Abstract
This study reports the results of a longitudinal study examining the effects of treatment for sentence processing deficits for a 70-year-old gentleman (DK) with the agrammatic variant of Primary Progressive Aphasia (PPA). On entry into the study, he presented with a 2-year history of impaired verb and sentence processing and concomitant neural atrophy in primarily subcortical regions. Spanning an 18-month period, treatment focused on improving comprehension and production of syntactically complex, passive and object cleft, structures, consecutively. Results, derived from extensive behavioral and neurocognitive testing, showed not only improved ability to comprehend and produce both trained and untrained, less complex, linguistically related structures in offline tasks, but also improved online sentence processing strategies as revealed by partially normalized eye movements in online comprehension (i.e., emergence of thematic prediction and thematic integration) and production (i.e., use of incremental processing) tasks. Changes in neural activation from pre- to post-treatment of both structures also were found, with upregulation of tissue in both the left and right hemispheres, overlapping with regions recruited by neurotypical adults performing the same task. These findings indicate that Treatment of Underlying Forms (TUF) is effective for treatment of patients with the agrammatic variant of PPA (as it is for those with stroke-induced agrammatism), and show that unaffected neural tissue in patients with PPA is malleable and may be recruited to support language, providing evidence of experience-based plasticity in neurodegenerative disease.
Keywords: neural plasticity, Primary Progressive Aphasia (PPA), agrammatic variant of PPA, Treatment of Underlying Forms (TUF), Wh-movement structures, NP-movement structures, eyetracking, visual world paradigms, functional neuroimaging
Introduction
Research examining the effects of language treatment in patients with primary progressive aphasia (PPA) has shown that behavioral intervention results in improved language ability. As shown in Table 1, which provides a review of 26 studies, including 131 participants across studies, most studies have focused on improving noun (or verb) naming (15 studies), and only one has directed treatment toward improving sentence-level deficits in PPA (Hameister, Nickels, Abel, & Croot, 2017). A few studies have also shown changes in neural processing resulting from treatment. Beeson et al. (2011) reported increased activation from pre- to post-naming treatment for a patient with logopenic PPA in the left dorsolateral prefrontal region using functional magnetic resonance imaging (fMRI). Another study, using anodal transcranial direct current stimulation (tDCS) applied to the left inferior frontal gyrus and posterior perisylvian regions in a patient with the nonfluent variant of PPA (nfvPPA), reported changes associated with language improvement in approximate entropy (measured by EEG recordings during a repetition task) in left Broca’s and bilateral Wernicke’s areas (Wang, Wu, Chen, Yuan, & Zhang, 2013). Bonakdarpour, Basu, Grasso, Schnyer, and Henry (2018) also found increased resting-state activity in left posterior perisylvian and bilateral anterior cortical regions in four participants with nfvPPA after 4–6 weeks of Video-Implemented Script Training for Aphasia (VISTA, Henry et al., 2018). These findings suggest that improvements in language ability in PPA are possible and that non-atrophic regions of the brain are sufficiently malleable to be recruited to support language.
Table 1. A.
Published studies investigating the effects of behavioral treatment by language domain for patients with primary progressive aphasia (PPA) from 2010 to 2019 (PPA-G: 24; PPA-L: 9; PPA-S: 15; mixed PPA: 2).
| Treatment Target | Number of Studies | # of participants with improved language | Author (year) |
|---|---|---|---|
| Naming | 11 | 21/25 | Beales et al. (2016) |
| Beeson et al. (2011) | |||
| Croot et al. (2015) | |||
| Croot et al. (2019) | |||
| Flanagan et al. (2016) | |||
| Henry, Rising et al. (2013) | |||
| Jafari et al. (2018) | |||
| Macoir et al. (2015) | |||
| Meyer et al. (2015) | |||
| Meyer et al. (2016) | |||
| Rebstock & Wallace (2018) | |||
| Narrative production | 1 | 10/10 | Henry, Hubbard et al. (2018) |
| Communication | 2 | 11/11 | Farrajota et al. (2012) |
| Goral-Polrola et al. (2016) | |||
| Spelling | 1 | 1/1 | Tsapkini & Hillis (2013) |
| Word retrieval & grammar | 1 | 2/2 | Hameister et al. (2017) |
| Apraxia of speech | 1 | 1/1 | Henry, Meese et al. (2013) |
| Total | 17 | 46/50 | |
Neuroplasticity is now recognized as a basic principle of cognitive neuroscience in that the human brain continues to create new neural pathways and alter existing ones as a result of experience and learning throughout the lifespan and following damage to the brain (e.g., stroke) (Kerr, Cheng, & Jones, 2011). A relatively large literature has emerged in stroke aphasia, showing that treatment-induced language improvement is associated with changes in neural activation patterns (see Kiran & Thompson, 2019, for a review). As in the PPA literature, most studies have focused on improving naming (e.g., Fridriksson, Richardson, Fillmore, & Cai, 2012; Kiran, Meier, Kapse, & Glynn, 2015), however, a few have also shown that sentence processing treatment improves both offline and online processing in people with aphasia and that these changes correlate with shifts in neural activation within regions supporting sentence processing and domain-general neural networks, even in patients with chronic aphasia (Barbieri, Mack, Chiappetta, Europa, & Thompson, 2019; Mack & Thompson, 2017; Mack, Nerantzini, & Thompson, 2017).
Still controversial in the stroke aphasia literature is whether left and/or right hemisphere regions are the best candidates for recruitment into the language network. This literature indicates that both are viable, although the regions recruited depend on several factors, including organism-internal (i.e., lesion related) and external (i.e., treatment and other environmental factors) variables (Kiran & Thompson, 2019). Similarly, it is likely that the neural tissue recruited to support language improvement in PPA is influenced by at least some of these factors (Bonakdarpour et al., 2018; Catani et al., 2013).
The present study examined the neurocognitive effects of treatment for sentence production and comprehension in one patient (DK) with the agrammatic variant of PPA (PPA-G) (Gorno-Tempini et al., 2011; Mesulam et al., 2009). DK presented with relatively spared word and sentence comprehension and semantic knowledge, and impaired verb and sentence production. Given this, the patient was enrolled in Treatment of Underlying Forms (TUF; Thompson & Shapiro, 2005), a metalinguistic intervention that has been shown to improve sentence processing in patients with stroke agrammatic aphasia (Thompson, 2019). Prior to and following treatment, we used eyetracking to chart changes in on-line sentence processing and fMRI to examine changes in neural activation. We predicted that treatment would improve processing of trained sentence structures and, based on the Complexity Account of Treatment Efficacy (CATE; Thompson, Shapiro, Kiran, & Sobecks, 2003), generalization to simpler, linguistically-related structures would occur. Also, as in stroke agrammatic aphasia, we predicted that treatment would impact on-line sentence processing strategies and that treatment-induced improvements would be associated with bilateral increases in BOLD signal activation, in regions overlapping with those engaged by healthy people.
Method
Participant
DK, a 70-year-old Caucasian, monolingual English-speaking, right-handed male, clinically diagnosed with PPA served as the participant in this study. Neurological examination indicated no evidence of stroke, tumor or history of neurological, psychiatric, or developmental speech, language, or learning impairments, and he showed normal hearing and corrected-to-normal vision. At study onset, he reported a two-year history of progressive language decline. As indicated in Table 2, upon study entry, DK’s comprehension of single words (nouns and verbs) was at ceiling, whereas, mild difficulties were noted in comprehension of non-canonical (and not canonical) sentences. Verb production showed a graded impairment according to verb argument structure complexity, and difficulties were noted in production of both canonical and non-canonical sentences. Narrative speech was characterized by production of short, often ungrammatical, sentences, with errors in production of verb argument structure and verb morphology. No evidence of motor speech deficits was found, and overall, language patterns were consistent with the agrammatic variant of PPA (PPA-G; Gorno-Tempini et al., 2011; Mesulam et al., 2009). Administration of the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2007) indicated an Aphasia Quotient (AQ) of 71.0, whereas memory, attention, executive function, abstract reasoning and conceptual flexibility test scores were within the average to very superior range. The study was approved by the Northwestern University Institutional Review Board (IRB). DK and four groups of healthy control participants, who participated in the structural (VBM) and functional (fMRI) neuroimaging, and in the active/passive and object cleft/subject cleft eye-tracking portions of the study, met MRI safety criteria and provided written informed consent according to IRB policies.
Table 2.
DK’s performance on language measures across phases of the study.
| Language Measure | Pre-Passive Treatment | Post-Passive Treatment | 6-Months Post-Passive Treatment | 12-Months Post-Passive/Pre-Object Cleft Treatment | Post-Object Cleft Treatment | 6-Months Post-Object Cleft Treatment | Healthy Adults’ Performance* |
|---|---|---|---|---|---|---|---|
| Northwestern Naming Battery | |||||||
| Noun Comprehension | 100% | 96.70% | 100% | NA | 90% | 97% | |
| Verb Comprehension | 100% | 100% | 100% | NA | 100% | 100% | |
| Noun Naming | 82% | 90% | 94% | NA | 70% | 66% | |
| Verb Naming | 100% | 81% | 88% | NA | 75% | 81% | |
| Pyramids and Palm Trees | 98% | 100% | 98% | NA | NA | 100% | |
| Northwestern Assessment of Verbs and Sentences | |||||||
| Verb Comprehension Test (Total) | 100% | 100% | 100% | 100% | 100% | 100% | |
| Verb Production Test (Total) | 91% | 95% | 95% | 72% | 69% | 86% | |
| Intransitive | 100% | 100% | 100% | 80% | 80% | 60% | |
| Transitive | 90% | 100% | 100% | 100% | 70% | 90% | |
| Ditransitive | 86% | 86% | 86% | 43% | 57% | 100% | |
| Argument Structure Production Test (Total) | 72% | 94% | 91% | 97% | 57% | 72% | |
| Intransitive | 100% | 100% | 100% | 100% | 40% | 100% | |
| Transitive | 87% | 100% | 93% | 100% | 80% | 80% | |
| Ditransitive | 42% | 83% | 83% | 92% | 33% | 50% | |
| Sentence Comprehension Test (Total) | 90% | 83% | 97% | 87% | 80% | 83% | |
| Canonical | 100% | 100% | 100% | 93% | 80% | 93% | |
| Noncanonical | 80% | 67% | 93% | 80% | 80% | 73% | |
| Sentence Production Test (Total) | 40% | 79% | 73% | 43% | 33% | 43% | |
| Canonical | 40% | 80% | 87% | 53% | 47% | 60% | |
| Noncanonical | 40% | 60% | 60% | 33% | 20% | 20% | |
| Northwestern Assessment of Verb Inflection | |||||||
| Total Score | 58% | 63% | 58% | 58% | NA | 43.33 | |
| Nonfinite | 85% | 100% | 80% | 80% | NA | 75% | |
| Finite | 30% | 50% | 40% | 40% | NA | 13.33% | |
| Cinderella Narrative Analysis | |||||||
| MLU (words) | 5.67 | 6.46 | 7.55 | 7.42 | 5.25 | 5.89 | 11.11 |
| WPM | 53.755 | 51.468 | 47.4 | 45.876 | 36.129 | 40.9 | 132.22 |
| Sentences with correct syntax | 55% | 73% | 30% | 38% | 33.33% | 39.29% | 93.02% |
| Open:Closed class ratio | 0.982 | 0.906 | 0.939 | 0.934 | 1.454 | 1.259 | 0.95 |
| Noun:Verb ratio | 1.381 | 0.983 | 1.79 | 1.423 | 1.407 | 1.457 | 1.21 |
| Verbs with correct argument structure | 76% | 95% | 93% | 96% | 92% | 93.94% | 98% |
| Correct grammatical morphology: verbs | 71% | 90% | 88% | 100% | 96% | 83.33% | 99% |
Older adult performance from Thompson et al., 2012. MLU=mean length of utterance; WPM=words per minute.
Sentence Structures and Stimuli
Sentence types used for training included long passives and object-cleft structures. Generalization to untrained linguistically-related short passives, active sentences with unaccusative or transitive verbs, and linguistically-unrelated cleft structures were tested during passive sentence training. During object cleft treatment, untrained object Wh-questions, and unrelated pronominal structures and passives were tested (see Table 3).
Table 3.
Sentence types and examples used for training (1, 5), generalization testing to linguistically-related structures during passive (2, 3, 4) and object-cleft treatment (6), and linguistically-unrelated structures tested during passive training (5) and object-cleft training (1,7).
| N | Sentence type | Example |
|---|---|---|
| 1 | Long Passives | The boy was shaved by the man in the barbershop |
| 2 | Short Passives | The boy was shaved by the man (a) |
| The boy was shaved in the barbershop (b) | ||
| 3 | Actives with unaccusative verbs | The woman was falling on the stairs |
| 4 | Actives with transitive verbs | The boy was shaving the man in the barbershop |
| 5 | Object Cleft | It was the boy who the man shaved |
| 6 | Object Wh-question | Who was the boy shaving? |
| 7 | Pronominal Structure | The man knew that the boy shaved him |
For all sentence types, except actives with unaccusatives, sentence/picture pairs with semantically reversible participant roles and transitive verbs were developed (n=20 each). Unaccusative intransitive verbs were non-alternating and selected for animate (Theme) arguments, with corresponding pictures depicting the same action performed by participants of the opposite sex. Unaccusative and transitive verbs were matched for length in syllables, frequency of usage as a verb based on the Corpus of Contemporary American English, and imageability based on the MRC database (Coltheart, 1981). The same six nouns were used as verb arguments across structures. Materials for training sentences (1) and (5) included word cards for the Action (in both the active and passive/object cleft form), Agent, Theme, and Location, as well as two sentence templates (one for the active and one for passive/object cleft forms).
Design
A longitudinal single-subject multiple-probe design was used to evaluate the effects of treatment. Following baseline testing (two consecutive probes), passive structures were trained, followed by repeat testing of all structures immediately following, six months, and one-year post treatment. Object-cleft structures then were trained after pre-treatment testing (three consecutive probe sessions), followed by post-testing of both structures immediately following and six months post-treatment. Both training periods spanned 12 weeks each (1.5-hour sessions; twice weekly). Sentence comprehension and production probe tasks (see Barbieri et al., 2019, for details) were administered throughout both training periods to evaluate the acquisition and generalization effects of treatment. Online eyetracking and neuroimaging data also were collected prior to and following each training period.
Two visual-world eyetracking tasks were administered prior to and following passive sentence treatment, using an ASL EYE-TRAC 6000 remote eye- tracker (Applied Science Laboratories, Bedford, MA), to test online processing of passive and active sentences. These included a sentence-picture matching task to evaluate on-line comprehension (after Mack and Thompson (2017)) and a syntactic priming task to evaluate production (after Mack et al., 2017). There was no overlap between the verbs/sentences used in training and the stimuli used for eyetracking. Prior to and following object cleft treatment, a sentence-picture matching task also was used to test online comprehension of object (e.g., It was the woman who the man lifted) and subject clefts (e.g., It was the man who lifted the woman). The verbs and nouns were the same as those used in the passive vs active task.
Brain images were acquired before and after each treatment phase using a Siemens 3T Prisma scanner, 64-channel head coil and echo-planar sequences for anatomical (3D MPRAGE, TR=2300ms; TE=2.91ms; flip angle=9°, FOV=256mm; voxel size=1×1×1mm) and functional (TR=2400ms; TE=20ms; flip angle=90°; FOV=220mm; voxel size=1.7×1.7×3mm) scans. Block-design fMRI picture-verification tasks (as in Europa, Kiran, Gitelman, & Thompson, 2019) were used to evaluate comprehension of (1) passive and active sentences, and (2) object-cleft and subject-cleft sentences, both running in E-Prime 2.0. Briefly, participants were presented with a picture, together with an auditory sentence, and were asked to determine if the two matched or not by pressing a button; to control for basic auditory and visual processes, a control condition consisting of scrambled pictures and digitally reversed speech was also included.
Treatment (TUF)
Both passive and object-cleft structures were trained using TUF. This approach uses a set of metalinguistic steps that emphasize the argument structure of verbs and thematic/syntactic mapping from canonical (active) to noncanonical forms (see Thompson, 2019).
Data analyses
Offline Probe Data
Performance on the weekly probe tasks was plotted over time to show acquisition of trained and untrained structures. We also calculated the mean percentage correct responses on baseline and post-treatment probes to calculate effect size (ES) using Cohen’s H formula.
Online Eyetracking Data
The eye data were analyzed by tallying fixations (i.e., a gaze of at least 100ms within one degree of visual angle) within areas of interest – for the target and foil pictures in the sentence-picture matching task and within rectangles surrounding the Agent and Theme for the syntactic priming task – using EYENAL (Applied Science Laboratories) and Data Viewer (SR Research Ltd). For sentence-picture matching tasks, data were aggregated into 50ms bins and time-locked to the onset of the picture + auditory sentence pair. For both tasks, data from groups of healthy controls were used for data analysis (n=10 for passive/active sentence-picture matching (from Mack & Thompson, 2017); n=12 for active/passive syntactic priming (from Mack et al., 2017); for object/subject relative sentence-picture matching n=6 participants were tested). Sentences were split into regions: for passive sentences (e.g., the woman was lifted by the man): noun 1 (NP1), verb, (V), noun 2 (NP2), and the first 1000ms following sentence end (S End); for object clefts (e.g., It was the man who the woman was lifting): NP1, NP2, V and S End regions. Thematic prediction (TP) and thematic integration (TI) scores were calculated, reflecting fixations during NP1 and NP2, respectively, and 1000ms after sentence end. In normal sentence processing, the proportion of fixations to the target picture during NP1 is low (<0.5), reflecting TP (i.e., prediction of an Agent thematic role, resulting in looks to the distractor picture for passive and object-cleft sentences), and high (>0.9) during NP2 and downstream, reflecting successful TI (i.e., correct thematic role assignment, resulting in looks to the target picture). Scores for DK were compared to those derived from healthy adults performing the same tasks, using Wilcoxon signed-rank tests.
For syntactic priming, 100 ms bins time-locked to the onset of target pictures were used to calculate the proportion of fixations to the Agent and Theme for each trial and sentence region (Onset, PreNP1, NP1, V, NP2, S End). These data were binarized: Agent advantage = greater fixations to the Agent; Theme advantage = greater fixations to the Theme.
Neuroimaging
Structural.
Anatomical scans, collected prior to each treatment period, were pre-processed using a custom-made pipeline (including re-orientation to the AC-PC line; segmentation; normalization to the VBM/DARTEL template), available through the Northwestern University Neuroimaging Data Archive (NUNDA). Modulated gray-matter maps for DK and a group of healthy controls (n=76; Age = 63.6±7.4, range: 50–80, scanned as part of two other projects (NIH-P50DC012283 and NIH-R01DC008552, using the same parameters) were thresholded at 20% intensity and entered into non-parametric Voxel-Based Morphometry (VBM) analysis (using SPM, Statistical Non-Parametric Mapping toolbox (SnPM, version 13.1.06, http://www.nisox.org/Software/SnPM13/) (see Scarpazza et al., 2016). Briefly, pseudo-t maps reflecting the differences in the amount of gray matter between DK and the control group were obtained by perfoming voxel-wise permutations of conditions (N=77, resulting in a smallest possible voxel-wise p-value of p=.013) with smoothed variance of 4×4×4mm, and thresholded using a cluster-level threshold of p<.001 (FWE p<.05 correction).
FMRI.
Pre-processing used the NUNDA RobustfMRI pipeline, with the same parameters as in Barbieri et al. (2019); motion correction regressed out volumes with FD (framewise displacement)>0.9mm. Fixed-effect General Linear Model (GLM) voxelwise analyses used SPM12 to determine activation for passive>control and object-cleft>control conditions at pre and post-treatment, as well for post>pre-treatment. Analyses were restricted to a set of brain regions that support sentence processing in healthy participants (see Walenski, Europa, Caplan, & Thompson, 2019), including the following (bilateral) regions: the inferior frontal gyrus (IFG), frontal operculum, insula, middle frontal gyrus (MFG), precentral gyrus (PCG), temporal pole, inferior temporal (ITG), middle temporal (MTG), and superior temporal gyri (STG), supramarginal gyrus (SMG) and angular gyrus (AG). For analyses of DK’s data, time and dispersion derivatives were introduced in the model to account for delayed hemodynamic response (Rombouts, Goekoop, Stam, Barkhof, & Scheltens, 2005) and T-maps were thresholded at p(unc.)<.001, with cluster-level FWE (p<.05) correction. For healthy individuals (N=23), second-level analyses were conducted using one-sample t-tests, with age as a covariate. Group T-maps were thresholded at p(unc.)<.001, with cluster size determined using AFNI’s 3dClustsim, a permutating testing function which simulates noise volumes to determine an appropriate cluster size to achieve FWE threshold of p<.05.
Results
Treatment Effects
Performance on Treatment Probe Tasks
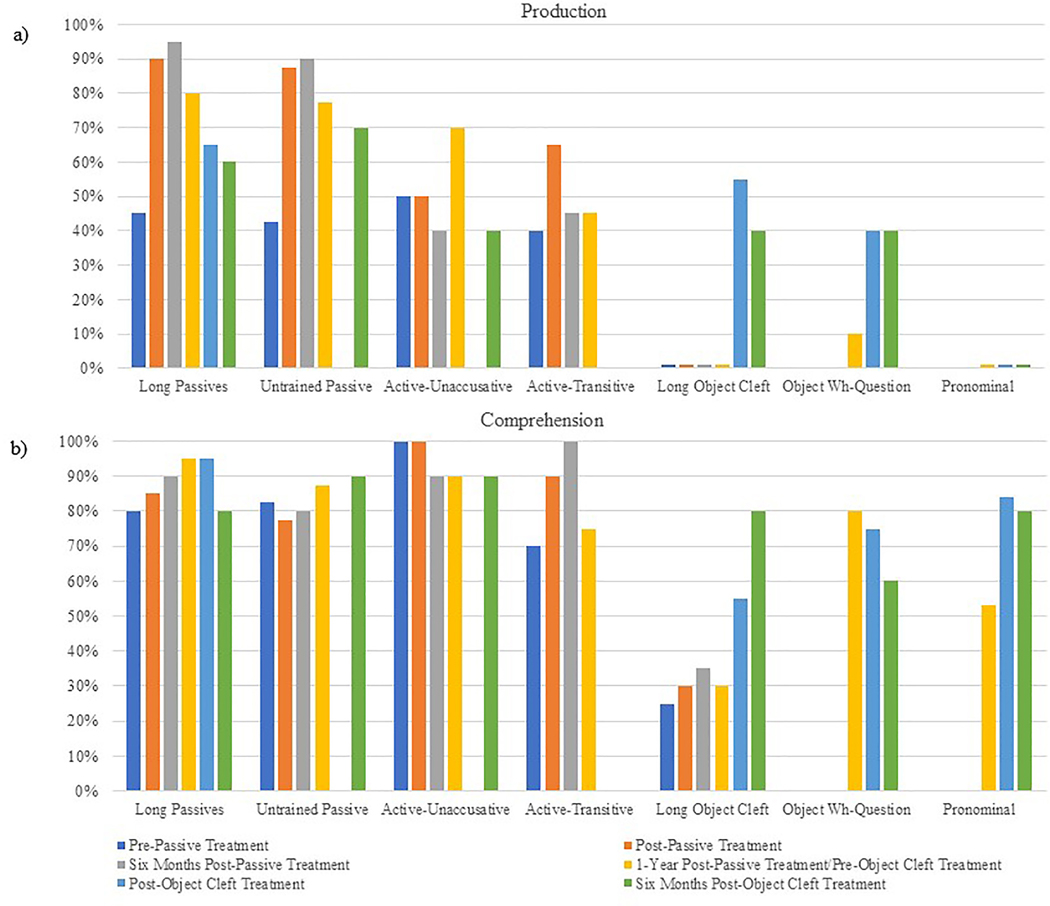
Weekly probe data (Figure 1, top) showed improved production of trained passives from pre- to post-testing (from 45% to 90% correct, ES=1.027); untrained passives (tested prior to and following each treatment phase) (Figure 2) also increased from 42.5% to 87.5%, both statistically significant (Table 4). Post-treatment increases in production accuracy for all passive structures were maintained at 6 months and 1-year post-passive treatment, while no significant changes in actives with unaccusative or transitive verbs, or object-cleft structures were noted.
Figure 1.
Proportion of correct passive (solid line) and object cleft (dashed line) responses on production (top) and comprehension (bottom) probes administered at baseline (i.e., pre-passive training: sessions 1 and 2; pre-object cleft training: sessions 18–20), during treatment phases (passives: sessions 2 – 14; object clefts: sessions 21 – 32), and on follow-up testing immediately following passive sentence treatment (session 15), six months post passive training (session 16), 1 year post passive training (session 17), immediately following object cleft training (session 33), and six months post object cleft training (session 34).
Figure 2.
Percentage correct production (a) and comprehension (b) of all sentence types across study phases.
Table 4.
Results of the mixed-effects regression analyses run on production and comprehension scores. For each comparison, regression parameter estimates (i.e., betas), standard error (SE), statistics and p-values are provided.
| Production | Comprehension | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Structure | Comparison | Estimate | SE | z-value | p-value | Estimate | SE | z-value | p-value |
| Passive Training Phase | Long Passive (trained) | post vs. pre-treatment | 2.398 | 0.870 | 2.755 | 0.006 | 0.348 | 0.839 | 0.415 | n.s. |
| 6months vs. pre-treatment | 3.145 | 1.120 | 2.808 | 0.005 | 0.811 | 0.932 | 0.87 | n.s. | ||
| 1year vs. pre-treatment | 1.587 | 0.717 | 2.212 | 0.027 | 1.558 | 1.168 | 1.334 | n.s. | ||
| Short Passive (untrained) | post vs. pre-treatment | 2.248 | 0.575 | 3.908 | <.0001 | −0.636 | 0.812 | 0.784 | n.s. | |
| 6months vs. pre-treatment | 2.499 | 0.616 | 4.055 | <.0001 | −0.348 | 0.839 | −0.415 | n.s. | ||
| 1year vs. pre-treatment | 1.539 | 0.496 | 3.105 | 0.002 | 0.463 | 0.973 | 0.475 | n.s. | ||
| Active Unaccusative (untrained) | post vs. pre-treatment | 0.000 | 0.000 | 0.000 | n.s. | ** | ||||
| 6months vs. pre-treatment | −0.405 | 0.904 | −0.449 | n.s. | ||||||
| 1year vs. pre-treatment | 0.847 | 0.936 | 0.905 | n.s. | ||||||
| Active Transitive (untrained) | post vs. pre-treatment | 1.025 | 0.654 | 1.566 | n.s. | 1.35 | 0.891 | 1.515 | n.s. | |
| 6months vs. pre-treatment | 0.205 | 0.641 | 0.32 | n.s. | 2.097 | 1.136 | 1.846 | 0.065 | ||
| 1year vs. pre-treatment | 0.205 | 0.641 | 0.32 | n.s. | 0.251 | 0.71 | 0.354 | n.s. | ||
| Object Cleft (untrained) | post vs. pre-treatment | *** | 0.251 | 0.71 | 0.354 | n.s. | ||||
| 6months vs. pre-treatment | 0.48 | 0.697 | 0.688 | n.s. | ||||||
| 1year vs. pre-treatment | 0.251 | 0.71 | 0.354 | n.s. | ||||||
| OC Training Phase | Object Cleft (trained) | post vs. pre-treatment | 3.145 | 1.12 | 2.808 | 0.005 | 1.048 | 0.663 | 1.58 | n.s. |
| 6months vs. pre-treatment | 2.539 | 1.212 | 2.095 | 0.036 | 2.234 | 0.929 | 2.404 | 0.016 | ||
| Object Wh-questions (untrained) | post vs. pre-treatment | 1.792 | 0.874 | 2.05 | 0.04 | −0.288 | 0.761 | −0.378 | n.s. | |
| 6months vs. pre-treatment | −1.792 | 0.986 | 1.817 | 0.069 | −0.981 | 0.854 | −1.149 | n.s. | ||
| Pronominal (untrained) | post vs. pre-treatment | *** | 1.253 | 0.762 | 1.644 | n.s. | ||||
| 6months vs. pre-treatment | 1.253 | 0.945 | 1.326 | n.s. | ||||||
| Long Passive (untrained) | post vs. pre-treatment | −0.767 | 0.723 | −1.052 | n.s. | 0.054 | 1.452 | 0.037 | n.s. | |
| 6months vs. pre-treatment | −0.901 | 0.854 | −1.149 | n.s. | 1.558 | 1.295 | 1.203 | n.s. | ||
| Active Unaccusative (untrained) | post vs. pre-treatment | 1.253 | 0.945 | 1.326 | n.s. | ** | ||||
= model did not converge because accuracy was the same across time points.
= model did not converge, as accuracy was 0% across time points.
These production patterns were maintained during the pre-object cleft multiple probe period, followed by significantly improved production of trained object clefts (from 0% to 55% correct, ES=1.671) and generalization to object wh-questions (from 10% to 40% correct) during object-cleft treatment. Improvements were largely maintained at 6 months post-treatment. Production of passive sentences also was maintained at 6 months following object-cleft treatment. No significant change in unrelated, untrained pronominal structures occurred, as expected.
Comprehension of trained and untrained passive and unaccusative structures was above chance at baseline whereas, that for object clefts was poorer (25% correct); all were unchanged throughout the passive training phase (ES=0.132; not significant) (Figure 1, bottom). Logistic regression analyses indicated no significant changes in comprehension of any sentence types (Table 4). However, comprehension of object-cleft structures improved during training, reaching 90% and decreasing to 55% correct on the post-treatment probe task, resulting in a small but significant effect size (ES=0.512). Throughout object-cleft training, comprehension of passive sentences was maintained, with no significant differences found across test points.
Longitudinal Language and Cognitive Performance
Language Measures.
Following passive treatment, improved production of grammatical sentences, nouns and verbs, verb argument structure, and verb inflection was noted both on language tests and in narrative production. Scores were largely unchanged on pre-object cleft treatment testing, with the exception that production of complex verbs and grammatical sentences showed mild decreases (Table 2). After object-cleft treatment, declines in verb (transitive>intransitive) and sentence (noncanonical>canonical), as well as in narrative production, were noted. Single-word comprehension (with the exception of complex verbs) and canonical sentence comprehension remained relatively preserved, whereas, noncanonical sentence comprehension was variable, throughout the study.
Cognitive Measures.
Results are shown in Table 5. On entry into the study, performance on non-language tests ranged from Average to Very Superior, with the exception of verbal learning memory (i.e., the Wechsler Memory Scale-Revised (WSM-R) Logical Memory (Part 1)), which showed Low Average performance (21st percentile) and further declined after passive and object-cleft treatment. Notably, DK’s performance on all other measures shifted only minimally across phases of the study and remained in the Average or above range.
Table 5.
Performance on tests of cognitive function administered prior to passive and object cleft treatment.
| Pre-Passive Treatment | Pre-Object Cleft Treatment | Post-Object Cleft Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive Process | Test | Score | %tile/z/ss | Class | Score | %tile/z/ss | Class | Score | %tile/z/ss | Class |
| Visual-Spatial Working Memory | WMS-R-IV Spatial Addition | N/A | N/A | N/A | 7/24 | 8 (ss) | Average | 7/24 | 8 (ss) | Average |
| Visual Working Memory | WMS-R-IV Symbol Span | N/A | N/A | N/A | 20/50 | 12 (ss) | High Average | 19/50 | 10 (ss) | Average |
| Verbal Learning Memory | WMS-R Logical Memory (Part I) | 15/50 | 21st % | Low Average | 11/50 | 7th % | Mild | 9/50 | 6th % | Mild |
| Visual Learning Memory | WMS-R Visual Rep (Part I) | 35/41 | 98th % | Very Superior | 30/41 | 76th % | High Average | 32/41 | 88th % | High Average |
| Spatial Learning Memory | WMS-R-IV Designs (Part I) | N/A | N/A | N/A | 62/120 | 10 (ss) | Average | 58/120 | 9 (ss) | Average |
| Verbal Delayed Recall | WMS-R Logical Memory (Part II) | 14/50 | 52nd % | Average | 14/50 | 52nd % | Average | 8/50 | 52nd % | Average |
| Visual Delayed Recall | WMS-R Visual Rep (Part II) | 35/41 | 99th % | Very Superior | 27/41 | 90th % | Superior | 25/41 | 84th % | High Average |
| Spatial Delayed Recall | WMS-R-IV Designs (Part II) | N/A | N/A | N/A | 56/120 | 12 (ss) | High Average | 51/120 | 11 (ss) | Average |
| Attention | Target Cancellation Test | time: 84s errors: 2 | --- | --- | time: 94s errors: 2 | --- | --- | time: 118s errors: 0 |
--- | --- |
| Trail Making Test (Part A) | time: 54s errors: 0 | --- | --- | time: 54s errors: 0 | --- | --- | time: 48s errors: 0 |
--- | --- | |
| Executive Attention | Trail Making Test (Part B) | time: 112s errors: 0 | --- | --- | time: 184s errors: 1 | --- | --- | time: 158s errors: 0 |
--- | --- |
| Abstract Reasoning/ Conceptual Flexibility |
Wisconsin Card Sorting Test (categories) | 5 | 2.18 (z) | Very Superior | 5 | 2.18 (z) | Very Superior | 5 | 2.18 (z) | Very Superior |
| Wisconsin Card Sorting Test (errors) | 8 | 2.467 (z) | Very Superior | 9 | 2.3 (z) | Very Superior | 10 | 2.13 (z) | Very Superior | |
| Raven Progressive Matrices | 29/36 | 90th % | Superior | 33/36 | >95th % | Superior | N/A | N/A | N/A | |
WMS-R=Weschler Memory Scale Revised; %=percentile; z=z-score; SS=standard score; Class=classification; N/A=not administered.
Online (Eyetracking) Results
Pre and Post Passive Sentence Treatment
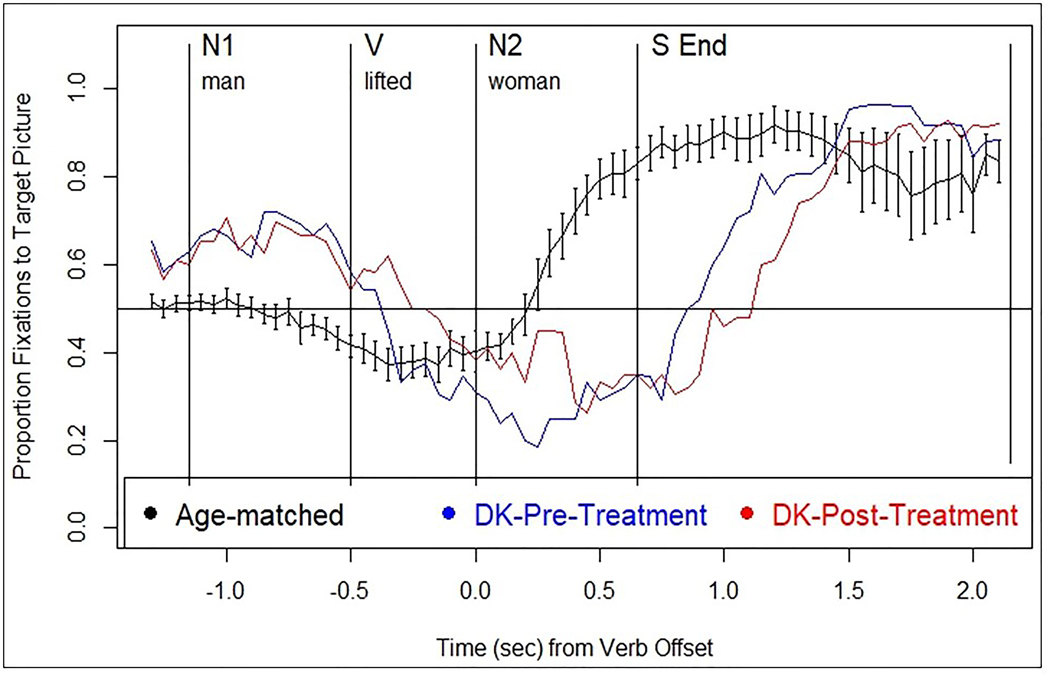
On the sentence-picture matching task, DK’s accuracy and eye movement patterns for passives were similar to healthy adults (Figure 3 and Table 6). Evidence of thematic prediction (TP) was seen at pre- and post-treatment in the same region as healthy controls (N1), whereas thematic integration (TI) emerged during the S End region (vs. N2 in healthy), as indicated by TP and TI scores (Table 6).
Figure 3.
Normal eye movement pattern while listening to passive sentences in the sentence-picture matching task (from Mack & Thompson, 2017) (black line) and DK’s patterns at pre- (blue line) and post-treatment (red line).
Table 6.
DK’s accuracy on the eye-tracking tasks, and thematic prediction and integration scores derived from performance of sentence-picture matching tasks administer at pre- and post- passive and object cleft treatment. Significant differences between DK’s data and the control group (Crawford-Howell t-test; significance levels:
| DK pre-tx | DK post-tx | Healthy Control Participants† | |
|---|---|---|---|
| Mean (SD) | |||
| Accuracy (%, Comprehension) | |||
| Active | 96 | 100 | |
| Passive | 96 | 100 | |
| Subject-cleft | 83 | 75 | |
| Object-cleft | 4 | 8 | |
| Accuracy (%, Production) | |||
| Active | 7 | 46# | |
| Passive | 54 | 71# | |
| Eye movements (Comprehension) | |||
| Passive sentence processing | |||
| Prediction (V region) | 0.41 | 0.5 | 0.40 (0.10) |
| Integration (N2 region) | 0.29* | 0.33* | 0.66 (0.10) |
| Integration (S End region) | 0.82 | 0.75 | 0.90 (0.14) |
| Object cleft sentence processing | |||
| Prediction (N1) | 0.44 | 0.37 | |
| Integration (V + S End) | 0.18 | 0.15 |
p< .05), as well as between pre- and post-treatment (mixed-effect logistic regression; significance levels:
p<.05) are indicated.
Data from Mack and Thompson (2017).
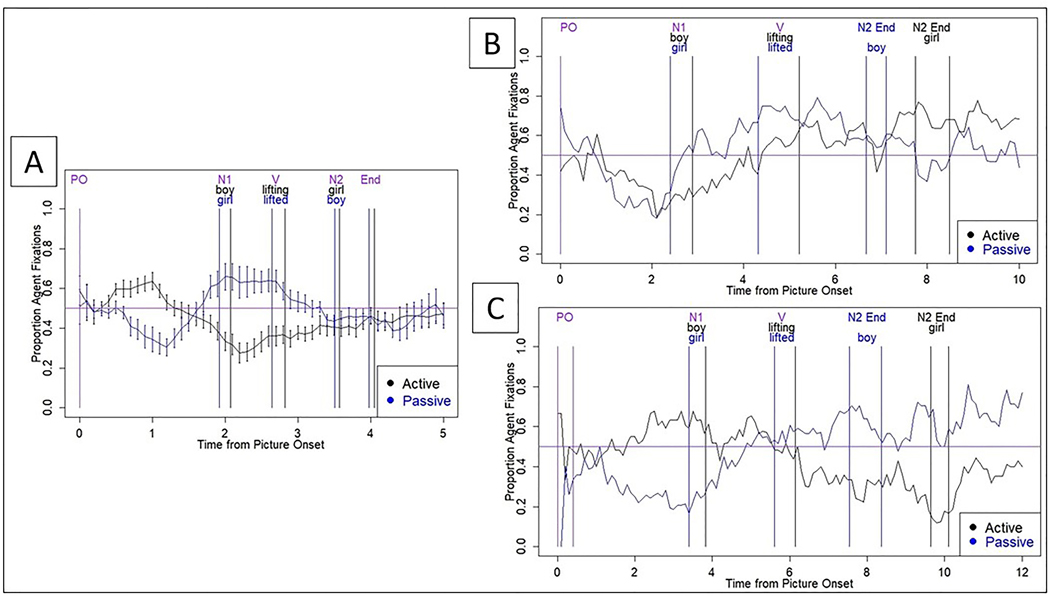
For the syntactic priming (production) task, DK’s accuracy was markedly impaired prior to, but improved after, passive sentence treatment (Table 6). Eye movements at pre-treatment also differed substantially from the incremental production patterns observed in neurotypical adults (Figure 4A), who showed Agent (for actives) or Theme (for passives) advantage in the PreN1 region and the reverse pattern in the N1 and V regions, indicating thematic role assignment to the sentence subject before producing it for both sentence types. For DK, pre-treatment eye movement patterns (Figure 4B) did not differ between actives and passives in any region, reflecting impaired thematic role assignment. However, eye movement patterns at post-treatment (Figure 4C) were more similar to healthy speakers: significant interactions (p’s < .05) were observed between study phase (pre-, post-treatment) and sentence type in the PreN1, V, and N2 regions.
Figure 4.
Eye movements for healthy older adults (A) (from Mack et al., 2017) and DK at pre- (B) and post-treatment (C) during active (The boy lifted the girl.) and passive (The girl was lifted by the boy.) sentence production. X axis = time PO in seconds; Y axis = proportion of fixations to the agent, out of all fixations. Black line = active; blue = passive. PO = picture onset; N1 = noun 1; V = verb; N2 = noun 2; End = sentence end. The horizontal line = at-chance fixation.
Pre and Post Object-Cleft Sentence Treatment
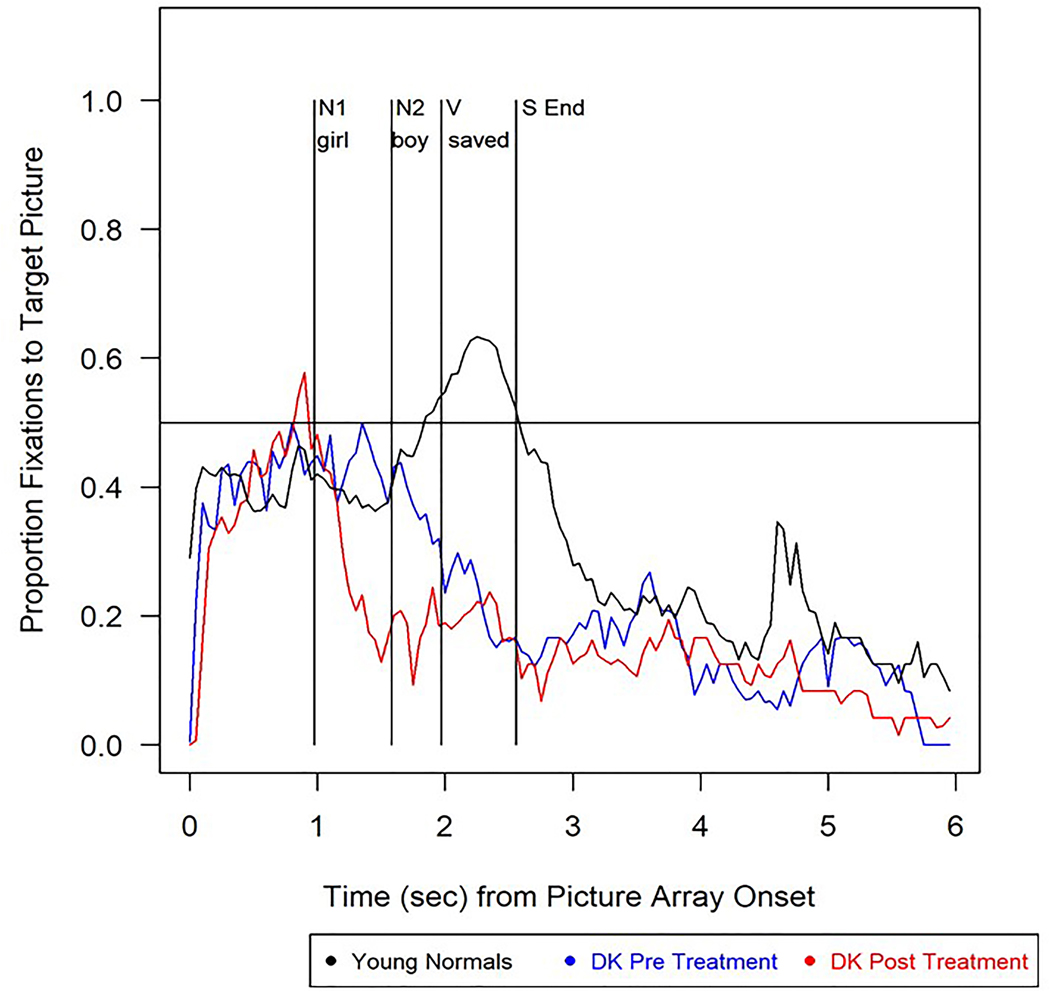
In the object-subject cleft eyetracking sentence-picture matching task, DK’s accuracy was lower than in healthy young adults and significantly poorer for object compared to subject-cleft structures at both pre- and post-treatment (Table 6). DK’s eye movements during object-cleft sentence processing are shown in Figure 5. Following, but not prior to, treatment he showed an Agent-first strategy upon hearing NP1, as reflected by a numerical (but not significant) change in thematic prediction (Table 6). No evidence of thematic integration was shown at either test point (Table 6).
Figure 5.
Eye movement patterns while listening to object cleft sentences such as It was the girl who the boy saved in the object cleft sentence-picture matching task for healthy young controls (black line) and DK pre-treatment (blue line) and post-treatment (red line). Note: N1=first noun and auxiliary; V=verb; N2=second noun; S End = sentence end.
Neuroimaging Results
Structural MRI
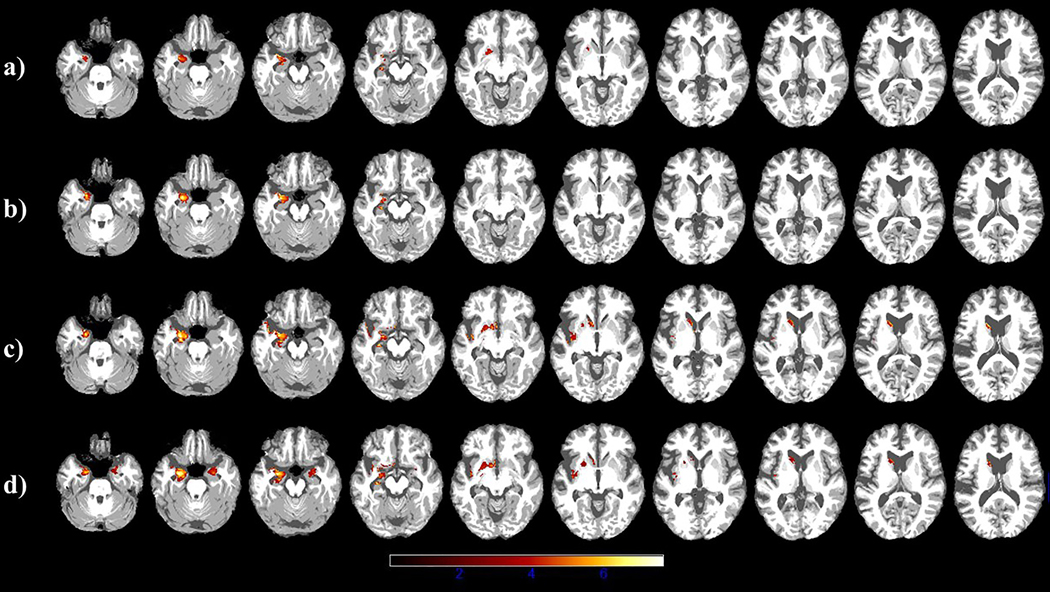
T1 images obtained from scans – prior to and following passive and object-cleft treatment – are shown in Figure 6 and the results of the VBM analyses of these data are presented in Table 7. Prior to passive sentence treatment, atrophy was constrained to the left hemisphere in the amygdala, hippocampus and anterior parahippocampal gyrus. Throughout the study, atrophy increased in these same areas and extended to left insula and temporal pole, to the left basal ganglia (caudate, putamen, and nucleus accumbens), and to the right hemisphere (including the amygdala, the anterior parahippocampal gyrus and the temporal pole) (Figure 6).
Figure 6.
Axial images showing changes in atrophy over time. Regions of significant cortical atrophy are shown prior to (a) and following (b) passive sentence treatment, and prior to (c) and following (d) object cleft treatment. Lighter colors indicate regions of greater atrophy in DK’s brain compared to a group of healthy participants (N=76).
Table 7.
Results of the nonparametric voxel-based morphometry (VBM) analysis comparing DK’s gray matter maps at each time point to a control group (N=76). The Table shows significant clusters at a cluster-level threshold of p<.001, FWE-corrected (p<.05), with labels derived from Harvard-Oxford atlas (Desikan et al., 2006).
| Pre- Passive Treatment | Post- Passive Treatment | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster Size | Pseudo-t | Peak coordinates |
Peak Region | Extent | L/R | Cluster Size | Pseudo-t | Peak coordinates |
Peak Region | Extent | L/R | ||||
| x | y | z | x | y | z | ||||||||||
| 557 | 5.92 | −30 | 2 | −20 | Amygdala | Hippocampus, Parahippocampal anterior | L | 758 | 6.85 | −29 | 0 | −18 | Amygdala | Hippocampus, Parahippocampal anterior, Insula, Temporal Pole | L |
| Pre- Cleft Treatment | Post- Cleft Treatment | ||||||||||||||
| Cluster Size | Pseudo-t | Peak coordinates |
Peak Region | Extent | L/R | Cluster Size | Pseudo-t | Peak coordinates |
Peak Region | Extent | L/R | ||||
| x | y | z | x | y | z | ||||||||||
| 1899 | 6.85 | −26 | −10 | −24 | Hippocampus | Parahippocampal anterior, Temporal Pole, Caudate, Insula, Amygdala, Putamen, Accumbens, Planum Polare | L | 1663 | 7.58 | −26 | −6 | −21 | Amygdala | Hippocampus, Parahippocampal anterior, Putamen, Accumbens, Insula, Temporal Pole, Temporal Fusiform anterior, Planum Polare | L |
| 240 | 5.63 | −14 | 12 | 12 | Caudate | Accumbens | L | ||||||||
| 467 | 5.14 | 27 | −4 | −24 | Amygdala | Hippocampus, Parahippocampal anterior, Temporal Pole | R | ||||||||
Functional MRI
Behavioral results.
Prior to treatment, DK’s in-scanner comprehension accuracy for passives and object-cleft sentences was relatively good (81.2% for both). Following passive treatment, accuracy for passives increased to 89.6%, whereas, no change in comprehension of object cleft structures was observed following object-cleft treatment.
Neuroimaging results.
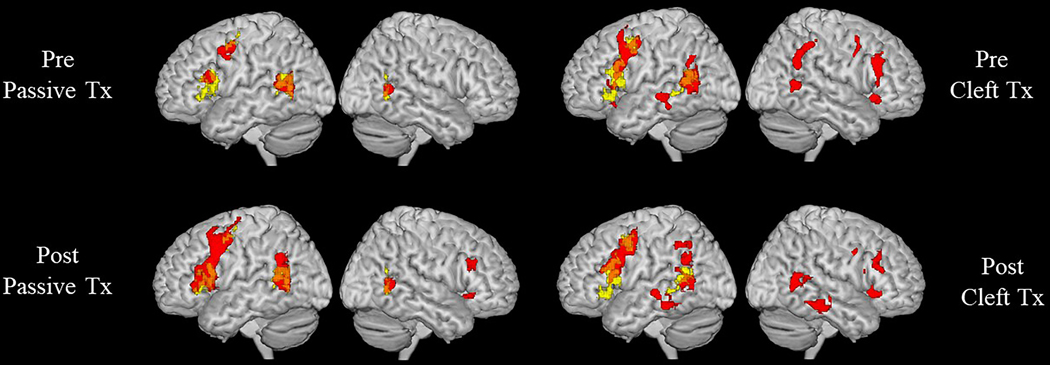
Regions of BOLD signal activation at pre- and post-treatment for DK are shown in Figure 7 (overlaid with neural activation derived from the control participants (n=23; age 24–64 years; M=37.1)) and coordinates/labels are provided in Tables 8 (for controls), 9 and 10 (for DK). Healthy controls showed mostly left-lateralized activation for both Passive>Control and Object-Cleft>Control contrasts, with significant clusters in the left frontal (IFG (opercularis and triangularis), MFG and PCG), posterior temporal and inferior parietal regions. A smaller cluster of activation for Passive>Control was also found in the right MTG temporo-occipital and AG. At baseline, DK showed activation for Passive>Control in the same clusters active in healthy controls (Figure 7, Table 9), with the exception that the left IFG was less extensively recruited in DK compared to healthy participants. Following passive sentence treatment, upregulation of activation (defined as increased activation from pre- to post-treatment at a voxel-wise threshold of p<.001, FWE p<.05 cluster-level correction) was observed in bilateral inferior frontal (left IFG opercularis, right IFG triangularis), right temporal (posterior and temporo-occipital MTG) and bilateral inferior parietal (posterior SMG and AG) regions. Post-treatment upregulation also was found bilaterally in the MFG and PCG. Turning to the Object-Cleft>Control contrast (Figure 7 and Table 10), activation patterns at baseline showed major overlap with healthy controls in the left hemisphere; in addition, activation was observed in homologous clusters in the right frontal (IFG, MFG, PCG), posterior temporal and inferior parietal (AG, pSMG) regions. Following object-cleft treatment, DK showed further, but smaller, shifts in activation, in left frontal (IFG triangularis and MFG) and right inferior parietal (SMG) regions.
Figure 7.
ROI analysis results. Regions of BOLD signal activation prior to and following passive (contrast: passive>control) or object cleft (contrast: object cleft>control) treatment are displayed in red for DK and overlaid onto healthy controls’ activation patterns for the same contrasts (yellow). The Figure reflects the results of voxel-wise analysis restricted to set of regions of interest (ROIs) that were selected (bilaterally) based on a recent meta-analysis of sentence processing studies (Walenski et al., 2019).
Table 8.
Significant clusters of activation found for passive>control and object clefts>control contrasts for heathy control participants (p<.001 uncorrected, cluster-level FWE<.05). Cluster size was determined by using AFNI’s 3dClustsim, a permutating test function that simulates noise volumes to determine the cluster size corresponding to a given FWE threshold (i.e., p<.05), and corresponded to k>46 for passive>control and k>211 for object clefts>control.
| Cluster size (k) | Peak T-value | Peak Coordinates | Peak Region | Extent | L/R | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
|
Passive>Control | |||||||
| 429 | 7.360 | −40 | −60 | 16 | AG | MTG (temporo-occipital), SMG (posterior) | L |
| 300 | 6.600 | −58 | 16 | 4 | IFG (pars opercularis) | IFG (pars triangularis) | L |
| 201 | 6.090 | −28 | −8 | 50 | PCG | L | |
| 155 | 8.170 | 46 | −58 | 4 | MTG (temporo-occipital) | AG | R |
|
Object Cleft>Control | |||||||
| 739 | 7.140 | −44 | 20 | 18 | IFG (pars opercularis) | IFG (pars orbitalis), Insula | L |
| 337 | 7.060 | −40 | 6 | 48 | MFG | PCG | L |
| 433 | 6.730 | −40 | −60 | 18 | AG | STG (posterior), MTG (posterior, temporo-occipital) | L |
Note. Labels were derived from the Harvard-Oxford atlas (Desikan et al., 2006). AG = Angular Gyrus; IFG = Inferior Frontal Gyrus; MFG = Middle Frontal Gyrus; MTG = Middle Temporal Gyrus; PCG = Precentral Gyrus; SMG = Supramarginal Gyrus; STG = Superior Temporal Gyrus.
Table 9.
Regions of significant activation (pre-treatment, post-treatment) and upregulation of activation (post- minus pre-treatment) for DK, derived from the sentence verification tasks for the contrast passive>control (p<.001 uncorrected, cluster-level FWE<.05).
| Passive>Control (Pre-Passive Treatment) | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Size | Peak T-value | Peak Coordinates | Peak Region | Extent | L/R | ||
| x | y | z | |||||
| 350 | 10.729 | −38 | 2 | 46 | MFG | PCG | L |
| 414 | 8.322 | −46 | −60 | 8 | MTG (temporo-occipital) | AG | L |
| 136 | 5.724 | −54 | 20 | 16 | IFG (pars opercularis) | IFG (pars triangularis) | L |
| 108 | 6.409 | 50 | −54 | 8 | MTG (temporo-occipital) | R | |
| Passive>Control (Post-Passive Treatment) | |||||||
| Cluster Size | Peak T-value | Peak Coordinates | Peak Region | Extent | L/R | ||
| x | y | z | |||||
| 1608 | 9.965 | −38 | 0 | 48 | MFG | IFG (pars opercularis, pars triangularis), PCG, Frontal Operculum | L |
| 757 | 6.672 | −54 | −52 | 8 | MTG (temporo-occipital) | AG, SMG (posterior) | L |
| 174 | 6.332 | 48 | −54 | 6 | MTG (temporo-occipital) | R | |
| 101 | 4.240 | 40 | 30 | −4 | IFG (pars orbitalis) | Insula, Frontal Operculum | R |
| 93 | 4.458 | 50 | 28 | 26 | MFG | IFG (pars triangularis) | R |
Note. Labels were derived from the Harvard-Oxford atlas (Desikan et al., 2006). AG = Angular Gyrus; IFG = Inferior Frontal Gyrus; MFG = Middle Frontal Gyrus; MTG = Middle Temporal Gyrus; PCG = Precentral Gyrus; SMG = Supramarginal Gyrus; STG = Superior Temporal Gyrus.
Table 10.
Regions of significant activation (pre-treatment, post-treatment) and upregulation of activation (post minus pre-treatment) for DK, derived from the sentence verification tasks for the contrast object cleft>control (p<.001 uncorrected, cluster-level FWE<.05).
| Object Cleft>Control (Pre-Cleft Treatment) | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Size | Peak T-value | Peak Coordinates | Peak Region | Extent | L/R | ||
| x | y | z | |||||
| 997 | 11.234 | −38 | 0 | 46 | MFG | IFG (pars opercularis, pars triangularis), PCG | L |
| 637 | 7.565 | −58 | −58 | 14 | AG | SMG (posterior), MTG (temporo-occipital) | L |
| 202 | 6.485 | −68 | −28 | −4 | MTG (posterior) | STG (posterior) | L |
| 106 | 4.069 | −28 | 24 | −8 | IFG (pars orbitalis) | Insula | L |
| 347 | 8.670 | 50 | 26 | 28 | MFG | IFG (pars triangularis) | R |
| 237 | 5.813 | 56 | −52 | 26 | AG | SMG (posterior) | R |
| 220 | 5.968 | 38 | 22 | −8 | IFG (pars orbitalis) | Insula, Frontal Operculum | R |
| 141 | 5.396 | 40 | 6 | 38 | MFG | PCG | R |
| 121 | 6.649 | 48 | −54 | 6 | MTG (temporo-occipital) | R | |
| Object Cleft>Control (Post-Cleft Treatment) | |||||||
| Cluster Size | Peak T-value | Peak Coordinates | Peak Region | Extent | L/R | ||
| x | y | z | |||||
| 1348 | 11.889 | −38 | 0 | 48 | MFG | IFG (pars opercularis, pars triangularis), PCG | L |
| 495 | 5.896 | −48 | −62 | 8 | MTG (temporo-occipital) | AG | L |
| 212 | 6.867 | −60 | −24 | −2 | STG (posterior) | MTG (posterior, temporo-occipital), ITG (posterior, temporo-occipital) | L |
| 188 | 7.491 | −38 | −58 | 44 | AG | SMG (posterior) | L |
| 235 | 6.611 | 50 | −60 | 2 | MTG (temporo-occipital) | AG | R |
| 229 | 6.711 | 38 | 24 | −6 | IFG (pars orbitalis) | Insula, Frontal Operculum | R |
| 196 | 7.212 | 62 | −38 | −16 | MTG (posterior) | ITG (posterior) | R |
| 196 | 6.944 | 42 | 24 | 22 | MFG | IFG (pars opercularis, pars triangularis) | R |
| 151 | 7.143 | 38 | 2 | 36 | PCG | IFG (pars opercularis), MFG | R |
Note. Labels were derived from the Harvard-Oxford atlas (Desikan et al., 2006). AG = Angular Gyrus; IFG = Inferior Frontal Gyrus; MFG = Middle Frontal Gyrus; MTG = Middle Temporal Gyrus; PCG = Precentral Gyrus; SMG = Supramarginal Gyrus; STG = Superior Temporal Gyrus.
Discussion
This paper examined the neurocognitive effects of treatment focused on production and comprehension of passive and object-cleft structures in a patient with the agrammatic variant of primary progressive aphasia (PPA-G). Following Treatment of Underlying Forms (TUF; Thompson & Shapiro, 2005), which exploits what is known about normal language representation and processing, DK showed improved comprehension and production of trained noncanonical sentences and generalization to untrained simpler, linguistically-related, structures, as seen in studies of treatment for stroke-induced aphasia (Thompson, 2019). Treatment-induced improvements also were largely maintained over time. These findings support the use of psycholinguistically-based treatment for sentence processing impairments in patients with PPA and provide additional support for the Complexity Account of Treatment Efficacy (CATE; Thompson et al., 2003): generalization to less complex structures occurs following treatment focused on more complex structures only when structures are linguistically related to one another. From a neural perspective, this suggests that treatment exploiting the psycholinguistic processes that underlie processing of complex forms boosts the neural circuitry that supports computation of these forms, which, in turn, supports processing of simpler, related structures.
Performance on language tests administered prior to and following treatment also reflected improved sentence processing. Improved scores on tests of verb morphology, verb-argument structure, and sentence production/comprehension, as well as improvements in spontaneous speech, were found following passive treatment; following object-cleft treatment, language test scores showed smaller improvements. However, production of complex verbs, noncanonical sentences and narratives declined following object-cleft treatment, reflecting the neurodegenerative nature of PPA. Nevertheless, comprehension/production of both trained sentence types was maintained over time. In addition, across the 18-month period of the study, scores on tests of cognitive function remained relatively stable, and within the normal range, with the exception of performance on the WSM-R Logical Memory test, which declined from pre to post object-cleft treatment. We note, however, that the WSM-R Logical Memory test assesses verbal memory; thus decline on this test likely reflects language, rather than general cognitive decline.
Notably, the offline behavioral improvements observed were aligned with changes in online processing. On the sentence-picture matching task for passive sentences, in line with his relatively unimpaired offline passive sentence comprehension ability, DK showed evidence of intact thematic prediction at both time points, with early looks to the incorrect picture, reflecting an “Agent-first” strategy as seen in neurotypical listeners. Evidence of partially normalized, albeit delayed, thematic integration was also noted. Similarly, his online comprehension of object-cleft structures paralleled offline performance. At pre-treatment, comprehension of object-cleft structures was quite impaired as he showed no evidence of thematic prediction or integration during the object-cleft/subject-cleft sentence-picture matching task. However, following object-cleft treatment, he showed timely thematic prediction (i.e., emergence of an Agent-first strategy), albeit thematic integration was not affected by treatment.
Eye movement changes from pre- to post-treatment also were noted on the syntactic priming (production) task. While pre-treatment eye movements showed significant abnormalities, mirroring DK’s impaired ability to produce passive sentences, partially normalized production patterns (i.e., use of an incremental processing strategy, Griffin & Bock, 2000; Mack et al., 2017) were found post-treatment. These findings indicate that behavioral treatment impacted DK’s real-time, automatic language processing abilities – not merely his ability to perform offline tests of sentence comprehension/production, which may rely on the use of processing strategies such as rehearsal and/or compensatory word retrieval. That DK’s eye movements reflected partially normalized processing strategies as a result of treatment is a strong indicator of treatment efficacy, as seen in our patients with stroke-induced agrammatism (see Barbieri, et al., 2019).
One of the primary aims of this study was to determine if/how the neural network for sentence processing reorganizes with treatment in PPA-G. We hypothesized that, due to the fact that the brain is an organ of plasticity and that much, or at least some, neural tissue remains intact in patients with PPA, treatment focused on improving sentence comprehension and production would result in experienced-based plasticity – that is, changes in neural activation from pre- to post-treatment. Notably, DK’s activation on the fMRI task at baseline showed major overlap with that of healthy participants, suggesting – in line with the relatively good off-line comprehension accuracy of passive structures, and with the evidence of limited cortical atrophy – well-preserved functionality of left-hemisphere language areas. Following passive treatment, upregulation was found, in both hemispheres, in brain regions both within the normal sentence processing network and within domain-general networks, in line with the increase in comprehension accuracy of passive structures and as seen in patients with agrammatic aphasia resulting from stroke (Barbieri, et al., 2019; see also DeMarco, Wilson, Rising, Rapcsak, & Beeson, 2018). Following object-cleft treatment, despite the change in off-line comprehension accuracy on the probe task but in line with the unchanged performance observed on the neuroimaging task, only minor changes in activation were noted, perhaps reflecting a reduction of neuroplasticity due to the progression of the disease. Notably, this observation is further supported by the smaller changes in online sentence processing observed following object cleft (compared to passive) sentence treatment.
Importantly, changes in both behavioral performance as well as brain activity were noted in the face of neural atrophy, which progressed throughout the course of the study. Of note is that DK showed atrophied tissue in regions not typically associated with PPA-G: portions of the left basal ganglia, hippocampus, and anterior parahippocampal gyrus, with the only affected cortical regions being the temporal pole, the planum polare and the anterior temporal fusiform. Over the course of the study atrophy increased in the left and spread to some of these regions in the right hemisphere. Studies on PPA-G have found peak atrophy primarily in left frontal regions, the temporoparietal junction and anterior superior temporal gyrus (Mesulam et al., 2009; Rogalski et al., 2011), with fewer reporting peak atrophy in the basal ganglia (Mandelli et al., 2016; Tetzloff et al., 2017). To our knowledge, no previous studies have identified striatal atrophy in the absence of concomitant frontal cortical pathology. However, our findings indicate that striatal atrophy alone may disrupt sentence processing. It is also possible, at least in early phases of the study, that in addition to subcortical atrophy, DK showed cortical atrophy that was not detectable. This interpretation, however, is unlikely given that atrophy within cortical regions associated with sentence processing was not seen on repeat scans obtained throughout the course of the study.
The role of the basal ganglia in language processing has been elucidated in patients with Parkinson’s disease, some of whom, in addition to characteristic motor impairments, exhibit deficits in sentence comprehension and production (Johari et al., 2019). Kotz, Frisch, Von Cramon, and Friederici (2003) also found abnormal ERP responses to sentences with verb-argument structure violations (i.e., the typical N400-P600 is lacking a P600 component) in patients with lesions within the basal ganglia, suggesting that such lesions impair temporal sequencing associated with procedural memory, which is required for processing hierarchical syntactic structure. FMRI studies with unimpaired adults have found striatal activation associated with syntactic comprehension, and models of language processing suggest that corticostriatal connections bind cortical representations of syntactic context, for example, in Broadmann’s area 47 to structure mapping representations (i.e., grammatical constructions in Broadmann’s area 44) during sentence comprehension (Dominey & Inui, 2009). Hence, it is not surprising that atrophied tissue in the basal ganglia may lead to agrammatic production and comprehension patterns in PPA as observed in our patient.
DK also showed atrophy within the hippocampal region. This observation on initial scans was somewhat surprising in that studies with both patients and cognitively healthy participants associate these regions with declarative memory and lexical learning (Tagarelli et al., 2019), rather than grammatical processes. Notably, however, increases in atrophy within the left hippocampus increased in concert with DK’s word retrieval difficulty.
Although a major strength of this study is that we were able to chart the progression of both on-line and offline sentence processing, as well as concomitant cognitive abilities, over time in a patient with the agrammatic variant of PPA, interpretation of our functional neuroimaging findings are limited in that DK did not undergo repeat scans prior to and following each treatment phase. Without repeat scans, allowing analysis of test-retest reliability of BOLD signal activation, it is possible that changes associated with the functional neuroimaging tasks may have resulted from scan-to-scan variability, rather than changes in functional activation over time. We also note that the lack of longitudinal data for healthy controls precludes the ability to rule out the possibility that changes in DK’s structural scans reflected normal aging, rather than disease progression.
Conclusion
This study illuminates changes in language and neurocognitive processes in a patient with PPA-G resulting from a course of psycholinguistically-based treatment of sentence deficits. The patient showed improved comprehension/production of trained structures, generalization to untrained, related structures of lesser complexity in keeping with the Complexity Account of Treatment Efficacy (CATE; Thompson et al., 2003), the emergence of partially normalized automatic online sentence processing strategies measured by tracking eye movements; and changes in neural activation from pre- to post-treatment of both NP- and Wh-movement structures, with upregulation of tissue in both the left and right hemispheres, overlapping with regions recruited by neurotypical adults performing the same task. These results were noted in the face of increased atrophy largely in subcortical regions, providing evidence of experience-based plasticity in neurodegenerative disease, and strongly supporting provision of behavioral treatment for patients with PPA.
Table 1. B.
Published studies examining the effects of noninvasive neural stimulation on language improvement across language domains in patients with primary progressive aphasia (PPA) from 2010 to 2018 (PPA-G: 44; PPA-L: 14; PPA-S: 22, unreported subtype: 1).
| Treatment Target | Number of Studies | # of participants with improved language | Authors (year) |
|---|---|---|---|
| tDCS | |||
| Naming | 1 | 8/8 treated | Cotelli et al. (2014) |
| 8/8 placebo | |||
| Language | 2 | 7/7 | Gervits et al. (2016) |
| Wang et al. (2013) | |||
| Spelling | 2 | 42/42 | Tsapkini et al. (2014) |
| Tsapkini et al. (2018) | |||
| rTMS | |||
| Naming | 1 | 10/10 nfvPPA | Cotelli et al. (2012) |
| 0/4 svPPA | |||
| Cognitive function | 1 | 1/1 participant (language only) | Trebbastoni et al. (2012) |
| Verb and noun inflection | 1 | 1/1 PPA (verbs only) | Finocchiaro et al. (2006) |
| Total | 9 | 77/81 | |
Acknowledgements
This study was supported by the National Institutes of Health under Grants R01-DC0148 and P50-DC012283, awarded to Cynthia K. Thompson, and Grant R01-DC008552, awarded to Marek-Marsel Mesulam. We are grateful to Dr. Kaitlyn Litcofsky for her contributions to the development and the analysis of the eye-tracking experiments, and to Brianne Chiappetta, Hannah Guion and Kristie Brockway for assistance with language training.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- Barbieri E, Mack JE, Chiappetta BM, Europa ER, & Thompson CK (2019). Recovery of offline and online sentence processing in aphasia: Language and domain-general network neuroplasticity. Cortex, 120, 394–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales A, Cartwright J, Whitworth A, & Panegyres PK (2016). Exploring generalization processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology, 18, 299–314. [DOI] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, & Rapcsak SZ (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience, 45, 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Basu AS, Grasso SM, Schnyer DM, & Henry ML (2018). Treatment-induced changes in resting brain activity in primary progressive aphasia. Alzheimer’s & Dementia, 14(7), P460–P461. [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, … & Rogalski E. (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136(8), 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M. (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology Section A, 33(4), 497–505. [Google Scholar]
- Cotelli M, Manenti R, Alberici A, Brambilla M, Cosseddu M, Zanetti O, … Borroni B. (2012). Prefrontal cotex rTMS enhances action naming in progressive non-fluent aphasia. European Journal of Neurology, 19, 1404–1412. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, … Borroni B. (2014). Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer’s Disease, 39 (4), 799–808. [DOI] [PubMed] [Google Scholar]
- Croot K, Raiser T, Taylor-Rubin C, Ruggero L, Ackl N, Wlasich E, … Nickels L. (2019). Lexical retrieval treatment in primary progressive aphasia: An investigation of treatment duration in a heterogeneous case series. Cortex, 115, 133–158. [DOI] [PubMed] [Google Scholar]
- Croot K, Taylor C, Abel S, Jones K, Krein L, Hameister I, … Nickels L. (2015). Measuring gains in connected speech following treatment for word retrieval: A study with two participants with primary progressive aphasia. Aphasiology, 29 (11), 1265–1288. [Google Scholar]
- DeMarco AT, Wilson SM, Rising K, Rapcsak SZ, Beeson PM (2018). The neural substrates of improved phonological processing following successful treatment in a case of phonological alexia and agraphia. Neurocase, 24:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … & Albert MS (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Dominey PF, & Inui T. (2009). Cortico-striatal function in sentence comprehension: Insights from neurophysiology and modeling. Cortex, 45(8), 1012–1018. [DOI] [PubMed] [Google Scholar]
- Europa E, Kiran S, Gitelman D, & Thompson CK (2019). Neural connectivity for processing noncanonical sentences with syntactic movement. Frontiers in Human Neuroscience, 13, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrajota L, Maruta C, Maroco J, Martins IP, Guerreiro M, & de Mendonca A. (2012). Speech therapy in primary progressive aphasia: A pilot study. Dementia and Geriatric Cognitive Disorders Extra, 2, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro C, Maimone M, Brighina F, Piccoli T, Giglia G, & Fierro B. (2006). A case study of primary progressive aphasia: Improvement on verbs after rTMS treatment. Neurocase, 12, 317–321. [DOI] [PubMed] [Google Scholar]
- Flanagan KJ, Copland DA, van Hees S, Byrne GJ, & Angwin AJ (2016). Semantic feature training for the treatment of anomia in Alzheimer disease: A preliminary investigation. Cognitive & Behavioral Neurology, 29 (1), 34–43. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, & Cai B. (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60(2), 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervits F, Ash S, Coslett HB, Rascovsky K. Grossman M, & Hamilton R. (2016). Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain and Language, 162, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral-Polrola JG, Polrola P, Mirska N, Mirski A, Herman-Sucharska I, & Pachalska M. (2016). Augmentative and alternative communication (AAC) for a patient with a nonfluent/agrammatic variant of PPA in the mutism stage. Annals of Agriculture and Environmental Medicine, 23 (1), 182–192. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ZM, & Bock K. (2000). What the eyes say about speaking. Psychological Science, 11(4), 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameister I, Nickels L, Abel S, & Croot K. (2017). Do you have mowing the lawn? Improvements in word retrieval and grammar following constraint-induced language therapy in primary progressive aphasia. Aphasiology, 31 (3), 308–331. [Google Scholar]
- Henry ML, Hubbard HI, Grasso SM, Mandelli ML, Wilson SM, Sathishkumar MT, … Gorno-Tempini ML (2018). Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain, 141(6), 1799–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Meese MV, Truong S, Babiak MC, Miller BL, & Gorno-Tempini ML (2013). Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behavioral Neurology, 26 (1–2), 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Rising K, DeMarco AT, Miller BL, Gorno-Tempini ML, & Beeson PM (2013). Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain and Language, 127 (2), 145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S, Khatoonabadi AR, Noroozian M, Mehri A, Ashayeri H, & Nickels L. (2018). The effect of word retrieval therapy in primary progressive aphasia: A single-case study. Archives of Neuroscience, 5 (4), e67577. [Google Scholar]
- Johari K, Walenski M, Reifegerste J, Ashrafi F, Behroozmand R, Daemi M, & Ullman MT (2019). A dissociation between syntactic and lexical processing in Parkinson’s disease. Journal of Neurolinguistics, 51, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AL, Cheng SY, & Jones TA (2011). Experience-dependent neural plasticity in the adult damaged brain. Journal of Communication Disorders, 44(5), 538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. (2007). Western Aphasia Battery (Revised) PsychCorp. San Antonio. [Google Scholar]
- Kiran S. (2007). Complexity in the treatment of naming deficits. American Journal of Speech-Language Pathology, 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Meier EL, Kapse KJ, & Glynn PA (2015). Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Frontiers in Human Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, & Thompson CK (2019). Neuroplasticity of language networks in aphasia: Advances, updates, and future challenges. Frontiers in Neurology: Stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz SA, Frisch S, von Cramon DY, & Friederici AD (2003). Syntactic language processing: ERP lesion data on the role of the basal ganglia. Journal of the International Neuropsychological Society, 9 (7), 1053–60. [DOI] [PubMed] [Google Scholar]
- Mack JE, Nerantzini M, & Thompson CK (2017). Recovery of sentence production processes following language treatment in aphasia: Evidence from eyetracking. Frontiers in Human Neuroscience, 11, article 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JE, & Thompson CK (2017). Recovery of online sentence processing in aphasia: Eye movement changes resulting from Treatment of Underlying Forms. Journal of Speech, Language, and Hearing Research, 60, 1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoir J, Leroy M, Routhier S, Auclair-Ouellet N, Houde M, & Laforce R. (2015). Improving verb anomia in the semantic variant of primary progressive aphasia: The effectiveness of semantic-phonological cueing treatment. Neurocase, 21 (4), 448–456. [DOI] [PubMed] [Google Scholar]
- Mandelli ML, Vilaplana E, Brown JA, Hubbard HI, Binney RJ, Attygalle S, … Gorno-Tempini ML (2016). Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain: A journal of neurology, 139 (Pt 10), 2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski R, Cobia D, Thompson CK, & Weintraub S. (2009). Quantitative template for subtyping primary progressive aphasia. Archives of Neurology, 66(12), 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Snider SF, Eckmann CB, & Friedman RB (2015). Prophylactic treatments for anomia in the logopenic variant of primary progressive aphasia: Cross-language transfer. Aphasiology, 29 (9), 1062–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan DM, Hu TM, & Friedman RB (2016). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology, 30 (4), 483–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebstock AM, Wallace SE (2018). Effects of a combined semantic feature analysis and multimodal treatment for primary progressive aphasia: Pilot study. Communication Disorders Quarterly. [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, & Mesulam M-M (2011). Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology, 76(21), 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Goekoop R, Stam CJ, Barkhof F, & Scheltens P. (2005). Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage, 26(4), 1078–1085. [DOI] [PubMed] [Google Scholar]
- Scarpazza C, Nichols TE, Seramondi D, Maumet C, Sartori G, & Mechelli A. (2016). When the single matters more than the group (II): addressing the problem of high false positive rates in single case voxel based morphometry using non-parametric statistics. Frontiers in neuroscience, 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagarelli KM, Shattuck KF, Turkeltaub PE, & Ullman MT (2019). Language learning in the adult brain: A neuroanatomical meta-analysis of lexical and grammatical learning. NeuroImage, 193, 178–200. [DOI] [PubMed] [Google Scholar]
- Tetzloff KA, Duffy JR, Clark HM, Strand EA, Machulda MM, Schwarz CG, … Lowe VJ (2017). Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain, 141(1), 302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK (2019). Neurocognitive recovery of sentence processing in aphasia. Journal of Speech, Language, and Hearing Research, 62(11), 3947–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, & Shapiro LP (2005). Treating agrammatic aphasia within a linguistic framework: Treatment of Underlying Forms. Aphasiology, 19(10–11), 1021–1036. PMC1847567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro L, Kiran S, & Sobecks J. (2003). The role of syntactic complexity in treatment of sentence deficits in agrammatic aphasia: The complexity account of treatment efficacy (CATE). Journal of Speech, Language, and Hearing Research, 46(3), 591–607. PMC1995234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebbastoni A, Raccah R, de Lena C, Zangen A, & Maurizio I. (2012). Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with primary progressive aphasia - logopenic variant (LPPA). Brain Stimulation, 6, 545–553. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, & Hillis AE (2013). Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behavioral Neurology, 26, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis C, Gomez Y, Davis C, Hillis AE (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology, 24 (8–9), 1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, … Hillis AE (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 4, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Europa E, Caplan D, & Thompson CK (2019). Neural networks for sentence comprehension and production: An ALE‐based meta‐analysis of neuroimaging studies. Human brain mapping, 40(8), 2275–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu D, Chen Y, Yuan Y, & Zhang M. (2013). Effects of transcranial direct current stimulation on language improvement and cortical activation in nonfluent variant primary progressive aphasia. Neuroscience Letters, 549, 29–33. [DOI] [PubMed] [Google Scholar]