Abstract
Estrogen has always been considered the female hormone and testosterone the male hormone. However, estrogen’s presence in the testis and deleterious effects of estrogen treatment during development have been known for nearly 90 years, long before estrogen receptors (ESRs) were discovered. Eventually it was learned that testes actually synthesize high levels of estradiol (E2) and sequester high concentrations in the reproductive tract lumen, which seems contradictory to the overwhelming number of studies showing reproductive pathology following exogenous estrogen exposures. For too long, the developmental pathology of estrogen has dominated our thinking, even resulting in the “estrogen hypothesis” as related to the testicular dysgenesis syndrome. However, these early studies and the development of an Esr1 knockout mouse led to a deluge of research into estrogen’s potential role in and disruption of development and function of the male reproductive system. What is new is that estrogen action in the male cannot be divorced from that of androgen. This paper presents what is known about components of the estrogen pathway, including its synthesis and target receptors, and the need to achieve a balance between androgen- and estrogen-action in male reproductive tract differentiation and adult functions. The review focuses on what is known regarding development of the male reproductive tract, from the rete testis to the vas deferens, and examines the expression of estrogen receptors and presence of aromatase in the male reproductive system, traces the evidence provided by estrogen-associated knockout and transgenic animal models and discusses the effects of fetal and postnatal exposures to estrogens. Hopefully, there will be enough here to stimulate discussions and new investigations of the androgen:estrogen balance that seems to be essential for development of the male reproductive tract.
Keywords: Estrogen, estrogen receptor, development, differentiation, fetal, neonatal, male reproduction, testis, rete testis, efferent ductule, epididymis, vas deferens, mesonephros, mesonephric tubule, Wolffian duct, rete cord, environmental estrogens, testicular dysgenesis syndrome
1. Introduction
We are currently at something of a crossroads in terms of our understanding of the importance of estrogens in the development and subsequent function of the male reproductive tract. To appreciate how we have reached this point, it is helpful to briefly review the history of the area. For many years, the scientific community assumed that estrogen was the female sex steroid, while testosterone (T) was the male sex steroid and, in females, was only used as an intermediate in the synthesis of estradiol (Eik-Nes, 1964). However, it has been known since 1921 that the testis produces a substance capable of increasing uterine weight and thus having a feminizing action (Fellner, 1921). Indeed, the first isolation of estradiol (E2) in the male was from the horse testis (Beall, 1940). Subsequently, numerous studies found high concentrations of estrogens, particularly estrone-sulfate, in the testis, rete testis fluid, semen and blood of the horse and other large domestic species (Amann and Ganjam, 1976; Claus et al., 1992; Claus and Hoffmann, 1980; Claus et al., 1985; Eiler and Graves, 1977; Setchell and Cox, 1982; Setchell et al., 1983). Even in the rodent, the concentration of E2 in rete testis fluid was reported to reach 510 pg/ml (Free and Jaffe, 1979), which is 5-fold higher than serum E2 in the female rat during proestrus (Döhler and Wuttke, 1975; Overpeck et al., 1978).
Long before the isolation and cloning of estrogen receptors (ESR), the actual presence of a ‘female’ hormone in the male raised curiosity about its source and potential function, but most research was focused on the effects of estrogen administration in males during development. Before the 1990s, most studies of estrogens in the male were devoted to describing reproductive pathologies induced by neonatal exposures to various types of estrogens, as well as to the synthetic, non-steroidal, but potent estrogen, diethylstilbestrol (DES) (Arai et al., 1978; Bern, 1951; Brown-Grant et al., 1975; Bullock et al., 1988; Dhar and Setty, 1976; Dunn and Green, 1963; Gill et al., 1977; Hendricks and Gerall, 1970; McLachlan et al., 1975; Newbold et al., 1985, 1987; Ohta and Takasugi, 1974; Takasugi, 1970; Yasuda et al., 1985; Zadina et al., 1979). The seeming contradiction between the many reports of physiologically high E2 production by the testis/reproductive tract on the one hand and, on the other hand, the overwhelming evidence of male reproductive pathology following exogenous estrogen exposures during development, might have been expected to cause researchers to pause and reflect. Instead, thinking was effectively steamrollered when the so-called ‘Oestrogen hypothesis’ was published in 1993 (Sharpe and Skakkebaek, 1993). This paper, which hypothesized that inappropriate estrogen exposure during perinatal development might underlie common human male reproductive pathologies (e.g., low/falling sperm count, undescended testis and hypospadias), established a new paradigm in developmental biology. The coincidence of this hypothesis with emerging studies showing that a variety of common environmental contaminant/pollutant chemicals possessed intrinsic (though weak) estrogenicity, was pivotal in kick-starting the era of endocrine-disrupting chemicals, which is still with us.
Whilst these developments served to hugely increase interest in estrogen’s adverse effects, they rather sidelined thinking about the physiological importance of estrogens in males. This changed with the discovery that knockout of the one known estrogen receptor (ESR1) at the time, resulted in dramatic changes in the male reproductive tract and infertility (Eddy et al., 1996; Hess et al., 1997a; Lubahn et al., 1993). Such developments led to an explosion in research that has provided an array of discoveries, including the following: a) an understanding of where and when E2 is produced in the male, and better antibodies for the localization of ESR (see previous reviews: Cooke et al., 2017; Hess and Cooke, 2018); b) discovery of a second receptor-ESR2 (Kuiper et al., 1996); c) the generation of several key knockout mice, which demonstrated an absolute requirement for the expression of ESR1 in male reproduction; and d) an abundance of environmental studies showing detrimental effects of synthetic chemicals on male reproductive tract development (Basak et al., 2020; Belcher et al., 2019; Conley et al., 2018; Lymperi and Giwercman, 2018).
Despite these discoveries, it is fair to say that the predominant research view at the start of this millennium was that developmental exposure of the male to estrogens in any form is likely to have negative consequences, largely ignoring the evidence to the contrary from Esr1 knockout (KO) mice. It is emphasized that this (imbalanced) perspective stemmed from research that had operated at the ‘extremes’, on the one hand exposing developing rodents to pharmacologically high doses of exogenous estrogens that induced adverse effects, while on the other reducing endogenous estrogen action (via Esr1KO) to zero, but also inducing adverse effects. The physiological importance of estrogens in the male is somehow lost in the middle of this ‘research at the extremes’. A further complication that then emerged was the discovery that the adverse developmental effects of high-dose DES on the male reproductive tract resulted from functional wipe-out of androgen action due to a double whammy effect of grossly reducing testicular production of T, at the same time it was wiping out expression of the androgen receptor (AR) at the protein level. This was proven by co-administration of T with the high dose DES, which essentially prevented all the adverse effects of DES (McKinnell et al 2001; Rivas et al 2003). These same authors went on to show that the adverse effects of DES resulted from disruption of the androgen-estrogen balance (Rivas et al 2002), which is discussed in more detail later.
What these new studies did was to highlight that estrogen action in males cannot be divorced from androgen action. Indeed, substantial research has gone on to identify that the pivotal event for normal reproductive development of the male is to be exposed to a sufficient level of androgens during a specific fetal period, the ‘masculinization programming window (MPW)’ (Sharpe, 2020). There is no current evidence that estrogens are involved in this process, but as this paper will show, all of the components for estrogen production and action are present in the developing male reproductive tract at this stage of development, so a physiological role for estradiol, or for the balance between androgen- and estrogen-action or their respective receptors is likely.
So, now we have come full circle and must ask again, “how important are estrogens in the development of the male reproductive tract and what are its specific physiological roles and contribution to male fertility?” As this review will show, we still have many more unanswered than answered questions in this regard. The review focuses on what is known regarding development of the male reproductive tract, from the rete testis to the vas deferens, examines the expression of estrogen receptors and the production of estrogen in the male reproductive system, traces the evidence provided by estrogen-associated knockout and transgenic animal models and discusses the effects of fetal and postnatal exposures to estrogens. Finally, we hope to stimulate the next generation of scientists by discussing where and when estrogen and/or an estrogen/androgen balance might play a role in the development of the male reproductive tract.
2. Morphogenesis of the embryonic mesonephric tubules/efferent ducts and mesonephric/Wolffian duct and their connections
Overview
Morphogenesis of the rete testis cord, its connection to mesonephric tubules, which will become the efferent ducts, and development of the Wolffian duct (mesonephric/nephric duct), which will become the epididymis and vas deferens, is complex and involves early expression of steroid receptors and synthesis of T and E2 (Fig. 1). The process involves a series of events that result in the efferent ducts having a unique branching pattern, which is quite different to the morphogenesis of classic branching tissues such as the prostate and mammary gland. On the other hand, the morphogenesis of the Wolffian duct is via non-branching morphogenesis, and the mechanisms by which this occurs may resemble that of the brain and intestine. It is unclear as to whether tissues that form biological tubules and then undergo their morphogenic events to form adult structures utilize unique mechanisms or there are overlapping mechanisms between the tissues (see further by: Lubarsky and Krasnow, 2003). It is possible both are used in that certain overall events are common, for example, cell proliferation and cell rearrangements but these events are differentially regulated. Although beyond the scope of this review for further discussion, it is also unclear as to the mechanisms that dictate the size and shape of these tissues. How does the Wolffian duct form the “dumbbell/hour glass-shape” seen in the adult epididymis of several species, and what limits its size?
Fig 1.
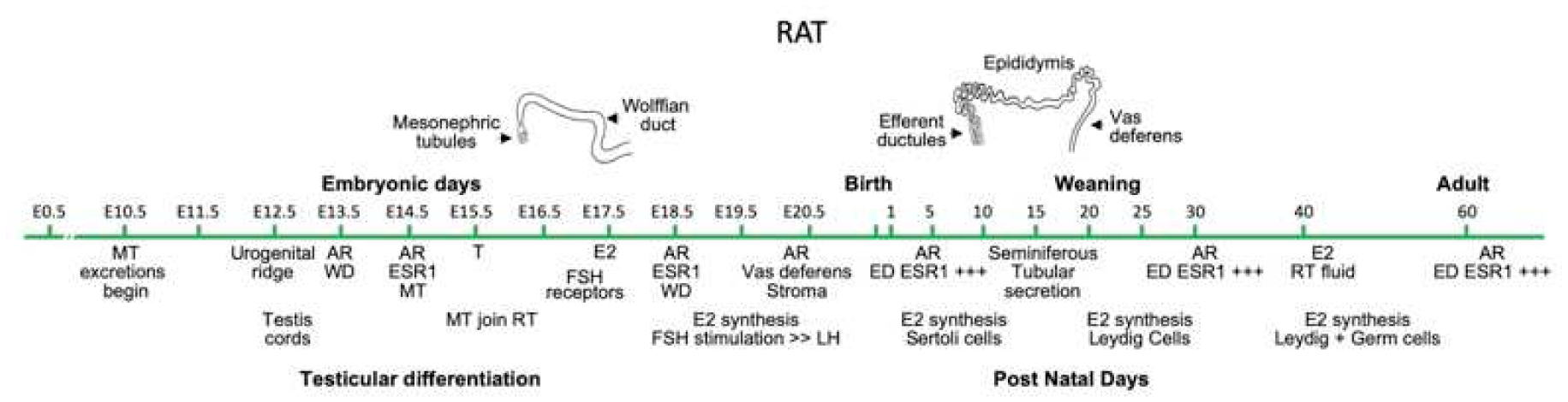
Time course major events in development and differentiation of the rat male reproductive system. Although efferent ductules were emphysized for ESR1 expression, epididymal epithelium has positive expression in select cell types (see section 3). MT, mesonephric tubules; AR, androgen receptor; ESR, estrogen receptor; WD, Wolffian duct; T, testosterone; E2, estrogen; FSH, Follicle Stimulating Hormone; LH, Luteinizing Hormone; ED, efferent ductules; RT, rete testis. Adapted from Hinton & Avellar (2018).
Following the development of the three germ layers, endoderm, mesoderm and ectoderm, the mesoderm (Greek: meso-middle, derma-skin) divides into three regions, paraxial, intermediate and lateral plate, of which the intermediate is the most important for this discussion. A series of events from anterior to posterior in sequence will form the pronephros, mesonephros and the metanephros, and it is the mesonephros that will go on to form the mesonephric tubules and duct.
However, the development of the mesonephric tubules, for example, is not “intermediate mesoderm/mesonephros autonomous” in that mid-line structures such as notochord and floor plate interact with the paraxial mesoderm via signaling pathways, which in turn again, via signaling pathways (e.g. Sonic hedgehog; Shh), regulate tubulogenesis and numbers of mesonephric tubules (Murashima et al., 2014).
Development of the mesonephric duct and mesonephric tubules
Within the most anterior region of the mesonephros a group of cells undergo specification through an unknown mechanism, take on a migratory phenotype and migrate towards the cloaca eventually forming the mesonephric/Wolffian duct. The migratory cells are under the control of a Fibroblast Growth Factor (FGF) gradient, at least in the chick (Atsuta and Takahashi, 2015; Attia et al., 2015) with the highest concentration of FGF being found at the cloacal end. As the animal grows, the cells within the proximal region find themselves surrounded by a lower concentration of FGF, which triggers signal transduction pathways leading to mesenchymal-epithelial transition, epithelial cell polarization and the eventual formation of a tube/duct. This continues until the entire group of migratory cells have undergone these processes, thereby forming a definitive duct. It is tempting to speculate that a similar mechanism is found during the formation of the human mesonephric duct. In the mouse, fusion of the Wolffian duct with the cloaca has been shown to be via a mutual apoptotic event (Hoshi et al., 2018). As the migratory Wolffian duct cells approach the cloaca, some of those cells together with a subset of cells within the cloaca undergo apoptosis, which allows the two structures to unite (See for a review on the mechanisms by which biological tubes fuse/join: Kao, 2013).
During migration of the Wolffian duct towards the cloaca, it produces Wnt9b (Carroll et al., 2005), which acts upon a group of cells within the surrounding mesenchyme to undergo mesenchymal-epithelial transition to form simple vesicles and then, S-shaped vesicles/bodies. Loss of Wnt9b results in loss of induction of the mesenchyme to form mesonephric tubules (Carroll et al., 2005). These vesicles are very similar to the vesicles that form renal nephrons. Renal vesicles that form within the metanephric mesenchyme undergo extensive patterning to generate S-shaped bodies having four regions: a region that connects to the Wolffian duct, distal, medial and proximal regions with each having overlapping and unique gene expression patterns along the length of the body (Georgas et al., 2009; Rumballe et al., 2011). Such detailed studies have not been performed examining the patterning of the S-shaped bodies that will eventually form the efferent ducts, but some specific markers have been identified in the proximal and distal regions of the S-shaped body (Sainio, 2003; Sainio et al., 1997). Studies have also shown some similar and unique overall gene expression profiles between the S-shaped bodies that will form metanephric renal nephrons and the efferent ducts. As mentioned above, Shh plays an indirect role in regulating the number of mesonephric tubules within the intermediate mesoderm. In addition to Wnt9b and Shh, Six1, Wt-1, Foxc1, Foxc2 and Robo2 are also important in regulating the morphogenesis of the mesonephric tubules (for extensive review see: Murashima et al., 2015). Most of the studies focusing on the development of human mesonephric tubules have been from a structural perspective. For example, human S-shaped bodies have been examined by late 19th century to early 20th century embryologists (Lewis, 1920; Meyer, 1890) and more recently by Ludwig & Landmann (2005). Lewis (1920) showed three-dimensional models of human mesonephric tubule development, with S-shaped bodies, being referred to as “double spirals,” connected to a glomerulus at one end and to the Wolffian duct at the other end. In addition to the models, Lewis (1920) suggested that the most proximal part of the tubule was secretory in function, whereas the most distal (closest to the testis) was a collecting part. It was shown that the secretory part of the tubule underwent regression, the collecting part then underwent elongation eventually joining the rete testis (Jacob et al., 2012). Some genes have been identified that are expressed in human mesonephric tubules and include Jagged1 (Crosnier et al., 2000), anosmin1 (Hardelin et al., 1999), EGF, TGF-alpha and receptors (Bernardini et al., 1996), although their role(s) are unknown. Interestingly, Lawrence and colleagues (Lawrence et al., 2018) have shown influx and efflux of organic anions and cations across mouse mesonephric tubule epithelium. This movement of anions and cations could reflect an excretory function, although further studies are needed to examine this possibility.
Mesonephric tubules destined to become the efferent ducts
As development proceeds, the caudal (posterior) mesonephric tubules undergo regression leaving 4–6 tubules in close apposition to the testis, which will form the efferent ducts. The manner by which those tubules survive is not entirely clear but some studies have suggested that the cells produce anti-apoptotic proteins whereas caudal tubule cells that undergo regression do not, but produce pro-apoptotic proteins (Sainio et al., 1997). Another study has shown that the caudal mesonephric tubules undergo regression via activation of cell senescence pathways, but the cranial tubules do not express the components of that pathway and are so, “protected” from regression (Muñoz-EspÌn et al., 2013). Presumably, the mechanisms by which the mesonephric tubules survive are dependent upon some factor(s) secreted by the testis, which in turn initiate survival pathways. Interestingly, Wt1 is important for the survival of the caudal tubules but not the cranial tubules, thereby showing differential regulation between the two sets of tubules (Kreidberg et al., 1993; Sainio et al., 1997).
In the mouse, the induced mesonephric tubules join the Wolffian duct between E9.5 and E11.5 (Vetter and Gibley, 1966), and then join the rete testis by E15.5 (Karl and Capel, 1995; Omotehara et al., 2020). Mesonephric tubules form lumens early in development, with small lumens at E11.5 in mice (Omotehara et al., 2020) and in humans at 9 weeks (Cunha et al., 2020). The mechanism(s) by which the tubules join with the rete testis and Wolffian duct are unclear and could certainly be similar to the manner by which the Wolffian duct joins the cloaca. Clues as to the mechanism(s) by which the mesonephric tubules join the rete testis have come from recent studies of several laboratories (Combes et al., 2009; Kulibin and Malolina, 2020; Omotehara et al., 2020). Combes et al. generated three-dimensional models of the testis and mesonephric tubules from E15.5 mouse embryo and showed the connection between the two tissues (Fig. 2). A plexus containing perforations was identified at the connecting points. Later, Omotehara et al (2020) showed very nicely the connection between mouse adrenal-4 binding protein/steroidogenic factor-1 positive gonadal somatic cells within the rete cords and the cells within the mesonephric tubules. Once contact was made between the two cell types, a common basement membrane containing collagen type IV surrounded the mesonephric tubules and the rete.
Fig 2.
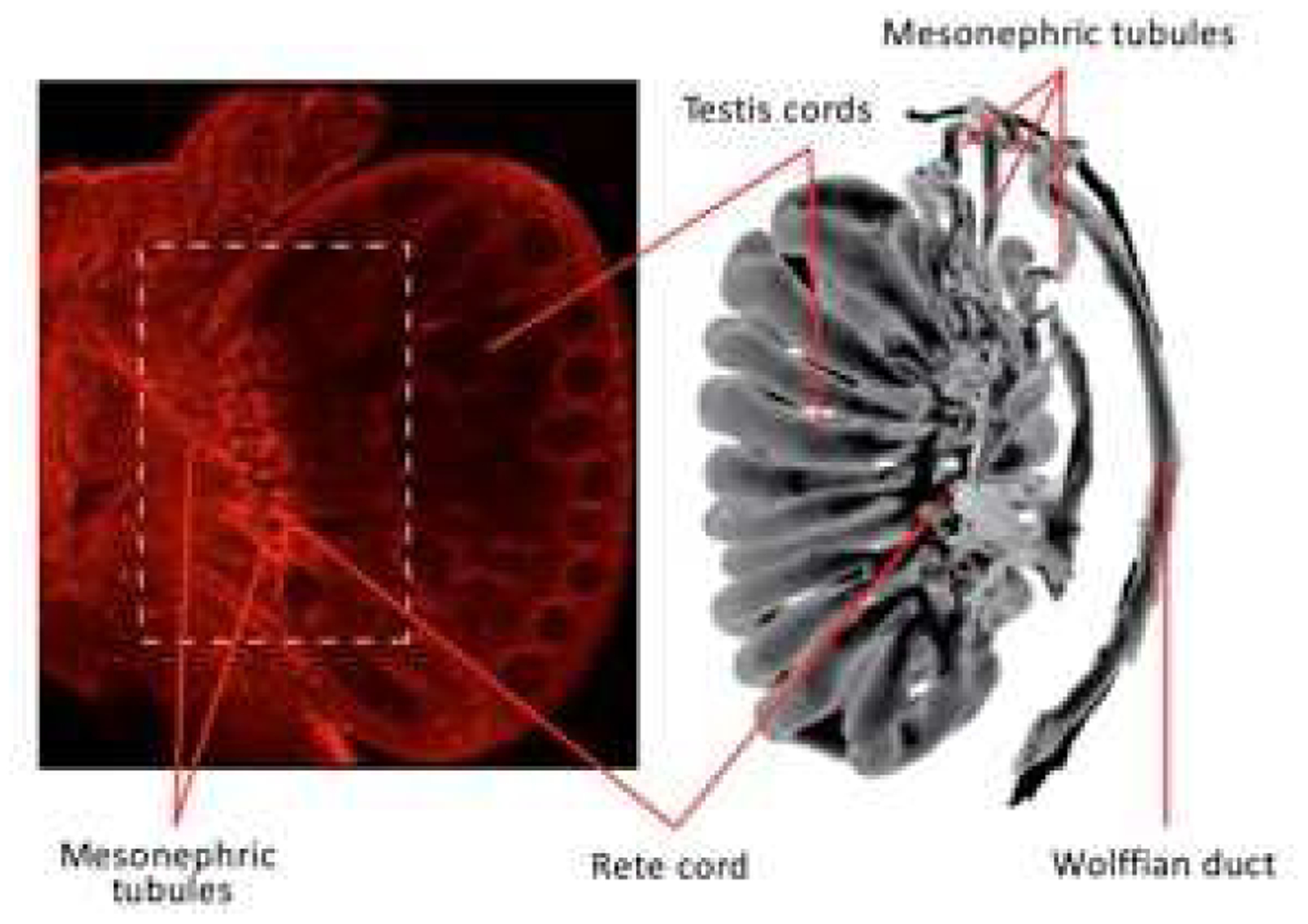
Connections in the E15.5 mouse embryo showing testis, rete testis cord, mesonephric tubules and Wolffian duct. On the right is a three-dimensional model. Photos were modified from Combes et al. (2009) and De Mello Santos et al. (2019) and reproduced by permission of Wiley Press.
The following question arose while writing this review: when do the mesonephric tubules become efferent ductules? It could be assumed that they remain mesonephric tubules while in utero, but that would suggest some magical differentiation taking place at birth, when in fact even the ciliated efferent ductal cells do not differentiate until PND5 in the mouse (Benoit, 1926). However, the general function of efferent ductules is to connect rete testis to the epididymis and as such this function occurs during fetal development and in mice at E15.5 (Karl and Capel, 1995; Omotehara et al., 2020). Therefore, we propose that this event be used for initial labeling of the tubules as efferent ductules.
From E15.5 onwards, the mesonephric tubules/efferent ductules elongate and coil, and unite/fuse/join with each other forming their characteristic branching pattern (can be reviewed in: Guttroff et al., 1992; Ilio and Hess, 1994). As mentioned in the Introduction, the morphogenesis of the branching pattern appears to be unusual in that it is not similar to that observed in tissues that undergo classical branching morphogenesis. We hypothesize that as the mesonephric tubules elongate and coil, they fuse with each other in a relatively non-stochastic pattern, that is, there is always a single communicating duct with the epididymis and at least 4–6 tubules (for rodents, for example) attached to the rete. However, it is partly stochastic in that the branching pattern can vary between males of the same species and also, between species. An excellent illustration of this point is from the work of Nakata & Iseki (2019), that showed in two mice of the same strain having the characteristic 4 tubules attached to the rete, which in turn attached to form pairs, which in turn attached to form a single pair, which in turn attached to form a single communicating duct/tubule. Blind ended tubules were not observed in these two samples. However, in another mouse, the pattern was similar but there were numerous blind-ending tubules. Blind-ending tubules were found in 60% and 40% of male rats and mice, respectively (Guttroff et al., 1992; Hess et al., 2000). In rodents, these small tubules exhibited abnormal epithelial morphology and had very small lumens that did not accumulate sperm, in contrast to larger mammals (Hess, 2002), which tend to form expanded sperm granulomas and cysts, as seen in the human (Mennemeyer and Mason, 1979). It is not clear how blind ended tubules form but presumably it is the failure of those tubules to fuse/join to a nearby tubule. Because the mesonephric tubules and differentiated efferent ductules express an abundance of ESR1 (section 3), it is possible that the estrogen receptor signaling pathway is involved in growth and branching of the mesonephric tubules. This hypothesis was supported by an unexpected observation in the Esr1KO mouse, which revealed 100% incidence of blind ended tubules compared to 40% in wild-type mice and the abnormal tubules were longer and contained bulbous endings in the Esr1KO males (Hess et al., 2000).
The mechanisms by which the mesonephric tubules elongate and coil are also not known but it is tempting to speculate that cell proliferation coupled with cell rearrangements as observed in the Wolffian duct (see below) are responsible. Interestingly, closer examination of the three-dimensional models of the adult mouse efferent ducts suggest that each tubule coils in isolation, they do not appear to coil around each other (Lambot et al., 2009; Nakata and Iseki, 2019). One clear distinction between human efferent ducts and the efferent ducts from other animals is that the efferent ducts in the human form the majority of the head (caput) region of the epididymis (Sullivan et al., 2019; Yeung et al., 1991). In rodent species, only the single common duct penetrates the epididymal capsule and coils near the initial segment (Hess, 2002, 2018a). This is the reason why the head of the human epididymis is of mesonephric tubule origin and not of mesonephric duct origin.
Update on the regulation of Wolffian duct elongation and coiling
For a more extensive discussion on the development of the Wolffian duct, the reader should refer to several reviews (de Mello Santos and Hinton, 2019; Georgas et al., 2015; Hannema and Hughes, 2006; Hannema et al., 2006; Hinton and Avellar, 2018; Hinton et al., 2011; Joseph et al., 2009; Murashima et al., 2014; Shaw and Renfree, 2014). At E14.5 in the mouse, the Wolffian duct is a single unconvoluted tubule approximately 1 mm in length, and will elongate to approximately 1 meter in the adult. The length of the human epididymal duct is 6 meters (Hinton et al., 2011; Sullivan et al., 2019). Coiling is observed at E15.5 beginning in the proximal region of the tubule in mice (Hinton and Avellar, 2018), which is mostly a simple sine-like wave. Very nice studies by (Hirashima, 2014, 2016) suggests that the biomechanical properties of the duct, the surrounding mesenchyme and capsule contribute to coiling. It is beyond the scope of this review to discuss this further, but this is an exciting finding since biomechanical properties play key roles during elongation and coiling of other tissues, for example the brain and heart. However, it is important to recognize that cell proliferation and cell rearrangements will contribute to the biomechanical properties of the elongating and coiling epididymal duct.
Certainly, cell proliferation is a major contributor to epididymal ductal elongation, and more recently, it was recognized that cell rearrangements, for example, convergent extension type of movement also contributes to duct elongation (Xu et al., 2016b). In the latter case, Protein Tyrosine Kinase 7, which is a member of the non-canonical Wnt pathway, is a major regulator (Xu et al., 2016a). Other members of the Wnt signaling pathway have also been shown to be major regulators of Wolffian duct elongation and coiling (Kumar et al., 2016) as have Fgf8 (Kitagaki et al., 2011), SPAG11C (Ribeiro et al., 2017), Hox genes (Branford et al., 2000; Raines et al., 2013; Zhao and Potter, 2002) and Inhbα (Tomaszewski et al., 2007). The potential involvement of estrogens in coiling and elongation of the Wolffian duct is suggested by studies showing that neonatal over-exposure to estrogens can reduce elongation/coiling within the epididymis and blurring of the epididymal-vas deferens transition as evidenced by coiling of the proximal vas, changes that are associated with altered epithelial and stromal expression of ESR1 (Atanassova et al., 2001; Atanassova et al., 2005b).
Perhaps the most well-recognized regulator of Wolffian duct development is testosterone and Fig. 1 shows key events of the action of androgens. The figure also shows the key events of the action of estrogen and despite the well-known action of this hormone on the postnatal development of the efferent ductules and epididymis (Hess and Cooke, 2018), it is unclear as to whether estrogen plays a significant role during the embryonic period (see sections 3–4). Data from the Esr1KO mouse strongly suggest that estrogen, or at least the expression of ESR1, has an important role in the differentiation of epithelia, in addition to potential effects on branching and the number of blind ended tubules formed. In wild-type males, the efferent duct epithelium terminates abruptly where the initial epididymal segment begins, but in the Esr1KO mice blotchy portions of initial segment epithelium were observed in the common efferent duct, as well as near the rete testis (Hess et al., 2000; Joseph et al., 2011), which suggests epithelial differentiation and the expression of region-specific genes may be under estrogen, as well as androgen, regulation.
Estrogen regulates several genes in breast cancer cells/tissue that are also expressed in the developing male reproductive tract, for example, Pax2 (Beauchemin et al., 2011) and Gata3 (Wilson and Giguère, 2008). Several genes appear to be highly expressed in mouse mesonephric tubules (Snyder et al., 2010) from E14.5 to P1 that are possibly regulated by estrogen, including the following: gene regulated by estrogen in breast cancer protein (Greb1), sulfotransferase 1E1 (Sult1e1 and Sult1c2), DEAD box 5 (Ddx5) an RNA helicase (Xing et al., 2019)) and UDP glucuronosyltransferase 1 family, polypeptide A1 (Ugt1a1). It is anticipated that a Wolffian duct and mesonephric tubule phenotype will be observed in Greb1 knockouts, because Greb1-like (Greb1l) knockouts (De Tomasi et al., 2017) clearly show a loss of the Wolffian duct and the most anterior mesonephric tubules that will form the efferent ductules. Expanding this thought further, it is tempting to speculate that Greb1 also plays a role in cell rearrangements during Wolffian duct and mesonephric tubule elongation because this gene is important during axial elongation in zebrafish embryos (Prajapati et al., 2019).
3. Estrogen receptors in the Male reproductive system
Estrogen receptor presence in the adult male reproductive tract has been thoroughly reviewed and the reader is urged to examine the following papers: (Carreau and Hess, 2010; Cooke et al., 2017; Hess, 2004; Hess and Carnes, 2004; Hess and Cooke, 2018; Hess et al., 2011; O’Donnell et al., 2013). Here we will focus on the fetal and neonatal presence of ESR1 and 2, as well as androgen receptor (AR) in the human, rat and mouse. However, for comparisons adult ages are included. Also, this section will include the testis, because the rete testis in man is mediastinal and penetrates approximately1/3 of the testicular core (Hess and Hermo, 2018).
The presence of high concentrations of E2 in the testis and rete testis fluid (Table 1) and the observation of pathological changes in the male reproductive system, following perinatal estrogen treatment (Brown-Grant et al., 1975; Dunn and Green, 1963; Emmens and Parkes, 1947; Greene et al., 1938; Hendricks and Gerall, 1970; Kincl et al., 1963; Kincl and Maqueo, 1972; McLachlan et al., 1975; Mori, 1967; Takasugi, 1970), suggested to many that estrogen receptors must be present in the male reproductive system, even during development (Iguchi, 1991). Evidence for ESR presence began to appear first in 3H-E2 binding studies that demonstrated distinct high affinity in interstitial cells of the testis and in whole epididymal extracts (Brinkmann et al., 1972; Danzo et al., 1975; Danzo et al., 1978; Danzo et al., 1977; Mulder et al., 1973; Mulder et al., 1974a; Mulder et al., 1974b; Schleicher et al., 1984; Stumpf, 1969; Stumpf and Sar, 1976; Toney and Danzo, 1988). At the time, it was assumed that only one ESR existed (Nilsson et al., 2001), but this original data, although encouraging for the general hypothesis, became less interpretable when a second ESR (ERβ) was cloned from the male rat prostate (Kuiper et al., 1996), as E2 binding could easily target ESR1 or ESR2 (see review: (Nilsson et al., 2001)). However, as it turns out, loss of ESR2 in the knockout mouse showed no major effects on the development of testes, efferent ducts and epididymis, although prominent long-term effects were seen in the prostate and an increase in neonatal gonocytes, but minor effects in the adult testis (Chen et al., 2010; Delbes et al., 2006; Delbes et al., 2004; Gustafsson et al., 2019; Krege et al., 1998; Prins and Korach, 2008). Therefore, the major focus here will be the presence and function of ESR1 in the male, during the fetal and neonatal periods.
Table 1.
Estradiol concentrations in the male
| Age | Source | Species | Concentration | References |
|---|---|---|---|---|
| Fetal | Amniotic fluid | Human | 64 pg/ml | 1 |
| Blood | Mice | 94 pg/ml | 2 | |
| Neonatal PND 0 | Umbilical cord artery | Human | 5,000–12,000 pg/ml | 3 |
| Neonatal PND >60 | Blood | Human | 5–95 pg/ml | 3 |
| Neonatal PND 1 | Blood | Rat | 300–325 pg/ml | 4 |
| Neonatal PND 2 | Blood | Rat | 150–175 pg/ml | 5 |
| Neonatal PND 3–11 | Blood | Rat | 80–130 pg/ml | 6 |
| Adult | Blood | Human/monkey | 3.6–145 pg/ml | 7 |
| Blood | Rat | 1.7–175 pg/ml | 8 | |
| Blood < | Mouse | 11.8–30.0 pg/ml | 9 | |
| Testis | Human | 5–20 ng/g | 10 | |
| Testis | Rat | 4.5–751 pg/g | 11 | |
| Rete testis | Monkey | 0.38 nmol/L | 12 | |
| Rete testis | Rat | 249 pg/ml | 13 |
References:
(Angsusingha et al., 1974; Bujan et al., 1993; Carreau et al., 2004; Cook et al., 1998; Dias et al., 2016; Doerr and Pirke, 1974; Finkelstein et al., 2013; Haug et al., 1974; Kley et al., 1976; Overpeck et al., 1978; Purvis et al., 1975; Waites and Einer-Jensen, 1974; Zhou et al., 2013)
(Butcher et al., 1974; Cook et al., 1998; de Jong et al., 1973; Döhler and Wuttke, 1975; Gorski et al., 1977; Hawkins et al., 1975; Jong et al., 1975; Södersten et al., 1974; Wichmann et al., 1984)
Carreau, 2004 #31563}
Determining specific expressions and localization of ESR1 and ESR2 in the male has been difficult and remains challenging today. Wide variation in reported presence or absence for steroid receptors in the male tract, including that of androgen receptor (AR), are seen in Tables 2–4. Differences in specificity of antibodies, as well as changes in results depending on tissue fixation and processing, including antigen retrieval methods (Andersson et al., 2017; Cooke et al., 2017; Gustafsson et al., 2019; Iwamura et al., 1994; Nelson et al., 2017; Shi et al., 1993), have led to these disparities. Thus, drawing conclusions and interpreting data from estrogen treatment-related effects, in specific male reproductive tissues, has become problematic. Some labs have reported no ESR1 mRNA in fetal and neonatal human testes (Berensztein et al., 2006; Gaskell et al., 2003), but an abundance of mRNA and protein were found in the mouse (Cederroth et al., 2007; Jefferson et al., 2000; Mowa and Iwanaga, 2001; Nielsen et al., 2000). Some laboratories find ESR1 expressed in neonatal rat Sertoli cells (Lucas et al., 2008), while others report none (Fisher et al., 1997; Sar and Welsch, 2000). Probably most troubling are conflicting reports for ESR mRNAs. However, despite this struggle, some patterns of receptor localization can be inferred. For example, it has been repeatedly confirmed in every species examined to date that efferent ductules express the highest concentration of ESR1 of any tissue (Cooke et al., 2017; Hess and Cooke, 2018), male or female, and does so starting during early fetal development of the mesonephric tubules (Fig. 3).
Table 2.
Fetal androgen and estrogen receptors in the human, rat and mouse male reproductive system
| Tissue | Receptor | Presence | Species | FetalAge/Type of Study1 | References |
|---|---|---|---|---|---|
| Whole testis | AR | − | Rat | <E15.5 | (Majdic et al., 1995) |
| +/− | Rat | E15 | (You and Sar, 1998) | ||
| ESR1 | − | Human | 23 wks 12–17 wks mRNA | (Gaskell et al., 2003) | |
| + | Human | 23 wks mRNA | (Brandenberger et al., 1997) | ||
| +++ | Mouse | E14, E18.5 mRNA | (Cederroth et al., 2007; Mowa and Iwanaga, 2001) | ||
| ESR2 | + | Human | 12–23 wks mRNA | (Brandenberger et al., 1997; Gaskell et al., 2003) | |
| +++ | Mouse | E18.5 mRNA | (Cederroth et al., 2007) | ||
| Sertoli cell | AR | − | Rat | E17–20.5 | (Majdic et al., 1995; Williams et al., 2001a) |
| − | Mouse | E14 | (Zhou et al., 1996) | ||
| ESR1 | − | Human | 12–22 wks | (Gaskell et al., 2003; Shapiro et al., 2005) | |
| − | Rat | E17.5, 18.5, 20.5 | (Fisher et al., 1997; Sar and Welsch, 2000; Saunders et al., 1998; Williams et al., 2001a) | ||
| − | Rat | E19 mRNA | (Mowa and Iwanaga, 2001) | ||
| − | Mouse | E13.5 | (Nielsen et al., 2000) | ||
| +/− | Mouse | E13–15 +; E17.5 +/− | (Greco et al., 1992) | ||
| ESR2 | +/− | Human | 14–22 wks +; 35wks − | (Boukari et al., 2007; Gaskell et al., 2003; Takeyama et al., 2001) | |
| +/++ | Rat | E16–20.5 | (Saunders et al., 1998; van Pelt et al., 1999; Williams et al., 2001a) | ||
| +/− | Rat | E19 | (Mowa and Iwanaga, 2001) | ||
| Germ cell/gonocyt e | AR | + | Rat | E20.5 | (Majdic et al., 1995) |
| ++ | Mouse | E14–18 | (Merlet et al., 2007; Zhou et al., 1996) | ||
| ESR1 | − | Human | 12->21 wks | (Gaskell et al., 2003; Magers et al., 2016; Shapiro et al., 2005) | |
| − | Rat | E17.5, 18.5, 20.5 | (Fisher et al., 1997; Sar and Welsch, 2000; Saunders et al., 1998) | ||
| −/+ | Rat | E14–17 mRNA −; E19 mRNA +/− | (Mowa and Iwanaga, 2001) | ||
| − | Mouse | E13.5 | (Nielsen et al., 2000) | ||
| + | Mouse | E15 | (Greco et al., 1992) | ||
| ESR2 | + | Human | 14–22 wks | (Boukari et al., 2007; Gaskell et al., 2003; Takeyama et al., 2001) | |
| − | Human | 35 wks | (Boukari et al., 2007) | ||
| ++ | Rat | E16, 20.5 | (Saunders et al., 1998; van Pelt et al., 1999) | ||
| ++ | Rat | E17–19 mRNA | (Mowa and Iwanaga, 2001) | ||
| + | Mouse | E16 | (Jefferson et al., 2000) | ||
| Leydig cell (Interstitial) | AR | +/+++ | Human | 12 −22 wks; increasing | (Shapiro et al., 2005) |
| −/+ | Rat | E15 | (You and Sar, 1998) | ||
| ++ | Rat | E17–19.5 increasing | (Majdic et al., 1995; You and Sar, 1998) | ||
| − | Mouse | E14–18 | (Zhou et al., 1996) | ||
| ESR | +++ | Rat | E17 mRNA | (Mowa and Iwanaga, 2001) | |
| − | Mouse | E4–13 Receptor binding | (Holderegger and Keefer, 1986) | ||
| + | Mouse | E14–17 Receptor binding | (Holderegger and Keefer, 1986; Stumpf et al., 1980) | ||
| ESR1 | ++/+ | Human | 12–22 wks; decreasing | (Shapiro et al., 2005) | |
| − | Human | 16–22 wks | (Gaskell et al., 2003; Magers et al., 2016) | ||
| +++ | Rat | E17.5–20.5 | (Fisher et al., 1997; Sar and Welsch, 2000; Saunders et al., 1998; Saunders et al., 1997) | ||
| ++/+ | Mouse | E13–15 decreasing | (Greco et al., 1993; Nielsen et al., 2000) | ||
| +/− | Mouse | E17–19 | (Greco et al., 1992) | ||
| ESR2 | ++/+ | Human | 12–22 wks; decreasing | (Boukari et al., 2007; Gaskell et al., 2003; Shapiro et al., 2005) | |
| − | Human | 35wks | (Boukari et al., 2007) | ||
| ++ | Rat | E20.5 | (Saunders et al., 1998) | ||
| +/− | Rat | E17–19 mRNA | (Mowa and Iwanaga, 2001) | ||
| Peritubular myoid cell | AR | +/+++ | Human | 7->21 wks; increasing | (Magers et al., 2016; Shapiro et al., 2005) |
| − | Rat | E15 | (You and Sar, 1998) | ||
| + | Rat | E17–20.5 increasing | (Majdic et al., 1995; You and Sar, 1998) | ||
| + | Mouse | E15.5 | (Merlet et al., 2007) | ||
| − | Mouse | E14–18 | (Zhou et al., 1996) | ||
| ESR1 | − | Human | 16 wks | (Gaskell et al., 2003; Magers et al., 2016) | |
| Rat | E17.5, 18.5, 20.5 | (Fisher et al., 1997; Sar and Welsch, 2000; Saunders et al., 1998) | |||
| +/− | Mouse | E13.5 | (Greco et al., 1992; Nielsen et al., 2000) | ||
| ++ | Mouse | E17–19 | (Greco et al., 1992) | ||
| ESR2 | ++ | Human | 17 wks | (Boukari et al., 2007; Gaskell et al., 2003) | |
| − | Human | 35 wks | (Boukari et al., 2007) | ||
| ++ | Rat | E20.5 | (Saunders et al., 1998) | ||
| Rete testis | AR | +++ | Human | >21 wks | (Magers et al., 2016) |
| ESR1 | − | Human | >21 wks | (Magers et al., 2016) | |
| − | Mouse | E15.5 Epithelium −; stroma − | (Nielsen et al., 2000) | ||
| Mesonephros/efferent ducts | AR | ++ | Human | 7->21 wks Epithelium ++; stroma ++ increasing | (Magers et al., 2016; Shapiro et al., 2005) |
| + | Rat | E16 Receptor binding: epithelium | (Cooke et al., 1991a) | ||
| + | Rat | E14 Epithelium +; stroma +/− | (Bentvelsen et al., 1995; You and Sar, 1998) | ||
| ++ | Rat | E17–19.5 Epithelium ++; stroma + | (Majdic et al., 1995; You and Sar, 1998) | ||
| + | Rat | E17.5, 18.5 stroma | (Fisher et al., 1997) | ||
| ++ | Mouse | E16–19 Receptor binding | (Cooke et al., 1991a) | ||
| ESR | ++ | Mouse | E16 Receptor binding: epithelium +++; stroma ++ | (Cooke et al., 1991b) | |
| ESR1 | − | Human | 8–9 wks Epithelium −; stroma − | (Cunha et al., 2020) | |
| +++ | Human | 12->21 wks Epithelium +++; stroma − | (Cunha et al., 2020; Gaskell et al., 2003; Magers et al., 2016) | ||
| − | Rat | E17.5–18.5 Epithelium −; stroma +++ | (Fisher et al., 1997) | ||
| +++ | Rat | E17 mRNA Epithelium +++; stroma + | (Mowa and Iwanaga, 2001) | ||
| − | Mouse | E11.5 Epithelium −; stroma −/+ | (Nielsen et al., 2000) | ||
| +/++ | Mouse | E13-E15; increasing | (Greco et al., 1992) | ||
| +/− | Mouse | E15.5 Epithelium +/−; stroma − | (Nielsen et al., 2000) | ||
| ESR2 | − | Human | 8–9 wks Epithelium −; stroma − | (Cunha et al., 2020) | |
| + | Human | 12–20 wks Epithelium +; stroma + | (Cunha et al., 2020) | ||
| − | Rat | E17–19 mRNA Epithelium stroma +/− | (Mowa and Iwanaga, 2001) | ||
| Wolffian duct/Epidid ymis | AR | ++ | Human | 7->21 wks Epithelium ++; stroma ++ | (Magers et al., 2016; Shapiro et al., 2005) |
| −/+ | Rat | E14–16.5 Epithelium −; stroma + | (Bentvelsen et al., 1995; You and Sar, 1998) | ||
| + | Rat | E17–18 Epithelium caput + | (Majdic et al., 1995) | ||
| − | Mouse | E14–16 Receptor binding: epithelium −; stroma ++ | (Cooke et al., 1991a) | ||
| ++ | Mouse | E19-PND 0 Receptor binding: epithelium ++/++ | (Cooke et al., 1991a) | ||
| ESR | − | Mouse | E4–10 Receptor binding: epithelium −; stroma − | (Holderegger and Keefer, 1986) | |
| −/++ | Mouse | E13–17 Receptor binding: epithelium −; stroma +/++ | (Holderegger and Keefer, 1986) | ||
| −/+++ | Mouse | E16 Receptor binding: epithelium −; stroma +++ | (Cooke et al., 1991b; Stumpf et al., 1980) | ||
| + | Mouse | E19 Receptor binding: epithelium + | (Cooke et al., 1991b) | ||
| ESR1 | − | Human | 8–9 wks Caput −; cauda − | (Cunha et al., 2020) | |
| + | Human | 12 wks Caput, cauda epithelium +; stroma + | (Cunha et al., 2020) | ||
| ++/− | Human | 7–22 wks Caput epithelium ++; Cauda − decreasing | (Cunha et al., 2020; Shapiro et al., 2005) | ||
| − | Rat | >21 wks Epithelium | (Magers et al., 2016) | ||
| −/++ | Mouse | E14–19 mRNA Epithelium −; stroma ++/+++ | (Mowa and Iwanaga, 2001) | ||
| +/++ | Mouse | E13–15 Increasing | (Greco et al., 1992; Nielsen et al., 2000) | ||
| +/− | Mouse | E17–19 Decreasing | (Greco et al., 1992) | ||
| ESR2 | ++ | Human | 7–22 wks Epithelium ++; stroma ++ | (Shapiro et al., 2005; Takeyama et al., 2001) | |
| − | Human | 8–9 wks Cauda | (Cunha et al., 2020) | ||
| + | Human | 12–20 wks Cauda | (Cunha et al., 2020) | ||
| − | Rat | E14–19 mRNA Epithelium −; stroma +/− | (Mowa and Iwanaga, 2001) | ||
| Vas deferens | AR | ++ | Human | >21 wks Epithelium ++; stroma +; smooth muscle − | (Magers et al., 2016) |
| + | Rat | E20 Stroma | (Bentvelsen et al., 1995) | ||
| + | Mouse | E19-PND 0 Receptor binding | (Cooke et al., 1991a) | ||
| ESR1 | − | Human | >21 wks | (Magers et al., 2016) | |
| −/++ | Rat | E14 mRNA Epithelium − ; stroma ++ | (Mowa and Iwanaga, 2001) | ||
| − | Mouse | E13.5 | (Nielsen et al., 2000) | ||
| ESR2 | − | Rat | E14–19 mRNA Epithelium −; stroma +/− | (Mowa and Iwanaga, 2001) |
Immunohistochemistry unless otherwise indicated; mRNA can be isolated RNA or insitu hybridization; Receptor binding can be autoradiography or biochemical assay binding
Table 4.
Early puberty and adult androgen and estrogen receptors in the human, rat and mouse male reproductive system
Estimate of expression. Immunohistochemistry (IM) unless otherwise indicated; mRNA can be isolated RNA or insitu hybridization; Receptor binding can be autoradiography or biochemical assay binding; PND, postnatal day; mo, months
Fig. 3.
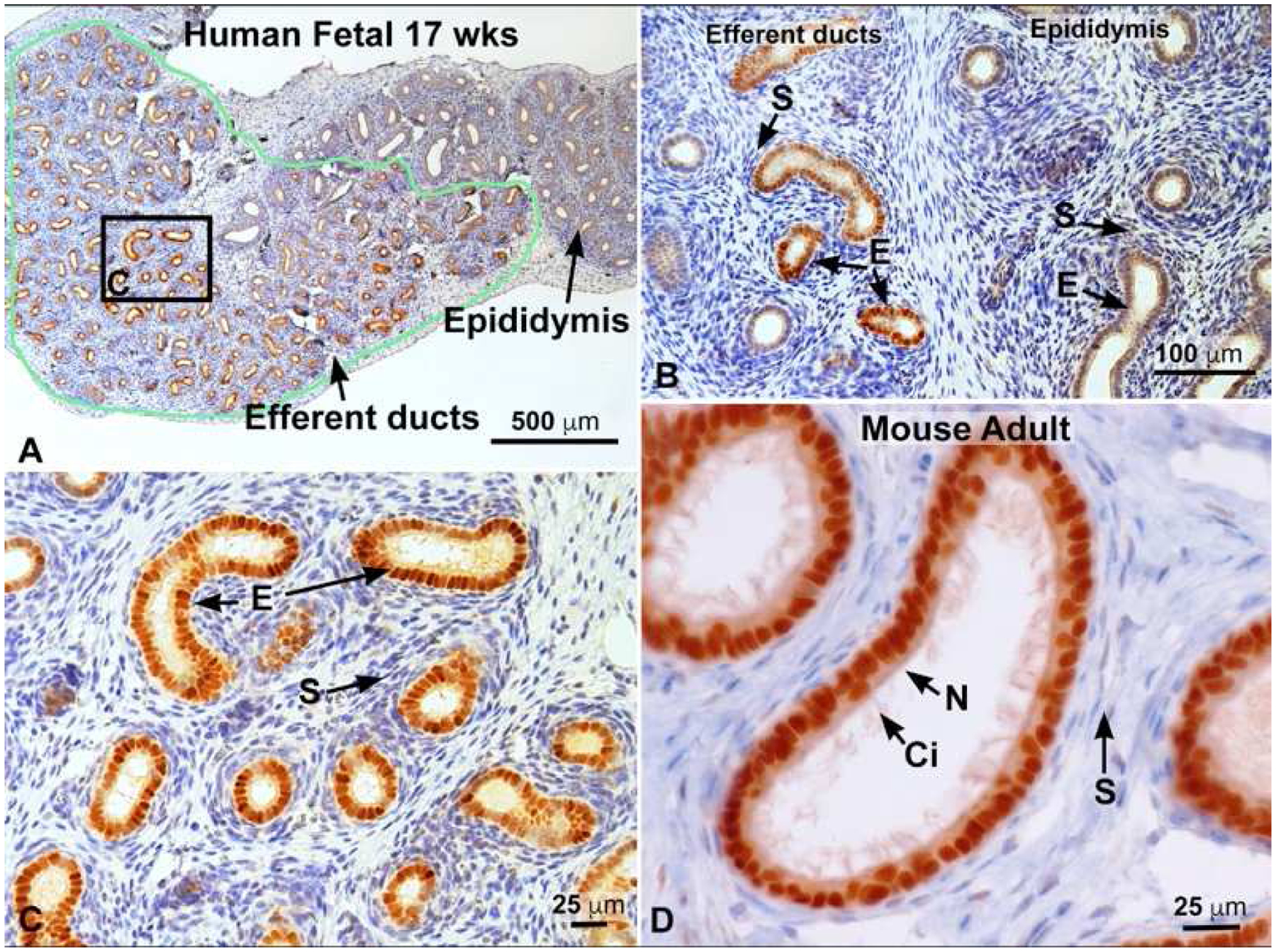
Estrogen receptor 1 (ESR1) immunostaining. A) 17 week fetal human efferent ductules in the head of the epididymis outlined in green and staining intensely for ESR1. To the right is the immature epididymal duct, which appears to have far less staining. B) Fetal 17 week human tissue showing efferent ductules to the left and epididymis to the right. Efferent duct stroma (S) is negative, but the epithelial cells (E) are strongly positive for ESR1, while epididymal epithelium is less positive and some stromal cells show slight staining. C) Higher magnification of area outlined in A. Efferent duct epithelial cells (E) are strongly positive for ESR1, but the stroma (S) is negative. D) Mouse adult efferent ductules showing intense staining for ESR1 in both nonciliated (N) resorptive epithelial cells, as well as the motile ciliated cells (Ci). Stromal (S) cells are mostly negative. Human fetal photos provided by Dr. Gerald Cunha, with approval by the Committee on Human Research at UCSF, IRB# 12–08813); Cunha et al. (2020).
Binding of E2 in male fetal reproductive tissues occurs prior to or simultaneous with androgen binding and occurs first in the efferent ductules (Cooke et al., 1991b). Subsequent immunohistochemical staining has shown that ESR1 appears more prominent in the efferent ductule epithelium, while ESR2 can be more prominent in the testis and epididymis (see Table 2 and reviewed in: Cooke et al., 2017), although specific cell types show more intense staining for ESR1 (Zhou et al., 2002). ESR1 expression is quite strong in the mesonephric/efferent ductule epithelium from the fetal and neonatal period through to adulthood, but the stromal areas are either negative or weakly positive (Fig. 3). In contrast, the rete testis appears to be either negative or weakly positive until adulthood, when the epithelium can show strongly positive staining. The Wolffian duct/epididymal epithelium is also negative to weakly positive for ESR1 during fetal and neonatal development, but then shows increased staining near puberty in some reports. A consistent observation for the epididymal epithelium is an intense expression of ESR1 in the apical and clear cells. For both the efferent ducts (in some species) and epididymis, it appears that the stromal areas can be more intensely stained during fetal development. The vas deferens epithelium has also been reported as negative for ESR1, while the stroma has shown some positivity during development.
The classical concept of estrogen-mediated signaling involves a ligand-dependent transcription factor or receptor that dimerizes, becomes phosphorylated and translocates to the nucleus, where the activated ESR binds to estrogen response elements in the DNA of specific genes. The modulation of transcription is achieved through its activation function domains (AF-1 and AF-2) that recruit coregulators in a complex interaction and docking on specific regions of the DNA (Arnal et al., 2017; Korach et al., 2019). This classical estrogen pathway requires both the production of E2 via the enzyme aromatase and the expression of nuclear ESRs. Therefore, great effort has been made to understand the developmental expressions of aromatase, as well as the receptors, ESR1 and 2 (Tables 2–4). However, this original pathway was found to be incomplete, as there have been new discoveries of non-genomic estrogen activity, including extranuclear or membrane ESR paths, as well as ligand-independent signaling, often involving growth factor activation of ESRs (Arnal et al., 2017; Levin, 2015; Stefkovich et al., 2017; Stellato et al., 2016).
One of the more surprising discoveries was that loss of the ability to produce E2, the natural ligand of ESR1, in the aromatase (Cyp19) KO (AromKO) mouse, had no effect on male reproductive tract development and physiology and produced relatively normal fertility until the mice began to age (Robertson et al., 1999; Robertson et al., 2002; Robertson et al., 2001). However, the expression of just the receptor, ESR1, was found to be essential for development and function of the male reproductive tract (Eddy et al., 1996; Hess et al., 1997a; Hess et al., 2000; Joseph et al., 2010a; Joseph et al., 2010b; Lee et al., 2000; Lee et al., 2009; Lubahn et al., 1993; Nakai et al., 2001; Toda et al., 2008; Zhou et al., 2001) and its expression was not altered in the AromKO male (Toda et al., 2008; Toda et al., 2001). This serendipitous discovery that the unliganded ESR1 can maintain efferent ductule structure and function demonstrated the regulation of essential genes in the absence of the steroid ligand (Caizzi et al., 2014; Sinkevicius et al., 2009; Stellato et al., 2016). Very little is known about estrogen regulation of early development of the testis and reproductive tract; however, during in utero development, ESR1 expression in the mesonephros appears to occur prior to the expression of aromatase in the testis. Thus, an unliganded ESR1 may have a role in the early differentiation and migration of mesonephric tubule epithelial cells. This conclusion is supported by an in vitro study demonstrating that ESR1 can bind to more than 4,000 chromatin sites in the absence of E2 (Caizzi et al., 2014). Importantly, these binding sites were shown to be related to genes linked to development and differentiation (Stellato et al., 2016).
In light of these stated complications and surprising discoveries related to ESR1, we are left with numerous questions, including the following. What is the precise timing of ESR1 and AR expression during fetal development and how do these compare across species? How do we successfully analyze ESR antibody data and determine which techniques are best for preservation of cellular localization? Will single-cell RNA sequencing and single cell proteomics finally resolve the conflicting data of ESR1 presence in the male reproductive tract (Green et al., 2018; Tan et al., 2020)? When will experiments finally determine the biochemical and physiological nature of co-localization of AR and ESR1 in the same cell? Also, why are the membrane and nuclear ESR1s equally essential for development of the efferent ductules and head of the epididymis (Cooke et al., 2019; Nanjappa et al., 2016)? Finally, how does the unliganded ESR1 actually function in efferent ductules? Is it possible that the expression of AR alongside an unliganded ESR1 in the presence of T provides cooperativity in the maintenance of specific genes? For example, both estrogen and androgen response elements are found in the promoter region of genes responsible for epithelial maintenance of fluid reabsorption in efferent ductules (Hess and Cooke, 2018; Snyder et al., 2009; Trepos-Pouplard et al., 2010; Yao et al., 2017). Many questions remain unanswered, but one intriguing fact stands out: ESR1 is essential and constitutively expressed in parts of the male reproductive tract (Oliveira et al., 2004), but the male reproductive system develops in the absence of its natural ligand E2 (Toda et al., 2008).
4. Aromatase and synthesis of estrogen in the male reproductive system
Aromatase cytochrome P450, CYP19A1, which catalyzes the final step of androgen conversion to 17β-estradiol (E2) and estrone (E1) (Corbin et al., 1988), is present in the testis from fetal development through to adulthood (Borday et al., 2013; Brodie and Inkster, 1993; Carreau and Hess, 2010; Dorrington and Armstrong, 1975; Guercio et al., 2020; Nitta et al., 1993; O’Donnell et al., 2001; Payne et al., 1987; Weniger and Zeis, 1988). During fetal development, serum E2 concentrations (Table 1) are relatively high in human males and females, as well as rodent species (Bonagura et al., 2011; Habert and Picon, 1984; Robinson and Bridson, 1978; vom Saal et al., 1997). In the male, this elevated level of estrogen continues neonatally (Table 1), when E2 is much higher than in the adult (Döhler and Wuttke, 1975; Gorski et al., 1977; Overpeck et al., 1978; Rommerts et al., 1982). In one rat study, E2 was reported to be over 300 pg/ml on postnatal day (PND) 1, but declined to near 50 pg/ml on day 3, followed by another rise just before weaning, and finally a steady decline toward ~25 pg/ml in the adult (Döhler and Wuttke, 1975), approximately the same concentration reported in adult men (Finkelstein et al., 2013). Thus, the fetal and neonatal periods of development in the male experience a significantly elevated level of estrogen, but also rising levels of T (Habert and Picon, 1984), which raises numerous questions regarding the sources and functions of E2 in organs that depend so much on androgens for activation and maintenance of functions programmed in fetal life during the ‘masculinization programming window’ (see review: Sharpe, 2020). Another question that needs attention, but cannot be addressed here, is the high levels of serum alpha-fetoprotein that binds E2 with high affinity in rodents and could limit the influence of circulating estrogen over target organs in the male (Cunha et al., 2019). However, this also places a greater emphasis on aromatase’s expression locally, such as the testis, which could diffuse throughout the developing gonad and mesonephros. Finally, the topic of sulfated or conjugated estrogens and estrogen- (SULT1E1) and hydroxysteroid- (SULT2A1) sulfotransferases also requires greater attention than has been given in the past. Sulfoconjugation can block ESR1 binding and thus the high concentration of unconjugated estrogens in the fetus could place an emphasis on local expressions of sulfotransferases, not only in liver but also the male reproductive tract (Duanmu et al., 2006; Fietz et al., 2013; Miki et al., 2002; Mutembei et al., 2008; Schuler et al., 2018a, b; Wood, 2014).
In the adult male, circulating estrogen is low (Table 1), while T is high, as would be expected if spermatogenesis is to be preserved and masculinity maintained (Finkelstein et al., 2013; Zirkin and Papadopoulos, 2018). However, intratesticular E2 is elevated, as there is a shift toward sequestration within the reproductive tract, which results in substantial increases in estrogen exiting the testis via rete testis fluid (see review: Cooke et al., 2017). In some species, such as the horse, the testis produces more estrone sulphate (5.4 μg/min) than testosterone (0.3 μg/min), much of which ends up in testicular lymph and rete testis fluid (Setchell and Cox, 1982). While the adult rat testis contributes about 21% of the blood E2 (de Jong et al., 1973), it contributes 100% to the rete testis fluid, where concentrations of E2 are reported to reach 510 pg/ml and average 249 pg/ml (Free and Jaffe, 1979). Although most of the E2 synthesis in the male reproductive system comes from the testis, the presence of aromatase has also been reported in the epithelium of efferent ductules and the epididymis (Carpino et al., 2004; Kim et al., 2008; Pereyra-Martinez et al., 2001; Rosati et al., 2020; Shayu and Rao, 2006; Swider-Al-Amawi et al., 2007). In addition, the external genitalia express aromatase (Jesmin et al., 2002).
The function of such high levels of estrogen in the lumen of the male tract remains unknown, but the efferent ductules have the highest expression of ESR1 of any tissue, male or female and presence of the receptor itself is absolutely required for fluid reabsorption physiology (Hess, 2018b; Hess and Cooke, 2018), while loss of luminal estrogen has no effect on the efferent ductules (Toda et al., 2008). Thus, it remains a mystery why such a high concentration of E2 would be found in the lumen of the rete testis in the first place, since it is not absolutely required for binding to the high concentration of ESR1 present downstream in the efferent ductule epithelium.
It is not known when, during development, the high concentration of E2 begins to appear in rete testis fluid. Estrogen is first produced in the testis during fetal life, with a significant increase in late gestation and the rete testis joins the developing mesonephric tubules around E15.5 in mice (Karl and Capel, 1995; Omotehara et al., 2020). However, seminiferous tubular lumens do not begin to open until PND10–15 in mice and rats (Auharek and de Franca, 2010), and full secretion of fluids is not achieved until PND30 in rats (Gondos and Berndston, 1993; Russell et al., 1989). Thus, fluid flow begins somewhere between 10–20 after birth and thereafter increases substantially. Therefore, a question arises regarding how testicular E2 might have direct influence on fetal development of the male reproductive tract? It is possible that prior to receiving fluid from the rete testis, the mesonephric tubules/early efferent ductules could transport circulating E2 into the lumen or synthesize E2 themselves. Mesonephric tubules form lumens as early as E11.5 (Omotehara et al., 2020) and thus are secretory. Because most studies of aromatase in the male have focused on the testis, only one paper has looked at the reproductive tract. Interestingly, they found high expression of aromatase mRNA in efferent ductules between PND7–14 but then a rapid drop in expression until there was no detectable presence at PND90 (Kim et al., 2008). This study should be extended into fetal development, as the mesonephros or the rete cord mesenchymal cells could be an interesting potential source of E2 for developmental influence over mesonephric tubule growth, fusion and branching.
For many years there has been a generally accepted hypothesis that Sertoli cells are the primary source of E2 production in the fetal and neonatal testis, while Leydig cells are the major source in the adult (Carreau et al., 1999; Carreau and Hess, 2010; Hess and Cooke, 2018; O’Donnell et al., 2001; Tsai-Morris et al., 1985). Some of the strongest support for this hypothesis came from 3H-T conversion studies in fetal testis tissue in cultures (Armstrong and Dorrington, 1977; Dorrington and Armstrong, 1975; Dorrington and Khan, 1993; Papadopoulos et al., 1986; Papadopoulos et al., 1987; Payne et al., 1987; Tsai-Morris et al., 1985; Valladares and Payne, 1979; Weniger and Zeis, 1983). Aromatase appears in the fetal mouse testis as early as E12.5 but around E17.5 shows a 20-fold increase in expression (Borday et al., 2013; Greco and Payne, 1994), although there was no E2 synthesis at E13.5. E2 synthesis was maximal in Sertoli cells from neonatal rats before the initiation of spermatogenesis and it was suggested that the release of E2 by Sertoli cells before puberty served to inhibit the synthesis of androgens by Leydig cells (Dorrington and Armstrong, 1979; Dorrington and Khan, 1993). A later report showed that endogenous E2 inhibited germ cell development in the fetal and neonatal mouse testis, which was thought to be acting through gonocyte expression of ESR2 (Delbes et al., 2004), but this could have also involved an inhibition of Leydig cell steroidogenic activity. After PND10, Sertoli cells are thought to produce very little E2 with or without FSH stimulation in the rat (Armstrong and Dorrington, 1977). In fact, Sertoli cell aromatase activity was found to be 7-fold higher in PND7–10 cells than at weaning and Leydig cells from animals older than PND15 were more capable of E2 synthesis than were Sertoli cells (Canick et al., 1979; Rommerts et al., 1982). However, one report showed no visible aromatase staining in the PND10 rat, but some staining of Leydig cells on PND18 (Lee et al., 2008). Nevertheless, in rodents the data appear to support the general statement that fetal and neonatal E2 is coming primarily from Sertoli cells, although Leydig cells appear to be capable of aromatase activity, if stimulated in culture, while the adult testis shows baseline Leydig cell activity (Borday et al., 2013; Greco and Payne, 1994; Warren et al., 1984; Weniger et al., 1993).
In humans the fetal and neonatal picture is not so clear. There are reports of low expressions of aromatase mRNA in the human fetal testis, as well as low E2 synthesis (Boukari et al., 2007; Tapanainen et al., 1989) and data showing E2 production at 8 mo postnatal and 3 yr that are nearly as high as that of adult testes (Inkster et al., 1995). Although aromatase immunostaining of Sertoli cells has been demonstrated at 14 wks prenatal (Boukari et al., 2007), in some studies Leydig and germ cells were also positive in the neonatal testis (Berensztein et al., 2006). However, by 35 wks of gestation, no staining could be found in the fetal testis, yet in the adult human, mRNA expression for aromatase was at least 4-fold higher than in the fetus (Boukari et al., 2007). Some studies have reported immunostaining for aromatase only in the adult interstitial area (Inkster et al., 1995). In the adult human testis, the major localization of aromatase has consistently been in Leydig cells (Brodie and Inkster, 1993; Payne et al., 1976), but germ cells, primarily elongating spermatids, also express the enzyme and they as well as sperm are capable of E2 synthesis and in some reports have a higher production than found in Leydig cells (Carreau et al., 2008; Carreau and Hess, 2010; Carreau et al., 2003; Guercio et al., 2020).
Aromatase in testicular germ cells was overlooked for decades, as it was generally accepted that Leydig cells were the primary site of E2 synthesis in the adult (Payne et al., 1976; Tsai-Morris et al., 1985; Valladares and Payne, 1979). However, this unanticipated discovery occurred when a very good aromatase antibody was used to localize the protein and the strong positive staining of elongating spermatids was not ignored and a curious graduate student asked the question: “Why is the center of the seminiferous tubule staining for aromatase?” (Hess et al., 1995; Nitta et al., 1993). Although we do not know why the concentration of E2 in rete testis fluid is so incredibly high (Free and Jaffe, 1979), it now appears that the source of this estrogen is testicular spermatids and cytoplasmic droplets of luminal spermatozoa (Aquila et al., 2003; Aquila et al., 2002; Carpino et al., 2007; Carreau, 2007; Carreau and Hess, 2010; Janulis et al., 1996a; Janulis et al., 1998; Janulis et al., 1996b; Lambard and Carreau, 2005; Lambard et al., 2003; Lambard et al., 2004; Nitta et al., 1993; Rago et al., 2003).
5. Estrogen-associated knockout and transgenic models
Esr1 Knockout mouse
The development of gene knockout and transgenic technology has been crucial in the study of estrogen in the male reproductive system and in particular the Esr1KO mouse (Table 5). However, despite an abundance of reports on the adult phenotypes in the Esr1KO male, fetal and newborn effects have yet to be published. For a more in-depth coverage of the adult phenotype, the reader can examine several prior reviews (Cooke et al., 2017; Hess and Cooke, 2018; Hess et al., 2011; Hess et al., 2002; Joseph et al., 2011). In general, although the Esr1KO males were infertile, spermatogenesis appeared normal until puberty, when testis weight began to increase and progressive degeneration of the seminiferous epithelium occurred, while plasma LH and T were significantly elevated (Eddy et al., 1996; Lubahn et al., 1993). However, when germ cells from the Esr1KO testis were transplanted into wild-type testes of busulfan-treated mice, there was normal proliferation, differentiation and production of sperm with fertilizing capability (Eddy et al., 1996; Mahato et al., 2001). Thus, the major pathological problem was not the testis, but rather downstream defects in the reproductive tract (Eddy et al., 1996; Hess et al., 1997a). Subsequent studies found dramatic dilation of the efferent ductules and disruption of their physiological function (Hess et al., 1997a). Efferent ductule epithelium expresses an abundance of ESR1 (Hess et al., 1997b) and are responsible for reabsorption of nearly 90% of the luminal fluid (Clulow et al., 1998; Hansen et al., 1999), which contains sperm transported from the rete testis in a dilute seminiferous tubular fluid. This activity is controlled by ESR1 through transcriptional regulation of epithelial ion transporters, AQP (aquaporin) water channels, and Na+/K+-ATPase (Huang et al., 2006; Joseph et al., 2011; Lee et al., 2008; Lee et al., 2001; Ruz et al., 2006; Zhou et al., 2001). Loss of ESR1, as well as blockage of receptor function by a pure antiestrogen, resulted in the inhibition of fluid reabsorption, causing extreme dilation of the ductules, rete testis and eventually the seminiferous tubules, as fluid produced by the testis accumulated in the lumen and overwhelmed the only exit via the single distal efferent ductule that enters the initial segment epididymis (Cooke et al., 2017; Hess, 2014). The inhibition of fluid reabsorption in the efferent ducts, which involves the loss of a differentiated epithelial morphology (Hess and Cooke, 2018), as well as effects on epididymal fluid pH and osmolality, caused sperm tail coiling and premature acrosome reaction and loss of fertilizing capability (Joseph et al., 2010a; Joseph et al., 2010b). Epididymal abnormalities were also reported in the apical and clear cells and abnormal growth of epithelium of the initial segment in areas of the efferent ductules (Hess et al., 2000; Joseph et al., 2011). Thus, numerous physiological pathways are regulated by ESR1 in the adult male reproductive tract and accomplished even in the absence of E2, the natural receptor ligand (see discussion below).
Table 5.
Male reproductive phenotypes in gene targeted and transgenic animal models for the estrogen receptor pathways
| Model | Name | Description | Key Phenotype | References |
|---|---|---|---|---|
| Esr1 (ERα)-null mouse | αERKO ERαKO Ex3 αERKO | Inactivation or deletion of Esr1 |
|
(Antonson et al., 2012; Couse et al., 2000; Delbes et al., 2005; Delbes et al., 2004; Dupont et al., 2000; Eddy et al., 1996; Gould et al., 2007; Goulding et al., 2010; Hess et al., 1997a; Hess et al., 2000; Hess and Carnes, 2004; Joseph et al., 2010a; Joseph et al., 2010b; Lee et al., 2001; Lee et al., 2000; Lee et al., 2009; Lubahn et al., 1993; Mahato et al., 2001; Nakai et al., 2001; Ruz et al., 2006; Shayu et al., 2007; Strauss et al., 2009; Toda et al., 2008; Toda et al., 2001; Weiss et al., 2008) |
| Esrl (ERα)-null rat | Ex3αERKO | Global deletion of Esrl |
|
(Rumi et al., 2014) |
| Esrl (ERα)- conditional deletion | ACTB-ERaKO | β-actin-Cre |
|
(Chen et al., 2009) |
| Esrl (ERα)- conditional deletion | fERαKO | Fibroblast-specific protein (FSP)-Cre |
|
(Chen et al., 2012a; Chen et al., 2012b; Vitkus et al., 2013) |
| Esrl (ERα)-conditional deletion | SmERαKO | Tgln (SM22α smooth muscle)-Cre |
|
(Vitkus et al., 2013) |
| Esrl/2 (ERα/ERβ)-null | Ex3ERαβKO DERKO | Double deletion of Esrl + Esr2 |
|
(Couse et al., 1999; Delbes et al., 2005; Dupont et al., 2000) |
| Esr2 (ERβ)-null | βERKO Ex3βERKO | Global deletion of Esr2 |
|
(Antal et al., 2008; Couse et al., 1999; Delbes et al., 2004; Dupont et al., 2000; Gould et al., 2007; Imamov et al., 2004; Krege et al., 1998; Prins et al., 2001a; Prins et al., 2001b; Risbridger et al., 2001; Strauss et al., 2009; Weihua et al., 2001) |
| ESR1 overexpression | ESR1+ | Transgenic ESR1 |
|
(Heath et al., 2011; Tomic et al., 2007) |
| Esr1 LBD mutant | ENERKI | Transgenic knock-in; agonist PPT activates |
|
(Sinkevicius et al., 2008; Sinkevicius et al., 2009) |
| DBD mutant | NERKI, aERKO (−/AA; AA), KIKO, ERαAA/− | DBD mutation on ERα-null background; precludes direct binding to ERE |
|
(Hewitt et al., 2014; Jakacka et al., 2002; McDevitt et al., 2008; McDevitt et al., 2007; Weiss et al., 2008) |
| DBD mutant | EAAE | ERα DBD mutation |
|
(Ahlbory-Dieker et al., 2009; Hewitt et al., 2014) |
| AF-1 mutant | ERα AF-10 | Deletion of AF-1 (ligandindependent) |
|
(Abot et al., 2013; Billon-Gales et al., 2009) |
| AF-2 mutant | ERα AF-20 | Deletion of AF-2 (LBD) |
|
(Billon-Gales et al., 2011) |
| AF-2 mutant | AF2ER (KI/KI) | AF-2 Mutation |
|
(Arao et al., 2012) |
| Esrl D-domain mutant | H2NES | Esrl D-domain Hinge 2 mutation with nuclear export signal |
|
(Burns et al., 2011; Stefkovich et al., 2017) |
| Nuclear-only ESR1 | NOER | Membrane ESR1 localization lacking |
|
(Nanjappa et al., 2016; Pedram et al., 2014) |
| Membrane-only ESR1 | MOER | Transgene Esrl LBD |
|
(Pedram et al., 2009) |
| Aromatase-null | ArKO +/− soy free Cyp 19KO | Global deletion Cyp19 |
|
(Cripps et al., 2019; Robertson et al., 1999; Robertson et al., 2002; Robertson et al., 2001; Toda et al., 2008; Toda et al., 2001) |
| Aromatase over-expression | Int-5/aromatase; AROM+ | Transgenic male overexpression |
|
(Fowler et al., 2000; Li and Rahman, 2008; Li et al., 2006) |
| GPER1-null | GPERKO | Deletion of GPER1 |
|
(Otto et al., 2009; Prossnitz and Hathaway, 2015) |
| Estrogen sulfotransferase-null | ESTKO | Global deletion |
|
(Qian et al., 2001) |
| Caput AR-null | CEARKO | Conditional deletion of caput epididymal epithelium AR |
|
(Krutskikh et al., 2011; O’Hara et al., 2011) |
| E2f4f/f;E2f5 +/− efferent ducts | E2f4f/f;E2f5 +/−;Vil-cre | Conditional deletion of efferent duct E2f4/5 transcription factors |
|
(Danielian et al., 2016) |
| LGR4-null and mutant | LGR4KO | Global deletion of LGR4/GPR48 |
|
(Hoshii et al., 2007; Li et al., 2010; Mendive et al., 2006) |
Abbreviations: wt, weight; mo, months; ESR1, estrogen receptor 1; ESR2, estrogen receptor 2; AR, androgen receptor; T, testosterone; FSH, follicle stimulating hormone; 4,4’,4′-(4-Propyl-[1H] pyrazole- 1,3,5-triyl), PPT; SLC9a3, sodium/hydrogen exchanger 3; AQP, aquaporin; CAR2 and 14, carbonic anhydrase 2 and 14; SLC4A4, sodium bicarbonate cotransporter; CFTR, cystic fibrosis transmembrane conductance regulator; GPER, G protein-coupled estrogen receptor 1; Cyp19, aromatase; LBD, ligand binding domain; DBD, DNA binding domain; HRE, hormone response element, ERE, estrogen response element; ARE, androgen response element; AF-1 and -2, activation functions 1 and 2 domains; DSP, daily sperm production; PND, postnatal day
The global deletion of Esr1 raised the possibility that the adult phenotype was only a developmental phenomenon, which of course is important because the initial interest in estrogen in the male came from studies showing developmental anomalies following fetal and neonatal estrogen treatments. However, to receive NIH funding for these studies in the early 1990s there was a general requirement that there actually had to be a known function for estrogen in the adult male reproductive system. For that reason, all efforts were focused on deciphering the physiological role of ESR1 by comparing the Esr1KO phenotype to adult males treated with the potent antiestrogen, ICI 182,780 (Cho et al., 2003; Hess et al., 1997a; Oliveira et al., 2005; Oliveira et al., 2004; Oliveira et al., 2003; Oliveira et al., 2002; Zhou et al., 2001). The antiestrogen treatment confirmed that the adult male reproductive tract requires ESR1 for normal function of the rete testis and efferent ductules and maintenance of fluid reabsorption. Without this steroid receptor, luminal sperm showed abnormal maturation and morphology and inability to fertilize an egg. So, now the question remains - what is the developmental role of ESR1 in the male reproductive track?
Only a handful of studies have looked at developmental aspects of ESR1 in male reproduction (Bartlett et al., 2001; Delbes et al., 2005; Eddy et al., 1996; Goulding et al., 2010; Lee et al., 2008; Lee et al., 2009). Delbes et al. (2005) has been the only one to examine testes of the fetal Esr1KO male. They found an increase in Leydig cell volume in E13.5 fetuses and PND2 neonates and increased expression of genes responsible for T synthesis (Delbes et al., 2005), which demonstrated that the increase in T in the adult was already established in the fetus. However, this increase in androgen was incapable of making up for the loss of ESR1 downstream in the efferent ducts and epididymis. Their study also contained a rather important experiment related to DES, which showed that the fetal testis “is more sensitive to estrogens than the neonatal testis…”, as DES treatment in vitro decreased basal T secretion in Esr1KO testes at E13.5 but had no effect on PND2 testes. However, in both fetal and neonatal wild-type testes, DES did decrease LH stimulation of T secretion, but had no effect in the Esr1KO. Thus, estrogen can have direct effects on the fetal and neonatal testes and decrease T synthesis, once there is LH production beginning around E16 in the mouse (O’Shaughnessy et al., 1998). Furthermore, this supports the idea that endogenous estrogen production by fetal Sertoli cells will have an inhibitory effect on fetal Leydig cells and T production and treatment with estrogens after E17.5 can greatly reduce androgen synthesis (Dorrington and Armstrong, 1975; Majdic et al., 1996).
Although treatment with the potent antiestrogen in adult rodents resulted in pathological changes nearly identical to those observed in the adult Esr1KO male (Cho et al., 2003; Hess et al., 1997a; Oliveira et al., 2005; Oliveira et al., 2004; Oliveira et al., 2003; Oliveira et al., 2002; Zhou et al., 2001), examination of PND10–20 tissues in Esr1KO mice confirmed the possibility that testicular and epididymal phenotypes were indeed developmental anomalies (Eddy et al., 1996; Lee et al., 2008; Lee et al., 2009). Between PND10–20, dilation of the rete testis had already begun, as well as luminal dilation in the seminiferous tubules. However, recently discovered archival images of Esr1KO testis revealed no differences in the seminiferous epithelium even closer to birth, as the lumens were closed and Sertoli and germ cells appeared normal at PND6 (Fig. 4).
Fig 4.
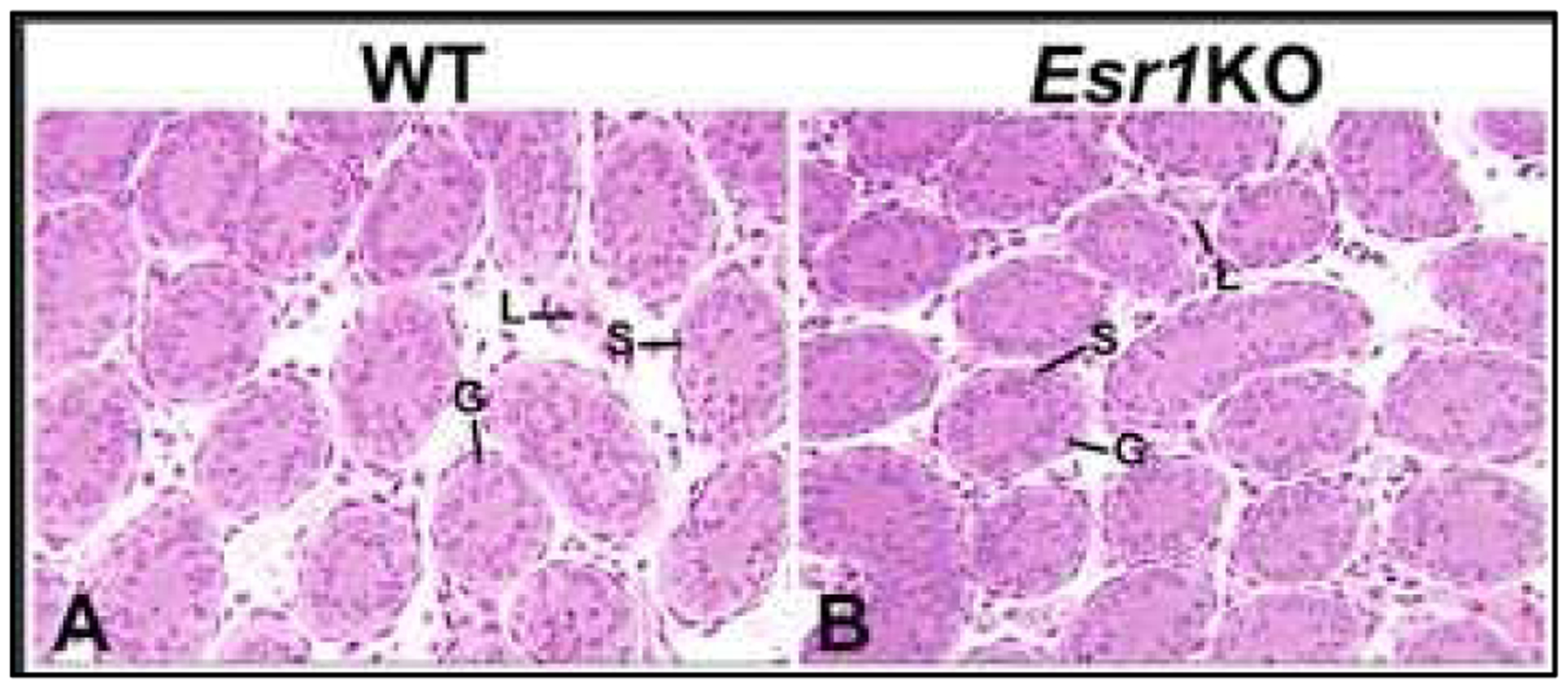
Testes from PND6 wild-type (WT) and Esr1KO mice. A. WT seminiferous tubules are small in diameter with no lumen and an epithelium consisting of Sertoli and germ cells. Leydig cells are seen in the interstitial space. B. Seminiferous tubules and interstitial space in the Esr1KO mouse are similar to WT at this age and show no formation of a lumen.
The original report on Esr1KO mice did not find effects in the efferent ductules and epididymis at any age from PND10–60, although there was extreme dilation of the rete testis and seminiferous tubule lumens (Eddy et al., 1996). However, subsequent studies found dramatic dilation of efferent ductule lumens in the adult mice (Fig. 5) and reduction in epithelial height of greater than 50% (Hess et al., 2000; Lee et al., 2000; Lee et al., 2009). Thus, the question remained whether the long-term pathological changes were of developmental origin, especially since antiestrogen treatment with ICI 182,780 in adult male mice and rats had nearly identical effects in the testis, rete testis and efferent ductules (Cho et al., 2003; Lee et al., 2000; Oliveira et al., 2005; Oliveira et al., 2001; Oliveira et al., 2002; Zhou et al., 2001). There were also epididymal effects in both the Esr1KO mice and antiestrogen-treated mice and rats (Cho et al., 2003; Hess et al., 2000; Hess et al., 2011). However, these epididymal effects have been ignored in the literature, but in reality, provide rather strong evidence that ESR1 has a presence and activity in specific cell types of the epididymis and maybe during fetal development, as discussed in section 3.
Fig 5.
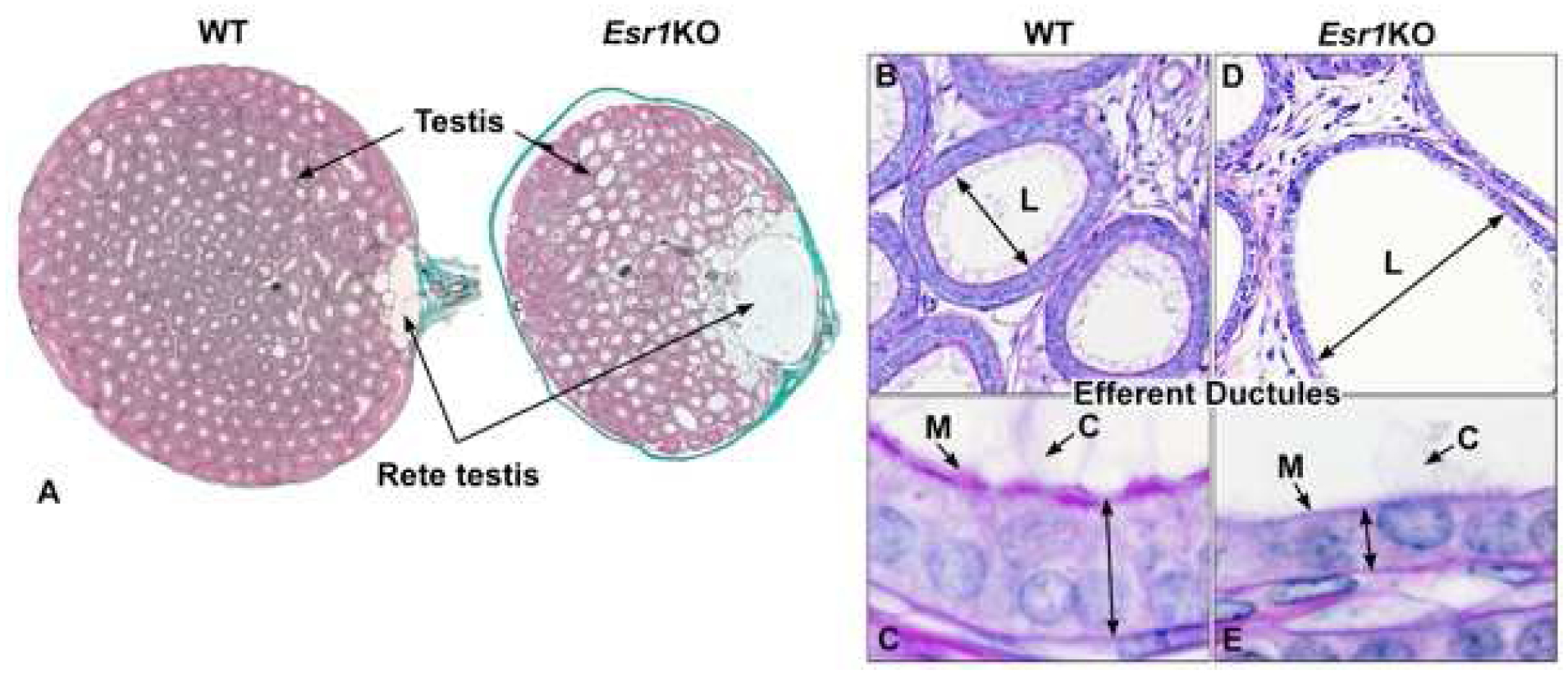
Testis, rete testis and efferent ductules in the adult wild-type (WT) and Esr1KO mice. A) WT testis shows normal seminiferous tubular lumens with a small flattened rete testis where sperm exit to enter the efferent ductules. In contrast, the rete testis in an Esr1KO mouse shows excessive dilation. B) WT efferent ductules showing a normal luminal (L) diameter. C) Enlargement of the WT epithelium to illustrate normal height, long motile cilia (C) extending into the lumen and adjacent thick microvillus boarder (M) of the nonciliated, resorptive cells that are responsible for reabsorption of nearly 90% of the luminal fluids. D) Esr1KO efferent ductule, showing hyperdilation of the lumen (L). E) Higher magnification of the Esr1KO epithelium, which shows the dramatic decrease in height and loss of apical cytoplasm and microvilli (M) in the resorptive cells. Motile cilia (C) extend into the lumen, but appear to be fewer in number and shorter in length. Adapted from figures in Nanjappa et al. (2016) and Hess et al. (2011) with permission from Oxford University Press and John Wiley and Sons.
In a subsequent study, PND10 and 18 were examined to determine if the observed adult phenotype, showing efferent ductule effects, was indeed present during postnatal development. Lee et al. (2008; 2009) found that as early as PND10 there was already luminal dilation of the ductules, as well as a reduction in epithelial height. They also found specific alteration in the expression of ion transporters necessary for the efferent ductules to perform their physiological function of fluid reabsorption. Recently discovered photos confirmed these published morphological findings and moved the efferent ductule dilation phenotype to as early as PND6 (Fig. 6). Thus, it appears that the male reproductive tract phenotype associated with the loss of ESR1 is an early developmental alteration, with early expansion of rete testis and efferent ductal lumens, with thinning of their respective epithelia (failure of differentiation). These neonatal morphological effects were accompanied by decreases in the expression of cystic fibrosis transmembrane conductance regulator (Cftr), chloride anion exchanger, Slc26a3, and ATPase-α1 (Lee et al., 2008). Although the water channels AQP1 and 9 were not studied in neonatal Esr1KO males, it is likely that these will also be found decreased in expression, because they are expressed even in the fetal and neonatal efferent ductules (Fisher et al., 1999; Fisher et al., 1998) and have major roles in the movement of water from lumen to the interstitium (Verkman, 2002).
Fig 6.
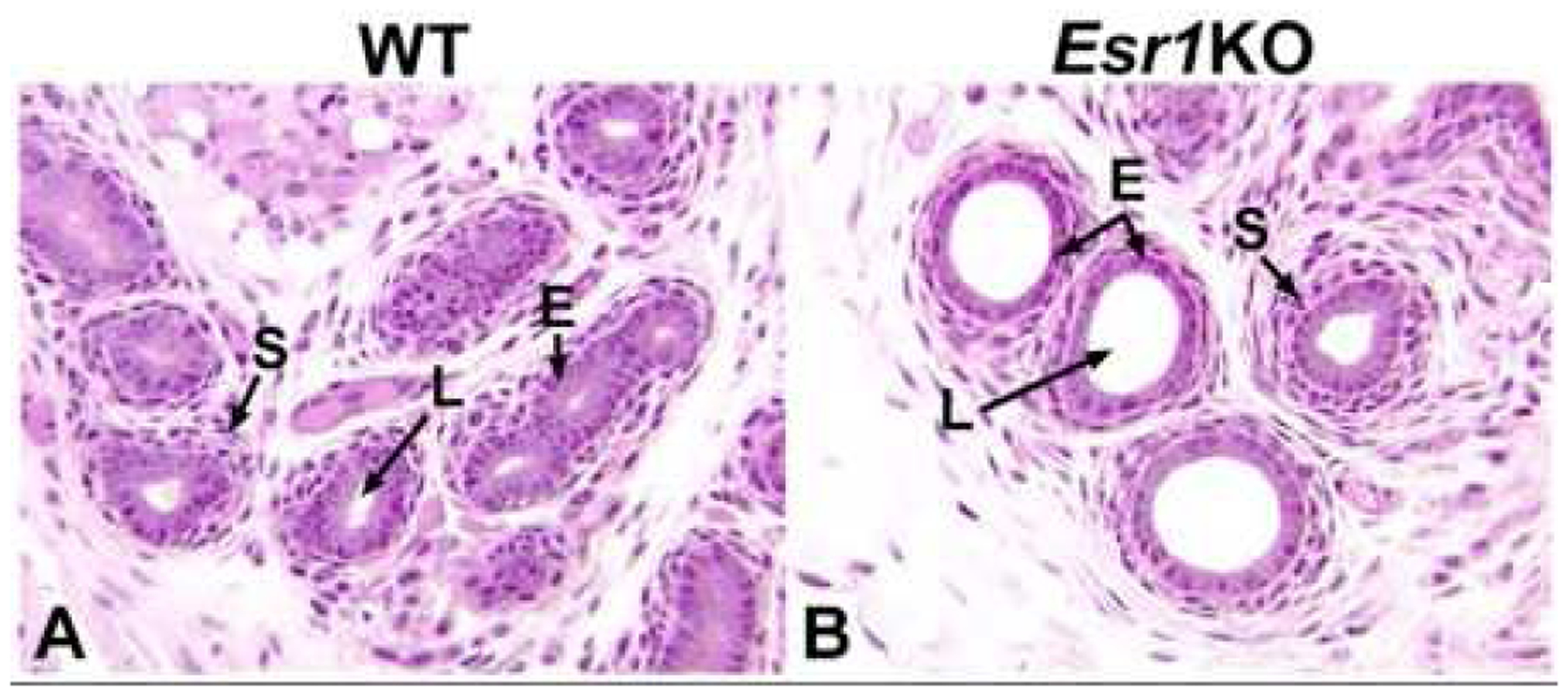
Efferent ductules from PND 6 wild-type (WT) and Esr1KO mice. A) WT ductules have small diameters with tiny, but open lumens (L). The epithelium is short but taller than in the Esr1KO and the surrounding stromal cells make up about two layers. B) Efferent ductules from the Esr1KO mouse show strong luminal (L) dilation at this early age and the epithelium (E) is already decreased in height. The stromal cells appear normal.
We do not know as yet if the rete testis expansion is due to the build-up of luminal fluid that is not reabsorbed by the efferent ductule epithelium or simply excessive proliferation of the epithelium. Further study of fetal and neonatal Esr1KO mice is required, as current publications are confusing and appear to support both hypotheses. For example, secretion by the seminiferous epithelium does not begin until approximately PND15 in the mouse (Auharek and de Franca, 2010; Gondos and Berndston, 1993; Nagano and Suzuki, 1976); therefore, the dilation of rete testis at or before birth due to accumulated seminiferous tubular fluid would not occur. Also, if fluid were to somehow leak into the rete testis lumen, an ESR1 mechanism seems missing because most studies have reported rete testis epithelium lacking ESR1 expression in both fetal and neonatal mice and rats (Nielsen et al., 2000; Sar and Welsch, 2000). However, during the developmental period, AR expression is abundant in rete testis epithelium (Magers et al., 2016; Williams et al., 2001a; You and Sar, 1998) and high doses of DES and other estrogens inhibit efferent ductule development (see section 6).
It was puzzling to find that perinatal treatments with DES or E2 induce the same expansion/dilation of rete testis lumen (Aceitero et al., 1998; Fisher et al., 1999; Fisher et al., 1998; Naito et al., 2014; Rivas et al., 2002; Rivas et al., 2003) as is found in the Esr1KO mouse and the anti-estrogen treated adult mice and rats (Cho et al., 2003; Hess, 2014; Hess et al., 1997a; Lee et al., 2000; Lee et al., 2009; Oliveira et al., 2002). Also, DES effects on prostate and seminal vesicles have been shown to be ESR1-dependent, but mediated through a permanent decrease in AR expression, as shown in DES-treated Esr1KO mice (Cederroth et al., 2007; Couse and Korach, 2004; Prins et al., 2001a; Walker et al., 2012). Therefore, DES effects on the rete testis must be further investigated, as there are no reports of ESR1 expression in the fetal mouse rete epithelium (Table 2). Table 6 lists prominent effects associated with efferent ducts and rete testis in the Esr1KO and antiestrogen treated rodents and other neonatal estrogen treatment models.
Table 6.
Comparison of Esr1KO and Neonatal Estrogen Treatment Models1
| Esr1 KO | Anti-estrogen ICI | High DES | 2High DES + T | High E2 | Low DES + GnRHa or Flutamide | GnRHa | Flutamide | |
|---|---|---|---|---|---|---|---|---|
| AR | − | − | ↓ | − | ↓ | ↓ | − | sl↓ |
| ESR1 | ↓ | ↓ | - | - | -/↓ | - | - | |
| T | ↑ | -- | ↓ | ↑ | ↓ | ↓ | ↑ | |
| AQP1 | ↓ | ↓ | ↓ | ↓ | - | |||
| AQP9 | ↓ | ↓ | ↓ | - | sl↓ | ↓ | ↓ | |
| Rete testis dilation | ↑ | ↑ | ↑ | - | ↑ | ↑ | - | - |
| ED dilation | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | - | - |
| References3 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
Abbreviations: -, no effect; ↓, decreased effect; ↑, increased effect; sl, slight effect; AR, androgen receptor; ESR1, estrogen receptor 1; T, testosterone; E2, estradiol; AQP1,9, aquaporin; ED, efferent ductule; KO, knockout mouse; ICI, antiestrogen ICI 182,780; DES, diethylstilbestrol; GnRH, gonadotrophin-releasing hormone antagonist
Testosterone treatment increases serum T, but also increases dihydrotestosterone (DHT) and E2, due to metabolic conversions (Amory et al., 2008; Robaire and Hamzeh, 2011). The increase in E2 would be nominal compared to the high dose of DES.
References:
Esr1KO: (Arao et al., 2012; Eddy et al., 1996; Goulding et al., 2010; Hess et al., 1997a; Hess et al., 2000; Lee et al., 2000; Lee et al., 2009; Ruz et al., 2006; Zhou et al., 2001)
ICI 182,780: (Cho et al., 2003; Lee et al., 2000; Oliveira et al., 2005; Oliveira et al., 2001; Oliveira et al., 2003; Oliveira et al., 2002)
High dose DES: (Atanassova et al., 2005b; Fisher et al., 2002; Fisher et al., 1999; McKinnell et al., 2001; Pastor-Soler et al., 2010; Rivas et al., 2002; Sharpe et al., 1998; Sharpe et al., 2003)
High dose DES + T: (Atanassova et al., 2005b; Pastor-Soler et al., 2010; Rivas et al., 2003)
High dose E2: effect may depend on age (Fisher et al., 2002; Fisher et al., 1999; Naito et al., 2014; Pastor-Soler et al., 2010)
Low dose DES + GnRHa or Flutamide: (Rivas et al., 2002)
GnRH antagonist: (Fisher et al., 2002; McKinnell et al., 2001; Pastor-Soler et al., 2010; Rivas et al., 2002; Sharpe et al., 2003)
Flutamide: (Atanassova et al., 2005b; Fisher et al., 2002; McKinnell et al., 2001; Rivas et al., 2002)
Neonatal high doses of DES work through its ability to down-regulate AR in the male reproductive system, along with a decrease in serum T, with no effect on ESR1 in efferent ductal epithelium, although changes in ESR1 expression in the cauda epididymis and initial part of the vas deferens have been observed (Williams et al., 2000). However, DES treatment may involve mechanisms that differ in the neonate compared to the adult male, because high doses of E2 in the adult result in nearly a total loss of both ESR1 and AR in the efferent duct epithelium (Oliveira et al., 2004) and down-regulation of AQP1(Fisher et al., 1998; Oliveira et al., 2005; Oliveira et al., 2004). These data are difficult to understand because castration alone removes efferent ductule AR expression but has no effect on the expression of AQP1, while causing a total loss of AQP9 (Oliveira et al., 2005). Although there is a loss of AR expression, castration also has no effect on ESR1. Interestingly, castration supplemented with either T or E2 rescued AQP9 and AR expressions (Oliveira et al., 2004). Thus, some genes in the male tract are responsive to both androgens and estrogens and some have both EREs and AREs (estrogen and androgen response elements) in their promoter regions, such as Slc9a3 and Slc9a3R1 (Snyder et al., 2009; Trepos-Pouplard et al., 2010; Yao et al., 2017). Both of these genes, as well as AQP9, are important for fluid reabsorption in the efferent ductules and it appears that their regulation requires some type of combination of AR and ESR1 expression and normal levels of only T, as E2 levels appear to be irrelevant for efferent duct development in the aromatase KO mouse (Robertson et al., 1999; Robertson et al., 2002; Toda et al., 2008).
Aromatase (Cyp19) Knockout mouse
The phenotype observed in the Esr1KO mouse (Hess et al., 1997a) pointed to the potential importance of “female hormones” in the male (Sharpe, 1997). We all assumed that E2 would be essential for development and differentiation of the male reproductive system, because aromatase is expressed in the testis from fetal life and an abundance of ESR1 and 2 are found throughout the system. It was assumed that the aromatase (Cyp19) knockout (AromKO) male mouse (Robertson et al., 1999; Robertson et al., 2002; Robertson et al., 2001) would have a phenotype comparable to that of the Esr1KO male (Table 5). However surprisingly, the AromKO male showed normal development of testes, rete testis, efferent ductules and epididymis (O’Donnell et al., 2001; Robertson et al., 1999; Robertson et al., 2001; Toda et al., 2001) and normal to subfertility due to impaired mounting behavior (Honda et al., 1998). Testicular effects were observed only after aging and with phytoestrogen-free diets (Robertson et al., 2002). The only developmental reproductive effect reported was mild hypospadias (Cripps et al., 2019). Even with aging, the rete testis, efferent ductules and epididymis remained normal, suggesting normal physiology in the reproductive tract.
The AromKO phenotype remained perplexing for many years, as the loss of E2 synthesis in the male had very little effect on the reproductive system. This caused some scientists to ignore the extensive work with the Esr1KO male and treatments with a potent antiestrogen (Tables 5 and 6). However, this unexpected phenotype also raised many more questions. Answers to these questions finally began to arrive when Toda et al. (Toda et al., 2008) discovered that ESR1 expression remained abundant in efferent ductules of the AromKO. Thus, it appears that expression of ESR1 in the absence of E2 is capable of rescuing the male reproductive tract morphology and physiology. This finding was consistent with a previous study showing that ESR1 expression was constitutive in these ducts and present even after bilateral castration (Oliveira et al., 2004). Oliveira et al. also showed that only a high dose of E2 in the adult male was able to reduce ESR1 expression after castration, as treatments with low and high doses of T or dihydrotestosterone (DHT) showed no effect on ESR1. Several plausible explanations for lack of abnormalities in the AromKO male reproductive tract have been presented (Hess and Cooke, 2018; Toda et al., 2008) and include the following: a) ligand-independent transcriptional activation of the ESR1; b) binding of a testosterone metabolite, 5α-androstane- 3β-17β-diol, (although it has higher affinity for ESR2 (Kuiper et al., 1997; Weihua et al., 2002) but was found to be as effective as E2 in maintaining post-castration efferent ductal function (Picciarelli-Lima et al., 2006); c) maternal estrogen during fetal development and the postnatal period through the milk (Toda et al., 2008); and d) androgen regulation of key factors that contain both EREs and AREs, as previously mentioned. Although we do not have a full explanation for the AromKO, the requirement of ESR1 expression in the male tract still remains, as the presence of AR alone is not sufficient for development and differentiation of these tissues.
Other animal models
Several other animal models either directly or indirectly affect the expressions of Esr1 or estrogen synthesis and metabolism and in some cases have phenotypes that resemble the Esr1KO male (Table 5). For example, in the CEARKO mouse there is conditional deletion of AR in the caput epididymal epithelium, which results in failure to develop the initial epididymal segment, but also ‘induces’ the expression of ESR1 throughout the caput epididymal epithelium (Krutskikh et al., 2011; O’Hara et al., 2011). It appears that this overexpression of ESR1 causes excessive fluid reabsorption leading to luminal occlusion and inflammation, fluid backup and dilation of rete testis and seminiferous tubules. As in the Esr1KO mouse, this results in long-term testicular atrophy. In the Lgr4 mutant and Lgr4KO mice the major effect was in the efferent ductules rather than epididymis. In this model, ESR1 expression was decreased, but there was no effect on AR, and as expected the phenotype also resembled that of the Esr1KO male, including decreased expression of AQP1, SLC9A3 and Na/K-ATPase α1 (Hoshii et al., 2007; Li et al., 2010; Mendive et al., 2006). In another model, conditional deletion of E2F4/5 transcription factors in only the efferent ductules, caused results that were also Esr1KO-like. Here again, there was a reduction in ESR1 expression and loss of AQP1 in the ductal epithelium (Danielian et al., 2016). Dilation of the rete testis in the E2F model was observed during the first week after birth, suggesting that this rete testis phenotype could be solely of efferent ductal origin. Although down regulation of ESR1 in the conditional E2f4/5 model was small, it is possible that the effect was mediated through non-genomic mechanisms involving the membrane ESR1. A new transgenic model, in which only a nuclear ESR1 is found (NOER) and membrane ESR1 localization is lost, has a phenotype that is essentially identical to that of global deletion of Esr1 (Nanjappa et al., 2016; Pedram et al., 2014). Thus, disruption of either the nuclear or membrane localization of ESR1 will inhibit development of the rete testis, efferent ductules and epididymis.
Summary of Animal Models
Disruption of ESR1 expression in efferent ductules and epididymis is clearly a major factor in development and differentiation of the male reproductive tract and in long-term production of sperm by the testis, which can lead to permanent infertility. However, loss of the natural ligand, E2, as shown in the AromKO mouse, does not equate to the Esr1KO and further study is required for a more complete understanding of the mechanisms involved with ligand-independent transcriptional activation of ESR1. Membrane ESR1 turns out to be as important as the nuclear localization of the receptor in efferent ductules and epididymis and its disruption requires further investigation to better understand how environmental factors could disrupt this pathway. Finally, the question of how fetal and neonatal DES exposure causes rete testis dilation has remained unanswered for many years. However, the E2f4/5 knockout model demonstrated that disruption of only the efferent ductules (using the villin-cre, which is specific for efferent ducts in the male tract) can cause rete testis dilation, beginning as early as the first week after birth, which is identical to that observed in the Esr1KO mouse. Thus, neonatal DES treatment effects in the efferent ductules alone could induce rete testis dilation, but the mechanisms involved require further investigation, as the source of fluid accumulation in the lumen in the perinatal period is not yet known.
6. Effects of developmental exposures to exogenous estrogens
Estrogen-induced male reproductive tract abnormalities
In males, E2 production and action must be considered primarily on a local basis, i.e., E2 is produced locally to act locally. Although E2 in the general circulation can have systemic effects in males (e.g., on the hypothalamus-pituitary unit), it is rare for circulating E2 levels to be high enough to be capable of such actions. Within the male reproductive tract there are several well-documented sites of E2 production and action, as detailed elsewhere in this paper, and there is no shortage of substrate (testosterone) for the aromatase enzyme as the level of T in the testis and along the male reproductive tract is higher than anywhere else in the body. Indeed, it is unlikely that lack of substrate will be a determining factor in local E2 levels in the male reproductive tract, at least in a normal adult male. Thus, the most important factor determining the local production of E2 will be the level of local expression of aromatase.
Interest in this area was awakened by evidence that prolonged treatment of pregnant women with DES in high doses (100–2300 μg/kg/day, depending on the stage of pregnancy (Palmer et al., 2009)) was associated with increased incidence of reproductive disorders in resulting sons; this included ~7-fold increase in incidence of urethral stenosis, ~4-fold increase in incidence of epididymal cysts and increased incidence of smaller testes and cryptorchidism, plus other evidence for penile and prostatic (squamous metaplasia) abnormalities (reviewed in: Toppari et al., 1996). However, a more recent follow-up cohort study of 1197 adult men who had been exposed chronically to DES in utero (Palmer et al., 2009), did not report such a high incidence of reproductive tract disorders compared with controls (n=1038), only finding significant (2- to 2.5-fold) increases in risk of cryptorchidism, epididymal cysts and inflammation of the testis.
Most of the experimental evidence for adverse effects of estrogens on the developing testis and male reproductive tract have involved exposure of rats and mice to high doses of the potent estrogen DES, and in some cases ethinyl estradiol (EE). Exposure has been either in pregnancy or immediately after birth in male neonates and, in general, comparable adverse changes have been reported in both developmental time periods. Before summarizing these effects, it is important to recognize some caveats. Administration of high doses of DES to pregnant rodents can cause adverse pregnancy outcomes, in particular reducing pup numbers and causing dystocia; the latter is particularly common in rats such that most studies of the effects of fetal exposure to DES on later reproductive structure and function in both males and females have used mice, which are less prone to dystocia. Because of this confounding effect, studies of DES effects on reproductive development in rats have generally used neonates.
High dose DES exposure of rats in neonatal life results in the following adverse changes in the reproductive tract: smaller testes (Atanassova et al., 2000; Atanassova et al., 1999; Goyal et al., 2003); reduced sperm count (Goyal et al., 2003) and fertility (Atanassova et al., 2000); fluid distension of the rete testis and efferent ducts (McKinnell et al., 2001; Rivas et al., 2002; Rivas et al., 2003) (Fig. 7); relative overgrowth of stromal tissue and relative undergrowth of epithelial tissue in the epididymis, vas deferens, prostate and seminal vesicles (Rivas et al., 2002; Rivas et al., 2003; Williams et al., 2001a; Williams et al., 2001b); smaller penis, hypospadias and reduced anogenital distance (Stewart et al., 2018). Some of these effects (e.g., prostate) are discussed in more detail elsewhere (Dobbs et al., 2019; Prins and Ho, 2010). Of all the DES-induced effects, perhaps the most dramatic is the gross distension of the rete and efferent ductules (Fig. 5), the latter change being comparable to what is also observed in Esr1KO mice (section 5).
Fig. 7.
Effect of neonatal treatment with vehicle (control; A), a low dose (0.1 μg; B) or a high dose (10 μg; C) of diethylstilbestrol (DES), a GnRH antagonist (GnRHa; D), 0.1 μg DES plus GnRHa (E) or 10 μg DES + 200 μg testosterone esters (TE) (F) on the size of the rete testis lumen (arrows) at postnatal age 15 d. Note that all sections were obtained in the region in which the efferent ducts (asterisks) emerged from the rete along the testis capsule. Sections were immunostained for ESR1, hence the dark staining of nuclei of epithelial cells in the efferent ducts. Scale bar, 400 μm. Adapted from figures in Rivas et al (2002) and McKinnell et al (2001) with permission from Oxford University Press and John Wiley and Sons.
Detailed analysis of the DES-induced abnormalities in the epididymis and vas has shown aberrant induction of epithelial ESR1 expression, coincident with gross reduction in epithelial cell height, a change that may indicate faulty cellular specification, i.e. epithelium appears flattened rather than columnar (Fig. 8). Consistent with this, the normally distinct boundary that demarcates the intense coiling of the distal cauda epididymis from the straight duct of the vas deferens is blurred in rats exposed neonatally to high dose DES, such that in most animals the initial part of the vas deferens is coiled rather than straight (Atanassova et al., 2005a; Atanassova et al., 2001). The aberrant changes in stromal and epithelial tissue in the epididymis and vas deferens of DES-exposed rats also predisposes to inflammatory changes in the epididymis (Atanassova et al., 2005a; Miyaso et al., 2014), similar to those seen in the prostate (Bianco et al., 2006; Nelles et al., 2011; Prins et al., 2001b; Prins et al., 2006) and in testes of transgenic mice that overexpress aromatase (Li et al., 2006). Although virtually all of these abnormal changes were only induced after exposure to very high doses of DES, and were not inducible by high doses of weaker environmental estrogens such as bisphenol A and octylphenol (Atanassova et al., 2000; Rivas et al., 2002; Rivas et al., 2003; Williams et al., 2001a; Williams et al., 2001b), the conclusion from such studies is that locally produced estrogens, in physiological levels, probably play a fundamental role together with androgens in orchestrating normal development of the male reproductive tract from fetal life through to adulthood.
Fig. 8.
Effect of neonatal treatment of rats with vehicle (controls) or a high dose of diethylstilbestrol (DES; 10 μg) on immunoexpression of ESR1 (left-hand panels) and AR (right-hand panels) on postnatal day 18 in the efferent ductules (top row), caput epididymis (middle row) and proximal cauda/proximal vas deferens (bottom row). Note that, unlike in controls (A), epithelial expression of ESR1 in DES-treated rats extends into the proximal (J) regions of the vas deferens. Immunoexpression of ESR1 was unaffected in the efferent ducts (A vs. B), caput (E vs. F), and proximal cauda (I vs. J). Note also the reduction in height of the epithelium in the efferent ducts (A vs. B) and proximal vas deferens of DES-treated rats (I vs. J and K vs.L). DES also induced luminal distension of the efferent ducts (asterisks; A vs B). DES treatment profoundly reduced both epithelial and stromal expression of AR in the efferent ducts (C vs. D), caput epididymis (G vs. H) and proximal cauda and vas (K vs. L). Insets in panels C and E show negative controls in which the primary antibody was preabsorbed with the appropriate recombinant protein. Arrowheads point to stromal immunoexpression of ESR1 in the proximal vas (I and J). Scale bars, 50 μm. Adapted from figures in Atanassova et al 2001 and McKinnell et al (2001) with permission from Oxford University Press and John Wiley and Sons.
Considering that in rodent perinatal studies only doses of DES in excess of ~10μg/kg/day seem to result in adverse reproductive tract changes (Fielden et al., 2002; Goyal et al., 2003; LaRocca et al., 2011; Rivas et al., 2002; Williams et al., 2001a), this would imply that, based solely on estrogenicity, exposure of the developing male fetus to human-relevant levels of estrogenic environmental chemicals would not induce adverse reproductive development, as these are uniformly very weak estrogens. Consistent with this interpretation, rodent experimental studies have shown that perinatal exposure to bisphenol A (Goodman et al., 2009; LaRocca et al., 2011; Rivas et al., 2002; Williams et al., 2001a; Williams et al., 2001b), octylphenol or butylphenol (Haavisto et al., 2003; Rivas et al., 2002; Williams et al., 2001a; Williams et al., 2001b) at doses far in excess of normal human exposure are unable to replicate any of adverse effects of DES on male reproductive tract development. However, this does not exclude the possibility that a weak environmental estrogen might cause effects in man via a non-estrogen dependent mechanism, such has been mooted for bisphenol A (N’Tumba-Byn et al., 2012).
Considering that the doses of DES administered to pregnant women (100–2300 μg/kg/day) are equivalent to or higher than doses that cause adverse changes in perinatal rats, it is perhaps surprising that far greater adverse human effects were not reported. For example, administration of 50 or 100 μg DES/kg/day to pregnant rats resulted in 91% and 89–96.5% suppression of intratesticular testosterone levels (Haavisto et al., 2003; Mitchell et al., 2013), changes which would grossly impair normal masculinization in rats with a >50% incidence of hypospadias and cryptorchidism (van den Driesche et al., 2017). In reality, chronic DES exposure of the human male fetus resulted in only a modest, but significant, increase in incidence of cryptorchidism (Palmer et al., 2009), an increased incidence of epididymal cysts and small testes (Palmer et al., 2009; Wilcox et al., 1995) but no apparent impact on fertility (Wilcox et al., 1995). These findings could be interpreted as evidence that the human male fetus is relatively resistant to high DES exposure in pregnancy, which may be because the human fetus, unlike the rodent fetus, is normally exposed to very high endogenous E2 levels as part of normal pregnancy and is presumably adapted to this. Thus, E2 levels in human pregnancy range from ~500pg/ml in early pregnancy to 20,000pg/ml in late pregnancy (Kuijper et al., 2013), whereas in rats and mice E2 levels are in the range 20–120 pg/ml throughout pregnancy (Camacho-Arroyo et al., 2018).
Mechanisms underlying estrogen-induced male reproductive tract abnormalities
There appear to be 3 changes that underlie DES-induced male reproductive tract abnormalities: elevated estrogen levels, reduced testosterone levels and reduced androgen receptor (AR) expression (Fig. 8).
Irrespective of whether DES exposure in rats has been before or after birth, the results show that high doses of DES markedly suppress testosterone production by the testis. Thus, administration of 50 or 100μg DES/kg/day to pregnant rats resulted in >90% suppression of fetal intratesticular testosterone levels (Haavisto et al., 2003; Mitchell et al., 2013), whereas treatment of neonatal rats from days 2–12 with ~370μg DES/kg/day resulted in ~75% suppression of plasma testosterone levels on postnatal day 15 (Rivas et al., 2002), a reduction that was still evident in adulthood (Atanassova et al., 1999; Goyal et al., 2003). Lower doses of DES caused proportionately smaller effects on testosterone levels; for example, 3.7μg DES/kg/day resulted in ~35% lowering of testosterone levels on day 15 and in adulthood. DES is equally able to suppress testosterone production by the fetal mouse testis (N’Tumba-Byn et al., 2012), but not by the human fetal testis, whether in culture (Habert et al., 2014; N’Tumba-Byn et al., 2012) or after xenografting into recipient mice (Mitchell et al., 2013); this species difference is discussed further below.
Mouse transgenic studies suggest that the adverse effects of DES/EE exposure on male reproductive tract development are mediated via ESR1. Thus, in Esr1KO mice, the adverse effects of perinatal DES exposure on development of the prostate (Couse and Korach, 2004) and seminal vesicles (Walker et al., 2012) do not occur, whereas they do in wild-type littermates. A more recent study (Nanjappa et al., 2019) suggested that selective knockout of membrane, but not cytosolic, ESR1 expression also protects against DES effects. From a physiological perspective, the evidence suggests that intratesticular E2 acts via ESR1 in fetal Leydig cells to negatively regulate Leydig cell size, steroidogenic enzyme expression and testosterone production, as all of these increase in Esr1KO mice (Delbes et al., 2005). This is reinforced by observations in the aromatase over-expressing mouse in which endogenous E2 levels are drastically elevated, as the males show grossly reduced testosterone levels, cryptorchidism and reproductive tract and prostate changes that are broadly similar to those observed in mice exposed developmentally to DES (Li et al., 2001).
The protective effect of Esr1KO against perinatal DES effects in mice has been studied in far more detail in female mice compared with male mice, but it is reasonable to presume that all of the adverse male reproductive tract effects of DES in mice are mediated via ESR1 (Couse and Korach, 2004). This is a pivotal observation, especially when assessing the translatability of mouse findings to the human. For example, as outlined above, one of the primary underlying causes for DES-induced adverse male reproductive effects in rodents is suppression of perinatal testicular testosterone production (Delbes et al., 2005; Haavisto et al., 2003; Mitchell et al., 2013; Rivas et al., 2002; Rivas et al., 2003), an effect presumed to be mediated via the expression of ESR1 in rodent Leydig cells (Tables 2, 3 and Mitchell et al., 2013). In contrast to the rodent testis, Leydig cells in the fetal human testis do not express ESR1 and, accordingly, are resistant to the adverse steroidogenic effects of DES seen in rodents; this has been demonstrated in human fetal testis xenografts (Mitchell et al., 2013) and in human fetal testis cultures (reviewed in: Habert et al., 2014). From a physiological perspective, it perhaps makes sense that human fetal Leydig cells lack sensitivity to estrogen negative effects because of the 10-to100-fold higher circulating levels of E2 during human versus rodent pregnancy as outlined above.
Table 3.
Neonatal androgen and estrogen receptors in the human, rat and mouse male reproductive system
| Tissue | Receptor | Presence | Species | Neonatal Age/Type of Study1 | References |
|---|---|---|---|---|---|
| Whole testis | ESR1 | − | Human | 12–60 mo: mRNA | (Berensztein et al., 2006) |
| +++ | Mouse | PND 1,5,12,19 mRNA | (Jefferson et al., 2000) | ||
| ESR2 | +++ | Human | 12–60 mo: mRNA | (Berensztein et al., 2006) | |
| +++/+ | Mouse | PND 1,5 +++; PND 12,19 + mRNA | (Jefferson et al., 2000) | ||
| Sertoli cell | AR | − | Human | <22d-7 mo | (Berensztein et al., 2006) |
| − | Rat | PND 2 | (You and Sar, 1998) | ||
| +/++ | Rat | PND 5–14 Increasing | (Bremner et al., 1994; Majdic et al., 1995; You and Sar, 1998) | ||
| ++ | Rat | PND 15–18 | (McKinnell et al., 2001; Suarez-Quian et al., 1996; Williams et al., 2001 a) | ||
| − | Mouse | PND 2 | (Tan et al., 2005) | ||
| ++ | Mouse | PND 10–12 | (De Gendt et al., 2009; Denolet et al., 2006; Tan et al., 2005) | ||
| ESR1 | − | Human | <22 wks | (Berensztein et al., 2006) | |
| − | Rat | PND 1–10 | (Fisher et al., 1997; Sar and Welsch, 2000) | ||
| ++ | Rat | PND 15 mRNA Primary culture | (Lucas et al., 2008) | ||
| + | Rat | PND 15 | (Lucas et al., 2008) | ||
| − | Mouse | PND 1–19 | (Jefferson et al., 2000; Nielsen et al., 2000) | ||
| ESR2 | +++/+ | Human | <22d-7 mo decreasing | (Berensztein et al., 2006) | |
| +/++ | Rat | PND 3–15 | (Lucas et al., 2008; Saunders et al., 1998; van Pelt et al., 1999) | ||
| ++ | Rat | PND 15 mRNA | (Lucas et al., 2008) | ||
| Germ cells/gonocytes | AR | − | Human | <22d-7 mo decreasing | (Berensztein et al., 2006) |
| − | Rat | PND 3–7 | (Majdic et al., 1995) | ||
| ESR1 | − | Human | <22d-7 mo | (Berensztein et al., 2006) | |
| + | Rat | PND 7 mRNA | (Mowa and Iwanaga, 2001) | ||
| − | Rat | PND 1–10 | (Fisher et al., 1997; Sar and Welsch, 2000) | ||
| − | Mouse | PND 1–12 | (Jefferson et al., 2000; Nielsen et al., 2000; Sato et al., 1994) | ||
| ESR2 | +++ | Human | <22d-7 mo | (Berensztein et al., 2006) | |
| ++/+++ | Rat | PND 3–4 | (Saunders et al., 1998; van Pelt et al., 1999) | ||
| ++ | Rat | PND 7 mRNA | (Mowa and Iwanaga, 2001) | ||
| +/++ | Mouse | PND 5 +; PND 12 ++ | (Jefferson et al., 2000) | ||
| Leydig cells/Interstitial | AR | + | Human | <22d-7 mo | (Berensztein et al., 2006) |
| +/++ | Rat | PND 2–18 increasing | (Bremner et al., 1994; McKinnell et al., 2001; Tan et al., 2005; Williams et al., 2001a; You and Sar, 1998) | ||
| ++ | Mouse | PND 2–12 | (De Gendt et al., 2009; Denolet et al., 2006; Tan et al., 2005) | ||
| ESR | + | Mouse | PND 1 Receptor binding | (Holderegger and Keefer, 1986) | |
| ESR1 | +/− | Human | <22d-7 mo | (Berensztein et al., 2006; Mitchell et al., 2013) | |
| ++ | Rat | PND 7 mRNA | (Mowa and Iwanaga, 2001) | ||
| ++ | Rat | PND 1–10 | (Fisher et al., 1997; Nielsen et al., 2000; Sar and Welsch, 2000) | ||
| + | Mouse | PND 0–12 | (Jefferson et al., 2000; Nielsen et al., 2000; Sato et al., 1994) | ||
| ESR2 | +++/+ | Human | <22d-7 mo | (Berensztein et al., 2006) | |
| ++ | Rat | PND 3,10 | (Saunders et al., 1998) | ||
| − | Rat | PND 4, 11 | (van Pelt et al., 1999) | ||
| +/− | Rat | PND 7 | (Mowa and Iwanaga, 2001) | ||
| Peritubular myoid | AR | +++ | Human | <22d-7 mo decreasing | (Berensztein et al., 2006) |
| +/++ | Rat | PND 2–18 increasing | (Bremner et al., 1994; McKinnell et al., 2001; Suarez-Quian et al., 1996; Williams et al., 2001a; You and Sar, 1998) | ||
| ++ | Mouse | PND 2–12 | (De Gendt et al., 2009; Denolet et al., 2006; Tan et al., 2005) | ||
| ESR1 | −/+ | Human | <22d-7mo | (Berensztein et al., 2006) | |
| − | Rat | PND 1–10 | (Fisher et al., 1997; Sar and Welsch, 2000) | ||
| + | Mouse | PND 0 | (Nielsen et al., 2000) | ||
| − | Mouse | PND 1–12 | (Jefferson et al., 2000) | ||
| ESR2 | +++/+ | Human | <22d-7mo decreasing | (Berensztein et al., 2006) | |
| ++ | Rat | PND 3,10 | (Saunders et al., 1998) | ||
| − | Rat | PND 4, 11 | (van Pelt et al., 1999) | ||
| Rete testis | AR | +++ | Rat | PND 5, 18 | (Williams et al., 2001a; You and Sar, 1998) |
| ESR1 | + | Rat | PND 4–10 Epithelium +; stroma +/− | (Fisher et al., 1997) | |
| − | Rat | PND 0–2 | (Nielsen et al., 2000; Sar and Welsch, 2000) | ||
| − | Mouse | PND 0 | (Nielsen et al., 2000) | ||
| Efferent ducts | AR | +++/++ | Rat | PND 15–18 Epithelium +++/++; stroma − | (McKinnell et al., 2001; Rivas et al., 2002; Williams et al., 2001a) |
| ++ | Mouse | PND 0–10 Receptor binding: epithelium ++; stroma ++ | (Cooke et al., 1991a) | ||
| +++ | Mouse | PND 11 | (O’Hara et al., 2011) | ||
| ESR1 | +++/− | Rat | PND 0–15 Epithelium +++; stroma − | (Atanassova et al., 2001; Fisher et al., 1997; Nielsen et al., 2000; Rivas et al., 2002; Rivas et al., 2003; Williams et al., 2000) | |
| +++/− | Rat | PND 7 mRNA Epithelium +++; stroma +/− | (Mowa and Iwanaga, 2001) | ||
| +++/− | Mouse | PND 0–10 Epithelium +++; stroma − | (Lee et al., 2008; Nielsen et al., 2000; Sar and Welsch, 2000; Sato et al., 1994) | ||
| ESR2 | ++/+ | Rat | PND 10 Epithelium ++/+; stroma −/+ | (Williams et al., 2000) | |
| −/+ | Rat | PND 7 mRNA Epithelium −; stroma +/− | (Mowa and Iwanaga, 2001) | ||
| +++/++ | Mouse | PND 1–10 Epithelium +++/++; stroma +++ | (Lee et al., 2008; Sar and Welsch, 2000) | ||
| Epididymis | AR | + | Rat | PND 1, 10 Epithelium −/+; stromal + shifting to Epithelium | (You and Sar, 1998) |
| + | Rat | PND 5,10 Epithelium +; stroma + | (Bentvelsen et al., 1995) | ||
| +++ | Rat | PND 18 Epithelium +++; stroma +/− | (McKinnell et al., 2001; Williams et al., 2001 a) | ||
| +++ | Mouse | PND 11 | (Hoshii et al., 2007; O’Hara et al., 2011) | ||
| ++ | Mouse | PND 0–10 Receptor binding: epithelium ++; stroma ++ | (Cooke et al., 1991a) | ||
| ESR | − | Mouse | PND 1 Receptor binding: epithelium −; stroma +++ | (Holderegger and Keefer, 1986) | |
| + | Mouse | PND 0–10 Receptor binding: epithelium +; stroma + | (Cooke et al., 1991a) | ||
| + | Mouse | PND 0–10 Initial segment > caput-cauda | (Cooke et al., 1991a) | ||
| ESR1 | ++/− | Rat | PND 1 −8 Initial segment Epithelium ++/−; stroma +/− | (Fisher et al., 1997; Sar and Welsch, 2000) | |
| Rat | PND 1 −8 Caput to cauda Epithelium −; stroma +/− | (Fisher et al., 1997; Sar and Welsch, 2000) | |||
| − | Rat | PND 10 Epithelium −; stroma − | (Atanassova et al., 2001; Williams et al., 2000) | ||
| ++/− | Rat | PND 18 Epithelium +/−; stroma −/++ region specific | (Atanassova et al., 2001) | ||
| +++ | Mouse | PND 1–12 mRNA; strongest d12 | (Jefferson et al., 2000) | ||
| ++/+ | Mouse | PND 0–12 Epithelium ++/+; stroma +/− | (Hoshii et al., 2007; Jefferson et al., 2000; Nielsen et al., 2000; Sato et al., 1994) | ||
| ESR2 | ++/− | Rat | Epithelium ++/−, gradient caput > cauda; stroma −/+ | (Sar and Welsch, 2000; Williams et al., 2000) | |
| +/− | Mouse | mRNA | (Jefferson et al., 2000) | ||
| ++/− | Mouse | PND 7−12 Epithelium ++/−; stroma ++ | (Hoshii et al., 2007; Jefferson et al., 2000) | ||
| Vas deferens | AR | + | Rat | PND 2 Epithelium +; stroma +; PND 5 basal cells − | (Bentvelsen et al., 1995) |
| +++ | Rat | PND 18 Epithelium +++; stroma +/− | (McKinnell et al., 2001; Williams et al., 2001a) | ||
| ++ | Mouse | PND 0–10 Receptor binding: epithelium ++; stroma ++ | (Cooke et al., 1991a) | ||
| ESR | + | Mouse | PND 0–10 Receptor binding: epithelium +; stroma + | (Cooke et al., 1991a) | |
| ESR1 | − | Rat | PND 10 Epithelium −; stroma − | (Atanassova et al., 2001; Williams et al., 2000) | |
| − | Rat | PND 18 Epithelium −; stroma +++ | (Atanassova et al., 2001; Rivas et al., 2003) | ||
| +++ | Mouse | PND 1–19 mRNA | (Jefferson et al., 2000) | ||
| − | Mouse | PND 0–12 Epithelium −; stroma −/+ | (Nielsen et al., 2000; Sato et al., 1994) | ||
| ESR2 | − | Mouse | PND 1–19 mRNA | (Jefferson et al., 2000) |
Immunohistochemistry unless otherwise indicated; mRNA can be isolated RNA or insitu hybridization; Receptor binding can be autoradiography or biochemical assay binding; PND, postnatal day; mo, months
In addition to drastically lowering endogenous testosterone levels, perinatal DES exposure in rats also reduces androgen action in target reproductive tract tissues by grossly suppressing AR protein expression (McKinnell et al., 2001; Williams et al., 2001a), an effect that occurs via increased proteosomic degradation of the AR protein rather than via altered AR gene expression (Woodham et al., 2003). Consequently, DES exposure effectively blocks normal androgen action by grossly reducing both the ligand (testosterone) and its target receptor. Considering the established pivotal role of androgen action in driving the normal development and function of the male reproductive tract, it is therefore unsurprising that exposure to high doses of DES causes multiple abnormalities of the male reproductive tract in rats and mice. Indeed, if neonatal rats are co-treated with high dose DES plus T, then virtually all of the adverse reproductive tract changes induced by DES alone are prevented (Atanassova et al., 2005a; McKinnell et al., 2001; Rivas et al., 2003). This poses the question as to whether DES is, effectively, acting just like an anti-androgen? The answer to this is probably no, because in neonatal rats treated with either a GnRH agonist, to reduce testosterone production, or the AR antagonist flutamide, to block androgen action, the adverse reproductive tract changes induced by DES are not reproduced, although there is a general retardation of reproductive development (McKinnell et al., 2001; Rivas et al., 2003; Williams et al., 2001a; Williams et al., 2001b). This suggests that for DES to induce adverse reproductive tract changes in the neonatal male rat, there has to be elevated estrogen action combined with lowered androgen action, namely alteration of the androgen-estrogen (A-E) balance.
7. The androgen-estrogen (A:E) balance
The notion of the A:E balance being important fits well with two fundamentally important factors. First, all cells/tissues that express AR in the male reproductive tract also express ESR1 and/or ESR2 at some stage of development (Tables 2–4). Second, testosterone supply is essential for estrogen action as it is the substrate for formation of E2 via the aromatase enzyme. Thus, differential regulation of the four key components (testosterone, AR, aromatase, ESR1/ESR2) that underpin the A:E balance would provide a simple but dynamic means of regulating reproductive tract development and function by locally modulating the balance in A:E action, via hormone levels or respective steroid receptor expression, according to local needs. It is also easy to see how gross distortion of the A:E balance (e.g., DES treatment, aromatase or ESR1 knockout) might disrupt normal development. But how do you prove that it is the A:E balance, as opposed to just hormone over- or under-exposure?
To elucidate if disturbance of the A:E balance is the mechanism underlying the adverse effects of high dose DES treatment in neonatal rats, researchers administered a DES dose that on its own caused no major adverse reproductive tract effects, but combined it with a treatment to either reduce endogenous testosterone production (GnRH agonist) or to block endogenous androgen action using an androgen receptor antagonist (flutamide). These experimental manipulations of the A:E balance resulted in a spectrum of adverse reproductive effects similar to those induced by high dose DES on its own, although the effects were of smaller magnitude (Rivas et al., 2002). The same study also showed that when a high dose of bisphenol A was substituted for the low dose of DES in combination with the GnRH agonist, no significant adverse reproductive tract changes were found, implying that weak environmental estrogens such as bisphenol A are unable to disrupt the A:E balance sufficient to cause adverse effects in this experimental situation. Convincing though such studies are, they do not identify how the A:E balance actually operates nor what exactly constitutes a normal A:E balance.
If the A:E balance is critical for normal reproductive tract development in males, then we should be aware that this may also explain changes reported in other situations involving altered estrogen production or action. For example, in the AromKO mouse, which has negligible endogenous E2, testosterone levels are chronically elevated ~6-fold (Amano et al., 2017), resulting in gross disturbance of the A:E balance, this time in favor of androgens rather than estrogens as was the case in the DES studies outlined above, but did not result in rete testis and efferent duct abnormalities because the AR:ESR1 balance was still intact (Toda et al., 2008). In the Esr1KO mouse, which effectively blocks endogenous E2 action via this receptor (which is established to be the most important estrogen action pathway in the male reproductive tract), there is also 2- to 3-fold elevation of endogenous testosterone levels with unchanged E2 levels and zero estrogen action via ESR1 (Couse and Korach, 1999b; Delbes et al., 2005; Walker et al., 2012), again resulting in gross disturbance of the A:E balance. The similar distension of the efferent ducts and rete testis seen in Esr1KO mice (Hess, 2014; Hess and Cooke, 2018) and high dose DES-treated rats, could be interpreted as evidence that maintenance of a normal balance in A:E action underpins normal fluid resorption in these ducts, but also includes an AR:ESR1 balance (see section 5).
If the A:E balance is critical for normal male reproductive tract development and later function, how might it operate physiologically? Perhaps it is easiest to view it as a classical endocrine system involving negative feedback control as follows. Testosterone is produced by testicular Leydig cells, generating high levels within the testis and along the reproductive tract via the movement of fluid from the testis along the male reproductive tract. This movement of fluid will be very much higher in the mature than in the developing male. As T levels increase, a particular level is reached at which the aromatase enzyme, expressed within the testis in Leydig, Sertoli and germ cells according to age and stage of development (see section 5, Fig. 1, Table 1), is able to convert some of the T into E2, which then acts via ESR1 in Leydig cells to reduce testosterone production. As Sertoli cells appear to be the main source of aromatase in the perinatal testis (Carreau et al., 1999; Carreau and Hess, 2010; Hess and Cooke, 2018; O’Donnell et al., 2001; Tsai-Morris et al., 1985), this would constitute a paracrine short-loop negative feedback system. Thus, inappropriately high estrogen exposure, (e.g., perinatal DES treatment) plugs into this feedback system and grossly suppresses testosterone production, at least in rodents, as detailed above. However, what this scenario does not explain is why estrogen over-exposure also negatively regulates AR protein expression, which effectively blocks androgen action. One way of interpreting this is that it is just another facet of regulating the balance between androgen-and estrogen-action and might be particularly important in the reproductive tract where it is not possible to negatively feedback control the supply of testosterone, so instead its ability to act via AR is regulated.
At puberty and beyond, the function of the reproductive tract changes dramatically, as germ cell numbers/types in the testis increase greatly leading up to the continual release of spermatozoa and their transport out of the testis and down the reproductive tract. The arrival of sperm demands new functions from the reproductive tract, as sperm need to undergo complex changes (e.g., membrane changes) associated with their functional maturation prior to their release from the body. The fact that sperm express aromatase and are immersed in testosterone in the surrounding fluid means that they can contribute to dynamic regulation of the A:E balance and can do so in a quantitative way as aromatase activity will increase in proportion to the number of sperm present. Thus, in theory, sperm can regulate their environment as they progress along the duct via manipulation of the relative action of testosterone and E2 on the neighboring ductal epithelium or underlying stroma.
Highlights:
Development of the male reproductive tract depends on fetal connections between mesonephric tubules, the Wolffian duct and rete testis cords
Perinatal development depends on both androgen and estrogen receptor pathways
Effects of Esr1KO on rete testis and efferent ducts begin during perinatal development
High dose of estrogen (including DES) is required to produce an Esr1KO-like phenotype by reducing testosterone synthesis and androgen receptor protein
An androgen:estrogen balance appears important for normal development/function
Acknowledgements:
Supported by the Eunice Shriver National Institutes of Child Health and Human Development Grant HD093703 (BTH).
Abbreviations:
- wt
weight
- mo
months
- ESR1
estrogen receptor 1
- ESR2
estrogen receptor 2
- AR
androgen receptor
- T
testosterone
- FSH
follicle stimulating hormone
- E2
estradiol
- ED
efferent ductule
- KO
knockout mouse
- ICI
antiestrogen ICI 182,780
- DES
diethylstilbestrol
- GnRH
gonadotrophin-releasing hormone antagonist
- PPT
4,4’,4′-(4-Propyl-[1H] pyrazole- 1,3,5-triyl)
- SLC9a3
sodium/hydrogen exchanger 3
- AQP
aquaporin
- CAR2 and 14
carbonic anhydrase 2 and 14
- SLC4A4
sodium bicarbonate cotransporter
- CFTR
cystic fibrosis transmembrane conductance regulator
- GPER
G protein-coupled estrogen receptor 1
- Cyp19
aromatase
- LBD
ligand binding domain
- DBD
DNA binding domain
- HRE
hormone response element
- ERE
estrogen response element
- ARE
androgen response element
- AF-1 and -2
activation functions 1 and 2 domains
- DSP
daily sperm production
- PND
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statements:
Rex Hess has an appointment as Director of Science at Epivara, Inc., a start-up company dedicated to research and development of animal sterilization methods. Epivara provided financial assistance for the literature review but had no influence on the preparation of the manuscript.
Richard Sharpe has no competing interests to declare.
Barry Hinton has no competing interests to declare
Contributor Information
Rex A. Hess, Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois Urbana-Champaign, IL, 61802 USA and Epivara, Inc., Research Park, 60 Hazelwood Dr., Suite 230G, Champaign, IL 61820, USA
Richard M. Sharpe, MRC Centre for Reproductive Health, The Queen’s Medical Research Institute, University of Edinburgh, 47 Little France Crescent, Edinburgh EH16 4TJ, U.K.
Barry T. Hinton, Department of Cell Biology, University of Virginia School of Medicine, Charlottesville, VA, USA
References
- Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, Lenfant F, Arnal JF, 2013. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology 154, 2222–2233. [DOI] [PubMed] [Google Scholar]
- Aceitero J, Llanero M, Parrado R, Pena E, Lopez-Beltran A, 1998. Neonatal exposure of male rats to estradiol benzoate causes rete testis dilation and backflow impairment of spermatogenesis. Anat Rec 252, 17–33. [DOI] [PubMed] [Google Scholar]
- Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trolenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schutz G, Wintermantel TM, 2009. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Mol Endocrinol 23, 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RP, Ganjam VK, 1976. Steroid production by the bovine testis and steroid transfer across the pampiniform plexus. Biol Reprod 15, 695–703. [DOI] [PubMed] [Google Scholar]
- Amano A, Kondo Y, Noda Y, Ohta M, Kawanishi N, Machida S, Mitsuhashi K, Senmaru T, Fukui M, Takaoka O, Mori T, Kitawaki J, Ono M, Saibara T, Obayashi H, Ishigami A, 2017. Abnormal lipid/lipoprotein metabolism and high plasma testosterone levels in male but not female aromatase-knockout mice. Archives of biochemistry and biophysics 622, 47–58. [DOI] [PubMed] [Google Scholar]
- Amory JK, Kalhorn TF, Page ST, 2008. Pharmacokinetics and pharmacodynamics of oral testosterone enanthate plus dutasteride for 4 weeks in normal men: implications for male hormonal contraception. J Androl 29, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A, 2010. Infants of Women with Polycystic Ovary Syndrome Have Lower Cord Blood Androstenedione and Estradiol Levels. The Journal of Clinical Endocrinology & Metabolism 95, 2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Sundberg M, Pristovsek N, Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramstrom M, Soderberg O, Williams C, Asplund A, 2017. Insufficient antibody validation challenges oestrogen receptor beta research. Nature communications 8, 15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsusingha K, Kenny FM, Nankin HR, Taylor FH, 1974. Unconjugated Estrone, Estradiol and FSH and LH in Prepubertal and Pubertal Males and Females. The Journal of Clinical Endocrinology & Metabolism 39, 63–68. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M, 2008. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A 105, 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson P, Omoto Y, Humire P, Gustafsson JA, 2012. Generation of ERalpha-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun 424, 710–716. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, Rago V, Ando S, 2003. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod 18, 1650–1659. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Ando S, 2002. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab 87, 3385–3390. [DOI] [PubMed] [Google Scholar]
- Arai Y, Chen CY, Nishizuka Y, 1978. Cancer development in male reproductive tract in rats given diethylstilbestrol at neonatal age. Gan 69, 861–862. [PubMed] [Google Scholar]
- Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, Korach KS, 2012. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor alpha is crucial to maintain male reproductive tract function. Proc Natl Acad Sci U S A 109, 21140–21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DT, Dorrington JH, 1977. Estrogen biosynthesis in the ovaries and testes. Advances in sex hormone research 3, 217–258. [PubMed] [Google Scholar]
- Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, Katzenellenbogen J, 2017. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiological reviews 97, 1045–1087. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Fisher J, Sharpe RM, 2005a. Neonatal treatment of rats with diethylstilboestrol (DES) induces stromal-epithelial abnormalities of the vas deferens and cauda epididymis in adulthood following delayed basal cell development. Reproduction (Cambridge, England) 129, 589–601. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM, 2000. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology 141, 3898–3907. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM, 1999. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology 140, 5364–5373. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Williams K, Turner KJ, Fisher JS, Saunders PT, Millar MR, Sharpe RM, 2001. Age-, cell- and region-specific immunoexpression of estrogen receptor alpha (but not estrogen receptor beta) during postnatal development of the epididymis and vas deferens of the rat and disruption of this pattern by neonatal treatment with diethylstilbestrol. Endocrinology 142, 874–886. [DOI] [PubMed] [Google Scholar]
- Atanassova NN, Walker M, McKinnell C, Fisher JS, Sharpe RM, 2005b. Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol 184, 107–117. [DOI] [PubMed] [Google Scholar]
- Atsuta Y, Takahashi Y, 2015. FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis. Development (Cambridge, England) 142, 2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia L, Schneider J, Yelin R, Schultheiss TM, 2015. Collective cell migration of the nephric duct requires FGF signaling. Dev Dyn 244, 157–167. [DOI] [PubMed] [Google Scholar]
- Auharek SA, de Franca LR, 2010. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. Journal of anatomy 216, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JE, Washburn T, Eddy EM, Korach KS, Temelcos C, Hutson JM, 2001. Early development of the gubernaculum and cremaster sac in estrogen receptor knockout mice. Urol Res 29, 163–167. [DOI] [PubMed] [Google Scholar]
- Basak S, Das MK, Duttaroy AK, 2020. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth defects research 112, 1308–1325. [DOI] [PubMed] [Google Scholar]
- Beall D, 1940. The isolation of a-oestradiol and oestrone from horse testes. The Biochemical journal 34, 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin D, Lacombe C, Van Themsche C, 2011. PAX2 is activated by estradiol in breast cancer cells of the luminal subgroup selectively, to confer a low invasive phenotype. Molecular cancer 10, 148–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Cline JM, Conley J, Groeters S, Jefferson WN, Law M, Mackey E, Suen AA, Williams CJ, Dixon D, Wolf JC, 2019. Endocrine Disruption and Reproductive Pathology. Toxicologic pathology 47, 1049–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit MJ, 1926. Recherches anatomiques; cytologiques et histophysiologiques sur les voies excretices du testicule; chez les mammifers. Arch d’anat d’histo et d’embryo 5, 173–403. [Google Scholar]
- Bentvelsen FM, Brinkmann AO, van der Schoot P, van der Linden JE, van der Kwast TH, Boersma WJ, Schroder FH, Nijman JM, 1995. Developmental pattern and regulation by androgens of androgen receptor expression in the urogenital tract of the rat. Mol Cell Endocrinol 113, 245–253. [DOI] [PubMed] [Google Scholar]
- Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, Rivarola MA, Belgorosky A, 2006. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res 60, 740–744. [DOI] [PubMed] [Google Scholar]
- Bern HA, 1951. Estrogen and alkaline phosphatase activity in the genital tract of the male mouse. Endocrinology 48, 25–33. [DOI] [PubMed] [Google Scholar]
- Bernardini N, Bianchi F, Lupetti M, Dolfi A, 1996. Immunohistochemical localization of the epidermal growth factor, transforming growth factor alpha, and their receptor in the human mesonephros and metanephros. Dev Dyn 206, 231–238. [DOI] [PubMed] [Google Scholar]
- Bianco JJ, McPherson SJ, Wang H, Prins GS, Risbridger GP, 2006. Transient neonatal estrogen exposure to estrogen-deficient mice (aromatase knockout) reduces prostate weight and induces inflammation in late life. The American journal of pathology 168, 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinska B, Kotula-Balak M, Gancarczyk M, Sadowska J, Tabarowski Z, Wojtusiak A, 2003. Androgen aromatization in cryptorchid mouse testis. Acta histochemica 105, 57–65. [DOI] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF, 2009. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. Proc Natl Acad Sci U S A 106, 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF, 2011. Activation function 2 (AF2) of estrogen receptor-alpha is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A 108, 13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg Jensen M, Lieben L, Nielsen JE, Willems A, Jorgensen A, Juul A, Toppari J, Carmeliet G, Rajpert-De Meyts E, 2013. Characterization of the testicular, epididymal and endocrine phenotypes in the Leuven Vdr-deficient mouse model: Targeting estrogen signalling. Mol Cell Endocrinol 377, 93–102. [DOI] [PubMed] [Google Scholar]
- Bonagura TW, Zhou H, Babischkin JS, Pepe GJ, Albrecht ED, 2011. Expression of P-450 aromatase, estrogen receptor α and β, and α-inhibin in the fetal baboon testis after estrogen suppression during the second half of gestation. Endocrine 39, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borday C, Merlet J, Racine C, Habert R, 2013. Expression and localization of aromatase during fetal mouse testis development. Basic and clinical andrology 23, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G, 2007. Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod 22, 1885–1892. [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB, 1997. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab 82, 3509–3512. [DOI] [PubMed] [Google Scholar]
- Branford WW, Benson GV, Ma L, Maas RL, Potter SS, 2000. Characterization of Hoxa-10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Dev Biol 224, 373–387. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Millar MR, Sharpe RM, Saunders PT, 1994. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinol 135, 1227–1234. [DOI] [PubMed] [Google Scholar]
- Brinkmann AO, Mulder E, Lamers-Stahlhofen GJ, Mechielsen MJ, van der Molen HJ, 1972. An oestradiol receptor in rat testis interstitial tissue. FEBS Lett 26, 301–305. [DOI] [PubMed] [Google Scholar]
- Brodie A, Inkster S, 1993. Aromatase in the human testis. J Steroid Biochem Molec Biol 44, 549–555. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Fink G, Greig F, Murray MA, 1975. Altered sexual development in male rats after oestrogen administration during the neonatal period. J Reprod Fertil 44, 25–42. [DOI] [PubMed] [Google Scholar]
- Bujan L, Mieusset R, Audran F, Lumbroso S, Sultan C, 1993. Increased oestradiol level in seminal plasma in infertile men. Hum Reprod 8, 74–77. [DOI] [PubMed] [Google Scholar]
- Bullock BC, Newbold RR, McLachlan JA, 1988. Lesions of testis and epididymis associated with prenatal diethylstilbestrol exposure. Environmental health perspectives 77, 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Li Y, Arao Y, Petrovich RM, Korach KS, 2011. Selective mutations in estrogen receptor alpha D-domain alters nuclear translocation and non-estrogen response element gene regulatory mechanisms. The Journal of biological chemistry 286, 12640–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW, 1974. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Caizzi L, Ferrero G, Cutrupi S, Cordero F, Ballaré C, Miano V, Reineri S, Ricci L, Friard O, Testori A, Corà D, Caselle M, Di Croce L, De Bortoli M, 2014. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci U S A 111, 4892–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, González-Arenas A, Jiménez-Arellano C, Morimoto S, Galván-Rosas A, Gómora-Arrati P, García-Juárez M, González-Flores O, 2018. Sex hormone levels and expression of their receptors in lactating and lactating pregnant rats. J Steroid Biochem Mol Biol 178, 213–220. [DOI] [PubMed] [Google Scholar]
- Canick JA, Makris A, Gunsalus GL, Ryan KJ, 1979. Testicular Aromatization in Immature Rats: Localization and Stimulation after Gonadotropin Administration in Vivo*. Endocrinology 104, 285–288. [DOI] [PubMed] [Google Scholar]
- Carpino A, Rago V, Pezzi V, Carani C, Ando S, 2007. Detection of aromatase and estrogen receptors (ERalpha, ERbeta1, ERbeta2) in human Leydig cell tumor. European journal of endocrinology 157, 239–244. [DOI] [PubMed] [Google Scholar]
- Carpino A, Romeo F, Rago V, 2004. Aromatase immunolocalization in human ductuli efferentes and proximal ductus epididymis. Journal of anatomy 204, 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, 2007. Leydig cell aromatase: from gene to physiological role, in: Payne AH, Hardy MP (Eds.), The Leydig Cell in Health and Disease. Human Press, Inc., Totowa, NJ, pp. 189–195. [Google Scholar]
- Carreau S, Bourguiba S, Marie E, 2004. Testicular and blood steroid levels in aged men. Reproductive biology 4, 299–304. [PubMed] [Google Scholar]
- Carreau S, de Vienne C, Galeraud-Denis I, 2008. Aromatase and estrogens in man reproduction: a review and latest advances. Adv Med Sci 53, 139–144. [DOI] [PubMed] [Google Scholar]
- Carreau S, Genissel C, Bilinska B, Levallet J, 1999. Sources of oestrogen in the testis and reproductive tract of the male. International journal of andrology 22, 211–223. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA, 2010. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365, 1517–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S, 2003. Aromatase expression and role of estrogens in male gonad : a review. Reprod Biol Endocrinol 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP, 2005. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Developmental cell 9, 283–292. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S, 2007. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology 148, 5507–5519. [DOI] [PubMed] [Google Scholar]
- Chen L, Bi J, Nakai M, Bunick D, Couse JF, Korach KS, Nowak RA, 2010. Expression of basigin in reproductive tissues of estrogen receptor-{alpha} or -{beta} null mice. Reproduction (Cambridge, England) 139, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S, 2009. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology 150, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yeh CR, Chang HC, Vitkus S, Wen XQ, Bhowmick NA, Wolfe A, Yeh S, 2012a. Loss of epithelial oestrogen receptor alpha inhibits oestrogen-stimulated prostate proliferation and squamous metaplasia via in vivo tissue selective knockout models. The Journal of pathology 226, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yeh CR, Shyr CR, Lin HH, Da J, Yeh S, 2012b. Reduced prostate branching morphogenesis in stromal fibroblast, but not in epithelial, estrogen receptor alpha knockout mice. Asian J Androl 14, 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Bunick D, Bahr JM, Klinefelter GR, Hess RA, 1998. Isolation and culture of epithelial cells from rat ductuli efferentes and initial segment epididymidis. Tissue Cell 30, 1–13. [DOI] [PubMed] [Google Scholar]
- Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA, 2003. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol 1, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Pearl CA, 2014. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst Biol Reprod Med 60, 89–97. [DOI] [PubMed] [Google Scholar]
- Claus R, Dimmick MA, Gimenez T, Hudson LW, 1992. Estrogens and prostaglandin F2alpha in the semen and blood plasma of stallions. Theriogenology 38, 687–693. [DOI] [PubMed] [Google Scholar]
- Claus R, Hoffmann B, 1980. Oestrogens, compared to other steroids of testicular origin, in blood plasma of boars. Acta endocrinologica 94, 404–411. [DOI] [PubMed] [Google Scholar]
- Claus R, Schopper D, Hoang-Vu C, 1985. Contribution of individual compartments of the genital tract to oestrogen and testosterone concentrations in ejaculates of the boar. Acta Endocrinol 109, 281–288. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY, 1998. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl 53, 1–14. [PubMed] [Google Scholar]
- Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D, Koopman P, 2009. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn 238, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Cardon M, Furr J, Wilson VS, Gray LE Jr., 2018. Mixed “Antiandrogenic” Chemicals at Low Individual Doses Produce Reproductive Tract Malformations in the Male Rat. Toxicol Sci 164, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JC, Johnson L, O’Connor JC, Biegel LB, Krams CH, Frame SR, Hurtt ME, 1998. Effects of dietary 17 beta-estradiol exposure on serum hormone concentrations and testicular parameters in male Crl:CD BR rats. Toxicol Sci 44, 155–168. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA, 2017. Estrogens in male physiology. Physiological reviews 97, 995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Nanjappa MK, Tevosian SG, Hess RA, 2019. Roles of membrane and nuclear estrogen receptors in spermatogenesis, in: Cheng CY(Ed.), Spermatogenesis: biology and clinical implications. Taylor & Francis Group, Boca Raton, FL, pp. 116–122. [Google Scholar]
- Cooke PS, Young P, Cunha GR, 1991a. Androgen receptor expression in developing male reproductive organs. Endocrinology 128, 2867–2873. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Hess RA, Cunha GR, 1991b. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology 128, 2874–2879. [DOI] [PubMed] [Google Scholar]
- Corbin C, Graham-Lorence S, McPhaul M, Mason J, Mendelson C, Simpson E, 1988. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P450 and its expression in nonsteroidogenic cells. Proc Natl Acad Sci (USA) 85, 8948–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Curtis Hewitt S, Korach KS, 2000. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol 74, 287–296. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS, 1999. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science (New York, N.Y.) 286, 2328–2331. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS, 1999a. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20, 358–417. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS, 1999b. Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Annales d’endocrinologie 60, 143–148. [PubMed] [Google Scholar]
- Couse JF, Korach KS, 2004. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology 205, 55–63. [DOI] [PubMed] [Google Scholar]
- Cripps SM, Mattiske DM, Black JR, Risbridger GP, Govers LC, Phillips TR, Pask AJ, 2019. A loss of estrogen signaling in the aromatase deficient mouse penis results in mild hypospadias. Differentiation; research in biological diversity 109, 42–52. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Attié-Bitach T, Encha-Razavi F, Audollent S, Soudy F, Hadchouel M, Meunier-Rotival M, Vekemans M, 2000. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 32, 574–581. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cao MYL, Derpinghaus A, Baskin LS, 2020. Ontogeny of estrogen receptors in human male and female fetal reproductive tracts. Differentiation; research in biological diversity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Ricke WA, Robboy SJ, Cao M, Baskin LS, 2019. Reproductive tract biology: Of mice and men. Differentiation; research in biological diversity 110, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Hess RA, Lees JA, 2016. E2f4 and E2f5 are essential for the development of the male reproductive system. Cell Cycle 15, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzo BJ, Eller BC, Judy LA, Trautman JR, Orgebin-Crist MC, 1975. Estradiol binding in cytosol from epididymides of immature rabbits. Mol Cell Endocrinol 2, 91–105. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Sutton W, Eller BC, 1978. Analysis of [3H]estradiol binding to nuclei prepared from epididymides of sexually immature intact rabbits. Mol Cell Endocrinol 9, 291–301. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Wolfe MS, Curry JB, 1977. The presence of an estradiol binding component in cytosol from immature rat epididymides. Mol Cell Endocrinol 6, 271–279. [DOI] [PubMed] [Google Scholar]
- De Gendt K, McKinnell C, Willems A, Saunders PT, Sharpe RM, Atanassova N, Swinnen JV, Verhoeven G, 2009. Organotypic cultures of prepubertal mouse testes: a method to study androgen action in sertoli cells while preserving their natural environment. Biol Reprod 81, 1083–1092. [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G, 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 101, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong F, Hey A, van der Molen H, 1973. Effect of gonadotrophins on the secretion of oestradiol-17b and testosterone by the rat testis. J Endocrinol 57, 277–284. [DOI] [PubMed] [Google Scholar]
- de Mello Santos T, Hinton BT, 2019. We, the developing rete testis, efferent ducts, and Wolffian duct, all hereby agree that we need to connect. Andrology 7, 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tomasi L, David P, Humbert C, Silbermann F, Arrondel C, Tores F, Fouquet S, Desgrange A, Niel O, Bole-Feysot C, Nitschké P, Roume J, Cordier MP, Pietrement C, Isidor B, Khau Van Kien P, Gonzales M, Saint-Frison MH, Martinovic J, Novo R, Piard J, Cabrol C, Verma IC, Puri R, Journel H, Aziza J, Gavard L, Said-Menthon MH, Heidet L, Saunier S, Jeanpierre C, 2017. Mutations in GREB1L Cause Bilateral Kidney Agenesis in Humans and Mice. Am J Hum Genet 101, 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R, 2005. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology 146, 2454–2461. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Habert R, 2006. Estrogen effects on fetal and neonatal testicular development. Reproduction (Cambridge, England) 132, 527–538. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R, 2004. Estrogen receptor beta-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology 145, 3395–3403. [DOI] [PubMed] [Google Scholar]
- Denger S, Reid G, Brand H, Kos M, Gannon F, 2001. Tissue-specific expression of human ERalpha and ERbeta in the male. Mol Cell Endocrinol 178, 155–160. [DOI] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, Verhoeven G, 2006. The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol 20, 321–334. [DOI] [PubMed] [Google Scholar]
- Dhar JD, Setty BS, 1976. Epididymal response to exogenous testosterone in rats sterilized neonatally by estrogen. Endokrinologie 68, 14–21. [PubMed] [Google Scholar]
- Dias JP, Melvin D, Simonsick EM, Carlson O, Shardell MD, Ferrucci L, Chia CW, Basaria S, Egan JM, 2016. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology 4, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs RW, Malhotra NR, Greenwald DT, Wang AY, Prins GS, Abern MR, 2019. Estrogens and prostate cancer. Prostate Cancer Prostatic Dis 22, 185–194. [DOI] [PubMed] [Google Scholar]
- Doerr P, Pirke KM, 1974. Regulation of plasma oestrogens in normal adult males. I. Response of oestradiol, oestrone and testosterone to HCG and fluoxymesterone administration. Acta endocrinologica 75, 617–624. [PubMed] [Google Scholar]
- Döhler KD, Wuttke W, 1975. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology 97, 898–907. [DOI] [PubMed] [Google Scholar]
- Dorrington JH, Armstrong DT, 1975. Follicle-stimulating hormone stimulates estradiol-17beta synthesis in cultured Sertoli cells. Proc Natl Acad Sci U S A 72, 2677–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington JH, Armstrong DT, 1979. Effects of FSH on gonadal functions. Recent progress in hormone research 35, 301–342. [DOI] [PubMed] [Google Scholar]
- Dorrington JH, Khan SA, 1993. Steroid production, metabolism, and release by Sertoli cells, in: Griswold MD, Russell LD (Eds.), The Sertoli Cell. Cache River Press, Clearwater, FL, pp. 537–550. [Google Scholar]
- Duanmu Z, Weckle A, Koukouritaki SB, Hines RN, Falany JL, Falany CN, Kocarek TA, Runge-Morris M, 2006. Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. The Journal of pharmacology and experimental therapeutics 316, 1310–1317. [DOI] [PubMed] [Google Scholar]
- Dunn TB, Green AW, 1963. Cysts of the epididymis, cancer of the cervix, granular cell myoblastoma, and other lesions after estrogen injection in newborn mice. J Natl Cancer Inst 31, 425–455. [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M, 2000. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development (Cambridge, England) 127, 4277–4291. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS, 1996. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinol 137, 4796–4805. [DOI] [PubMed] [Google Scholar]
- Eik-Nes KB, 1964. Effects of gonadotrophins on secretion of steroids by the testis and ovary. Physiological reviews 44, 609–630. [DOI] [PubMed] [Google Scholar]
- Eiler H, Graves C, 1977. Oestrogen content of semen and the effect of exogenous oestradiol-17beta on the oestrogen and androgen concentration in semen and blood plasma of bulls. J Reprod Fert 50, 17–21. [DOI] [PubMed] [Google Scholar]
- Emmens CW, Parkes AS, 1947. Effect of Exogenous Estrogens on the Male Mammal, in: Harris RS, Thimann KV (Eds.), Vitamins & Hormones. Academic Press, pp. 233–272. [Google Scholar]
- Ergun S, Ungefroren H, Holstein AF, Davidoff MS, 1997. Estrogen and progesterone receptors and estrogen receptor-related antigen (ER-D5) in human epididymis. Mol Reprod Dev 47, 448–455. [DOI] [PubMed] [Google Scholar]
- Fellner OO, 1921. Ueber die Wirkung des Placentar- und Hodenlipoids auf die männlichen und weiblichen Sexualorgane. Arch. f. d. ges. Physiol 189, 199–214. [Google Scholar]
- Fielden MR, Halgren RG, Fong CJ, Staub C, Johnson L, Chou K, Zacharewski TR, 2002. Gestational and lactational exposure of male mice to diethylstilbestrol causes long-term effects on the testis, sperm fertilizing ability in vitro, and testicular gene expression. Endocrinology 143, 3044–3059. [DOI] [PubMed] [Google Scholar]
- Fietz D, Bakhaus K, Wapelhorst B, Grosser G, Gunther S, Alber J, Doring B, Kliesch S, Weidner W, Galuska CE, Hartmann MF, Wudy SA, Bergmann M, Geyer J, 2013. Membrane transporters for sulfated steroids in the human testis - cellular localization, expression pattern and functional analysis. PloS one 8, e62638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz D, Bergmann M, Hartmann K, 2016. In Situ Hybridization of Estrogen Receptors alpha and beta and GPER in the Human Testis. Methods in molecular biology (Clifton, N.J.) 1366, 189–205. [DOI] [PubMed] [Google Scholar]
- Fietz D, Ratzenbock C, Hartmann K, Raabe O, Kliesch S, Weidner W, Klug J, Bergmann M, 2014. Expression pattern of estrogen receptors alpha and beta and G-protein-coupled estrogen receptor 1 in the human testis. Histochemistry and cell biology 142, 421–432. [DOI] [PubMed] [Google Scholar]
- Figueiredo AF, Franca LR, Hess RA, Costa GM, 2016. Sertoli cells are capable of proliferation into adulthood in the transition region between the seminiferous tubules and the rete testis in Wistar rats. Cell Cycle 15, 2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ, 2013. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM, 1997. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol 153, 485–495. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Pastor-Soler N, Sharpe RM, Breton S, 2002. Modulation of the onset of postnatal development of H(+)-ATPase-rich cells by steroid hormones in rat epididymis. Biol Reprod 67, 1106–1114. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Brown D, Sharpe RM, 1999. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environmental health perspectives 107, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Fraser HM, Saunders PT, Brown D, Sharpe RM, 1998. Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 139, 3935–3945. [DOI] [PubMed] [Google Scholar]
- Fowler KA, Gill K, Kirma N, Dillehay DL, Tekmal RR, 2000. Overexpression of aromatase leads to development of testicular leydig cell tumors : an in vivo model for hormone-mediated TesticularCancer. The American journal of pathology 156, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free MJ, Jaffe RA, 1979. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod 20, 269–278. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Robinson LL, Groome NP, Anderson RA, Saunders PT, 2003. Differential Expression of Two Estrogen Receptor beta Isoforms in the Human Fetal Testis during the Second Trimester of Pregnancy. J Clin Endocrinol Metab 88, 424–432. [DOI] [PubMed] [Google Scholar]
- Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH, 2009. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332, 273–286. [DOI] [PubMed] [Google Scholar]
- Georgas KM, Armstrong J, Keast JR, Larkins CE, McHugh KM, Southard-Smith EM, Cohn MJ, Batourina E, Dan H, Schneider K, Buehler DP, Wiese CB, Brennan J, Davies JA, Harding SD, Baldock RA, Little MH, Vezina CM, Mendelsohn C, 2015. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development (Cambridge, England) 142, 1893–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Saunders PT, 2012. Estrogen dependent signaling in reproductive tissues - a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol 348, 361–372. [DOI] [PubMed] [Google Scholar]
- Gill WB, Schumacher GF, Bibbo M, 1977. Pathological semen and anatomical abnormalities of the genital tract in human male subjects exposed to diethylstilbestrol in utero. J Urol 117, 477–480. [DOI] [PubMed] [Google Scholar]
- Gondos B, Berndston WE, 1993. Postnatal and pubertal development, in: Russell LD, Griswold MD (Eds.), The Sertoli Cell. Cache River Press, Clearwater, FL, pp. 115–154. [Google Scholar]
- Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, Franz AM, Rhomberg LR, 2009. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Critical reviews in toxicology 39, 1–75. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Christensen LW, 1977. Perinatal hormonal exposure and the development of neuroendocrine regulatory processes. Journal of Toxicology and Environmental Health 3, 97–121. [DOI] [PubMed] [Google Scholar]
- Gould ML, Hurst PR, Nicholson HD, 2007. The effects of oestrogen receptors alpha and beta on testicular cell number and steroidogenesis in mice. Reproduction (Cambridge, England) 134, 271–279. [DOI] [PubMed] [Google Scholar]
- Goulding EH, Curtis-Hewitt S, Nakamura N, Hamilton K, Korach KS, Eddy EM, 2010. Ex3alphaERKO male infertility phenotype recapitulates the alphaERKO phenotype. J Endocrinol 207, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal HO, Robateau A, Braden TD, Williams CS, Srivastava KK, Ali K, 2003. Neonatal estrogen exposure of male rats alters reproductive functions at adulthood. Biol Reprod 68, 2081–2091. [DOI] [PubMed] [Google Scholar]
- Greco TL, Duello TM, Gorski J, 1993. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev 14, 59–71. [DOI] [PubMed] [Google Scholar]
- Greco TL, Furlow JD, Duello TM, Gorski J, 1992. Immunodetection of estrogen receptors in fetal and neonatal male mouse reproductive tracts. Endocrinology 130, 421–429. [DOI] [PubMed] [Google Scholar]
- Greco TL, Payne AH, 1994. Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3 beta-hydroxysteroid dehydrogenase, P450 17 alpha-hydroxylase/C17–20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 135, 262–268. [DOI] [PubMed] [Google Scholar]
- Green CD, Ma Q, Manske GL, Shami AN, Zheng X, Marini S, Moritz L, Sultan C, Gurczynski SJ, Moore BB, Tallquist MD, Li JZ, Hammoud SS, 2018. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Developmental cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RR, Burrill MW, Ivy AC, 1938. Experimental Intersexuality: The Production of Feminized Male Rats by Antenatal Treatment with Estrogens. Science (New York, N.Y.) 88, 130–131. [DOI] [PubMed] [Google Scholar]
- Guercio G, Saraco N, Costanzo M, Marino R, Ramirez P, Berensztein E, Rivarola MA, Belgorosky A, 2020. Estrogens in Human Male Gonadotropin Secretion and Testicular Physiology From Infancy to Late Puberty. Frontiers in Endocrinology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur FM, Aktas I, 2020. The localization of ER alpha and ER beta in rat testis and epididymis. Ann Med Res 27, 2534–2539. [Google Scholar]
- Gustafsson JA, Strom A, Warner M, 2019. Update on ERbeta. J Steroid Biochem Mol Biol 191, 105312. [DOI] [PubMed] [Google Scholar]
- Guttroff RF, Cooke PS, Hess RA, 1992. Blind-ending tubules and branching patterns of the rat ductuli efferentes. Anat Rec 232, 423–431. [DOI] [PubMed] [Google Scholar]
- Haavisto TE, Adamsson NA, Myllymäki SA, Toppari J, Paranko J, 2003. Effects of 4-tert-octylphenol, 4-tert-butylphenol, and diethylstilbestrol on prenatal testosterone surge in the rat. Reprod Toxicol 17, 593–605. [DOI] [PubMed] [Google Scholar]
- Habert R, Muczynski V, Grisin T, Moison D, Messiaen S, Frydman R, Benachi A, Delbes G, Lambrot R, Lehraiki A, N’Tumba-Byn T, Guerquin MJ, Levacher C, Rouiller-Fabre V, Livera G, 2014. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction (Cambridge, England) 147, R119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habert R, Picon R, 1984. Testosterone, dihydrotestosterone and estradiol-17β levels in maternal and fetal plasma and in fetal testes in the rat. Journal of Steroid Biochemistry 21, 193–198. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA, 2006. Regulation of Wolffian Duct Development. Horm Res 67, 142–151. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Print CG, Charnock-Jones DS, Coleman N, Hughes IA, 2006. Changes in gene expression during Wolffian duct development. Horm Res 65, 200–209. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Clulow J, Jones RC, 1999. The role of Na+-H+ exchange in fluid and solute transport in the rat efferent ducts. Exp Physiol 84, 521–527. [PubMed] [Google Scholar]
- Hardelin JP, Julliard AK, Moniot B, Soussi-Yanicostas N, Verney C, Schwanzel-Fukuda M, Ayer-Le Lievre C, Petit C, 1999. Anosmin-1 is a regionally restricted component of basement membranes and interstitial matrices during organogenesis: implications for the developmental anomalies of X chromosome-linked Kallmann syndrome. Dev Dyn 215, 26–44. [DOI] [PubMed] [Google Scholar]
- Haug E, Aakvaag A, Sand T, Torjesen PA, 1974. The gonadotrophin response to synthetic gonadotrophin-releasing hormone in males in relation to age, dose, and basal serum levels of testosterone, oestradiol-17beta and gonadotrophins. Acta endocrinologica 77, 625–635. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Freedman B, Marshall A, Killen E, 1975. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. British journal of cancer 32, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J, Abdelmageed Y, Braden TD, Williams CS, Williams JW, Paulose T, Hernandez-Ochoa I, Gupta R, Flaws JA, Goyal HO, 2011. Genetically induced estrogen receptor alpha mRNA (Esr1) overexpression does not adversely affect fertility or penile development in male mice. J Androl 32, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks SE, Gerall AA, 1970. Effect of Neonatally Administered Estrogen on Development of Male and Female Rats. Endocrinology 87, 435–439. [DOI] [PubMed] [Google Scholar]
- Hess RA, 2002. The efferent ductules: structure and functions, in: Robaire B, Hinton B (Eds.), The Epididymis: from Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers, New York, pp. 49–80. [Google Scholar]
- Hess RA, 2004. Estrogen in the adult male: from a curiosity to absolute necessity. Ann Rev Biomed Sci 6, 1–12. [Google Scholar]
- Hess RA, 2014. Disruption of estrogen receptor signaling and similar pathways in the efferent ductules and initial segments of the epididymis. Spermatogenesis 4, e979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, 2018a. Efferent ductules: Structure and function, in: Skinner MK (Ed.), Encyclopedia of Reproduction (Second Edition). Academic Press, Oxford, pp. 270–278. [Google Scholar]
- Hess RA, 2018b. Endocrinology and pathology of rete testis and efferent ductules, in: Skinner MK (Ed.), Encyclopedia of Reproduction (Second Edition). Academic Press, Oxford, pp. 279–285. [Google Scholar]
- Hess RA, Bunick D, Bahr JM, 1995. Sperm, a source of estrogen. Environmental health perspectives 103 Suppl 7, 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB, 1997a. A role for oestrogens in the male reproductive system. Nature 390, 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J, 2000. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. Journal of Andrology 21, 107–121. [PubMed] [Google Scholar]
- Hess RA, Carnes K, 2004. The role of estrogen in testis and the male reproductive tract: a review and species comparison. Anim. Reprod 1, 5–30. [Google Scholar]
- Hess RA, Cooke PS, 2018. Estrogen in the male: a historical perspective. Biol Reprod 99, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Fernandes SA, Gomes GR, Oliveira CA, Lazari MF, Porto CS, 2011. Estrogen and its receptors in efferent ductules and epididymis. J Androl 32, 600–613. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL, 1997b. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl 18, 602–611. [PubMed] [Google Scholar]
- Hess RA, Hermo L, 2018. Rete testis: structure, cell biology and site for stem cell transplantation, in: Skinner MK (Ed.), Encyclopedia of Reproduction (Second Edition). Academic Press, Oxford, pp. 263–269. [Google Scholar]
- Hess RA, Zhou Q, Nie R, 2002. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens, in: Robaire B, Hinton BT (Eds.), The Epididymis: from Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers, New York, pp. 317–338. [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schutz G, Korach KS, 2014. Novel DNA motif binding activity observed in vivo with an estrogen receptor alpha mutant mouse. Mol Endocrinol 28, 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton BT, Avellar MCW, 2018. Wolffian duct development, in: Skinner MK (Ed.), Encyclopedia of Reproduction, 2nd ed. Elsevier, Inc., Amsterdam, Netherlands, pp. 256–262. [Google Scholar]
- Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R, Yang L, 2011. How do you get six meters of epididymis inside a human scrotum? J Androl 32, 558–564. [DOI] [PubMed] [Google Scholar]
- Hirashima T, 2014. Pattern formation of an epithelial tubule by mechanical instability during epididymal development. Cell reports 9, 866–873. [DOI] [PubMed] [Google Scholar]
- Hirashima T, 2016. Mathematical study on robust tissue pattern formation in growing epididymal tubule. Journal of theoretical biology 407, 71–80. [DOI] [PubMed] [Google Scholar]
- Holderegger C, Keefer D, 1986. The ontogeny of the mouse estrogen receptor: The pelvic region. American Journal of Anatomy 177, 285–297. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S, 1998. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun 252, 445–449. [DOI] [PubMed] [Google Scholar]
- Hoshi M, Reginensi A, Joens MS, Fitzpatrick JAJ, McNeill H, Jain S, 2018. Reciprocal Spatiotemporally Controlled Apoptosis Regulates Wolffian Duct Cloaca Fusion. Journal of the American Society of Nephrology : JASN 29, 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K, 2007. LGR4 Regulates the Postnatal Development and Integrity of Male Reproductive Tracts in Mice. Biol Reprod 76, 303–313. [DOI] [PubMed] [Google Scholar]
- Huang B, Butler R, Miao Y, Dai Y, Wu W, Su W, Fujii-Kuriyama Y, Warner M, Gustafsson JA, 2016. Dysregulation of Notch and ERalpha signaling in AhR−/− male mice. Proc Natl Acad Sci U S A 113, 11883–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HF, He RH, Sun CC, Zhang Y, Meng QX, Ma YY, 2006. Function of aquaporins in female and male reproductive systems. Hum Reprod Update 12, 785–795. [DOI] [PubMed] [Google Scholar]
- Iguchi T, 1991. Developmental pattern of estrogen receptor expression in male mouse genital organs. Mol. Androl 3, 109–120. [Google Scholar]
- Ilio KY, Hess RA, 1994. Structure and function of the ductuli efferentes: a review. Microsc Res Tech 29, 432–467. [DOI] [PubMed] [Google Scholar]
- Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson JA, 2004. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc Natl Acad Sci U S A 101, 9375–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster S, Yue W, Brodie A, 1995. Human testicular aromatase: immunocytochemical and biochemical studies. J Clin Endocrinol Metab 80, 1941–1947. [DOI] [PubMed] [Google Scholar]
- Iwamura M, Abrahamsson PA, Benning CM, Cockett AT, di Sant’Agnese PA, 1994. Androgen receptor immunostaining and its tissue distribution in formalin-fixed, paraffin-embedded sections after microwave treatment. Journal of Histochemistry & Cytochemistry 42, 783–788. [DOI] [PubMed] [Google Scholar]
- Jacob M, Yusuf F, Jacob HJ, 2012. Development, differentiation and derivatives of the Wolffian and Müellerian ducts, in: Yamada S (Ed.), The Human Embryo. InTech, London, UK., pp. 143–166. [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL, 2002. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16, 2188–2201. [DOI] [PubMed] [Google Scholar]
- Janulis L, Bahr JM, Hess RA, Bunick D, 1996a. P450 aromatase messenger ribonucleic acid expression in male rat germ cells: detection by reverse transcription-polymerase chain reaction amplification. J Androl 17, 651–658. [PubMed] [Google Scholar]
- Janulis L, Bahr JM, Hess RA, Janssen S, Osawa Y, Bunick D, 1998. Rat testicular germ cells and epididymal sperm contain active P450 aromatase. J Androl 19, 65–71. [PubMed] [Google Scholar]
- Janulis L, Hess RA, Bunick D, Nitta H, Janssen S, Osawa Y, Bahr JM, 1996b. Mouse epididymal sperm contain active P450 aromatase which decreases as sperm traverse the epididymis. J Androl 17, 111–116. [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR, 2000. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62, 310–317. [DOI] [PubMed] [Google Scholar]
- Jensen MB, 2014. Vitamin D and male reproduction. Nat Rev Endocrinol 10, 175–186. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, Sakuma I, Hattori Y, Kitabatake A, 2002. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology 143, 4764–4774. [DOI] [PubMed] [Google Scholar]
- Jong FH, Uilenbroek J, Molen HJ, 1975. Oestradiol-17beta, testosterone and gonadotrophins in oestradiol-17beta-treated intact adult male rats. J Endocrinol 65, 281–282. [DOI] [PubMed] [Google Scholar]
- Joseph A, Hess RA, Schaeffer DJ, Ko C, Hudgin-Spivey S, Chambon P, Shur BD, 2010a. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biol Reprod 82, 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Hess RA, 2011. Estrogen, efferent ductules, and the epididymis. Biol Reprod 84, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Shur BD, Ko C, Chambon P, Hess RA, 2010b. Epididymal hypo-osmolality induces abnormal sperm morphology and function in the estrogen receptor alpha knockout mouse. Biol Reprod 82, 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Yao H, Hinton BT, 2009. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol 325, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao RM, 2013. The luminal connection: from animal development to lumopathies. Organogenesis 9, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl J, Capel B, 1995. Three-dimensional structure of the developing mouse genital ridge. Philos Trans R Soc Lond B Biol Sci 350, 235–242. [DOI] [PubMed] [Google Scholar]
- Kim JY, Seo HJ, Kim OS, Kim BJ, Lee SK, Baik HW, Lee KH, 2008. Analysis of Differential Expression of Cytochrome P450 Aromatase (Cyp19) in The Efferent Ductules and The Epididymis of Male Rats During Postnatal Development. Korean Society of Animal Science and Technology 50, 783–792. [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H, 1993. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 41, 671–678. [DOI] [PubMed] [Google Scholar]
- Kincl FA, Folch Pi A, Herrera Lasso L, 1963. Effect of estradiol benzoate treatment in the newborn male rat. Endocrinology 72, 966–968. [DOI] [PubMed] [Google Scholar]
- Kincl FA, Maqueo M, 1972. Neonatal sterilization of rodents with steroid hormones. 4. The influence of estradiol benzoate injected into five day old male rats on the weight of testes and ventral prostate and histology of the seminiferous epithelium and the pituitary gland during sexual maturation. Endocrinol Exp 6, 11–15. [PubMed] [Google Scholar]
- Kitagaki J, Ueda Y, Chi X, Sharma N, Elder CM, Truffer E, Costantini F, Lewandoski M, Perantoni AO, 2011. FGF8 is essential for formation of the ductal system in the male reproductive tract. Development (Cambridge, England) 138, 5369–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley HK, Nieschlag E, Wiegelmann W, Krüskemper HL, 1976. Oestrone, oestradiol and testosterone in normal and hypogonadal men following LH-RH or HCG stimulation. Acta endocrinologica 81, 616–622. [DOI] [PubMed] [Google Scholar]
- Kolasa A, Wiszniewska B, Marchlewicz M, Wenda-Rozewicka L, 2003. Localisation of oestrogen receptors (ERalpha and ERbeta) in the human and rat epididymides. Folia morphologica 62, 467–469. [PubMed] [Google Scholar]
- Korach KS, Hewitt SC, Hamilton KJ, Li Y, Ramsey JT, Garcia M, Mathura E, Arao Y, 2019. Physiological and Pathological Roles of Estrogen Receptor, in: Zhang X (Ed.), Estrogen Receptor and Breast Cancer: Celebrating the 60th Anniversary of the Discovery of ER (Cancer Drug Discovery and Development), 1st ed. Humana Press, Cham, Switzerland. [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O, 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95, 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R, 1993. WT-1 is required for early kidney development. Cell 74, 679–691. [DOI] [PubMed] [Google Scholar]
- Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I, 2011. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology 152, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper EA, Ket JC, Caanen MR, Lambalk CB, 2013. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 27, 33–63. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA, 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138, 863–870. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA, 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A 93, 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulibin AY, Malolina EA, 2020. Formation of the rete testis during mouse embryonic development. Dev Dyn. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dumasia K, Deshpande S, Raut S, Balasinor NH, 2018. Delineating the regulation of estrogen and androgen receptor expression by sex steroids during rat spermatogenesis. J Steroid Biochem Mol Biol 182, 127–136. [DOI] [PubMed] [Google Scholar]
- Kumar M, Syed SM, Taketo MM, Tanwar PS, 2016. Epithelial Wnt/betacatenin signalling is essential for epididymal coiling. Dev Biol. [DOI] [PubMed] [Google Scholar]
- Lambard S, Carreau S, 2005. Aromatase and oestrogens in human male germ cells. International journal of andrology 28, 254–259. [DOI] [PubMed] [Google Scholar]
- Lambard S, Galeraud-Denis I, Bouraima H, Bourguiba S, Chocat A, Carreau S, 2003. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Molecular human reproduction 9, 117–124. [DOI] [PubMed] [Google Scholar]
- Lambard S, Galeraud-Denis I, Saunders PT, Carreau S, 2004. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. Journal of molecular endocrinology 32, 279–289. [DOI] [PubMed] [Google Scholar]
- Lambot MA, Mendive F, Laurent P, Van Schoore G, Noel JC, Vanderhaeghen P, Vassart G, 2009. Three-dimensional reconstruction of efferent ducts in wild-type and Lgr4 knock-out mice. Anat Rec (Hoboken) 292, 595–603. [DOI] [PubMed] [Google Scholar]
- LaRocca J, Boyajian A, Brown C, Smith SD, Hixon M, 2011. Effects of in utero exposure to Bisphenol A or diethylstilbestrol on the adult male reproductive system. Birth Defects Res B Dev Reprod Toxicol 92, 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence ML, Smith JR, Davies JA, 2018. Functional transport of organic anions and cations in the murine mesonephros. American journal of physiology. Renal physiology 315, F130–f137. [DOI] [PubMed] [Google Scholar]
- Lee KH, Bunick D, Lamprecht G, Choi I, Bahr JM, 2008. Differential expression of genes important to efferent ductules ion homeostasis across postnatal development in estrogen receptor-alpha knockout and wildtype mice. Asian Australas. J. Anim. Sci 21, 510–522. [Google Scholar]
- Lee KH, Finnigan-Bunick C, Bahr J, Bunick D, 2001. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta. Biol Reprod 65, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D, 2000. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod 63, 1873–1880. [DOI] [PubMed] [Google Scholar]
- Lee KH, Park JH, Bunick D, Lubahn DB, Bahr JM, 2009. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor alpha knockout mice during postnatal development. Journal of anatomy 214, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré C, Sullivan R, 2020. Differential gene expression profiles of human efferent ducts and proximal epididymis. Andrology 8, 625–636. [DOI] [PubMed] [Google Scholar]
- Levin ER, 2015. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med 66, 271–280. [DOI] [PubMed] [Google Scholar]
- Lewis FT, 1920. The course of the Wolffian tubules in mammalian embryos. American Journal of Anatomy 26, 422–435. [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M, 2001. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology 142, 2435–2442. [DOI] [PubMed] [Google Scholar]
- Li X, Rahman N, 2008. Impact of androgen/estrogen ratio: lessons learned from the aromatase over-expression mice. Gen Comp Endocrinol 159, 1–9. [DOI] [PubMed] [Google Scholar]
- Li X, Strauss L, Kaatrasalo A, Mayerhofer A, Huhtaniemi I, Santti R, Makela S, Poutanen M, 2006. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology 147, 1271–1277. [DOI] [PubMed] [Google Scholar]
- Li XY, Lu Y, Sun HY, Wang JQ, Yang J, Zhang HJ, Fan NG, Xu J, Jiang JJ, Liu RY, Li DL, Liu MY, Ning G, 2010. G protein-coupled receptor 48 upregulates estrogen receptor {alpha} expression via cAMP/PKA signaling in the male reproductive tract. Development (Cambridge, England) 137, 151–157. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O, 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90, 11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA, 2003. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28. [DOI] [PubMed] [Google Scholar]
- Lucas TF, Siu ER, Esteves CA, Monteiro HP, Oliveira CA, Porto CS, Lazari MF, 2008. 17beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol Reprod 78, 101–114. [DOI] [PubMed] [Google Scholar]
- Ludwig KS, Landmann L, 2005. Early development of the human mesonephros. Anat Embryol (Berl) 209, 439–447. [DOI] [PubMed] [Google Scholar]
- Lymperi S, Giwercman A, 2018. Endocrine disruptors and testicular function. Metabolism: clinical and experimental 86, 79–90. [DOI] [PubMed] [Google Scholar]
- Magers MJ, Udager AM, Chinnaiyan AM, French D, Myers JL, Jentzen JM, McHugh JB, Heider A, Mehra R, 2016. Comprehensive Immunophenotypic Characterization of Adult and Fetal Testes, the Excretory Duct System, and Testicular and Epididymal Appendages. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry 24, e50–68. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM, 2001. Estrogen receptor-alpha is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol 178, 57–63. [DOI] [PubMed] [Google Scholar]
- Majdic G, Millar MR, Saunders PT, 1995. Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J Endocrinol 147, 285–293. [DOI] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, O’Shaughnessy PJ, Saunders PT, 1996. Expression of cytochrome P450 17alpha-hydroxylase/C17–20 lyase in the fetal rat testis is reduced by maternal exposure to exogenous estrogens. Endocrinology 137, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Mäkinen S, Makela S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson JA, 2001. Localization of oestrogen receptors alpha and beta in human testis. Molecular human reproduction 7, 497–503. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE, 2008. New insights into the classical and non-classical actions of estrogen: Evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol 290, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE, 2007. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology 148, 5288–5294. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Atanassova N, Williams K, Fisher JS, Walker M, Turner KJ, Saunders TK, Sharpe RM, 2001. Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Androl 22, 323–338. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock B, 1975. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science (New York, N.Y.) 190, 991–992. [DOI] [PubMed] [Google Scholar]
- Menad R, Smai S, Bonnet X, Gernigon-Spychalowicz T, Moudilou E, Khammar F, Exbrayat JM, 2017. Seasonal variations of aromatase and estrogen receptors expression in the testis of free-ranging sand rats. Acta histochemica 119, 382–391. [DOI] [PubMed] [Google Scholar]
- Menad R, Smai S, Moudilou E, Khammar F, Exbrayat JM, Gernigon-Spychalowicz T, 2014. Immunolocalization of estrogen and androgen receptors in the caput epididymidis of the fat sand rat (Psammomys obesus): Effects of seasonal variations, castration and efferent duct ligation. Acta histochemica 116, 559–569. [DOI] [PubMed] [Google Scholar]
- Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G, 2006. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol 290, 421–434. [DOI] [PubMed] [Google Scholar]
- Mennemeyer RP, Mason JT, 1979. Non-neoplastic cystic lesions of the tunica albuginea: an electron microscopic and clinical study of 2 cases. J Urol 121, 373–375. [DOI] [PubMed] [Google Scholar]
- Merlet J, Racine C, Moreau E, Moreno SG, Habert R, 2007. Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc Natl Acad Sci U S A 104, 3615–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HH, 1890. Die entwickelung der urnieren beim menschen. Arch. F. Mikr. Anat 36, 137–172. [Google Scholar]
- Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H, 2002. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab 87, 5760–5768. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Sharpe RM, Anderson RA, McKinnell C, Macpherson S, Smith LB, Wallace WH, Kelnar CJ, van den Driesche S, 2013. Diethylstilboestrol exposure does not reduce testosterone production in human fetal testis xenografts. PloS one 8, e61726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaso H, Naito M, Hirai S, Matsuno Y, Komiyama M, Itoh M, Mori C, 2014. Neonatal exposure to diethylstilbestrol causes granulomatous orchitis via epididymal inflammation. Anatomical science international 89, 215–223. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Kojima Y, Kurokawa S, Kamisawa H, Kohri K, Hayashi Y, 2011. Altered expression and localization of estrogen receptors alpha and beta in the testes of a cryptorchid rat model. Urology 77, 251 e251–256. [DOI] [PubMed] [Google Scholar]
- Mori T, 1967. Effects of early postnatal injections of estrogen on endocrine organs and sex accessories in male C3H/MS mice. J Fac Sci Univ Tokyo 11, 244–254. [Google Scholar]
- Mowa CN, Iwanaga T, 2001. Expression of estrogen receptor-alpha and -beta mRNAs in the male reproductive system of the rat as revealed by in situ hybridization. Journal of molecular endocrinology 26, 165–174. [DOI] [PubMed] [Google Scholar]
- Mulder E, Brinkmann AO, Lamers-Stahlhofen GJ, van der Molen HJ, 1973. Binding of oestradiol by the nuclear fraction of rat testis interstitial tissue. FEBS Lett 31, 131–136. [DOI] [PubMed] [Google Scholar]
- Mulder E, van Beurden-Lamers WM, De Boer W, Brinkman AO, van der Molen HJ, 1974a. Testicular estradiol receptors in the rat. Curr Top Mol Endocrinol 1, 343–355. [DOI] [PubMed] [Google Scholar]
- Mulder JB, Van Beurden-Lamers WHO, Brinkmann AO, Mechielsen MJ, Van der Molen HJ, 1974b. High affinity binding of oestradiol by rat testis interstitial tissue and by several other tissues of the male rat. J Steroid Biochem 5, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-EspÌn D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, RodrÌguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M, 2013. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 155, 1104–1118. [DOI] [PubMed] [Google Scholar]
- Murashima A, Akita H, Okazawa M, Kishigami S, Nakagata N, Nishinakamura R, Yamada G, 2014. Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Dev Biol 386, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashima A, Xu B, Hinton B, 2015. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl 17, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB, Emmott RC, Hicks LL, Walsh PC, 1980. Estrogen receptors in the human prostate, seminal vesicle, epididymis, testis, and genital skin: a marker for estrogen-responsive tissues? J Clin Endocrinol Metab 50, 938–948. [DOI] [PubMed] [Google Scholar]
- Mutembei H, Kowalewski M, Ugele B, Schuler G, Hoffmann B, 2008. Expression and activity of steroid sulphatase in the boar testis. Reproduction in domestic animals = Zuchthygiene 44, 17–23. [DOI] [PubMed] [Google Scholar]
- N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, Rouiller-Fabre V, Habert R, 2012. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PloS one 7, e51579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Suzuki F, 1976. The postnatal development of the junctional complexes of the mouse Sertoli cells as revealed by freeze-fracture. Anat Rec 185, 403–417. [DOI] [PubMed] [Google Scholar]
- Nagata C, Iwasa S, Shiraki M, Shimizu H, 2006. Estrogen and α-Fetoprotein Levels in Maternal and Umbilical Cord Blood Samples in Relation to Birth Weight. Cancer Epidemiology Biomarkers & Prevention 15, 1469–1472. [DOI] [PubMed] [Google Scholar]
- Naito M, Hirai S, Terayama H, Qu N, Hayashi S, Hatayama N, Kawamura H, Nakano T, Itoh M, 2014. Neonatal estrogen treatment with beta-estradiol 17-cypionate induces in post-pubertal mice inflammation in the ductuli efferentes, epididymis, and vas deferens, but not in the testis, provoking obstructive azoospermia. Medical molecular morphology 47, 21–30. [DOI] [PubMed] [Google Scholar]
- Nakai M, Bouma J, Nie R, Zhou Q, Carnes K, Jassim E, Lubahn DB, Hess RA, 2001. Morphological analysis of endocytosis in efferent ductules of estrogen receptor-alpha knockout male mouse. Anat Rec 263, 10–18. [DOI] [PubMed] [Google Scholar]
- Nakata H, Iseki S, 2019. Three-dimensional structure of efferent and epididymal ducts in mice. Journal of anatomy 235, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa MK, Hess RA, Medrano TI, Locker SH, Levin ER, Cooke PS, 2016. Membrane-localized estrogen receptor 1 Is required for normal male reproductive development and function in mice. Endocrinology 157, 2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa MK, Medrano TI, Mesa AM, Ortega MT, Caldo PD, Mao J, Kinkade JA, Levin ER, Rosenfeld CS, Cooke PS, 2019. Mice lacking membrane estrogen receptor 1 are protected from reproductive pathologies resulting from developmental estrogen exposure. Biol Reprod. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles JL, Hu WY, Prins GS, 2011. Estrogen action and prostate cancer. Expert review of endocrinology & metabolism 6, 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AW, Groen AJ, Miller JL, Warren AY, Holmes KA, Tarulli GA, Tilley WD, Katzenellenbogen BS, Hawse JR, Gnanapragasam VJ, Carroll JS, 2017. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol. Cell. Endocrinol 440, 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA, 1985. Lesions of the rete testis in mice exposed prenatally to diethylstilbestrol. Cancer Res 45, 5145–5150. [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA, 1987. Testicular tumors in mice exposed in utero to diethylstilbestrol. J Urol 138, 1446–1450. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Bjornsdottir S, Hoyer PE, Byskov AG, 2000. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. J Reprod Fertil 118, 195–204. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J, 2001. Mechanisms of estrogen action. Physiological reviews 81, 1535–1565. [DOI] [PubMed] [Google Scholar]
- Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM, 1993. Germ cells of the mouse testis express P450 aromatase. Endocrinology 132, 1396–1401. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, Simpson ER, 2001. Estrogen and spermatogenesis. Endocr Rev 22, 289–318. [DOI] [PubMed] [Google Scholar]
- O’Donnell L, Stanton P, de Kretser DM, 2013. Endocrinology of the Male Reproductive System and Spermatogenesis, in: McLachlan R (Ed.), Endocrinology of the Male Reproductive System. Endotext, South Dartmouth, MA, pp. 1–57. [Google Scholar]
- O’Hara L, Smith LB, 2015. Androgen receptor roles in spermatogenesis and infertility. Best practice & research. Clinical endocrinology & metabolism 29, 595–605. [DOI] [PubMed] [Google Scholar]
- O’Hara L, Welsh M, Saunders PT, Smith LB, 2011. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology 152, 718–729. [DOI] [PubMed] [Google Scholar]
- O’Hara L, Smith LB, 2016. Development and Characterization of Cell-Specific Androgen Receptor Knockout Mice, in: McEwan PIJ (Ed.), The Nuclear Receptor Superfamily: Methods and Protocols. Springer New York, New York, NY, pp. 219–248. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Baker P, Sohnius U, Haavisto A-M, Charlton HM, Huhtaniemi I, 1998. Fetal Development of Leydig Cell Activity in the Mouse Is Independent of Pituitary Gonadotroph Function*. Endocrinology 139, 1141–1146. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Takasugi N, 1974. Ultrastructural changes in the testis of mice given neonatal injections of estrogen. Endocrinol Jpn 21, 183–190. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hermo L, Hess RA, 2005. Aquaporin-1 and −9 are differentially regulated by estrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell 97, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hess RA, 2001. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol Reprod 65, 913–920. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, Franca LR, Hess RA, 2004. Differential hormonal regulation of estrogen receptors ER alpha and ER beta and androgen receptor expression in rat efferent ductules. Reproduction (Cambridge, England) 128, 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Nie R, Carnes K, Franca LR, Prins GS, Saunders PT, Hess RA, 2003. The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod Biol Endocrinol 1, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA, 2002. ER function in the adult male rat: short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology 143, 2399–2409. [DOI] [PubMed] [Google Scholar]
- Omotehara T, Wu X, Kuramasu M, Itoh M, 2020. Connection between seminiferous tubules and epididymal duct is originally induced before sex differentiation in a sex-independent manner. Developmental Dynamics n/a. [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH, 2009. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80, 34–41. [DOI] [PubMed] [Google Scholar]
- Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF, 1978. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health 4, 785–803. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Herbst AL, Noller KL, Boggs DA, Troisi R, Titus-Ernstoff L, Hatch EE, Wise LA, Strohsnitter WC, Hoover RN, 2009. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environmental health : a global access science source 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Carreau S, Szerman-Joly E, Drosdowsky MA, Dehennin L, Scholler R, 1986. Rat testis 17 beta-estradiol: identification by gas chromatography-mass spectrometry and age related cellular distribution. J Steroid Biochem 24, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Kamtchouing P, Drosdowsky MA, Hochereau de Reviers MT, Carreau S, 1987. Adult rat Sertoli cells secrete a factor or factors which modulate Leydig cell function. J Endocrinol 114, 459–467. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler NM, Fisher JS, Sharpe R, Hill E, Van Hoek A, Brown D, Breton S, 2010. Aquaporin 9 expression in the developing rat epididymis is modulated by steroid hormones. Reproduction (Cambridge, England) 139, 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AH, Kelch RP, Musich SS, Halpern ME, 1976. Intratesticular site of aromatization in the human. J Clin Endocrinol Metab 42, 1081–1087. [DOI] [PubMed] [Google Scholar]
- Payne AH, Perkins LM, Georgiou M, Quinn PG, 1987. Intratesticular site of aromatase activity and possible function of testicular estradiol. Steroids 50, 435–448. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EY, Luderer U, Levin ER, 2009. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. The Journal of biological chemistry 284, 3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lewis M, Hammes S, Levin ER, 2014. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Developmental cell 29, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, 2000. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 15, 1261–1270. [DOI] [PubMed] [Google Scholar]
- Pelletier G, El-Alfy M, 2000. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab 85, 4835–4840. [DOI] [PubMed] [Google Scholar]
- Pereyra-Martinez AC, Roselli CE, Stadelman HL, Resko JA, 2001. Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine 16, 15–19. [DOI] [PubMed] [Google Scholar]
- Picciarelli-Lima P, Oliveira AG, Reis AM, Kalapothakis E, Mahecha GA, Hess RA, Oliveira CA, 2006. Effects of 3-beta-diol, an androgen metabolite with intrinsic estrogen-like effects, in modulating the aquaporin-9 expression in the rat efferent ductules. Reprod Biol Endocrinol 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilutin A, Misiakiewicz-Has K, Kolasa A, Baranowska-Bosiacka I, Marchlewicz M, Wiszniewska B, 2014. The immunoexpression of androgen receptor, estrogen receptors alpha and beta, vanilloid type 1 receptor and cytochrome p450 aromatase in rats testis chronically treated with letrozole, an aromatase inhibitor. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society 52, 206–217. [DOI] [PubMed] [Google Scholar]
- Prajapati R, Mitter R, Vezzaro A, Ish-Horowicz D, 2019. Greb1 is required for axial elongation and segmentation in vertebrate embryos. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS, 2001a. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res 61, 6089–6097. [PubMed] [Google Scholar]
- Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C, 2001b. Influence of neonatal estrogens on rat prostate development. Reprod Fertil Dev 13, 241–252. [DOI] [PubMed] [Google Scholar]
- Prins GS, Ho SM, 2010. Early-life estrogens and prostate cancer in an animal model. J Dev Orig Health Dis 1, 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y, 2006. The role of estrogens in normal and abnormal development of the prostate gland. Ann N Y Acad Sci 1089, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Korach KS, 2008. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Hathaway HJ, 2015. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol 153, 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis K, Brenner PF, Landgren BM, Cekan Z, Diczfalusy E, 1975. Indices of gonadal function in the human male. I. Plasma levels of unconjugated steroids and gonadotrophins under normal and pathological conditions. Clin Endocrinol (Oxf) 4, 237–246. [DOI] [PubMed] [Google Scholar]
- Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC, 2001. Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142, 5342–5350. [DOI] [PubMed] [Google Scholar]
- Rago V, Bilinska B, Palma A, Ando S, Carpino A, 2003. Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society 41, 23–27. [PubMed] [Google Scholar]
- Rago V, Romeo F, Giordano F, Malivindi R, Pezzi V, Casaburi I, Carpino A, 2018. Expression of oestrogen receptors (GPER, ESR1, ESR2) in human ductuli efferentes and proximal epididymis. Andrology 6, 192–198. [DOI] [PubMed] [Google Scholar]
- Raines AM, Adam M, Magella B, Meyer SE, Grimes HL, Dey SK, Potter SS, 2013. Recombineering-based dissection of flanking and paralogous Hox gene functions in mouse reproductive tracts. Development (Cambridge, England) 140, 2942–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CM, Ferreira LG, Thimoteo DS, Smith LB, Hinton BT, Avellar MC, 2017. Novel androgen-induced activity of an antimicrobial beta-defensin: Regulation of Wolffian duct morphogenesis. Mol Cell Endocrinol 442, 142–152. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wong YZ, Lubahn D, Gustafsson JA, Cunha G, 2001. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol 229, 432–442. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM, 2002. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology 143, 4797–4808. [DOI] [PubMed] [Google Scholar]
- Rivas A, McKinnell C, Fisher JS, Atanassova N, Williams K, Sharpe RM, 2003. Neonatal coadministration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. J Androl 24, 557–567. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hamzeh M, 2011. Androgen action in the epididymis. J Androl 32, 592–599. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER, 1999. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci U S A 96, 7986–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Simpson ER, Jones ME, 2002. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology 143, 2913–2921. [DOI] [PubMed] [Google Scholar]
- Robertson KM, Simpson ER, Lacham-Kaplan O, Jones ME, 2001. Characterization of the fertility of male aromatase knockout mice. J Androl 22, 825–830. [PubMed] [Google Scholar]
- Robinson JA, Bridson WE, 1978. Neonatal Hormone Patterns In the Macaque. I. Steroids1. Biology of reproduction 19, 773–778. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Judd HL, Young PE, Jones OW, Yen SS, 1977. Amniotic fluid androgens and estrogens in midgestation. J Clin Endocrinol Metab 45, 755–761. [DOI] [PubMed] [Google Scholar]
- Rommerts FF, de Jong FH, Brinkmann AO, van der Molen HJ, 1982. Development and cellular localization of rat testicular aromatase activity. J Reprod Fertil 65, 281–288. [DOI] [PubMed] [Google Scholar]
- Rosati L, Prisco M, Di Lorenzo M, De Falco M, Andreuccetti P, 2020. Immunolocalization of aromatase P450 in the epididymis of Podarcis sicula and Rattus rattus. European journal of histochemistry : EJH 64, 3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumballe BA, Georgas KM, Combes AN, Ju AL, Gilbert T, Little MH, 2011. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev Biol 360, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ, 2014. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology 155, 1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Bartke A, Goh JC, 1989. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat 184, 179–189. [DOI] [PubMed] [Google Scholar]
- Ruz R, Gregory M, Smith CE, Cyr DG, Lubahn DB, Hess RA, Hermo L, 2006. Expression of aquaporins in the efferent ductules, sperm counts, and sperm motility in estrogen receptor-alpha deficient mice fed lab chow versus casein. Mol Reprod Dev 73, 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio K, 2003. Development of the Mesonephric Kidney, in: Vize PD, Woolf AS, JBL Bard JBL(Eds.), The Kidney. Academic Press, Inc, , Cambridge, MA., pp. 75–86. [Google Scholar]
- Sainio K, Hellstedt P, Kreidberg JA, Saxen L, Sariola H, 1997. Differential regulation of two sets of mesonephric tubules by WT-1. Development (Cambridge, England) 124, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Sar M, Welsch F, 2000. Oestrogen receptor alpha and beta in rat prostate and epididymis. Andrologia 32, 295–301. [DOI] [PubMed] [Google Scholar]
- Sato T, Chiba A, Hayashi S, Okamura H, Ohta Y, Takasugi N, Iguchi T, 1994. Induction of estrogen receptor and cell division in genital tracts of male mice by neonatal exposure to diethylstilbestrol. Reprod Toxicol 8, 145–153. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Fisher JS, Sharpe RM, Millar MR, 1998. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J Endocrinol 156, R13–17. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Majdic G, Parte P, Millar MR, Fisher JS, Turner KJ, Sharpe RM, 1997. Fetal and perinatal influence of xenoestrogens on testis gene expression. Adv Exp Med Biol 424, 99–110. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA, 2002. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab 87, 2706–2715. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR, 2001. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Molecular human reproduction 7, 227–236. [DOI] [PubMed] [Google Scholar]
- Saunders PTK, Sierens JE, Groome NP, Millar MR, 2003. Oestrogen receptors in the human and primate testis and reproductive tract. andrologie 13, 43–50. [Google Scholar]
- Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F, 2007. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A 104, 4961–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M, 1984. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry 81, 139–147. [DOI] [PubMed] [Google Scholar]
- Schuler G, Dezhkam Y, Tenbusch L, Klymiuk MC, Zimmer B, Hoffmann B, 2018a. Formation and hydrolysis of sulfonated estrogens in the porcine testis and epididymis. Journal of molecular endocrinology. [DOI] [PubMed] [Google Scholar]
- Schuler G, Dezhkam Y, Tenbusch L, Klymiuk MC, Zimmer B, Hoffmann B, 2018b. SULFATION PATHWAYS: Formation and hydrolysis of sulfonated estrogens in the porcine testis and epididymis. Journal of molecular endocrinology 61, M13–m25. [DOI] [PubMed] [Google Scholar]
- Scobie GA, Macpherson S, Millar MR, Groome NP, Romana PG, Saunders PT, 2002. Human oestrogen receptors: differential expression of ERalpha and beta and the identification of ERbeta variants. Steroids 67, 985–992. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Cox JE, 1982. Secretion of free and conjugated steroids by the horse testis into lymph and venous blood. J Reprod Fertil Suppl 32, 123–127. [PubMed] [Google Scholar]
- Setchell BP, Laurie MS, Flint AP, Heap RB, 1983. Transport of free and conjugated steroids from the boar testis in lymph, venous blood and rete testis fluid. J Endocrinol 96, 127–136. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Huang H, Masch RJ, McFadden DE, Wu XR, Ostrer H, 2005. Immunolocalization of androgen receptor and estrogen receptors alpha and beta in human fetal testis and epididymis. J Urol 174, 1695–1698; discussion 1698. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, 1997. Do males rely on female hormones? [news; comment]. Nature 390, 447–448. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, 2020. Androgens and the masculinization programming window: human-rodent differences. Biochemical Society transactions 48, 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Atanassova N, McKinnell C, Parte P, Turner KJ, Fisher JS, Kerr JB, Groome NP, Macpherson S, Millar MR, Saunders PT, 1998. Abnormalities in functional development of the Sertoli cells in rats treated neonatally with diethylstilbestrol: a possible role for estrogens in Sertoli cell development. Biol Reprod 59, 1084–1094. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Rivas A, Walker M, McKinnell C, Fisher JS, 2003. Effect of neonatal treatment of rats with potent or weak (environmental) oestrogens, or with a GnRH antagonist, on Leydig cell development and function through puberty into adulthood. International journal of andrology 26, 26–36. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE, 1993. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? [see comments]. Lancet 341, 1392–1395. [DOI] [PubMed] [Google Scholar]
- Shaw G, Renfree MB, 2014. Wolffian duct development. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation 8, 273–280. [DOI] [PubMed] [Google Scholar]
- Shayu D, Hardy MP, Rao AJ, 2007. Delineating the role of estrogen in regulating epididymal gene expression. Society of Reproduction and Fertility supplement 63, 31–43. [PubMed] [Google Scholar]
- Shayu D, Rao AJ, 2006. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol Cell Endocrinol 249, 40–50. [DOI] [PubMed] [Google Scholar]
- Shi SR, Chaiwun B, Young L, Cote RJ, Taylor CR, 1993. Antigen retrieval technique utilizing citrate buffer or urea solution for immunohistochemical demonstration of androgen receptor in formalin-fixed paraffin sections. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 41, 1599–1604. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL, 2008. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology 149, 2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius KW, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL, 2009. Estrogen-dependent and -independent estrogen receptor-alpha signaling separately regulate male fertility. Endocrinology 150, 2898–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Walker WH, O’Donnell L, 2015. Hormonal regulation of spermatogenesis through Sertoli cells by androgens and estrogens, in: Griswold M (Ed.), Sertoli Cell Biology, 2nd ed. Elsevier Academic Press, Oxford, pp. 175–200. [Google Scholar]
- Snyder EM, Small CL, Bomgardner D, Xu B, Evanoff R, Griswold MD, Hinton BT, 2010. Gene expression in the efferent ducts, epididymis, and vas deferens during embryonic development of the mouse. Dev Dyn 239, 2479–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Small CL, Li Y, Griswold MD, 2009. Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod 81, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Södersten P, de Jong FH, Vreeburg JT, Baum MJ, 1974. Lordosis behavior in intact male rats: absence of correlation with mounting behavior or testicular secretion of estradiol-17 beta and testosterone. Physiol Behav 13, 803–808. [DOI] [PubMed] [Google Scholar]
- Stefkovich ML, Arao Y, Hamilton KJ, Korach KS, 2017. Experimental models for evaluating non-genomic estrogen signaling. Steroids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato C, Porreca I, Cuomo D, Tarallo R, Nassa G, Ambrosino C, 2016. The “busy life” of unliganded estrogen receptors. Proteomics 16, 288–300. [DOI] [PubMed] [Google Scholar]
- Stewart MK, Mattiske DM, Pask AJ, 2018. In utero exposure to both high- and low-dose diethylsalbestrol disrupts mouse genital tubercle development†. Biology of reproducaon 99, 1184–1193. [DOI] [PubMed] [Google Scholar]
- Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, Albrecht M, Makela S, Mayerhofer A, Poutanen M, 2009. Increased exposure to estrogens disturbs maturation, steroidogenesis, and cholesterol homeostasis via estrogen receptor alpha in adult mouse Leydig cells. Endocrinology 150, 2865–2872. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, 1969. Nuclear concentration of 3H-estradiol in target tissues. Dry-mount autoradiography of vagina, oviduct, ovary, testis, mammary tumor, liver and adrenal. Endocrinology 85, 31–37. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Narbaitz R, Sar M, 1980. Estrogen receptors in the fetal mouse. J Steroid Biochem 12, 55–64. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, 1976. Steroid hormone target sites in the brain: the differential distribution of estrogen, progestin, androgen and glucocorticosteroid. J Steroid Biochem 7, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Suarez-Quian CA, Oke BO, Vornberger W, Prins G, Xiao S, Musto NA, 1996. Androgen Receptor Distribution in the Testis, in: Desjardins C (Ed.), Cellular and Molecular Regulation of Testicular Cells. Springer-Verlag, New York, pp. 189–212. [Google Scholar]
- Sullivan R, Legare C, Lamontagne-Proulx J, Breton S, Soulet D, 2019. Revisiting structure/functions of the human epididymis. Andrology 7, 748–757. [DOI] [PubMed] [Google Scholar]
- Swider-Al-Amawi M, Marchlewicz M, Kolasa A, Wenda-Rozewicka L, Wiszniewska B, 2007. Rat epididymal epithelial cells and 17beta-estradiol synthesis under hCG stimulation in vitro. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society 45, 255–263. [PubMed] [Google Scholar]
- Takasugi N, 1970. Testicular damages in neonatally estrogenized adult mice. Endocrinol Jpn 17, 277–281. [DOI] [PubMed] [Google Scholar]
- Takeda H, Chodak G, Mutchnik S, Nakamoto T, Chang C, 1990. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J Endocrinol 126, 17–25. [DOI] [PubMed] [Google Scholar]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H, 2001. Expression and Cellular Localization of Estrogen Receptors alpha and beta in the Human Fetus. J Clin Endocrinol Metab 86, 2258–2262. [DOI] [PubMed] [Google Scholar]
- Tan K, Song H-W, Wilkinson MF, 2020. Single-cell RNAseq analysis of testicular germ and somatic cell development during the perinatal period. Development (Cambridge, England), dev.183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G, 2005. The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146, 2674–2683. [DOI] [PubMed] [Google Scholar]
- Tapanainen J, Voutilainen R, Jaffe RB, 1989. Low aromatase activity and gene expression in human fetal testes. J Steroid Biochem 33, 7–11. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Jones MB, Besch-Williford CL, Berendzen AF, Ricke WA, F SVS, 2020. Interactive Effects of Perinatal BPA or DES and Adult Testosterone and Estradiol Exposure on Adult Urethral Obstruction and Bladder, Kidney, and Prostate Pathology in Male Mice. International journal of molecular sciences 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K, Okada T, Hayashi Y, Saibara T, 2008. Preserved tissue structure of efferent ductules in aromatase-deficient mice. J Endocrinol 199, 137–146. [DOI] [PubMed] [Google Scholar]
- Toda K, Okada T, Takeda K, Akira S, Saibara T, Shiraishi M, Onishi S, Shizuta Y, 2001. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17beta- oestradiol to aromatase gene (Cyp19) knockout mice. J Endocrinol 168, 455–463. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HH, 2007. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci U S A 104, 11322–11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, Flaws JA, 2007. Effects of ERalpha overexpression on female reproduction in mice. Reprod Toxicol 23, 317–325. [DOI] [PubMed] [Google Scholar]
- Toney TW, Danzo BJ, 1988. Developmental changes in and hormonal regulation of estrogen and androgen receptors present in the rabbit epididymis. Biol Reprod 39, 818–828. [DOI] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr., Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE, 1996. Male reproductive health and environmental xenoestrogens. Environmental health perspectives 104 Suppl 4, 741–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepos-Pouplard M, Lardenois A, Staub C, Guitton N, Dorval-Coiffec I, Pineau C, Primig M, Jegou B, 2010. Proteome analysis and genome-wide regulatory motif prediction identify novel potentially sex-hormone regulated proteins in rat efferent ducts. International journal of andrology 33, 661–674. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris CH, Aquilano DR, Dufau ML, 1985. Cellular localization of rat testicular aromatase activity during development. Endocrinology 116, 38–46. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Morley M, MacPherson S, Millar MR, Wilson JA, Sharpe RM, Saunders PT, 2001. Modulation of gene expression by androgen and oestrogens in the testis and prostate of the adult rat following androgen withdrawal. Mol Cell Endocrinol 178, 73–87. [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Ivell R, Ergun S, 1997. Region-specific expression of the androgen receptor in the human epididymis. Molecular human reproduction 3, 933–940. [DOI] [PubMed] [Google Scholar]
- Valladares L, Payne A, 1979. Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinol 105, 431–436. [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Kilcoyne KR, Wagner I, Rebourcet D, Boyle A, Mitchell R, McKinnell C, Macpherson S, Donat R, Shukla CJ, Jorgensen A, Meyts ER, Skakkebaek NE, Sharpe RM, 2017. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI insight 2, e91204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG, van der Burg B, van der Saag PT, Gustafsson JA, Kuiper GG, 1999. Ontogeny of estrogen receptor-beta expression in rat testis. Endocrinology 140, 478–483. [DOI] [PubMed] [Google Scholar]
- Verkman AS, 2002. Physiological importance of aquaporin water channels. Ann Med 34, 192–200. [PubMed] [Google Scholar]
- Vetter MR, Gibley CW Jr., 1966. Morphogenesis and histochemistry of the developing mouse kidney. Journal of morphology 120, 135–155. [DOI] [PubMed] [Google Scholar]
- Vitkus S, Yeh CR, Lin HH, Hsu I, Yu J, Chen M, Yeh S, 2013. Distinct function of estrogen receptor alpha in smooth muscle and fibroblast cells in prostate development. Mol Endocrinol 27, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV, 1997. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A 94, 2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA, 1994. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology 134, 2307–2316. [DOI] [PubMed] [Google Scholar]
- Waites GM, Einer-Jensen N, 1974. Collection and analysis of rete testis fluid from macaque monkeys. J Reprod Fertil 41, 505–508. [DOI] [PubMed] [Google Scholar]
- Walker VR, Jefferson WN, Couse JF, Korach KS, 2012. Estrogen receptor-alpha mediates diethylstilbestrol-induced feminization of the seminal vesicle in male mice. Environmental health perspectives 120, 560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DW, Huhtaniemi IT, Tapanainen J, Dufau ML, Catt KJ, 1984. Ontogeny of gonadotropin receptors in the fetal and neonatal rat testis. Endocrinology 114, 470–476. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Lathe R, Warner M, Gustafsson JA, 2002. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci U S A 99, 13589–13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA, 2001. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A 98, 6330–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenney C, Pillai S, Tong M, Korach KS, Jameson JL, 2008. Estrogen actions in the male reproductive system involve estrogen response element-independent pathways. Endocrinology 149, 6198–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB, 2009. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 23, 4218–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Sharpe RM, Moffat L, Atanassova N, Saunders PT, Kilter S, Bergh A, Smith LB, 2010. Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PloS one 5, e13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger JP, Chouraqui J, Zeis A, 1993. Production of estradiol by the fetal rat testis. Reproduction, nutrition, development 33, 121–127. [DOI] [PubMed] [Google Scholar]
- Weniger JP, Zeis A, 1983. [Aromatization of testosterone by the rat embryo testis]. C R Seances Acad Sci III 296, 293–296. [PubMed] [Google Scholar]
- Weniger JP, Zeis A, 1988. Stimulation of aromatase activity in the fetal rat testis by cyclic AMP and FSH. J Endocrinol 118, 485–489. [DOI] [PubMed] [Google Scholar]
- Wichmann U, Wichmann G, Krause W, 1984. Serum levels of testosterone precursors, testosterone and estradiol in 10 animal species. Exp Clin Endocrinol 83, 283–290. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL, 1995. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med 332, 1411–1416. [DOI] [PubMed] [Google Scholar]
- Williams K, Fisher JS, Turner KJ, McKinnell C, Saunders PT, Sharpe RM, 2001a. Relationship between expression of sex steroid receptors and structure of the seminal vesicles after neonatal treatment of rats with potent or weak estrogens. Environmental health perspectives 109, 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M, 2001b. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update 7, 236–247. [DOI] [PubMed] [Google Scholar]
- Williams K, Saunders PTK, Atanassova N, Fisher JS, Turner KJ, Millar MR, McKinnell C, Sharpe RM, 2000. Induction of progesterone receptor immunoexpression in stromal tissue throughout the male reproductive tract after neonatal oestrogen treatment of rats. Mol Cell Endocrinol 164, 117–131. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Giguère V, 2008. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Molecular cancer 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, 2014. Estrogen in the fetus. Adv Exp Med Biol 814, 217–228. [DOI] [PubMed] [Google Scholar]
- Woodham C, Birch L, Prins GS, 2003. Neonatal estrogen down-regulates prostatic androgen receptor through a proteosome-mediated protein degradation pathway. Endocrinology 144, 4841–4850. [DOI] [PubMed] [Google Scholar]
- Xing Z, Ma WK, Tran EJ, 2019. The DDX5/Dbp2 subfamily of DEAD-box RNA helicases. Wiley Interdiscip Rev RNA 10, e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Washington AM, Domeniconi RF, Ferreira Souza AC, Lu X, Sutherland A, Hinton BT, 2016a. Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct. Dev Biol 412, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Washington AM, Hinton BT, 2016b. Initial Segment Differentiation Begins During a Critical Window and Is Dependent upon Lumicrine Factors and SRC Proto-Oncogene (SRC) in the Mouse. Biol Reprod 95, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, 2004. Localization of estrogen and androgen receptors in male reproductive tissues of mice and rats. Anat Rec 279A, 768–778. [DOI] [PubMed] [Google Scholar]
- Yao G, Hu S, Yu L, Ru Y, Chen CD, Liu Q, Zhang Y, 2017. Genome-wide mapping of in vivo ERα-binding sites in male mouse efferent ductules. Endocrinology 158, 3724–3737. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kihara T, Tanimura T, 1985. Effect of ethinyl estradiol on the differentiation of mouse fetal testis. Teratology 32, 113–118. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, Bergmann M, Schulze H, 1991. Organization of tubules in the human caput epididymidis and the ultrastructure of their epithelia. Am J Anat 191, 261–279. [DOI] [PubMed] [Google Scholar]
- You L, Sar M, 1998. Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine 9, 253–261. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Dunlap JL, Gerall AA, 1979. Modifications induced by neonatal steroids in reproductive organs and behavior of male rats. J Comp Physiol Psychol 93, 314–322. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Potter SS, 2002. Functional comparison of the Hoxa 4, Hoxa 10, and Hoxa 11 homeoboxes. Dev Biol 244, 21–36. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA, 2001. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci U S A 98, 14132–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Miao M, Ran M, Ding L, Bai L, Wu T, Yuan W, Gao E, Wang J, Li G, Li DK, 2013. Serum bisphenol-A concentration and sex hormone levels in men. Fertility and sterility 100, 478–482. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA, 2002. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23, 870–881. [PubMed] [Google Scholar]
- Zhou X, Kudo A, Kawakami H, Hirano H, 1996. Immunohistochemical localization of androgen receptor in mouse testicular germ cells during fetal and postnatal development. The Anatomical record 245, 509–518. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Hardy MP, Inigo IV, Huhtaniemi I, Bardin CW, Moo-Young AJ, 2000. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: a quantitative immunohistochemical study. Biol Reprod 63, 368–376. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Papadopoulos V, 2018. Leydig cells: formation, function, and regulation. Biol Reprod 99, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


