Abstract
Paired Box 8 (PAX8) is a lineage-specific transcription factor that has essential roles during embryogenesis and tumorigenesis. The importance of PAX8 in the development of the reproductive system is highlighted by abnormalities observed upon the loss or mutation of this PAX family member. In cancer, PAX8 expression is deregulated in a key set of neoplasms, including those arising from the Müllerian ducts. The roles of PAX8 in oncogenesis are diverse and include epigenetic remodeling, stimulation of proliferation, inhibition of apoptosis, and regulation of angiogenesis. PAX8 can interact with different protein partners during cancer progression and may exhibit significant function-altering alternative splicing. Moreover, expression of PAX8 in cancer can also serve as biomarker for diagnostic and prognostic purposes. In this review, we focus on the roles of PAX8 in cancers of the reproductive system. Understanding the diverse mechanisms of action of PAX8 in development and oncogenesis may identify new vulnerabilities in malignancies that currently lack effective therapies.
Keywords: PAX8, Reproductive system, endometrial cancer, ovarian cancer, testicular cancer
INTRODUCTION
The establishment of cell lineages that give rise to specific tissues during embryogenesis requires the strict regulation of gene expression. The genesis of tissue stem cells, their maintenance, and terminal differentiation is essential for successful development and normal tissue homeostasis. These events are highly organized and require the coordinated expression of multiple factors, including the PAX family of transcription factors (1). PAX genes were initially described in the common fruit fly, Drosophila melanogaster, and subsequently identified in many different species (2). Their ancestral role in Drosophila is in embryonic development where they influence segmentation of the larvae and promote proper eye and brain development (3,4).
The PAX family is composed of nine genes in mammals, which can be sub-classified into four classes and it is described in details in table 1 (5). These genes encode nuclear transcription factors that include a combination of up to four functional domains: an N-terminal paired domain with DNA-binding activity, a conserved octapeptide motif, a homeodomain (which can serve as a second independent DNA-binding region), and a transactivation domain at the C-terminus (1). The paired domain, which consists of 128 amino acids, is the most conserved and most studied. It is folded into two β-sheets and six α-helices divided into two subdomains, PAI and RED. Mutations in PAI or RED subdomains disrupt the DNA-binding activity and lead to congenital abnormalities (6). Furthermore, PAX members can form heterodimers by interacting with homeodomain-containing partners, such as HOX and SOX members (7). This cooperativity alters their binding specificity and changes the set of regulated target genes that determine cell type and temporal activation. Moreover, it was also demonstrated that PAX members have pioneering activity, i.e. they can directly bind to condensed chromatin and recruit other transcription factors and histone modification enzymes that contribute to transcription factors hierarchical regulation of chromatin landscape and accessibility (8,9).
Table 1:
Overview of the PAX family.
| Class | Gene | Chromosomal location |
Structure | Post-translation modification |
Isoforms | Translocations | Mutations |
|---|---|---|---|---|---|---|---|
| I | PAX1 | 20p11 | Paired domain Octapeptide |
Amidation Hydroxylation Glycosylation |
3 isoforms | A277V A277T |
|
| PAX9 | 14q12 | Sumoylation or Ubiquitination Phosphorylation |
1 isoform | A24D/T G96D/S |
|||
| II | PAX2 | 10q24 | Paired domain Octapeptide Partial homeodomain |
Methylation Amidation Acetilation Phosphorylation Glycosylation |
6 isoforms | V26G R403G |
|
| PAX5 | 9p13 | Sulfatation Sumoylation or Ubiquitination Glutathionylation |
11 isoforms | PAX5-ETV6 PAX5-FOXP1 PAX5-ZNF521 |
V26F/G R104C/H/L R239C/H |
||
| PAX8 | 2q12 | 6 isoforms | PAX8-PPARG | G41V D94N R64S |
|||
| III | PAX3 | 2q35 | Paired domain Octapeptide Homeodomain |
ADP-ribosylation Amidation Phosphorylation Carboxylation Glycosylation |
8 isoforms | PAX3-FOXO1 PAX3-NCOA1 |
R220S F294S A411E |
| PAX7 | 1p36 | 4 isoforms | PAX7-FOXO1 | S155L P408L/S/T G459A/C/S |
|||
| IV | PAX4 | 7q32 | Paired domain Homeodomain |
ADP-ribosylation Glycosylation Amidation Ubiquitination Phosphorylation |
3 isoforms | R166L/Q/W E180K |
|
| PAX6 | 11p13 | 4 isoforms | H376T H390T P389H |
Data obtained and modified from https://www.ncbi.nlm.nih.gov/, http://dbptm.mbc.nctu.edu.tw/, and https://www.cbioportal.org/.
Dysregulation of embryogenesis programs (through genetic or epigenetic changes) can lead to tumorigenesis (10). Some of these aberrant changes reactivate PAX factors and drive the emergence of a more embryonic undifferentiated state with loss of regulated growth, survival, and migratory programs (11). PAX proteins contribute to these neoplastic processes through their ability to regulate cellular networks by controlling transcription and chromatin remodeling activities (12,13). Herein, we focused on the transcription factor PAX8 to provide a comprehensive assessment of its importance during reproductive system development and malignant transformation.
ROLES OF PAX8 DURING DEVELOPMENT
During embryogenesis, the PAX proteins maintain epithelial progenitor cells mitotically active before fully committing to their fate (1). The first indication that PAX8 is vital during embryonic development came from the identification of hypothyroidism due to thyroid dysgenesis or agenesis in newborns with point mutations or deletions in the PAX8 gene (14). Of the PAX8 loss-of-function mutations reported in different populations of patients with congenital hypothyroidism and concomitant urogenital malformation, only the G41V and D94N mutations in the critical DNA-binding domain of PAX8 have been functionally characterized. The PAX8-G41V mutant demonstrated loss of DNA-binding activity and the inability to bind and activate the promoters of the Thyroglobulin and Thyroid Peroxidase genes (15).
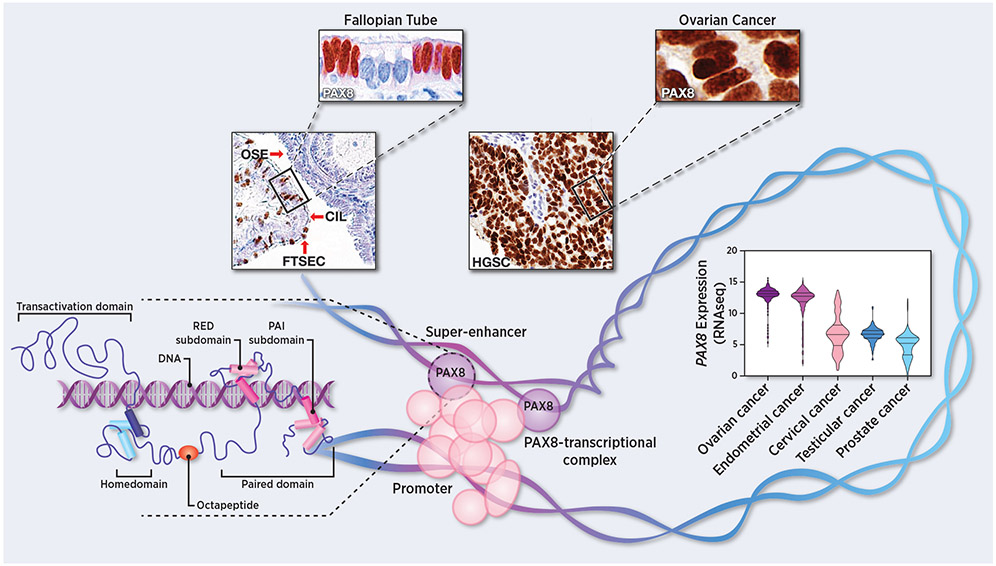
Subsequent genetic studies showed that Pax8−/− mice presented with pronounced thyroid hypoplasia, were drastically underdeveloped and died shortly after weaning (16). Early thyroid hormone replacement allowed female Pax8−/− mice to survive; however, they were infertile. The mice also showed abnormalities in the Müllerian duct-derived tissues, such as atresia of the uterus, vaginal opening, and impairment of the oviducts due to hydrosalpinx (17). During development, Pax8 expression in wildtype mice is present in the kidney, the thyroid gland, and the normal epithelium of the Müllerian ducts, the origin of the female reproductive system (18). In adult tissues, PAX8 expression is found in the kidney, thyroid gland, Müllerian derived tissues (cervix, fallopian tube, and endometrium), and male reproductive tissues (seminal vesicle and epididymis) (19). Interestingly, PAX8 staining is localized in the nuclei of healthy fallopian tube secretory epithelial cells (Figure 1), which can self-renew and differentiate into normal fallopian tube epithelial ciliated cells to preserve the functional oviduct structure (20). PAX8 is expressed in human and mouse oviductal epithelial progenitor cells, which are the cell-of-origin of the majority of high-grade serous carcinomas (21,22).
Figure 1:
Unraveling the mysteries of PAX8. Top, representative images of PAX8 immunostaining of normal fallopian tube epithelium (FTSEC = fallopian tube secretory epithelial cells and CIL = ciliated cells) normal ovary (OSE = ovarian surface epithelial cells), and invasive high-grade serous carcinoma (HGSC). Bottom, PAX8 transcript expression levels across different reproductive tract cancers (TCGA dataset). Insertion, graphic representation of PAX8-transcriptional complex highlighting the PAX8 structural domains.
As suggested by its expression pattern, PAX8 also plays a pivotal role in the development of the male genital system and is expressed in the Wolffian ducts during the embryonic period (23). Treatment of male Pax8−/− mice with thyroxine restored the general deficits of congenital hypothyroidism, but these mice were infertile due to the absence of efferent ducts, epididymis, and spermatozoa (24). Therefore, PAX8 plays an important role during mouse development to guide the proper formation of the reproductive organs of both sexes.
ALTERATIONS OF PAX8 EXPRESSION IN THE CANCERS OF THE REPRODUCTIVE SYSTEM
PAX8 is over-expressed in genital malignancies, enhancing both the survival and the proliferation of tumor cells (25). The upregulation of PAX8 in endometrial and ovarian cancer tissues is accompanied by a higher risk of death, (lower 5-year survival), and disease recurrence (26,27). Pan-cancer analysis of PAX8 expression showed higher expression in ovarian and endometrial cancer than in cervical, testicular, or prostate cancers (Figure 1). In particular, PAX8 expression is higher in ovarian and uterine cancers of serous histology than those of endometrioid histology and in testicular seminomas compared to non-seminoma. Interestingly, PAX8 expression trends higher as disease stage increases (Supplementary Figure 1 A-D).
To begin deciphering the function of PAX8 in ovarian cancer, RNA-Seq analysis of high-grade serous ovarian carcinoma (HGSOC) cell lines following PAX8 knockdown were performed to define the transcriptional network directly regulated by PAX8 (12). Surprisingly, this analysis revealed a negligible impact of PAX8 knockdown on the transcriptomes of immortalized fallopian tube secretory epithelial cell lines (FTSEC) with very few transcripts significantly affected by PAX8 loss. Moreover, PAX8 knockdown had negligible effect on viability or cellular proliferation, consistent with results in murine FTSECs (28). This was not due to functional redundancy between PAX8 and other PAX family members because PAX8 was the only PAX gene significantly expressed in these cells. In contrast, PAX8 loss in ovarian cancer cell lines had a marked impact on the transcriptional profile of the cells, with PAX8 target genes enriched in cell proliferation, angiogenesis and cell adhesion pathways, processes that are crucial for tumor progression (12).
To determine whether there were differences in how PAX8 interacts with chromatin between the benign and malignant state, ChIP-Seq analyses were performed showing that PAX8 binding sites are re-distributed in the genome of cancer cells (12). Notably, only a small percentage of peaks (~6%) were near promoter regions. The vast majority of binding peaks were located in either intronic or intergenic regions, including super-enhancers. Corroboration of this findings were observed after the discovery that PAX8, SOX17, and MECOM are tumor-driving master transcription factors required for cell viability and lied proximal to super-enhancers in HGSOC cells. ChIP-Seq analyses also revealed that these factors co-occupy HGSOC regulatory elements globally and co-bind at critical target genes (13).
PAX8 re-distribution in cancer cells was shown to be in part due to changes in the PAX8 network. Interestingly, purification of the PAX8 protein complex from ovarian cancer cells revealed that PAX8 is part of a 600 kilodalton chromatin-remodeling complex, called NurD. Among the protein partners that PAX8 associates with is SOX17, another master transcription factor that helps to orchestrate ovarian cancer angiogenesis and progression (29).
PAX8 is highly expressed in fallopian tube epithelia, a precursor of HGSOC and is commonly amplified in ovarian cancer, emphasizing its essential role in tumor development (30). The PAX8 super-enhancer was detected in all ovarian cancers histotypes and was most active in serous and endometrioid ovarian cancers. Moreover, 90% of ovarian cancers harbor an alteration in the PAX8 pathway, either by somatic amplification of the PAX8 locus, mutation of enhancers upstream of PAX8, or somatic mutations in PAX8 or TEAD binding sites, all of which can lead to the deregulation of PAX8 and its gene targets (31).
While our understanding of the molecular functions of PAX8 are still evolving, recent studies indicate that PAX8 governs transcriptional programs involved in the regulation of tumor-stromal interactions, cell adhesion, proliferation, survival, and metastasis (25,32-34). PAX8, as a master transcription factor, has a complex oncogenic mechanism that depends on cell type identity. Adding further complexity, Kozmik and colleagues showed the expression of six different isoforms of PAX8 that are usually co-expressed with PAX2, and their expression levels change during neoplasia (35). The exact roles of the various isoforms during transformation are not completely understood at this time. In addition, a chromosomal translocation between chromosomes 2 and 13 that results in a PAX8-PPARG fusion protein that affects PAX8 transcriptional regulation has been reported in thyroid cancer, but is not common in other cancers (36).
Experimental evidence suggests both a direct and an indirect role for PAX8 in tumorigenesis. PAX8 functions as a transcription factor that drives activation of specific target genes and also associates with different interacting partners that regulate cell fate and identity.
IMPLICATIONS FOR DRUG TARGETING
Targeting PAX transcription factors as a strategy to inhibit cancer growth could prove highly useful for treating specific subsets of PAX-driven malignancies. Since PAX factors are tissue-specific, this therapeutic strategy is expected to have lower levels of toxicity than approaches that perturb targets with pervasive expression. In addition, while a number of normal tissues express some levels of PAX8 (such as the kidney and the thyroid gland) there is evidence that cancers that overexpress PAX8 become “addicted” to its activity, while normal tissues do not appear to exhibit the same dependency (12,28,30,32,37-40). This dependency may be related to the specific set of interactions observed in cancer (described above). Importantly, this strong dependency may help mitigate the issue of intra-tumoral heterogeneity of PAX8 expression, as even cells with low expression would be expected to be dependent on its activity. These issues are currently under investigation.
Strategies to inhibit PAX8 can be grouped in three main categories: 1) those that reduce PAX8 levels, 2) those that inhibit its DNA binding activity, and 3) those that inhibit its binding to crucial interacting protein(s). As a proof of concept, Hardy and colleagues showed that reducing PAX8 levels, either genetically or by treatment with the thiostrepton, a natural cyclic oligopeptide antibiotic, leads to a reduction in tumor burden (34). Moreover, Shi and colleagues demonstrated that blockade of HDAC1, a PAX8-interacting partner, perturbed the super-enhancer topology associated with PAX8 gene locus, resulting in epigenetic downregulation of PAX8 transcripts and related targets, efficaciously suppressing ovarian cancer growth (39).
Interestingly, the targeting of the paired domain has been shown to inhibit DNA-binding activity. For this purpose, Grimley and colleagues identified a small-molecule, EG1, that was able to abrogate the transcription factor DNA-binding activity and also slowed the growth of ovarian cancer cells (41). As an example of targeting protein-protein interaction, disrupting super-enhancers as therapeutic strategy could complement traditional approaches and/or lead to new drug discovery. Pharmacologic inhibition of CDK7 impairs phosphorylation of RNA polymerase II, and results in preferential down-regulation of super-enhancer-regulated genes (such as PAX8) (42). High-grade serous ovarian cancer models are exquisitely sensitive to pharmacologic inhibition of CDK7 by THZ1. PAX8 and SOX17 levels were particularly sensitive to low-doses of THZ1 treatment and are among the 10%-most sensitive protein-coding transcripts. In addition, PAX8 and SOX17 knockdowns both phenocopy effects of low-dose THZ1 treatment, suggesting these factors, at least in part, explain the anti-cancer effect of this drug in ovarian cancer cells. PAX8 and SOX17 target genes are largely overlapping, with some of the most downregulated genes falling into cell cycle, DNA replication, and DNA division pathways, including cell-cycle regulators in the retinoblastoma pathway such as known ovarian cancer oncogene CCNE1 (13,29).
Because of its restricted expression patterns in normal tissues, and the strong dependency of certain cancers to its expression, PAX8 and its interacting-partners represent promising targets for cancer therapy. If successful, the current efforts focused at inhibiting PAX8 are expected to have a significant impact on individuals with PAX8-dependent cancers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kate Lawrenson for her suggestions and critical reading of the manuscript and Lauren Schwartz, Levon Katsakhyan, and Xiaoming Zhang for assistance with histology and image development. This work was supported by NIH SPORE P50 CA228991 (R.D.), the US Department of Defense (OC170094, OC180420), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (R.D.), the Honorable Tina Brozman Foundation for Ovarian Cancer Research (R.D.), The V Foundation (R.D.), The Basser Center for BRCA (R.D.), the Claneil Foundation (R.D.), and an Ann and Sol Schreiber Mentored Investigator Award from the Ovarian Cancer Research Fund Alliance (D. C-M).
Footnotes
Disclosure of Potential Conflicts of Interest:
R. Drapkin serves on the scientific advisory board of Repare Therapeutics and VOC Health, and has received consulting fees from Boehringer Laboratories, Cedilla Therapeutics, and TwoXAR. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Robson EJ, He SJ, Eccles MR. A PANorama of PAX genes in cancer and development. Nat Rev Cancer 2006;6:52–62 [DOI] [PubMed] [Google Scholar]
- 2.Paixao-Cortes VR, Salzano FM, Bortolini MC. Origins and evolvability of the PAX family. Semin Cell Dev Biol 2015;44:64–74 [DOI] [PubMed] [Google Scholar]
- 3.Kozmik Z The role of Pax genes in eye evolution. Brain Res Bull 2008;75:335–9 [DOI] [PubMed] [Google Scholar]
- 4.Dahl E, Koseki H, Balling R. Pax genes and organogenesis. Bioessays 1997;19:755–65 [DOI] [PubMed] [Google Scholar]
- 5.Mayran A, Pelletier A, Drouin J. Pax factors in transcription and epigenetic remodelling. Semin Cell Dev Biol 2015;44:135–44 [DOI] [PubMed] [Google Scholar]
- 6.Campagnolo M, Pesaresi A, Zelezetsky I, Geremia S, Randaccio L, Bisca A, et al. Structural studies on Pax-8 Prd domain/DNA complex. J Biomol Struct Dyn 2007;24:429–41 [DOI] [PubMed] [Google Scholar]
- 7.Jolma A, Yin Y, Nitta KR, Dave K, Popov A, Taipale M, et al. DNA-dependent formation of transcription factor pairs alters their binding specificity. Nature 2015;527:384–8 [DOI] [PubMed] [Google Scholar]
- 8.Mayran A, Sochodolsky K, Khetchoumian K, Harris J, Gauthier Y, Bemmo A, et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun 2019;10:3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015;161:555–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 11.Maulbecker CC, Gruss P The oncogenic potential of Pax genes. EMBO J 1993;12:2361–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias KM, Emori MM, Westerling T, Long H, Budina-Kolomets A, Li F, et al. Epigenetic remodeling regulates transcriptional changes between ovarian cancer and benign precursors. JCI Insight 2016;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy J, Fonseca MAS, Corona RI, Nameki R, Segato Dezem F, Klein IA, et al. Predicting master transcription factors from pan-cancer expression data. bioRxiv 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet 1998;19:83–6 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Wang X, Zou H, Ge Y, Wang F, Wang Y, et al. Identification and characterization of novel PAX8 mutations in Congenital Hypothyroidism(CH) in a Chinese population. Oncotarget 2017;8:8707–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedrichsen S, Christ S, Heuer H, Schafer MK, Parlow AF, Visser TJ, et al. Expression of pituitary hormones in the Pax8−/− mouse model of congenital hypothyroidism. Endocrinology 2004;145:1276–83 [DOI] [PubMed] [Google Scholar]
- 17.Mittag J, Winterhager E, Bauer K, Grummer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology 2007;148:719–25 [DOI] [PubMed] [Google Scholar]
- 18.Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 1990;110:643–51 [DOI] [PubMed] [Google Scholar]
- 19.Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol 2011;24:751–64 [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Syed SM, Tanwar PS. In vivo genetic cell lineage tracing reveals that oviductal secretory cells self-renew and give rise to ciliated cells. Development 2017;144:3031–41 [DOI] [PubMed] [Google Scholar]
- 21.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 2013;24:751–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soong TR, Howitt BE, Horowitz N, Nucci MR, Crum CP. The fallopian tube, "precursor escape" and narrowing the knowledge gap to the origins of high-grade serous carcinoma. Gynecol Oncol 2019;152:426–33 [DOI] [PubMed] [Google Scholar]
- 23.Magers MJ, Udager AM, Chinnaiyan AM, French D, Myers JL, Jentzen JM, et al. Comprehensive Immunophenotypic Characterization of Adult and Fetal Testes, the Excretory Duct System, and Testicular and Epididymal Appendages. Appl Immunohistochem Mol Morphol 2016;24:e50–68 [DOI] [PubMed] [Google Scholar]
- 24.Wistuba J, Mittag J, Luetjens CM, Cooper TG, Yeung CH, Nieschlag E, et al. Male congenital hypothyroid Pax8−/− mice are infertile despite adequate treatment with thyroid hormone. J Endocrinol 2007;192:99–109 [DOI] [PubMed] [Google Scholar]
- 25.Di Palma T, Filippone MG, Pierantoni GM, Fusco A, Soddu S, Zannini M. Pax8 has a critical role in epithelial cell survival and proliferation. Cell Death Dis 2013;4:e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mhawech-Fauceglia P, Wang D, Samrao D, Godoy H, Pejovic T, Liu S, et al. Pair-Box (PAX8) protein-positive expression is associated with poor disease outcome in women with endometrial cancer. Br J Cancer 2012;107:370–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai HJ, Ren Q, Fan Q, Ye L, Du GY, Du HW, et al. PAX8 is a potential marker for the diagnosis of primary epithelial ovarian cancer. Oncol Lett 2017;14:5871–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodgers LH, E Oh, Young AN, Burdette JE. Loss of PAX8 in high-grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget 2016;7:32785–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaves-Moreira D, Mitchell M, Arruza C, Rawat P, Sidoli S, Nameki R, et al. PAX8 orchestrates an angiogenic program through interaction with SOX17. bioRxiv 2020 [Google Scholar]
- 30.Cheung HW, Cowley GS, Weir BA, Boehm JS, Rusin S, Scott JA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci U S A 2011;108:12372–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corona RI, Seo JH, Lin X, Hazelett DJ, Reddy J, Fonseca MAS, et al. Non-coding somatic mutations converge on the PAX8 pathway in ovarian cancer. Nat Commun 2020;11:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghannam-Shahbari D, Jacob E, Kakun RR, Wasserman T, Korsensky L, Sternfeld O, et al. PAX8 activates a p53-p21-dependent pro-proliferative effect in high grade serous ovarian carcinoma. Oncogene 2018;37:2213–24 [DOI] [PubMed] [Google Scholar]
- 33.Soriano AA, de Cristofaro T, Di Palma T, Dotolo S, Gokulnath P, Izzo A, et al. PAX8 expression in high-grade serous ovarian cancer positively regulates attachment to ECM via Integrin beta3. Cancer Cell Int 2019;19:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy LR, Pergande MR, Esparza K, Heath KN, Onyuksel H, Cologna SM, et al. Proteomic analysis reveals a role for PAX8 in peritoneal colonization of high grade serous ovarian cancer that can be targeted with micelle encapsulated thiostrepton. Oncogene 2019;38:6003–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozmik Z, Kurzbauer R, Dorfler P, Busslinger M. Alternative splicing of Pax-8 gene transcripts is developmentally regulated and generates isoforms with different transactivation properties. Mol Cell Biol 1993;13:6024–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science 2000;289:1357–60 [DOI] [PubMed] [Google Scholar]
- 37.Di Palma T, Lucci V, de Cristofaro T, Filippone MG, Zannini M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. BMC Cancer 2014;14:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adler EK, Corona RI, Lee JM, Rodriguez-Malave N, Mhawech-Fauceglia P, Sowter H, et al. The PAX8 cistrome in epithelial ovarian cancer. Oncotarget 2017;8:108316–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi K, Yin X, Cai MC, Yan Y, Jia C, Ma P, et al. PAX8 regulon in human ovarian cancer links lineage dependency with epigenetic vulnerability to HDAC inhibitors. Elife 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koumarianou P, Gomez-Lopez G, Santisteban P. Pax8 controls thyroid follicular polarity through cadherin-16. J Cell Sci 2017;130:219–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimley E, Liao C, Ranghini EJ, Nikolovska-Coleska Z, Dressler GR. Inhibition of Pax2 Transcription Activation with a Small Molecule that Targets the DNA Binding Domain. ACS Chem Biol 2017;12:724–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y, Long W, Liu Q. Targeting Super-Enhancers as a Therapeutic Strategy for Cancer Treatment. Front Pharmacol 2019;10:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.