Abstract
Background:
Indications for heart-liver transplantation (HLT) for Fontan patients are not well-defined. We compared listing characteristics, post-operative complications, and post-transplant outcomes of Fontan patients with HLT to those with heart-only transplant (HT). We hypothesized HLT patients have increased post-operative complications, but superior survival outcomes compared to HT patients.
Methods:
We performed a retrospective review of Fontan patients who underwent HLT or HT at a single institution. Characteristics at time of listing, including extent of liver disease determined by laboratory, imaging, and biopsy data, were compared. Post-operative complications were assessed, and the Kaplan-Meier survival method was used to compare post-transplant survival. Univariate regression analyses were performed to identify risk factors for increased mortality and morbidity among HT-only patients.
Results:
Forty-seven patients (9 HLT, 38 HT) were included. HLT patients were older, more likely to be on dual inotrope therapy, and had evidence of worse liver disease. While ischemic time was longer for the HLT group, post-operative complications were similar. Over a median post-transplant follow-up of 17 (IQR 5, 52) months, overall mortality for the cohort was 17%; only one HLT patient died (11%) versus 7 (18%) HT patients (p = 0.64). Among HT patients, cirrhosis on pre-transplant imaging was associated with worse outcomes.
Conclusion:
Despite greater inotrope need and more severe liver disease at the time of listing, Fontan patients undergoing HLT have post-transplant outcomes comparable to patients with HT. HLT may offer a survival benefit for Fontan patients with liver disease.
INTRODUCTION
The Fontan procedure, the final surgical palliation to a total cavopulmonary anastomosis, is the current standard practice for patients with single ventricle heart disease. While surgical results and outcomes have improved greatly since its inception in 1971, the Fontan procedure leaves patients with abnormal hemodynamics that may result in heart failure, multisystem end-organ damage, and need for heart-only transplantation (HT). Additionally, Fontan-associated liver disease (FALD) is a well-recognized risk in this population, where longstanding hypoxia, decreased cardiac output, and hepatic congestion can lead to advanced liver fibrosis, cirrhosis, portal hypertension, and even hepatocellular carcinoma1.
Combined heart-liver transplantation (HLT) is offered for select Fontan patients with failing Fontan physiology and hepatic dysfunction2. Literature shows that HLT is feasible and has comparable outcomes to HT in both the heart failure patient population3–8 and the congenital heart disease population9–13. In addition, HLT has been shown to confer protection from rejection in these patients14,15. There is currently no accepted threshold for liver disease severity which necessitates listing for combined HLT over HT. Indications, perioperative complications, and outcomes of HLT in Fontan patients remain incompletely defined.
In this study, we compare pre-transplant listing characteristics, perioperative complications, and post-transplant outcomes between Fontan patients who underwent HT versus combined HLT. We hypothesized Fontan patients requiring HLT will have increased perioperative complications but superior intermediate-term outcomes and freedom from rejection. A secondary aim was to identify risk factors for worse outcome among Fontan patients who receive HT only to better elucidate which Fontan patients should receive heart-only versus heart-liver transplantation.
METHODS
Study cohort and definitions
This is a retrospective, single center study. All patients with single ventricle heart disease palliated to a Fontan, who subsequently received either HT or HLT at Lucile Packard Children’s Hospital (Palo Alto, CA) from July 2006 through December 2019, were included. Patients who underwent other multi-organ transplants (heart-kidney) were excluded. Pre-transplant characteristics were collected from the electronic medical record and included data at the time of transplant listing and at the time of transplant. At our institution, all Fontan patients evaluated for heart transplantation undergo a formal evaluation by our pediatric hepatology team. Indications for HLT at our institution have been previously described and include imaging and/or biopsy evidence of cirrhosis and portal hypertension 13. All combined heart -liver transplants were performed en-bloc16.
Data from liver biopsy results and imaging studies were extracted from final reports in the medical record. The definition of cirrhosis on imaging included a nodular liver surface with heterogeneous density, alteration in hepatic or portal vein flow, portal vein dilation, the presence of any porto-systemic collaterals, and/or ascites13. Fibrosis on liver biopsies was defined as: stage 0: no fibrosis; stage 1: pericellular fibrosis; stage 2: bridging fibrosis; and stage 3: bridging fibrosis with regenerative nodules17. The Model of End-stage Liver Disease eXcluding INR (MELD-XI) score (5.11 x ln[bilirubin] + 11.6 x ln[creatinine] +9.44)18, Child-Pugh score (which accounts for serum bilirubin, serum albumin, INR, ascites, and encephalopathy)19 and VAST (varices, ascites, splenomegaly, and thrombocytopenia) score20 were calculated using respective data at time of listing. Cumulative panel reactive antibodies for human leukocyte antigens (cPRAs) were determined by the clinical laboratory using Luminex technology for IgG and C1q antibodies (One Lambda, Thermo Fisher Scientific, USA).
Peri-operative complication data collection focused on the post-operative period, defined as the time from return from the operating room to the cardiac intensive care unit until time of discharge. Complications reviewed included mortality (overall and prior to hospital discharge), transplant rejection defined as ISHLT cellular rejection grade 2R, or antibody-mediated rejection grade pAMR 2, or greater, unplanned reintubation, need for tracheostomy, unplanned surgical procedure, infection, acute kidney injury (defined as an increase in serum creatinine by at least 1.5 times baseline per KDIGO criteria)21, need for dialysis, need for mechanical circulatory support, and neurologic insult. Infection was defined as positive bacterial or fungal culture and/or elevated inflammatory markers in the setting of clinical signs of sepsis requiring a treatment course with antimicrobials for 5 or more days. Neurologic insult was defined as a new head CT scan finding of infarct or hemorrhage, and/or new onset seizure.
Immunosuppression and cardiac biopsy surveillance
All patients received induction therapy with methylprednisolone and rabbit antithymocyte globulin (or basiliximab or daclizumab if before 2014). Patients with a positive virtual crossmatch (typically with pretransplant donor-specific IgG antibodies [MFI>1000]) underwent plasmapheresis intra-operatively (1.5x volume exchange) and treatment with IVIG on POD 0; patients with persistent donor-specific antibodies following cardiac transplantation continued to receive IVIG at the discretion of the transplant cardiologist. For select HLT patients, Rituximab (375 mg/m2/dose) was given in the early postoperative period at the discretion of the liver transplant team. Standard maintenance immunosuppression included mycophenolate mofetil and a calcineurin inhibitor; corticosteroids were usually discontinued within the first post-transplant month. Endomyocardial biopsies were performed per our institution’s standard surveillance protocol, with additional biopsies performed when rejection was clinically suspected. Surveillance liver biopsies were not routinely performed and were only obtained in the setting of clinical concern for liver rejection. Immunohistochemical staining was performed at the discretion of the cardiac pathologist or at the request of the transplant cardiologist.
Analysis
The primary outcome was overall post-transplant survival. Secondary outcomes included survival to hospital discharge, post-operative complications as defined above, and freedom from rejection. The Kaplan-Meier method was used to assess survival and freedom from rejection, with risk between HT and HLT groups compared using the Cox proportional method. Comparisons of patient characteristics at listing and post-operative complications between HT and HLT groups were performed using Fisher’s exact test or Wilcoxon-Rank-Sum test, as appropriate. To identify risk factors for worse outcome within the HT group, univariate regression analyses were performed using Cox proportional hazards method for survival and logistic regression for post-operative complications, with variables collected at time of transplant listing.
Study data were collected using REDCap electronic data capture tools hosted at Stanford University22,23. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies. Continuous data are presented as median (25th, 75th percentile). All statistical analyses were conducted using Stata/IC version 13.1 (College Station, Texas, USA). P-values < 0.05 were considered significant. This study was approved by Stanford University’s Institutional Review Board Panel.
RESULTS
Forty-seven Fontan patients were included; 38 (81%) received HT only and 9 (19%) received HLT. Compared to HT patients at time of listing, HLT patients were older (19 years [16, 21] versus 14 years [10, 19], p=0.01) and more were on dual inotrope therapy (3 [33%], versus 1 [2.6%], p=0.02). Total bilirubin was higher (1.2 mg/dL [0.9, 1.3] versus 0.7 mg/dL [0.5, 1.1], p=0.03), and platelet count was lower (118 x 103/uL [103, 195] versus 204 x 103/uL [145, 272], p=0.04). More HLT patients had evidence of varices, ascites, splenomegaly, and cirrhosis on imaging, and MELD-XI, Child Pugh, and VAST scores were higher, indicating more severe liver disease (Table 1). Fifteen patients (32%) underwent liver biopsies; 3/6 (50%) in the HLT group and 3/9 (33%) in the HT group had Stage 3 fibrosis (p=0.62).
Table 1.
Characteristics at time of listing
| All (n=47) | HT only (n=38) | Combined HLT (n=9) | p-value | |
|---|---|---|---|---|
| Age (years) | 15 (11, 20) | 14 (10, 19) | 19 (16, 21) | 0.01 |
| Weight (kg) | 43 (27, 62) | 38 (25, 59) | 60 (57, 65) | 0.02 |
| Male gender | 29 (62) | 26 (68) | 3 (33) | 0.06 |
| Diagnosis | 0.21 | |||
| HLHS | 17 (36) | 16 (42) | 1 (11) | |
| TA | 4 (9) | 3 (8) | 1 (11) | |
| AVC | 13 (28) | 11 (29) | 2 (22) | |
| Heterotaxy | 11 (23) | 9 (23) | 2 (22) | 1.0 |
| PLE | 17 (36) | 14 (37) | 3 (33) | 1.0 |
| Listing status | 0.19 | |||
| 1A | 13 (28) | 10 (26) | 3 (33) | |
| 1B | 12 (25) | 12 (31) | 0 (0) | |
| 2 | 21 (45) | 15 (39) | 6 (67) | |
| Adult 4 | 1 (2) | 1 (3) | 0 (0) | |
| Panel Reactive Antibodies | ||||
| IgG (%) | 22 (0.5, 56.5) | 26 (0, 54) | 4 (3, 66) | 0.84 |
| C1q (%) | 4 (0, 24) | 4 (0, 24) | 5.5 (1.5, 20.5) | 0.93 |
| IV inotropic support | 14 (30) | 10 (26) | 4 (44) | 0.42 |
| Milrinone | 14 (30) | 10 (26) | 4 (44) | 0.42 |
| Dopamine | 4 (9) | 1 (3) | 3 (33) | 0.02 |
| VAD (at transplant) | 6 (13) | 6 (16) | 0 (0) | 0.58 |
| Hospitalized | 16 (34) | 12 (32) | 4 (44) | 0.46 |
| Transplant Era2 | 0.69 | |||
| < 2014 | 13 (28) | 10 (26) | 3 (33) | |
| ≥ 2014 | 34 (72) | 28 (73) | 6 (67) | |
| Cardiac catheterization | ||||
| Fontan pressure (mmHg) | 16 (13, 18) | 15.5 (13, 17.5) | 17 (14,18) | 0.36 |
| Filling pressure3 (mmHg) | 11 (9, 14) | 11 (9, 13.5) | 13 (11, 14) | 0.28 |
| Aortic saturation (%) | 90 (85, 93) | 91 (85, 93) | 87 (84.5, 93.5) | 0.96 |
| Cardiac index (L/min/m2) | 3.1 (2.7, 3.8) | 3.1 (2.6, 3.7) | 3 (2.9, 3.8) | 0.73 |
| Pulmonary vascular resistance (iWU) | 2 (1.3, 2.5) | 2 (1.4, 2.5) | 1.9 (1.3, 2.3) | 0.63 |
| Laboratory results | ||||
| Sodium | 135 (133, 138) | 135 (133, 137) | 138 (131, 139) | 0.18 |
| Creatinine | 0.7 (0.53, 0.9) | 0.60 (0.45, 0.9) | 0.82 (0.73, 1.0) | 0.08 |
| Total bilirubin | 0.8 (0.5, 1.2) | 0.7 (0.5, 1.1) | 1.2 (0.9, 1.3) | 0.03 |
| Albumin | 3.5 (3.0, 4.2) | 3.5 (3.0, 4.2) | 4 (3.5, 4.2) | 0.43 |
| Platelets | 183 (123, 266) | 204 (145, 272) | 118 (103, 195) | 0.04 |
| INR1 | 1.3 (1.2, 1.5) | 1.3 (1.2, 1.6) | 1.4 (1.3, 1.5) | 0.58 |
| Radiology findings (n=41) | ||||
| Cirrhosis | 18 (44) | 10 (31) | 8 (89) | 0.005 |
| Varices | 9 (21) | 3 (9) | 6 (67) | 0.001 |
| Ascites | 15 (37) | 7 (22) | 8 (89) | 0.000 |
| Splenomegaly | 13 (36) | 6 (22) | 7 (78) | 0.006 |
| Liver biopsy findings (n=15) | 0.89 | |||
| Stage 0: No fibrosis | 1 (7) | 1 (11) | 0 (0) | |
| Stage 1: Perivascular fibrosis | 4 (27) | 3 (33) | 1 (17) | |
| Stage 2: Bridging fibrosis | 4 (27) | 2 (22) | 2 (33) | |
| Stage 3: Nodules | 6 (40) | 3 (33) | 3 (50) | |
| Liver disease scores | ||||
| MELD-XI | 9 (9, 11) | 9 (9, 10) | 10 (9, 11) | 0.17 |
| Child Pugh | 6.5 (6, 8) | 6 (6, 7) | 8 (7, 9) | 0.007 |
| VAST | 1 (0, 2) | 1 (0, 1) | 3 (2, 4) | 0.0001 |
Data are presented as median (25th, 75th percentiles) or n (%)
For patients not on coumadin (n=15)
Patients transplanted prior to 2014 received anti-interleukin-2 receptor antibodies (basiliximab or daclizumab) during induction, patients transplanted in 2014 or later received rabbit antithymocyte globulin
Filling pressure was the highest available number of right or left pulmonary capillary wedge pressure, direct left atrial pressure, or direct right atrial pressure PLE = protein losing enteropathy. VAD = ventricular assist device.
Post-transplant survival
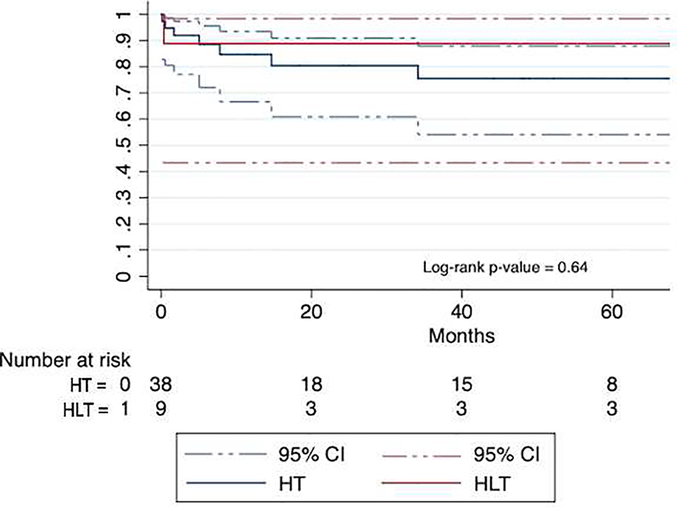
Median waitlist time was 131 days (76, 455), with no difference in waitlist time between the two groups (131 days [109, 149] for HLT versus 135 days [76, 483] for HT, p=0.74). Over a median post-transplant follow up of 17 (5, 52) months, overall mortality for the entire cohort was 17%. The Kaplan Meier estimated survival at 1-year, 5-years and 10-years was 85% (95% CI 70–93), 78% (59–89), and 78% (59–89). Median post-transplant follow up was 17 (4, 49) months for HT patients and 17 (5, 77) months for HLT patients (p=0.73). There was no difference in overall post-transplant mortality between the two groups; one (11%) HLT patient died versus 7 (18%) HT patients (p = 0.64, Figure 1). Survival to hospital discharge post-transplant was also similar between the two groups (p=0.67). For the one HLT patient who died, death occurred before hospital discharge on POD 11, due to profound neurologic injury from a left middle cerebral artery embolic infarct sustained intra-operatively. Four HT patients died before discharge, at a median of 34 [9.5, 103] days post-transplant. Notably, all four of these patients had protein-losing enteropathy (PLE) pre-transplant; two died from multi-organ failure related to sepsis (POD 52 and 154), one developed ventricular fibrillation while receiving dialysis for post-operative renal failure (POD 16), and one died from a large intracranial bleed after massive intra-operative hemorrhage with multi-system organ injury requiring VA-ECMO support to leave the operating room (POD 3).
Figure 1.
Post-transplant Survival
Peri-operative complications
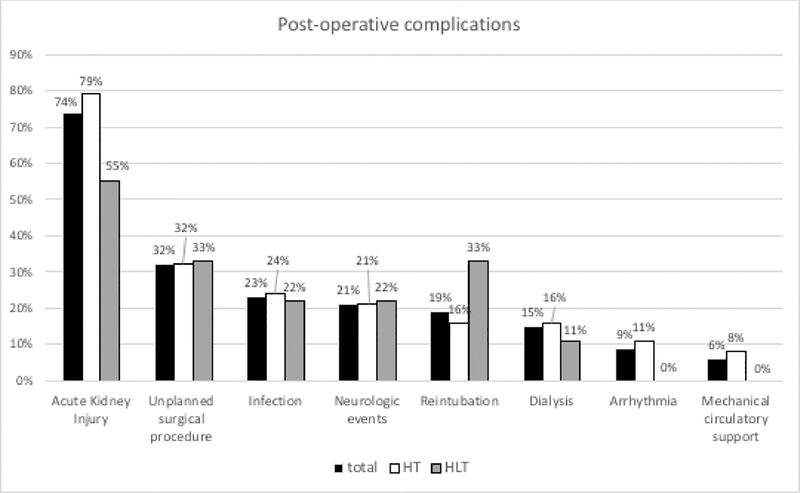
At transplant, ischemic time was longer for the HLT group, but bypass and cross-clamp time were similar (Table 2). Duration of intubation, ICU stay, and post-transplant hospital length of stay (LOS) were similar for both groups, and there was no significant difference in the number of post-operative complications (Figure 2). Acute kidney injury occurred in the majority of patients in both groups (79% HT and 55% HLT). Other common complications included infection (37% HT, 56% HLT), unplanned surgical procedures (32% HT, 33% HLT), and neurological events (21% HT, 22% HLT). Types of infections were similar, with bacterial infections (n=9 HT, n=2 HLT), Clostridium dificile colitis (n=1 HT, n=1 HLT), and culture negative sepsis (n=6 HT, n=2 HLT). Unplanned surgical procedures included chest washout for bleeding or infection (n=4 HT, n=1 HLT), peritoneal drain placement (n=4 HT, n=1 HLT), dialysis catheter placement (n=2 HT), craniotomy, colonoscopy with biopsy, tracheostomy (n=1 HT), and bile duct reconstruction (n=1 HLT). Neurological events included intracranial hemorrhage (n=5 HT, n=1 HLT), ischemic infarcts (n=2 HT, n=1 HT) and seizures (n=4 HT).
Table 2.
Post-operative course and complications
| All (n=47) | HT only (n=38) | Combined HLT (n=9) | p-value | |
|---|---|---|---|---|
| Ischemic time (min) | 223 (188, 260) | 216 (184, 245) | 293 (255, 336) | 0.004 |
| CPB time (min) | 271 (216, 320) | 277 (205, 320) | 264 (243, 327) | 0.67 |
| Cross clamp time (min) | 178 (120, 206) | 175 (120, 204) | 200 (120, 216) | 0.32 |
| Length of intubation (days) | 2 (1, 4) | 2 (1, 4) | 2 (1, 3) | 0.48 |
| Length of ICU stay (days) | 9.5 (6, 20) | 10 (6, 22) | 8 (6, 19) | 0.62 |
| Length of post-transplant stay (days) | 28.5 (17, 47) | 26 (18, 47) | 29 (16, 42) | 0.90 |
Data are presented as median (25th, 75th percentiles) or n (%)
CPB = cardiopulmonary bypass
Figure 2.
Post-operative complications. HLT, heart–liver transplantation; HT, heart-only transplantation.
Risk factors for mortality and morbidity in HT patients
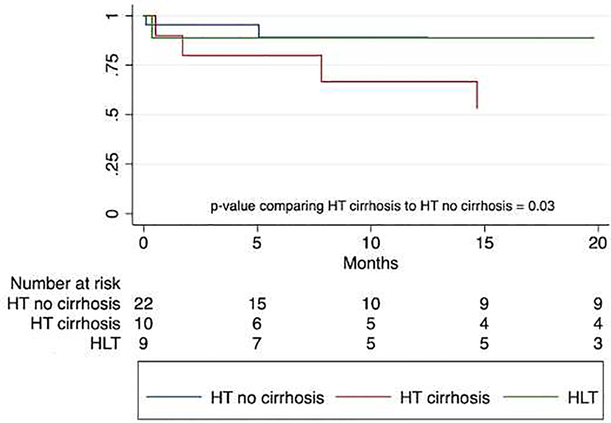
Univariate regression analyses were performed to identify risk factors for increased mortality and morbidity among the patients who received HT (Table 3). Of 32 HT patients with imaging at time of listing, 10 had findings of cirrhosis and 5 of these patients (50%) died at a median 7.8 (1.7–14.7) months post-transplant. The estimated 1-year survival for HT patients with cirrhosis was 67% (95% CI 23–88), compared to 89% (95% CI 62–97) of HT patients without cirrhosis and 89% (95% CI 43–98) of HLT patients (Figure 3). The hazard ratio for post-transplant survival for HT patients with cirrhosis was near significance (HR 5.1, 95% CI 0.98–26.3, p=0.052).
Table 3.
Univariate regression for mortality and morbidity in HT only patients (N = 32)
| Characteristic at time of listing | Post-transplant survival HR (95th CI) | p-value | Unplanned surgical procedure OR (95th CI) | p-value | Dialysis OR (95th CI) | p-value |
|---|---|---|---|---|---|---|
| Age | 1.01 (0.88–1.17) | 0.850 | 1.08 (0.95–1.22) | 0.252 | 1.06 (0.90–1.24) | 0.503 |
| Male gender | 2.30 (0.27–19.22) | 0.443 | 8.07 (0.90–72.08) | 0.062 | 2.62 (0.27–25.29) | 0.405 |
| Heterotaxy | 0.75 (0.88–6.34) | 0.791 | 21 (0.45–9.86) | 0.347 | 0.60 (0.06–5.93) | 0.662 |
| PLE | 5.76 (1.11–29.88) | 0.037 | 3.8 (0.90–16.00) | 0.069 | 4.40 (0.69–28.11) | 0.117 |
| Sodium | 0.97 (0.81–1.15) | 0.684 | 0.72 (0.54–0.96) | 0.027 | 0.90 (0.72–1.13) | 0.359 |
| Creatinine | 0.70 (0.06–8.12) | 0.778 | 1.42 (0.15–13.25) | 0.757 | 2.95 (0.19–45.52) | 0.439 |
| Total bilirubin | 0.96 (0.19–4.87) | 0.958 | 0.46 (0.08–2.64) | 0.388 | 0.50 (0.53–4.74) | 0.549 |
| Albumin | 1.18 (0.39–3.59) | 0.769 | 0.81 (0.31–2.14) | 0.668 | 0.89 (0.26–3.04) | 0.859 |
| Platelets | 0.99 (0.99–1.01) | 0.455 | 1.00 (1.00–1.01) | 0.518 | 0.99 (0.98–1.01) | 0.311 |
| Cirrhosis | 5.08 (0.98–26.27) | 0.052 | 10.5 (1.86–59.40) | 0.008 | 6.67 (0.97–45.79) | 0.054 |
| Varices | 1.90 (0.23–15.96) | 0.552 | 4.44 (0.36–55.58) | 0.247 | 2.40 (0.18–31.88) | 0.507 |
| Ascites | 0.41 (0.05–3.45) | 0.408 | 3.43 (0.61–19.40) | 0.163 | 2.10 (0.30–14.87) | 0.458 |
| Splenomegaly | 1.23 (0.24–6.35) | 0.805 | 2.43 (0.47–12.54) | 0.289 | 1.67 (0.24–11.44) | 0.603 |
| MELD-XI | 0.92 (0.62–1.37) | 0.696 | 0.83 (0.54–1.27) | 0.392 | 1.05 (0.69–1.58) | 0.824 |
| Child Pugh | 0.62 (0.29–1.37) | 0.237 | 1.29 (0.69–2.43) | 0.426 | 0.96 (0.42–2.16) | 0.915 |
| VAST | 0.73 (0.27–1.94) | 0.525 | 2.35 (0.98–5.65) | 0.056 | 1.28 (0.48–3.42) | 0.614 |
Cox-proportional method was used for survival outcomes, and logistic regression method for post-transplant morbidities.
Other variables included in analysis but not significant are: listing status, VAD, hospitalization, milrinone, dopamine, fibrosis on biopsy, stage 3 fibrosis on biopsy.
Figure 3.
Post-transplant Survival
HT patients with cirrhosis were at increased risk of unscheduled surgical procedure in the peri-operative period (HR 10.5, 95% CI 1.9–59.4, p=0.008), and the risk for post-transplant dialysis was near significance (HR 6.7, 95% CI 0.97–45.8, p=0.054). Post-operative complications compared between HT patients without cirrhosis (n=22), HT patients with cirrhosis (n=10), and HLT patient (n=9) are shown in Table 4; notably, 70% of HT patients with cirrhosis had unplanned surgical procedures (compared to 18% HT without cirrhosis and 33% HLT, p=0.009) and 40% required post-transplant dialysis (compared to 9% HT without cirrhosis and 11% HLT, p=0.09).
Table 4.
Post-operative complications
| HT −cirrhosis (n=22) | HT +cirrhosis (n=10) | HLT (n=9) | p-value | |
|---|---|---|---|---|
| Reintubation | 2 (9) | 1 (10) | 3 (33) | 0.15 |
| Acute kidney injury | 16 (73) | 10 (100) | 5 (55) | 0.16 |
| Dialysis | 2 (9) | 4 (40) | 1 (11) | 0.09 |
| Infection | 6 (27) | 5 (50) | 5 (56) | 0.23 |
| Unplanned surgical procedure* | 4 (18) | 7 (70) | 3 (33) | 0.02 |
| Mechanical circulatory support | 2 (9) | 1 (10) | 0 (0) | 1.00 |
| Arrhythmia | 3 (14) | 0 (0) | 0 (0) | 0.40 |
| Neurologic event | 4 (18) | 3 (30) | 2 (22) | 0.88 |
Unplanned surgical procedures included chest washout, peritoneal drain placement, dialysis catheter placement, craniotomy, colonoscopy with biopsy, tracheostomy, and bile duct reconstruction.
Rejection
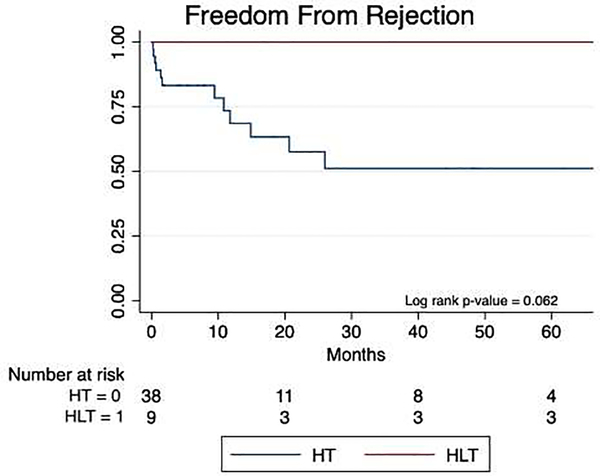
At the time of listing, the median cPRA by IgG was 26% (0, 54) for HT patients and 4% (3, 66) for HLT patients (p=0.71). Induction regimens were similar between HT and HLT patients (26% vs 33% received anti-interleukin-2 receptor antagonists, p=0.69). One HLT patient received rituximab in the immediate post-operative period at the discretion of the liver transplant team. The retrospective crossmatch, available for 45 patients, was positive for 8 of 37 HT patients and none of 8 HLT patients. No HLT patients experienced cardiac rejection, whereas 12 (32%) HT patients had rejection (p = 0.062, Figure 4), with first rejection episode occurring at a median 5.5 (0.6–13.3) months from transplant. Of the 12 HT patients with rejection, four had a positive retrospective crossmatch and eight had a negative crossmatch; 10 had cellular rejection, 1 had humoral rejection only, and 1 patient had both. One HLT patient had moderate acute cellular rejection of the liver which was successfully treated. This patient had a liver biopsy performed at 11 months post-transplant due to rising liver enzymes; concurrent cardiac biopsy did not show rejection.
Figure 4.
Freedom from rejection. HLT, heart–liver transplantation; HT, heart-only transplantation.
DISCUSSION
Overall, our single center experience suggests similar peri-operative and survival outcomes between pediatric and young adult Fontan patients receiving HT versus HLT. While sample size could have prevented the detection of a true difference in outcomes, our findings are consistent with prior studies which show comparable survival for HLT recipients for all indications (e.g. amyloidosis) and HT recipients 3,6 as well as those with congenital heart disease9–11. Bryant et al. conducted a UNOS database analysis (1987 – 2015) comparing outcomes between 41 patients with congenital heart disease who underwent HLT with a matched cohort who underwent HT. In this study, the HLT group actually had a survival advantage. They also showed similar rates of stroke and need for dialysis in the post-operative period10. Here, we add to the growing collection of evidence that HLT is a reasonable option for congenital heart disease patients, and more specifically, we show comparable outcomes between Fontan patients who undergo HT versus HLT.
Whereas we directly compared outcomes between HT and HLT groups, it is difficult to predict how the HLT cohort would have done if they had undergone HT only. Organs remain a limited resource, and transplant clinicians must be thoughtful when advocating for their responsible allocation. The Fontan patients who received HLT in our study had more advanced liver disease (as evidenced by findings on imaging studies and higher liver disease scores), but we do not yet fully understand what level of liver disease in the Fontan population necessitates HLT for optimal outcomes. FALD is known to be a progressive condition, one which may even begin prior to completion of the Fontan cavopulmonary connection24,25. There is a known direct correlation between time from Fontan procedure and the extent of liver fibrosis. Whereas liver biopsy is the gold standard for evaluating liver fibrosis, fibrosis is typically heterogenous, and thus liver biopsy sampling error may underestimate the extent of liver involvement in FALD. Laboratory markers of liver injury (AST, ALT, bilirubin) are typically normal or only mildly deranged, and synthetic function may be preserved26. With only mild, or no, laboratory derangements and variable findings on liver biopsy, FALD remains difficult to characterize, and thus markers to determine which Fontan patient should receive HLT are still not clear.
To address the question of which Fontan patient warrants HLT, we looked at risk factors associated with worsen outcomes within the HT group. While it is possible that clinical status may change over the course of waitlist time, the decision to list for heart versus heart-liver is made at the time of listing. We thus deliberately chose to use clinical data at the time of transplant listing, rather than at time of transplant, as we believe this would be most useful to clinicians who are deciding at the time of transplant evaluation whether to list for heart or to list for heart-liver. We found that cirrhosis on imaging was significantly associated with an unplanned surgical procedure in the post-operative period, and neared significance for post-transplant mortality and increased need for dialysis. Multivariable analyses were not performed due to small sample size, but this finding suggests that cirrhosis on imaging in a failing Fontan patient should prompt strong consideration for HLT rather than HT. It is possible that cirrhosis on imaging is a risk factor because it is the variable that best correlates with overall FALD progression. Alternatively, it is possible that cirrhosis on imaging is a marker for a longer period of time spent in a failing Fontan physiology with abnormal hemodynamics, and thus patients undergoing HT who have cirrhosis on imaging have a generally more fragile pre-transplant state and worse outcomes.
Interestingly, Simpson et al. evaluated 32 patients with Fontan physiology and cirrhosis on CT imaging who underwent HT evaluation27. Of the 20 patients in their cohort who underwent heart transplantation, 1-year survival was 80%, and there was no difference in survival between those with and those without cirrhosis. Our definition of cirrhosis on imaging differed from theirs and included findings of portal hypertension (e.g. ascites or varices). Thus, it is possible that our patients with cirrhosis had more severe liver disease than theirs, explaining the difference in our findings. Again, this illustrates the difficulty in defining the extent of FALD. There certainly are patients with some degree of FALD who may do well with HT only. It is our challenge as a field to further refine that extent of liver involvement which necessitates HLT and what clinical, imaging, and pathologic markers will help make that determination.
Historically, there has been debate about whether the outcomes for patients with Fontan physiology after HT are inferior or comparable to patients with other forms of congenital heart disease28, 29, 30. However, more recent publications show better outcomes for Fontan patients after HT31. As post-transplant mortality improves for patients with failing Fontan physiology, attention should be paid to limiting post-operative morbidities. Because of increased acuity, significantly longer operative ischemic times, and large volume transfusion requirement at the time of transplant, we initially hypothesized that HLT patients would have greater post-operative complications. Our hypothesis turned out to be incorrect; both HLT and HT patients experienced similar rates of post-operative complications. Acute kidney injury was the most common post-operative complication in all Fontan patients. There is a known association between the Fontan circulation and renal dysfunction, driven by renal venous congestion and low cardiac output, and surgery and cardiopulmonary bypass are the insult that lead to AKI1. Careful attention to optimizing hemodynamics and fluid management, as well as avoidance of nephrotoxic medications, are needed to avoid AKI in the vulnerable Fontan population. Infection and unplanned surgical procedures were also commonly seen. Interestingly, 5 of the 19 unplanned surgeries were mediastinal or abdominal washouts related to infection, highlighting the gravity of this particular morbidity. Fontan HT patients are known to have an increased likelihood of infection and bleeding28. It was recently reported that Fontan HT patients have a higher risk of infection and a more pronounced suppression of CD3 counts in the post-operative period32. It is notable that there were no rejection events in the HLT cohort in our study, despite no significant difference in cPRA at listing, and this is consistent with what has been reported by others10. The lack of rejection raises the possibility of decreasing standard immunosuppression in HLT patients to decrease the risk of infection.
CONCLUSION
This single pediatric-center study of patients with failing Fontan physiology who underwent HT or HLT shows similar survival and post-operative complication outcomes between the two groups. Overall mortality was low, and rejection was rare in both groups, with the potential benefit of less rejection in the HLT group. Common post-operative complications were acute kidney injury, need for unplanned surgical procedure, and infection. Cirrhosis on pre-transplant listing imaging was a risk factor for increased post-transplant complications among the heart-only transplant patient cohort. While markers to identify which patient warrants HLT over HT still need to be refined, HLT appears to be a safe and viable option for failing Fontan patients with advanced Fontan-associated liver disease.
Acknowledgments
FUNDING SOURCES: Research IT grant support (Stanford CTSA award number UL1 TR001085 from NIH/NCRR)
Footnotes
CONFLICTS OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lui GK, Saidi A, Bhatt AB, et al. Diagnosis and Management of Noncardiac Complications in Adults With Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation. 2017;136(20):e348–e392. [DOI] [PubMed] [Google Scholar]
- 2.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122(2):173–183. [DOI] [PubMed] [Google Scholar]
- 3.Te HS, Anderson AS, Millis JM, Jeevanandam V, Jensen DM. Current state of combined heart -liver transplantation in the United States. J Heart Lung Transplant. 2008;27(7):753–759. [DOI] [PubMed] [Google Scholar]
- 4.Reich HJ, Awad M, Ruzza A,et al. Combined Heart and Liver Transplantation: The Cedars-Sinai Experience. Transplant Proc. 2015;47(9):2722–2726. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal AD, Chamogeorgakis T, Shafii AE, et al. Combined heart and liver transplantation:the Cleveland Clinic experience. Ann Thorac Surg. 2013;95(1):179–182. [DOI] [PubMed] [Google Scholar]
- 6.Cannon RM, Hughes MG, Jones CM, Eng M, Marvin MR. A review of the United States experience with combined heart-liver transplantation. Transpl Int. 2012;25(12):1223–1228. [DOI] [PubMed] [Google Scholar]
- 7.Beal EW, Mumtaz K, Hayes D, Jr., Whitson BA, Black SM. Combined heart-liver transplantation:Indications, outcomes and current experience. Transplant Rev (Orlando). 2016;30(4):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atluri P, Gaffey A, Howard J, et al. Combined heartand liver transplantation can be safely performed with excellent short-and long-term results. Ann Thorac Surg. 2014;98(3):858–862. [DOI] [PubMed] [Google Scholar]
- 9.Bradley EA, Pinyoluksana KO, Moore-Clingenpeel M, Miao Y, Daniels C. Isolated heart transplant and combined heart-liver transplant in adult congenital heart disease patients: Insights from the united network of organ sharing. Int J Cardiol. 2017;228:790–795. [DOI] [PubMed] [Google Scholar]
- 10.Bryant R, 3rd, Rizwan R, Zafar F,et al. Contemporary Outcomes of Combined Heart-Liver Transplant in Patients With Congenital Heart Disease. Transplantation. 2018;102(2):e67–e73. [DOI] [PubMed] [Google Scholar]
- 11.D'Souza BA, Fuller S, Gleason LP, et al. Single-center out comes of combined heartand liver transplantation in the failing Fontan. Clin Transplant. 2017;31(3). [DOI] [PubMed] [Google Scholar]
- 12.Hollander SA, Reinhartz O, Maeda K, Hurwitz M, DNR, Bernstein D. Intermediate -term outcomes after combined heart-liver transplantation in children with a univentricular heart. J Heart Lung Transplant. 2013;32(3):368–370. [DOI] [PubMed] [Google Scholar]
- 13.Vaikunth SS, Concepcion W, Daugherty T, et al. Short-term out comes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant. 2019;33(6):e13540. [DOI] [PubMed] [Google Scholar]
- 14.Rana A, Robles S, Russo MJ, et al. The combined organ effect:protection against rejection? Ann Surg. 2008;248(5):871–879. [DOI] [PubMed] [Google Scholar]
- 15.Chou AS, Habertheuer A, Chin AL, Sultan I, Vallabhajosyula P. Heart-Kidney and Heart-Liver Transplantation Provide Immunoprotection to the Cardiac Allograft. Ann Thorac Surg 2019;108(2):458–466. [DOI] [PubMed] [Google Scholar]
- 16.Hill AL, Maeda K, Bonham CA, Concepcion W. Pediatric combined heart-liver transplantation performed en bloc:a single-center experience. Pediatr Transplant. 2012;16(4):392–397. [DOI] [PubMed] [Google Scholar]
- 17.Louie CY, Pham MX, Daugherty TJ, Kambham N, Higgins JP. The liver in heart failure:a biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod Pathol. 2015;28(7):932–943. [DOI] [PubMed] [Google Scholar]
- 18.Heuman DM, Mihas AA, Habib A, et al. MELD-XI:arational approach to "sickest first" liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007;13(1):30–37. [DOI] [PubMed] [Google Scholar]
- 19.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60(8):646–649. [DOI] [PubMed] [Google Scholar]
- 20.Elder RW, McCabe NM, Hebson C, et al.Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol. 2013;168(4):3764–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, & Uchino S Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2(1):1–138. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture(REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium:Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz MC, Glatz AC, Daniels K, et al. Hepatic Abnormalities Are Present Before and Early After the Fontan Operation. Ann Thorac Surg 2015;100(6):2298–2304. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MC, Sullivan L, Cohen MS, et al. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg. 2012;143(4):904–909. [DOI] [PubMed] [Google Scholar]
- 26.Greenway SC, Crossland DS, Hudson M, et al. Fontan-associated liver disease: Implications for heart transplantation. J Heart Lung Transplant. 2016;35(1):26–33. [DOI] [PubMed] [Google Scholar]
- 27.Simpson KE, Esmaeeli A, Khanna G, et al. Liver cirrhosis in Fontan patients does not affect 1-year post-heart transplant mortality or markers of liver function. J Heart Lung Transplant 2014;33(2):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation. 2006;114(4):273–280. [DOI] [PubMed] [Google Scholar]
- 29.Kovach JR, Naftel DC, Pearce FB, et al. Comparison of risk factors and outcomes for pediatric patients listed for heart transplantation after bidirectional Glenn and after Fontan: an analysis from the Pediatric Heart Transplant Study. J Heart Lung Transplant. 2012;31(2):133–139. [DOI] [PubMed] [Google Scholar]
- 30.Lamour JM, Kanter KR, Naftel DC, et al. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol. 2009;54(2):16Q–165. [DOI] [PubMed] [Google Scholar]
- 31.Simpson KE, Pruitt E, Kirklin JK, et al. Fontan Patient Survival After Pediatric Heart Transplantation Has Improved in the Current Era. Ann Thorac Surg. 2017;103(4):1315–1320. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed JHL; Profita E; Dykes J; Hollander S; Kaufman B; Bernstein D; Rosenthal DN; Barkoff L; Lee D; Chen S Heightened Immune Response and Increased Risk of Infections in Pediatric Fontan Patients after Heart Transplantation. The Journal of Heart and Lung Transplantation. 2020;39(4):S452–S453 [DOI] [PubMed] [Google Scholar]