syndrome is defined as a state of decreased mobility due to musculoskeletal organ impairment. As locomotive syndrome progresses, the risk of needing long-term care increases.
Locomotive syndrome differs markedly from metabolic syndrome, which involves lipid metabolism. However, these two syndromes overlap, such as in LSS. This review aimed to provide an overview of LSS from the perspective of locomotive syndrome and metabolic syndrome, along with findings by our research group.
2.Lumbar Spinal Canal Stenosis from the Perspective of Locomotive Syndrome
2. 1.Locomotive syndrome
Functional mobility impairment can greatly affect health and happiness. Japan has seen an increase in the number of patients complaining of symptoms associated with disorders of the joints, spine, and other musculoskeletal organs as the average age of its population has increased. Thus, prevention, early detection, and early treatment of these disorders are needed. The locomotive syndrome concept can be shown by systematic monitoring of relevant features. Diagnosis of locomotive syndrome is made by conducting locomotive syndrome risk tests, including the stand-up test, two-step test, and self-administered 25-question Geriatric Locomotive Function Scale (GLFS)20). Patients who exceed the reference value for at least one of the above three tests are diagnosed as having locomotive syndrome. Locomotive syndrome has two stages. Stage 1 is indicated by the beginning of a decline in mobility function, and Stage 2 is defined by a progressive decrease in mobility and an increased risk of losing the ability to live independently. Osteoarthritis and osteoporosis are two other major musculoskeletal diseases that cause locomotive syndrome.
2. 2.Lumbar spinal canal stenosis and locomotive syndrome
We previously performed locomotive syndrome risk tests for 200 patients aged ≥65 years with LSS who were scheduled to undergo surgery21). All 200 patients were diagnosed as having locomotive syndrome, and 97% of the patients were Stage 2. As LSS became more severe, their locomotive syndrome risk test scores worsened, demonstrating a correlation between the stage of locomotive syndrome and severity of LSS21); this relationship was also observed by Kasukawa et al.22) and is consistent with the correlation between the 25-question GLFS and the Zurich Claudication Questionnaire reported by Araki et al.23). We determined that among the locomotive syndrome risk tests, the two-step test is particularly strongly correlated with the severity of LSS21). Importantly, we and others recently found that surgical treatment improves locomotive syndrome severity in patients with LSS24,25), with Shimizu et al. noting that risk factors associated with non-improvement of neurological symptoms by surgical treatment were late elderly age and postoperative sagittal imbalance25). To summarize, LSS exacerbates locomotive syndrome, whereas surgical treatment is one method for improving it.
2. 3.Short stride length and risk for falls
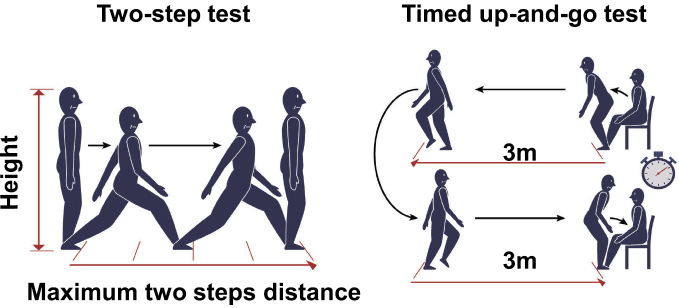
Among the elderly, fractures sustained in falls can lead to the necessity of long-term care26). Short stride length is correlated with the risk for falls among the elderly27,28) and is a clinical symptom of LSS29,30); consistent with this correlation, the risk for falls increases in LSS31,32). We examined the risk for falls in 357 LSS patients aged ≥65 years by conducting the two-step test33), which is a very simple test for measuring maximum stride length and is part of the locomotive syndrome examination20). In this test, the patient stands and moves two strides forward with maximum stride from a start line, after which the distance is normalized by the patient's height (Fig. 1). We found strong associations of the two-step test results for the risk for falls with age of ≥80 years, motor deficits of the lower extremities, and forward-bent posture33). Our previous study also showed that the results of the two-step test agreed well with those of the timed up-and-go test (TUGT) in patients with LSS33). In the TUGT, physical therapists usually measure the time required for getting up from a standard chair, walking 3 m, turning around, and walking back to sit down again34) (Fig. 1). The TUGT has been validated as an appropriate method for predicting the risk for falls in elderly individuals35), and a TUGT score cutoff value was established as indicative of a high risk for falls in elderly individuals36). Hence, in LSS patients, the two-step test and the TUGT are effective tools for assessing the risk for falls and the severity of LSS.
Figure 1.
The two-step test (left panel) and timed up-and-go test (right panel).
3.Lumbar Spinal Canal Stenosisfrom the Perspective of Metabolic Syndrome
3. 1.Lumbar spinal epidural lipomatosis
LSEL was first reported by Lee et al. in 1975 in patients who were continuously administered steroids as immunosuppressants following kidney transplantation37). Subsequently, steroid use and endocrine disorders that result in an excess of endogenous steroids have been suggested to be involved in causing LSEL38-41). In 1982, Badami et al. reported a case of idiopathic LSEL unrelated to steroids10). Since then, LSEL has frequently been reported in obese patients, mostly men11-15). A diagnosis of LSEL is mainly based on MRI. Abnormally large masses with high signal intensity on both T1- and T2-weighted MRIs are observed in the spinal epidural space of LSEL patients. The characteristic “Y-sign” is often seen on axial MRI42). When we examined epidural fat tissues from 16 patients with LSEL, histological analysis revealed hypertrophy of adipocytes in LSEL patients who demonstrated a significantly larger area of adipocytes than the control group43). In a case report, Ohba et al. also histologically examined the epidural fat of a patient with LSEL with underlying obesity and reported adipocyte hypertrophy, a finding that is consistent with our own results15). In our study, we used real-time polymerase chain reaction to examine mRNA expression levels of cytokines in adipocytes. Although we did not observe significant differences for the adipocytokines adiponectin or leptin, expressions of the inflammatory cytokines TNF-α and IL-1β were significantly elevated in LSEL patients. Visceral fat in obese patients with metabolic syndrome also demonstrates adipocyte hypertrophy and elevated expressions of inflammatory cytokines, a pathology known to be involved in chronic inflammation44). The above findings indicate that epidural and visceral fat may share similar cellular biological properties. Given that inflammatory cytokines are involved in discogenic low back pain45) and that inflammatory cytokines induce expression of molecules involved in pain, such as prostaglandin E2 and substance P46), elevated expressions of inflammatory cytokines are presumed to be involved in pain in patients with LSEL.
3. 2.Epidural fat accumulation and pain
In our previous study, we used epidural fat thickness as the basis to create three groups from 166 male patients with LSS who had undergone surgery and retrospectively compared body mass index (BMI), blood test results (including lipid markers), and ankle-brachial pressure index among these groups47). We found that as the epidural fat thickened, BMI significantly increased, as did total cholesterol and triglyceride levels. We also found that, as epidural fat thickened, scores worsened for the pain-related disorder and walking ability domains of the self-administered Japanese Orthopedic Association Back Pain Evaluation Scale. These results suggest that, in patients with LSEL, obesity and hyperlipidemia are related to epidural fat thickness and that thicker epidural fat further exacerbates clinical symptoms. The exacerbation of pain associated with thicker epidural fat may be caused in part by elevated expression of inflammatory cytokines produced by hypertrophic adipocytes (Fig. 2). Obese patients tend to have severe pain, the mechanism of which may involve chronic inflammation48). In obese patients with LSEL, however, mechanical stress on the lumbar spine associated with a high BMI is commonly viewed as the cause of their pain. However, based on the above results, pain in obese patients with LSEL may be caused not only by mechanical stress but also by chronic inflammation associated with hypertrophic adipocytes. Further basic research is required regarding the relationship between pain and epidural fat hypertrophy in patients with LSEL.
Figure 2.
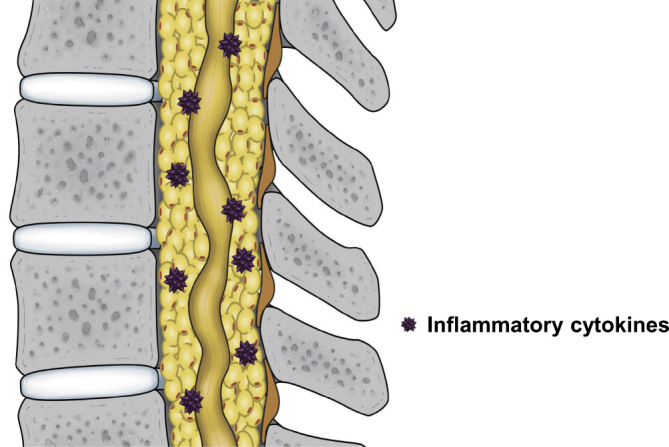
Schematic model for the mechanisms of pain in patients with lumbar spinal epidural lipomatosis.
3. 3.Lumbar spinal epidural lipomatosis and metabolic syndrome
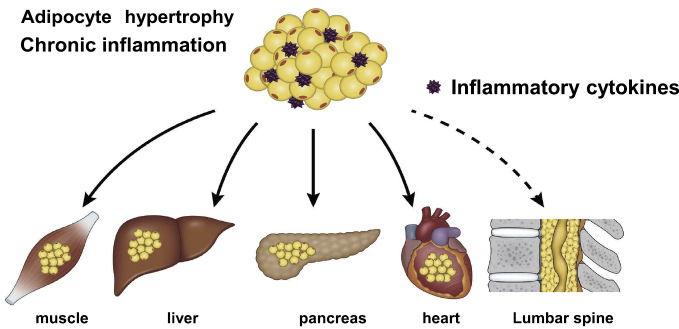
In a previous study we conducted, multivariate analysis of LSEL and lifestyle-related diseases demonstrated an association between LSEL and hyperlipidemia47). The subjects participating in this study were LSS patients, and the number of subjects was relatively low, meaning that the relationship between LSEL and hyperlipidemia remains debatable. However, the results suggested that LSEL may be associated with metabolic syndrome. Thus, we examined the correlations among epidural fat accumulation in the lumbar spinal canal and BMI, abdominal circumference, visceral fat area by computed tomography, and blood test results in 324 healthy subjects who underwent medical checkups16). Such analysis demonstrated the following: BMI, abdominal circumference, and visceral fat area were all significantly correlated with epidural fat accumulation, and the incidence of LSEL was significantly higher in the individuals with metabolic syndrome than in those without metabolic syndrome. Other research groups also have investigated such correlations, with Morishita et al. reporting that LSEL was strongly correlated with visceral fat49) and Abe et al. reporting that epidural fat accumulation was strongly correlated with accumulation of fat in the liver50). Recently, the concept of ectopic fat has been proposed as a third type of fat, separate from subcutaneous fat and visceral fat, and excessive storage of ectopic fat in organs can diminish their functions51). Ectopic fat primarily occurs in the pancreas, muscles, liver, and heart. Because LSEL is also associated with lifestyle-related diseases and metabolic syndrome, excess accumulation of epidural fat may also be considered a type of ectopic fat (Fig. 3). Intriguingly, quite unlike ectopic fat in other organs, ectopic fat in the epidural space appears to be associated with pain.
Figure 3.
Schematic representation of ectopic fat.
Excess accumulation of epidural fat can also be considered as a type of ectopic fat.
3. 4.Treatment for lumbar spinal epidural lipomatosis
Treatments for LSEL include conservative approaches, such as weight reduction, and surgical treatments, such as decompression surgery. Given the pathology of LSEL combined with obesity, weight reduction would be predicted to reduce the excess epidural fat. In fact, weight reduction has been shown to be effective in reducing symptoms of LSEL in several case reports12,52,53). Regarding the efficacy of weight reduction in treating LSEL with obesity, prospective interventional studies will be critical for establishing strategies.
The efficacy of surgical treatment on patients with LSEL was indicated by Ishikawa et al.54) and Ferlic et al.55) in retrospective studies. However, in both studies, the sample size was limited and no control group was included. By conducting a case-control study, Bayerl et al. demonstrated that the effect of surgical treatment on LSEL was nearly identical to its effect in patients with LSS without LSEL56). Recently, we also conducted a multicenter retrospective study on the efficacy of surgical treatment for LSEL57). Unlike the results from Bayerl et al., ours demonstrated that, although surgical treatment significantly improved clinical symptoms in patients with LSEL, the outcomes of surgical treatment for LSEL were significantly inferior to therapeutic outcomes among patients with LSS without LSEL. The differences between the results in these two studies are conceivably due to differences in the definition of LSEL and the methods of statistical analysis between the groups. However, importantly, the results of both studies show that surgical treatment can be recommended for patients with LSEL. If chronic inflammation is involved in LSEL43), then biopharmaceuticals that target inflammatory cytokines could be used to treat LSEL in the future. However, because of their adverse drug reactions and relatively high cost, biopharmaceuticals are unlikely to become a first-line treatment for LSEL. Presently, weight reduction, combined with surgical treatment, is likely the most reasonable treatment for LSEL with obesity or metabolic syndrome.
3. 5.Lumbar spinal canal stenosis and metabolic syndrome
As mentioned above, LSEL has been demonstrated to be associated with lifestyle diseases and metabolic syndrome16,43,47). As other research groups have reported, the whole LSS is also associated with lifestyle-related diseases, such as diabetes and hypertension58,59). In a basic study, Luo et al. demonstrated that ligamentum flavum hypertrophy, a cause of LSS, was affected with hyperglycemia60). Given that blood glucose is included in the diagnostic criteria for metabolic syndrome61), these study results suggest that the whole LSS also can be associated with metabolic syndrome. Although the causal relationship remains unclear, it can be inferred that LSS and metabolic syndrome mutually affect each other.
4.Future Tasks
LSS, which includes elements of locomotive syndrome and metabolic syndrome, can be viewed as an interaction between the two (Fig. 4). We and others have demonstrated that LSS was associated with locomotive syndrome and that surgical treatment for LSS was effective in decreasing the severity of locomotive syndrome21-25). However, there has not yet been a direct assessment of (i) the extent to which LSS affects healthy life expectancy and (ii) whether surgical treatment for LSS extends healthy life expectancy. In the future, large-scale study data should be used to determine the relationship between LSS and healthy life expectancy.
Figure 4.
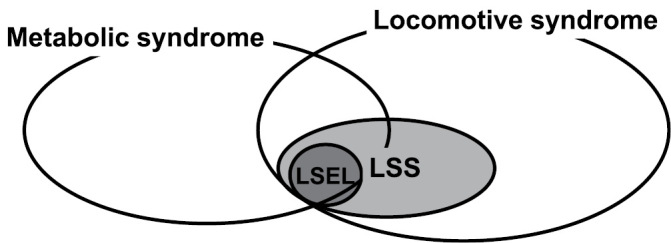
Lumbar spinal canal stenosis (LSS), which includes lumbar spinal epidural lipomatosis (LSEL), can be considered as an interaction between locomotive syndrome and metabolic syndrome.
Although LSEL has been recognized as being associated with lifestyle-related diseases and metabolic syndrome16,43,47), a consistent definition or diagnosis of LSEL is still needed. A standardized LSEL definition would likely accelerate basic and clinical research. Furthermore, large-scale studies must be conducted to investigate the extent to which not only LSEL but also the whole LSS is involved with metabolic syndrome. Going forward, approaching LSS from the perspectives of both locomotive syndrome and metabolic syndrome may lead to novel methods for prevention and treatment of LSS. Conversely, research on LSS is anticipated to yield clues useful in developing treatments for both syndromes.
Conflicts of Interest: The author declares that there are no relevant conflicts of interest.
Ethical Approval: unnecessary
Acknowledgement
This study was supported by a research grant (2019-3) from the Japanese Orthopaedic Association. The author thanks Dr. Morio Matsumoto, Dr. Masaya Nakamura, Dr. Kota Watanabe, Dr. Shinichi Ishihara, Dr. Hans Dijkstra, Ms. Aiko Sakurai, and Mr. Azusa Miyamoto for their contributions to this review.
References
- 1.Bell GR, Rothman RH, Booth RE, et al. A study of computer-assisted tomography. II. Comparison of metrizamide myelography and computed tomography in the diagnosis of herniated lumbar disc and spinal stenosis. Spine. 1984;9(6):552-6. [PubMed] [Google Scholar]
- 2.Bolender NF, Schönström NS, Spengler DM. Role of computed tomography and myelography in the diagnosis of central spinal stenosis. J Bone Joint Surg Am. 1985;67(2):240-6. [PubMed] [Google Scholar]
- 3.Bischoff RJ, Rodriguez RP, Gupta K, et al. A comparison of computed tomography-myelography, magnetic resonance imaging, and myelography in the diagnosis of herniated nucleus pulposus and spinal stenosis. J Spinal Disord. 1993;6(4):289-95. [DOI] [PubMed] [Google Scholar]
- 4.Matsudaira K, Seichi A, Kunogi J, et al. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. The efficacy of prostaglandin E1 derivative in patients with lumbar spinal stenosis. Spine. 2009;34(2):115-20. [DOI] [PubMed] [Google Scholar]
- 5.Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82(11):1589-93. [DOI] [PubMed] [Google Scholar]
- 6.Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81(12):898-905. [DOI] [PubMed] [Google Scholar]
- 7.Delport EG, Cucuzzella AR, Marley JK, et al. Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil. 2004;85(3):479-84. [DOI] [PubMed] [Google Scholar]
- 8.Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double-blind, controlled trial. Spine. 2005;30(8):857-62. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badami JP, Hinck VC. Symptomatic deposition of epidural fat in a morbidity obese woman. AJNR Am J Neuroradiol. 1982;3(6):664-5. [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson SC, Traynelis VC, Follett KA, et al. Idiopathic spinal epidural lipomatosis. Neurosurgery. 1997;41(1):68-74. [DOI] [PubMed] [Google Scholar]
- 12.Maillot F, Mulleman D, Mammou S, et al. Is epidural lipomatosis associated with abnormality of body fat distribution? A case report. Eur Spine J. 2006;15(1):105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar K, Nath RK, Nair CP, et al. Symptomatic epidural lipomatosis secondary to obesity: case report. J Neurosurg. 1996;85(2):348-50. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya H, Tanaka T, Ogawa T, et al. Spinal epidural lipomatosis in lumbar magnetic resonance imaging scans. Orthopedics. 2014;37(4):e362-6. [DOI] [PubMed] [Google Scholar]
- 15.Ohba T, Saito T, Kawasaki N, et al. Symptomatic spinal epidural lipomatosis with severe obesity at a young age. Orthopedics. 2011;34(6):233. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara S, Fujita N, Azuma K, et al. Spinal epidural lipomatosis is a previously unrecognized manifestation of metabolic syndrome. Spine J. 2019;19(3):493-500. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K. Locomotive syndrome: disability-free life expectancy and locomotive organ health in a “super-aged” society. J Orthop Sci. 2009;14(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura K. The concept and treatment of locomotive syndrome: its acceptance and spread in Japan. J Orthop Sci. 2011;16(5):489-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura K, Ogata T. Locomotive syndrome: definition and management. Clin Rev Bone Miner Metab. 2016;14:56-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita N, Sakurai A, Miyamoto A, et al. Lumbar spinal canal stenosis leads to locomotive syndrome in elderly patients. J Orthop Sci. 2019;24(1):19-23. [DOI] [PubMed] [Google Scholar]
- 22.Kasukawa Y, Miyakoshi N, Hongo M, et al. Lumbar spinal stenosis associated with progression of locomotive syndrome and lower extremity muscle weakness. Clin Interv Aging. 2019;14:1399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki M, Nonoshita H, Kitano S, et al. The critical cutoff point of the Zurich Claudication Questionnaire and the Japanese Orthopaedic Association score indicating locomotive syndrome in patients with lumbar spinal canal stenosis. J Orthop Sci. In press. [DOI] [PubMed] [Google Scholar]
- 24.Fujita N, Michikawa T, Miyamoto A, et al. Lumbar spinal surgery improves locomotive syndrome in elderly patients with lumbar spinal canal stenosis: a multicenter prospective study. J Orthop Sci. 2020;25(2):213-8. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu T, Kato S, Demura S, et al. The efficacy of surgical treatment on locomotive syndrome and physical function in patients with lumbar spinal canal stenosis. J Orthop Sci. In press. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35 Suppl 2:ii37-41. [DOI] [PubMed] [Google Scholar]
- 27.Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86(11):1501-10. [DOI] [PubMed] [Google Scholar]
- 28.Menz HB, Lord SR, Fitzpatrick RC. A structural equation model relating impaired sensorimotor function, fear of falling and gait patterns in older people. Gait Posture. 2007;25(2):243-9. [DOI] [PubMed] [Google Scholar]
- 29.Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38(4):987-92. [DOI] [PubMed] [Google Scholar]
- 30.Suda Y, Saitou M, Shibasaki K, et al. Gait analysis of patients with neurogenic intermittent claudication. Spine. 2002;27(22):2509-13. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Chun HJ, Han CD, et al. The risk assessment of a fall in patients with lumbar spinal stenosis. Spine. 2011;36(9):E588-92. [DOI] [PubMed] [Google Scholar]
- 32.Lee BH, Kim TH, Park MS, et al. Comparison of effects of nonoperative treatment and decompression surgery on risk of patients with lumbar spinal stenosis falling. J Bone Joint Surg Am. 2014;96(13):e110. [DOI] [PubMed] [Google Scholar]
- 33.Fujita N, Sakurai A, Miyamoto A, et al. Stride length of elderly patients with lumbar spinal stenosis: multi-center study using the Two-Step test. J Orthop Sci. 2019;24(5):787-92. [DOI] [PubMed] [Google Scholar]
- 34.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142e8. [DOI] [PubMed] [Google Scholar]
- 35.American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc. 2001;49(5):664e72. [PubMed] [Google Scholar]
- 36.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed Up & Go test. Phys Ther. 2000;80(9):896e903. [PubMed] [Google Scholar]
- 37.Lee M, Lekias J, Gubbay SS, et al. Spinal cord compression by extradural fat after renal transplantation. Med J Aust. 1975;1(7):201-3. [DOI] [PubMed] [Google Scholar]
- 38.Fessler RG, Johnson DL, Brown FD, et al. Epidural lipomatosis in steroid-treated patients. Spine. 1992;17(2):183-8. [DOI] [PubMed] [Google Scholar]
- 39.George Jr WE, Wilmot M, Greenhouse A, et al. Medical management of steroid-induced epidural lipomatosis. N Engl J Med. 1983;308(6):316-9. [DOI] [PubMed] [Google Scholar]
- 40.Noël P, Pepersack T, Vanbinst A, et al. Spinal epidural lipomatosis in Cushing's syndrome secondary to an adrenal tumor. Neurology. 1992;42(6):1250-1. [DOI] [PubMed] [Google Scholar]
- 41.Al-Khawaja D, Seex K, Eslick GD. Spinal epidural lipomatosis-a brief review. J Clin Neurosci. 2008;15(12):1323-6. [DOI] [PubMed] [Google Scholar]
- 42.Borré DG, Borré GE, Aude F, et al. Lumbosacral epidural lipomatosis: MRI grading. Eur Radiol. 2003;13(7):1709-21. [DOI] [PubMed] [Google Scholar]
- 43.Fujita N, Hosogane N, Hikata T, et al. Potential involvement of obesity-associated chronic inflammation in the pathogenesis of idiopathic spinal epidural lipomatosis. Spine. 2016;41(23):E1402-7. [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtori S, Inoue G, Miyagi M, et al. Pathomechanism of discogenic low back pain in humans and animal models. Spine J. 2015;15(6):1347-55. [DOI] [PubMed] [Google Scholar]
- 46.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishihara S, Fujita N, Yagi M, et al. Idiopathic spinal epidural fat accumulation is associated with hyperlipidemia. Spine. 2018;43(8):E468-73. [DOI] [PubMed] [Google Scholar]
- 48.Ray L, Lipton RB, Zimmerman ME, et al. Mechanisms of association between obesity and chronic pain in the elderly. Pain. 2011;152(1):53-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morishita S, Arai Y, Yoshii T, et al. Lumbar epidural lipomatosis is associated with visceral fat and metabolic disorders. Eur Spine J. 2018;27(7):1653-61. [DOI] [PubMed] [Google Scholar]
- 50.Abe T, Miyazaki M, Ishihara T, et al. Spinal epidural lipomatosis is associated with liver fat deposition and dysfunction. Clin Neurol Neurosurg. 2019;185:105480. [DOI] [PubMed] [Google Scholar]
- 51.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371(12):1131-41. [DOI] [PubMed] [Google Scholar]
- 52.Kniprath K, Farooque M. Drastic weight reduction decrease in epidural fat and concomitant improvement of neurogenic claudicatory symptoms of spinal epidural lipomatosis. Pain Med. 2017;18(6):1204-6. [DOI] [PubMed] [Google Scholar]
- 53.Beckworth WJ, McCarty EJ, Garcia-Corrada JE, et al. Epidural lipomatosis and associated spinal stenosis-the impact of weight loss: a case report. Am J Lifestyle Med. 2017;11(6):511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa Y, Shimada Y, Miyakoshi N, et al. Decompression of idiopathic lumbar epidural lipomatosis: diagnostic magnetic resonance imaging evaluation and review of the literature. J Neurosurg Spine. 2006;4(1):24-30. [DOI] [PubMed] [Google Scholar]
- 55.Ferlic PW, Mannion AF, Jeszenszky D, et al. Patient-reported outcome of surgical treatment for lumbar spinal epidural lipomatosis. Spine J. 2016;16(11):1333-41. [DOI] [PubMed] [Google Scholar]
- 56.Bayerl SH, Dinkelbach M, Heiden P, et al. Treatment results for lumbar epidural lipomatosis: does fat matter? Eur Spine J. 2019;28(1):69-77. [DOI] [PubMed] [Google Scholar]
- 57.Fujita N, Ishihara S, Michikawa T, et al. Negative impact of spinal epidural lipomatosis on the surgical outcome of posterior lumbar spinous-splitting decompression surgery: a multicenter retrospective study. Spine J. 2019;19(12):1977-85. [DOI] [PubMed] [Google Scholar]
- 58.Lotan R, Oron A, Anekstein Y, et al. Lumbar stenosis and systemic diseases: is there any relevance? J Spinal Disord Tech. 2008;21(4):247-51. [DOI] [PubMed] [Google Scholar]
- 59.Uesugi K, Sekiguchi M, Kikuchi S, et al. Relationship between lumbar spinal stenosis and lifestyle-related disorders: a cross-sectional multicenter observational study. Spine. 2013;38(9):E540-5. [DOI] [PubMed] [Google Scholar]
- 60.Luo J, Huang L, Chen Z, et al. Increased sorbitol levels in the hypertrophic ligamentum flavum of diabetic patients with lumbar spinal canal stenosis. J Orthop Res. 2017;35(5):1058-66. [DOI] [PubMed] [Google Scholar]
- 61.Metabolic Syndrome Diagnostic Criteria Exploratory Committee. Definition and the diagnostic standard for metabolic syndrome-Committee to evaluate diagnostic standards for metabolic syndrome. J Jpn Soc Intern Med. 2005;94(4):794-809. [PubMed] [Google Scholar]