Abstract
Purpose
We investigated the impact of infusion duration (30 and 60 min) on the pharmacokinetic profile of ramucirumab using a population pharmacokinetic (PopPK) modeling approach. We also assessed the relationship between infusion rate and incidence of immediate infusion-related reactions (IRRs; occurring on the day of administration) using ramucirumab phase II/III study data.
Methods
The impact of different infusion durations (30 vs. 60 min) on the time-course of ramucirumab concentration profiles were evaluated using a PopPK model, established using ramucirumab pharmacokinetic data from 2522 patients. Logistic regression was used to evaluate the association between ramucirumab infusion rate and incidence of immediate IRRs in clinical trials.
Results
Ramucirumab time-course concentration profiles were equivalent following a 30- or 60-min infusion. In the pooled clinical study dataset, 254 of 3216 (7.9%) patients receiving ramucirumab experienced at least one immediate IRR (any grade). When grouped according to infusion rate quartile, the incidence of immediate IRRs (any grade or grade ≥ 3) was similar across quartiles; findings were confirmed in sensitivity analyses. The risk of immediate IRRs was not found to be associated with infusion rate based on multivariate logistic analysis.
Conclusion
Shortening the infusion duration of ramucirumab from 60 to 30 min has no impact on ramucirumab exposure. Analysis of trial data found no relationship between an increased risk of immediate IRRs and a faster infusion rate. Such a change in infusion duration is unlikely to affect the clinical efficacy or overall safety profile of ramucirumab.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-020-04223-9.
Keywords: Ramucirumab, Infusion-related reactions, Infusion duration, Pharmacokinetics, Vascular endothelial growth factor receptor-2 antagonist
Introduction
Lengthy intravenous infusions are burdensome and inconvenient for patients. Patients with cancer have indicated a strong preference for receiving drugs with shorter infusion times as they are less disruptive to their lives [1]. Infusions are usually administered in an outpatient setting, and long infusion times necessitate lengthy observation periods with increased nursing and administration staff workloads. Shorter infusion times have been associated with notable reductions in healthcare resources, medical personnel time [2, 3], and time in care; potentially allowing more patients to be treated in a day [1].
Ramucirumab is a human recombinant immunoglobin (Ig)G1 monoclonal antibody (mAb) antagonist of vascular endothelial growth factor receptor-2 currently approved for the second-line treatment of advanced or metastatic gastric or gastroesophageal junction adenocarcinoma, metastatic non-small-cell lung cancer [in combination with docetaxel or erlotinib in patients with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) mutations], metastatic colorectal cancer, and hepatocellular carcinoma with elevated alpha fetoprotein levels. The recommended initial dosing regimen for ramucirumab, either as monotherapy or in combination with chemotherapy, is 8 mg/kg or 10 mg/kg every 2 weeks (Q2W) or 10 mg/kg every 3 weeks (Q3W) administered as an intravenous infusion over 60 min following premedication with a histamine-1 receptor antagonist [4].
The efficacy and overall safety of antibody therapies such as ramucirumab have been linked to exposure to the drug [5, 6]. Hence, while a shortened ramucirumab infusion duration could benefit both patients and healthcare staff, it is also important to consider the impact such a change may have on the pharmacokinetic (PK) profile of the drug. Population PK (PopPK) modeling is widely used during drug development to describe and predict the concentration profile of a drug over time [7, 8]. The pharmacokinetics of ramucirumab have previously been well-described by a linear two-compartment pharmacostatistical model which incorporated the effect of body weight on ramucirumab clearance and central compartment volume [9]. Other patient-related factors such as age, sex, race, cancer type, various measures of liver and renal function as well as baseline measures of tumor burden were not found to be statistically and/or clinically relevant factors in the disposition of ramucirumab [9].
PopPK modeling was applied to predict exposure parameters for individual patients for use in determining PK/pharmacodynamic relationships for ramucirumab from both safety and efficacy perspectives [5, 6, 10].
A faster infusion rate is also perceived as a potential risk factor for infusion-related reactions (IRRs), common adverse events associated with mAb infusions [11]. In patients who have previously experienced a grade 1–2 IRR, standard clinical practice is to reduce the infusion rate during drug rechallenge [11]. However, the relationship between infusion rate and IRR incidence is not well defined.
We developed a PopPK model to simulate and compare ramucirumab PK profiles following either a 30- or 60-min infusion at the two approved dosing regimens to evaluate the potential impact of a change in infusion duration on the clinical efficacy and overall safety profile of the drug. Additionally, the relationship between ramucirumab infusion rate and the incidence of immediate IRRs was investigated using data from key phase II and III studies of ramucirumab.
Materials and methods
PopPK model development and simulation
Emerging evidence has suggested that monoclonal antibodies may exhibit time-dependent clearance (CL) due to changes in oncologic disease status [12–14]. In this updated PopPK analysis, temporal changes in CL were incorporated into the two-compartment linear model developed by O’Brien et al. [9] with a sigmoidal function as described for nivolumab [12]:
where CL0 represents clearance (CL) at time of first dose administration, Emax represents maximal change in CL, T50 is the time at which the change in CL is 50% of Emax, and ƴ represents the sigmoidicity of the relationship with time.
The effect of body weight on both CL and central volume of distribution (V1) was included in the base pharmacostatistical model. Additional patient-related factors including age, sex, race, Eastern Cooperative Oncology Group (ECOG) performance status, cancer type, and various measures of liver and renal function were also assessed. Covariate evaluation and final model development were performed as described in O’Brien [9]. In addition to standard goodness-of-fit plots, model evaluation also included a visual predictive check (VPC) to assess the general predictability of the model by visually examining the observed versus predicted profiles. The distribution (median, 5th, and 95th percentiles) of simulated profiles was overlaid on observed data. Plotted data were stratified by duration of treatment (< 27 days and ≥ 27 days), corresponding to estimated T50, to assess model fit of early timepoints and later timepoints.
The model was developed using concentration–time data (for pharmacokinetic parameters see online Supplementary Material, Table S1) collected from 2522 patients in 17 clinical studies (data on file, Eli Lilly and Company; see online Supplementary Material, Table S2). All patients provided written informed consent and the trials were designed and conducted to follow Good Clinical Practice guidelines and in accordance with the Declaration of Helsinki. Ramucirumab was administered as an intravenous infusion over approximately 60 min. Patients included in the pooled analysis received one of five treatment regimens: ramucirumab 8 mg/kg every 2 weeks (Q2W), 12 mg/kg Q2W, 6 mg/kg once weekly (QW), 8 mg/kg on days 1 and 8 every 3 weeks (Q3W), or 10 mg/kg Q3W.
The final PK model was used to simulate concentration–time profiles and exposure parameters following a 30- or 60-min infusion with ramucirumab 8 mg/kg Q2W or 10 mg/kg Q3W. The simulation dataset included 500 patients for each infusion–regimen combination. Dose amounts were determined using 500 weights randomly sampled without replacement from the baseline weights of the 2522 patients included in the PopPK analysis. Modeling and simulations were performed using NONMEM 7.4.2 [15].
Immediate IRRs
Clinical data from all eight phase III trials evaluating commercial formulations and two phase II studies evaluating ramucirumab 12 mg/kg (Table 1) were included in analyses to evaluate the association between ramucirumab infusion rate and the incidence of immediate IRRs (IRRs occurring on the day of ramucirumab infusion). All included trials required premedication. As dosing of ramucirumab was weight based, the total amount of ramucirumab administered varied for each patient, making it possible to observe different ramucirumab infusion rates. While the recommended infusion time was approximately 60 min, several studies allowed some flexibility in initial infusion time (± ≤ 15 min), and infusions could be longer if needed to maintain an infusion rate of ≤ 25 mg/min. Sensitivity analyses were therefore performed using infusion rates derived from a standard 60-min infusion. (For details of the methodology used to identify immediate IRRs, calculate immediate IRR incidence, and investigate the relationship between immediate IRR incidence and infusion rate, see the online Supplementary Material.)
Table 1.
Summary of immediate infusion-related reactions in ramucirumab-treated patients by study and overall
| Studya | Phase | Indication | RAM dosing regimen | Patients with ≥ 1 immediate IRR (narrow termsb) | |
|---|---|---|---|---|---|
| n/N | % | ||||
|
REGARD [21] |
III | Second-line GC | 8 mg/kg Q2W | 6/236 | 2.5 |
|
RAINBOWc [22] |
III | Second-line GC | 8 mg/kg Q2W | 45/327 | 13.8 |
|
REVELc [23] |
III | Second-line NSCLC | 10 mg/kg Q3W | 51/626 | 8.1 |
|
RAISEc [24] |
III | Second-line CRC | 8 mg/kg Q2W | 46/528 | 8.7 |
|
REACH [25] |
III | Second-line HCC | 8 mg/kg Q2W | 41/317 | 12.9 |
|
REACH-2 [26] |
III | Second-line HCC | 8 mg/kg Q2W | 17/197 | 8.6 |
|
RAINFALLc [27] |
III | First-line GC | 8 mg/kg D1D8 Q3W | 15/323 | 4.6 |
|
RANGEc [28] |
III | Second-line UC | 10 mg/kg Q3W | 18/258 | 7.0 |
| NCT02443883 (I4T-MC-JVDB) | II | Second-line GC |
8 mg/kg Q2W 12 mg/kg Q2W 6 mg/kg QW 8 mg/kg D1D8 Q3W |
4/161 | 2.5 |
| NCT02514551c (I4T-MC-JVCZ) | II | Second-line GC |
8 mg/kg Q2W 12 mg/kg Q2W |
11/243 | 4.5 |
| All studies | 254/3216 | 7.9 | |||
CAP capecitabine, CIS cisplatin, CRC colorectal cancer, D day, DOC docetaxel, FOLFIRI irinotecan, folinic acid, and 5FU, GC gastric cancer, HCC hepatocellular carcinoma, IRR infusion-related reaction, n number of patients in specified category, N total number of patients, NSCLC non-small-cell lung cancer, PAC paclitaxel, QW weekly, Q2W every 2 weeks, Q3W every 3 weeks, RAM ramucirumab, UC urothelial carcinoma, 5FU 5-fluorouracil
aPer protocol, all studies required RAM to be delivered over approximately 60 min (or longer, if needed to maintain an infusion rate ≤ 25 mg/min). All trial protocols required premedication to reduce the risk of infusion-related reactions
bBroad- and narrow-scope preferred terms were used within the Standardized Medical Dictionary for Regulatory Activities (MedDRA) queries. “Narrow” preferred terms included terms that are highly likely to represent the condition of interest and were considered sufficient to identify immediate IRRs with reasonable precision and to appropriately reflect the incidence rate of immediate IRRs
cStudies in combination with chemotherapy (RAINBOW: RAM + PAC; REVEL: RAM + DOC; RAISE: RAM + FOLFIRI; RAINFALL: RAM + CAP [or 5FU] + CIS; RANGE: RAM + DOC; Study JVCZ: RAM + PAC)
Any potential association between infusion rate and an increased risk of an immediate IRR was investigated using multivariate logistic regression analysis.
Statistical analyses were performed using SAS software (SAS, version 9.4). %SEE is defined as the % standard error of the estimate. It is calculated as Standard Error/Estimate × 100%.
Results
Pharmacokinetic model development and simulation result
The ramucirumab concentration–time data were well described by a two-compartment structural model parameterized in terms of CL, V1, peripheral volume of distribution (V2), and inter-compartmental CL. Drug CL was found to be time-varying and was incorporated into the model using a sigmoid function. The effect of body weight on CL and V1 was included and exponential interpatient variability terms were included for CL, V1, and V2. An additive inter-patient variability term was included on Emax, with covariance between CL and V1, and CL and Emax. Residual variability was accounted for by an additive/proportional error structure. Aside from body weight, none of the additional patient factors investigated for influence on the PK parameters were found to satisfy the criteria defined in a previous publication [9]. Model parameters are shown in Table S1 (online Supplementary Material).
VPC data for the 8 mg/kg Q2W and 10 mg/kg Q3W regimens are provided in Fig. S1 (online Supplementary Material). Visual examination ensured general concordance of the estimates and supported the validity of this model to describe ramucirumab concentrations in this patient population.
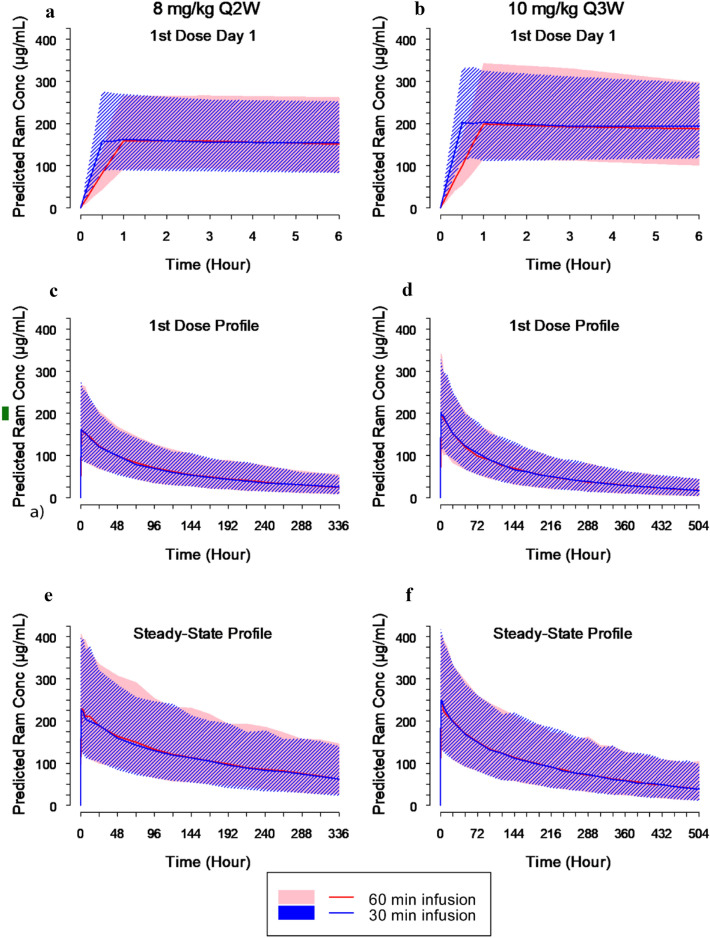
The final PopPK model with time-varying CL was used to simulate concentration–time profiles and predict ramucirumab exposure parameters for the 8 mg/kg Q2W and 10 mg/kg Q3W regimens. Figure 1 compares the predicted concentration–time profiles following 30- and 60-min infusions. Predicted ramucirumab exposure estimates are shown in Table S3 (online Supplementary Material). These results indicate that ramucirumab PK profiles, and hence systemic exposure, are equivalent following infusions of either 30 or 60 min.
Fig. 1.
Predicted ramucirumab concentration (µg/ml) time profiles following administration of 8 mg/kg every 2 weeks (a, c, e) or 10 mg/kg every 3 weeks (b, d, f) based on a time-varying clearance population pharmacokinetic model (established using ramucirumab pharmacokinetic data from 17 studies (2522 patients). Data following the first dose (a–d) and at steady state (e, f) are shown. CL clearance, conc concentration, Q2W every 2 weeks, Q3W every 3 weeks, Ram ramucirumab. Shaded regions represent the 5th and 95th percentile ramucirumab concentrations calculated from 500 simulation iterations
Incidence of immediate IRRs
Table 1 summarizes the incidence of immediate IRRs in each of the ten clinical studies included in IRR analyses.
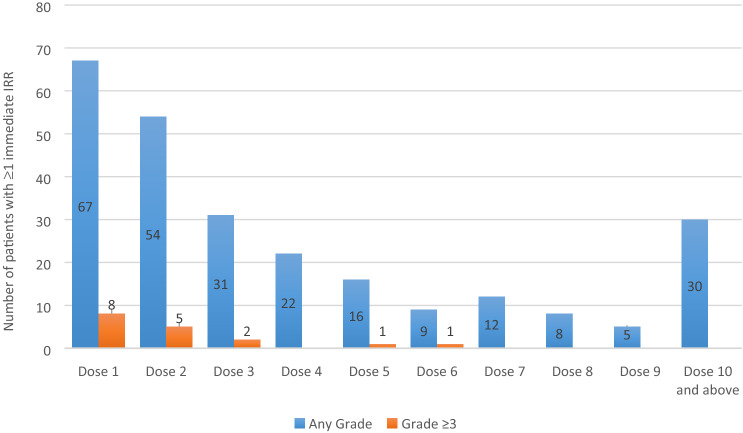
In the pooled dataset, 254/3216 (7.9%) patients receiving ramucirumab experienced at least one immediate any-grade IRR (Table 1). The number of patients who experienced immediate IRRs for the first time decreased as the dose number increased (Fig. 2). Grade ≥ 3 immediate IRRs were seen in 17/3216 patients (0.5%); most occurred during the first two infusions (Fig. 2). No fatal IRRs were identified.
Fig. 2.
Plot of immediate infusion-related reactions (IRRs) (narrow termsa) by infusion number. Data shown are the number of patients with a first immediate IRR (any grade) occurring at the dose specified (blue bars), and the number of patients with a first IRR (grade ≥ 3) occurring at the dose specified (yellow bars). aBroad- and narrow-scope preferred terms were used within the Standardized Medical Dictionary for Regulatory Activities (MedDRA) queries. “Narrow” preferred terms included terms that are highly likely to represent the condition of interest and were considered sufficient to identify immediate IRRs with reasonable precision and to appropriately reflect the incidence rate of immediate IRRs
Relationship between infusion rate and immediate IRR incidence
Within each study and in pooled data, the summary statistics of ramucirumab infusion rates were similar for patients with or without any immediate IRR (Table 2).
Table 2.
Summary of infusion rates for patients with or without immediate infusion-related reactions (narrow termsa) by study and overall in ramucirumab-treated patients
| Study | Infusion rate, mg/min | |||
|---|---|---|---|---|
| Pts with ≥ 1 IRR | Pts with no IRR | |||
|
REGARD [21] |
N | 5 | 224 | |
| Mean (SD) | 9.8 (2.69) | 8.5 (2.64) | ||
| Median (IQR) | 9.2 (8.1–12.1) | 8.4 (6.9–9.6) | ||
| Min–Max | 6.6–13.1 | 2.4–32.5 | ||
|
RAINBOWb [22] |
N | 45 | 279 | |
| Mean (SD) | 7.5 (1.89) | 8.4 (2.15) | ||
| Median (IQR) | 7.6 (6.7–8.5) | 8.0 (6.9–9.6) | ||
| Min–Max | 3.0–13.2 | 4.3–17.9 | ||
|
REVELb [23] |
N | 51 | 574 | |
| Mean (SD) | 16.2 (25.90) | 12.6 (7.72) | ||
| Median (IQR) | 12.0 (9.4–13.2) | 12.0 (10.3–13.9) | ||
| Min–Max | 0.5–190.0 | 3.5–182.0 | ||
|
RAISEb [24] |
N | 45 | 481 | |
| Mean (SD) | 9.5 (4.84) | 9.5 (2.33) | ||
| Median (IQR) | 8.8 (7.3–10.7) | 9.1 (7.7–10.9) | ||
| Min–Max | 3.1–36.0 | 4.0–19.9 | ||
|
REACH [25] |
N | 41 | 275 | |
| Mean (SD) | 8.3 (2.09) | 8.9 (1.95) | ||
| Median (IQR) | 8.3 (7.4–9.2) | 8.5 (7.5–10.0) | ||
| Min–Max | 3.2–13.0 | 3.2–15.3 | ||
|
REACH-2 [26] |
N | 17 | 179 | |
| Mean (SD) | 7.1 (2.90) | 8.6 (2.09) | ||
| Median (IQR) | 7.1 (5.7–8.4) | 8.4 (7.2–9.7) | ||
| Min–Max | 1.0–11.9 | 3.2–17.0 | ||
|
RAINFALLb [27] |
N | 15 | 306 | |
| Mean (SD) | 10.4 (3.04) | 8.8 (2.27) | ||
| Median (IQR) | 9.7 (7.5–13.9) | 8.6 (6.9–10.3) | ||
| Min–Max | 6.7–15.0 | 4.3–18.0 | ||
|
RANGEb [28] |
N | 18 | 237 | |
| Mean (SD) | 11.0 (3.42) | 11.9 (2.84) | ||
| Median (IQR) | 11.2 (9.6–12.9) | 11.8 (10.0–13.7) | ||
| Min–Max | 3.3–16.0 | 5.3–23.2 | ||
|
(I4T-MC-JVDB) |
N | 4 | 156 | |
| Mean (SD) | 4.8 (1.48) | 9.5 (3.30) | ||
| Median (IQR) | 4.7 (3.6–6.1) | 8.4 (7.1–11.4) | ||
| Min–Max | 3.4–6.4 | 4.0–19.5 | ||
|
(I4T-MC-JVCZ) |
N | 11 | 230 | |
| Mean (SD) | 11.1 (5.09) | 11.1 (3.30) | ||
| Median (IQR) | 12.1 (7.3–16.0) | 10.6 (8.7–13.0) | ||
| Min–Max | 2.5–18.6 | 4.6–25.0 | ||
| All studiesc | N | 252 | 2941 | |
| Mean (SD) | 10.3 (12.35) | 10.1 (4.39) | ||
| Median (IQR) | 8.7 (7.3–11.4) | 9.6 (7.9–11.7) | ||
| Min–Max | 0.5–190.0 | 2.4–182.0 | ||
Infusion rates were calculated as total dose/duration of infusion on day of earliest IRR event for patients with at least one immediate IRR and as total dose/infusion time on the first ramucirumab dose for patients with no IRR events
Two outliers of infusion data (one with infusion rate = 182 mg/min calculated from 910 mg total dose infused within 5 min; one with infusion rate = 190 mg/min calculated from 950 mg total dose infused within 5 min; both in REVEAL) were considered due to data entry issues
CAP capecitabine, CIS cisplatin, DOC docetaxel, FOLFIRI irinotecan, folinic acid, and 5FU, IQR interquartile range, IRR infusion-related reaction, Max maximum, Min minimum, N total number of patients, PAC paclitaxel, pts patients, Q quartile, RAM ramucirumab, SD standard deviation, 5FU 5-fluorouracil
aBroad- and narrow-scope preferred terms were used within the Standardized Medical Dictionary for Regulatory Activities (MedDRA) queries. “Narrow” preferred terms included terms that are highly likely to represent the condition of interest and were considered sufficient to identify immediate IRRs with reasonable precision and to appropriately reflect the incidence rate of immediate IRRs
bStudies in combination with chemotherapy (RAINBOW: RAM + PAC; REVEL: RAM + DOC; RAISE: RAM + FOLFIRI; RAINFALL: RAM + CAP [or 5FU] + CIS; RANGE: RAM + DOC; Study JVCZ: RAM + PAC)
cInfusion times were missing for 23 patients across the studies
When grouped according to infusion rate quartile, the incidence of immediate IRRs (any grade or grade ≥ 3) was similar across quartiles. There was no obvious increasing trend in IRR incidence from the lowest to the highest quartile (Table 3); similar findings were obtained in sensitivity analyses (see online Supplementary Material, including Table S4).
Table 3.
Summary of immediate infusion-rate reactions (narrow termsa) by infusion rate quartile groups in ramucirumab-treated patients
| Infusion rate Q1b (N = 799) | Infusion rate Q2b (N = 803) | Infusion rate Q3b (N = 797) | Infusion rate Q4b (N = 794) | Infusion rate missing (N = 23) | Total (N = 3216) | ||
|---|---|---|---|---|---|---|---|
| Patients with ≥ 1 event on the day of ramucirumab administration | Any grade | 87 (10.9) | 65 (8.1) | 40 (5.0) | 60 (7.6) | 2 (8.7) | 254 (7.9) |
| Grade ≥ 3 | 5 (0.6) | 5 (0.6) | 5 (0.6) | 2 (0.3) | 0 | 17 (0.5) | |
| Anaphylactic reaction SMQ (narrow) | Any grade | 0 | 1 (0.1) | 1 (0.1) | 1 (0.1) | 0 | 3 (0.1) |
| Grade ≥ 3 | 0 | 1 (0.1) | 1 (0.1) | 0 | 0 | 2 (0.1) | |
| Angioedema SMQ (narrow) | Any grade | 16 (2.0) | 12 (1.5) | 4 (0.5) | 8 (1.0) | 0 | 40 (1.2) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hypersensitivity SMQ (narrow) | Any grade | 54 (6.8) | 45 (5.6) | 28 (3.5) | 45 (5.7) | 2 (8.7) | 174 (5.4) |
| Grade ≥ 3 | 2 (0.3) | 1 (0.1) | 4 (0.5) | 0 | 0 | 7 (0.2) | |
| Cytokine release syndrome (PT) | Any grade | 2 (0.3) | 2 (0.2) | 0 | 0 | 0 | 4 (0.1) |
| Grade ≥ 3 | 1 (0.1) | 0 | 0 | 0 | 0 | 1 (0.0) | |
| Infusion-related reaction (PT) | Any grade | 38 (4.8) | 19 (2.4) | 12 (1.5) | 17 (2.1) | 0 | 86 (2.7) |
| Grade ≥ 3 | 4 (0.5) | 4 (0.5) | 1 (0.1) | 2 (0.3) | 0 | 11 (0.3) | |
Data are presented as n (%)
IRR infusion-related reaction, n number of patients in specified category, N total number of patients, PT preferred term, Q quartile, SMQ Standardized Medical Dictionary for Regulatory Activities queries
aBroad- and narrow-scope preferred terms were used within the Standardized Medical Dictionary for Regulatory Activities (MedDRA) queries. “Narrow” preferred terms included terms that are highly likely to represent the condition of interest and were considered sufficient to identify immediate IRRs with reasonable precision and to appropriately reflect the incidence rate of immediate IRRs
bRange (minimum–maximum, mg/min) within each quartile group: Q1 = 0.50–7.76; Q2 = 7.76–9.50, Q3 = 9.50–11.67; Q4 = 11.67–40.29; and two outliers (182.00, 190.00)
Under multivariate logistic analysis that included infusion rate as a continuous variable together with other factors, infusion rate was not significantly associated with an increased risk of an immediate IRR (odds ratio per 1 mg/min increase 1.014; P = 0.071; Table 4). Identified risk factors for an immediate IRR included residing in Asia, receiving chemotherapy, and no premedication.
Table 4.
Multivariate logistic analysesa of relationships between infusion rates (continuous variable) and incidence of immediate infusion-related reactions (narrow termsb) in ramucirumab-treated patients
| Factor | Comparison | OR (95% CI) for risk of immediate IRR | p value |
|---|---|---|---|
| Infusion rate | 1-unit increase, mg/min | 1.014 (0.999, 1.030) | 0.071 |
| Age | ≥ 65 vs. < 65 years | 0.831 (0.633, 1.090) | 0.181 |
| Sex | Female vs. male | 1.127 (0.848, 1.497) | 0.409 |
| Region | Asia vs. rest of the world | 2.151 (1.596, 2.898) | < 0.001 |
| Premedication | Yes vs. no | 0.519 (0.389, 0.691) | < 0.001 |
| Chemotherapy | Yes vs. no | 1.443 (1.033, 2.016) | 0.032 |
| Dosing regimen | 10 mg/kg Q3W vs. 8 mg/kg Q2W | 0.790 (0.565, 1.106) | 0.170 |
| 12 mg/kg Q2W vs. 8 mg/kg Q2W | 0.456 (0.196, 1.061) | 0.068 | |
| 6 mg/kg QW vs. 8 mg/kg Q2W | 0.922 (0.215, 3.951) | 0.913 | |
| 8 mg/kg D1D8 Q3W vs. 8 mg/kg Q2W | 0.505 (0.289, 0.884) | 0.017 |
CI confidence interval, D day, IRR infusion-related reaction, OR odds ratio, QW weekly, Q2W every 2 weeks, Q3W every 3 weeks
aIncluding infusion rate as a continuous variable and adjusting for other factors that might affect the incidence of immediate infusion-related reactions (age, sex, premedication, chemotherapy, region, and dosing regimen). Baseline bodyweight was not included as a covariate because of its strong correlation with infusion rate
bBroad- and narrow-scope preferred terms were used within the Standardized Medical Dictionary for Regulatory Activities (MedDRA) queries. “Narrow” preferred terms included terms that are highly likely to represent the condition of interest and were considered sufficient to identify immediate IRRs with reasonable precision and to appropriately reflect the incidence rate of immediate IRRs
Discussion
Results of simulations from a PopPK model with time-varying CL established using ramucirumab PK data from 17 studies (2522 patients) found the PK profiles of ramucirumab following either a 30- or 60-min infusion to be equivalent. This finding of no change in ramucirumab exposure suggests that shortening the infusion duration of ramucirumab from 60 to 30 min would not impact efficacy or overall safety outcomes related to systemic exposure to ramucirumab.
General management strategies to reduce the risk of IRRs to mAb therapies include premedication with antihistamines and/or corticosteroids and reducing the infusion rate [11]. We investigated the perceived association between immediate IRRs and infusion rate, specifically the impact of infusion rate on the incidence of immediate IRRs. The incidence of any-grade immediate IRRs following the infusion of ramucirumab was low, with 7.9% of 3216 patients experiencing at least one immediate IRR in the ten included studies. Most immediate IRRs were grade 1 or 2; incidence of grade ≥ 3 events (requiring permanent discontinuation of ramucirumab) was low (0.5%), and most such events occurred during the first two infusions.
Although ramucirumab was administered at a range of infusion rates in the ten studies analyzed, no difference in infusion rate distributions was apparent between patients with and without at least one immediate IRR, either within each individual study or in the pooled dataset. The incidence of immediate IRRs across infusion rate quartile groups further suggested that a faster infusion rate was not associated with a higher incidence of immediate IRRs within the range of the observed infusion rate data. In fact, the highest incidence of immediate IRRs occurred in the quartile with the lowest infusion rate (Q1; 10.9% of patients had any-grade immediate IRR, 0.6% had grade ≥ 3 immediate IRR; Table 3), potentially because many of the patients who experienced an IRR would have had their infusions interrupted and infusion times prolonged (as per usual clinical practice and current labeling [4]). Sensitivity analyses, assuming a 60-min infusion for all patients, found no association between infusion rate and immediate IRR incidence. Multivariate logistic regression analyses also demonstrated that immediate IRR occurrence was not significantly associated with dose or infusion rate (mg/min) in the range of observed infusion rate data investigated. These analyses indicated that residing in Asia was associated with an increased risk of immediate IRRs. Reasons are unclear, but as we found a higher incidence of immediate IRRs in placebo-treated Asian patients than in placebo-treated non-Asian patients (data not shown) it is thought that local medical management or more stringent reporting of IRRs are more likely contributory factors to this association than ramucirumab treatment.
The mechanisms by which mAbs induce IRRs are imperfectly understood. Mechanisms proposed for rituximab (direct binding of the antibody to its antigen, resulting in cytokine release) and cetuximab (formation of IgE antidrug antibody complexes leading to allergic reactions or anaphylaxis [16, 17]) are not thought to be implicated in IRRs to ramucirumab. Retrospective and prospective studies of various therapeutic antibodies (e.g., ramucirumab, bevacizumab, rituximab, daratumumab, and infliximab), with IRR incidence as an endpoint, have found that a more rapid rate of infusion/shortened infusion time does not appear to increase IRR incidence. For example, a recent small prospective study found no immediate IRRs in patients receiving ramucirumab infused over 20 min following at least one initial 60-min infusion without evidence of immediate IRRs [18]. Hence, the commonly held perception that immediate IRR incidence is related to infusion rate warrants further investigation. Furthermore, shorter infusion times for other monoclonal antibody treatments are known to improve patient [19, 20] and clinic personal satisfaction [20] as well as lower the cost of treatment [19].
Strengths/limitations
PopPK modeling/simulation is an established tool used during the drug development process to help predict drug concentration profiles with changing dosing regimens. The PopPK model used in these analyses was based on ramucirumab PK data from 2522 patients enrolled in 17 studies; its findings can therefore be considered robust.
The ten phase II/III clinical studies included in the evaluation of the association between ramucirumab infusion rate and the incidence of immediate IRRs enabled a thorough exploration of any potential relationship between infusion rate and immediate IRRs. Although data for higher infusion rates are sparse, IRR incidences in the literature from shorter infusion durations of therapeutic antibodies (including ramucirumab) do not seem to support a strong relationship between infusion rate and IRR incidence. We therefore consider it unlikely that increasing the infusion rate of ramucirumab (by shortening the infusion time) would impact the incidence of immediate IRRs.
This was a retrospective analysis of clinical data from historical studies and was not designed to confirm whether a shorter infusion time would affect the incidence of IRRs. The observed infusion rates that patients received might have been affected by interventions such as appropriate infusion interruptions or longer infusion times resulting from immediate IRRs, although sensitivity analyses to account for such interventions did not reveal an association. A shortened ramucirumab infusion duration could reduce overall waiting times for patients, allowing more patients to be treated, improving patient satisfaction, and lowering resource utilization. In this study, PK profiles for ramucirumab were equivalent (showing no change in drug exposure) following either a 30- or 60-min infusion. Additionally, these analyses found no evidence to suggest that decreasing the infusion time for ramucirumab would result in an increased incidence of any grade or grade ≥ 3 immediate IRRs. Given these findings, it is considered that shortening the ramucirumab infusion duration from 60 to 30 min is unlikely to impact the efficacy or safety profiles of ramucirumab. A reasonable approach would therefore be to administer the initial infusion over the currently approved 60 min and, if no IRR is observed, reduce this duration to 30 min for subsequent infusions. As of publication, the USA and Japanese regulatory authorities have updated their label to allow this dosing regimen for all approved cancer indications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Gill Gummer and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Funding
This work was supported by Eli Lilly and Company.
Compliance with ethical standards
Conflict of interest
PA, Y-KL, LG, L O’B, AL, YP and RW are current employees of Eli Lilly and Company and own stock in Eli Lilly and Company.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chern B, Joseph D, Joshua D, Pittman K, Richardson G, Schou M, Lowe S, Copeman M, De Abreu LR, Lynch K. Bisphosphonate infusions: patient preference, safety and clinic use. Support Care Cancer. 2004;12(6):463–466. doi: 10.1007/s00520-004-0628-z. [DOI] [PubMed] [Google Scholar]
- 2.Chiang J, Chan A, Shih V, Hee SW, Tao M, Lim ST. A prospective study to evaluate the feasibility and economic benefits of rapid infusion rituximab at an Asian cancer center. Int J Hematol. 2010;91(5):826–830. doi: 10.1007/s12185-010-0583-z. [DOI] [PubMed] [Google Scholar]
- 3.Dranitsaris G, Yu B, Wang L, Sun W, Zhou Y, King J, Kaura S, Zhang A, Yuan P. Abraxane(R) versus Taxol(R) for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205–211. doi: 10.1177/1078155214556008. [DOI] [PubMed] [Google Scholar]
- 4.Lilly Cyramza prescribing information [online]. http://pi.lilly.com/us/cyramza-pi.pdf. Accessed 5 Dec 2019
- 5.Cohn AL, Yoshino T, Heinemann V, Obermannova R, Bodoky G, Prausova J, Garcia-Carbonero R, Ciuleanu T, Garcia-Alfonso P, Portnoy DC, Van Cutsem E, Yamazaki K, Clingan PR, Polikoff J, Lonardi S, O'Brien LM, Gao L, Yang L, Ferry D, Nasroulah F, Tabernero J. Exposure–response relationship of ramucirumab in patients with advanced second-line colorectal cancer: exploratory analysis of the RAISE trial. Cancer Chemother Pharmacol. 2017;80(3):599–608. doi: 10.1007/s00280-017-3380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabernero J, Ohtsu A, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Ajani JA, Tomasek J, Safran H, Chandrawansa K, Hsu Y, Heathman M, Khan A, Ni L, Melemed AS, Gao L, Ferry D, Fuchs CS. Exposure-response analyses of ramucirumab from two randomized, Phase III trials of second-line treatment for advanced gastric or gastroesophageal junction cancer. Mol Cancer Ther. 2017;16(10):2215–2222. doi: 10.1158/1535-7163.MCT-16-0895. [DOI] [PubMed] [Google Scholar]
- 7.Sheiner LB, Ludden TM. Population pharmacokinetics/dynamics. Annu Rev Pharmacol Toxicol. 1992;32:185–209. doi: 10.1146/annurev.pa.32.040192.001153. [DOI] [PubMed] [Google Scholar]
- 8.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacometr Syst Pharmacol. 2012;1:e6. doi: 10.1038/psp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien L, Westwood P, Gao L, Heathman M. Population pharmacokinetic meta-analysis of ramucirumab in cancer patients. Br J Clin Pharmacol. 2017;83(12):2741–2751. doi: 10.1111/bcp.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smit EF, Garon EB, Reck M, Cappuzzo F, Bidoli P, Cohen RB, Gao L, O'Brien LM, Lee P, Zimmermann A, Ferry DR, Melemed AS, Perol M. Exposure-response relationship for ramucirumab from the randomized, double-blind, phase 3 REVEL trial (docetaxel versus docetaxel plus ramucirumab) in second-line treatment of metastatic non-small cell lung cancer. Cancer Chemother Pharmacol. 2018;82(1):77–86. doi: 10.1007/s00280-018-3560-5. [DOI] [PubMed] [Google Scholar]
- 11.Rosello S, Blasco I, Garcia Fabregat L, Cervantes A, Jordan K, clinicalguidelines@esmo.org EGCEa Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2017;28(Suppl 4):100–118. doi: 10.1093/annonc/mdx216. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-based population pharmacokinetic analysis of Nivolumab in patients with solid tumors. CPT Pharmacomet Syst Pharmacol. 2017;6(1):58–66. doi: 10.1002/psp4.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Yu J, Li H, Liu J, Xu Y, Song P, Liu Q, Zhao H, Xu J, Maher VE, Booth BP, Kim G, Rahman A, Wang Y. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther. 2017;101(5):657–666. doi: 10.1002/cpt.656. [DOI] [PubMed] [Google Scholar]
- 14.Baverel PG, Dubois VFS, Jin CY, Zheng Y, Song X, Jin X, Mukhopadhyay P, Gupta A, Dennis PA, Ben Y, Vicini P, Roskos L, Narwal R. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther. 2018;103(4):631–642. doi: 10.1002/cpt.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beal SSL, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989–2009) Ellicott City: Icon Development Solution; 2009. [Google Scholar]
- 16.Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, James H, van Berkel PH, van de Winkel JG, Platts-Mills TA, Parren PW. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574–576. doi: 10.1038/nbt.1912. [DOI] [PubMed] [Google Scholar]
- 17.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto N, Mitani S, Taniguchi H, Narita Y, Kato K, Masuishi T, Kadowaki S, Onishi S, Tajika M, Takahashi S, Shimomura K, Takahata C, Hotta E, Kobara M, Muro K. A prospective trial evaluating the safety of a shortened infusion of ramucirumab in patients with gastrointestinal cancer. Oncologist. 2019;24(2):159–e166. doi: 10.1634/theoncologist.2018-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer S, Fadeyi M, Chan S. A study of tolerability, satisfaction, and cost reduction using a 10% immunoglobulin product at higher administration rates. J Infus Nurs. 2019;42(6):297–302. doi: 10.1097/NAN.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 20.Daniel SR, McDermott JD, Jr, Le C, Pierce CA, Ziskind MA, Ellis LA. A real-world, multi-site, observational study of infusion time and treatment satisfaction with rheumatoid arthritis patients treated with intravenous golimumab or infliximab. J Med Econ. 2018;21(7):724–731. doi: 10.1080/13696998.2018.1472098. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Investigators RT. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 22.Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, Group RS. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Paz-Ares L, Bidoli P, Cappuzzo F, Dakhil S, Moro-Sibilot D, Borghaei H, Johnson M, Jotte R, Pennell NA, Shepherd FA, Tsao A, Thomas M, Carter GC, Chan-Diehl F, Alexandris E, Lee P, Zimmermann A, Sashegyi A, Perol M. Outcomes in patients with aggressive or refractory disease from REVEL: a randomized phase III study of docetaxel with ramucirumab or placebo for second-line treatment of stage IV non-small-cell lung cancer. Lung Cancer. 2017;112:181–187. doi: 10.1016/j.lungcan.2017.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausova J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F, Investigators RS. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M, Investigators RT. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- 26.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M, Investigators R-s Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Shitara K, Di Bartolomeo M, Lonardi S, Al-Batran SE, Van Cutsem E, Ilson DH, Alsina M, Chau I, Lacy J, Ducreux M, Mendez GA, Alavez AM, Takahari D, Mansoor W, Enzinger PC, Gorbounova V, Wainberg ZA, Hegewisch-Becker S, Ferry D, Lin J, Carlesi R, Das M, Shah MA, Group RS. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420–435. doi: 10.1016/S1470-2045(18)30791-5. [DOI] [PubMed] [Google Scholar]
- 28.Petrylak DP, de Wit R, Chi KN, Drakaki A, Sternberg CN, Nishiyama H, Castellano D, Hussain S, Flechon A, Bamias A, Yu EY, van der Heijden MS, Matsubara N, Alekseev B, Necchi A, Geczi L, Ou YC, Coskun HS, Su WP, Hegemann M, Percent IJ, Lee JL, Tucci M, Semenov A, Laestadius F, Peer A, Tortora G, Safina S, Del Muro XG, Rodriguez-Vida A, Cicin I, Harputluoglu H, Widau RC, Liepa AM, Walgren RA, Hamid O, Zimmermann AH, Bell-McGuinn KM, Powles T, Investigators Rs Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet. 2017;390(10109):2266–2277. doi: 10.1016/S0140-6736(17)32365-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.