Abstract
Several studies show associations between gut bacterial dysbiosis and chronic liver diseases, but causative mechanisms are largely unclear. We recently identified cytolysin, a bacterial exotoxin expressed and secreted by Enterococcus faecalis to cause liver damage in the setting of alcohol-related liver disease. Cytolysin was increased and highly correlated with liver disease severity and mortality in alcoholic hepatitis patients. In this study, we investigated if fecal cytolysin-positivity can be linked to non-alcoholic fatty liver disease, a highly prevalent disease where new biomarkers and treatment targets are urgently needed. In contrast to what we observed in alcoholic hepatitis, only seven out of 96 NAFLD patients were cytolysin positive, and these patients did not have increased liver disease activity compared to cytolysin-negative patients. These results indicate, that the association of cytolysin carriage with worse clinical outcome might be specific for alcoholic hepatitis.
Keywords: microbiome, microbiota, virulence factors
INTRODUCTION
Gut bacterial dysbiosis has been associated with the development and progression of chronic liver diseases but the mechanisms are largely unclear1. We recently found that cytolysin, a bacterial toxin expressed and secreted by Enterococcus faecalis (E. faecalis), causes direct lysis of hepatocytes during alcohol-related liver disease, likely through its ability to form pores2. Alcoholic hepatitis is a severe form of alcohol-related liver disease and is associated with high mortality rates3. Cytolysin-positive E. faecalis was present in 30% of the fecal samples from patients with alcoholic hepatitis (cytolysin-positive), and 89% of cytolysin-positive patients with alcoholic hepatitis died within 180 days, compared to only 3.8% of cytolysin-negative patients. This indicated that besides the possible causative links, cytolysin might also be a prognostic marker for worse outcome in these patients2. However, the question remains if this new finding is specific for alcoholic hepatitis, or if cytolysin might be also relevant in the disease spectrum of non-alcoholic fatty liver disease (NAFLD). NAFLD is a global disease burden with growing incidence and non-invasive diagnostic tools to predict disease activity as well as treatment targets are urgently needed. Therefore, the aim of this study was to determine the prevalence of cytolysin-positive E. faecalis in fecal samples from a well-described cohort of NAFLD patients with various stages of liver disease, and correlate cytolysin-positivity with clinical and histological characteristics.
METHODS
Patients
A total of 96 NAFLD patients and 19 healthy controls were prospectively enrolled between March 2015 and December 2018 in the outpatient liver department of the Clinic for Gastroenterology and Hepatology, University Hospital of Cologne, Germany. The protocol was approved by the local Ethics Committee and written informed consent was obtained from each patient. NAFLD was diagnosed if the following conditions were met: hepatic steatosis on liver imaging (ultrasound and/or magnetic resonance imaging) and/or the presence of >5% fat in histological analysis of liver biopsy, daily alcohol consumption of less than 10g in women and less than 20g in men, absence of steatogenic drugs and other diseases causing secondary steatosis.
Inclusion criteria for healthy controls were: absence of any known chronic disease, body mass index <25 kg/m2, daily alcohol consumption <10g in women and <20g in men, abdominal ultrasound without abnormalities, all laboratory parameters within the reference ranges. Exclusion criteria for all study subjects were oral- or intravenous antibiotic treatment within the last 6 months prior to the study, known malignancy, pregnancy, and age <18 years.
Abdominal ultrasound was performed for all patients. All blood samples were collected under fasting conditions. Anthropometric measurements were carried out by trained physicians or research assistant nurses.
Type 2 diabetes was defined as glycated hemoglobin (HbA1c) ≥6.5% and/or fasting glucose ≥126 mg/dL and/or use of antidiabetic medications. Metabolic syndrome was defined following the International Diabetes Foundation (IDF) criteria.
Liver biopsies
If liver biopsy was performed, samples were evaluated by an experienced liver pathologist who was blinded for all clinical and laboratory patient data. The NASH CRN histological scoring system4 was used to evaluate disease activity and severity. Accordingly, steatosis was graded 0–3, ballooning was graded 0–2, lobular inflammation was graded 0–3. Fibrosis was staged from 0–4. Stages 1a, 1b and 1c were summarized as stage 1. The NAFLD activity score (NAS) was obtained for each biopsy. This score is defined as the unweighted sum of the scores for steatosis, (0–3), lobular inflammation (0–3), and ballooning (0–2); thus ranging from 0 to 8. Definite non-alcoholic steatohepatitis (NASH) was defined as NAS 5–8.
Non-invasive diagnosis of cirrhosis
If the following criteria were present, patients were staged as histological F4 fibrosis without determination of histological grading: liver imaging consistent with cirrhosis (e.g. nodular hepatic contour, changes in volume distribution indicating portal hypertension in the absence of portal vein thrombosis, secondary phenomena of portal hypertension such as splenomegaly, enlarged caudate lobe and left lobe lateral segment, regenerative nodules) together with clinical and laboratory signs of portal hypertension/cirrhosis (e.g. low platelets, albumin and prothrombin time, esophageal varices).
Bacterial DNA extraction and real-time quantitative PCR
Fecal samples were immediately frozen at −80°C. DNA from human stool samples was extracted as described before5. Real-time quantitative PCR for E. faecalis and cytolysin was done as in our recent publication2. Only cylLL and cylLS genes were used.
The following primer sequences were used: E. faecalis forward: 5’-CGCTTCTTTCCTCCCGAGT-3’; reverse: 5’-GCCATGCGGCATAAACTG-3’. E. faecalis cylLL forward: 5’-CTGTTGCGGCGACAGCT-3’; reverse: 5’-CCACCAACCCAGCCACAA-3’. E. faecalis cylLS forward: 5’-GTAAAATAAGTAAAATCAAGAAAACTATTACTC-3’; reverse: 5’-CAAAAGAAGGACCAACAAGTTCTAATT-3’.
Statistical analysis
Results are expressed as median and interquartile range (IQR) in brackets for each continuous outcome and as actual number and percentage for factor variables. A P value of equal or less than 0.05 was considered as statistically significant. Two groups were compared using the Wilcoxon-Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables. Statistical analysis was performed using R statistical software, R version 3.5.1, 2018 the R Foundation for Statistical Computing.
RESULTS
The median age of NAFLD patients was 54 years and 52% were female. The median body mass index (BMI) was 30 kg/m2 and 23% had type 2 diabetes (Table 1). Fecal samples from NAFLD patients did not contain more E. faecalis compared to healthy controls (Fig. 1a). 13 (13.5%) out of 96 NAFLD patients used proton pump inhibitors on a daily basis, but fecal samples from these patients did not contain significantly higher numbers of E. faecalis (P=0.067). Genomic DNA of cytolysin-positive E. faecalis was detected in two (10.5%) out of 19 healthy controls (Fig. 1b). NAFLD patients were not more frequently cytolysin-positive (7/96 (7.3%), patients cytolysin positive (P=0.64, Fig.1b). Of 64 biopsy-proven NAFLD patients, 48% had definite NASH (defined as NAFLD activity score 5–8). 31% of the NAFLD patients had advanced liver fibrosis (fibrosis stage 3–4), which included patients with the diagnosis of cirrhosis based on clinical findings (see Methods). Patients with definite NASH or patients with NASH-associated advanced fibrosis were not more frequently cytolysin-positive compared with patients who did not have NASH or fibrosis (P-values 0.67 and 1, respectively, Fig. 1c,d). Further, NAFLD patients with type 2 diabetes were not more often cytolysin-positive (P=0.62). Proton pump inhibitor use in NAFLD patients was not associated with cytolysin positivity (P=0.59). We also did not find a significant association between cytolysin and alanine-aminotransferase, ferritin levels and the body mass index (Fig. e–g).
Table 1:
Clinical characteristics of the study cohort
| N/A | Healthy controls | NAFLD | |
|---|---|---|---|
| Total n | 19 | 96 | |
| Demographics | |||
| Age, years | 31.6 (7.9) | 54.0 (21.8) | |
| Gender female, n (%) | 11 (57.9) | 50 (52.1) | |
| Body mass index, kg/m2 | 20.4 (3.6) | 30.2 (6.3) | |
| Type 2 diabetes, n (%) | 22 (23.4) | ||
| Arterial hypertension, n (%) | 62 (64.6) | ||
| Metabolic syndrome (IDF criteria), n (%) | 3 | 40 (42.1) | |
| Waist circumference (cm) | 21 | 83.0 (5.5) | 108.0 (21.0) |
| Proton pump inhibitor use, n (%) | 0 | 13 (13.5) | |
| Laboratory parameters | |||
| Albumin, g/L | 5 | 45.0 (1.2) | 44.0 (4.0) |
| Creatinine, mg/dL | 5 | 0.7 (0.3) | 0.8 (0.3) |
| Urea, mg/dL | 5 | 23.5 (12.8) | 29.0 (13.5) |
| Uric acid, mg/dL | 6 | 4.1 (1.2) | 5.8 (2.1) |
| AST, U/L | 4 | 24.0 (7.0) | 35.5 (23.8) |
| ALT, U/L | 4 | 13.0 (11.0) | 44.5 (43.8) |
| GGT, U/L | 4 | 15.0 (9.0) | 74.0 (79.5) |
| Alkaline phosphatase, U/L | 5 | 55.5 (14.0) | 73.0 (30.8) |
| Bilirubin, mg/dL | 6 | 0.5 (0.5) | 0.5 (0.5) |
| Ferritin, μg/L | 8 | 54.0 (58.5) | 191.5 (184.2) |
| Triglycerides, mg/dL | 6 | 67.0 (95.0) | 141.0 (121.8) |
| Total cholesterol, mg/dL | 6 | 152.0 (27.0) | 195.5 (55.2) |
| HDL cholesterol mg/dL | 15 | 64.0 (15.0) | 49.0 (18.0) |
| LDL cholesterol mg/dL | 19 | 70.0 (19.0) | 120.0 (55.0) |
| Platelet count, x1E9/L | 4 | 239.0 (33.0) | 231.0 (97.2) |
| INR | 7 | 1.0 (0.0) | 1.0 (0.1) |
| HbA1c, % | 26 | 4.9 (0.2) | 5.5 (0.9) |
| Fasting glucose, mg/dL | 4 | 82.0 (10.0) | 96.0 (28.0) |
| Alpha-fetoprotein kU/L | 10 | 3.0 (2.0) | |
| Liver histology features NAFLD cohort | Scoring | Classification | Number |
| Grade of steatosis, n (%) | 0 | <5% | 0 (0.0) |
| 1 | 5%−33% | 19 (29.7) | |
| 2 | >33%−66% | 27 (42.2) | |
| 3 | >66% | 18 (28.1) | |
| Ballooning, n (%) | 0 | None | 14 (21.9) |
| 1 | Few balloon cells | 31 (48.4) | |
| 2 | Prominent ballooning | 19 (29.7) | |
| Grade of inflammation, n (%) | 0 | No foci | 8 (12.5) |
| 1 | <2 foci | 33 (51.6) | |
| 2 | 2–4 foci | 22 (34.4) | |
| 3 | >4 foci | 1 (1.6) | |
| Stage of fibrosis, n (%) | 0 | None | 15 (20.3) |
| 1 | Perisinusoidal or periportal | 22 (29.7) | |
| 2 | Perisinusoidal and portal/periportal | 14 (18.9) | |
| 3 | Bridging fibrosis | 6 (8.1) | |
| 4 | Cirrhosis | 17 (23.0) |
Values are presented as median and interquartile range (IQR) in parentheses. The number of missing values within the overall cohort is indicated in the third column (“N/A”). Liver histology data is presented as number and percentage in parentheses. If liver biopsy was performed (n=64 NAFLD patients), samples were evaluated by an experienced liver pathologist who was blinded for all clinical and laboratory patient data. The NASH CRN histological scoring system was used to evaluate disease activity and severity. The NAFLD activity score (NAS) was obtained for each biopsy. This score is defined as the unweighted sum of the scores for steatosis, (0–3), lobular inflammation (0–3), and ballooning (0–2); thus ranging from 0 to 8. 10 patients were staged as histological F4 fibrosis without determination of histological grading if the following criteria were present: liver imaging consistent with cirrhosis (e.g. nodular hepatic contour, changes in volume distribution indicating portal hypertension in the absence of portal vein thrombosis, secondary phenomena of portal hypertension such as splenomegaly, enlarged caudate lobe and left lobe lateral segment, regenerative nodules) together with clinical and laboratory signs of portal hypertension/cirrhosis (e.g. low platelets, albumin and prothrombin time, esophageal varices). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma-glutamyl-transferase; HbA1c, glycated hemoglobin; INR, international normalized ratio; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; N/A, not applicable.
Figure 1. Association of faecal cytolysin-positivity with disease activity in NAFLD patients.
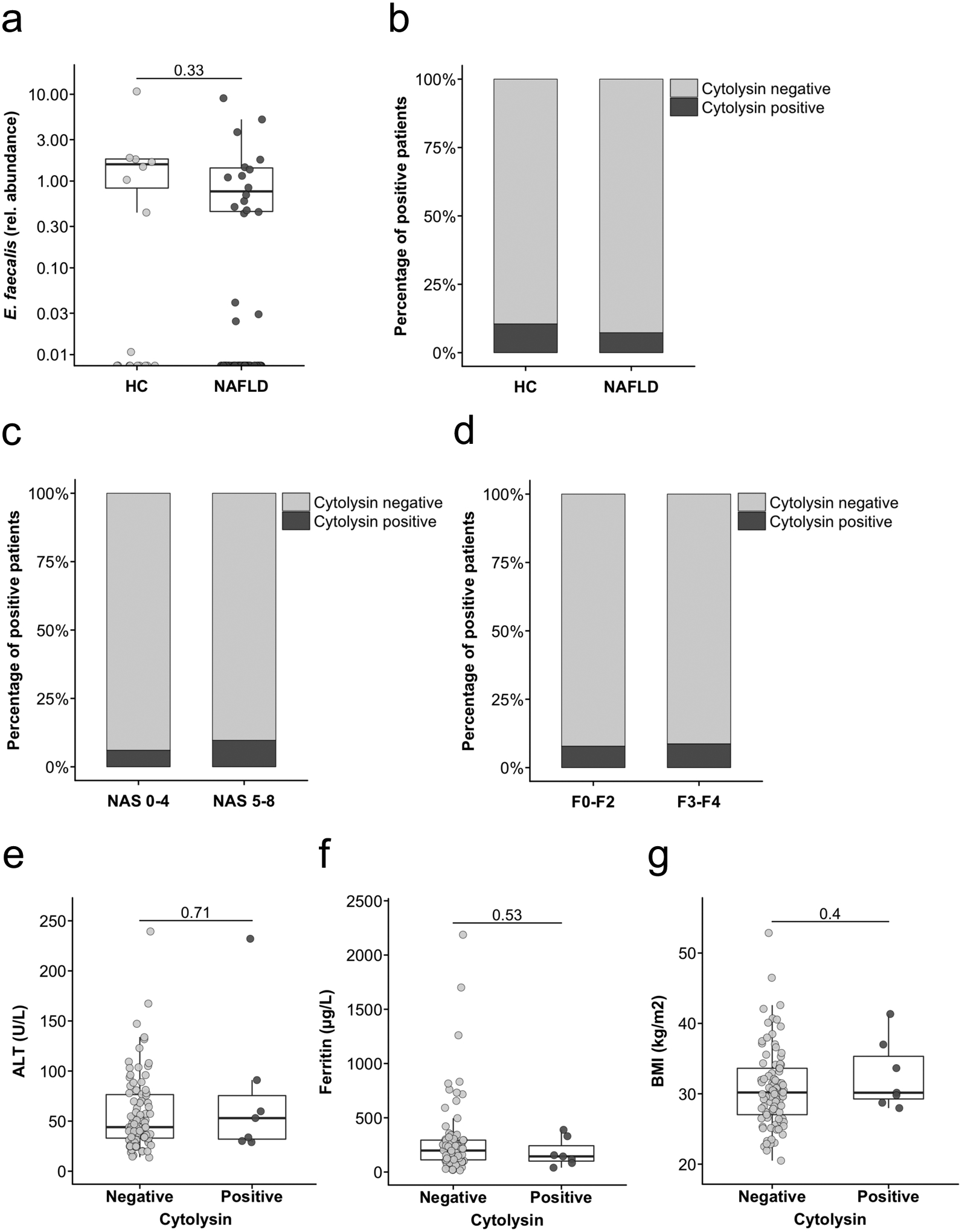
(a) E. faecalis in faecal samples from healthy controls (n=19) or NAFLD patients (n=96), assessed by qPCR. (b) Proportion of cytolysin-positive faecal samples among 19 healthy controls and 96 NAFLD patients. P=0.64. (c) Proportion of cytolysin-positive faecal samples among 64 biopsy proven NAFLD patients. 31 of these patients had definite non-alcoholic steatohepatitis (NASH). NASH was defined as NAFLD activity score (NAS) of 5–8 on liver biopsy. P=0.67. (d) Proportion of cytolysin-positive faecal samples among 51 NAFLD patients with no to moderate (F0-F2) versus 23 patients with advanced (F3-F4) fibrosis. P=1. In 10 of these patients, NASH-cirrhosis was diagnosed based on clinical findings (see Methods). (e) Comparison of alanine amino-transferase (ALT) levels between 87 cytolysin-negative and 7 cytolysin-positive NAFLD patients. (f) Comparison of total ferritin levels between 83 cytolysin-negative and 7 cytolysin-positive NAFLD patients. (g) Comparison of body mass index (BMI) between 89 cytolysin-negative and 7 cytolysin-positive NAFLD patients. Two-sided Fisher’s exact test (b,c,d) or Mann-Whitney-Wilcoxon rank-sum test (a,e,f,g). For the box and whisker plots in a,e,f and g, the box extends from the 25th to 75th percentiles, and the centre line represents the median. The upper whisker extends from the box to the largest value no further than 1.5 * IQR from the box (where IQR is the inter-quartile range, or distance between the first and third quartiles). The lower whisker extends from the box to the smallest value at most 1.5 * IQR of the box. Data beyond the end of the whiskers are plotted individually.
DISCUSSION
We did not detect an increased prevalence of fecal cytolysin-positive E. faecalis or association of cytolysin-positivity and worse outcome in NAFLD patients. This is in contrast to what we observed in alcoholic hepatitis patients, were cytolysin-positivity was detected in one third of the patients and strongly associated with mortality2. In our previous study, we investigated mechanisms of how cytolysin might cause liver damage. In vitro, we could show that cytolysin causes direct death of primary mouse hepatocytes. But most importantly, ethanol-induced changes in the gut barrier are needed for the translocation of cytolytic E. faecalis from the intestine to the liver. The role of gut barrier dysfunction in NAFLD patients is less clear as compared with patients suffering from alcohol-related liver disease. It appears that only a subset of NAFLD patients has increased intestinal permeability6. This might be an explanation why the carriage of cytolysin in our NAFLD cohort did not lead to increased liver damage. It is unknown why cytolysin-positive E. faecalis colonizes the gut of patients with alcoholic hepatitis. Profound changes in the bile acid metabolism that we observed in these patients7 might contribute but future experiments are required to explore causal relationships.
In summary, only a small proportion of NAFLD patients carried cytolysin-positive E. faecalis in fecal samples. These cytolysin-positive patients did not have increased liver damage, indicating that the association of cytolysin carriage with worse clinical outcome might be specific for alcoholic hepatitis.
Funding statement:
This study was supported in part by the “Marga und Walter Boll-Stiftung”, project number 210-03-2016, and the “Köln Fortune” research pool, Faculty of Medicine, University of Cologne, Germany, project number 160/2014 (to M.D.), by NIH grants R01 AA24726, U01 AA026939 (to B.S.) and by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). S.L. was supported by a DFG fellowship (LA 4286/1-1).
Abbreviations:
- ALT
alanine amino-transferase
- E. faecalis
Enterococcus faecalis
- INR
international normalized ratio
- qPCR
quantitative polymerase chain reaction
Footnotes
Conflicts of interests: B.S. consults for the Ferring Research Institute, no other authors have financial or personal conflicting interests to declare.
Ethics approval and patient consent statement: Approval of the local Ethics Committee was obtained and written informed consent was obtained from each study subject.
REFERENCES
- 1.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65(12):2035–2044. [DOI] [PubMed] [Google Scholar]
- 2.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. The New England journal of medicine. 2009;360(26):2758–2769. [DOI] [PubMed] [Google Scholar]
- 4.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 5.Chu H, Duan Y, Lang S, et al. The Candida albicans exotoxin Candidalysin promotes alcohol-associated liver disease. J Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu H, Duan Y, Yang L, Schnabl B. Small metabolites, possible big changes: a microbiota-centered view of non-alcoholic fatty liver disease. Gut. 2019;68(2):359–370. [DOI] [PubMed] [Google Scholar]
- 7.Brandl K, Hartmann P, Jih LJ, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol. 2018;69(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


