Abstract
Importance
Rectal cancers occupy the eighth position worldwide for new cases and deaths for both men and women. These cancers have a high tendency to form metastases in the mesorectum but also in the lateral lymph nodes. The therapeutic approach for the involved lateral lymph nodes remains controversial.
Objective
We performed a systematic review and meta-analysis to assess the prevalence of metastatic lateral lymph nodes in patients with lateral lymph node dissection (LLND) for rectal cancer, which seems to be a fundamental and necessary criterion to discuss any possible indications for LLND.
Methods
Data sources–study selection–data extraction and synthesis–main outcome and measures. We searched MEDLINE, EMBASE and COCHRANE from November 1, 2018, to November 19, 2018, for studies reporting the presence of metastatic lateral lymph nodes (iliac, obturator and middle sacral nodes) among patients undergoing rectal surgery with LLND. Pooled prevalence values were obtained by random effects models, and the robustness was tested by leave-one-out sensitivity analyses. Heterogeneity was assessed using the Q-test, quantified based on the I2 value and explored by subgroup analyses.
Results
Our final analysis included 31 studies from Asian countries, comprising 7599 patients. The pooled prevalence of metastatic lateral lymph nodes was 17.3% (95% CI: 14.6–20.5). The inter-study variability (heterogeneity) was high (I2 = 89%). The pooled prevalence was, however, robust and varied between 16.6% and 17.9% according to leave-one-out sensitivity analysis. The pooled prevalence of metastatic lymph nodes was not significantly different when pooling only studies including patients who received neoadjuvant treatment or those without neoadjuvant treatment (p = 0.44). Meta-regression showed that the pooled prevalence was associated with the sample size of studies (p < 0.05), as the prevalence decreased when the sample size increased.
Conclusion
The pooled prevalence of metastatic lateral lymph nodes was 17.3% among patients who underwent rectal surgery with LLND in Asian countries. Further studies are necessary to determine whether this finding could impact the therapeutic strategy (total mesorectal excision with LLND versus total mesorectal excision with neoadjuvant radiochemotherapy).
Supplementary Information
The online version contains supplementary material available at(10.1007/s00268-021-05956-1)
Introduction
Rectal cancer metastasizes to the perirectal lymph nodes contained within the mesorectum and along the iliac arteries [1]. Assessment of lymph node involvement is a strong predictor of recurrence-free survival and overall survival in patients with rectal cancer [2]. Currently, total mesorectal excision constitutes the gold standard for removing perirectal lymph nodes [3]. However, the therapeutic strategy regarding lymph nodes, notably those located along the iliac arteries, may include neoadjuvant radiochemotherapy, as performed in Western countries [3], or lateral lymph node dissection (LLND), as performed in Japan when the lower border of the tumour is located under the reflection of the peritoneum and when it has passed the muscularis propria [4].
However, to date, only two randomized controlled trials (RCTs) [5, 6] have compared the outcomes of the two therapeutic strategies in terms of survival, looking at local and distant recurrences and complications. Furthermore, the choice of the best strategy, either LLND or radiochemotherapy or the combination of the two treatments, is difficult to identify, as the prevalence of metastatic lateral lymph nodes in patients with rectal cancer is poorly documented. Indeed, before comparing two therapeutic strategies and their outcomes, it is necessary and more relevant to know if it is legitimate to propose them as a treatment; in other words, if they would even correctly target metastatic lateral lymph nodes (LLNs).
Therefore, our objective was to perform a systematic review and meta-analysis reporting on the prevalence of metastatic lateral lymph nodes in patients with rectal cancer with the hypothesis that this meta-analysis will elucidate the best therapeutic strategy in the current absence of reliable assessment of tumour aggressiveness.
Materials and methods
The present methodology is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (Table S1).
Literature search and study selection
A literature search was conducted in MEDLINE, EMBASE and COCHRANE from inception (DATE??) until November 19, 2018. Keyword combinations are reported in Table S2. Additional records were identified by manually searching the reference lists of the included publications. To be included, studies had to be written in English or French and to report the prevalence of metastatic lateral lymph nodes among patients with rectal cancer who underwent surgery with LLND. LLND was defined by the dissection of nodes included in areas such as the common iliac (IC), internal iliac (II), external iliac (EI), obturator (O), middle sacral (MS) and aorta bifurcation (Ao).
We excluded case series, conference abstracts, letters to the editor and secondary analyses of previously published papers.
Data extraction
Two independent reviewers (NC and JM) independently selected articles for inclusion and extracted the data according to a pre-established data collection form. Discrepancies were resolved by reaching a consensus with the senior authors (NCB and FR). The following data were extracted: first author; publication year; country where the study took place; study period, after and before 2010 due to modifications of the Japanese Guidelines for LLND; study design; number of patients who underwent LLND, prophylactic: dissection and removal of nodes that seem invaded (enlarged) based on pre- and peri-operative examinations, versus therapeutic/curative: removal of all nodes in the “lateral lymph area”; sex; number of patients with metastatic lateral lymph nodes; number of patients who underwent pre-operative radio- and/or chemotherapy; type of neoadjuvant treatment in those patients; and oncological stages of included patients.
Statistical analysis
Models with random effects (DerSimonian and Laird’s approach [7]) were used to combine the prevalence of metastatic lateral lymph nodes across the studies. A logit transformation was applied to prevalence before statistical pooling, and the pooled logit of prevalence was then transformed back. Heterogeneity was assessed by using the I2 statistic, and a leave-one-out sensitivity analysis was conducted to check the robustness of the pooled prevalence. Potential sources of heterogeneity were investigated by comparing the pooled prevalence between subgroups of studies. A sensitivity analysis was also conducted excluding stage 4 patients from the denominator and from the numerator of the prevalence because stage 4 patients were assumed to have metastatic lateral lymph nodes (model with random effects). In addition, a meta-regression analysis was conducted to assess the relationship between the sample size of studies and the prevalence with the restricted maximum likelihood method. [8] All analyses were performed with the Meta and Metafor packages for R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Literature search and study characteristics
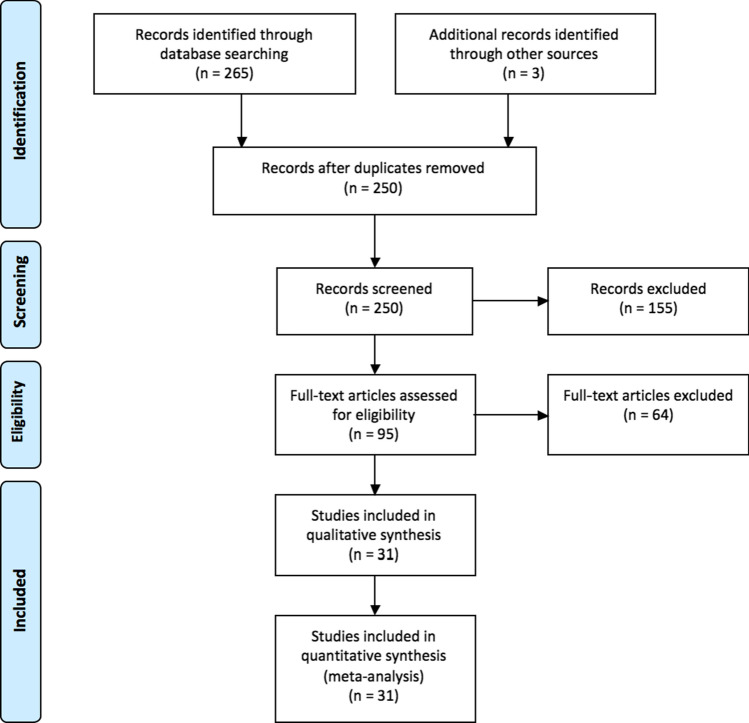
Two hundred and sixty-five publications were identified in MEDLINE, EMBASE and COCHRANE. Three publications were identified from other sources. Fifteen duplicates were removed. Of the 250 publications that were identified as eligible, 155 were excluded after title/abstract screening, and 64 were excluded after full-text screening (44 publications did not report the number of patients with metastatic lateral lymph nodes and 20 publications did not distinguish patients with metastatic lateral lymph nodes from those with metastatic lymph nodes from other areas). Ultimately, 31 publications [5, 6, 9–37] were included in the quantitative synthesis, all coming from Japan except one from China (Fig. 1, Table 1).
Fig. 1.
Flow chart showing the selection of publications for quantitative review. Forest plot of the prevalence of metastatic lateral lymph nodes in patients who underwent lateral lymph node dissection. Each horizontal bar summarizes a study. The bars represent 95% confidence intervals. The grey squares represent each of the studies’ weights in the meta-analysis. The diamond in the lower part of the graph depicts the pooled estimate along with 95% confidence intervals. Events = number of patients with metastatic lateral lymph nodes, total = number of patients who underwent lateral lymph node dissection for rectal cancer
Table 1.
Characteristics of included studies
| Author | Year | Cohort dates | Design | Mono-/multicentric | Country |
|---|---|---|---|---|---|
| Fujita et al | 2012 | 2010–2003 | RCT | Multicentric | Japan |
| Hara et al | 2007 | 1987–1999 | Retrospective cohort | Monocentric | Japan |
| Ishibe et al | 2016 | 2008–2012 | Retrospective cohort | Monocentric | Japan |
| Kanemitsu et al | 2017 | 1975–2009 | Retrospective cohort | Multicentric | Japan |
| Ishida et al | 2012 | 1997–2011 | Retrospective cohort | Monocentric | Japan |
| Kagawa et al | 2015 | 2012–2013 | Retrospective cohort | Monocentric | Japan |
| Masaki et al | 2010 | 2000–2009 | RCT | Monocentric | Japan |
| Matsuoka et al | 2007 | 1997–2005 | Retrospective cohort | Monocentric | Japan |
| Miyake et al | 2017 | 2014–206 | Retrospective cohort | Monocentric | Japan |
| Min et al | 2009 | 1996–2006 | Retrospective cohort | Monocentric | Japan |
| Sato et al | 2011 | 2005–2007 | Prospective cohort | Monocentric | Japan |
| Sato et al | 2011 | 1990–2005 | Retrospective cohort | Monocentric | Japan |
| Shimoyama et al | 2003 | 1981–1994 | Retrospective cohort | Monocentric | Japan |
| Yokoyama et al | 2014 | 2000–2008 | Retrospective cohort | Monocentric | Japan |
| Komori et al | 2013 | 1979–2001 | Retrospective cohort | Monocentric | Japan |
| Masaki et al | 2008 | 2000–2007 | Retrospective cohort | Monocentric | Japan |
| Wu et al | 2007 | ns | Retrospective cohort | Monocentric | Japan |
| Ueno et al | 2007 | 1985–2000 | Retrospective cohort | Monocentric | Japan |
| Steup et al | 2002 | 1974–1990 | Retrospective cohort | Monocentric | Japan |
| Mori et al | 1998 | 1975–1996 | Retrospective cohort | Monocentric | Japan |
| Tan et al | 2010 | 1980–2008 | Retrospective cohort | Monocentric | Japan |
| Nagasaki et al | 2017 | 1985–2012 | Retrospective cohort | Monocentric | Japan |
| Yamaoka et al | 2017 | 2013–2015 | Retrospective cohort | Monocentric | Japan |
| Numata et al | 2017 | 2002–2013 | Retrospective cohort | Monocentric | Japan |
| Yamaguchi et al | 2016 | 2010–2014 | Retrospective cohort | Monocentric | Japan |
| Yu et al | 2011 | 2006–2007 | Retrospective cohort | Monocentric | China |
| Kobayashi et al | 2009 | 1991–1998 | Retrospective cohort | Multicentric | Japan |
| Yano et al | 2007 | 1995–2013 | Prospective cohort | Monocentric | Japan |
| Kinugasa et al | 2013 | 1975–2004 | Retrospective cohort | Monocentric | Japan |
| Ueno et al | 2005 | 1985–1999 | Retrospective cohort | Monocentric | Japan |
| Hida et al | 1997 | 1979–1988 | Retrospective cohort | Monocentric | Japan |
Among the included publications, 28 were retrospective cohort studies [9–35, 37], one was a prospective cohort study [36] and two were RCTs [5, 6]. Thirty studies were performed in Japan [5, 6, 9–33, 35–37] and one in China [34]. Eleven studies only included patients with TNM cancer stages 2 and 3 [5, 9–13, 15, 31, 32, 35, 38]. In 24 studies [5, 6, 9–11, 15, 17–28, 31–35, 37], systematic LLND was performed in all included patients, whereas in six studies [12–14, 16, 29, 30, 36], LLND was performed only in patients with enlarged lateral lymph nodes on pre-operative imaging, which distinguished these patients from others who had confirmation by histopathological results. In one study, the type of LLND was not described [28]. In two studies, patients underwent systematic bilateral dissection [22, 32]; in 27 studies, uni- or bilateral dissection was defined according to clinical and/or imaging findings [6, 12–21, 23, 25, 26, 28–31, 37–39] and in four studies, lateralization of dissection was not documented [24, 34–36]. Nine studies included patients who received neoadjuvant treatment [6, 11, 13–17, 30, 31]. Indications for neoadjuvant treatment were not documented in four studies [11, 15–17] and depended on tumour stage in five studies [13, 14, 16, 30, 31]. The characteristics of the included studies are reported in Tables 2, 3, S3, S4. It is worth noting that the study of Yano et al. has been classified above as a study with “curative LLND” because pre-imaging with enlarged nodes was considered the main decision-making criterion for LLND. However, in fact, after the flow chart analysis, dissection was also performed in a systematic way (Table S4). Thus, for statistical analysis, we classified it as a “prophylactic study”.
Table 2.
Demographics of included studies
| Authors | Patients, n | Women, n (%) | Men, n (%) | Age (median) | T1, n (%) | T2, n (%) | T3, n (%) | T4, n (%) | Stage 1, n (%) | Stage 2, n (%) | Stage 3, n (%) | Stage 4, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujita et al | 351 | 115 (32.8%) | 236 (67.2%) | 61 | n/s | n/s | n/s | n/s | n/s | 188 (53.6%) | 163 (46.4%) | n/s |
| Hara et al | 177 | 65 (36.7%) | 112 (63.3%) | 56.0 | n/s | 60 (33.9%) | 102 (57.6%) | 15 (8.5%) | 32 (18.1%) | 36 (20.3%) | 109 (61.6%) | n/s |
| Ishibe et al | 84 | 31 (36.9%) | 53 (63.1%) | 62 | n/s | n/s | n/s | n/s | 8 (9.5%) | 25 (29.8%) | 39 (46.4%) | 12 (14.3%) |
| Kanemitsu et al | 1191 | 419 (35.2%) | 772 (64.9%) | 57 | 26 (2.2%) | 348 (29.2%) | 731 (61.4%) | 86 (7.2%) | 244 (20.5%) | 351 (29.5%) | 596 (50.0%) | n/s |
| Ishida et al | 47 | 16 (34%) | 31 (66%) | 62 | n/s | n/s | n/s | n/s | 11 (23.4%) | 13 (27.7%) | 23 (48.9%) | n/s |
| Kagawa et al | 50 | 8 (16%) | 42 (84%) | 62 | n/s | n/s | n/s | n/s | 6 (12%) | 11 (22.0%) | 28 (56.0%) | 5 (10.0%) |
| Masaki et al | 55 | 16 (29.1%) | 39 (70.9%) | n/s | n/s | n/s | 46 (83.6%) | n/s | n/s | n/s | n/s | n/s |
| Matsuoka et al | 51 | 16 (31.4%) | 35 (68.6%) | 63 | n/s | n/s | 46 (90.2%) | 5 (9.8%) | n/s | n/s | n/s | n/s |
| Miyake et al | 25 | 8 (32%) | 17 (68%) | 67 | 2 (8.0%) | 10 (40.0%) | 8 (32.0%) | 1 (4.0%) | 10 (40.0%) | 3 (12.0%) | 3 (12.0%) | 5 (20.0%) |
| Min et al | 151 | 75 (49.7%) | 76 (50.3%) | 52.6 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Sato et al | 67 | 24 (35.8%) | 43 (64.2%) | 63 | n/s | n/s | 56 (83.6%) | 11 (16.4%) | n/s | n/s | n/s | n/s |
| Sato et al | 149 | 51 (34.2%) | 98 (65.8%) | n/s | n/s | 13 (8.7%) | 125 (83.9%) | 11 (7.4%) | n/s | n/s | n/s | n/s |
| Shimoyama et al | 66 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | ||
| Yokoyama et al | 131 | 40 (30.5%) | 91 (69.5%) | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Komori et al | 88 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | ||
| Masaki et al | 41 | 12 (29.3%) | 29 (70.7%) | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Wu et al | 96 | 50 (52.1%) | 46 (47.9%) | 65 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Ueno et al | 244 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Steup et al | 605 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Mori et al | 157 | 62 (39.5%) | 95 (60.5%) | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Tan et al | 1046 | 340 (32.5%) | 706 (67.5%) | 58.6 | 189 (18.1%) | 301 (28.8%) | 516 (49.3%) | 32 (3.1%) | n/s | n/s | n/s | n/s |
| Nagasaki et al | 371 | 27 (7.3%) | 46 (12.4%) | 61 | n/s | n/s | 66 (17.8%) | n/s | n/s | n/s | n/s | n/s |
| Yamaoka et al | 150 | 35 (23.3%) | 115 (76.7%) | 61.5 | 6 (4.0%) | 33 (22.0%) | 88 (58.7%) | 23 (15.3%) | n/s | 30 (20.0%) | 79 (52.6%) | 17 (11.3%) |
| Numata et al | 229 | 65 (28.4%) | 164 (71.6%) | 61 | n/s | 41 (17.9%) | 160 (69.9%) | 28 (12.2%) | 30 (13.1%) | 75 (32.8%) | 119 (52%) | 5 (2.2%) |
| Yamaguchi et al | 173 | 39 (22.5%) | 134 (77.5%) | n/s | n/s | n/s | n/s | n/s | 23 (13.3%) | 41 (23.7%) | 93 (53.4%) | 16 (9.2%) |
| Yu et al | 96 | 42 (43.4%) | 54 (56.3%) | 60 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Kobayashi et al | 784 | 277 (35.3%) | 507 (64.7%) | n/s | 37 (4.7%) | 207 (26.4%) | 497 (63.4%) | 43 (5.5%) | 179 (22.8%) | 224 (28.6%) | 381 (48.6%) | n/s |
| Yano et al | 109 | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s | n/s |
| Kinugasa et al | 994 | 349 (35.1%) | 645 (64.9%) | n/s | 142 (14.3%) | 260 (26.2%) | 658 (66.2%) | 384 (38.6%) | n/s | n/s | n/s | n/s |
| Ueno et al | 237 | 91 (38.4%) | 146 (61.6%) | 58 | 224 (94.5%) | 13 (5.5%) | n/s | n/s | n/s | n/s | ||
| Hida et al | 198 | n/s | n/s | n/s | 8 (4.0%) | 38 (19.2%) | 128 (64.6%) | 24 (12.1%) | n/s | n/s | n/s | n/s |
Table 3.
Characteristics of studies according to the type of LLND surgery and neoadjuvant treatment
| Authors | Neoadjuvant treatment | Surgical approach, n | Areas of LLND | Unilateral/Bilateral | Metastatic lateral lymph nodes, n (%) | R0 resection, n (%) |
|---|---|---|---|---|---|---|
| Fujita et al | No | Open | IC + II + IE + O + MS | Mixed | 26 (7.4%) | n/s |
| Hara et al | No | n/s | II + MS + O ± Ao bifurcation ± IC ± EI | Mixed | 32 (18.1%) | n/s |
| Ishibe et al | No | n/s | IC + II + IE + O | Mixed | 16 (19%) | n/s |
| Kanemitsu et al | Selected patients | n/s | Ao bifurcation + IC + II + IE + 0 | Mixed | 92 (7.7%) | n/s |
| Ishida et al | No | n/s | O + II | Mixed | 11 (23.4%) | n/s |
| Kagawa et al | Selected patients | Robotic-assisted | IC + II + O | Mixed | 10 (20%) | n/s |
| Masaki et al | Yes | Open | IC + II + O | Mixed | 11 (20%) | 50 (90.9%) |
| Matsuoka et al | Yes | n/s | Mixed | 15 (29.4%) | n/s | |
| Miyake et al | Selected patients | n/s | IC + II + IE + O | Bilateral | 4 (16%) | n/s |
| Min et al | Selected patients | n/s | Ao + I + O | Mixed | 36 (23.8%) | 133 (88.1%) |
| Sato et al | Yes | n/s | II + IE + O | Mixed | 6 (9%) | n/s |
| Sato et al | No | n/s | Mixed | 64 (43%) | n/s | |
| Shimoyama et al | No | n/s | Ao + II + IE + IC | Mixed | 20 (30.3%) | n/s |
| Yokoyama et al | No | n/s | IC + II + IE + O | Mixed | 26 (19.8%) | n/s |
| Komori et al | No | n/s | IC + II + IE + O | Mixed | 14 (15.9%) | n/s |
| Masaki et al | No | n/s | IC + II + IE + O | Bilateral | 10 (24.4%) | n/s |
| Wu et al | No | n/s | Mixed | 14 (14.6%) | n/s | |
| Ueno et al | No | n/s | internal pudendal + IC + II + IE + O + MS | Mixed | 41 (16.8%) | n/s |
| Steup et al | No | n/s | IC + II + O | Mixed | 83 (13.7%) | n/s |
| Mori et al | No | Open | II + EI + O | Mixed | 40 (25.5%) | n/s |
| Tan et al | No | n/s | n/s | Mixed | 113 (10.8%) | n/s |
| Nagasaki et al | Selected patients | n/s | II + EI + O + CI | Mixed | 73 (19.7%) | 68 (18.3%) |
| Yamaoka et al | Selected patients |
Open: 27 Robot: 115 Laparoscopy: 8 |
CI, II, O | Mixed | 34 (22.7%) | n/s |
| Numata et al | No |
Open: 199 Robot: 28 Laparoscopy: 2 |
CI, II, O | Bilateral | 32 (14%) | 229 (100%) |
| Yamaguchi et al | No | Open: 88, Robot: 85 | CI, II, O | Mixed | 32 (18.5%) | n/s |
| Yu et al | No | n/s | CI, II, MS, O, EI | n/s | 14 (14.6%) | n/s |
| Kobayashi et al | n/s | n/s | n/s | n/s | 117 (14.9%) | n/s |
| Yano et al | n/s | n/s | n/s | n/s | 21 (53.8%) | 103 (94.5%) |
| Kinugasa et al | No | n/s | n/s | Mixed | 59 (13.1) | n/s |
| Ueno et al | No | n/s | CI, IE, II, middle rectal | Mixed | 41 (17.3%) | 237 (100%) |
| Hida et al | No | n/s | CI, O, II, middle rectal | n/s | 22 (11.1%) | n/s |
Common iliac (IC), internal iliac node (II), external iliac node (EI), obturator node (O), middle sacral node (MS), aorta (Ao)
Prevalence of metastatic lateral lymph nodes
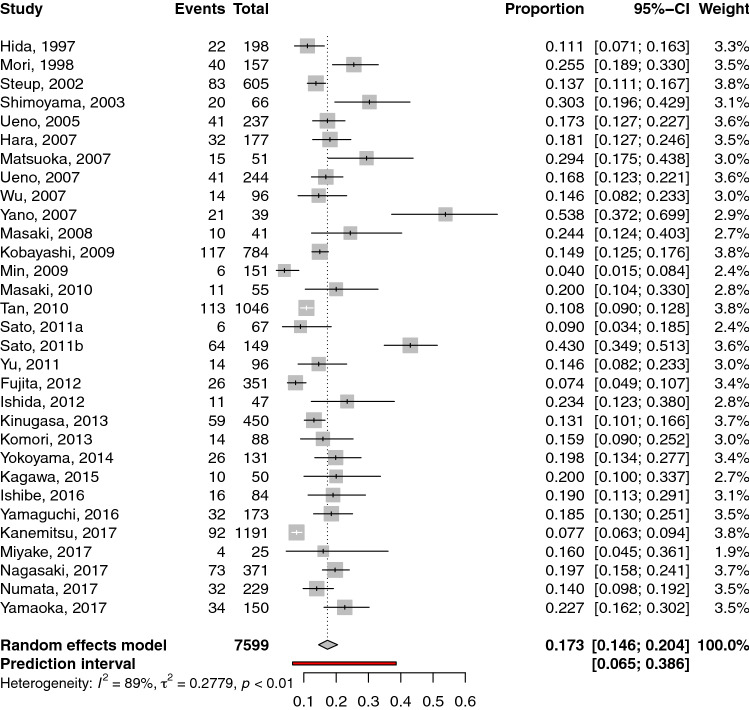
We obtained a pooled prevalence of metastatic lateral lymph nodes of 17.3% (95% CI: 14.6–20.5) (31 studies, 7599 patients) (Fig. 2). The inter-study variability (heterogeneity) was noteworthy, with I2 = 89%. In other words, the variation in study outcome (prevalence) between studies was high. Furthermore, the prediction interval was 6.5 to 38.6%. This means that if a new study is conducted, the assessed prevalence is likely, with a probability of 95%, to fall between 6.5 and 38.6%. However, the pooled prevalence was robust according to leave-one-out sensitivity analysis. The pooled prevalence varied from 16.6% (14.0 to 19.5%), when the study by Yano et al. [36] was omitted, to 17.9% (15.1 to 21.1%), when the study by Min et al. [16] was omitted (Fig. S1). The heterogeneity, however, remained stable, as the I2 statistic for heterogeneity varied from 85%, when the study by Sato et al. [18] was omitted, to 89.1%, when one of the following studies was omitted [6, 10, 13, 15, 21, 23, 25, 26, 32, 34, 35].
Fig. 2.
Meta-analysis of the prevalence of metastatic lateral lymph nodes
To determine whether neoadjuvant treatment could alter the prevalence of metastatic lateral lymph nodes, we performed subgroup analyses by separately pooling studies reporting patients who received neoadjuvant treatment from others (Fig. S2). The difference in pooled prevalence of metastatic lateral lymph nodes between studies with versus without neoadjuvant treatment was 16.9% (95% CI: 14.1–20.2), but the inter-study variability (heterogeneity) was high important, with I2 = 88%.
Then, we explored whether the heterogeneity could be explained by changes in the therapeutic strategies across the years. The pooled prevalence of metastatic lateral lymph nodes between studies published before and after 2010 was 17.3% (95% CI: 14.6–20.4), but the inter-study variability (heterogeneity) was noteworthy, with I2 = 89% (Fig. S3).
Furthermore, we excluded patients with TNM stage IV (in six studies). After exclusion of these patients, the pooled prevalence of metastatic lateral lymph nodes was 15.8% (95% CI 13.1 to 18.9) (Fig. S4).
Then, we separately pooled studies according to the type of LLND performed (systematic versus curative). The difference in the pooled prevalence of metastatic lateral lymph nodes between studies with systematic or curative dissection was not statistically significant (p = 0.7111) (Fig. S5).
However, the pooled prevalence of metastatic lateral lymph nodes appeared to be associated with the sample size of studies (p = 0.005): the prevalence decreased when the sample size increased (Fig. S6). Meta-regression showed that, on average, a study with a sample size that was 10 times larger than that of some other study had an estimated odds ratio for prevalence that was reduced by approximately 50% (Fig. S7).
Discussion
We performed a systematic review and meta-analysis estimating the prevalence of metastatic lateral lymph nodes in patients with rectal cancer. By pooling 31 studies of Asian origin (30 from Japan and one from China) totalling 7599 patients, we found a prevalence of metastatic lateral lymph nodes of 17.3%, thereby underlining the importance of further examination of the treatment of these node areas to avoid cancer recurrence.
Six studies included patients with TNM stage IV rectal cancer. According to the most recent Japanese guidelines, metastatic disease is not the most important stage to indicate LLND [4]. However, within this stage, some patients may present both primary tumour and resectable distant metastases, thus proceeding to the possibility of undergoing LLND. As a result, to understand the prevalence of metastatic lymph nodes within the most common indications, we excluded patients with TNM stage IV disease by considering them to have metastatic lateral lymph nodes. After exclusion, the pooled prevalence of metastatic lateral lymph nodes remained stable (15.8%).
In the present systematic review and meta-analysis, we were not able to show any improvement in neoadjuvant treatment in terms of lateral lymph node metastasis. However, it should be noted that the power of this subgroup analysis might be insufficient. Additionally, Asian protocols differed from Western protocols, and only a small proportion of patients among studies with neoadjuvant treatment benefited from that modality. Therefore, it is not possible to draw any conclusions about the efficacy of neoadjuvant treatment in terms of the prevalence of metastatic lateral lymph nodes based on our results. Despite this, it is worth noting that neoadjuvant treatments between the studies were quite similar using 5-fluorouracil, oxaliplatin and irinotecan (Table S3).
Some centres performed systematic LLND, whereas others performed LLND in patients with enlarged lateral lymph nodes diagnosed by pre-operative imaging. We pooled these studies separately and found no difference in terms of metastatic lateral lymph nodes. After 2010, as Japanese guidelines changed and recommended limiting LLND for all cT3-T4 tumours with a lower edge below the peritoneal reflection, we reported a subgroup analysis of the pooled prevalence of metastatic LLN according to the publication date. Due to the high heterogeneity, we cannot interpret this result as significant.
Furthermore, we note that meta-regression showed that the pooled prevalence of metastatic lymph nodes was associated with the sample size of studies, as the prevalence of metastatic lymph nodes decreased when the sample size increased. We think that this might be explained by more selective indications for LLND in studies including fewer patients with LLND. Thus, even if the number of studies between curative and systematic LLND intention was not the same, the tendency of the sample size of studies with curative LLND was lower. This indirectly shows that smaller studies were more likely to have a selected population. As a consequence, studies with larger sample sizes (in other words, studies with prophylactic LLND) were more likely to represent the “real” proportion of patients with rectal cancer with LLN metastasis.
Our meta-analysis is the first to examine the prevalence of metastatic lateral lymph nodes, which seems to be an essential prerequisite before considering any treatment. Indeed, recent literature [40–42] directly questions the oncological and functional results of LLND without even first investigating whether it is necessary.
Advantages of the study
The strengths of our systematic review and meta-analysis are as follows: 1) the inclusion of a large number of studies reporting the prevalence of metastatic lateral lymph nodes based on histopathological analysis and to clarify, for the first time to our knowledge, its subsequent effects in patients operated on for rectal cancer; and 2) the highlighting of the necessity of systematic treatment of lateral lymph nodes due to the high prevalence (17.3%) of LLN metastasis. Indeed, despite the heterogeneity of the studies, which could have impacted the aim of our work, we were able to make some sound calculations due to a specific statistical analysis methodology.
Limitations of the study
The limitations of the present study are as follows: 1) the high heterogeneity of the calculated pooled prevalence of metastatic lateral lymph nodes, resulting from the quality and heterogeneity of the included publications; and 2) the final analysis included 31 papers, most of which were from Japan and were retrospective studies. Therefore, some patients might have overlapped because these papers were published from some limited leading hospitals. Moreover, it is worth noting that by focussing on patients who have benefited from LLND, a surgical procedure that is most common in Asian countries, selection has been carried out. As a result, it is expected that the selected studies tend to oversample patients with indications for LLND, with these patients being more likely to have advanced disease. Thus, a bias could have been introduced. The percentage of 17.3% of metastatic LLNs could have been overestimated. This element can explain the counter-intuitive (but non-significant) results of a higher prevalence of metastasis in the prophylactic LLND group (17.3%) versus the curative group (15.9%).
Conclusion
In conclusion, this systematic review and meta-analysis of Asian studies indicates that the prevalence of metastatic lateral lymph nodes in patients with low rectal cancer who underwent surgery is 17.3%. This prevalence is of clinical importance, as it may result in cancer recurrence and calls for the systematic treatment of these lymph node areas. Future randomized controlled trials should determine which therapeutic strategy, whether neoadjuvant radiochemotherapy versus systematic or imaging-guided lateral lymph node dissection, offers the best improvement in overall and recurrence-free survival.
Supplementary Information
Abbreviations
- LLND
Lateral lymph node dissection
- RCT
Randomized controlled trial
Author contributions
JM conceived and designed the study. JM and NC acquired the data. CC analysed the data. NC, JM, CC, AB, NCB and FR interpreted the data. NC, JM, CC, AB, JRY, NCB and FR contributed to the writing of the manuscript and to its critical revision. NC, JM, CC, AB, JRY, NCB and FR approved the final version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Funahashi K, Koike J, Shimada M, Okamoto K, Goto T, Teramoto T. A preliminary study of the draining lymph node basin in advanced lower rectal cancer using a radioactive tracer. Dis Colon Rectum. 2006;49(10 Suppl):S53–58. doi: 10.1007/s10350-006-0659-2. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Benhamiche AM, Meny B, Cheynel N, Rat P, Faivre J. Population-based study of factors influencing occurrence and prognosis of local recurrence after surgery for rectal cancer. Br J Surg. 2001;88(9):1221–1227. doi: 10.1046/j.0007-1323.2001.01861.x. [DOI] [PubMed] [Google Scholar]
- 3.van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon and rectum. Eur J Cancer. 2014;50(1):1–e1. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita S, Akasu T, Mizusawa J, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13(6):616–621. doi: 10.1016/S1470-2045(12)70158-4. [DOI] [PubMed] [Google Scholar]
- 6.Masaki T, Matsuoka H, Kobayashi T, et al. Quality assurance of pelvic autonomic nerve-preserving surgery for advanced lower rectal cancer–preliminary results of a randomized controlled trial. Langenbecks Arch Surg. 2010;395(6):607–613. doi: 10.1007/s00423-010-0655-9. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Viechtbauer W, Lopez-Lopez JA, Sanchez-Meca J, Marin-Martinez F. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol Methods. 2015;20(3):360–374. doi: 10.1037/met0000023. [DOI] [PubMed] [Google Scholar]
- 9.Hara M, Hirai T, Nakanishi H, et al. Isolated tumor cell in lateral lymph node has no influences on the prognosis of rectal cancer patients. Int J Colorectal Dis. 2007;22(8):911–917. doi: 10.1007/s00384-007-0280-4. [DOI] [PubMed] [Google Scholar]
- 10.Ishibe A, Ota M, Watanabe J, et al. Prediction of lateral pelvic lymph-node metastasis in low rectal cancer by magnetic resonance imaging. World J Surg. 2016;40(4):995–1001. doi: 10.1007/s00268-015-3299-7. [DOI] [PubMed] [Google Scholar]
- 11.Kanemitsu Y, Komori K, Shida D, et al. Potential impact of lateral lymph node dissection (LLND) for low rectal cancer on prognoses and local control: a comparison of 2 high-volume centers in Japan that employ different policies concerning LLND. Surgery. 2017;162(2):303–314. doi: 10.1016/j.surg.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Ishida H, Hatano S, Ishiguro T, Kumamoto K, Ishibashi K, Haga N. Prediction of lateral lymph node metastasis in lower rectal cancer: analysis of paraffin-embedded sections. Jpn J Clin Oncol. 2012;42(6):485–490. doi: 10.1093/jjco/hys041. [DOI] [PubMed] [Google Scholar]
- 13.Kagawa H, Kinugasa Y, Shiomi A, et al. Robotic-assisted lateral lymph node dissection for lower rectal cancer: short-term outcomes in 50 consecutive patients. Surg Endosc. 2015;29(4):995–1000. doi: 10.1007/s00464-014-3760-y. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka H, Nakamura A, Masaki T, et al. Optimal diagnostic criteria for lateral pelvic lymph node metastasis in rectal carcinoma. Anticancer Res. 2007;27(5B):3529–3533. [PubMed] [Google Scholar]
- 15.Miyake Y, Mizushima T, Hata T, et al. Inspection of perirectal lymph nodes by one-step nucleic acid amplification predicts lateral lymph node metastasis in advanced rectal cancer. Ann Surg Oncol. 2017;24(13):3850–3856. doi: 10.1245/s10434-017-6069-y. [DOI] [PubMed] [Google Scholar]
- 16.Min BS, Kim JS, Kim NK, et al. Extended lymph node dissection for rectal cancer with radiologically diagnosed extramesenteric lymph node metastasis. Ann Surg Oncol. 2009;16(12):3271–3278. doi: 10.1245/s10434-009-0692-1. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Ozawa H, Hatate K, et al. A Phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys. 2011;79(3):677–683. doi: 10.1016/j.ijrobp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Sato H, Maeda K, Maruta M. Prognostic significance of lateral lymph node dissection in node positive low rectal carcinoma. Int J Colorectal Dis. 2011;26(7):881–889. doi: 10.1007/s00384-011-1170-3. [DOI] [PubMed] [Google Scholar]
- 19.Shimoyama M, Yamazaki T, Suda T, Hatakeyama K. Prognostic significance of lateral lymph node micrometastases in lower rectal cancer: an immunohistochemical study with CAM5.2. Dis Colon Rectum. 2003;46(3):333–339. doi: 10.1007/s10350-004-6552-y. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama S, Takifuji K, Hotta T, et al. Survival benefit of lateral lymph node dissection according to the region of involvement and the number of lateral lymph nodes involved. Surg Today. 2014;44(6):1097–1103. doi: 10.1007/s00595-013-0815-y. [DOI] [PubMed] [Google Scholar]
- 21.Komori K, Kanemitsu Y, Kimura K, et al. Detailed stratification of TNM stage III rectal cancer based on the presence/absence of extracapsular invasion of the metastatic lymph nodes. Dis Colon Rectum. 2013;56(6):726–732. doi: 10.1097/DCR.0b013e318286c518. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T, Takayama M, Matsuoka H, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg. 2008;393(2):173–180. doi: 10.1007/s00423-007-0260-8. [DOI] [PubMed] [Google Scholar]
- 23.Wu ZY, Wan J, Li JH, et al. Prognostic value of lateral lymph node metastasis for advanced low rectal cancer. World J Gastroenterol. 2007;13(45):6048–6052. doi: 10.3748/wjg.v13.45.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hida J, Yasutomi M, Fujimoto K, Maruyama T, Okuno K, Shindo K. Does lateral lymph node dissection improve survival in rectal carcinoma? examination of node metastases by the clearing method. J Am Coll Surg. 1997;184(5):475–480. [PubMed] [Google Scholar]
- 25.Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. 2005;92(6):756–763. doi: 10.1002/bjs.4975. [DOI] [PubMed] [Google Scholar]
- 26.Ueno H, Mochizuki H, Hashiguchi Y, et al. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. 2007;245(1):80–87. doi: 10.1097/01.sla.0000225359.72553.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steup WH, Hojo K, Moriya Y, et al. An analysis on the effect of blood transfusion on recurrence and survival in patients undergoing extended lymphadenectomy for colorectal cancer. Hepatogastroenterology. 1994;41(3):253–259. [PubMed] [Google Scholar]
- 28.Mori T, Takahashi K, Yasuno M. Radical resection with autonomic nerve preservation and lymph node dissection techniques in lower rectal cancer surgery and its results: the impact of lateral lymph node dissection. Langenbecks Arch Surg. 1998;383(6):409–415. doi: 10.1007/s004230050153. [DOI] [PubMed] [Google Scholar]
- 29.Tan KY, Yamamoto S, Fujita S, Akasu T, Moriya Y. Improving prediction of lateral node spread in low rectal cancers–multivariate analysis of clinicopathological factors in 1046 cases. Langenbecks Arch Surg. 2010;395(5):545–549. doi: 10.1007/s00423-010-0642-1. [DOI] [PubMed] [Google Scholar]
- 30.Nagasaki T, Akiyoshi T, Fujimoto Y, et al. Preoperative chemoradiotherapy might improve the prognosis of patients with locally advanced low rectal cancer and lateral pelvic lymph node metastases. World J Surg. 2017;41(3):876–883. doi: 10.1007/s00268-016-3748-y. [DOI] [PubMed] [Google Scholar]
- 31.Yamaoka Y, Kinugasa Y, Shiomi A, et al. Preoperative chemoradiotherapy changes the size criterion for predicting lateral lymph node metastasis in lower rectal cancer. Int J Colorectal Dis. 2017;32(11):1631–1637. doi: 10.1007/s00384-017-2873-x. [DOI] [PubMed] [Google Scholar]
- 32.Numata M, Yamaguchi T, Kinugasa Y, et al. Index of estimated benefit from lateral lymph node dissection for middle and lower rectal cancer. Anticancer Res. 2017;37(5):2549–2555. doi: 10.21873/anticanres.11598. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H, Yamakawa Y. Robotic-assisted vs. conventional laparoscopic surgery for rectal cancer: short-term outcomes at a single center. Surg Today. 2016;46(8):957–962. doi: 10.1007/s00595-015-1266-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu YY, Wang C, Xu D, Shen XG, Ding SQ, Zhou ZG. Mesorectal and lateral node metastasis and micrometastasis in lower rectal cancer. Hepatogastroenterology. 2011;58(107–108):745–748. [PubMed] [Google Scholar]
- 35.Kobayashi H, Mochizuki H, Kato T, et al. Outcomes of surgery alone for lower rectal cancer with and without pelvic sidewall dissection. Dis Colon Rectum. 2009;52(4):567–576. doi: 10.1007/DCR.0b013e3181a1d994. [DOI] [PubMed] [Google Scholar]
- 36.Yano H, Saito Y, Takeshita E, Miyake O, Ishizuka N. Prediction of lateral pelvic node involvement in low rectal cancer by conventional computed tomography. Br J Surg. 2007;94(8):1014–1019. doi: 10.1002/bjs.5665. [DOI] [PubMed] [Google Scholar]
- 37.Kinugasa T, Akagi Y, Ochi T, et al. Lateral lymph-node dissection for rectal cancer: meta-analysis of all 944 cases undergoing surgery during 1975–2004. Anticancer Res. 2013;33(7):2921–2927. [PubMed] [Google Scholar]
- 38.Yamaguchi T, Kinugasa Y, Shiomi A, Tomioka H, Kagawa H. Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer. Surg Endosc. 2016;30(2):721–728. doi: 10.1007/s00464-015-4266-y. [DOI] [PubMed] [Google Scholar]
- 39.Steup WH, Moriya Y, van de Velde CJ. Patterns of lymphatic spread in rectal cancer. a topographical analysis on lymph node metastases. Eur J Cancer. 2002;38(7):911–918. doi: 10.1016/S0959-8049(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 40.Du R, Zhou J, Li D, Zhang Q, Liu J, Ma C, et al. (2020) Postoperative morbidity and mortality after mesorectal excision with laparoscopic versus conventional open lateral lymph node dissection for advanced rectal cancer: a meta-analysis. Asian J Surg [Internet]. 11 août 2020; Disponible sur: http://www.sciencedirect.com/science/article/pii/S1015958420301950 [DOI] [PubMed]
- 41.Yang X, Yang S, Hu T, Gu C, Wei M, Deng X, et al. What is the role of lateral lymph node dissection in rectal cancer patients with clinically suspected lateral lymph node metastasis after preoperative chemoradiotherapy? a meta-analysis and systematic review. Cancer Med. 2020;9(13):4477–4489. doi: 10.1002/cam4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao X, Wang C, Yu Y, Yang L, Zhou Z (2020) Lateral lymph node dissection reduces local recurrence of locally advanced lower rectal cancer in the absence of preoperative neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Disponible sur: https://europepmc.org/article/ppr/ppr216100 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.