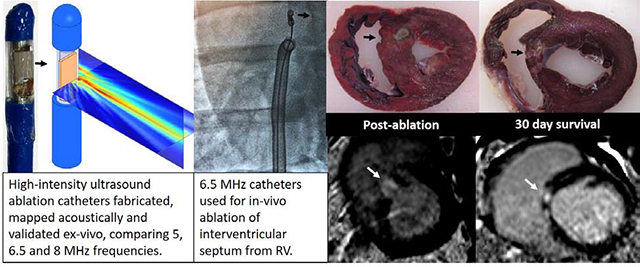
Abstract
Background:
Radiofrequency (RF) ablation of epicardial and mid-myocardial ventricular arrhythmias (VAs) is limited by lesion depth.
Objectives:
To generate deep, mid-interventricular septal (IVS) lesions using high-intensity ultrasound (US) from an endocardial catheter-based approach.
Methods:
Irrigated US catheters (12 Fr) were fabricated with 3×5 mm transducers of 5.0, 6.5 and 8.0 MHz frequencies, and compared in an ex vivo perfused myocardial ablation model. In vivo septal ablation in swine (n=12) was performed via femoral venous access to the right ventricle. Lesions were characterized by echocardiography, cardiac MRI and electroanatomic voltage mapping pre- and post-ablation, and at 30 days. Four animals were euthanized immediately post-ablation to compare acute and chronic lesion histology and gross pathology.
Results:
In ex vivo models, maximal lesion depth and volume was achieved by 6.5 MHz catheters, which were used in vivo. Lesion depth by gross pathology was similar post-ablation (10.8[95% CI:9.9,12.4] mm) and at 30 days (11.2[CI:10.6,12.4] mm, p=0.56). Lesion volume decreased post-ablation to 30 days (255[CI:198,440] to 162[CI:133,234] mm3 p=0.05), yet transmurality increased 58%(CI:50,76) to 81%(CI:74,93), attributable to a reduction in IVS thickness (16.0±1.7 to 10.6±2.4 mm, p=0.007). MRI confirmed dense septal ablation by delayed enhancement, with increased T1 time post-ablation and at 30 days, and increased T2 time only post-ablation. Voltage mapping of both sides of IVS demonstrated reduced unipolar (but not bipolar) voltage along the IVS.
Conclusions:
High-intensity US catheter ablation may be an effective treatment for mid-myocardial or epicardial VAs from an endocardial approach.
Keywords: ventricular tachycardia, ventricular arrhythmia, mid-myocardial, ultrasound, catheter ablation
Graphical Abstract
Introduction:
Ventricular arrhythmias (VA) often arise from deep mid-myocardial or epicardial sites, particularly in patients with non-ischemic cardiomyopathy1,2, or those with idiopathic VAs from the left ventricular (LV) summit3. Radiofrequency ablation (RF) is limited by shallow depth (1.9 – 6.7 mm)4–6, often precluding mid-myocardial ablation, or epicardial ablation from an endocardial approach. Furthermore, RF ablation of the basal inter-ventricular septum (IVS) from a right ventricular (RV) approach may increase risk of damage to the His-Purkinje system which is often sub-endocardial in location7.
Therapeutic high-intensity ultrasound (HIU) is achieved by applying ultrasound energy, often in the diagnostic frequency range (1–10 MHz) but at sufficiently high amplitude (~10 MPa) to generate localized tissue heating and thermal necrosis8. By virtue of the natural focusing and deep tissue penetration of ultrasound (US) energy, HIU ablation has been shown to demonstrate deep, nearly transmural lesions from an epicardial approach, while sparing the immediately adjacent tissue9. The application of HIU for arrhythmias has focused primarily on epicardial ultrasound device placement which allows ablation without near-field injury to epicardial vessels9,10. HIU ablation from an endocardial approach has not been fully explored, but has the potential to make deep, mid-myocardial lesions from an endocardial catheter location. The sparing of the near-field during HIU ablation may also be advantageous for avoiding damage to the His-Purkinje system when performing ablations of the IVS from an RV approach.
In this study, we designed, fabricated, acoustically characterized, optimized and tested HIU ablation catheters suitable for ablation of the IVS from a femoral venous approach to the RV. We hypothesized that this approach could produce deep, mid-myocardial lesions without His-Purkinje injury and iatrogenic atrio-ventricular (AV) block.
Methods:
Catheter assembly and acoustic testing
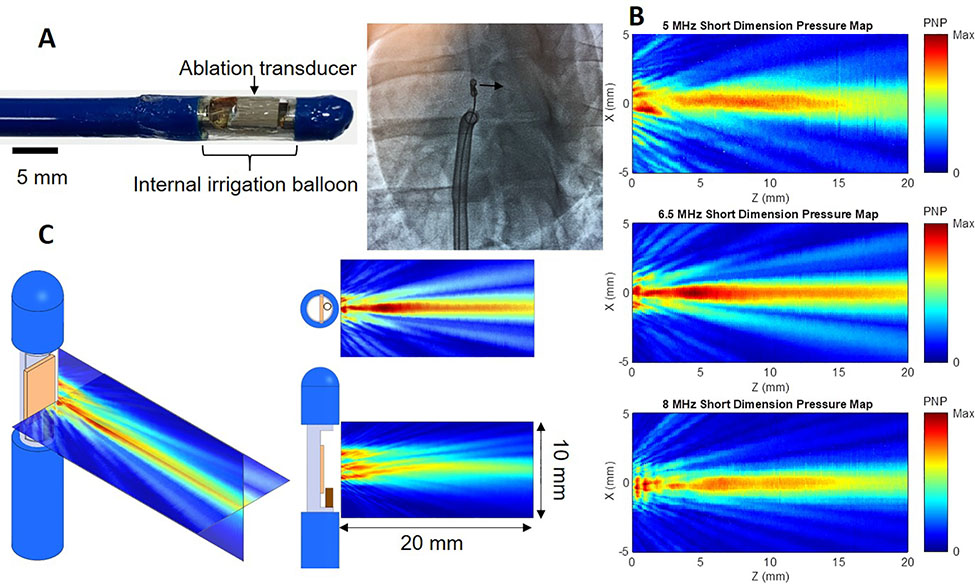
Side-facing HIU ablation catheters were fabricated from piezoelectric crystals, electrical cable, modified stainless steel tubing, nylon tubing and electrical connectors (Fig. 1A). Each catheter contained a planar 5.0, 6.5 and 8.0 MHz single-element PZT-4 piezoceramic crystal 3 × 5 mm in dimension (EBL Products Inc.; East Hartford, CT) which was soldered to 34 AWG micro coaxial electrical cable (Alphawire; Elizabeth, NJ) and a BNC connector (Amphenol; Wallingford, CT). Edges of the US crystal were bonded to a partially cut stainless steel hypodermic tubing that served as a rigid transducer platform with air backing. The transducer platform assembly was then bonded to 12 French (Fr) flexible nylon catheter tubing (Freelin-Wade Inc.; McMinnville, OR). The transducer assemblies were encapsulated within a thin-walled polyester balloon, through which closed-loop internal irrigation was performed with deionized and degassed water at 10° C with flow rate of 50 ml/min supplied by a peristaltic pump. Finally, impedance matching networks were built to achieve >99% electrical power transmission.
Figure 1.
High-intensity ultrasound (HIU) catheter and acoustic fields. A: 12 Fr internally-irrigated HIU catheters (A) were built and directed to the right ventricular septum under transthoracic echocardiogram and fluoroscopic guidance (left anterior oblique 15° view demonstrates transducer in profile and aiming toward the septum). B: Acoustic field maps measured using a needle hydrophone in degassed water, where the catheter surface is at Z = 0 mm and HIU pressure propagates in the positive Z direction, indicating the 6.5 MHz (middle) catheter to generate the deepest lesions and largest acoustic field. C: Further characterization of the 6.5 MHz HIU catheter’s acoustic field in two orthogonal planes.
PNP: peak negative pressure.
Catheters were operated in continuous wave mode using signals produced by a waveform generator (Agilent 33250A; Santa Clara, CA), amplified by a power amplifier (Amplifier Research 600A225; Souderton, PA) and monitored using a high-voltage passive detection US probe (LeCroy PPE 1.2 kV; Chestnut Ridge, NY) connected to an oscilloscope (LeCroy Waverunner 44 MXi-A; Chestnut Ridge, NY). After fabrication, the ultrasound pressure field for each catheter was characterized in filtered, deionized and degassed water at room temperature using a needle hydrophone (75 μm, Precision Acoustics; Dorchester, United Kingdom) to map peak negative acoustic pressures.
Ex Vivo Myocardial Ablation Protocol
Ovine hearts were obtained from animals receiving intravenous heparin immediately prior to euthanasia. Hearts were studied within 30 minutes of explant. A 5 Fr perfusion catheter was sutured in the proximal left anterior descending coronary artery (LAD) to perfuse the anterior myocardium. The heart was suspended in a degassed waterbath at room temperature. A solution of 1% bovine serum albumin in normal saline was perfused through the LAD at 30 ml/min using a non-peristaltic syringe pump. Epicardial HIU lesions were applied at a power of 30 W for 60 seconds. Eight separate lesions were made in the LAD perfusion territory: one anteroseptal line of 4 extending from basal to apical anteroseptum 1 cm lateral and parallel to the LAD, and a second anterior line of 4 just 1 cm lateral to the first line. Ultrasound frequency (n=32 sonications for each frequency) was randomized on a per-heart basis.
In Vivo Myocardial Ablation Protocol
This study was approved and monitored by the Oregon Health & Science University Institutional Animal Care and Use Committee under guidelines set forth by the Association for the Assessment and Accreditation of Laboratory Animal Care, and consistent with the Guide for the Care and Use of Laboratory Animals. Female farm swine (sus domesticus) (40–45 kg) were anesthetized with tiletamine and zolazepam and intubated. Anesthesia was maintained with inhaled isoflurane (1.5–3.5% via 2 L/min O2). Isothermia was maintained with water-heated blankets. The HIU catheter was advanced via femoral venous access through a 14 Fr steerable sheath to the RV, and guided to the RV septum using fluoroscopic and trans-thoracic echocardiogram (TTE) guidance. Eight sonications at a power of 30 W were performed for 60 seconds, approximately 1 cm apart in a 4 × 2 pattern with 4 lesions extending from the basal to mid anteroseptum, and a second row from basal to mid inferoseptum. Continuous ECG monitoring was performed during the procedure, with attention to AV conduction, QRS width and morphology. Four swine were euthanized immediately post-ablation per protocol for assessment of acute lesions. The remainder were survived for 30 days.
Imaging and Electroanatomic Mapping
TTE (Epiq CV, Philips Ultrasound, Andover, MA) was performed with a phased-array probe and tissue harmonic filtering pre-ablation, immediately post-ablation, and when applicable, at 30 days. Parasternal short-axis views at the basal and mid-ventricular planes, and apical 4-chamber views were obtained at a transmit frequency of 1.6 MHz.
A subset of swine underwent cardiac MRI pre-ablation (4), immediately post-ablation (6) and at 30 days (6). MRI was performed on a 3.0T Siemens Prisma system (Erlangen, Germany) using the body coil for RF transmitting and spine- and body-matrix coils for signal receiving, and with ECG gating. After standard T1- and T2- weighted scans, 0.1 mmol/kg Prohance gadolinium contrast agent (Bracco; Milan, Italy) was administered intravenously. First-pass perfusion as well as T1-weighted delayed-enhanced images were acquired ~ 20 minutes post-injection with phase-sensitive inversion recovery reconstruction. MRI analysis was performed using dedicated MRI software (Circle Cardiovascular Imaging; Calgary, Canada). The number of lesions and their locations were first assessed on short axis slices of delayed enhancement images. The corresponding regions on the native (pre-contrast) T1 and T2 maps were sampled for T1 and T2 times at lesion locations. We used MOdified Look Locker Inversion recovery (MOLLI sequences) to measure T1. These measurements were also normalized to their respective T1 and T2 times directly opposite in the un-ablated, inferolateral LV myocardium to account for variations between swine. T1 and T2 times were made in the middle-third of the ventricular myocardium to avoid partial volume effects.
Electroanatomic voltage mapping of the RV and LV septum was also performed in a subset of 4 swine using a PentaRay (Biosense Webster International; Diamond Bar, CA) pre- and post-ablation, and at 30 days. Bipolar voltage mapping was assessed at standard threshold of 0.5 and 1.5 mV, and unipolar voltage at 1.0 and 5.5 mV for RV and 1.0 and 8.3 mV for LV septum.
Gross Pathology and Histology
For both ex vivo and in vivo studies, the heart was perfused with 10 gm 2,3,5-Triphenyl-2H-tetrazolium chloride (TTC) in 50 mL saline (through the perfusion catheter for ex vivo studies and via central venous access for in vivo studies) to aid visual inspection of myocardial necrosis. Hearts were fixed in 10% formalin at 4°C for 1 week, then sectioned in 3 mm thick short axis segments. Segments containing lesions were further sectioned to measure individual lesion sizes in 3 dimensions, then further fixed and stained with Masson’s trichrome and hematoxylin & eosin (H&E).
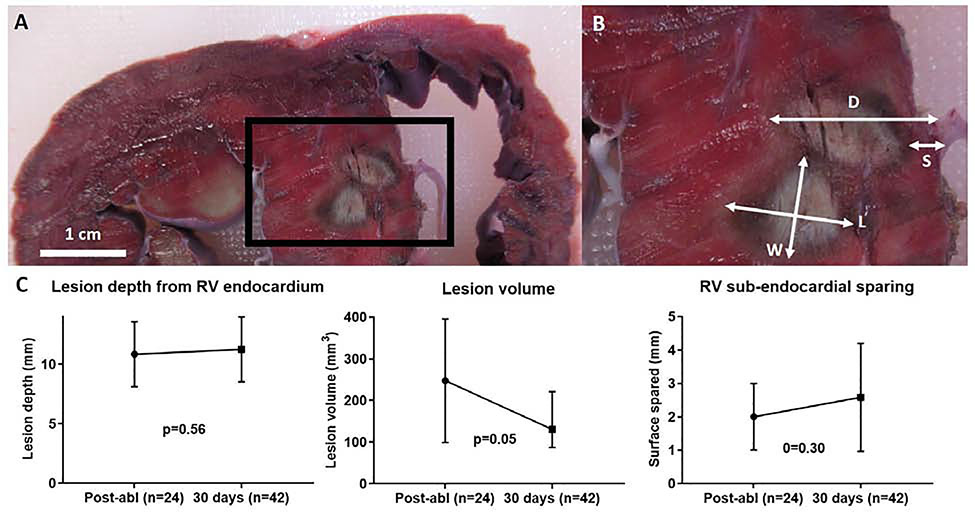
Lesion depth was measured starting from and orthogonal to the RV endocardium, and ending at the distal (toward the LV) end of the lesion (Fig. 2B). RV sub-endocardial sparing measurement also started at the RV endocardium, but extended toward the proximal (toward the RV) end of the lesion (Fig. 2B). Lesion volume was calculated using visual observation of lesion boundaries as input to the revolved ellipsoid volume formula (4/3 × π × L/2 × W/2 × T/2) where L = lesion length (measured in the axis going away from the transducer from RV-to-LV endocardium), W = width (from antero- to infero-septum), and T = thickness (along basal-to-apical axis). This equation has been validated for assessment of ablation lesions11. Sparing of the tissue immediately adjacent to the transducer (“subendocardial sparing”) was defined as the distance from the RV endocardial surface to the beginning of the lesion along the “lesion length” axis above. Lesion transmurality (defined as % of the IVS thickness bounded by the lesion at its cross-section) and near- (RV side of mid-septum) vs far-field (LV side of mid-septum) deposition of necrosis was quantified by determining the IVS midline using a custom image processing algorithm utilizing spline analysis (Supplemental materials).
Figure 2.
In-vivo myocardial lesion characteristics. A: short-axis gross pathology of two adjacent lesions in the IVS. B: magnification of IVS demonstrating lesion measurements (d, depth; s, sub-endocardial surface sparing; l, length; w, width; t, thickness is not shown, but was measured by sectioning serial short-axis cross-sections). C: Quantification of gross pathologic lesion characteristics. Graph bars are means with error bars as standard deviation.
IVS: inter-ventricular septum
For histologic analysis of necrosis, inflammatory infiltrate, hemorrhage and fibrosis, lesion blocks were further sectioned at a thickness of 10 μm, and stained with hematoxylin and eosin (H&E) and Masson’s trichrome.
Statistical Analysis
Data were analyzed using Prism GraphPad Software (version 8.4.1) and SAS (version 9.4). Group-wise differences were assessed by Mann-Whitney U test for data that were determined to be non-normally distributed.. For data with normal distribution, group-wise differences were assessed by student’s t-test (paired for comparisons at different timepoints). These data presented as mean ± SD. A one-way analysis of variance (ANOVA) test was used when comparing multiple groups/timepoints. One survival swine died at 14 days. Gross pathology data from this swine was included with the 30 day survival swine. When comparing repeated measures (multiple ablation lesions per animal) among multiple swine, fixed-effects regression models were fit and standard errors adjusted to address correlation among measurements from the same animal. Means with bootstrapped 95% confidence intervals (CI) based on 500 replications are reported from these models. All statistical tests were two-sided with p≤0.05 considered significant.
Results:
Comparison of Ultrasound Frequencies
The acoustic fields of HIU catheters with fundamental frequencies of 5.0, 6.5 and 8.0 MHz were mapped (Fig. 1) and predicted that 6.5 MHz would generate the largest and deepest acoustic field. Ablation at 5 MHz did not reproducibly result in necrotic lesions (32 sonications yielded only 4 identifiable small lesions on gross pathology), likely due to reduced thermal effects of US at lower frequencies having fewer harmonics formed during nonlinear acoustic propagation12,13. Ablation at 6.5 MHz generated lesions that were deeper (10.7±3.2 vs 8.3±2.6 mm from the RV endocardium, p=0.0015) and larger (223±171 vs 137±95 mm3 in volume, p=0.028) than at 8 MHz (Supplemental Fig. 1). Sparing of the RV sub-endocardial surface tissue immediately adjacent to the transducer was similar between both frequencies (1.6±1.2 vs 1.5±0.6 mm distance from RV endocardial surface to ablation lesion, p=0.797). To increase study efficiency, in vivo studies were performed only with 6.5 MHz HIU catheters.
In Vivo Ablation: Pathologic Analysis
In the 4 animals euthanized after post-ablation imaging, histology detected 24 necrotic lesions out of 32 HIU ablation attempts (75% efficiency); while 42 necrotic lesions were found out of 64 HIU ablation attempts (66% efficiency) in survival animals. Sonication locations that were more apically located were least likely to result in visible lesions on gross pathology, largely due to inability of the custom steerable sheath to reach the apical septum. HIU lesions were typically ellipsoid in shape (Fig. 2), similar to prior descriptions of HIU epicardial LV lesions, and in contrast to the typical half-ellipsoid shape of RF lesions9. Mean lesion depth was similar immediately post-ablation (10.8[95% CI:9.9,12.4] mm) and at 30 days (11.2[CI:10.6,12.4]mm, p=0.56), although lesion volume decreased over time (255[CI:198,440] to 162[CI:133,234] mm3 p=0.05; Fig. 2). The RV sub-endocardial surface was similarly spared at both timepoints (2.2[CI:1.7,3.3] vs 2.7[CI:2.3,3.4] mm, p=0.3). Similar to lesion depth, lesion length (L, going away from the transducer from RV-to-LV endocardium and excluding RV sub-endocardial sparing) was similar at both timepoints. However, lesion width (W) decreased from 6.4(CI:5.9,7.0) to 5.5(CI:5.0,6.1) mm (p=0.006) and lesion thickness (T) decreased from 7.8(CI:7.2,8.4) to 6.2(CI:5.6,6.8) mm at 30 days (p<0.0001).
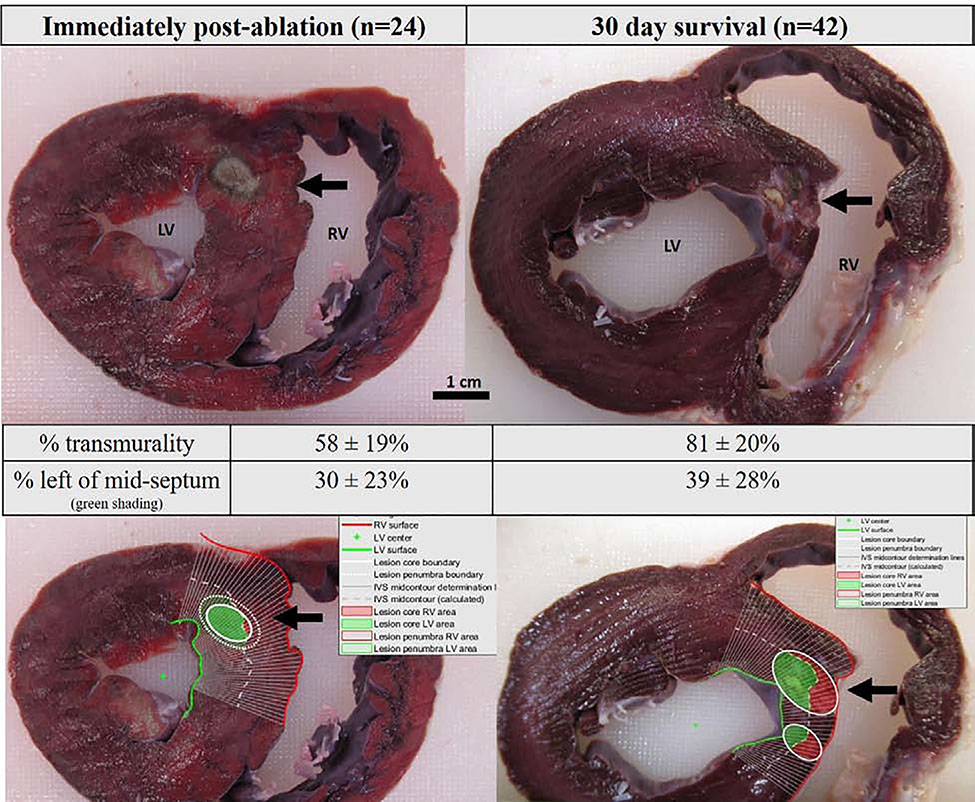
Based on spline analysis image processing, a similar portion of lesion surface area was deposited on the LV side of the IVS (30%[CI:22,46] post-ablation vs 39%[CI:33,51] at 30 days, p=0.29; Fig. 3). Spline analysis showed that lesion transmurality increased from 58%(CI:50,76) to 81%(CI:74,93) over 30 days (p=0.002). Increased transmurality despite decreased lesion volume at 30 days compared with immediately post-ablation was attributable to IVS remodeling resulting in remodeling in thickness: IVS thickness at the level of the lesions decreased from 16.0±1.7 mm post-ablation to 10.6±2.4 mm (p=0.0074) at 30 days (Fig. 3).
Figure 3.
Gross pathology images of in-vivo lesions immediately post-ablation (left) and at 30 days (right). Bottom row images demonstrate image processing with spline analysis defining the portion of lesion surface area to the right (red) and left ventricular (green) side of the septum, with the mid-septum defined as the white dotted line.
Histology demonstrated myocyte disruption without fibrosis or inflammation immediately post-ablation. At 30 days, dense mid-septal fibrosis was noted without surrounding inflammatory infiltrate (Supplemental Figure 2).
TTE, MRI and Electroanatomic Mapping
TTE confirmed gross pathology findings: diastolic IVS thickness by TTE increased from 11.8±1.3 mm at baseline to 13.4±1.6 mm immediately post-ablation (presumed due to edema), but decreased to 10.0±1.3 mm at 30 days (p=0.0003) despite swine growth. LV ejection fraction at baseline was 58±6%, decreased to 49±4% immediately post-ablation (largely due to hypokinesis of the basal IVS), and recovered to 58±5% at 30 days (p=0.0002 by ANOVA for between-group differences; p=0.92 for comparison between pre-ablation and 30 days post-ablation).
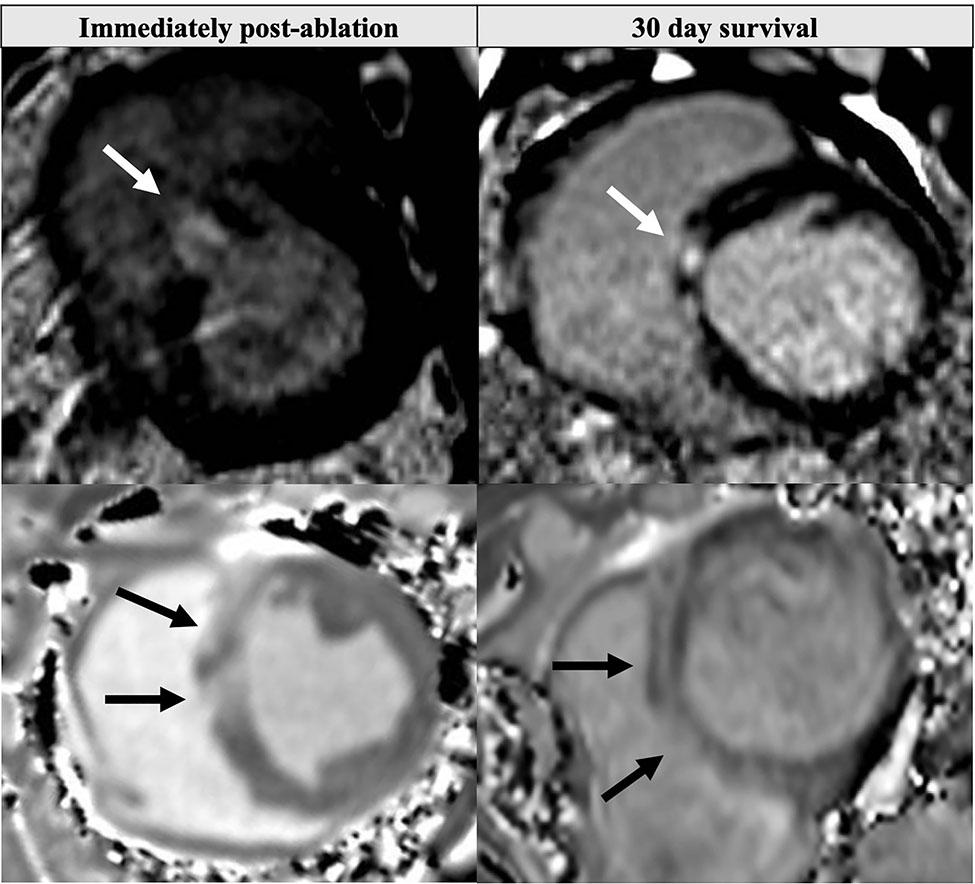
MRI also confirmed IVS remodeling: IVS thickness increased from 11.0±0.8 mm at baseline to 13.8±1.7 mm immediately post-ablation (presumed due to edema) but decreased to 10.6±1.1 mm at 30 days (p=0.003) despite swine growth. First-pass MRI contrast perfusion imaging demonstrated dense perfusion defects in the basal-mid IVS immediately post-ablation (Supplemental Videos 1–2). Late gadolinium enhancement detected areas of HIU ablation in the IVS, but resolution was insufficient to quantify individual lesion size on a per-lesion basis: 3.6±1.1 regions of LGE were present post-ablation and 3.4±1.1 regions at 30 days (Fig. 4; Supplemental Fig. 3), likely due to coalescence of multiple HIU lesions into single LGE regions. T1 time at the basal IVS HIU ablation sites increased from 1176±18 ms pre-ablation to 1408±87 ms post-ablation, and remained elevated at 30 days (1319±128 ms, p=0.008). T2 time increased from 40.4±5.1 to 64.2±6.0 post-ablation, but then returned to near baseline 47.1±5.8 ms at 30 days (p=0.1169 for comparison with pre-ablation; p<0.0001 for group-wise comparison). Data were similar when normalizing septal ablation site T1 and T2 values with basal inferolateral (non-ablated) T1 and T2 values.
Figure 4.
Short-axis MRI images of HIU lesions immediately post-ablation (left) and at 30 days (right). Late gadolinium enhancement in the septum (top row) corresponded to increased pre-contrast T1 time (bottom row). Ablation had been performed from the RV in the direction of the white arrows.
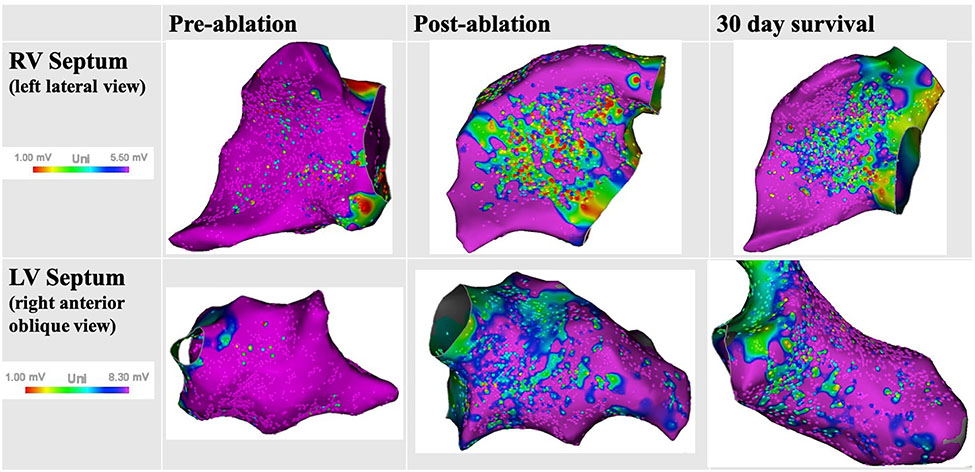
Electroanatomic voltage mapping did not reveal any bipolar voltage abnormalities at standard voltage thresholds, but demonstrated new areas of reduced unipolar voltage along the basal and mid RV septum immediately post-ablation (10.6±6.1 cm2) and at 30 days (7.0±3.5 cm2, p=0.35). Similar unipolar voltage findings were present on the LV septum (7.6±0.5 cm2 vs 6.0±1.8 cm2, p=0.31) confirming deep lesions (Fig. 5). No fractionated or late electrograms were noted, suggesting fairly homogenous, deep ablation.
Figure 5.
Electroanatomic unipolar voltage mapping of the right and left ventricular septum. New unipolar voltage abnormalities noted post-ablation and at 30 days, confirming presence of deep mid-myocardial lesions.
Procedural Complications
No AV block, QRS widening, pericardial effusions or ventricular septal defects were noted intra- or post-procedure. All survival swine recovered uneventfully from anesthesia after the procedure. One survival swine died suddenly at 14 days post-procedure with no structural abnormalities determined at autopsy.
Discussion:
There are spatial challenges related to catheter ablation of VAs. Mid-myocardial sites of VA origin and epicardial sites of origin in patients unsuitable for percutaneous subxiphoid pericardial access pose significant challenges for RF ablation. Several novel strategies have arisen to improve upon shallow lesion depth of standard RF ablation, including an RF needle catheter14, stereotactic body radiation therapy15, use of hypotonic irrigated RF ablation16, bipolar RF ablation17,18, and alcohol venous ablation19, although access to these therapies is limited, and recurrence and complication rates remain high.
There are several potential advantages of using HIU over RF for targeting mid-myocardial and epicardial sites from the endocardium. Lower US absorption in tissue allows deeper penetration. Moreover, ultrasound transducers can be geometrically focused, or the natural focus of flat US element may permit an “offset” which, with catheter cooling by irrigation, can spare tissue immediately adjacent to the transducer, thereby avoiding the sub-endocardial His-Purkinje system with septal ablation. Finally, ultrasound-based ablation may permit the real-time monitoring of the region of thermal injury which could help guide lesion placement or size.
In our study, HIU ablation from the RV side of the IVS resulted in lesions of approximately 11 mm depth, initially spanning 58% of the IVS acutely increasing to 81% transmural at 30 days. This increase in transmurality was, in part, explained by remodeling of the IVS, which was noted to markedly decrease in thickness in response to septal ablation. Indeed, 38% of the lesion surface area was deposited on the far-field, LV side of the IVS, with the first 2–3 mm of the RV septal sub-endocardium spared. No QRS widening, bundle branch block or AV block were noted, suggesting protection of the sub-endocardial His-Purkinje system. However, it should be noted that while extensive ablation of the basal-mid RV antero-septum was performed, sonications were not performed in a dedicated manner over the His and right bundle potentials, but simply in its general region. Furthermore, we did not histopathologically define the His-Purkinje system after sacrifice, and thus were not able define the proximity of our ablation lesions to the conduction system. No acute complications were noted, but there was one autopsy-negative sudden death event at 14 days that we presume was due to ventricular arrhythmia secondary to heterogenous necrosis in the IVS with lesions spaced 1 cm apart, although late AV block cannot be excluded20.
HIU lesions were successfully identified by cardiac MRI, which may serve as a means of assessing lesion extent clinically either using delayed gadolinium enhancement, or non-contrast T1 imaging, as has previously been shown for RF21. In our study, T1 and T2 times at the IVS were both elevated immediately post-ablation, whereas only T1 remained elevated at 30 days, suggesting an acute edematous +/− inflammatory reaction (although significant inflammation not noted histologically post-ablation), resolving to dense, mid-septal fibrosis without edema or inflammation at 30 days (which was confirmed by histology). It should be noted that our MRI imaging lacked the resolution to confidently measure individual lesion dimensions for correlation with gross pathology.
Limitations of our study include lack of an RF control group, although the shallow depth of RF ablation has been well-documented4–6, including in our prior study where it was 4.7±4.0 mm9 and significantly less deep than epicardial HIU. Swine were not monitored with telemetry or implantable loop recorder monitoring over the 30 day survival period, we cannot exclude delayed, transient AV block. We also did not test a large range of frequencies, transducer sizes (restricted to 3 × 5 mm transducers that would accommodate a 12 Fr catheter), or differences in acoustic power or lesion duration time. Instead, we used HIU frequencies within a range known to promote thermal necrosis22 and our previous experience to narrow our range of HIU variables, compared 5, 6.5 and 8 MHz in ex vivo models, which identified 6.5 MHz as the optimal frequency for the transducer size under in vivo study.
Another limitation is the large variability in lesion sizes. While TTE was used to confirm placement against the RV endocardial surface, we cannot exclude the periodic non-contact which could explain the variability, as well as why lesion efficiency was not 100% (i.e. fewer histologic lesions than ablations performed; most evident for sonications made in the most apical portions of the RV), and also why lesion size varied. Furthermore, our side-facing transducer may not have always been exactly parallel to the IVS. Future iterations of the catheter should employ a contact- and orientation-sensing mechanism. Integration of the catheter into electroanatomic mapping may also aid in assessing contact, as well as allow us to guide HIU ablation directly over the His-Purkinje system to formally demonstrate sparing of the conduction system with surface-sparing lesions. Finally, our studies were not performed in a model of arrhythmia or cardiomyopathy. It has been shown that RF applications within myocardial scar are less likely to generate reliable and large necrotic lesions compared with applications performed in healthy myocardium due to heterogeneous electrical impedance of scar tissue23. HIU thermal necrosis relies on tissue acoustic impedance, which is more homogenous across tissue types than electrical impedance24. Thus, HIU lesions may perform more efficiently in scar, although this has yet to be tested.
Future investigations of HIU should assess lesions in large animal cardiomyopathy models, use long-term ambulatory rhythm monitoring for pro-arrhythmia, and assess endocardial-to-epicardial lesions with ablation over the non-septal LV endocardium. While we previously demonstrated a dose-response relationship between HIU powers of 15–30W and lesion depth and volume9 (and thus chose 30 W for this study as it is the maximal acoustic power that the transducer and circuitry can tolerate in continuous wave mode), further control of lesion depth and size may also be achieved with adjusting transducer size or ablation time, and should be investigated. Our finding of reduction in IVS thickness is intriguing, and warrants further investigation with a consolidated lesion set over the basal septum to determine if HIU may be a suitable treatment for septal reduction therapy in hypertrophic cardiomyopathy.
Conclusion
This first study of HIU endocardial ventricular ablation demonstrates deep, nearly transmural mid-myocardial lesion formation with sparing of the sub-endocardium and His-Purkinje system.
Supplementary Material
Acknowledgements:
We would like to thank Xin Li, PhD, William Woodward, MS, Chris Diederich, PhD and Peter Douglas Jones, BS for their technical assistance. We would like to thank Traci L Schaller BS, LVT, LAT for veterinary and anesthesia care. We would like to thank Thomas Murphy for assistance with electroanatomic mapping.
Funding: Dr. Nazer is supported by a grant (K08-HL138156) from the National Institutes of Health (NIH); and, Dr. Nazer and Mr. Giraud are supported by an E21 Physician-Engineer Partnership Award from the American Society of Echocardiography, Durham, NC. Dr. Lindner is supported by grants (R01-HL078610, R01-HL130046, and P51-OD011092) from the NIH.
Conflicts of Interest: This study was partially supported by an investigator-initiated grant from Biosense-Webster International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oloriz T, Silberbauer J, MacCabelli G, et al. : Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: Anteroseptal versus inferolateral scar sub-types. Circulation: Arrhythmia and Electrophysiology 2014; 7:414–423. [DOI] [PubMed] [Google Scholar]
- 2.Haqqani HM, Tschabrunn CM, Tzou WS, et al. : Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart rhythm 2011; 8:1169–76. [DOI] [PubMed] [Google Scholar]
- 3.Romero J, Shivkumar K, Valderrabano M, et al. : Modern mapping and ablation techniques to treat ventricular arrhythmias from the left ventricular summit and interventricular septum. Heart Rhythm 2020; 17:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4.Jauregui-Abularach ME, Campos B, Betensky BP, Michele J, Gerstenfeld EP: Comparison of epicardial cryoablation and irrigated radiofrequency ablation in a Swine infarct model. Journal of cardiovascular electrophysiology 2012; 23:1016–23. [DOI] [PubMed] [Google Scholar]
- 5.John RM, Connell J, Termin P, et al. : Characterization of Warm Saline-Enhanced Radiofrequency Ablation Lesions in the Infarcted Porcine Ventricular Myocardium. Journal of cardiovascular electrophysiology 2014; 25:309–316. [DOI] [PubMed] [Google Scholar]
- 6.D’Avila A, Houghtaling C, Gutierrez P, et al. : Catheter ablation of ventricular epicardial tissue: A comparison of standard and cooled-tip radiofrequency energy. Circulation 2004; 109:2363–2369. [DOI] [PubMed] [Google Scholar]
- 7.Nagarajan VD, Ho SY, Ernst S: Anatomical Considerations for His Bundle Pacing. Circ: Arrhythmia and Electrophysiology 2019; 12. Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.118.006897 [DOI] [PubMed] [Google Scholar]
- 8.Nazer B, Gerstenfeld EP, Hata A, Crum L a., Matula TJ: Cardiovascular applications of therapeutic ultrasound. Journal of Interventional Cardiac Electrophysiology 2014; 39:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazer B, Salgaonkar V, Diederich CJ, et al. : Epicardial Catheter Ablation Using High-Intensity Ultrasound: Validation in a Swine Model. Circulation: Arrhythmia and Electrophysiology 2015; 8:1491–1497. [DOI] [PubMed] [Google Scholar]
- 10.Koruth JS, Dukkipati S, Carrillo RG, et al. : Safety and efficacy of high-intensity focused ultrasound atop coronary arteries during epicardial catheter ablation. Journal of cardiovascular electrophysiology 2011; 22:1274–80. [DOI] [PubMed] [Google Scholar]
- 11.Koruth JS, Dukkipati S, Carrillo RG, et al. : Safety and efficacy of high-intensity focused ultrasound atop coronary arteries during epicardial catheter ablation. Journal of cardiovascular electrophysiology 2011; 22:1274–1280. [DOI] [PubMed] [Google Scholar]
- 12.Swindell W: A theoretical study of nonlinear effects with focused ultrasound in tissues: An “acoustic bragg peak.” Ultrasound in Medicine & Biology 1985; 11:121–130. [DOI] [PubMed] [Google Scholar]
- 13.Hynynen K, Roemer R, Anhalt D, et al. : A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. International Journal of Hyperthermia Taylor & Francis, 2010; 26:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson WG, Tedrow UB, Reddy V, et al. : Infusion Needle Radiofrequency Ablation for Treatment of Refractory Ventricular Arrhythmias. Journal of the American College of Cardiology 2019; 73:1413–1425. [DOI] [PubMed] [Google Scholar]
- 15.Cuculich PS, Schill MR, Kashani R, et al. : Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. New England Journal of Medicine 2017; 377:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DT, Tzou WS, Sandhu A, et al. : Prospective Multicenter Experience With Cooled Radiofrequency Ablation Using High Impedance Irrigant to Target Deep Myocardial Substrate Refractory to Standard Ablation. JACC: Clinical Electrophysiology 2018; 4:1176–1185. [DOI] [PubMed] [Google Scholar]
- 17.Koruth JS, Dukkipati S, Miller MA, Neuzil P, D’Avila A, Reddy VY: Bipolar irrigated radiofrequency ablation: A therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm 2012; 9:1932–1941. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen DT, Tzou WS, Brunnquell M, et al. : Clinical and biophysical evaluation of variable bipolar configurations during radiofrequency ablation for treatment of ventricular arrhythmias. Heart Rhythm 2016; 13:2161–2171. [DOI] [PubMed] [Google Scholar]
- 19.Kreidieh B, Rodríguez-Mañero M, Schurmann A P, Ibarra-Cortez SH, Dave AS, Valderrábano M: Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Ventricular Tachycardia. Circ Arrhythm Electrophysiol 2016; 9. Available from: https://www.ahajournals.org/doi/10.1161/CIRCEP.116.004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleszynski PA, Goldenberg I, Fernandez G, et al. : Risk of arrhythmic events following alcohol septal ablation for hypertrophic cardiomyopathy using continuous implantable cardiac monitoring. Heart Rhythm 2020; 10.1016/j.hrthm.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Kholmovski EG, Silvernagel J, Angel N, et al. : Acute noncontrast T1-weighted magnetic resonance imaging predicts chronic radiofrequency ablation lesions. J Cardiovasc Electrophysiol 2018; 29:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgaonkar VA, Diederich CJ: Catheter-based ultrasound technology for image-guided thermal therapy: Current technology and applications. International Journal of Hyperthermia Taylor & Francis, 2015; 31:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barkagan M, Leshem E, Shapira-Daniels A, et al. : Histopathological Characterization of Radiofrequency Ablation in Ventricular Scar Tissue. JACC: Clinical Electrophysiology 2019; 5:920–931. [DOI] [PubMed] [Google Scholar]
- 24.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY: Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound in Medicine & Biology 1998; 24:1419–1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.