Abstract
Bacterial coinfections increase the severity of respiratory viral infections and were frequent causes of mortality in influenza pandemics but have not been well characterized in patients with coronavirus disease 2019 (COVID-19). The aim of this review was to identify the frequency and microbial etiologies of bacterial coinfections that are present upon admission to the hospital and that occur during hospitalization for COVID-19. We found that bacterial coinfections were present in <4% of patients upon admission and the yield of routine diagnostic tests for pneumonia was low. When bacterial coinfections did occur, Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae were the most common pathogens and atypical bacteria were rare. Although uncommon upon admission, bacterial infections frequently occurred in patients with prolonged hospitalization, and Pseudomonas aeruginosa, Klebsiella spp., and S. aureus were common pathogens. Antibacterial therapy and diagnostic testing for bacterial infections are unnecessary upon admission in most patients hospitalized with COVID-19, but clinicians should be vigilant for nosocomial bacterial infections.
Keywords: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), bacterial coinfection, hospital-acquired infections, multidrug resistance (MDR)
The Threat of Bacterial Coinfections in COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of a devastating pandemic of COVID-19 and has led to more than 100 million cases and 2 million deaths globally in a span of 12 monthsi. Pandemics have plagued humans throughout history but are now becoming increasingly common. Influenza was responsible for the 1918 pandemic that resulted in ~50 million deaths worldwide, and more recently pandemics in 1957, 1968, and 2009 [1]. In 2003, a near-pandemic of severe acute respiratory syndrome coronavirus (SARS-CoV) occurred, closely followed by the emergence of another lethal coronavirus, Middle East respiratory syndrome coronavirus (MERS-CoV), in 2012 [2].
Bacterial coinfection is a common complication of many viral respiratory tract infections and leads to significantly increased morbidity and mortality [3]. During the 1918 pandemic, bacterial coinfection was a significant contributor in nearly all influenza deaths, with common upper respiratory tract bacteria such as S. pneumoniae, β-hemolytic streptococci, H. influenzae, and S. aureus being the most common pathogens [4]. Bacterial coinfection was also common in the 2009 influenza pandemic, occurring in 18–30% of cases admitted to the intensive care unit (ICU) and up to 55% of published autopsy series [5., 6., 7.]. Bacterial coinfections were associated with increased risk of shock and respiratory failure, prolonged ICU length of stay, and mortality [5,6]. The frequency of bacterial coinfection with coronaviruses SARS-CoV and MERS-CoV is not clearly defined because of the relatively small numbers of cases, but a multicenter study of patients admitted to ICUs in Saudi Arabia identified that 19% of patients with MERS had bacterial coinfection [8]. The high frequency and clinical significance of bacterial coinfections in influenza and other novel coronaviruses raises concern that bacterial coinfection could be an important complication of SARS-CoV-2 infection.
Although initial reports described the clinical presentations and outcomes of patients hospitalized with COVID-19 [9,10], few reports have focused on the frequency and microbiological etiologies of bacterial coinfections. Furthermore, these reports did not distinguish secondary infections that were present upon admission from those that were acquired in the hospital. Thus, initial reviews on secondary infections complicating COVID-19 relied on limited data [11,12]. However, since those initial reports, numerous studies have reported on secondary infections in COVID-19 patients, and thus our understanding of bacterial coinfections has evolved. Therefore, the focus of this review is to highlight the frequency, risk factors, etiologies, and outcomes of bacterial coinfections in COVID-19, separating those that are present on admission from those that are acquired in the hospital, evaluate the yield of diagnostics, assess the emergence of multidrug-resistant (MDR) bacterial infections, and provide practical guidance to clinicians.
Bacterial Coinfections at the Time of Initial Presentation to the Hospital
Frequency of Bacterial Coinfection and Associated Risk Factors
We reviewed publications in which the primary objective of the study was to evaluate bacterial coinfections upon hospital admission in patients with COVID-19. We identified ten studies that evaluated a minimum of 100 patients (Table 1 ), and in nearly all of these studies fewer than 4% of hospitalized patients with COVID-19 had a documented bacterial coinfection [13., 14., 15., 16., 17., 18., 19., 20., 21.].
Table 1.
Bacterial Coinfections in Patients Admitted to the Hospital with COVID-19
| Refs | Location | No. of patients | Prevalence of bacterial coinfection | Proportion of patients who received antibacterial therapy |
|---|---|---|---|---|
| [13] | Michigan, USA (38 hospitals) | 1705 | 3.5% | 57% (median: 3 days) |
| [14] | London, England (2 hospitals) | 1396 | 2.7% | 98%a |
| [15] | Barcelona, Spain (1 hospital) | 989 | 2.5% | NR |
| [16] | The Netherlands (4 hospitals) | 925 | 0.8% | 60% (median: 2 days) |
| [17] | London, England (2 hospitals) | 836 | 3.2% | NR |
| [18] | Chicago, USA (1 hospital) | 321 | 1.2% | 69% |
| [19] | Liverpool, England (1 hospital) | 195 | 2.6% | NR |
| [20] | Hong Kong (1 hospital) | 147 | 2.7% | 35%b |
| [21] | Hangzhou, China (1 hospital) | 101 | 0% | NR |
| [22] | Paris, France (1 hospital) | 101 (ICU patients only) | 19.8% | 58% |
Abbreviations: ICU, intensive care unit; No., number; NR, not reported.
The denominator for this proportion includes 37 patients with bacterial coinfection and 100 randomly selected patients without bacterial coinfection.
Of the 35% of patients who received empirical antibiotics, 37% received them for more than 1 week.
Despite the low prevalence of bacterial coinfection, the majority of patients in these studies received empirical antibacterial therapy. For example, of 1705 patients with COVID-19 presenting to 38 Michigan hospitals, 57% received empirical antibacterial therapy for a median of 3 days (interquartile range: 2–6 days); however, only 3.5% were found to have a bacterial infection upon admission [13]. Furthermore, 15% of patients received agents targeting methicillin-resistant Staphylococcus aureus (MRSA) and 15% received agents targeting P. aeruginosa.
The wide disparities between the proportion of hospitalized COVID-19 patients who are treated with antibacterial agents and the proportion of patients who actually have a bacterial coinfection highlights substantial unnecessary use of antibacterial agents among COVID-19 patients. Antibiotic overuse early in the pandemic was driven by uncertainty around the course of a new infectious disease, extrapolation from experiences of bacterial coinfection with influenza [23], a surge in critically ill patients, and the lack of effective therapies for SARS-CoV-2. As our knowledge of COVID-19 grows, understanding presenting factors associated with bacterial coinfection is essential so that clinicians can target empirical antibacterial therapy to these high-risk patients. Advanced age and other comorbidities, such as chronic kidney disease, diabetes, and chronic heart disease, have been associated with bacterial coinfections in some but not in other studies [13., 14., 15.]. Leukocytosis is more common in patients presenting with bacterial coinfections. In two of the largest studies, the median white blood cell (WBC) counts were higher in patients with bacterial coinfection compared to those without coinfection (median of approximately 10.0×109 to 11.3×109 cells/μl versus 7×109 cells/μl, respectively) [13,14]. Wang and colleagues found that the absolute neutrophil count of patients with bacterial coinfections was 9.2×109 cells/μl, compared to 5.5×109 cells/μl in patients without bacterial coinfection (P <0.0001) [14]. Vaughn and colleagues found that procalcitonin levels are also higher in patients with bacterial coinfection compared to those without coinfection [13]. However, leukocytosis, neutrophilia, and elevated procalcitonin levels do not have sufficient sensitivity, specificity, or positive predictive value to accurately diagnose bacterial coinfection as stand-alone tests [13,14]. For example, Vaughn et al. found that the positive predictive value of a procalcitonin level >0.5 ng/ml was only 9.3% [13]. Conversely, the negative predictive values of a WBC count <8.8×109 cells/μl, absolute neutrophil count <6.8×109 cells/μl, and procalcitonin level ≤0.5 ng/ml are ≥98%.
While bacterial coinfections are overall rare among patients who are hospitalized for COVID-19, they are more common among patients admitted to an ICU, occurring in 6–29% of these patients [13,22,24., 25., 26., 27., 28.]. Although some of the increased frequency of bacterial coinfections in patients admitted to an ICU may be related to increasing use of blood and respiratory tract cultures to diagnose bacterial infections [17], these patients may also be more likely to have positive cultures [13,29,30].
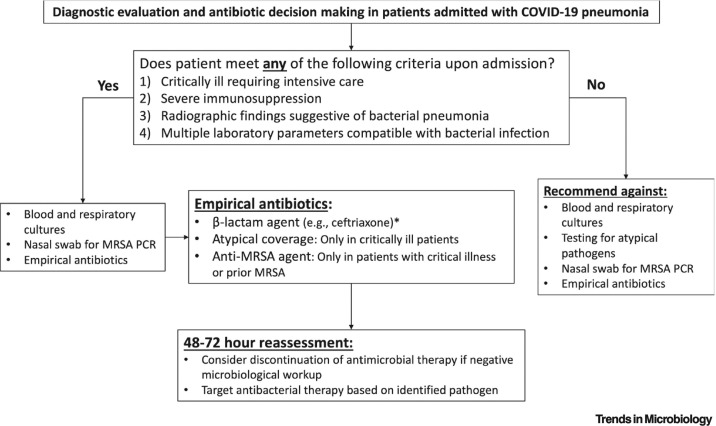
Clinicians face a difficult challenge in deciding which hospitalized patients with COVID-19 to treat with antibacterial therapy. Figure 1 illustrates a recommended approach for diagnosing bacterial coinfection and antibiotic management upon hospital admission in patients with COVID-19. We recommend pursing microbiologic diagnostic tests and initiating empirical antibiotic therapy only in patients who are critically ill, severely immunocompromised, or have radiographic or multiple laboratory findings suggestive of bacterial coinfection. When empirical therapy is warranted, we recommend β-lactam agents and provide guidance for when coverage of P. aeruginosa, atypical bacteria, and/or MRSA is warranted.
Figure 1.
Algorithm to Guide Diagnostic Evaluations and Antibiotic Decision Making in Patients who are Admitted to the Hospital with COVID-19 Pneumonia.
This flow diagram provides criteria for clinicians to help identify patients for whom a diagnostic work-up should be pursued and for whom empirical antibiotic therapy is warranted. It also provides guidance for the selection of antibiotics. *Consider anti-pseudomonal β-lactam agents in patients with prior Pseudomonas aeruginosa, bronchiectasis, or recent use of parenteral antibiotics. MRSA, methicillin-resistant Staphylococcus aureus.
Yield of Diagnostic Tests for Bacterial Pathogens in Patients Hospitalized for COVID-19
Studies of hospitalized patients with COVID-19 have demonstrated that blood cultures upon admission are positive for pathogens in 1.2–4.2% of cases (Table 2 ) [13,14,17,19,30]. Furthermore, at least half of positive blood cultures in hospitalized patients with COVID-19 represent skin contaminants [14,17,30., 31., 32.], perhaps in part due to technical challenges for healthcare personnel collecting blood cultures while wearing personal protective equipment (PPE) required for SARS-CoV-2. Hospitals in New York City (NYC) noted a surge in blood culture utilization during the peak of the pandemic, with 35% more cultures analyzed than the same time during the previous year, that at times overwhelmed the capacity of blood culture instruments [31]. Moreover, the rate of positive blood cultures in patients with COVID-19 has been shown to be significantly lower than in patients without COVID-19 [32]. As with the initiation of empirical antibacterial therapy, blood cultures should not be routinely ordered in hospitalized patients with COVID-19. Instead, they should be selectively ordered in patients with a suspicion for bacterial coinfection, which would include patients for whom empirical antibacterial therapy is being initiated.
Table 2.
Yield of Diagnostic Tests for Bacterial Coinfection in Patients Presenting to the Hospital with COVID-19
| Blood cultures: the yield of blood cultures is low among patients who present to the hospital with COVID-19 and many of the positive results represent skin contaminants. | ||
|---|---|---|
| Refs | Yield for pathogen | Yield for contaminant |
| [13] | 31/1063 (2.9%) | Not reported |
| [14] | 12/969 (1.2%) | 65/969 (6.7%) |
| [17] | 21/643 (3.2%) | 39/643 (6.1%) |
| [19] | 2/128 (1.6%) | Not reported |
| [30] | 5/118 (4.2%) | 4/118 (4.2%) |
| Respiratory tract cultures: the yield of respiratory-tract cultures is higher than the yield of blood cultures in patients presenting to the hospital with COVID-19; however, respiratory tract cultures are frequently not obtained because many patients do not have a productive cough. | ||
| Refs | Patient type | Yield for respiratory pathogen |
| [13] | All hospitalized patients | 25/131 (19.1%) |
| [18] | All hospitalized patients | 2/66 (3.0%) |
| [14] | All hospitalized patients | 8/48 (16.7%) |
| [29] | Intensive care unit (ICU) patients | 9/43 (20.9%) |
| [26] | ICU patients | 3/30 (10.0%) |
| [30] | Mostly ICU patients | 3/24 (12.5%) |
| [19] | All hospitalized patients | 0/25 (0%) |
| Urine antigen tests: the yield of urine pneumococcal and urine Legionella antigen tests is low in patients with COVID-19. Additionally, none of the 965 patients in six different studies who had nasopharyngeal or bronchoalveolar lavage samples analyzed for Mycoplasma pneumoniae and Chlamydia pneumoniae by PCR had positive test results [18,27,29,35., 36., 37.]. Thus, coinfections with atypical bacteria are extremely rare. | ||
| Refs | Yield of pneumococcal antigen | Yield of Legionella antigen |
| [14] | 3/296 (1.0%) | 0/308 (0%) |
| [17] | 0/249 (0%) | 0/246 (0%) |
| [18] | 1/236 (0.4%) | 0/240 (0%) |
| [16] | 1/189 (0.5%) | 0/187 (0%) |
| [30] | 0/107 (0%) | 0/117 (0%) |
| [27] | 1/88 (1.1%) | 0/88 (0%) |
| [26] | 2/43 (5.7%) | 0/46 (0%) |
| [19] | 3/29 (10.3%) | 1/31 (3.2%) |
| Total | 11/1237 (0.9%) | 1/1263 (0.08%) |
Most patients who present to the hospital with COVID-19 do not have a productive cough and thus are not able to produce sputum for culture [13,33]. Studies report that respiratory tract cultures yielded a pathogen in 3–19% of the general population of hospitalized patients with COVID-19 and in 10–21% of patients admitted to ICUs (Table 2) [13,14,18,19,26,29,30]. Based on these data, we recommend the use of respiratory-tract cultures only in the minority of patients who have a productive cough and for whom empirical antibacterial therapy is being considered or in patients who are admitted to the ICU and intubated for respiratory failure.
Only 11 (0.9%) out of 1237 patients had a positive urine pneumococcal antigen test result (Table 2) [14,16., 17., 18., 19.,26,27,30]. Thus, we do not recommend routine use of urine pneumococcal antigens in patients hospitalized with COVID-19. However, this test may be considered in patients with severe disease for whom antibacterial therapy is being considered, which is in line with guidelines of the American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) for community-acquired pneumonia (CAP) [34]. The yield of urine legionella antigen tests is even lower, with only one (0.08%) positive test in 1263 patients (Table 2) [14,16., 17., 18., 19.,26,27,30]. None of the samples that were sent for Mycoplasma pneumoniae and Chlamydia pneumoniae PCR were positive (Table 2) [18,27,29,35., 36., 37.]. Thus, we do not recommend the routine use of urine legionella antigens or atypical pneumonia PCR panels in patients with COVID-19.
Etiologies of Bacterial Coinfection on Admission, and Implications for Empirical Antibacterial Therapy
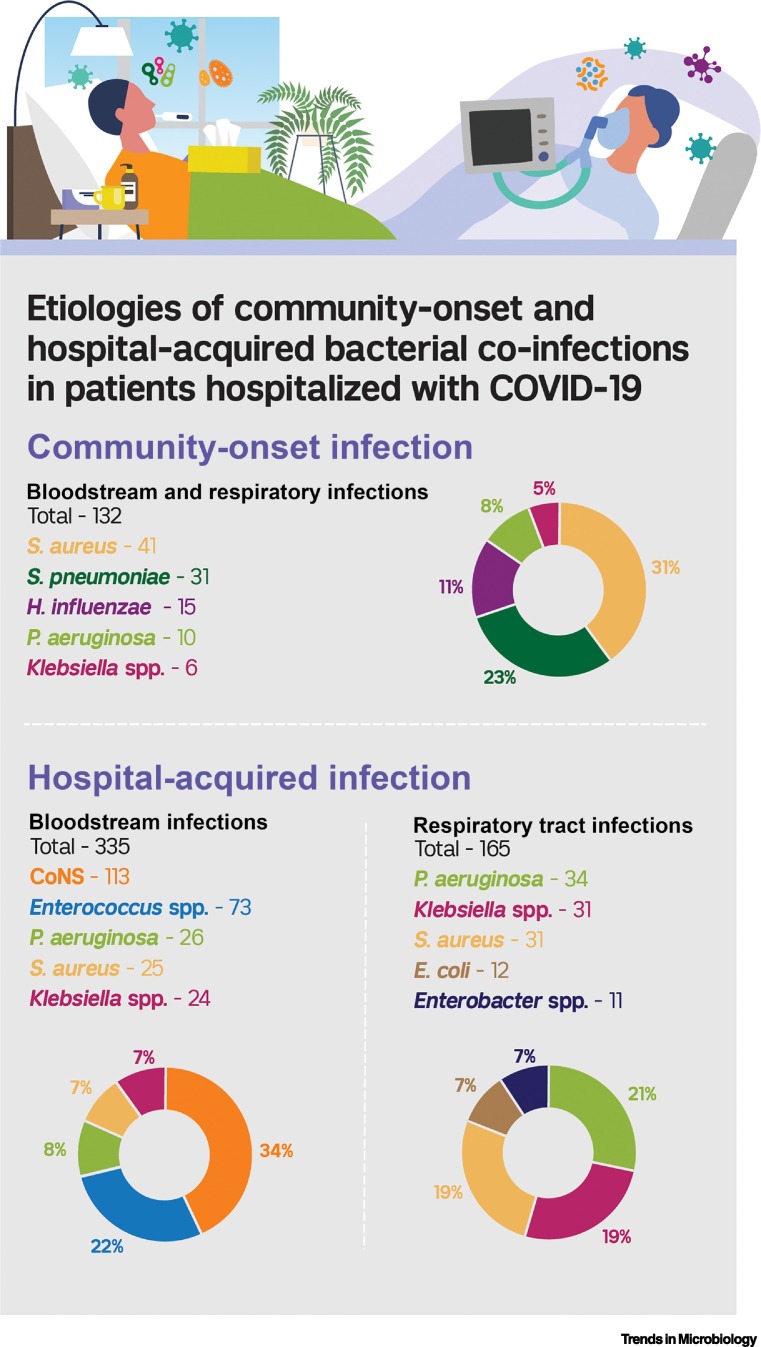
The most common bacterial respiratory and bloodstream pathogens that caused coinfection upon admission with COVID-19 are outlined in Figure 2 . Of 132 total bacterial respiratory and bloodstream pathogens, the most common were S. aureus (n = 41, 31%), S. pneumoniae (n = 31, 23%), and H. influenzae (n = 15, 11%). Thus, for patients hospitalized with COVID-19 for whom empirical antibacterial therapy is indicated, coverage of these organisms is recommended (Figure 1: empirical antibiotic regimens).
Figure 2.
Etiologies of Community-onset and Hospital-acquired Bacterial Coinfections in Patients Hospitalized with COVID-19.
Community-onset bacterial infections are those that occurred within 2 days of hospital admission in 10 studies [15,16,19,20,23,25., 26., 27., 28., 29.], and hospital-acquired bacterial infections are those that occurred after the second day of hospitalization in six studies [16,31,48., 49., 50.,52]. Other bacterial etiologies of community-onset bloodstream or respiratory infections included Escherichia coli (n =5), Proteus spp. (n = 4), Legionella pneumophila (n = 4), and Citrobacter spp. (n = 2). Other hospital-onset bloodstream infections included E. coli (n = 14), Acinetobacter spp. (n = 8), Enterobacter spp. (n = 7), Serratia marcescens (n = 3), and Stenotrophomonas maltophilia (n = 3). Other respiratory tract infections included S. maltophilia (n = 9), S. marcescens (n = 8), Acinetobacter spp. (n = 7), Citrobacter spp. (n = 6), and Proteus spp. (n = 5). CoNS, coagulase-negative staphylococci; H. influenzae, Haemophilus influenzae; P. aeruginosa, Pseudomonas aeruginosa; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae.
Initial treatment strategies for CAP, as recommended by the ATS and IDSA, consist of combination therapy with a β-lactam and a macrolide or a respiratory fluoroquinolone [34]. These regimens will generally cover S. aureus, S. pneumoniae, and H. influenzae, with the exception of MRSA. The choice of whether to include anti-MRSA therapy in the empirical treatment of bacterial coinfection should be influenced by the local geographic prevalence of MRSA and/or prior isolation of MRSA from the patient (Figure 1). Furthermore, collecting a nares swab for MRSA PCR can guide the use of anti-MRSA therapies. This test has high specificity for MRSA pneumonia and a high negative predictive value for ruling out MRSA in patients with and without COVID-19 [38,39]. Thus, a reasonable strategy to pursue in critically ill patients admitted with COVID-19 pneumonia to an ICU is to begin anti-MRSA coverage, collect a nares swab for MRSA PCR, and discontinue anti-MRSA coverage if the PCR is negative.
P. aeruginosa was identified as a bacterial respiratory pathogen in 10 (8%) of the 132 coinfections outlined in Figure 2. Thus, routine empirical coverage of P. aeruginosa is generally unnecessary, unless the patient has had a history of infection due to this organism, a chronic lung disease associated with P. aeruginosa pneumonia, such as bronchiectasis [40], or recent parenteral antibiotic exposure (Figure 1). As with MRSA, if P. aeruginosa is not isolated from culture within 48–72 h, anti-pseudomonal therapy should be discontinued if it was started empirically.
The extremely low yield of PCR and urine antigen tests for atypical bacteria suggests that these organisms rarely cause secondary infections in patients with COVID-19, and thus the inclusion of coverage for atypical pathogens with a macrolide or fluoroquinolone may not be necessary in patients with suspected bacterial pneumonia. This conclusion contrasts with findings from a review by Lansbury et al. that highlighted M. pneumoniae as a common cause of bacterial coinfection [12]. However, this review reported studies that detected M. pneumoniae infection based on positive serologic IgM tests, a diagnostic test that lacks specificity for infection [41]. Legionella pneumophila may be more common in critically ill patients requiring ICU admission. Although only a single urine legionella antigen test was positive, L. pneumophila was identified by culture or PCR in 3 of 50 bacterial coinfections in studies that evaluated only those patients who were admitted to an ICU [24., 25., 26., 27., 28.].
Although guidelines prefer combination therapy with β-lactam/macrolide antibiotics to monotherapy with just β-lactam antibiotics for CAP in hospitalized patients [34], a recent randomized trial found that the latter is not inferior when compared to the former in patients with CAP who are admitted to non-ICU wards [42]. Another randomized trial of β-lactam/macrolide combination therapy vs. β-lactam monotherapy for CAP also found similar outcomes between groups, except in patients who were infected with atypical pathogens or who were critically ill [43]. A potential benefit of including macrolides in the treatment of CAP is that they may have beneficial immunomodulatory properties [44]. However, randomized trials have not identified a clinical benefit for the use of macrolides in hospitalized patients with COVID-19 [45,46]. Based on available data, we do not recommend adding macrolides to β-lactam agents for patients being treated empirically for bacterial coinfection with COVID-19, with the exception of critically ill patients where L. pneumophila coinfection has been observed (Figure 1).
Outcomes of Hospitalized Patients with COVID-19 who Present with a Bacterial Coinfection
Most studies of bacterial coinfections in patients with COVID-19 did not have a sufficient number of patients to identify differences in outcomes between patients with and without bacterial coinfection. The largest study of bacterial coinfections on admission in our review, however, found that patients with bacterial coinfection had a longer length of hospital stay (median 7 days vs. 5 days; P = 0.003) and increased in-hospital mortality (48% vs. 18%; P <0.001) compared to patients without bacterial coinfection [13]. However, other smaller studies have not found the same association, possibly due to insufficient power or nonrespiratory sources of infection [14,15].
Hospital-acquired Bacterial Infections that Occur in Patients with COVID-19
Frequency and Risk Factors for Hospital-acquired Bacterial Infections
Table 3 outlines results from nine studies in which the primary objective was to assess hospital-acquired bacterial infections in patients with COVID-19 and which evaluated a minimum of 100 patients [15,20,30,47., 48., 49., 50., 51.]. Hospital-acquired bacterial infections occurred in 3.7–21.9% of patients admitted with COVID-19. Two studies that evaluated only patients admitted to an ICU found that 38.6% and 47.5% of patients developed a hospital-acquired bacterial infection, respectively. The median time from admission until hospital-acquired bacterial infection was typically 1–2 weeks. Pneumonia and bloodstream infections (BSIs) were the most common sources of hospital-acquired bacterial infections.
Table 3.
Hospital-acquired Bacterial Infections that Occur in Patients with COVID-19
| Refs | Setting | No. of patients | Incidence of bacterial HAI | Median days of HAI from admission | Type of infection | Risk factors for hospital-acquired infection in multivariate models | Mortality: HAI vs. no secondary infection |
|---|---|---|---|---|---|---|---|
| [47] | Georgia, USA (1 hospital) | 1565 | 3.7% (85% bacterial) | NR | PNA 48% BSI 38% UTI 12% |
Tocilizumab; corticosteroids; hydroxychloroquine AKI requiring dialysis |
40.7% vs. 11.8% (P <0.001) |
| [15] | Barcelona, Spain (1 hospital) | 989 | 3.8% | 11 | PNA 34% BSI 36% UTI 27% |
NR | 18.6% vs. 9.4% (P = 0.047) |
| [48] | Milan, Italy (1 hospital) | 731 | 9.3% | 12 | BSI 72% PNA 28% |
Baseline lymphopenia; hypoxia on admission; ICU admission in first 48 h |
NR |
| [49] | Pisa, Italy (1 hospital) | 315 | 21.9% | 19 | BSI 40% UTI 28% PNA 26% |
CRE intestinal colonization; mechanical ventilation; IL-6 or JAK inhibitors; elevated CRP; piperacillin-tazobactam |
18.8% vs. 23.2% (P = 0.45) |
| [50] | Huangshi, China (1 hospital) | 212 | 14.6% | NR | PNA 39% UTI 32% BSI 23% |
Urinary catheterization; leukocytosis; elevated PCT |
NR |
| [20] | Hong Kong (2 hospitals) | 147 | 5.4% | NR | NR | NR | NR |
| [30] | Munich, Germany (1 hospital) | 140 | 7.1% (BSI only) | NR | PNA 67% BSI 33% |
NR | NR |
| [51] | Madrid, Spain (1 hospital) | 140 (ICU patients only) | 38.6% | 9 | BSI 56% PNA 33% UTI 8% |
APACHE II score | 54% vs. 24% (P <0.001) |
| [22] | Paris, France (1 hospital) | 101 (ICU patients only) | 47.5% | 8 | NR | NR | NR |
Abbreviations: AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; BSI, bloodstream infection; CRP, C-reactive protein; HAI, hospital-acquired infection; ICU, intensive care unit; IL-6, interleukin-6; JAK, Janus-associated kinase; No., number; NR, not reported; PCT, procalcitonin; PNA, pneumonia; UTI, urinary-tract infection.
A substantial proportion of hospitalized patients with COVID-19 require mechanical ventilation [10,33]. Thus, an important clinical question is whether patients with COVID-19 have higher risks of ventilator-associated pneumonia (VAP) than other patients receiving mechanical ventilation. Maes and colleagues compared the incidence and etiologies of VAP between 81 mechanically ventilated patients with COVID-19 and 144 mechanically ventilated patients without COVID-19 [52]. They found that COVID-19 patients had 28 VAP events per 1000 ventilator days, compared to 13/1000 for patients without COVID-19 (P = 0.009). Although COVID-19 patients were more likely to develop VAP, the organisms causing infection were similar to patients without COVID-19. Another group compared 90 patients with COVID-19-related acute respiratory distress syndrome (ARDS) to 82 ARDS patients without COVID-19 and found that COVID-19 patients were almost twice as likely to develop VAP [53].
Independent risk factors for hospital-acquired bacterial infections varied across studies. Associations between COVID-19 or antibacterial therapies and risk of infection are likely confounded by the fact that sicker patients are more likely to receive these therapies. Measures of acuity of illness upon presentation were consistently identified as risk factors for hospital-acquired bacterial infection, including hypoxia upon admission, need for mechanical ventilation, and ICU admission within the first 48 h [48,49]. Baseline laboratory results were not consistently identified as risk factors, but lymphopenia, leukocytosis, elevated procalcitonin and C-reactive protein levels, acute kidney injury requiring dialysis, and Acute Physiology and Chronic Health Evaluation II score were identified as independent risk factors in individual studies [47., 48., 49., 50., 51.].
Etiologic Agents of Bacteria that Cause Hospital-acquired Bacterial Infections
Figure 2 outlines the most common etiologies of hospital-acquired bacterial infections in patients with COVID-19. We reviewed data from seven studies that characterized hospital-acquired bacterial infections and identified 556 microbiologically confirmed infections [15,30,47., 48., 49.,51,54]. The five most common bacterial pathogens were coagulase-negative staphylococci (n = 115, 21%), Enterococcus spp. (n = 86, 15%), Klebsiella spp. (n = 84, 15%), P. aeruginosa (n = 72, 13%), and S. aureus (n = 50, 9%). Coagulase-negative staphylococci and enterococci were primarily causes of BSIs; whereas, P. aeruginosa, Klebsiella spp., and S. aureus were the predominant causes of respiratory tract infections.
Outcomes of Patients with COVID-19 and Hospital-acquired Bacterial Infections
Three of the four studies that compared outcomes of patients with and without hospital-acquired bacterial infections found an increased mortality rate in the former group [15,47,49,51]. Garcia-Vidal et al. found that patients with hospital-acquired bacterial infections had a longer length of hospital stay (20 days vs. 9 days, P <0.001) [15]. Bhatt and colleagues found that 53.1% of patients with a secondary BSI died during the hospitalization, compared to 32.8% of controls (P = 0.0001) [54]. These findings are similar to those observed in patients hospitalized with influenza, where in one study in-hospital mortality was 45.7% in patients who developed a hospital-acquired bacterial or fungal infection and 11.8% in patients who did not develop a hospital-acquired infection [55].
Carbapenem-resistant Gram-negative Infections in Patients with COVID-19
Although patients with COVID-19 rarely have bacterial coinfections upon admission to the hospital, many receive antibacterial therapies, have prolonged hospitalizations that often require mechanical ventilation, and develop hospital-acquired infections. Thus, it is not surprising that MDR bacterial infections have been reported in patients with COVID-19. One NYC hospital reported 13 patients infected with carbapenemase-producing Enterobacterales, including K. pneumoniae carbapenemase (KPC)-producing K. pneumoniae and New Delhi metallo-β-lactamase (NDM)-producing Enterobacter cloacae, during the height of the COVID-19 pandemic in the city [56]. All but one of these patients were receiving mechanical ventilation at the time of infection and 5 of the 13 patients died. Genomic sequencing identified multiple lineages, suggesting that this was not an outbreak caused by a single strain. Another NYC hospital reported five patients with NDM-producing E. cloacae infections who did not have recent international travel history and were admitted from the community [57]. Four of these five patients died of septic shock.
A hospital in New Jersey reported a large increase in the number of patients with carbapenem-resistant Acinetobacter baumannii (CRAB) during a surge in COVID-19 admissions [58]. They identified 34 patients infected or colonized with CRAB, none of whom had prior CRAB infection or colonization, and most were admitted from home and were mechanically ventilated at the time the bacteria were identified. Ten of the 20 CRAB-infected patients died. Twenty-six of the isolates harbored an OXA-23 carbapenemase and three had an NDM. Upon investigation, deviations from typical infection prevention and control practices related to the COVID-19 surge were thought to be responsible for this outbreak, including discontinuation of contact precautions for certain MDR pathogens to preserve PPE, decreases in the frequency of changes of suctioning catheters, ventilator circuits, masks, and face shields, less frequent chlorhexidine bathing, suspension of typical activities to monitor the use of central venous and urinary catheters, increased patient-to-staff ratios, and use of staff without experience in an ICU setting. This report highlights how hospitals experiencing surges of patients with COVID-19 might be vulnerable to outbreaks of MDR bacteria (see Outstanding Questions).
Outstanding Questions.
Which clinical features and laboratory tests can reliably identify the small proportion of hospitalized patients with COVID-19 who have bacterial coinfection and who therefore should receive empirical antibacterial therapy and diagnostic testing for bacterial infections?
Will the prevalence of bacterial coinfection upon hospital admission for COVID-19 change in subsequent waves of the pandemic, particularly with the emergence of new SARS-CoV-2 variants?
How will the routine use of corticosteroids change the spectrum of hospital-acquired bacterial infections in patients requiring prolonged hospitalization for COVID-19?
How will the increase in COVID-19 patients who require intensive care around the world impact the emergence of multidrug-resistant Gram-negative bacterial infections?
Alt-text: Outstanding Questions
Increasing reports of carbapenem-resistant Gram-negative bacterial infections have also been reported in European hospitals during COVID-19 surges. In one Italian hospital, 19% of blood cultures were positive for KPC-producing K. pneumoniae in an ICU devoted to COVID-19 patients [59]. Other hospitals in Italy reported increases in transmission of KPC-producing K. pneumoniae that were attributed to similar factors as in the above CRAB outbreak, as well as to the large number of healthcare workers required to pronate hypoxic COVID-19 patients [60,61]. A hospital in France reported an outbreak of NDM-producing Escherichia coli in an ICU dedicated to COVID-19 patients that was attributed to a lack of compliance with standard and contact precautions, overworked and inexperienced ICU staff, and lack of typical screening for carriage of MDR Gram-negative pathogens [62].
Concluding Remarks
Unlike in prior influenza pandemics, a relatively small proportion of patients hospitalized with COVID-19 initially presented with bacterial coinfection. Based on currently available data, we recommend that empirical antibacterial therapy and blood and respiratory tract cultures should be reserved for patients who are critically ill, severely immunocompromised, or who have multiple clinical features suggestive of bacterial pneumonia; however, additional research is needed to more accurately identify patients who present with bacterial coinfection (see Outstanding Questions). When bacterial coinfections are present on admission, S. aureus, S. pneumoniae, and H. influenzae are the most common pathogens, and atypical bacterial infections are rare. While bacterial coinfections are rare upon admission, they are common among patients requiring prolonged hospitalization for COVID-19, particularly those who require mechanical ventilation in an ICU. In this setting, P. aeruginosa, Klebsiella spp., and S. aureus are the most common causative agents of hospital-acquired pneumonia. Furthermore, frequent antibiotic use and changes in infection-control practices related to COVID-19 may have also contributed to the emergence of carbapenem-resistant Gram-negative infections.
Current data are primarily from experiences in the first wave of the pandemic. Evaluations of bacterial coinfections during subsequent waves of the pandemic are critical, particularly given changes in the care of these patients, such as the routine use of corticosteroids [63], and the emergence of new SARS-CoV-2 variants [64] (see Outstanding Questions). Optimizing antibacterial therapies in patients with COVID-19 will hopefully minimize the risks of increasing antimicrobial resistance while appropriately treating bacterial coinfections when they occur.
Acknowledgments
Acknowledgments
We would like to acknowledge Pinki Bhatt, Navaneeth Narayanan, and Stephanie Shiau for providing data on hospital-onset bloodstream infections from their manuscript to include in our analysis [54].
Declaration of Interests
There are no interests to declare.
Resources
ihttps://covid19.who.int/References
- 1.Morens D.M., et al. Pandemic COVID-19 Joins History’s Pandemic Legion. mBio. 2020;11 doi: 10.1128/mBio.00812-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memish Z.A., et al. Middle East respiratory syndrome. Lancet. 2020;395:1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta R.K., et al. Bacterial pneumonia and pandemic influenza planning. Emerg. Infect. Dis. 2008;14:1187–1192. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens D.M., et al. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martín-Loeches I., et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A (H1N1) virus. Chest. 2011;139:555–562. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 6.Rice T.W., et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill J.R., et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch. Pathol. Lab. Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arabi Y.M., et al. Critically ill patients with the Middle East Respiratory Syndrome: A multicenter retrospective cohort study. Crit. Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 9.Guan W.J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal P., et al. Clinical characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawson T.M., et al. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support Covid-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lansbury L., et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughn V.M., et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: A multi-hospital cohort study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1239. Published online August 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021;76:796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Vidal C., et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karami Z., et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. (Lond) 2021;53:102–110. doi: 10.1080/23744235.2020.1839672. [DOI] [PubMed] [Google Scholar]
- 17.Hughes S., et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann C.J., et al. Community acquired co-infection in COVID-19: A retrospective observational experience. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa902. Published online July 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler H., et al. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L.S.-K., et al. Bacterial co-infections and antibiotic prescribing practice in adults with COVID-19: experience from a single hospital cluster. Ther. Adv. Infect. Dis. 2020;7 doi: 10.1177/2049936120978095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu Y., et al. Secondary bacterial infections in critically ill patients with coronavirus disease 2019. Open Forum Infect. Dis. 2020;5 doi: 10.1093/ofid/ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elabbadi A., et al. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021 doi: 10.1007/s15010-020-01553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertow D.S., Memoli M.J. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013;309:275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 24.Kolenda C., et al. Assessment of respiratory bacterial coinfections among severe acute respiratory syndrome coronavirus 2-positive patients hospitalized in intensive care units using conventional culture and BioFire, FilmArray Pneumonia Panel Plus Assay. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano M.C., et al. Low incidence of co-infection, but high incidence of ICU-acquired infections in critically ill patients with COVID-19. J. Infect. 2021;82:e20–e21. doi: 10.1016/j.jinf.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson D.R., et al. Improving antimicrobial stewardship in critically-ill patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1559. Published online October 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contou D., et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudoignon E., et al. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin. Infect. Dis. 2021;72:905–906. doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caméléna F., et al. Performance of multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn. Microbiol. Infect. Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothe K., et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2020;40:859–869. doi: 10.1007/s10096-020-04063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sepulveda J., et al. Bacteremia and blood culture utilization during COVID-19 surge in New York City. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00875-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu D., et al. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argenziano M.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369 doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metlay J.P., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2020;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D., et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harza A., et al. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect. Control Hosp. Epidemiol. 2020;41:1228–1229. doi: 10.1017/ice.2020.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oster Y., et al. Decreased prevalence rate of respiratory pathogens in hospitalized patients during the COVID-19 pandemic: possible role for public health containment measures? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.12.007. Published online December 31, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parente D.M., et al. The clinical utility of methicillin-resistant Staphylococcus aureus (MRSA) nasal screening to rule out MRSA pneumonia: A diagnostic meta-analysis with antimicrobial stewardship implications. Clin. Infect. Dis. 2018;67:1–7. doi: 10.1093/cid/ciy024. [DOI] [PubMed] [Google Scholar]
- 39.Punjabi C.D., et al. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in respiratory cultures and diagnostic performance of the MRSA nasal polymerase chain reaction (PCR) in patients hospitalized with coronavirus disease 2019 (COVID-19) pneumonia. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.440. Published online August 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restrepo M.I., et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalized patients. Eur. Respir. J. 2018;9 doi: 10.1183/13993003.01190-2017. [DOI] [PubMed] [Google Scholar]
- 41.Nir-Paz R., et al. Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin. Microbiol. Infect. 2006;12:685–688. doi: 10.1111/j.1469-0691.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 42.Postma D.F., et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N. Engl. J. Med. 2015;372:1312–1323. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- 43.Garin N., et al. β-lactam monotherapy vs. β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern. Med. 2014;174:1894–1901. doi: 10.1001/jamainternmed.2014.4887. [DOI] [PubMed] [Google Scholar]
- 44.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furtado R.H.M., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalcanti A.B., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar G., et al. Predictors and outcomes of HAIs in COVID-19 patients. Int. J. Infect. Dis. 2020;104:287–292. doi: 10.1016/j.ijid.2020.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ripa M., et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin. Microbiol. Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falcone M., et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J. Antimicrob. Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng K., et al. Analysis of the risk factors for nosocomial bacterial infection in patients with COVID-19 in a tertiary care hospital. Risk Manag. Healthc. Policy. 2020;13:2593–2599. doi: 10.2147/RMHP.S277963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardi T., et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maes M., et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care. 2021;25:25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razazi K., et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to coronavirus 19 disease. Crit. Care. 2020;24:699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatt P.J., et al. Risk factors and outcomes of hospitalized patients with severe COVID-19 and secondary bloodstream infections: A multicenter, case-control study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1748. Published online November 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou F., et al. Risk factors for nosocomial infection among hospitalized patients with severe influenza A(H1N1)pdm09 patients. Respir. Med. 2018;134:86–91. doi: 10.1016/j.rmed.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 56.Gomez-Simmonds A., et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J. Antimicrob. Chemother. 2021;76:380–384. doi: 10.1093/jac/dkaa466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nori P., et al. Emerging co-pathogens: New Delhi Metallo-beta-lactamase producing Enterobacterales infections in New York City COVID-19 patients. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez S., et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions – New Jersey, February–July 2020. Morb. Mortal. Wkly Rep. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arcari G., et al. Klebsiella pneumoniae infections in COVID-19 patients: a 2-month retrospective analysis in an Italian hospital. Int. J. Antimicrob. Agents. 2021;57:106245. doi: 10.1016/j.ijantimicag.2020.106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiri B., et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020;25:2744. doi: 10.3390/jcm9092744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belvisi V., et al. Impact of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) pandemic on carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) prevention and control program: convergent or divergent action? J. Hosp. Infect. 2020;109:29–31. doi: 10.1016/j.jhin.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farfour E., et al. Carbapenemase-producing Enterobacterales outbreak: Another dark side of COVID-19. Am. J. Infect. Control. 2020;48:1533–1536. doi: 10.1016/j.ajic.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.RECOVERY Collaborative Group, et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lauring A.S., Hodcroft E.D. Genetic variants of SARS-COV-2-What do they mean? JAMA. 2021;325:529–531. doi: 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]