Abstract
Aims
G protein-coupled receptor kinase 4 (GRK4) has been reported to play an important role in hypertension, but little is known about its role in cardiomyocytes and myocardial infarction (MI). The goal of present study is to explore the role of GRK4 in the pathogenesis and progression of MI.
Methods and results
We studied the expression and distribution pattern of GRK4 in mouse heart after MI. GRK4 A486V transgenic mice, inducible cardiomyocyte-specific GRK4 knockout mice, were generated and subjected to MI with their control mice. Cardiac infarction, cardiac function, cardiomyocyte apoptosis, autophagic activity, and HDAC4 phosphorylation were assessed. The mRNA and protein levels of GRK4 in the heart were increased after MI. Transgenic mice with the overexpression of human GRK4 wild type (WT) or human GRK4 A486V variant had increased cardiac infarction, exaggerated cardiac dysfunction and remodelling. In contrast, the MI-induced cardiac dysfunction and remodelling were ameliorated in cardiomyocyte-specific GRK4 knockout mice. GRK4 overexpression in cardiomyocytes aggravated apoptosis, repressed autophagy, and decreased beclin-1 expression, which were partially rescued by the autophagy agonist rapamycin. MI also induced the nuclear translocation of GRK4, which inhibited autophagy by increasing HDAC4 phosphorylation and decreasing its binding to the beclin-1 promoter. HDAC4 S632A mutation partially restored the GRK4-induced inhibition of autophagy. MI caused greater impairment of cardiac function in patients carrying the GRK4 A486V variant than in WT carriers.
Conclusion
GRK4 increases cardiomyocyte injury during MI by inhibiting autophagy and promoting cardiomyocyte apoptosis. These effects are mediated by the phosphorylation of HDAC4 and a decrease in beclin-1 expression.
Keywords: GRK4, Myocardial infarction, HDAC4, Autophagy, Apoptosis
Graphical Abstract
See page 1431 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa847)
Translational Perspective
Myocardial infarction (MI) causes marked loss of functional cardiomyocytes, which is the fundamental reason for leading to cardiac remodelling and heart failure. The apoptosis and autophagy of cardiomyocytes are critical mechanisms involved in cardiomyocyte loss. The present study identified the role of G protein-coupled receptor kinase 4 (GRK4) in cardiomyocyte apoptosis and autophagy. It showed that GRK4 is up-regulated in the heart and aggravated cardiac remodelling post-MI, while GRK4 deficiency exerted a protective effect. MI patient carrying GRK4 gain-of-function variant A486V exhibited deteriorated cardiac function than wild-type control. These findings suggested that GRK4 polymorphism is associated with the prognosis of cardiac injury and that GRK4 might be an important therapeutic target for treating MI and heart failure.
Introduction
Myocardial infarction (MI) and heart failure are the leading causes of human death worldwide. Despite current treatment, mortality and heart failure due to MI are still substantial.1 MI causes marked loss of functional cardiomyocytes, which is the fundamental pathological process that triggers heart failure. Experimental studies over the last few decades have identified complex signal transduction processes regulating cardiomyocyte death, but novel therapies are still lacking.
G protein-coupled receptor kinases (GRKs) are involved in cardiac injury and failure and are, therefore, promising therapeutic targets for these diseases. Increased GRK2 expression is central to the pathogenesis of heart failure, via desensitization of β-adrenergic receptors, which leads to the loss of contractile reserve.2 GRK2-mediated phosphorylation of adiponectin receptor 1 in failing cardiomyocytes contributes to post-MI remodelling and progression of heart failure.3 GRK5, functioning as a kinase for histone deacetylase (HDAC), exaggerates pathological cardiac hypertrophy by enhancing the activation of myocyte enhancer factor-24 and nuclear factor of activated T cells.5
GRK4, another member of the GRK family, plays an important role in the development of essential hypertension.6 Among the three GRK4 gene variants, R65L, A486V, and A142V, constitutively active A486V and A142V were demonstrated to increase the activity of GRK4, independent of protein abundance.6 Transgenic mice overexpressing human GRK4 A486V have high GRK4 activity and develop hypertension when sodium intake is increased.7 These GRK4 gene variants are associated with human essential hypertension.8 GRK4 has also been reported to be expressed in the heart of rodents8 and humans9, but whether GRK4 plays a role in pathological processes after MI is unknown. The expression of GRK4 in normal heart is low9 , 10 and its role in the heart has not been well studied. The current experiments were performed to determine if and how GRK4 influences myocardial injury.
Methods
Detailed methods are described in the Supplementary material online.
Results
Myocardial infarction increases G protein-coupled receptor kinase 4 expression and nuclear translocation in cardiomyocytes of the infarcted heart
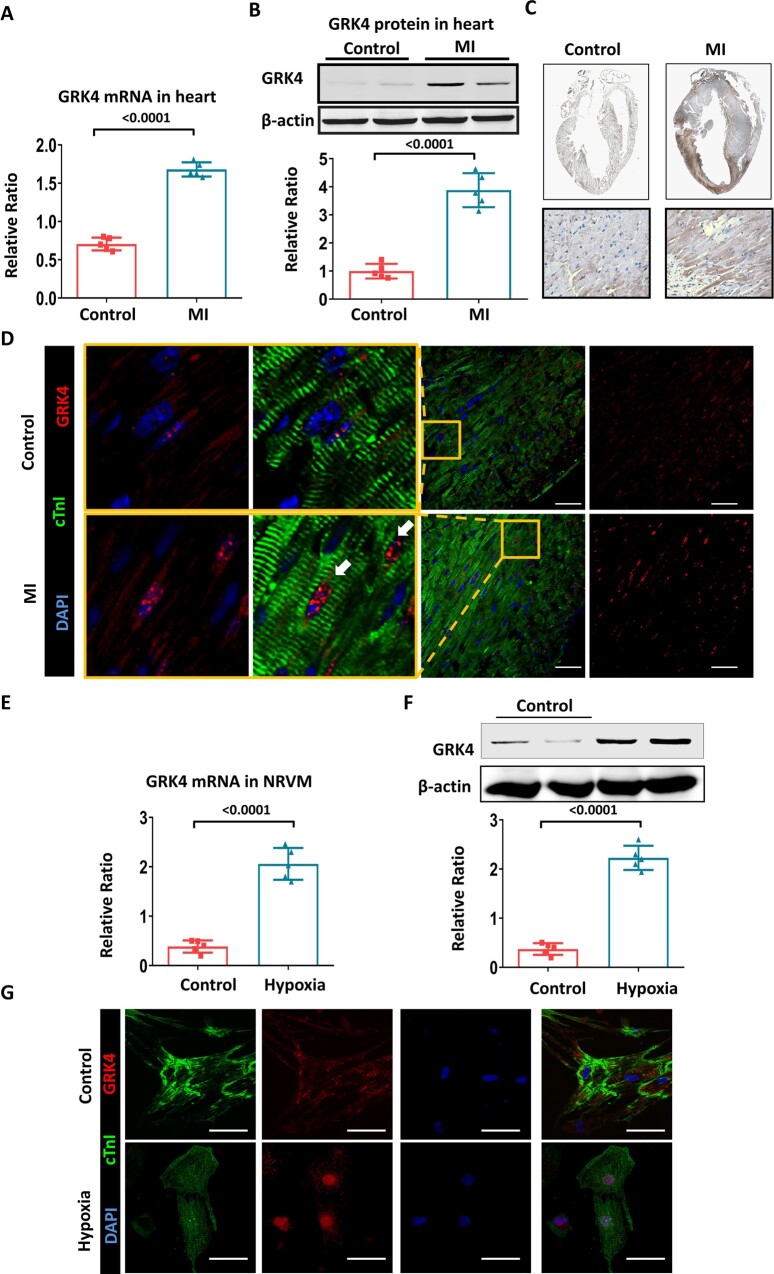
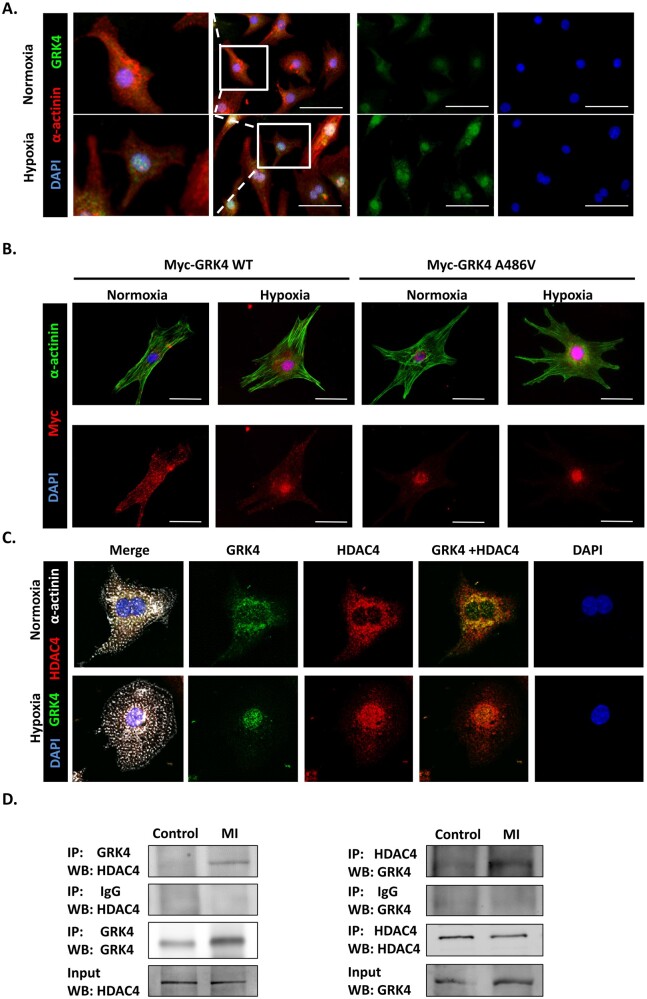
C57BL/6 mice were subjected to MI injury at the age of 2 months. Under normal condition, GRK4 was modestly expressed in the heart; MI significantly increased the mRNA and protein levels of GRK4 (Figure 1A and B). Cardiac GRK4 protein expression after MI increased in a time-dependent manner; it was significantly elevated at 6 h after MI, peaked at 24-48 h, and lasted for 7 days ( Supplementary material online, Figure S1A ). The GRK4 protein was mainly found in the infarct border zone (Figure 1C and Supplementary material online, Figure S1B and C1) and principally located in cardiomyocytes, although weak GRK4 expression was also found in non-cardiomyocytes, including smooth muscle cells, endothelial cells, and fibroblasts but with much lower levels (Supplementary material online, Figure S1C2–C5). MI induced the translocation of GRK4 from the cytosol to nucleus of cardiomyocytes (Figure 1D). In cultured cardiomyocytes [neonatal rat ventricular myocytes (NRVMs)], hypoxia significantly increased GRK4 mRNA and protein levels (Figure 1E and F). Immunostaining confirmed the increased expression and nuclear translocation of GRK4 protein in hypoxic NRVMs (Figure 1G).
Figure 1.
The expression and distribution of G protein-coupled receptor kinase 4 in heart subjected to MI. (A) G protein-coupled receptor kinase 4 mRNA in the hearts of C57BL/6 mice before and 2 days after myocardial infarction, n = 5 per group. (B) Representative G protein-coupled receptor kinase 4 protein bands and their quantification in the heart before and 2 days after myocardial infarction, n = 5 per group. (C) G protein-coupled receptor kinase 4 expression in the heart, before and 2 days after myocardial infarction, detected by immunohistochemistry. (D) G protein-coupled receptor kinase 4 expression and distribution in infarct border zone, yellow boxes indicate the magnified areas. (E) G protein-coupled receptor kinase 4 mRNA in NRVMs with or without hypoxia, n = 5 per group. (F) Representative G protein-coupled receptor kinase 4 protein bands and their quantification in neonatal rat ventricular myocytes with or without hypoxia, n = 5 per group. (G) G protein-coupled receptor kinase 4 expression and distribution in cultured neonatal rat ventricular myocytes; scale bar = 50 μm. Data are expressed as mean ± standard deviation; number above the overbraces in the figures shows P-values. GRK4, G protein-coupled receptor kinase 4; MI, myocardial infarction; NRVMs, neonatal rat ventricular myocytes.
Overexpressing human G protein-coupled receptor kinase 4 A486V caused a greater injury and cardiac dysfunction than overexpressing wild-type human G protein-coupled receptor kinase 4 in transgenic mice
We generated transgenic mice with the overexpression of wild-type (WT) GRK4 or GRK4 A486V variant. Higher GRK4 activity in GRK4 A486V mice was identified by assessing the phosphorylation of a downstream protein target, dopamine D1 receptor (DRD1)6 (Supplementary material online, Figure S2A and B). The high GRK4 activity did not cause pathological changes in major organs, including the heart, kidney, lung, liver, and aorta. The heart rate, cardiac function, blood pressure, and cardiac, kidney, and lung histology of GRK4 WT and GRK4 A486V transgenic mice were comparable to control mice (Supplementary material online, Figure S3A–L).
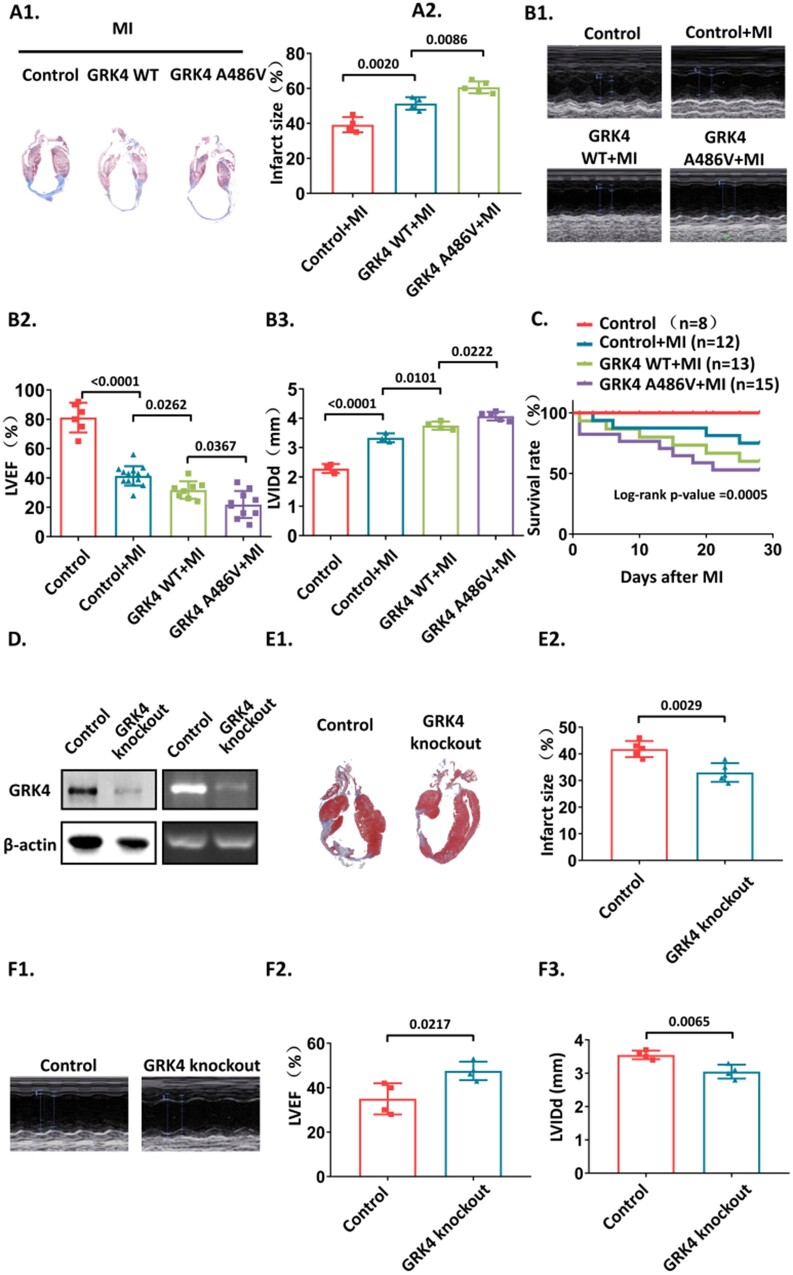
The infarct size and cardiac function were evaluated 4 weeks post-MI. Compared with control mice, GRK4 WT and GRK4 A486V transgenic mice had increased infarct size (Figure 2A), ventricular dilation, and cardiac dysfunction (Figure 2B1–B3) post-MI. The survival rates of GRK4 WT and GRK4 A486V transgenic mice were lower than control mice subjected to MI (Figure 2C). Moreover, the infarction size, cardiac function and left ventricular dilation, and survival rate were worse in GRK4 A486V mice than in GRK4 WT transgenic mice (Figure 2A–C). We also generated inducible cardiomyocyte-specific GRK4 knockout mice (GRK4flox/flox/myh6-MerCreMer mice induced by 4-OH tamoxifen). Myh6-MerCreMer mice with 4-OH tamoxifen injection were used as control (Supplementary material online, Figure S4). Impaired GRK4 activity in heart tissue of conditional cardiomyocyte specific GRK4 knockout mice was revealed by detecting DRD1 phosphorylation (Supplementary material online, Figure S2C). The GRK4 protein levels in the hearts of conditional cardiomyocyte-specific GRK4 knockout mice were significantly lower than that in control mice (Figure 2D). The hearts of conditional cardiomyocyte specific GRK4 knockout mice had smaller infarct size (Figure 2E), better cardiac function, and lesser left ventricular dilation post-MI than control mice (Figure 2F1–F3).
Figure 2.
The effect of G protein-coupled receptor kinase 4 gain and loss of function on myocardial infarction. (A) Infarct size in the hearts of the indicated group was detected by Masson's trichrome staining 4 weeks post-myocardial infarction, n = 4–5 per group. (B) left ventricular ejection fraction (LVEF) and left ventricular internal diameter end diastole (LVIDd) were measured by echocardiography, n = 4–12 per group. (C) The Kaplan–Meier survival rate of the mice subjected to myocardial infarction. The methodology used for statistical analysis is log-rank (Mantel–Cox) test, n = 8–15 per group. (D) G protein-coupled receptor kinase 4 protein and mRNA levels from control (myh6-MerCreMer) and inducible cardiomyocyte-specific G protein-coupled receptor kinase 4 knockout mice post-myocardial infarction. (E) The infarct size in the hearts from control and inducible cardiomyocyte-specific knockout mice 4 weeks post-myocardial infarction, n = 5 per group. (F) LVEF and LVIDd of control and inducible cardiomyocyte-specific G protein-coupled receptor kinase 4 knockout mice 4 weeks post-myocardial infarction, n = 4–5 per group. Data are expressed as mean ± standard deviation; number above the overbraces in the figures shows P-values. GRK4, G protein-coupled receptor kinase 4; MI, myocardial infarction; WT, wild type.
These data suggest that GRK4 enhanced MI-induced cardiac injury, which was further aggravated by the GRK4 A486V variant with high GRK4 activity. GRK4 deficiency ameliorates MI-induced cardiac injury.
G protein-coupled receptor kinase 4 increased cardiomyocyte apoptosis and decreased cardiomyocyte autophagy post-myocardial infarction
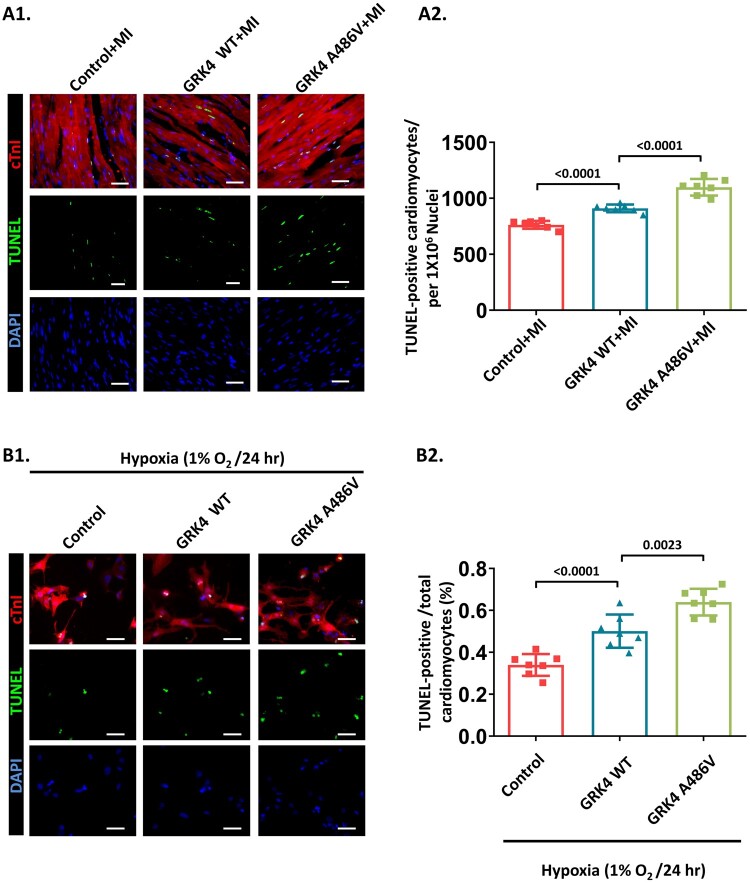
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay was performed on heart tissue sections containing border zones collected at 2 days post-MI. Compared with control mice, GRK4 transgenic mice had increased TUNEL+ cardiomyocytes that were greater in GRK4 A486V mice than in GRK4 WT transgenic mice (Figure 3A), although the GRK4 protein levels in GRK4 WT and GRK4 A486V groups were comparable (Supplementary material online, Figure S5A). In in vitro experiments, NRVMs with the indicated GRK4 adenovirus transfection were subjected to hypoxia. WT GRK4 overexpression increased cardiomyocyte apoptosis, which was augmented by GRK4 A486V overexpression (Figure 3B), while GRK4 levels and transfection efficiency in GRK4 WT- and GRK4 A486V-transfected NRVMs were also comparable (Supplementary material online, Figure S5B1 and B2). These results indicate that the GRK4-augmented cardiac injury is associated with increased cardiomyocyte apoptosis.
Figure 3.
G protein-coupled receptor kinase 4 increases cardiomyocyte apoptosis in post-myocardial infarction heart. (A) Representative images and quantification of the cardiomyocytes apoptosis (TUNEL staining) in border zone of heart tissue 2 days post-myocardial infarction, n = 7 per group. (B) Hypoxia-induced apoptosis in neonatal rat ventricular myocytes with G protein-coupled receptor kinase 4 WT or G protein-coupled receptor kinase 4 A486V overexpression assessed by terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay, n = 7 per group. Scale bar = 50 μm. Data are expressed as mean ± standard deviation; number above the overbraces in figures shows P-values. GRK4, G protein-coupled receptor kinase 4; MI, myocardial infarction; WT, wild type.
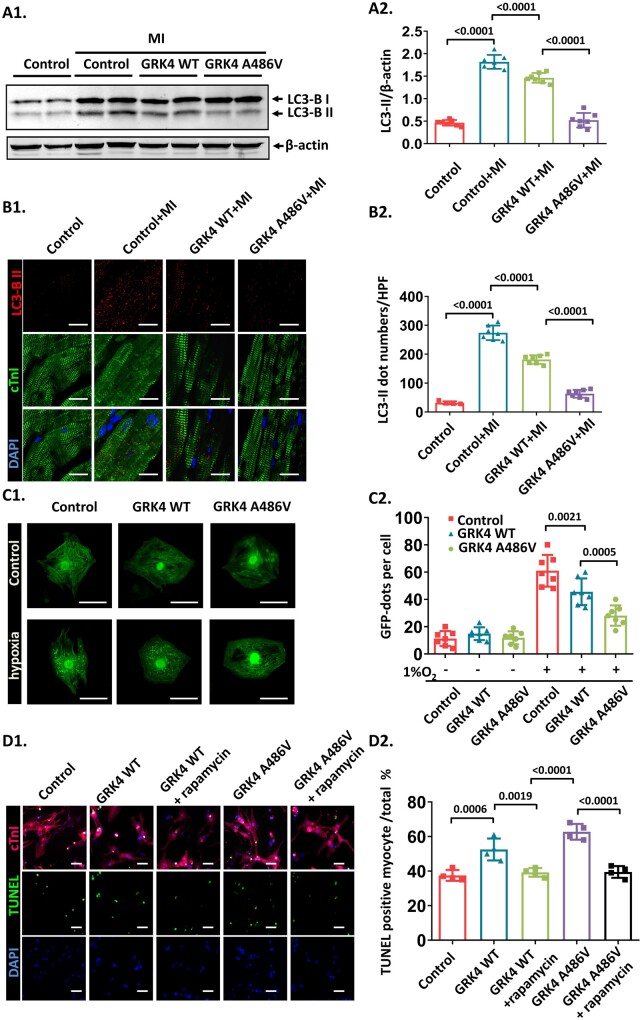
Autophagy coordinates and affects apoptosis that determines cardiomyocyte fate during ischaemic cardiac injury.11 The expression of microtubule-associated protein-1 light chain 3-II (LC3-II), a marker of autophagy,11 , 12 was increased by MI, which was dampened in GRK4 transgenic mice and to a greater extent in GRK4 A486V mice than in GRK4 WT transgenic mice (Figure 4A). Immunostaining showed increased the LC3-II in infarct border zone of the post-MI hearts, which was blunted in GRK4 transgenic mice (to a greater extent in GRK4 A486V mice than in GRK4 WT mice) (Figure 4B). We also quantified the protein expressions of other autophagy markers ATG5, ATG7, and p62 in the mouse heart. ATG5 and ATG7 were up-regulated after MI in control mice; the increases in ATG5 and ATG7 proteins after MI were blunted in GRK4 WT and GRK4 A486V mice. By contrast, p62, an autophagy adaptor protein, which accumulates when autophagy is inhibited,12 was decreased after MI in control mice and to a lesser extent in GRK4 WT mice but increased in GRK4 A486V mice (Supplementary material online, Figure S6). Electron microscopy showed that MI induced the formation of autophagy vacuoles in cardiomyocytes in control (or sham mice). The quantity of autophagy vacuoles was decreased in GRK4 WT and GRK4 A486V mice after MI (Supplementary material online, Figure S7). These data show that GRK4 (to a greater extent in GRK4 A486V mice than in GRK4 WT mice) represses autophagy in the heart after MI.
Figure 4.
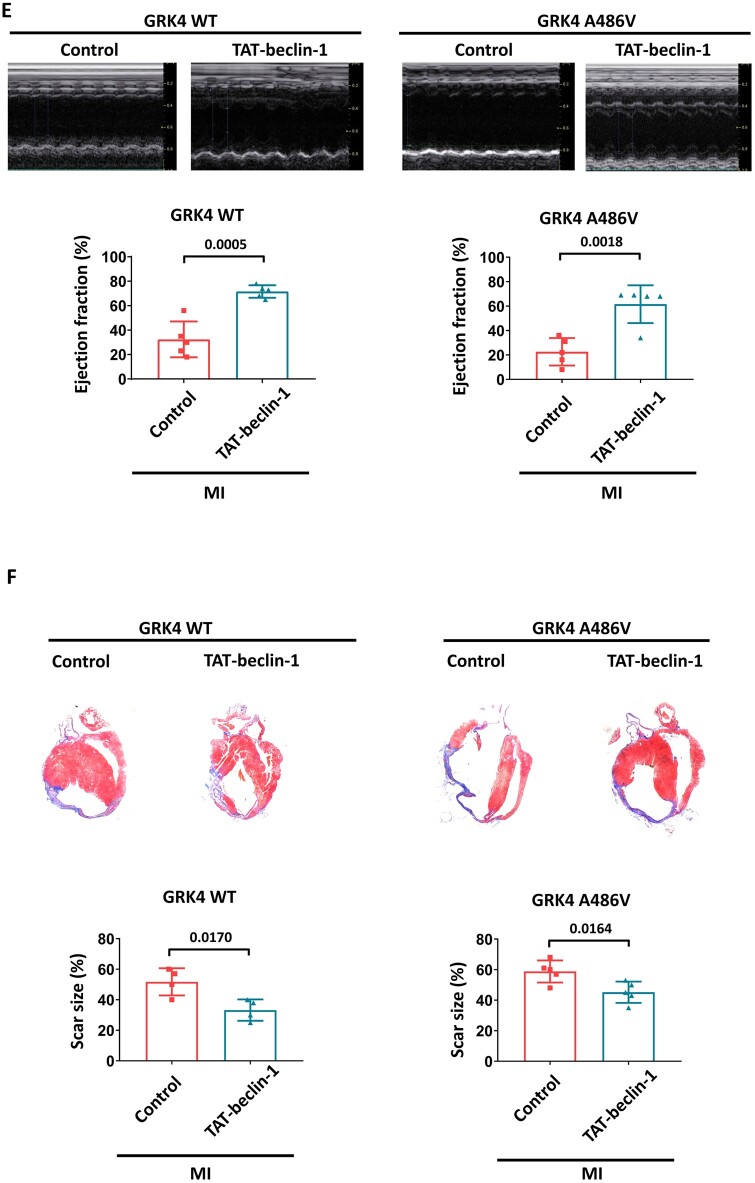
G protein-coupled receptor kinase 4 decreases cardiomyocyte autophagy in post-myocardial infarction heart. (A) LC3-BII protein levels in heart tissue 1 week post-myocardial infarction, n = 7 per group. (B) LC3-BII dots analysed by immunostaining in heart tissue 1 week post-myocardial infarction, n = 7 per group. Scale bar = 20 μm. (C) Neonatal rat ventricular myocytes transfected with Ad-GFP-LC3 and indicated adenovirus with or without hypoxia treatment. The representative images and quantification of GFP dots are shown, n = 7 per group. (D) The effect of rapamycin on hypoxia-induced apoptosis in neonatal rat ventricular myocytes with G protein-coupled receptor kinase 4 WT or A486V overexpression, n = 4 per group. Scale bar = 50 μm. (E) The representative images and quantification of echocardiographic data of mice 4 weeks after myocardial infarction treated with control (TAT-scramble) or TAT-beclin-1, n = 4–5 per group. (F) The scar size of the infarcts in the hearts of the indicated groups, measured by Masson staining, n = 4–5 per group. Data are expressed as mean ± standard deviation; numbers above the overbraces show P-values. GRK4, G protein-coupled receptor kinase 4; MI, myocardial infarction; WT, wild type.
We also found that hypoxia induced GFP-LC3-II dot formation and protein expression, which was blunted in cardiomyocytes overexpressing GRK4 WT or GRK4 A486V (Figure 4C and Supplementary material online, Figure S8). In conditional cardiomyocyte-specific GRK4 knockout mice, the LC3-II formation was significantly increased after MI compared with control (Supplementary material online, Figure S9). Bafilomycin A1, a lysosomal inhibitor that can block the degradation of LC3-II in autophagy flux, did not affect the LC3-II levels repressed by GRK4 in vitro and in vivo (Supplementary material online, Figures S8 and S10), suggesting that the decreased LC3-II formation was not due to the accelerated degradation in autophagy flux. In addition, the autophagy agonist rapamycin12 reversed the GRK4 WT- and GRK4 A486V-induced repression of LC3-II (Supplementary material online, Figure S11) and normalized the apoptosis (TUNEL positive) in cardiomyocytes expressing GRK4 WT and GRK4 A486V (Figure 4D1 and D2). Treatment with the autophagy inducer TAT-beclin-1 rescued the impaired cardiac function and decreased the enlarged scar size caused by GRK4 A486V variant (Figure 4E and F). These data support autophagy as the mechanism by which GRK4 aggravates MI injury, rather than just serving as an injury marker.
G protein-coupled receptor kinase 4 regulated beclin-1 expression and autophagy in cardiomyocytes via an HDAC4-dependent mechanism
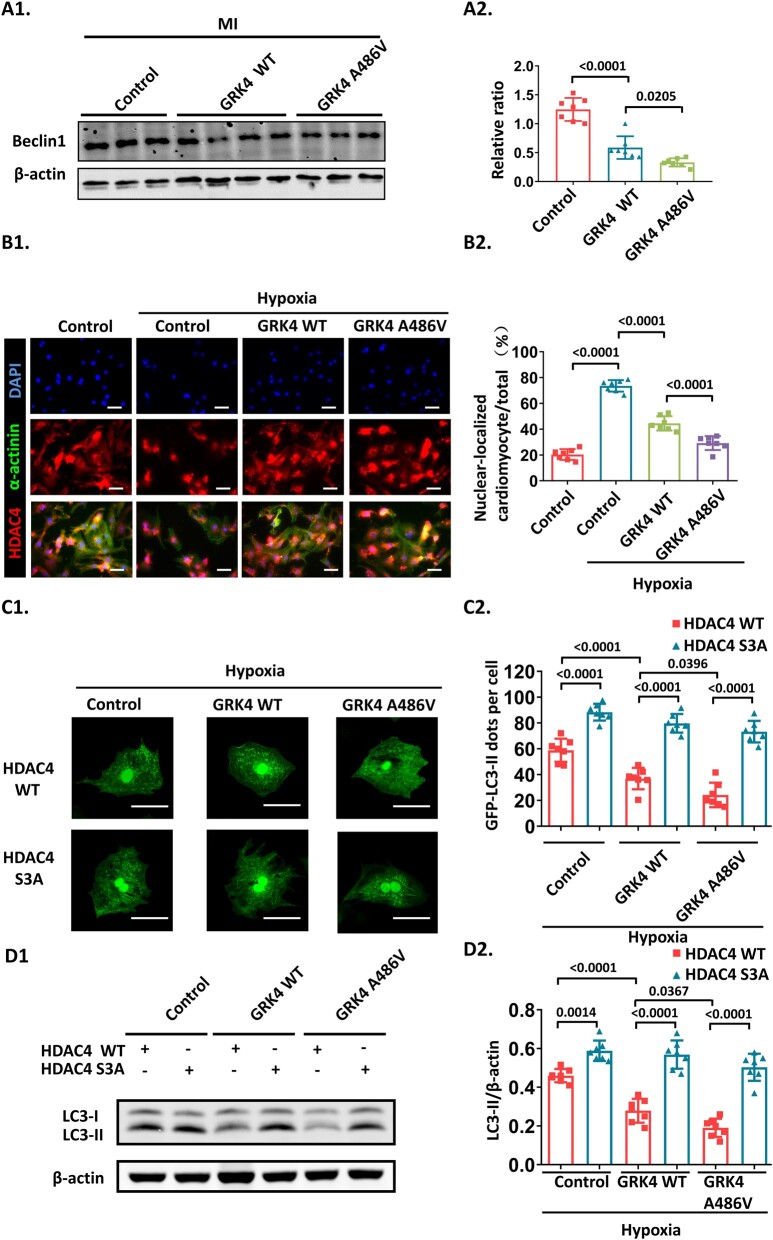
Beclin-1 is essential for initiating autophagosome nucleation; inhibition of beclin-1 decreases LC3-II protein levels and impairs autophagy.12 Compared with post-MI control mice, cardiac beclin-1 protein expression was deceased in GRK4 transgenic mice (to a greater extent in GRK4 A486V mice than in GRK4 WT mice) (Figure 5A). How GRK4 impairs beclin-1 expression is unknown. Previous reports showed that GRKs may regulate HDAC function, thereby influencing gene expression.4 , 13 Moreover, HDAC4 participates in autophagy regulation by up-regulating beclin-1 in rodent vascular endothelial and smooth muscle cells.14 Our data showed that hypoxia induced HDAC4 nuclear translocation in NRVMs, which was blunted by GRK4 WT or to a greater extent by GRK4 A486V overexpression (Figure 5B). In addition, the LC3-II formation in cardiomyocyte-specific GRK4 knockout mice post-MI was impaired by HDAC4 knockdown with HDAC4 siRNA (Supplementary material online, Figure S9), indicating that HDAC4 was involved in GRK4-mediated inhibition of autophagy.
Figure 5.
G protein-coupled receptor kinase 4 decreases beclin-1 and inhibits autophagy by impairing HDAC4 nuclear localization. (A) Beclin-1 protein levels in heart tissue at 1 week post-myocardial infarction, n = 7 per group. (B) The nuclear localization of HDAC4 observed by immunostaining, n = 7 per group. (C) The representative images and quantification of GFP-light chain 3-II dots in neonatal rat ventricular myocytes transfected with Ad-GFP-LC3, indicated adenovirus, and cultured under hypoxia, n = 7 per group. (D) Light chain 3-II protein in neonatal rat ventricular myocytes transfected with indicated adenovirus and cultured under hypoxia, n = 7 per group. (E) The hypoxia-induced apoptosis in neonatal rat ventricular myocytes transfected with indicated adenovirus, n = 7 per group. Scale bar = 50 μm. Data are expressed as mean ± standard deviation; numbers above the overbraces show P-values. GRK4, G protein-coupled receptor kinase 4; LC3-I, light chain 3-I; LC3-II, light chain 3-II; MI, myocardial infarction; WT, wild type.
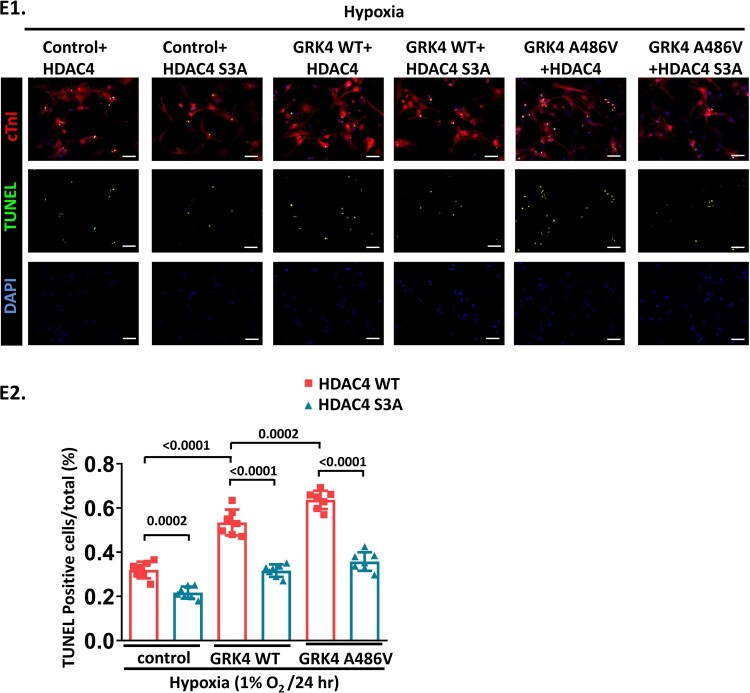
A previous study showed that the nuclear-cytosol shuffling of HDAC4 is regulated by the phosphorylation of 246/467/632 serine.15 HDAC4 S3A, the 246, 467, and 632 serine to alanine mutant, which is resistant to phosphorylation, caused a constitutive nuclear localization of HDAC4 and partially rescued the decrease in LC3-II expression and aggravation of apoptosis induced by GRK4 in NRVMs under hypoxia. The GFP-LC3 dots repressed by GRK4 in NRMVs subjected to hypoxia were increased in adenovirus-mediated HDAC4 S3A overexpressing cardiomyocytes (Figure 5C–E), while comparable exogenous amount and transfection efficiency of HDAC4 were verified (Supplementary material online, Figure S5C1–C4). In NRVMs overexpressing GRK4/HDAC4 and in post-MI heart infarct border zone, the phosphorylation of HDAC4 S632, along with the interaction between 14-3-3 and HDAC4 (which correlates with HDAC4 phosphorylation and is a surrogate marker of HDAC4 phosphorylation), was significantly increased by GRK4 WT and further increased by GRK4 A486V (Supplementary material online, Figure S12A–C). In NRVMs, the increased HDAC4 phosphorylation and the decreased LC3-II protein expression, with GRK4 A486V overexpression in HDAC4 WT, were blocked by adenovirus overexpressing HDAC4-S632A (resistant to serine 632 phosphorylation) (Supplementary material online, Figure S12D). These data indicate that HDAC4 phosphorylation at S632 is necessary for the GRK4-mediated regulation of cardiomyocyte autophagy. By contrast, HDAC phosphorylation at S246 is not affected by GRK4 WT or GRK4 486V (Supplementary material online, Figure S12C1 and C2).
G protein-coupled receptor kinase 4 interacts with HDAC4 and reduces its binding to beclin-1 promoter
We further investigated how GRK4 regulates HDAC4 in cardiomyocytes. In NRVMs, GRK4 was distributed in both the cytoplasm and the nucleus under normal conditions. However, GRK4 aggregated into the nucleus after hypoxia (Figure 6A), and significantly more in NRVMs transfected with Myc-GRK4 A486V (Figure 6B). Moreover, the GRK4 expression in the nuclei was increased several hours post-MI that peaked at 24–48 h (Supplementary material online, Figure S13A), jibing with the nuclear HDAC4 localization time course (Supplementary material online, Figure S13B). Confocal microscopy revealed that GRK4 co-localized with HDAC4 in the nuclei in NRVMs, which was increased by hypoxia (Figure 6C). In the hearts from the in vivo studies, GRK4 co-immunoprecipitated with HDAC4 (Figure 6D). Proximity ligation assay on heart sections showed that the fluorescence signal was observed in the nuclei of post-MI heart, confirming the nuclear co-localization of GRK4 and HDAC4 (Supplementary material online, Figure S14). These results support the assumption that GRK4 translocated into the nucleus, interacted with HDAC4, and affected its function in MI/hypoxia. Furthermore, ChIP assay showed that the HDAC4 bound to the beclin-1 promoter (−1218 to 1211 bp of the beclin-1 promoter, containing FOXO3 binding site ‘GGAAAACA’). The binding was significantly reduced in GRK4 WT and GRK4 A486V mouse heart, to a greater extent in the latter than in the former, demonstrated by polymerase chain reaction (PCR) and quantified by real-time PCR (Supplementary material online, Figure S15A). There was no ChIP in negative control (3′-untranslated region) (Supplementary material online, Figure S15B). These results indicate that GRK4 can translocate into the nucleus and phosphorylate HDAC4 and, thus, decrease its binding to beclin-1 promoter.
Figure 6.
The interaction between G protein-coupled receptor kinase 4 and HDAC4 in hypoxic cardiomyocytes. (A) The distribution of G protein-coupled receptor kinase 4 in neonatal rat ventricular myocytes in normoxia/hypoxia, scale bar = 50 μm. (B) The G protein-coupled receptor kinase 4 distribution in neonatal rat ventricular myocytes transfected with indicated adenovirus in normoxia/hypoxia, scale bar = 25 μm. (C) The co-localization of G protein-coupled receptor kinase 4 and HDAC4 in neonatal rat ventricular myocytes analysed by confocal microscopy. (D) The interaction between HDAC4 and G protein-coupled receptor kinase 4 in mouse heart determined by co-immunoprecipitation. Left panel: the protein sample enriched by anti-G protein-coupled receptor kinase 4 antibody and probed with the indicated antibodies. Right panel: the protein sample enriched by anti-HDAC4 antibody and probed with the indicated antibodies. The experiment was repeated for three times. GRK4, G protein-coupled receptor kinase 4; WT, wild type.
Patients with G protein-coupled receptor kinase 4 A486V mutant have augmented myocardial infarction-induced injury
Constitutively active GRK4 A486V has increased activity that is independent of its protein abundance.8–10 A total of 550 patients were enrolled to investigate the effect of GRK4 on the cardiac function in patients with MI, and multivariate linear regression analyses were performed. The ejection fraction (EF) % (mean ± SD) of GRK4 486 A:A (WT), GRK4 486 A:V (heterozygous variant), and GRK4 486 V:V (homozygous variant) were 63.49 ± 9.3 (n = 111), 60.83 ± 10.81 (n = 295), and 57.9 ± 11.17 (n = 144), respectively. Potential covariates considered in the regression model included age, sex, history of hypertension and diabetes, history of heart failure and MI, smoking, serum lipids, serum creatinine, serum glycated haemoglobin, ST-segment elevation myocardial infarction (STEMI)/non-STEMI, culprit vessel and stent number, diseased vessel number, thrombolysis In Myocardial Infarction (TIMI) flow grade, and time of symptom onset to percutaneous coronary intervention (Table 1). The data showed that GRK4 A486V (486 A:V and V:V) genotypes were independent factors, that predicted a lower EF% than GRK4 WT (486 A:A) (Table 2).
Table 1.
Basic characteristics of myocardial infarction patients carrying G protein-coupled receptor kinase 4 wild type or G protein-coupled receptor kinase 4 A486V
| N | GRK4 A486V polymorphism |
P-value | ||
|---|---|---|---|---|
| A:A | A:V | V:V | ||
| 111 | 295 | 144 | ||
| Age | 62.91 ± 11.12 | 63.63 ± 12.11 | 63.5 ± 11.21 | 0.851 |
| Male sex, n (%) | 92 (83%) | 248 (84%) | 125 (86%) | 0.427 |
| BMI | 24.47 ± 3.33 | 24.24 ± 3.33 | 24.03 ± 2.79 | 0.782 |
| Systolic blood pressure (mmHg) | 125.6 ± 16.3 | 129.4 ± 22.10 | 127.1 ± 21 | 0.669 |
| Diastolic blood pressure (mmHg) | 77.4 ± 17.5 | 77.32 ± 15.02 | 77.84 ± 14.53 | 0.937 |
| History of hypertension, n (%) | 51 (46%) | 157 (53.2%) | 74 (51.3%) | 0.393 |
| History of diabetes, n (%) | 22 (20%) | 62 (21%) | 27 (19%) | 0.231 |
| History of smoking, n (%) | 44 (40%) | 130 (44%) | 60 (42%) | 0.335 |
| History of MI, n (%) | 6 (5.4%) | 11 (1.6%) | 5 (4%) | 0.413 |
| History of heart failure, n (%) | 6 (5.4%) | 10 (3%) | 4 (3%) | 0.214 |
| Cholesterol (mmol/L) | 4.9 ± 1.4 | 4.8 ± 1.2 | 4.8 ± 1.5 | 0.468 |
| Triglyceride (mmol/L) | 1.54 ± 0.79 | 1.53 ± 0.8 | 1.55 ± 0.75 | 0.310 |
| HDL (mmol/L) | 1.15 ± 0.32 | 1.17 ± 0.30 | 1.16 ± 0.29 | 0.282 |
| LDL (mmol/L) | 2.68 ± 1.18 | 2.57 ± 1.18 | 2.43 ± 1.18 | 0.274 |
| BUN (mmol/L) | 5.58 ± 2.13 | 5.51 ± 2.13 | 5.64 ± 1.95 | 0.391 |
| Creatinine (mmol/L) | 87.40 ± 33.34 | 89.40 ± 31.14 | 88.30 ± 32.24 | 0.246 |
| HbA1c (%) | 6.47 ± 1.2 | 6.37 ± 1.1 | 6.41 ± 1.3 | 0.439 |
| Culprit vessel | 0.468 | |||
| LAD | 61 (55%) | 160 (54%) | 81 (56%) | |
| LCX | 27 (24%) | 77 (26%) | 33 (22%) | |
| RCA | 23 (21%) | 58 (20%) | 31 (22%) | |
| Stent number implanted | 1.17 ± 0.91 | 1.22 ± 0.92 | 1.3 ± 0.85 | 0.292 |
| Number of diseased vessels | ||||
| 1 vessel | 53 (48%) | 138 (47%) | 66 (46%) | 0.410 |
| 2 vessels | 51 (46%) | 141 (48%) | 70 (48%) | 0.377 |
| 3 vessels | 7 (6%) | 16 (5%) | 8 (6%) | 0.323 |
| TIMI flow grade before PCI | 0.522 | |||
| TIMI 0, n (%) | 34 (30.6%) | 71 (24%) | 40 (27%) | |
| TIMI 1, n (%) | 2 (1.8%) | 9 (3%) | 4 (2.7%) | |
| TIMI 2, n (%) | 3 (2.7%) | 11 (3.7%) | 7 (4.8%) | |
| TIMI 3, n (%) | 72 (64.8%) | 202 (68.4%) | 93 (64.5%) | |
| TIMI flow grade after PCI | 0.517 | |||
| TIMI 0, n (%) | 2 (12%) | 5 (1.6%) | 3 (2%) | |
| TIMI 1, n (%) | 0 (0%) | 1 (0.003%) | 0 (0%) | |
| TIMI 2, n (%) | 0 (0%) | 2 (0.006%) | 3 (2%) | |
| TIMI 3, n (%) | 109 (98%) | 287 (97%) | 138 (96%) | |
| STEMI, n (%) | 45 (40.07%) | 116 (39.32) | 64 (44.0%) | 0.609 |
| Time of symptom onset to PCI (h) | 95 ± 138 | 86 ± 123 | 90 ± 117 | 0.441 |
The patients diagnosed with MI were divided into GRK4 486 A:A (GRK4 WT), GRK4 486 A:V (GRK4 486, heterozygotes), and GRK4 486 V:V (GRK4 486V homozygotes) groups, according to genotyping results. These data of patients are presented as mean ± standard deviation and analysed by Pearson χ 2, Kruskal–Wallis tests, or analysis of variance (ANOVA) according to the type of variables. HbA1c, hemoglobin A1c; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery.
GRK4, G protein-coupled receptor kinase 4, MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery.
Table 2.
Ejection fraction % of G protein-coupled receptor kinase 4 wild type and A486V variants carrier
| Unstandardized coefficient |
Standard coefficient | P * | ||
|---|---|---|---|---|
| β | Standard error | |||
| GRK4 486 A:V | −3.953 | 1.782 | −1.81 | 0.028 |
| GRK4 486 V:V | −5.39 | 2.19 | −1.98 | 0.015 |
The EF% of GRK4 486 A:A, GRK4 486 A:V, and GRK4 486 V:V patients were analysed by multivariable linear regression adjusted for multivariables, indicated in Table 1. GRK4 486 A:A (GRK4 WT) served as reference.
EF, ejection fraction; GRK4, G protein-coupled receptor kinase 4.
Adjusted for basic characteristics described in Table 1, GRK 486 A:V and GRK4 486 V:V were compared with GRK4 486 A:A.
Discussion
In the present study, we found that GRK4 expression and nuclear localization are increased by MI in cardiomyocytes. GRK4 overexpression exaggerated MI injury while inducible cardiomyocyte-specific GRK4 knockout ameliorated MI-induced cardiac dysfunction and remodelling. In cardiomyocytes, GRK4 inhibited autophagy and promoted apoptosis, through a mechanism that involved the phosphorylation of HDAC4 and a decrease in beclin-1 expression. The GRK4 A486V variant with constitutive activity further exaggerated those detrimental effects. These data suggest that GRK4 may be a therapeutic target in the treatment of MI.
GRK4 plays a critical role in essential hypertension because its increased expression or variants impair the ability to excrete a salt load by desensitizing dopamine receptors and increasing AT1 receptor expression in the kidney.8–10 In humans, GRK4 activity is constitutively increased by GRK4 R65L, GRK4 A142V, and GRK4 A486V,6 among which GRK4 A486V is most frequent in the Asian population.6 However, little is known about the role of GRK4 and its polymorphisms in cardiac ischaemic injury. Indeed, GRK4 expression has not been consistently shown in normal heart,8–10 , 16 and minimally expressed in healthy left ventricles in humans.9 Based on clinical data, a total of 550 patients was enrolled to investigate the effect of GRK4 on cardiac function in patients with MI. Potential covariates were considered in the regression model and multivariate linear regression analyses were performed. The data showed that GRK4 A486V genotypes were independent risk factors of EF% post-MI and indicated that patients carrying GRK4 A486V may suffer more severe myocardial injury after MI.
The two members of the GRK family, GRK2 and GRK5, are abundantly expressed in the heart and elevated under ischemia2 and ventricular overload.4 However, the characteristics of GRK4 expression in ischaemic heart have not been reported. The present study is the first to describe the expression and distribution of GRK4 in heart tissue before and after MI. GRK4 was modestly expressed in the normal mouse heart, but its expression was increased by MI, mainly in the infarct border zone, in a time-dependent manner. Hypoxia could stimulate GRK4 expression in cultured cardiomyocytes. GRK4 was located in both cytoplasm and nucleus of cardiomyocytes, while MI or hypoxia induced its nuclear translocation. We further demonstrated the effect of GRK4 on cardiac function in human GRK4 WT or GRK4 A486V transgenic mice and inducible cardiomyocyte-specific GRK4 knockout mice. Our data showed that human GRK4 positively regulates cardiomyocyte apoptosis in the ischaemic myocardium. Either high expression or high activity of GRK4 was harmful to the heart subjected to MI. Transgenic mice expressing human GRK4 WT or human GRK4 A486V did not develop hypertension and cardiomyopathy; thus, the augmented ischaemic injury in human GRK4 WT or human GRK4 A486V mice was not a secondary effect of elevated blood pressure.
Autophagy, which is required to maintain normal heart function, is up-regulated after MI. Activation of autophagy can reduce MI-induced cardiomyocyte apoptosis and cardiac dysfunction.12 Our data showed that GRK4 overexpression significantly attenuated autophagy after MI. Moreover, the autophagy agonist rapamycin rescued the aggregated apoptosis in GRK4-overexpressing cardiomyocytes. This result is in agreement with a previous study showing that rapamycin protects cardiomyocytes from apoptosis induced by ischemia/reperfusion injury.17
How GRK4 influences cardiomyocyte autophagy is unknown. Previous studies showed that GRK can target HDACs, thereby regulating gene expressions.13 Among the HDAC family members, HDAC4 can both positively regulate autophagy and ameliorate ischaemic injury. HDAC4 has been reported to induce the expression of beclin-1 and other autophagy-related genes by the deacetylation of transcription factors, such as FOXO3a.14 , 18 In our experiments, GRK4 attenuated the HDAC4 nuclear translocation in cardiomyocytes. The HDAC4 phosphorylation-resistant mutant S3A (serine 246/467/632 to alanine mutation), which leads to constitutive nuclear localization of HDAC4, restored the GRK4-induced autophagy inhibition and apoptosis induction in cardiomyocytes, indicating that the GRK4’s effect on cardiac injury is dependent on HDAC4 phosphorylation. Our result also suggested that, among the serine 246, 467, and 632 phosphorylation sites, serine 632 was phosphorylated by GRK4. Moreover, hypoxia stimulated the co-localization of GRK4 and HDAC4 in cardiomyocytes; co-immunoprecipitation and proximal ligation assay suggested the interaction between the two proteins. ChIP assay showed HDAC4 binding with beclin-1 promoter, which was blocked by GRK4 variants. These results indicate that GRK4 can translocate into the nucleus and phosphorylate HDAC4, thus decrease its binding with beclin-1 promoter, thereby influencing autophagy.
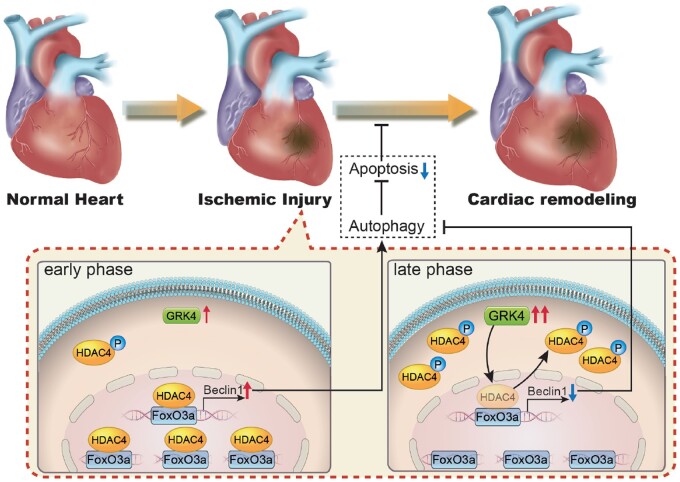
In conclusion, high expression or activity of GRK4 increased cardiomyocyte death and cardiac dysfunction after MI, while inhibiting GRK4 exerts a therapeutic effect against MI. GRK4 inhibits autophagy by blunting HDAC4 function and subsequently decreasing beclin-1 expression (Take home figure). GRK4 may be a potential therapeutic target for ischaemic heart injury.
Take home figure.
GRK4 aggravates cardiac ischemic injury via inhibiting autophagy in a HDAC4-dependent way. Autophagy activity is protective for cardiomyocyte apoptosis in post-myocardial infarction heart. Nuclear accumulated HDAC4 regulates the binding of FoxO3a with beclin1 promoter, thereby increasing the expression of beclin1 and autophagy. Ischaemic injury stimulates GRK4 expression in cardiomyocyte. Elevated GRK4 translocate into nuclei, phosphorylates HDAC4 and promotes its nuclear export. This inhibits the effect of HDAC4 on autophagy, thereby aggravating cardiomyocyte apoptosis and cardiac remodeling.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81922005, 81930008, 81970306), National Key Research & Development Program of China (2018YFC1312700, 2018YFA0107403), Program of Innovative Research Team by National Natural Science Foundation (81721001), Program for Changjiang Scholars and Innovative Research Team in University; IRT1216), and National Institutes of Health (USA), R01DK039308, R01HL119652, and P01HL74940.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Contributor Information
Liangpeng Li, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Wenbin Fu, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Xue Gong, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Zhi Chen, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Luxun Tang, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Dezhong Yang, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Qiao Liao, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Xuewei Xia, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Hao Wu, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Chao Liu, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Miao Tian, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Andi Zeng, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Lin Zhou, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Pedro A Jose, Division of Renal Disease & Hypertension, The George Washington University School of Medicine and Health Sciences, Walter G. Ross Hall, Suite 745C, 2300 I Street, N.W. Washington, DC 20037, USA.
Ken Chen, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China.
Wei Eric Wang, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China; State Key Laboratory of Trauma, Burns and Combined Injury, Daping Hospital, The Third Military Medical University, Chongqing, China.
Chunyu Zeng, Department of Cardiology, Daping Hospital, Third Military Medical University, Chongqing, China; Chongqing Key Laboratory for Hypertension Research, Chongqing Cardiovascular Clinical Research Center, Chongqing Institute of Cardiology, Chongqing, China; State Key Laboratory of Trauma, Burns and Combined Injury, Daping Hospital, The Third Military Medical University, Chongqing, China; Cardiovascular Research Center of Chongqing College, Department of Cardiology of Chongqing General Hospital, University of Chinese Academy of Sciences, Chongqing, China.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, Collet J-P, Kristensen SD, Aboyans V, Baumbach A, Bugiardini R, Coman IM, Delgado V, Fitzsimons D, Gaemperli O, Gershlick AH, Gielen S, Harjola V-P, Katus HA, Knuuti J, Kolh P, Leclercq C, Lip GYH, Morais J, Neskovic AN, Neumann F-J, Niessner A, Piepoli MF, Richter DJ, Shlyakhto E, Simpson IA, Steg PG, Terkelsen CJ, Thygesen K, Windecker S, Zamorano JL, Zeymer U, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J-P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Chettibi M, Hayrapetyan HG, Metzler B, Ibrahimov F, Sujayeva V, Beauloye C, Dizdarevic-Hudic L, Karamfiloff K, Skoric B, Antoniades L, Tousek P, Terkelsen PJ, Shaheen SM, Marandi T, Niemelä M, Kedev S, Gilard M, Aladashvili A, Elsaesser A, Kanakakis IG, Merkely B, Gudnason T, Iakobishvili Z, Bolognese L, Berkinbayev S, Bajraktari G, Beishenkulov M, Zake I, Lamin HB, Gustiene O, Pereira B, Xuereb RG, Ztot S, Juliebø V, Legutko J, Timóteo AT, Tatu-Chiţoiu G, Yakovlev A, Bertelli L, Nedeljkovic M, Studenčan M, Bunc M, García de Castro AM, Petursson P, Jeger R, Mourali MS, Yildirir A, Parkhomenko A, Gale CP; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 2. Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR Jr., Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res 2008;103:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Gao E, Lau WB, Wang Y, Liu G, Li J-J, Wang X, Yuan Y, Koch WJ, Ma X-L. G-protein-coupled receptor kinase 2-mediated desensitization of adiponectin receptor 1 in failing heart. Circulation 2015;131:1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martini JS, Raake P, Vinge LE, DeGeorge BR Jr., Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci USA 2008;105:12457–12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hullmann JE, Grisanti LA, Makarewich CA, Gao E, Gold JI, Chuprun JK, Tilley DG, Houser SR, Koch WJ. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ Res 2014;115:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci USA 2002;99:3872–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diao Z, Asico LD, Villar VAM, Zheng X, Cuevas S, Armando I, Jose PA, Wang X. Increased renal oxidative stress in salt-sensitive human GRK4gamma486V transgenic mice. Free Radic Biol Med 2017;106:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension 2006;47:1131–1139. [DOI] [PubMed] [Google Scholar]
- 9. Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol 2004;489:167–177. [DOI] [PubMed] [Google Scholar]
- 10. Virlon B, Firsov D, Cheval L, Reiter E, Troispoux C, Guillou F, Elalouf JM. Rat G protein-coupled receptor kinase GRK4: identification, functional expression, and differential tissue distribution of two splice variants. Endocrinology 1998;139:2784–2795. [DOI] [PubMed] [Google Scholar]
- 11. Kanamori H, Takemura G, Goto K, Maruyama R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res 2011;91:330–339. [DOI] [PubMed] [Google Scholar]
- 12. Sciarretta S, Yee D, Nagarajan N, Bianchi F, Saito T, Valenti V, Tong M, Del Re DP, Vecchione C, Schirone L, Forte M, Rubattu S, Shirakabe A, Boppana VS, Volpe M, Frati G, Zhai P, Sadoshima J. Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J Am Coll Cardiol 2018;71:1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Zeng C, Villar VA, Chen SY, Konkalmatt P, Wang X, Asico LD, Jones JE, Yang Y, Sanada H, Felder RA, Eisner GM, Weir MR, Armando I, Jose PA. Human GRK4gamma142V variant promotes angiotensin II type I receptor-mediated hypertension via renal histone deacetylase type 1 inhibition. Hypertension 2016;67:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang D, Xiao C, Long F, Su Z, Jia W, Qin M, Huang M, Wu W, Suguro R, Liu X, Zhu Y. HDAC4 regulates vascular inflammation via activation of autophagy. Cardiovasc Res 2018;114:1016–1028. [DOI] [PubMed] [Google Scholar]
- 15. Ling S, Sun Q, Li Y, Zhang L, Zhang P, Wang X, Tian C, Li Q, Song J, Liu H, Kan G, Cao H, Huang Z, Nie J, Bai Y, Chen S, Li Y, He F, Zhang L, Li Y. CKIP-1 inhibits cardiac hypertrophy by regulating class II histone deacetylase phosphorylation through recruiting PP2A. Circulation 2012;126:3028–3040. [DOI] [PubMed] [Google Scholar]
- 16. Premont RT, Macrae AD, Aparicio SA, Kendall HE, Welch JE, Lefkowitz RJ. The GRK4 subfamily of G protein-coupled receptor kinases. Alternative splicing, gene organization, and sequence conservation. J Biol Chem 1999;274:29381–29389. [DOI] [PubMed] [Google Scholar]
- 17. Das A, Salloum FN, Filippone SM, Durrant DE, Rokosh G, Bolli R, Kukreja RC. Inhibition of mammalian target of rapamycin protects against reperfusion injury in diabetic heart through STAT3 signaling. Basic Res Cardiol 2015;110:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, Zviti R, Ha C, Wang ZV, Hill JA, Lin F. Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J Biol Chem 2017;292:13774–13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.