Abstract
Cardiovascular diseases (CVD) are the leading cause of death worldwide. Overweight and obesity are strongly associated with comorbidities such as hypertension and insulin resistance, which collectively contribute to the development of cardiovascular diseases and resultant morbidity and mortality. 42% of adults in the US are obese and a total of 1.9 billion adults worldwide are overweight or obese. These alarming numbers, which continue to climb, represent a major health and economic burden. Adipose tissue is a highly dynamic organ that can be classified based on the cellular composition of different depots and their distinct anatomic localization. Massive expansion and remodeling of adipose tissue during obesity differentially affects specific adipose tissue depots and significantly contributes to vascular dysfunction and CVD. Visceral adipose tissue accumulation results in increased immune cell infiltration and secretion of vasoconstrictor mediators, whereas expansion of subcutaneous AT is less harmful. Therefore, fat distribution more than overall body weight is a key determinant of the risk for CVD. Thermogenic brown and beige adipose tissue, in contrast to white adipose tissue, is associated with beneficial effects on the vasculature. The relationship between the type of adipose tissue and its influence on vascular function becomes particularly evident in the context of the heterogenous phenotype of perivascular adipose tissue that is strongly location dependent. In this review, we address the abnormal remodeling of specific adipose tissue depots during obesity and how this critically contributes to the development of hypertension, endothelial dysfunction and vascular stiffness. We also discuss the local and systemic roles of adipose tissue derived secreted factors and increased systemic inflammation during obesity and highlight their detrimental impact on cardiovascular health.
Keywords: Adipose Tissue, Hypertension, Aortic Stiffness, Obesity, Cardiovascular diseases
Subject Terms: Cardiovascular Disease, Hypertension, Obesity
I. Obesity and Cardiovascular Disease
The worldwide prevalence of obesity has tripled since 1975, with a parallel trend in type 2 diabetes1,2. Globally, over 1.9 billion adults were overweight or obese in 2016 and more than 60% of people with obesity live in developing countries3. Today, about two out of three adults (69%) are overweight or obese in the United States and current projections suggest that nearly 50% of adults in the US will be obese by 20304. Predictions made in 2008, estimated up to 3.3 billion individuals to become overweight and obese by 2030, if adjusted for secular trends5. Non-adjusted predictions for 2030 generated by the same study predicted only 1.35 billion overweight and 573 million obese individuals for 20305, a number that was outdated already by 20163. While it is well documented that genetic and epigenetic factors contribute to obesity, environmental factors such as diet, physical activity and environmental toxins also play a major role in the increased prevalence of this disorder (Figure 1). For example, the increase in obesity in the US and other industrialized nations is closely related to increased consumption of high fructose corn syrup and saturated fat and to reduced physical activity3,4 (Figure 1). Further, there is emerging evidence that consumption of high fructose corn syrup diets by pregnant women programs the offspring for the subsequent development of obesity and associated cardiometabolic and cardiovascular disease (CVD) in later life (Figure 2)6. These maternal influences appear to be mediated through adverse effects of metabolic factors such as impaired insulin signaling, dyslipidemia and altered blood supply on placental function and resultant fetal nutrition as well as epigenetic influences that originate from maternal obesity6.
Figure 1. Obesity, vascular stiffness and cardiovascular disease (CVD): genetic/epigenetic and environment interactions.
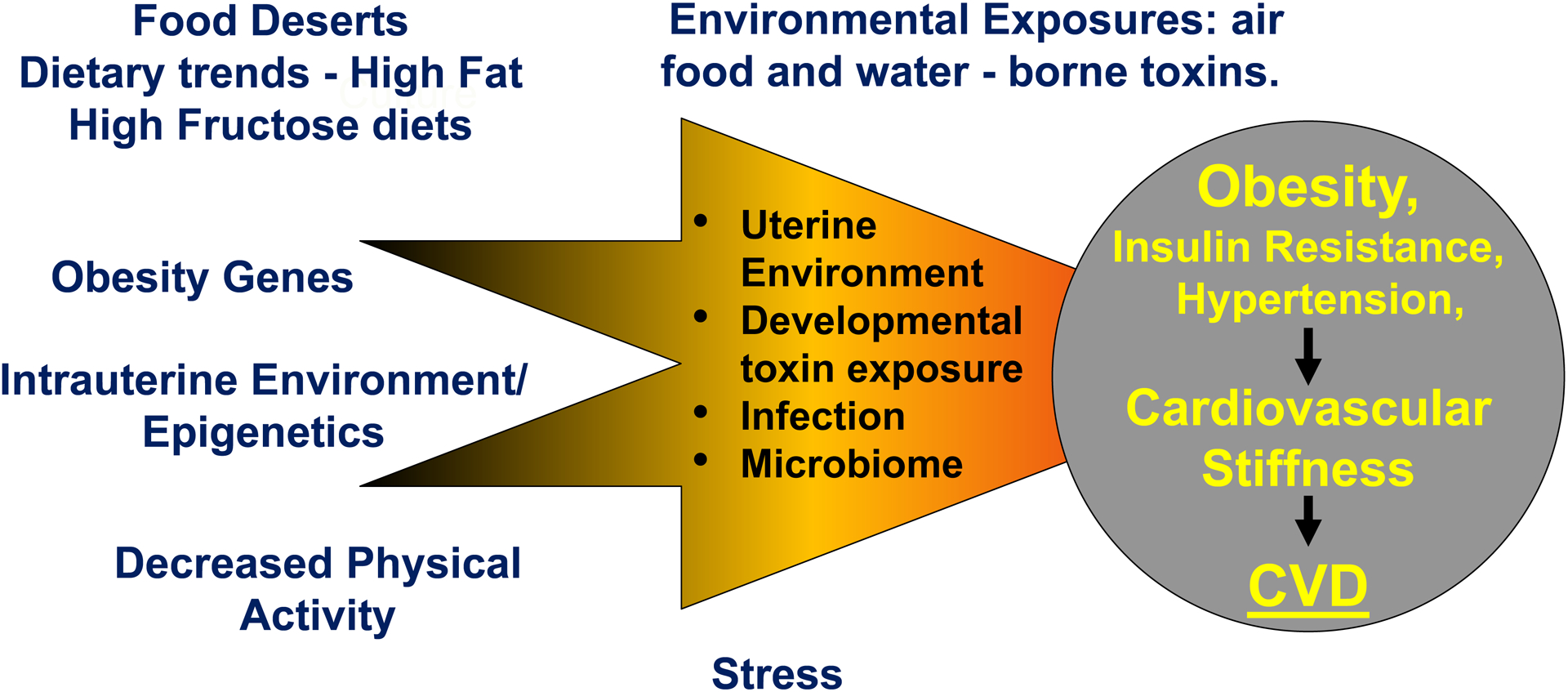
A food desert refers to an area with limited access to nutritious, affordable food.
Figure 2. Prenatal programming and epigenetics in the genesis of obesity and cardiovascular disease (CVD) in offspring.
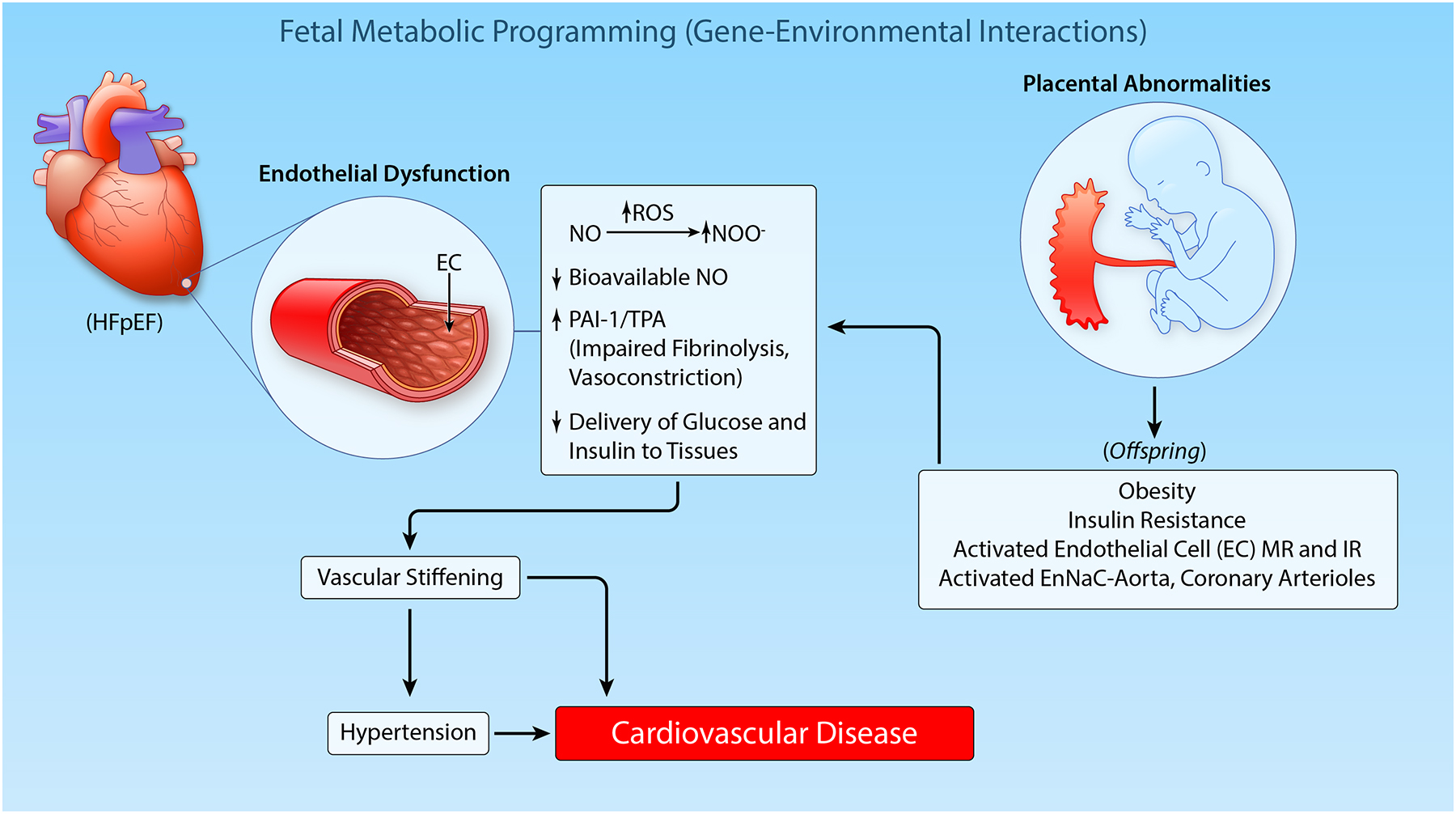
(Illustration credit: Ben Smith)
There is considerable evidence that overweight and obesity and their comorbidities, hypertension and insulin resistance, increase CVD and overall morbidity and mortality rates7–12. Indeed, a positive association even exists between a progressive increase in body mass index (BMI) within the normal and overweight range and the risk of CVD6,7. In this regard, an analysis of the Framingham Heart Study showed a positive association between overweight (BMI 25–29.9 kg/m2) and the relative risks of hypertension and CVD8. In addition, the presence of childhood obesity has been shown to increase the risk for development of type 2 diabetes, hypertension, dyslipidemia, and atherosclerosis and related CVD in adulthood9–11. This review discusses the various factors that promote vascular dysfunction and CVD in obesity, with a focus on the role of dysfunctional adipose tissue.
II. Types of Adipose Tissue
Functionally distinct adipose tissue depots in mice and humans
Adipose tissue (AT) is a dynamic organ distributed throughout the body with an almost unlimited capacity to expand during obesity. Several distinct depots can be defined by their location, size, cellular composition and function. While many functions of AT are conserved between mouse models and humans, their location and abundance can vary broadly. Mammals possess two major types of AT: white and brown (Figure 3). White adipose tissue (WAT) represents the largest proportion of whole-body AT and can be found around major organs and blood vessels in the abdominal cavity and subcutaneously (Figure 4). WAT stores excess energy in the form of triglycerides, and increased accumulation of WAT, particularly in visceral depots, is a key determinant of the relative risk for cardiometabolic disorders, hypertension and CVD12–17. To this point, fat distribution dictates CVD risk such that individuals with higher visceral AT and ectopic fat deposition have an increased prevalence of cardiometabolic disorders including hypertension18,19, dyslipidemia and insulin resistance15–17 compared to equally obese individuals with less visceral AT and relatively more subcutaneous fat. Thus, measurements limited to determination of BMI do not reflect the actual risk for CVD conferred by obesity.
Figure 3. Function and localization of different adipose tissue depots.
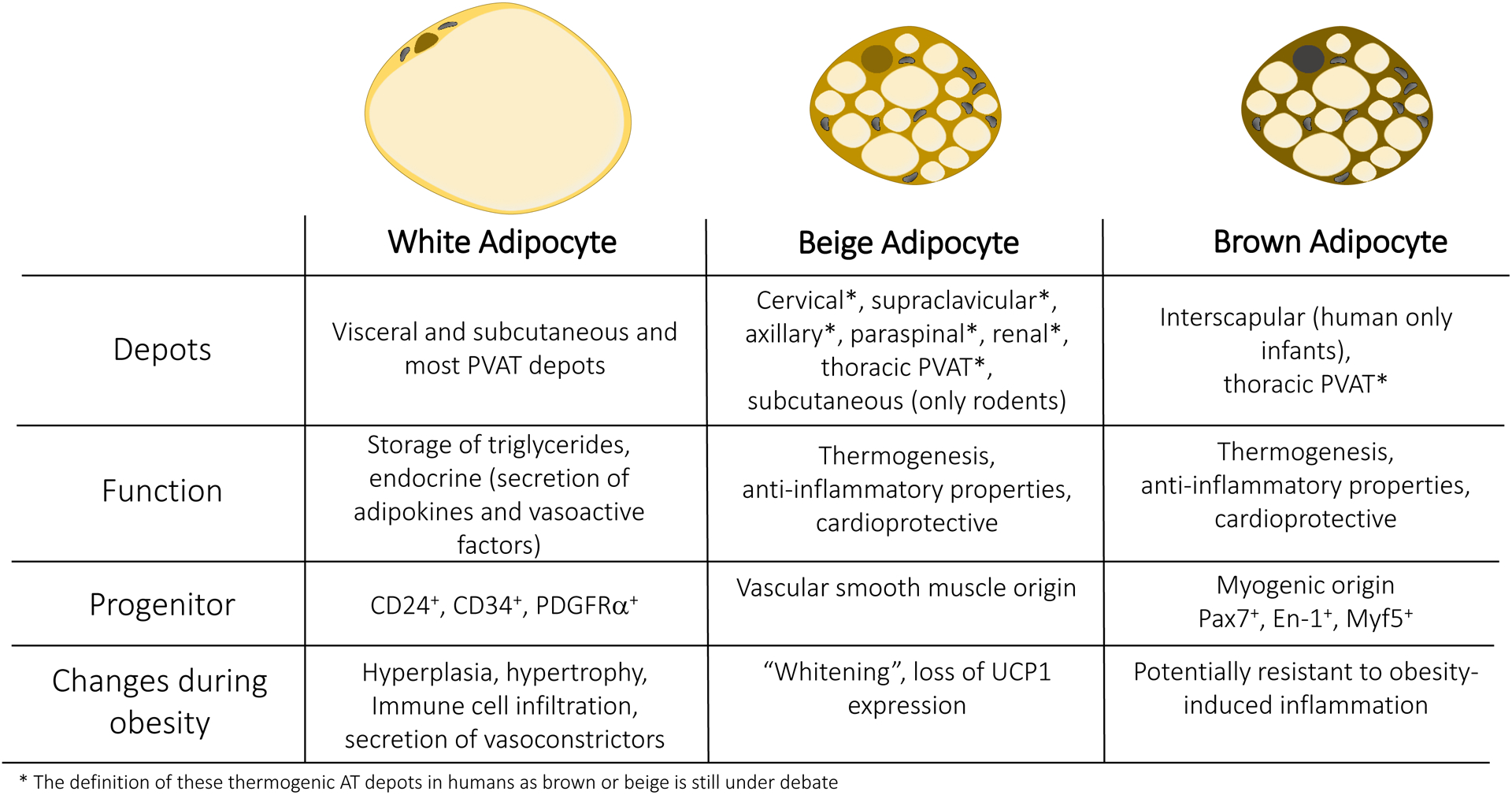
Comparison of white, beige and brown adipocytes in regard to their localization in specific depots in human and mice. Their major functions and progenitor cells are depicted. Major changes occurring during adipose tissue remodeling in obesity are highlighted. PVAT, Perivascular adipose tissue; CD, Cluster of differentiation; PDGFRα: Platelet-derived growth factor receptor alpha; Pax7, Paired box 7; En-1, Engrailed-1; Myf5, Myogenic factor 5;Ucp-1, Uncoupling protein-1; AT, Adipose tissue.
Figure 4. Changes in different adipose tissue depots in homeostasis and during obesity.
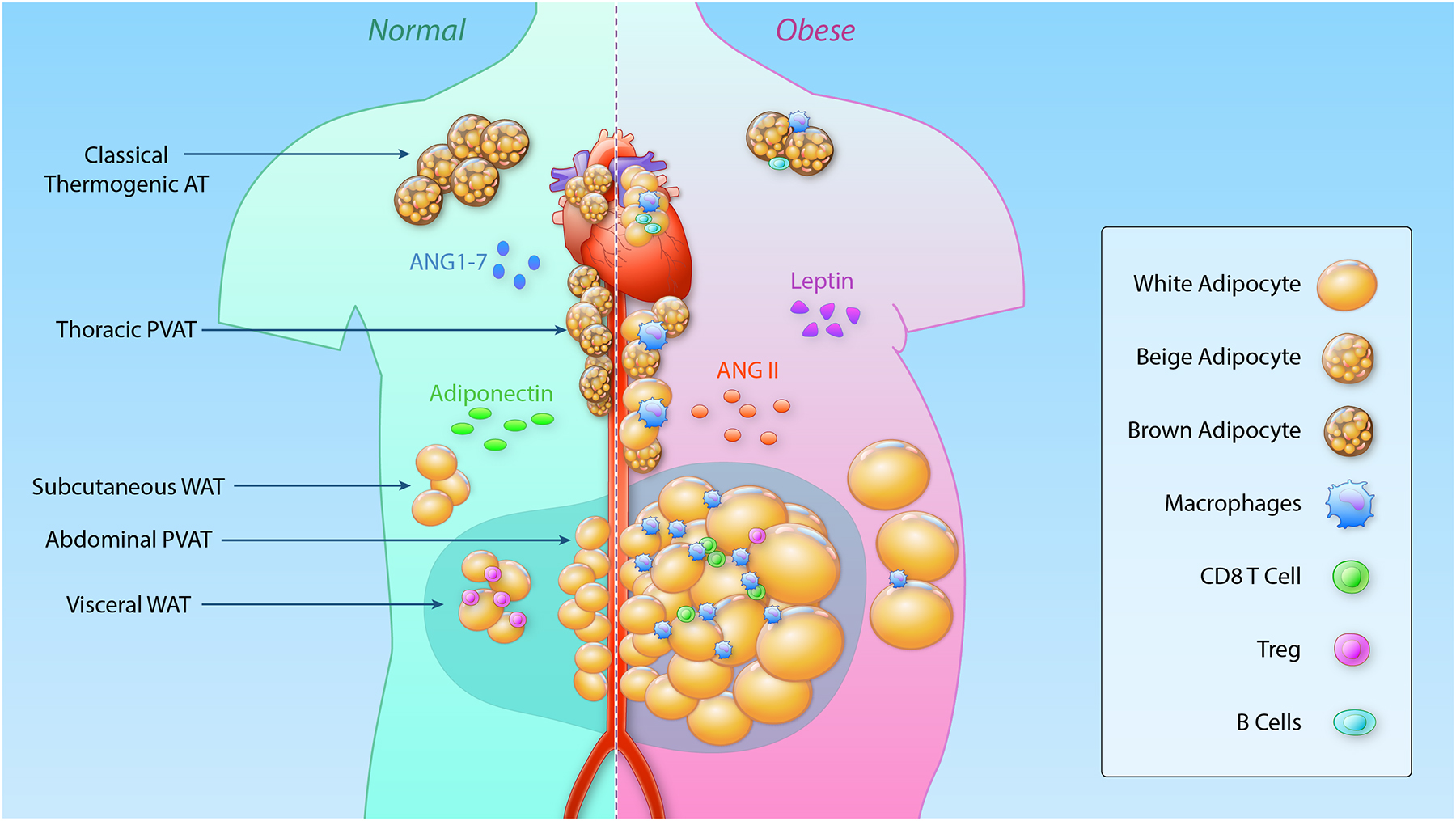
In states of normal body weight (left), thermogenic brown and beige adipocytes are found surrounding the thoracic aorta (PVAT) and can be detected in the cervical, supraclavicular, axillary, paraspinal, renal and epicardial area and in infants in the interscapular depot. These cells have a multilocular appearance and due to the high density of mitochondria appear brown. The abdominal aorta and mesenteric vasculature are surrounded by white adipocytes. These unilocular adipocytes are also found in visceral and subcutaneous adipose depots. Adiponectin and angiotensin 1–7 are secreted by adipocytes and have a vasodilating effect on the vasculature. In the lean state, adipose tissue is populated with different immune cells important for homeostasis, that change dramatically during obesity. During obesity (right), T regulatory cells (Treg) are lost in visceral adipose tissue and inflammatory CD8 T cells and macrophages infiltrate the visceral, mesenteric and to a lesser extent subcutaneous adipose depot. Thermogenic adipose tissue in proximity to the heart and the aorta downregulates thermogenic gene expression and becomes infiltrated with immune cells. Classical brown adipose tissue is potentially protected against obesity-induced immune cell infiltration. Secretion of vasodilatory factors from adipocytes are downregulated whereas leptin and angiotensin II (ANGII) are predominantly secreted, resulting in elevations in blood pressure. PVAT, Perivascular adipose tissue; WAT, white adipose tissue; ANG II, Angiotensin II; ANG 1–7, Angiotensin 1–7; CD, Cluster of differentiation. (Illustration credit: Ben Smith)
In contrast to WAT, brown AT (BAT) represents only approximately 4.3% of all AT in adult humans and can be found in cervical, supraclavicular, axillary, paraspinal, mediastinal and abdominal depots20–22 (Figure 4). In addition, newborns possess interscapular BAT that decreases in size over time and is no longer detectable in adults23. BAT protects animals from hypothermia by dissipating energy as heat, via a process called non-shivering thermogenesis, and has more recently been found to also have anti-obesity and anti-diabetes properties and to confer broad cardiometabolic health benefits24.
The main functional cell type of AT is the adipocyte or fat cell. White adipocytes contain a single large lipid droplet (unilocular) and only possess a small number of mitochondria. Brown adipocytes, on the other hand, have multilocular lipid droplets and contain a large number of cristae-dense mitochondria, which uniquely express uncoupling protein-1 (UCP1) in the inner mitochondrial membrane (Figure 3). UCP1 uncouples oxidative phosphorylation from ATP production, ultimately resulting in the generation of heat25. More recently, several UCP1-independent thermogenic mechanisms have also been described26.
In addition to developmentally preformed brown adipocytes, mice and humans also have inducible brown adipocytes, referred to as beige or brite adipocytes. These multilocular fat cells come from a distinct developmental lineage and tend to be interspersed within WAT, but also express UCP127 (Figure 3). At baseline or during thermoneutrality, beige adipocytes display a more white-like phenotype with large lipid droplets and low expression of thermogenic genes28, but activation by cold exposure, beta-adrenergic stimulation or exercise results in the robust upregulation of a thermogenic program in a process commonly called “browning.” While these cold-inducible brown-like adipocytes were first described almost 40 years ago28–32, their developmental origin, molecular properties, and physiological roles have only more recently been investigated. In mice, beige adipocytes are enriched within subcutaneous fat depots, and are rarely detected in visceral depots. Intriguingly, due to their temperature dependent epigenomic plasticity, beige adipocytes also have the capacity to “whiten” in a warm environment33.
In light of their morphological and functional differences, it is not surprising that white and thermogenic brown/beige adipocytes are derived from distinct precursors31,34–37. White adipocytes arise from mural precursors that are CD24, CD3438 and PDGFRα positive12,39, and subcutaneous and visceral white adipocytes appear to originate from distinct progenitor populations40. Developmentally preformed or classical brown fat is derived from a myogenic precursor expressing Pax7, Engrailed-1 and Myf5 around embryonic days 9.5–11.5 in mice, even before white adipocytes develop35,37,41. Beige adipocytes, in contrast, originate from a vascular smooth muscle lineage42. Despite their distinct origins, the development of both brown and beige adipocytes is dependent on the transcriptional coregulatory protein PRDM16. Adult humans also have inducible thermogenic adipocytes, and evidence suggests that these cells share properties with both murine brown and beige adipocytes23,27,29,36,43. The relative proportion of brown vs. beige adipocytes in different human depots in various contexts remains to be fully clarified44.
Stromal cell composition of AT and impact on physiology
Although adipocytes account for most of the volume of AT, they only make up about 50% of the cellular content45,46. Other cell types include immune cells such as macrophages47–49, lymphocytes50–53, eosinophils54,55 and mast cells49, as well as fibroblasts, adipocyte precursors, vascular cells45, multipotent mesenchymal stem-like cells56 and nerve processes57,58. Visceral AT, in contrast to subcutaneous AT, tends to have a higher content of macrophages49, regulatory T cells52, natural killer T cells51 and eosinophils54. Further, visceral and subcutaneous AT display differences in angiogenesis59–63 and sympathetic innervation58,64,65, which can modulate the propensity for energy storage vs. dissipation. Finally, changes in macrophages66, eosinophils66–68 and group two innate lymphoid cells (ILC2)69 can regulate the browning of AT.
Perivascular and epicardial adipose tissue
In addition to the well-described white and brown adipose depots, AT is also located around most large blood vessels including the aorta and mesenteric vessels, but not the pulmonary and brain vasculature or the microcirculation70 (Figure 4). Perivascular adipose tissue (PVAT) is a specialized local deposit of adipose tissue surrounding blood vessels that also provides mechanical protection and regulation of blood vessel tone71–73. Ex vivo aortic ring experiments revealed a role for PVAT in relaxation after stretch-mediated stress in mesenteric arteries and the thoracic aorta of rats74. The contractile response of isolated murine mesenteric arteries towards norepinephrine is significantly reduced in the presence of PVAT75. Further, electrical field stimulation assays of mesenteric arteries demonstrated a role for sympathetic nerve activation76 and sensory neurons77 in the vasodilatory effects of PVAT. The anti-contractile effects of sympathetic stimulation are mediated by the stimulation of β3-adrenoreceptors in PVAT, and treatment with an antagonist of β3-adrenoreceptors reduces these effects76.
Interestingly, PVAT is itself heterogeneous, with its phenotype strongly location-dependent78–80. Due to its close proximity to the vasculature and direct contact with the adventitia81, PVAT is thought to play a role in vascular function and pathology. PVAT surrounding the abdominal aorta and the mesenteric arteries displays a mostly white phenotype in humans82 and mice, with almost no UCP1 expressing thermogenic adipocytes28. On the other hand, rodent PVAT surrounding the thoracic aorta has a brown-like phenotype with multilocular adipocytes and UCP1 expression similar to classical brown adipocytes83–86. This is supported by patterns of BAT detected by positron emission tomography – computed tomography (PET-CT) in the para-aortic area and around the heart of humans87. In addition, autopsy studies of Siberian adults revealed clear UCP1 expression and multilocular and paucilocular appearance of about 40% of mediastinal periaortic vascular AT, with some individuals displaying up to 73%88. Long-term moderate cold exposure (16°C) of mice results in further browning of thoracic PVAT with a markedly increased expression of Ucp1 and Pgc1α and β84.
Thermogenesis of PVAT through cold exposure or genetic manipulation in mice supports a protective role of thoracic PVAT in inflammation and atherosclerosis. Overexpression of the mitochondrial membrane protein MitoNEET induces browning of WAT and thermogenic gene expression89,90. Ucp1-driven overexpression of MitoNEET in BAT and PVAT prevented mice from an intravascular temperature drop during cold exposure and increased energy expenditure even after removal of interscapular BAT90. Further, cold exposure of atherosclerosis-prone ApoE-deficient or ApoE-MitoNEET double deficient mice with removed interscapular BAT resulted in reduced atherosclerotic lesion sizes84,90. Likewise, lack of PVAT in ApoE-deficient mice with an additional smooth muscle-specific deletion of PPARy (peroxisome proliferator-activated receptor y) had increased atherosclerotic lesions and cold exposure had no protective effect84. Although, the potential contribution of cold-induced browning of WAT was not excluded, these studies imply a contribution of PVAT to whole-body thermogenesis and protection from atherosclerosis.
Several studies in humans have examined the phenotype of perivascular fat surrounding the internal thoracic arteries. While human internal thoracic artery PVAT has been reported to have a white phenotype in one study, importantly 84% of the individuals were overweight or obese, which might affect the appearance of AT91. Nevertheless, PVAT of human internal thoracic arteries attenuated the contractile response to the thromboxane A2/prostaglandin H2 receptor agonist U46619 and phenylephrine91. Similar effects were observed in PVAT stripped arteries through the transfer of PVAT-incubated supernatant91. Detailed analysis of human thoracic PVAT is limited due to difficulties with sample acquisition and is often isolated from patients with underlying cardiovascular complications, complicating phenotypic assessment.
Despite the close morphological relationship between BAT and tPVAT in mice, proteomics data revealed a depot specific clustering and an only 43% overlap of their proteome on a standard diet92. This is comparable to the overlap of 44% of detected proteins between tPVAT with visceral WAT or the overlap of 53% between visceral WAT and BAT, two very distinct depots with different functions92 suggesting a potentially unique PVAT composition. Interestingly, PVAT has been shown to regulate vascular tone83,93 through contact dependent and paracrine functions that are impaired during obesity in mice and humans91,94,95. The contractile response of mesenteric arteries to norepinephrine, for example, is reduced in the presence of PVAT but compromised in diet-induced obesity95. Further, the expression of vasodilatory factors, such as angiotensin (1–7)96–98, adiponectin75,76 and nitric oxide99 is inhibited during obesity94,95,99,100, and the expression of the vasoconstrictor angiotensin II is induced in PVAT70. Finally, a recent single cell RNA sequencing study demonstrated the existence of two main clusters of mesenchymal stem/stromal cells in PVAT of the thoracic aorta of mice101. One of the clusters was associated with angiogenic and adipogenic potential, whereas the other cluster was enriched for genes associated with vascular smooth muscle cell differentiation101. Transplantation of those PVAT-derived mesenchymal stem/stromal cells to a vein graft model significantly promoted neointima formation demonstrating a possible role of PVAT in vascular remodeling101.
PVAT is an important contributing factor to hypertension18,19, endothelial dysfunction102 and other vascular abnormalities in obesity71–73,94,103,104 (Figure 5). PVAT normally releases vasodilatory mediators, including adiponectin75,76, and yet to be fully characterized molecules often acting on K+ channels, that exert an anti-contractile activity and promote vascular relaxation70. However, in the setting of obesity and insulin resistance, oxidative stress and inflammation are increased in PVAT, thereby resulting in an increase in pro-inflammatory adipokines including tumor necrosis factor alpha (TNF-α), and interleukins (IL-6 and IL-8), leading to vascular insulin resistance, impaired relaxation, and vascular stiffness71. Il-6 and TNF-α also attenuate the vasodilation of mesenteric arteries ex vivo94. Other cytokines such as interleukin-18 (IL-18) are thought to have protective effects on PVAT and vascular function, and loss of IL-18 results in elevated blood pressure in mice associated with the whitening of thoracic PVAT105. However, the specific impact of IL-18 in PVAT needs to be addressed in AT-specific conditional knock out animals. The Framingham Offspring and Third Generation cohort studies showed that increased PVAT volume is associated with higher thoracic and abdominal aortic dimensions and increased arterial stiffness, even after adjusting for age and CVD risk factors including BMI and visceral AT volume104.
Figure 5. Effects of obesity on the vasculature which promote dysfunctional remodeling and stiffness of the vasculature.
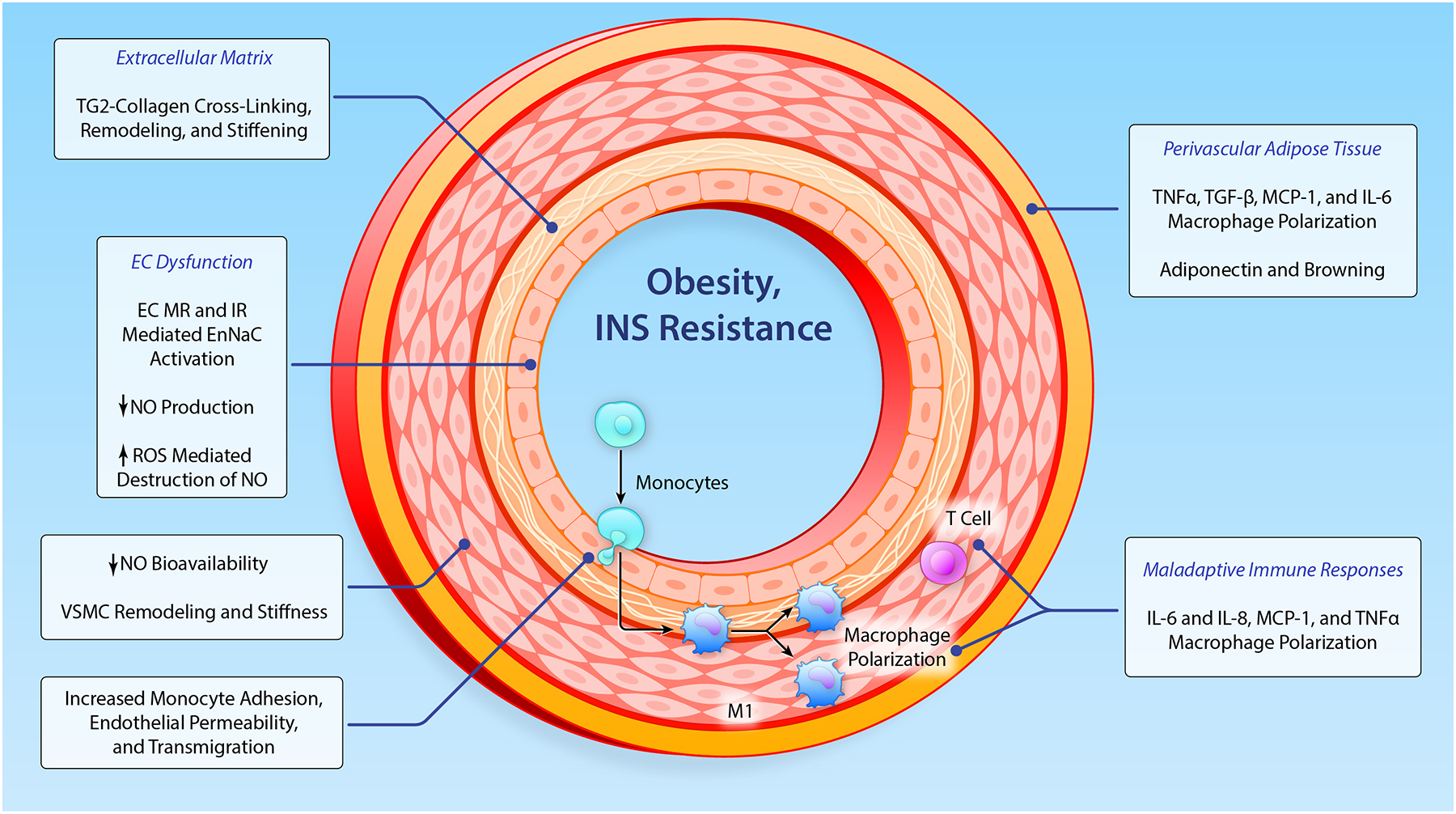
EC, endothelial cell; VSMC, vascular smooth muscle cell; MMPs, matrix metalloproteinase; TG2, tissue transglutaminase; Ang II, angiotensin II; MR, mineralocorticoid receptor; TxA2, thromboxane A2; ENaC, epithelial Na+ channel; IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide; MCP-1, monocyte chemotactic protein-1; CRP, C- reactive protein; TGF-β, transforming growth factor-β. (Illustration credit: Ben Smith)
The heart is also directly associated with specific AT depots. Epicardial AT is located on the surface of the myocardium in direct contact with the coronary arteries, and pericardial AT is in contact with the pericardial sac106. Under physiological conditions, epicardial AT may supply energetic substrates to the heart and has a greater capacity for free fatty acid turnover than other visceral AT depots107. Although its cold-induced UCP1 expression does not reach levels of classical BAT28, human epicardial AT has a thermogenic phenotype and has been suggested to regulate the temperature of the myocardium108. Other studies described the portion of epicardial AT surrounding the coronary arteries in humans as a white-like depot despite the expression of some classical brown fat marker genes such as UCP1, PRDM16 and CPT1β109. The same study found a lower expression of adipogenic marker genes PPARγ, FABP4 and C/EBPα but an increased expression of pro-inflammatory cytokines compared to subcutaneous AT109. This discrepancy might be explained by the reported whitening of epicardial AT after birth in humans, with only distinct subset of multilocular UCP1 positive cells110. Epicardial AT secretes polypeptides, such as adiponectin111 and adrenomedullin112, which have cardioprotective effects, with low expression of adiponectin in epicardial AT being associated with hypertension113. Healthy epicardial AT accounts for approximately 5–20% of the heart weight114, and the thickness of epicardial AT is increased in hypertensive individuals115–117. Under pathological conditions, epicardial AT becomes infiltrated with immune cells expressing pro-inflammatory genes (IL-1β, Il-6 and TNF-α)118 and can contribute to structural changes in the heart119–121. Studies from epicardial AT derived from coronary artery bypass grafts showed significantly lower adiponectin expression compared to other visceral adipose depots and a marked increase in CD45 expression, suggesting increased immune cell infiltration compared to omental AT122. Studies of mild cold exposure in humans and the analysis of epicardial AT could be beneficial to understanding the role of epicardial AT thermogenesis for CVD110. Since mice do not have a comparable epicardial AT depot, a mechanistic understanding of how epicardial AT contributes to blood pressure modulation is lacking.
III. Cardiovascular consequences of obesity and adipose tissue dysfunction
Impact of AT on blood pressure regulation
One of the central modes of blood pressure regulation is via the renin-angiotensin-aldosterone system (RAAS). The major bioactive component angiotensin II is produced from its precursor angiotensinogen by the activation of angiotensin-converting enzymes 1 and 2. Angiotensin-converting enzyme 2 can further process angiotensin II to generate angiotensin 1–7, which has vasodilatory properties96–98. Angiotensin II83 and aldosterone are also secreted by adipocytes and can directly activate vascular smooth muscle cells (VSMC) via the angiotensin type 1 receptor123. Angiotensin II is a prominent regulator of vascular tone,124 and its expression is spatially regulated in PVAT, with higher expression in mesenteric PVAT compared to thoracic PVAT83. Interestingly, studies in rats have demonstrated that fasting reduces angiotensinogen expression in visceral AT, whereas refeeding significantly induces its expression and results in elevated blood pressure125. A similar effect can be observed by overexpression of angiotensinogen in mice, which also results in hypertension126.
All of the components of the RAAS are also secreted by human WAT127. However, there are conflicting data as to whether the basal expression of RAAS components differ in visceral and subcutaneous AT in lean individuals. One study reported a higher general expression of angiotensinogen, the precursor of angiotensin II, in visceral AT compared to subcutaneous AT128. A more recent, larger study, however, reported no changes in angiotensinogen expression between the two depots in lean individuals129. Nevertheless, visceral AT expressed higher amounts of renin, angiotensin-converting enzyme 2 and both angiotensin receptor types 1 and 2 in the same study, whereas ACE1 was not changed129. In rats, mesenteric PVAT expresses higher levels of angiotensin II and both angiotensin receptor subtypes than thermogenic thoracic PVAT83. This is in line with the reported downregulation of angiotensinogen after beta-adrenergic stimulation of murine adipocytes in vitro130.
Thermogenic brown and beige AT is considered to have protective effects on the vasculature, as individuals with detectable thermogenic AT have lower odds for hypertension and coronary artery disease relative to individuals without thermogenic AT24. Moreover, coding variants in PRDM16, the master regulator of thermogenic AT, are associated with hypertension in humans131. Interestingly, components of the RAAS cascade can directly affect AT, and angiotensin 1–7, besides its vasodilatory actions96–98 also induces BAT and reduces diet-induced obesity in mice132,133. Surprisingly, pharmacological activation of angiotensin receptor 2 and angiotensin II treatment can induce browning of subcutaneous white adipocytes in vivo and stimulation of brown precursor differentiation in vitro134,135. This protective impact on BAT is assumed to be either mediated by increased sympathetic nerve activation135 or through increased conversion of angiotensin II to angiotensin 1–7. Moreover, deletion of the type 1 angiotensin receptor results in increased appearance of multilocular beige adipocytes136. Taken together, it appears that angiotensin 1–7 and activation of the angiotensin receptor 2 or inhibition of the type 1 angiotensin receptor can stimulate BAT, which in turn has beneficial effects on blood pressure and attenuates development of CVD. Further studies will be needed to investigate the direct impact and molecular basis of the protective impact of thermogenic AT on hypertension.
Adipose tissue remodeling during obesity
Obesity results in a chronic low-grade inflammatory state in adipose tissue137,138. Visceral obesity in particular, is strongly associated with the development of CVD13,14. Defining and understanding remodeling of different AT depots during obesity is thus of utmost importance to ultimately preventing deleterious sequelae. During obesity, AT can expand by either enlargement of existing adipocytes (hypertrophy) or by increasing the number of adipocytes (hyperplasia) (Figure 3), with the relative importance of either mechanism varying based on depot, sex and age31. At baseline, fed a standard diet, neither visceral nor subcutaneous AT exhibit significant new adipogenesis in adult humans or mice31,139. Long-term high fat feeding of mice, on the other hand, resulted in increased adipogenesis and hypertrophy in the visceral AT, including mesenteric PVAT, whereas subcutaneous AT adapts to the higher energy intake by hypertrophy31. The individual impact of hypertrophy versus hyperplasia in the development of the metabolic syndrome is still under debate140. Maximum hypertrophy in adipocytes in established obese conditions can result in the exhaustion of the lipid storing capacity in adipocytes, which in turn can induce ectopic storage of fat in other organs such as the liver, supporting the development of the metabolic syndrom141. On the other hand, visceral AT is more susceptible to AT inflammation, which in turn contributes to metabolic and CVD outcomes142. Sex-dependent differences in AT distribution have been reviewed elsewhere143–145, but in short, females most often accumulate AT in the subcutaneous depot, whereas men and post-menopausal women tend to accumulate AT in central visceral depots143. Hormone replacement therapy in postmenopausal women prevents this central AT distribution146, highlighting the role of sex hormones in fat distribution. However, recent studies, using an elegant separation of gonadal sex and sex chromosomes demonstrated that the XX chromosomal sex results in increased weight gain independent of the gonadal sex147,148. This was mediated through the X-chromosome-escaped dose-dependent expression differences of the histone demethylase KDM5C in females compared to males, and lowering KDM5C levels in females to the same extend seen in males resulted in weight loss and body fat content148.
In obesity, the immune cell composition of different AT depots demonstrates dynamic changes70,142,149 (Figure 4). For example, adipose tissue macrophages increase in obesity and their ablation improves insulin sensitivity and reduces inflammation47,150–152. The recruitment47 and proliferation153 of pro-inflammatory macrophages during obesity is greater in visceral than in subcutaneous AT154,155. Obesity further results in the loss of protective CD4 helper156 and regulatory T cells (Tregs)52,157 and in the enrichment of CD8 T cells in visceral AT53. These variations in immune cell infiltration between visceral and subcutaneous AT results in a low-grade inflammatory environment that can contribute to CVD158,159. Recently, eosinophils have gained attention for their role in promoting beige adipocyte activation67,68, and their loss during obesity, especially in visceral and mesenteric AT, renders mice susceptible to diet-induced obesity54 and abolishes the anti-contractile effect of PVAT to norepinephrine95. However, some of these findings require further clarification and together with detailed information on PVAT immune cell content and changes during obesity are discussed elsewhere70.
Thermogenic brown and beige fat, on the other hand, have anti-obesity effects in humans160,161, and depletion of UCP1 itself or ablation of UCP1 expressing thermogenic AT results in weight gain162,163. In contrast to WAT, classical BAT of obese mice expresses lower levels of genes associated with immune cells, suggesting that thermogenic AT is resistant to diet-induced inflammation86. However, other studies have shown that macrophages164 and B lymphocytes165 infiltrate thermogenic AT during obesity, and together with increased inflammatory cytokines109 are thought to suppress UCP1 expression in brown adipocytes164. Further, mice fed a HFD for 12 weeks, show reduced expression of some thermogenic marker genes, and adipocytes shifted from a multilocular to an unilocular appearance with increased lipid accumulation in BAT and thoracic PVAT92. The increased body and PVAT weight also impair anti-contractile effects of PVAT91. High fat feeding further results in a tPVAT-specific upregulation of Notch1 compared to WAT or BAT92. Genetic adipocyte-specific induction of Notch1 resulted in morphological changes of tPVAT comparable to HFD induced effects92. This is supported by another study showing that adipocyte-specific overexpression of Notch1 impairs thermogenesis and insulin sensitivity and results in whitening of classical BAT, whereas pharmacological inhibition of Notch1 results in browning of WAT and ameliorates HFD-induced obesity166.
Remodeling of AT during obesity and its impact on blood pressure homeostasis
Obesity is strongly associated with the development of hypertension13, a major risk factor for CVD morbidity and mortality167,168. Compared to normal weight individuals, obese individuals also carry a greater risk for coronary artery calcification, carotid artery intimal media thickening and left ventricular hypertrophy, even after adjustment for traditional CVD risk factors169. Weight reduction significantly improves blood pressure19,170,171, and therefore, suggests a direct link between AT phenotype and odds of developing CVD and hypertension. Visceral obesity in rodents and humans is particularly associated with the metabolic syndrome172, which consists of several risk factors for CVD, including hypertension173. On the other hand, humans with thermogenic AT have lower odds for hypertension, coronary artery disease and congestive heart failure, even when obese24.
Angiotensinogen expression is significantly elevated in obese individuals and is also higher in visceral AT compared to subcutaneous AT128,174,175 (Figure 4). Interestingly, expression of angiotensin II is increased in subcutaneous AT in obese individuals with hypertension compared to normotensive obese individuals128. Diet-induced obesity did not affect angiotensinogen levels in BAT, liver, kidney or heart in wild-type mice or in mice expressing the human angiotensinogen gene under its own promoter175. Importantly, adipocyte-specific deletion of angiotensinogen prevents increased angiotensin II in the circulation and blocks elevation of BP in obese mice176, suggesting a direct impact of AT-derived angiotensinogen on blood pressure. Moreover, angiotensin receptor type 1 inhibition reverses obesity-induced blood pressure elevation in rats177. Finally, angiotensinogen levels are negatively regulated by PRDM16, and deletion of PRDM16 and ablation of beige adipocytes results in increased angiotensinogen expression178,179. Ablation of BAT in mice results in obesity as well as elevated blood pressure180; however, whether this is a consequence of obesity induced changes in RAAS or can be directly linked to factors secreted by brown AT needs to be further determined. Aldosterone, another component of the RAAS secreted by adipocytes123, also positively correlates with BMI, and weight loss reduces serum aldosterone levels and reduces hypertension181. Components of the RAAS can therefore affect VSMC and endothelial dependent regulation of vascular tone, both of which are adversely affected during obesity.
Leptin, an adipocyte-derived hormone that regulates food intake and energy expenditure, is significantly increased in obesity in mice and humans182,183 (Figure 4). In contrast to angiotensinogen, it may be expressed at higher levels in subcutaneous than in visceral AT184–186, and its expression is correlated with adipocyte size185. Nevertheless, diet-induced obesity results in elevated leptin levels and attendant increases in heart rate and blood pressure in rodents92,187,188. This induction is mediated by a leptin-stimulated increase in sympathetic nerve activity189,190, and antibody blockade of leptin or inhibition of leptin receptors on hypothalamic neurons normalized blood pressure in obese rodents187. Finally, leptin deficient mice191 and humans with loss of function mutations in leptin or the leptin receptor have lower blood pressure despite severe obesity187. It is not well understood how the chronic increase of leptin in obese subjects results in leptin-resistance192 and whether this affects blood pressure. Based on the above-mentioned data, reduced leptin signaling ameliorates blood pressure in mice, and therefore, leptin-resistant obesity should be beneficial in regard to blood pressure. Indeed, leptin also has some vasodilatory effects in healthy rodents, via induction of nitric oxide expression in endothelial cells77,193 and in healthy humans by a mechanism independent of nitric oxide194. Further, leptin resistance was demonstrated to selectively affect neurons in the hypothalamus that regulate food intake, while affecting other neuronal circuits to a lesser extent195,196, which could explain how obese individuals do not have beneficial effects on blood pressure when leptin resistant. In detail, agouti obese mice were resistant to food intake and body weight effects of systemic leptin administration, but had a preserved induction of leptin-induced renal sympathetic activation196,197. Similar results in diet-induced obese mice showed the preservation of leptin-induced renal sympathetic activation and blood pressure regulation despite the resistance to weight-reducing actions of leptin188.
Resistin is enriched in visceral AT198, including epicardial AT199 and PVAT200, and is markedly increased during obesity200,201. Resistin has an important role in type 2 diabetes and insulin resistance in mice201. In humans with type 2 diabetes, resistin expression was only elevated in combination with hypertension and not in patients without hypertension202. In hypertensive patients without type two diabetes, resistin levels did not correlate with blood pressure indicating a more complex connection of obesity, insulin resistance and blood pressure regulation by resistin. In mice, resistin treatment induced hypertension through the induction of angiotensinogen203. Finally, resistin treatment of isolated human VSMC similar to angiotensin, resulted in increased proliferation204.
Visfatin is also expressed in visceral AT, including PVAT200, and increased through hypoxia induced expression of HIF1α205 in obesity200. Hypertensive patients have elevated serum visfatin levels206 however, newly diagnosed, non-obese hypertensive men did not show any association of plasma visfatin levels and hypertension207. Importantly, visfatin is mostly enriched in adipose tissue macrophages in mice200 and humans,208 and therefore, its role in adipocyte specific regulation of blood pressure might be a secondary cause of increased immune cell infiltration in obesity. Nevertheless, it was shown that hypoxic conditions can induce visfatin in murine adipocyte cell lines and its adipocyte specific role in blood pressure regulation should be determined by adipocyte-specific deletion of visfatin.
Adiponectin is another endocrine factor secreted by AT that tends to be reduced during obesity209,210 (Figure 4). In humans, visceral adiposity inversely correlates with adiponectin secretion, whereas secretion of adiponectin by subcutaneous AT is not affected by adiposity209. Serum adiponectin levels are reduced in obese individuals with hypertension211, and lifestyle intervention212 or anti-hypertensive therapy211 resulted in increased adiponectin levels and improved blood pressure212. In addition, lower adiponectin levels correlate with the risk for development of hypertension in humans213,214, independent of body fat distribution215. Mice on a standard diet that lack adiponectin display elevated blood pressure despite similar body weight76, whereas adiponectin overexpression in obese mice ameliorates elevated blood pressure210. To understand the direct impact of adiponectin without secondary metabolic effects such as insulin resistance, mice lacking adiponectin were fed a high salt diet. These mice developed hypertension, which could be rescued by adiponectin administration210. The observed elevation in blood pressure was associated with reduced endothelial eNOS and prostaglandin I2 synthase210, indicating a role for adiponectin in endothelial cell mediated vasodilation216. Further, ex vivo stimulation of murine mesenteric arteries with norepinephrine was significantly reduced in the presence of PVAT or PVAT-derived supernatant and could be blocked by adiponectin blocking peptide or in vessels derived from adiponectin-deficient mice95. Adiponectin blocking peptide also blocked electrical field stimulation of mesenteric arteries depending on the presence of PVAT76. Adiponectin treatment of isolated mesenteric arteries stripped of PVAT restores the anti-contractile effects75,76, depending on the vascular large-conductance Ca2+-activated K+ channel on VSMC75. Finally, AMPKα1-deficient mice secrete less adiponectin, and ex vivo stimulation of thoracic aortic rings from these mice displayed an impaired vasodilatory effect of PVAT after U46619 treatment217.
Another factor enriched in human omental AT and detected in human serum is omentin218. Like adiponectin, it is reduced in obese conditions219 and induced through weight reduction220. In rats, omentin treatment ameliorates angiotensin II or noradrenalin-induced hypertension and reduces blood pressure in normotensive rats221,222. Interestingly, omentin suppressed inflammatory mediators in various vascular cell types222–224 and induced adiponectin levels, which might result in the indirect regulation of blood pressure. This is also the case for adipolin225, which is reduced in obese mice226 and has a protective role in vascular remodeling through the inhibition of VSMC proliferation and macrophage activation227, and although associated with protective effects on CVD, its role in regulation of blood pressure needs to be further determined.
Several other factors secreted by different adipose tissue depots have been associated with a role in blood pressure regulation; however, functional and mechanistical proof is still sparse and will be required to understand the independent impact of those AT-derived mediators in the regulation of hypertension. Interleukin-33 (IL-33), for example, plays a pivotal role in the activation of eosinophils, and genetic loss or obesity-induced reduction of eosinophils in PVAT results in a reduced anti-contractile response95. Further, activation of eosinophils by IL-33 treatment rescues obesity-induced high blood pressure to the level of control mice, dependent on an endothelial cell and nitric oxide synthase-mediated effect228. Of note, patients with pulmonary hypertension showed elevated IL-33 levels229, and deficiency of the IL-33 receptor attenuates the progression of pulmonary arterial hypertension in mice230. Therefore IL-33 could play a differential role in blood pressure regulation of vasculature with and without PVAT.
Vascular stiffening and CVD risk
While vascular stiffening is a normal phenomenon with increasing age, obesity and associated insulin resistance accelerates this process. To this point, a population study showed that skin-fold thickness is a predictor of arterial stiffness in hypertensive patients231. Another study found an association between abdominal obesity and increased vascular stiffness232,233. Epidemiological studies have demonstrated that hyperinsulinemia or insulin resistance, as exists in overweight and obese individuals, is an independent risk factor for vascular stiffening. This vascular stiffening in association with obesity and insulin resistance has been observed in all age groups, including children234,235.
There is considerable evidence that the vascular stiffening that is increased in obesity is a powerful risk factor for CVD. Data from the Framingham Heart Study have established an increased incidence of CVD events with increasing weight in both men and women8, and CVD has been strongly associated with vascular stiffness235,236. Importantly, arterial stiffening is especially striking in obese and diabetic premenopausal females who tend to lose the normal protection afforded by female sex hormones against vascular disease and show an increase in CVD events relative to lean, non-diabetic, age-matched women237. Indeed, vascular stiffness independently predicts heart disease, cerebrovascular disease and renal disease, as increased vascular stiffness is significantly associated with damage to target organs such as the heart, kidney, and brain238. For example, stiffening of central arteries increases systolic pressure and decreases diastolic pressure, resulting in increased pulse pressure and afterload leading to an increase in left ventricular mass and myocardial oxygen demand. Further, the decrease in diastolic pressure is associated with reduced coronary blood flow during diastole. These changes have been consistently associated with left ventricular remodeling and fibrosis together with left ventricular diastolic dysfunction and associated heart failure with preserved systolic function (HFpEF)239,240 (Figure 5). While early detection of arterial stiffening in obese individuals certainly helps to identify a powerful risk factor for CVD, definitive studies on the impact of weight loss on reversal of vascular stiffness have yet to be conducted.
Mechanisms in CV stiffness with Obesity
Development of arterial stiffness is a complex process that is driven by the interaction of endocrine factors and AT-derived cytokines, as well as interactions between different vascular cellular components, the extracellular matrix (ECM), PVAT, and immune cells in the vasculature6,94. The paragraphs that follow focus on mechanisms involved in CV stiffness in conditions of overnutrition and obesity. This includes a discussion of the role of vascular endothelial abnormalities which lead to impaired endothelial nitric oxide (NO) synthase (eNOS) activation and associated increases in vascular stiffness. We also discuss the emerging role of vascular cell-specific mineralocorticoid (MR) and insulin receptor (IR) activation in promoting endothelial stiffness via endothelial Na+ channel (EnNaC) activation, and the impact of a decrease in bioavailable NO in mediating vascular stiffness in diet induced obesity (Figure 5).
Arterial stiffness in obesity is associated with structural and functional changes in the intimal, medial, and adventitial layers of the vasculature241. Arterial stiffness is regulated by plasma factors such as aldosterone and insulin, as well as factors derived from the different layers of the vascular wall. Moreover, interactive signaling between different cells of the vascular wall modulates structure and function of cellular and non-cellular components. Increased arterial stiffness in obese and insulin resistant states has been related to mechanisms related to both endothelial cell (EC) and VSMC stiffness, leading to the use of such terms as the “stiff endothelial cell syndrome”241–243 and the “smooth muscle stiffness syndrome”242. In addition to the role of ECs and VSMCs, vascular adipose and immune cell dysfunction and ECM remodeling contribute to obesity-associated arterial stiffness. This underscores the importance of understanding the complex cellular and ECM interactions that contribute to obesity-associated arterial stiffness243,244.
Increased plasma insulin and aldosterone levels lead to heightened activation of vascular MRs and IRs in obesity and insulin resistance states239–243. Further, a downstream mediator of MR and IR activation, the ion channel EnNaC, has recently been identified as a key molecular determinant of endothelial dysfunction and CV fibrosis and stiffening239,243. Increased activity of EnNaC results in a number of negative consequences including stiffening of the cortical actin cytoskeleton in ECs, impaired endothelial nitric oxide (NO) release, increased oxidative stress meditated NO destruction, increased vascular permeability and stimulation of an inflammatory environment. Such endothelial alterations impact vascular function and stiffening through increases in vascular constriction and stimulation of tissue remodeling including fibrosis. In the case of the myocardium, obesity and associated elevations in aldosterone and insulin are associated with coronary vascular endothelial stiffening and related reductions in bioavailable NO leading to heart failure with preserved systolic function (HFpEF).
Recent studies, conducted in female mice fed a diet high in refined carbohydrates and saturated fat showed increased endothelial and aortic stiffness, impaired endothelial-dependent vasorelaxation, aortic fibrosis, aortic oxidative stress and increased vascular expression of EnNaC239–241. To gain further insight into the vascular role played by EnNaC, we have characterized a mouse model with endothelial cell-specific deletion of the α, pore-forming, subunit of EnNaC241. Obesogenic diet induced abnormalities, along with vascular and cardiac remodeling and fibrosis, were all significantly attenuated in mice with deletion of EnNaC241–243. From a mechanistic standpoint, these studies showed that diet induced obesity resulted in a heightened inflammatory response that was associated with reduced endothelial NO synthase (eNOS) activation and NO production and bioavailability. These latter events likely emanated from increased EnNaC activity leading to polymerization of cortical actin fibers, subsequently reducing eNOS activity, and decreasing NO production leading to increased vascular stiffness (Figure 5). This research has further revealed that activation of the endothelial Na+ channel by aldosterone and insulin leads to endothelial cortical stiffening, impaired NO production and subsequent vascular fibrosis and stiffening in diet induced obesity244,245. Additionally, these observations in this obese mouse model also suggest that activation of the endothelial Na+ channel in the coronary vasculature promotes myocardial fibrosis, myocardial stiffening and impaired diastolic relaxation and HFpEF, a condition that is especially pronounced in obese and insulin resistant females.
Studies performed in epithelial cells have shown that both aldosterone and insulin increase ENaC activity via activation of the ubiquitously expressed serum and glucocorticoid regulated kinase 1 (SGK-1)246. Very recent work has shown that SGK-1 represents a point of convergence for insulin and aldosterone signaling in endothelial cells244. Consistent with this notion, our preliminary studies have shown that aldosterone and insulin induced increases in EnNaC activity are diminished in isolated ECs from SGK-1 global knock-out mice compared to those of wild-type controls244. It is also of relevance that evidence exists in humans for SGK-1 playing an important integrative role in the development of the cardiometabolic syndrome. Specifically, an SGK-1 gain of function gene variant that exists in 5 percent of the population is associated with increased blood pressure and obesity247 and has a particularly strong effect in increasing blood pressure in states of hyperinsulinemia and obesity247. Further, in rodent models, hyperinsulinism sensitizes the blood pressure to high fructose and salt intake, an effect involving increased activity of SGK-1248. Indeed, SGK-1-knockout mice are protected against salt-induced hypertension in the context of obesity caused by a high-fat and high-fructose diet248. Finally, increased SGK-1 activity in obesity and hypertension has also been demonstrated in adipocytes249 and immune cells250. Thus, multiple lines of evidence point towards important contributions of SGK-1 signaling in promoting the cardiometabolic syndrome, vascular stiffness and associated CVD in obesity.
In summary, obesity is increasing in prevalence and these increases in obesity are associated with increased consumption of refined carbohydrates and saturated fat and reduced physical activity. These and other environmental factors interact with genetic and epigenetic factors to promote obesity and related CVD (Figure 1). Obesity also negates the CVD protection normally afforded in premenopausal women. The earliest sign of obesity related CVD is impaired NO mediated relaxation which leads to CV stiffness. Recent studies indicate that insulin and mineralocorticoid receptor activation of the EnNaC is important in the pathogenesis of CV stiffness, especially in obese females who lose the protection against CVD normally afforded in premenstrual women.
IV. Unanswered questions and future directions
While recent research has highlighted key links between obesity, adipose tissue, and vascular function, a number of important unanswered questions remain. From a basic standpoint, a more complete understanding of the developmental origin and cellular and molecular components of perivascular fat is necessary. Moreover, a comprehensive inventory of the secreted polypeptides and metabolites released by adipose tissues in normal physiology and the obese state will help further illuminate how excess adiposity contributes generally to vascular dysfunction and more specifically to the pathogenesis of hypertension and vascular stiffening. Future studies will also need to uncover the role of environment, genetics, epigenetics, and the microbiome on modulating the interactions between adipose tissues and the vasculature.
Financial support for the author(s):
M.K. was supported by the Women & Science Initiative at Rockefeller University; P.C. was supported by the Sinsheimer Foundation; M.A.H. and J.R.S. were supported by the NIH.
Non-standard Abbreviations and Acronyms
- AMPKα1
5’AMP-activated protein kinase catalytic subunit alpha 1
- ANG 1–7
Angiotensin 1–7
- ANG II
Angiotensin II
- ApoE
Apolipoprotein E
- AT
Adipose tissue
- ATP
Adenosine triphosphate
- BAT
Brown adipose tissue
- BMI
Body mass index
- CD
Cluster of differentiation
- C/EBPα
CCAAT-enhancer binding protein alpha
- CVD
Cardiovascular disease
- EC
Endothelial cells
- ECM
Extracellular matrix
- EnNaC
Endothelial Na2+ channel
- eNOS
Endothelial nitric oxide synthase
- FABP4
Fatty acid-binding protein
- HFD
High fat diet
- HFpEF
Heart failure with preserved ejection fraction
- HIF1α
Hypoxia-inducible factor 1 alpha
- IL
Interleukin
- ILC2
Group 2 innate lymphoid cells
- IR
Insulin receptor
- KDM5C
Lysine-specific demethylase 5C
- MR
Mineralocorticoid receptor
- Myf5
Myogenic factor 5
- NO
Nitric oxide
- Pax7
Paired box 7
- PET-CT
Positron emission tomography-computed tomography
- PDGFRα
Platelet-derived growth factor receptor alpha
- PGC1α
Pparg coactivator 1 alpha
- PRDM16
PR domain containing 16
- PVAT
Perivascular adipose tissue
- RAAS
Renin-Angiotensin-Aldosterone-System
- SGK-1
Serum/Glucocorticoid regulated kinase 1
- TNFα
Tumor necrosis factor alpha
- Treg
Regulatory T cells
- UCP1
Uncoupling protein 1
- US
United States
- VSMC
Vascular smooth muscle cell
- WAT
White adipose tissue
Footnotes
Disclosures:
None of the authors have any financial, personal or professional relationships with other people or organizations that could reasonably be perceived as conflicts of interest or as potentially influencing or biasing this work.
References
- 1.GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377:80–81. [DOI] [PubMed] [Google Scholar]
- 3.Obesity and overweight. Accessed January 11, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 4.Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. New England Journal of Medicine. 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 5.Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity. 2008;32:1431–1437. [DOI] [PubMed] [Google Scholar]
- 6.Guanghong Jia, Hill Michael A., Sowers James R. Maternal Exposure to High Fructose and Offspring Health. Hypertension. 2019;74:499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. [DOI] [PubMed] [Google Scholar]
- 8.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 9.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juonala M, Magnussen CG, Berenson GS, et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. New England Journal of Medicine. 2011;365:1876–1885. [DOI] [PubMed] [Google Scholar]
- 11.Cote AT, Harris KC, Panagiotopoulos C, Sandor GGS, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. [DOI] [PubMed] [Google Scholar]
- 12.Lee M-J, Wu Y, Fried SK. Adipose Tissue Heterogeneity: Implication of depot differences in adipose tissue for Obesity Complications. Mol Aspects Med. 2013;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: The Framingham offspring study. Preventive Medicine. 1987;16:235–251. [DOI] [PubMed] [Google Scholar]
- 14.Bhupathiraju Shilpa N, Hu Frank B Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circulation Research. 2016;118:1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia G, Martinez-Lemus LA, Sowers JR. Interaction of Adipogenesis and Angiogenesis in Dietary-Induced Obesity. Diabetes. 2015;64:2326–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piché Marie-Eve, Tchernof André, Després Jean-Pierre. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circulation Research. 126:1477–1500. [DOI] [PubMed] [Google Scholar]
- 18.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall John E, do Carmo Jussara M, da Silva Alexandre A, Wang Zhen, Hall Michael E. Obesity-Induced Hypertension. Circulation Research. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen KA, Lidell ME, Orava J, et al. Functional Brown Adipose Tissue in Healthy Adults. New England Journal of Medicine. 2009;360:1518–1525. [DOI] [PubMed] [Google Scholar]
- 21.Leitner BP, Huang S, Brychta RJ, et al. Mapping of human brown adipose tissue in lean and obese young men. PNAS. 2017;114:8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Hao G, Shao M, et al. An Adipose Tissue Atlas: An Image Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018;27:252–262.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidell ME, Betz MJ, Leinhard OD, et al. Evidence for two types of brown adipose tissue in humans. Nature Medicine. 2013;19:631–634. [DOI] [PubMed] [Google Scholar]
- 24.Becher T, Palanisamy S, Kramer DJ, et al. Brown adipose tissue is associated with cardiometabolic health. Nature Medicine. Published online January 4, 2021:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaus S, Casteilla L, Bouillaud F, Ricquier D. The uncoupling protein UCP: a membraneous mitochondrial ion carrier exclusively expressed in brown adipose tissue. Int J Biochem. 1991;23:791–801. [DOI] [PubMed] [Google Scholar]
- 26.Roesler A, Kazak L. UCP1-independent thermogenesis. Biochem J. 2020;477:709–725. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Boström P, Sparks LM, et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. American Journal of Physiology-Endocrinology and Metabolism. 2011;302:E19–E31. [DOI] [PubMed] [Google Scholar]
- 29.Young P, Arch JRS, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Letters. 1984;167:10–14. [DOI] [PubMed] [Google Scholar]
- 30.Orava J, Nuutila P, Lidell ME, et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metabolism. 2011;14:272–279. [DOI] [PubMed] [Google Scholar]
- 31.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013;19:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. [DOI] [PubMed] [Google Scholar]
- 33.Roh HC, Tsai LTY, Shao M, et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metabolism. 2018;27:1121–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulin K, Truel N, André M, et al. Emergence during development of the white-adipocyte cell phenotype is independent of the brown-adipocyte cell phenotype. Biochem J. 2001;356:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue B, Rim J-S, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. [DOI] [PubMed] [Google Scholar]
- 37.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodeheffer MS, Birsoy K, Friedman JM. Identification of White Adipocyte Progenitor Cells In Vivo. Cell. 2008;135:240–249. [DOI] [PubMed] [Google Scholar]
- 39.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gesta S, Blüher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. PNAS. 2006;103:6676–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atit R, Sgaier SK, Mohamed OA, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. [DOI] [PubMed] [Google Scholar]
- 42.Long JZ, Svensson KJ, Tsai L, et al. A Smooth Muscle-Like Origin for Beige Adipocytes. Cell Metabolism. 2014;19:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannon B, Jong JMA de, Fischer AW, Nedergaard J, Petrovic N Human brown adipose tissue: Classical brown rather than brite/beige? Experimental Physiology. 2020;105:1191–1200. [DOI] [PubMed] [Google Scholar]
- 45.Silva KR, Côrtes I, Liechocki S, et al. Characterization of stromal vascular fraction and adipose stem cells from subcutaneous, preperitoneal and visceral morbidly obese human adipose tissue depots. PLOS ONE. 2017;12:e0174115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar RK, Jin Y, Watts SW, Rockwell CE. Naïve, Regulatory, Activated, and Memory Immune Cells Co-exist in PVATs That Are Comparable in Density to Non-PVAT Fats in Health. Front Physiol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altintas MM, Azad A, Nayer B, et al. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 2011;52:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caspar-Bauguil S, Cousin B, Galinier A, et al. Adipose tissues as an ancestral immune organ: Site-specific change in obesity. FEBS Letters. 2005;579:3487–3492. [DOI] [PubMed] [Google Scholar]
- 51.Schipper HS, Rakhshandehroo M, Graaf SFJ van de, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Medicine. 2009;15:930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. [DOI] [PubMed] [Google Scholar]
- 54.Wu D, Molofsky AB, Liang H-E, et al. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011;332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brigger D, Riether C, van Brummelen R, et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nature Metabolism. 2020;2:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. Curr Opin Clin Nutr Metab Care. 2005;8:440–444. [DOI] [PubMed] [Google Scholar]
- 58.Chi J, Wu Z, Choi CHJ, et al. Three-Dimensional Adipose Tissue Imaging Reveals Regional Variation in Beige Fat Biogenesis and PRDM16-Dependent Sympathetic Neurite Density. Cell Metabolism. 2018;27:226–236 [DOI] [PubMed] [Google Scholar]
- 59.Xue Y, Petrovic N, Cao R, et al. Hypoxia-Independent Angiogenesis in Adipose Tissues during Cold Acclimation. Cell Metabolism. 2009;9:99–109. [DOI] [PubMed] [Google Scholar]
- 60.Villaret A, Galitzky J, Decaunes P, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elias I, Franckhauser S, Ferré T, et al. Adipose Tissue Overexpression of Vascular Endothelial Growth Factor Protects Against Diet-Induced Obesity and Insulin Resistance. Diabetes. 2012;61:1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, D’Amore PA. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J. 2013;27:3257–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu I, Aprahamian T, Kikuchi R, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124:2099–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat. 2009;214:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, Loh KH, Wu M, et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583:839–844. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell. 2014;157:1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knights AJ, Vohralik EJ, Houweling PJ, et al. Eosinophil function in adipose tissue is regulated by Krüppel-like factor 3 (KLF3). Nature Communications. 2020;11:2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiological Reviews. 2019;99:1701–1763. [DOI] [PubMed] [Google Scholar]
- 71.Padilla J, Vieira-Potter VJ, Jia G, Sowers JR. Role of perivascular adipose tissue on vascular reactive oxygen species in type 2 diabetes: a give-and-take relationship. Diabetes. 2015;64:1904–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia G, Durante W, Sowers JR. Endothelium-Derived Hyperpolarizing Factors: A Potential Therapeutic Target for Vascular Dysfunction in Obesity and Insulin Resistance. Diabetes. 2016;65:2118–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia G, Aroor AR, Sowers JR. The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall. Cardiovasc Res. 2017;113:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watts SW, Flood ED, Garver H, Fink GD, Roccabianca S. A New Function for Perivascular Adipose Tissue (PVAT): Assistance of Arterial Stress Relaxation. Scientific Reports. 2020;10:1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lynch FM, Withers SB, Yao Z, et al. Perivascular adipose tissue-derived adiponectin activates BKCa channels to induce anticontractile responses. American Journal of Physiology-Heart and Circulatory Physiology. 2013;304:H786–H795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saxton Sophie N, Ryding Katie E, Aldous Robert G, Withers Sarah B, Ohanian Jacqueline, Heagerty Anthony M. Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38:880–891. [DOI] [PubMed] [Google Scholar]
- 77.Abu Bakar H, Robert Dunn W, Daly C, Ralevic V. Sensory innervation of perivascular adipose tissue: a crucial role in artery vasodilatation and leptin release. Cardiovascular Research. 2017;113:962–972. [DOI] [PubMed] [Google Scholar]
- 78.Police Sara B, Thatcher Sean E, Charnigo Richard, Daugherty Alan, Cassis Lisa A. Obesity Promotes Inflammation in Periaortic Adipose Tissue and Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi X-Y, Qu S-L, Xiong W-H, Rom O, Chang L, Jiang Z-S. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CDA. Regulation of Vascular Function and Insulin Sensitivity by Adipose Tissue: Focus on Perivascular Adipose Tissue. Microcirculation. 2007;14:389–402. [DOI] [PubMed] [Google Scholar]
- 82.Kwok KHM, Lam KSL, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Experimental & Molecular Medicine. 2016;48:e215–e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gálvez-Prieto B, Bolbrinker J, Stucchi P, et al. Comparative expression analysis of the renin–angiotensin system components between white and brown perivascular adipose tissue. Journal of Endocrinology. 2008;197:55–64. [DOI] [PubMed] [Google Scholar]
- 84.Chang L, Villacorta L, Li R, et al. Loss of Perivascular Adipose Tissue upon PPARγ Deletion in Smooth Muscle Cells Impairs Intravascular Thermoregulation and Enhances Atherosclerosis. Circulation. 2012;126:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elvire Henrichot, Juge-Aubry Cristiana E., Pernin Agnès, et al. Production of Chemokines by Perivascular Adipose Tissue. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:2594–2599. [DOI] [PubMed] [Google Scholar]
- 86.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301:H1425–H1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E444–E452. [DOI] [PubMed] [Google Scholar]
- 88.Efremova A, Senzacqua M, Venema W, et al. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 2020;76:185–192. [DOI] [PubMed] [Google Scholar]
- 89.Kusminski CM, Park J, Scherer PE. MitoNEET-mediated effects on browning of white adipose tissue. Nature Communications. 2014;5(:3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiong W, Zhao X, Garcia-Barrio MT, et al. MitoNEET in Perivascular Adipose Tissue Blunts Atherosclerosis under Mild Cold Condition in Mice. Front Physiol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Y-J, Zeng Z, Teoh K, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. The Journal of Thoracic and Cardiovascular Surgery. 2005;130:1130–1136. [DOI] [PubMed] [Google Scholar]
- 92.Boucher Joshua M, Ryzhova Larisa, Harrington Anne, et al. Pathological Conversion of Mouse Perivascular Adipose Tissue by Notch Activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40:2227–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord. 2013;14:49–58. [DOI] [PubMed] [Google Scholar]
- 94.Greenstein Adam S, Khavandi Kaivan, Withers Sarah B., et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation. 2009;119:1661–1670. [DOI] [PubMed] [Google Scholar]
- 95.Withers SB, Forman R, Meza-Perez S, et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Scientific Reports. 2017;7:44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bridget Brosnihan K., Ping Li, Ferrario Carlos M. Angiotensin-(1–7) Dilates Canine Coronary Arteries Through Kinins and Nitric Oxide. Hypertension. 1996;27:523–528. [DOI] [PubMed] [Google Scholar]
- 97.Durand MJ, Zinkevich NS, Riedel M, et al. Vascular Actions of Angiotensin 1–7 in the Human Microcirculation: Novel Role for Telomerase. Arterioscler Thromb Vasc Biol. 2016;36:1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bujak-Gizycka B, Madej J, Wołkow PP, et al. Measurement of angiotensin metabolites in organ bath and cell culture experiments by liquid chromatography - electrospray ionization - mass spectrometry (LC-ESI-MS). J Physiol Pharmacol. 2007;58:529–540. [PubMed] [Google Scholar]
- 99.Gao Y-J, Lu C, Su L-Y, Sharma AM, Lee RMKW. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. British Journal of Pharmacology. 2007;151:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- 101.Wenduo Gu, Nowak Witold N., Xie Yao, et al. Single-Cell RNA-Sequencing and Metabolomics Analyses Reveal the Contribution of Perivascular Adipose Tissue Stem Cells to Vascular Remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:2049–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guanghong Jia, Sowers James R. Endothelial Dysfunction Potentially Interacts With Impaired Glucose Metabolism to Increase Cardiovascular Risk. Hypertension. 2014;64:1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thanassoulis G, Massaro JM, Corsini E, et al. Periaortic Adipose Tissue and Aortic Dimensions in the Framingham Heart Study. J Am Heart Assoc. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li W, Jin D, Takai S, et al. Impaired function of aorta and perivascular adipose tissue in IL-18-deficient mice. American Journal of Physiology-Heart and Circulatory Physiology. 2019;317:H1142–H1156. [DOI] [PubMed] [Google Scholar]
- 106.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DTL. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–232. [DOI] [PubMed] [Google Scholar]
- 108.Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. [DOI] [PubMed] [Google Scholar]
- 109.Chatterjee Tapan K, Stoll Lynn L, Denning Gerene M, et al. Proinflammatory Phenotype of Perivascular Adipocytes. Circulation Research. 2009;104:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. International Journal of Cardiology. 2017;228:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. [DOI] [PubMed] [Google Scholar]
- 112.Silaghi A, Achard V, Paulmyer-Lacroix O, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–1450. [DOI] [PubMed] [Google Scholar]
- 113.Teijeira-Fernandez E, Eiras S, Grigorian-Shamagian L, Fernandez A, Adrio B, Gonzalez-Juanatey JR. Epicardial adipose tissue expression of adiponectin is lower in patients with hypertension. Journal of Human Hypertension. 2008;22:856–863. [DOI] [PubMed] [Google Scholar]
- 114.Le Jemtel TH, Samson R, Ayinapudi K, Singh T, Oparil S. Epicardial Adipose Tissue and Cardiovascular Disease. Curr Hypertens Rep. 2019;21:36. [DOI] [PubMed] [Google Scholar]
- 115.Dicker D, Atar E, Kornowski R, Bachar GN. Increased Epicardial Adipose Tissue Thickness as a Predictor for Hypertension: A Cross-Sectional Observational Study. The Journal of Clinical Hypertension. 2013;15:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eroğlu S, Sade LE, Yıldırır A, Demir O, Müderrisoğlu H. Association of epicardial adipose tissue thickness by echocardiography and hypertension. Turk Kardiyol Dern Ars. 2013;41:115–122. [DOI] [PubMed] [Google Scholar]
- 117.Austys D, Dobrovolskij A, Jablonskienė V, Dobrovolskij V, Valevičienė N, Stukas R. Epicardial Adipose Tissue Accumulation and Essential Hypertension in Non-Obese Adults. Medicina (Kaunas). 2019;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mazurek Tomasz, Zhang LiFeng, Zalewski Andrew, et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 119.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–1087. [DOI] [PubMed] [Google Scholar]
- 120.Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. International Journal of Cardiology. 2007;115:272–273. [DOI] [PubMed] [Google Scholar]
- 121.George Thanassoulis, Massaro Joseph M., O’Donnell Christopher J., et al. Pericardial Fat Is Associated With Prevalent Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology. 2010;3:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baker AR, da Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovascular Diabetology. 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen Dinh Cat A, Briones AM, Callera GE, et al. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension. 2011;58:479–488. [DOI] [PubMed] [Google Scholar]
- 124.Nguyen Dinh Cat A, Touyz RM. A new look at the renin–angiotensin system—Focusing on the vascular system. Peptides. 2011;32:2141–2150. [DOI] [PubMed] [Google Scholar]
- 125.Frederich RC, Kahn BB, Peach MJ, Flier JS. Tissue-specific nutritional regulation of angiotensinogen in adipose tissue. Hypertension. 1992;19:339–344. [DOI] [PubMed] [Google Scholar]
- 126.Massiéra F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. [DOI] [PubMed] [Google Scholar]
- 127.Engeli S, Gorzelniak K, Kreutz R, Runkel N, Distler A, Sharma AM. Co-expression of renin-angiotensin system genes in human adipose tissue. Journal of Hypertension. 1999;17:555–560. [DOI] [PubMed] [Google Scholar]
- 128.Giacchetti G, Faloia E, Sardu C, et al. Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes. 2000;24:S142–S143. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y, Somers KR, Becari C, et al. Comparative Expression of Renin-Angiotensin Pathway Proteins in Visceral Versus Subcutaneous Fat. Front Physiol. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones BH, Standridge MK, Taylor JW, Moustaid N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1997;273:R236–R242. [DOI] [PubMed] [Google Scholar]
- 131.Liu C, Kraja AT, Smith JA, et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet. 2016;48:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morimoto H, Mori J, Nakajima H, et al. Angiotensin 1–7 stimulates brown adipose tissue and reduces diet-induced obesity. Am J Physiol Endocrinol Metab. 2018;314:E131–E138. [DOI] [PubMed] [Google Scholar]
- 133.Kawabe Y, Mori J, Morimoto H, et al. ACE2 exerts anti-obesity effect via stimulating brown adipose tissue and induction of browning in white adipose tissue. American Journal of Physiology-Endocrinology and Metabolism. 2019;317:E1140–E1149. [DOI] [PubMed] [Google Scholar]
- 134.Than A, Xu S, Li R, Leow M-S, Sun L, Chen P. Angiotensin type 2 receptor activation promotes browning of white adipose tissue and brown adipogenesis. Signal Transduction and Targeted Therapy. 2017;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Kloet AD, Krause EG, Scott KA, et al. Central angiotensin II has catabolic action at white and brown adipose tissue. American Journal of Physiology-Endocrinology and Metabolism. 2011;301:E1081–E1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsukuda K, Mogi M, Iwanami J, et al. Enhancement of Adipocyte Browning by Angiotensin II Type 1 Receptor Blockade. PLoS One. 2016;11:e0167704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 138.Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends in Molecular Medicine. 2013;19:487–500. [DOI] [PubMed] [Google Scholar]
- 139.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. [DOI] [PubMed] [Google Scholar]
- 140.Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moreno-Indias I, Tinahones FJ. Impaired Adipose Tissue Expandability and Lipogenic Capacities as Ones of the Main Causes of Metabolic Disorders. J Diabetes Res. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fried SK, Lee M-J, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring). 2015;23:1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee M-J, Fried SK. Sex-dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids. J Obes Metab Syndr. 2017;26:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323–1326. [DOI] [PubMed] [Google Scholar]
- 147.Chen X, McClusky R, Chen J, et al. The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice. PLOS Genetics. 2012;8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Link JC, Wiese CB, Chen X, et al. X chromosome dosage of histone demethylase KDM5C determines sex differences in adiposity. J Clin Invest. 2020;130:5688–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu R, Nikolajczyk BS. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patsouris D, Li P-P, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-Positive Cells Normalizes Insulin Sensitivity in Obese Insulin Resistant Animals. Cell Metabolism. 2008;8:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wentworth JM, Naselli G, Brown WA, et al. Pro-Inflammatory CD11c+CD206+ Adipose Tissue Macrophages Are Associated With Insulin Resistance in Human Obesity. Diabetes. 2010;59:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amano SU, Cohen JL, Vangala P, et al. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell Metabolism. 2014;19:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cancello R, Tordjman J, Poitou C, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. [DOI] [PubMed] [Google Scholar]
- 155.Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. [DOI] [PubMed] [Google Scholar]
- 156.Winer S, Chan Y, Paltser G, et al. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy: CD4+ T Cells Control Glucose Homeostasis. Nat Med. 2009;15:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cipolletta D, Feuerer M, Li A, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res. 2016;118:1786–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Medicine. 2012;18:363–374. [DOI] [PubMed] [Google Scholar]
- 160.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans: Effects of Cold Exposure and Adiposity. Diabetes. 2009;58:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lowell BB S-Susulic V, Hamann A, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. [DOI] [PubMed] [Google Scholar]
- 163.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. [DOI] [PubMed] [Google Scholar]
- 164.Sakamoto T, Nitta T, Maruno K, et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. American Journal of Physiology-Endocrinology and Metabolism. 2016;310:E676–E687. [DOI] [PubMed] [Google Scholar]
- 165.Peterson KR, Flaherty DK, Hasty AH. Obesity alters B cell and macrophage populations in brown adipose tissue. Obesity (Silver Spring). 2017;25:1881–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bi P, Shan T, Liu W, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nature Medicine. 2014;20:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lawes CM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. The Lancet. 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 168.Zhou D, Xi B, Zhao M, Wang L, Veeranki SP. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III Linked Mortality Study. Sci Rep. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stevens VJ, Obarzanek E, Cook NR, et al. Long-Term Weight Loss and Changes in Blood Pressure: Results of the Trials of Hypertension Prevention, Phase II. Ann Intern Med. 2001;134:1–11. [DOI] [PubMed] [Google Scholar]
- 171.Lee D, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in Fitness and Fatness on the Development of Cardiovascular Disease Risk Factors: Hypertension, Metabolic Syndrome, and Hypercholesterolemia. Journal of the American College of Cardiology. 2012;59:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18:629–639. [DOI] [PubMed] [Google Scholar]
- 173.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van Harmelen V, Elizalde M, Ariapart P, et al. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. International Journal of Obesity. 2000;24:673–678. [DOI] [PubMed] [Google Scholar]
- 175.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. American Journal of Physiology-Endocrinology and Metabolism. 2004;286:E891–E895. [DOI] [PubMed] [Google Scholar]
- 176.Frederique Yiannikouris, Manisha Gupte, Kelly Putnam, et al. Adipocyte Deficiency of Angiotensinogen Prevents Obesity-Induced Hypertension in Male Mice. Hypertension. 2012;60:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2005;289:R181–R186. [DOI] [PubMed] [Google Scholar]
- 178.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Harms MJ, Lim H-W, Ho Y, et al. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes Dev. 2015;29:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Antonio Cittadini, Mantzoros Christos S., Hampton Thomas G., et al. Cardiovascular Abnormalities in Transgenic Mice With Reduced Brown Fat. Circulation. 1999;100:2177–2183. [DOI] [PubMed] [Google Scholar]
- 181.Dall’Asta C, Vedani P, Manunta P, et al. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutrition, Metabolism and Cardiovascular Diseases. 2009;19:110–114. [DOI] [PubMed] [Google Scholar]
- 182.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine. 1995;1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 183.Considine RV, Sinha MK, Heiman ML, et al. Serum Immunoreactive-Leptin Concentrations in Normal-Weight and Obese Humans. New England Journal of Medicine. 1996;334:292–295. [DOI] [PubMed] [Google Scholar]
- 184.Russell CD, Petersen RN, Rao SP, et al. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. American Journal of Physiology-Endocrinology and Metabolism. 1998;275:E507–E515. [DOI] [PubMed] [Google Scholar]
- 185.Harmelen VV, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. [DOI] [PubMed] [Google Scholar]
- 186.Schoof E, Stuppy A, Harig F, et al. Comparison of leptin gene expression in different adipose tissues in children and adults. Eur J Endocrinol. 2004;150:579–584. [DOI] [PubMed] [Google Scholar]
- 187.Simonds SE, Pryor JT, Ravussin E, et al. Leptin Mediates the Increase in Blood Pressure Associated with Obesity. Cell. 2014;159:1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of Selective Leptin Resistance in Diet-Induced Obesity Hypertension. Diabetes. 2005;54:2012–2018. [DOI] [PubMed] [Google Scholar]
- 189.Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension. 1999;33:537–541. [DOI] [PubMed] [Google Scholar]
- 190.Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Current Science Inc. 2000;2:311–318. [DOI] [PubMed] [Google Scholar]
- 191.Mark AL, Shaffer RA, Correia MLG, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. Journal of Hypertension. 1999;17:1949–1953. [DOI] [PubMed] [Google Scholar]
- 192.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. PNAS. 1997;94:8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lembo G, Vecchione C, Fratta L, et al. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. [DOI] [PubMed] [Google Scholar]
- 194.Nakagawa K, Higashi Y, Sasaki S, Oshima T, Matsuura H, Chayama K. Leptin Causes Vasodilation in Humans. Hypertension Research. 2002;25:161–165. [DOI] [PubMed] [Google Scholar]
- 195.Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and Hypertension in Obesity. Vasc Health Risk Manag. 2006;2:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mark AL. Selective leptin resistance revisited. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2013;305:R566–R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Correia MLG, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The Concept of Selective Leptin Resistance: Evidence From Agouti Yellow Obese Mice. Diabetes. 2002;51:439–442. [DOI] [PubMed] [Google Scholar]
- 198.Gabriely I, Ma XH, Yang XM, et al. Removal of Visceral Fat Prevents Insulin Resistance and Glucose Intolerance of Aging: An Adipokine-Mediated Process? Diabetes. 2002;51:2951–2958. [DOI] [PubMed] [Google Scholar]
- 199.Langheim S, Dreas L, Veschini L, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. American Journal of Physiology-Heart and Circulatory Physiology. 2010;298:H746–H753. [DOI] [PubMed] [Google Scholar]
- 200.Park SY, Kim KH, Seo KW, et al. Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. The Journal of Pathology. 2014;232:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. [DOI] [PubMed] [Google Scholar]
- 202.Yasunori Takata, Haruhiko Osawa, Mie Kurata, et al. Hyperresistinemia Is Associated With Coexistence of Hypertension and Type 2 Diabetes. Hypertension. 2008;51:534–539. [DOI] [PubMed] [Google Scholar]
- 203.Jiang Y, Lu L, Hu Y, et al. Resistin Induces Hypertension and Insulin Resistance in Mice via a TLR4-Dependent Pathway. Scientific Reports. 2016;6:22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Calabro P, Samudio I, Willerson JT, Yeh ETH. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. [DOI] [PubMed] [Google Scholar]
- 205.Segawa K, Fukuhara A, Hosogai N, et al. Visfatin in adipocytes is upregulated by hypoxia through HIF1α-dependent mechanism. Biochemical and Biophysical Research Communications. 2006;349:875–882. [DOI] [PubMed] [Google Scholar]
- 206.Gunes F, Akbal E, Cakir E, Akyurek O, Altunbas M, Ozbek M. Visfatin may be a novel marker for identifying stages of essential hypertension in advanced age patients. Intern Med. 2012;51:553–557. [DOI] [PubMed] [Google Scholar]
- 207.Dogru T, Sonmez A, Tasci I, et al. Plasma visfatin levels in young male patients with uncomplicated and newly diagnosed hypertension. Journal of Human Hypertension. 2007;21:173–175. [DOI] [PubMed] [Google Scholar]
- 208.Curat CA, Wegner V, Sengenès C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744. [DOI] [PubMed] [Google Scholar]
- 209.Reneau J, Goldblatt M, Gould J, et al. Effect of adiposity on tissue-specific adiponectin secretion. PLOS ONE. 2018;13:e0198889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ohashi K, Kihara S, Ouchi N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. [DOI] [PubMed] [Google Scholar]
- 211.Yilmaz MI, Sonmez A, Caglar K, et al. Effect of antihypertensive agents on plasma adiponectin levels in hypertensive patients with metabolic syndrome. Nephrology. 2007;12:147–153. [DOI] [PubMed] [Google Scholar]
- 212.Yin C, Chu H, Li H, Xiao Y. Plasma Sfrp5 and adiponectin levels in relation to blood pressure among obese children. J Hum Hypertens. 2017;31:284–291. [DOI] [PubMed] [Google Scholar]
- 213.Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H. Adiponectin Levels Associated with the Development of Hypertension: A Prospective Study. Hypertension Research. 2008;31:229–233. [DOI] [PubMed] [Google Scholar]
- 214.Shatat IF, Freeman KD, Vuguin PM, Dimartino-Nardi JR, Flynn JT. Relationship Between Adiponectin and Ambulatory Blood Pressure in Obese Adolescents. Pediatric Research. 2009;65:691–695. [DOI] [PubMed] [Google Scholar]
- 215.Peri-Okonny PA, Ayers C, Maalouf N, et al. Adiponectin protects against incident hypertension independent of body fat distribution: observations from the Dallas Heart Study. Diabetes Metab Res Rev. 2017;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Noriyuki Ouchi, Mitsuru Ohishi, Shinji Kihara, et al. Association of Hypoadiponectinemia With Impaired Vasoreactivity. Hypertension. 2003;42:231–234. [DOI] [PubMed] [Google Scholar]
- 217.Almabrouk TAM, Ugusman AB, Katwan OJ, Salt IP, Kennedy S. Deletion of AMPKα1 attenuates the anticontractile effect of perivascular adipose tissue (PVAT) and reduces adiponectin release. Br J Pharmacol. 2017;174:3398–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yang R-Z, Lee M-J, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. American Journal of Physiology-Endocrinology and Metabolism. 2006;290:E1253–E1261. [DOI] [PubMed] [Google Scholar]
- 219.de Souza Batista CM, Yang R-Z, Lee M-J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. [DOI] [PubMed] [Google Scholar]
- 220.Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond). 2010;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kazama K, Okada M, Hara Y, Yamawaki H. A Novel Adipocytokine, Omentin, Inhibits Agonists-Induced Increases of Blood Pressure in Rats. Journal of Veterinary Medical Science. 2013;advpub. [DOI] [PubMed] [Google Scholar]
- 222.Brunetti L, Leone S, Orlando G, et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacological Reports. 2014;66:991–995. [DOI] [PubMed] [Google Scholar]
- 223.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends in Endocrinology & Metabolism. 2014;25:348–355. [DOI] [PubMed] [Google Scholar]
- 224.Zabetian-Targhi F, Mirzaei K, Keshavarz SA, Hossein-Nezhad A. Modulatory Role of Omentin-1 in Inflammation: Cytokines and Dietary Intake. J Am Coll Nutr. 2016;35:670–678. [DOI] [PubMed] [Google Scholar]
- 225.Sargolzaei J, Chamani E, Kazemi T, Fallah S, Soori H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clinical Biochemistry. 2018;54:1–10. [DOI] [PubMed] [Google Scholar]
- 226.Enomoto T, Ohashi K, Shibata R, et al. Adipolin/C1qdc2/CTRP12 Protein Functions as an Adipokine That Improves Glucose Metabolism*. Journal of Biological Chemistry. 2011;286:34552–34558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ogawa H, Ohashi K, Ito M, et al. Adipolin/CTRP12 protects against pathological vascular remodelling through suppression of smooth muscle cell growth and macrophage inflammatory response. Cardiovascular Research. 2020;116:237–249. [DOI] [PubMed] [Google Scholar]
- 228.Saxton SN, Whitley AS, Potter RJ, Withers SB, Grencis R, Heagerty AM. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am J Physiol Heart Circ Physiol. 2020;319:H1387–H1397. [DOI] [PubMed] [Google Scholar]
- 229.Titone D, Aroonsakool N, Li J, et al. Increased Serum Interleukin-33 In Patients With Pulmonary Arterial Hypertension: A Role For IL-33/ST2 In Disease Pathogenesis. In: B95. NOVEL THERAPEUTIC TARGETS IN PULMONARY HYPERTENSION: INSIGHTS FROM TRANSLATIONAL AND PRECLINICAL STUDIES American Thoracic Society International Conference Abstracts. American Thoracic Society; 2014:A3636–A3636. [Google Scholar]
- 230.Gutierrez AK. The Role of Interleukin-33 in the Progression of Pulmonary Arterial Hypertension Through an ST2/MyD88 Pathway. 2018.
- 231.Selcuk A, Bulucu F, Kalafat F, et al. Skinfold thickness as a predictor of arterial stiffness: obesity and fatness linked to higher stiffness measurements in hypertensive patients. Clin Exp Hypertens. 2013;35:459–464. [DOI] [PubMed] [Google Scholar]
- 232.Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25:495–502. [DOI] [PubMed] [Google Scholar]
- 233.Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. [DOI] [PubMed] [Google Scholar]
- 234.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. [DOI] [PubMed] [Google Scholar]
- 235.Cote AT, Phillips AA, Harris KC, Sandor GGS, Panagiotopoulos C, Devlin AM. Obesity and arterial stiffness in children: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35:1038–1044. [DOI] [PubMed] [Google Scholar]
- 236.Mitchell GF, Hwang S-J, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kim H-L, Lee J-M, Seo J-B, et al. The effects of metabolic syndrome and its components on arterial stiffness in relation to gender. Journal of Cardiology. 2015;65:243–249. [DOI] [PubMed] [Google Scholar]
- 238.Cooper LL, Palmisano JN, Benjamin EJ, et al. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events: the Framingham Heart Study. Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.B B J H, Vg D, et al. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2015;308:H1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jia G, Habibi J, Aroor AR, et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sowers JR, Habibi J, Aroor AR, et al. Epithelial sodium channels in endothelial cells mediate diet-induced endothelium stiffness and impaired vascular relaxation in obese female mice. Metabolism. 2019;99:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sehgel NL, Vatner SF, Meininger GA. “Smooth Muscle Cell Stiffness Syndrome”—Revisiting the Structural Basis of Arterial Stiffness. Front Physiol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sowers JR, Habibi J, Jia G, et al. Endothelial sodium channel activation promotes cardiac stiffness and diastolic dysfunction in Western diet fed female mice. Metabolism. 2020;109:154223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovascular Research. 2020;(cvaa326). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Leopold Jane A Cellular and Molecular Mechanisms of Arterial Stiffness Associated With Obesity. Hypertension. 2013;62:1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang J, Barbry P, Maiyar AC, et al. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. American Journal of Physiology-Renal Physiology. 2001;280:F303–F313. [DOI] [PubMed] [Google Scholar]
- 247.von Wowern F, Berglund G, Carlson J, Månsson H, Hedblad B, Melander O. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 2005;68:2164–2172. [DOI] [PubMed] [Google Scholar]
- 248.Huang DY, Boini KM, Osswald H, et al. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. American Journal of Physiology-Renal Physiology. 2006;291:F1264–F1273. [DOI] [PubMed] [Google Scholar]
- 249.Li P, Pan F, Hao Y, Feng W, Song H, Zhu D. SGK1 is regulated by metabolic-related factors in 3T3-L1 adipocytes and overexpressed in the adipose tissue of subjects with obesity and diabetes. Diabetes Res Clin Pract. 2013;102:35–42. [DOI] [PubMed] [Google Scholar]
- 250.Norlander AE, Saleh MA, Pandey AK, et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2018;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


