Abstract
Background:
Expired-air carbon monoxide (CO) is commonly used to biochemically verify smoking status. The CO cutoff and CO monitor brand may affect the probability of classifying smokers as abstinent, thus influencing conclusions about the efficacy of cessation trials. No systematic reviews have tested this hypothesis. Therefore, we performed a meta-analysis examining whether the likelihood of smoking cessation classification varied due to CO cutoff and monitor brand.
Methods:
Eligible studies (k=122) longitudinally assessed CO-verified cessation in adult smokers in randomized trials. Primary meta-regressions separately assessed differences in quit classification likelihood due to continuous and categorical CO cutoffs (Low, 3–4 parts per million [ppm]; [SRNT] Recommended, 5–6 ppm; Moderate, 7–8 ppm; and High, 9–10 ppm); exploratory analyses compared likelihood outcomes between monitor brands: Bedfont and Vitalograph.
Results:
The likelihood of quit classification increased 18% with each 1 ppm increase above the lowest cutoff (3 ppm). Odds of classification as quit significantly increased between each cutoff category and High: 261% increase from Low; 162% increase from Recommended; and 150% increase from Moderate. There were no differences in cessation classification between monitor brands.
Conclusions:
As expected, higher CO cutoffs were associated with greater likelihood of cessation classification. The lack of CO monitor brand differences may have been due to model-level variance not able to be followed up in the present dataset. Researchers are advised to report outcomes using a range of cutoffs—including the recommended range (5–6 ppm)—and the CO monitor brand/model used. Using higher CO cutoffs significantly increases likelihood of quit classification, possibly artificially elevating treatment strategies.
Keywords: Smoking Cessation, Bioverification, Expired-air Carbon Monoxide, Meta-analysis
1. INTRODUCTION
Smoking cessation randomized clinical trials are the gold standard for determining the efficacy of interventions to promote quitting. Biochemical verification of abstinence in smoking cessation trials adds rigor to study methods and is strongly encouraged for use, when possible (Benowitz et al., 2020). In other words, verifying smoking status objectively—rather than by self-report—is critical for accurately determining the efficacy of cessation treatments. Self-reported smoking status can be subject to bias—including recall bias and social desirability reporting—in which smokers unintentionally or intentionally misreport their smoking behavior (Gorber et al., 2009).
Cotinine and expired-air carbon monoxide (CO) testing are two of the most common forms of biochemical verification of smoking status. While both methods are feasible to do as point-of-care testing, expired-air CO has the advantage of being less costly, less invasive to obtain, and not affected by the use of nicotine replacement products (Benowitz et al., 2020, 2002). Historically, the common threshold (i.e., cutoff) of < 8–10 parts per million (ppm) for CO was considered indicative of abstinence, and was the gold standard of detection endorsed by Society for Research on Nicotine and Tobacco (SRNT) (Benowitz et al., 2002).
However, that cutoff recommendation was made in 2002 and since that time, there has been increasing skepticism about its validity. More recently, research has supported lowering the threshold to 6 ppm and potentially to as low as 3 ppm as a means of increasing specificity (Cropsey et al., 2006, 2014; Deveci et al., 2004; Javors et al., 2005; Low et al., 2004; MacLaren et al., 2010; Perkins et al., 2013). Single sample non-randomized studies assessing the impact of reducing the CO threshold on cessation prevalence found mixed results, depending on which CO cutoff was employed (Brose et al., 2013; Cropsey et al., 2008). For example, Cropsey et al. (2008) found that the prevalence of cessation doubled when comparing a cutoff of 3 ppm versus the standard 10 ppm (18.4% vs. 37.2% at end of treatment). Further, while Brose et al. (2013) indicated no significant differences when using < 10 ppm vs. < 8 ppm (35% vs. 34.7%), they found that the prevalence of cessation reduced significantly when compared to a < 3 ppm cutoff (26.3%). Taken together, these findings suggest that the CO cutoff utilized to determine abstinence does impact cessation prevalence. Yet another important confounding variable to consider is the type of CO monitor used—prior research has demonstrated that CO results from two commonly used monitor brands differ significantly (Karelitz et al., 2017).
Based on findings from these studies, SRNT recently updated biomarker verification recommendations for tobacco use and abstinence and reduced the CO threshold recommendation for smoking cessation to 5–6 ppm, while also recommending that all studies report which model CO monitor was utilized (Benowitz et al., 2020). However, these recommendations are based on a narrative synthesis of the literature and did not distinctly measure the impact of using different CO cutoffs on smoking cessation prevalence. The purpose of this study was to conduct a meta-analysis of published smoking cessation randomized trials to examine whether the likelihood of being classified as quit varies due to use of different CO cutoffs, ranging 3–10 ppm. We also explored whether cessation outcomes differed by the brand of CO monitor used.
2. METHODS
2.1. Search strategy and selection criteria
We conducted a literature search in April 2020 in PubMed using the following combination of keywords/terms:
(“Bupropion”[MeSH Terms] OR “varenicline”[MeSH Terms] OR “Tobacco Use Cessation Devices”[MeSH Terms]) AND (“smoking cessation”[MeSH Terms] OR (“smoking”[All Fields] AND “cessation”[All Fields]) OR “smoking cessation”[All Fields]) AND Clinical Trial[ptyp] AND (“2010/01/01”[PDAT] : “2020/12/31”[PDAT]) AND “humans”[MeSH Terms] AND English[lang] AND “adult”[MeSH Terms]
In addition, we also solicited articles via the SRNT Treatment Research Network listserv to obtain additional studies for inclusion.
Article titles and abstracts were reviewed by the authors to determine eligibility. Studies were eligible for inclusion if they: (a) longitudinally assessed cessation in adult smokers (i.e., ages ≥18 years); (b) randomized participants to treatment groups; (c) recruited ≥ 50 participants (Nüesch et al., 2010); (d) used expired-air CO to confirm abstinence; (e) reported the CO cutoff that was used; (f) presented original data (i.e., secondary analyses were not included) published no earlier than 2010 in a peer-reviewed journal; and (g) were written in English. Our goal was not to provide a comprehensive review of the entire smoking cessation trial literature, therefore we limited inclusion of studies to only those published since 2010. We expected a literature search within this ten year period to identify an adequate number of relevant studies to allow us to thoroughly examine our research question using the meta-analytic procedures outlined below.
2.2. Study coding and data extraction
Nine coders—three authors (JLK, EAM, CWC) and six assistants—were trained on procedures for independently coding data from eligible studies. In order to standardize data extraction, data were coded into an online database via a survey programmed in Qualtrics (Qualtrics, 2020). Data from all studies were double-entered; issues encountered during data coding and discrepancies between coders were resolved in consultation with authors JLK and EAM. Extracted variables included: sample size, intervention/treatment type, follow-up period (in weeks), CO cutoff, brand of CO monitor used, and the proportion of participants who were classified as quit (i.e., the dependent measure). Cessation outcomes from all follow up periods that were reported were coded separately for each intervention type (Lipsey and Wilson, 2001).
2.3. Data synthesis and analysis plan
Data were analyzed using meta-regression with restricted maximum likelihood (REML) estimation in Comprehensive Meta-Analysis 3.0 (CMA) (Borenstein et al., 2013). CMA software converted cessation proportions (i.e., percent of each treatment group with CO below the respective criterion) into logits for use as the effect size in all analyses. These normally distributed logits are preferred over proportions, as the latter are constrained between 0 and 1. Analysis of proportion data often results in underestimated confidence intervals and overestimated levels of heterogeneity (Lipsey and Wilson, 2001). For all results, β indicate logit regression coefficients and ‘odds’ refer to exponentiated β’s.
As our aim was to examine whether the likelihood of classifying smokers as abstinent varied due to the CO cutoff used, and not to compare specific intervention or treatment efficacy, analyses collapsed across treatment subgroups and used study as the level of analysis (Borenstein et al., 2001). Effect sizes were averaged across follow-up periods, where applicable (due to limitations of CMA software, analyzing effect of follow up period would require treating this repeated measures data as independent, increasing risk of Type I error). All analyses used random-effects models which allowed for estimations of both between-study (T2) and within-study sources of variance (Nikolakopoulou et al., 2014). To quantify the proportion of variance explained for each model with covariates, we calculated R2 using the following formula:
Effect size heterogeneity was assessed using Cochran’s Q and I2 statistics. When significant, Cochran’s Q indicates that the heterogeneity in effect sizes between studies is not due to random error (Higgins and Thompson, 2002). The I2 statistic reflects the amount of variance between studies due to real differences (i.e., not sampling error). In other words, I2 quantifies the proportion of heterogeneity that may possibly be explained by covariates—values of 0, <30, and >50% indicate no, moderate, and high levels of heterogeneity, respectively (Higgins and Thompson, 2002).
2.3.1. Preliminary analysis and publication bias
A preliminary intercept-only meta-regression model was first estimated to determine whether the overall prevalence of cessation was greater than zero. Publication bias was then assessed across all studies using Kendall’s tau (Begg and Mazumdar, 1994) and Duval and Tweedie’s trim and fill method (Duval, 2005; Duval and Tweedie, 2000). Kendall’s tau looks for an inverse correlation between effect size and sample size, which would indicate publication bias (i.e., whether large studies tended to have small effect sizes and small studies tended to have large effect sizes). Duval and Tweedie’s trim and fill method imputes values for missing studies needed to balance the funnel plot and tests whether these imputed values affect the overall effect size.
2.3.2. Primary analyses
Primary analyses used random effects meta-regression to assess whether the CO cutoff used to determine quit status was related to the cessation effect size. We separately examined CO cutoff as a continuous (centered at 3 ppm) and categorical covariate. Categories for CO cutoffs were guided by the most recent recommendations for using CO to verify smoking abstinence (Benowitz et al., 2020): Low (3–4 ppm; k = 13), Recommended (5–6 ppm; k = 16), Moderate (7–8 ppm; k = 29), and High (9–10 ppm; k = 64).
2.3.3. Secondary analyses
As previously discussed, earlier research by Karelitz et al. (2017) found that CO values can vary due to the brand of CO monitor used. Therefore, secondary analyses examined the potential moderating effect of CO monitor brand (Vitalograph or Bedfont only) on cessation effect size with the inclusion of dichotomous covariate, CO monitor, and the interaction of CO monitor by continuous CO cutoff.
2.3.4. Exploratory analyses
Exploratory analyses examined whether adjusting CO cutoffs based on monitor brand would affect associations with cessation effect size. We used data from an earlier study of 654 pairs of consecutively obtained CO values from Vitalograph and Bedfont monitors (Karelitz et al., 2017) to derive equivalent CO values between these monitor brands. Vitalograph CO values were regressed on Bedfont CO values to obtain conversion equation:
Using this conversion equation to adjust Vitalograph CO cutoffs resulted in values < 5 ppm each being increased by 1 ppm and those 6–10 ppm increasing by 2 ppm.
3. RESULTS
3.1. Characteristics of included studies
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Moher et al., 2009) is displayed in Figure 1. The literature search identified 2,279 studies and an additional 14 publications were found through searching reference lists and responses to our listserv request. Following removal of duplicates, titles and abstracts of 2,173 articles were screened and 596 full-texts were assessed for eligibility. Overall, a total of 122 individual studies provided 605 effect sizes.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection.
3.1.1. Study-level characteristics
Study characteristics are presented in Supplementary Table 1. Most studies (116 out of 122) provided data for more than one follow-up period. Duration of follow-up periods (in weeks) ranged from 1 to 64, with a mean (SD) of 20.5 (14.9) (results of sensitivity analyses excluding follow-up periods < 4 weeks (11 effect sizes excluded) were not different from those of analyses including all follow-up periods; all follow-up periods were included in results detailed below). The number of subgroups per study ranged 2 to 6, with a mean (SD) of 2.3 (0.8). Less than half of the studies reported the brand of CO monitor used (k=51); 52 authors responded to our email inquiry with the missing CO monitor information. Most studies used a Bedfont CO monitor (k=73), while others used Vitalograph (k=19), a mix of Bedfont and Vitalograph (k=2), other brands (k=7), or did not know which brand they used (k=3). The number of participants analyzed per study ranged from 20 to 1841 (combined n = 46,949), with a mean (SD) of 384.8 (368.0). Due to participant attrition or exclusion following randomization to treatment groups, two studies analyzed fewer than the n ≥ 50 recruited (sensitivity analyses excluding these two studies provided results consistent with analyses including all studies, therefore these two studies were included in results reported in subsequent sections).
3.2. Preliminary analyses
3.2.1. Overall cessation classification
The overall proportion classified as quit, 27.86% (SE = 1.08), was significantly greater than zero when collapsing across follow up periods and interventions, β = −1.28, 95% CI [−1.43, −1.12], t(121) = −16.22, p < 0.001. Cessation effect sizes varied significantly, Q(121) = 3584.65, p < 0.001, with between-study variance, T2, estimated at 0.70. Almost all observed variance (I2 = 96.62%) reflected differences in study effects.
3.2.2. Publication bias
Kendall’s tau was not significant, τ = −0.12, Zτ = 1.95, p > 0.05, suggesting no publication bias. Duval and Tweedie’s trim and fill method found no missing studies to the left of the mean, further supporting an absence of publication bias.
3.3. Primary meta-regression analyses
3.3.1. Continuous CO cutoff
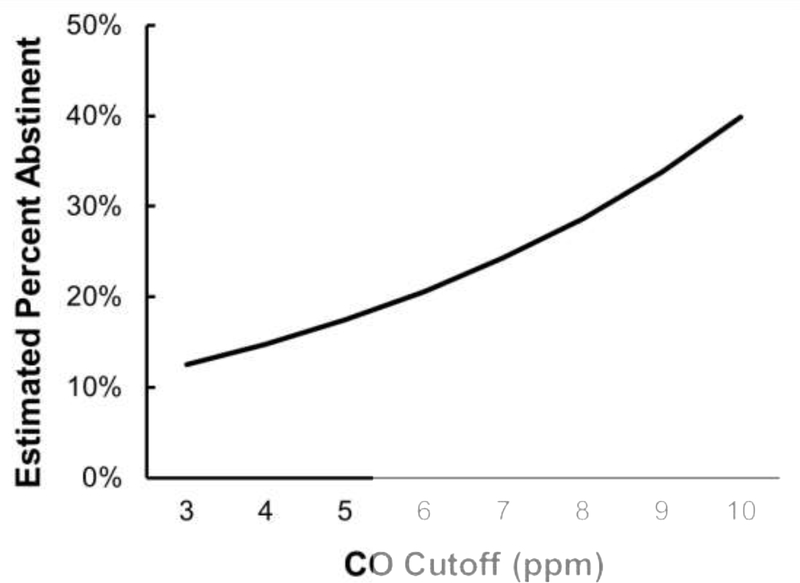
CO cutoff (centered at 3 ppm) was significantly associated with cessation effect sizes, β = 0.17, SE = 0.04, 95% CI [0.10, 0.24], t(120) = 4.66, p < 0.001. As illustrated in Figure 2, for each one unit increase in CO cutoff above 3 ppm, the odds of being classified as abstinent increased by 18%. CO cutoff explained 15% of the variance in effect sizes, R2 = 0.15. There was significant heterogeneity across effect sizes, Q(120) = 3226.76, p < 0.001, with between-study variance, T2, estimated at 0.59 and I2 of 96.28%.
Figure 2.
Meta-regression estimated likelihood of being classified as abstinent by the expired-air carbon monoxide cutoff (CO; in ppm units) used to determine smoking status. Each one ppm increase in CO cutoff resulted in an 18% increase in likelihood of being classified as abstinent.
3.3.2. Categorical CO criteria
Regression-estimated percent abstinent (with 95% CIs) by CO cutoff category are presented in Table 1. Overall, there was significant heterogeneity in effect sizes between CO cutoff categories, F(3, 118) = 5.70, p = 0.001. The odds of being classified as abstinent significantly increased between each cutoff category and High (9–10 ppm): 261% increase from Low (3–4 ppm), p < 0.001; 162% increase from Recommended (5–6 ppm), p = 0.04; and a 150% increase from Moderate (7–8 ppm), p = 0.03.
Table 1.
Meta-regression estimated percent abstinent and 95% confidence intervals by CO cutoff categories.
| CO Cutoff Category | k | Percent Abstinent | 95% Confidence Interval |
|
|---|---|---|---|---|
| LL | UL | |||
| Low (3–4 ppm) | 13 | 13.78 | 8.57 | 22.15 |
| Recommended (5–6 ppm) | 16 | 22.21 | 14.77 | 33.19 |
| Moderate (7–8 ppm) | 29 | 23.91 | 17.67 | 32.36 |
| High (9–10 ppm) | 64 | 35.94 | 29.38 | 43.97 |
Note. Values estimated using meta-regression; CO is expired-air carbon monoxide; k is number of studies; LL is lower limit; UL is upper limit; ppm is parts per million of CO.
Relative to the Low category (3–4 ppm), the odds of being classified as abstinent were not significantly different than Recommended (5–6 ppm), p = 0.13, or Moderate (7–8 ppm) categories, p = 0.05. Similarly, cessation classification odds were not different between the Recommended (5–6 ppm) and Moderate (7–8 ppm) categories, p = 0.77.
3.4. Secondary analyses
Analyses involving CO monitor brands were restricted to studies reporting having exclusively used one of the two most commonly reported brands: Bedfont (k = 73) and Vitalograph (k = 19). There was no difference in effect sizes between CO monitor brands, β = 0.31, 95% CI [−0.82, 1.43], t(88) = 0.54, p = 0.59. Similarly, the interaction of CO cutoff by CO monitor brand was not significant, β = −0.05, 95% CI [−0.26, 0.15], t(88) = −0.52, p = 0.60.
3.5. Exploratory analyses
To equate CO cutoffs between monitor brands, Vitalograph cutoffs ranging 1–5 ppm were increased by 1 ppm and those 6–10 ppm were adjusted upwards by 2 ppm. Only studies having reported using Vitalograph or Bedfont monitors were included in exploratory analyses (k = 92). Using the adjusted CO cutoffs, neither the main effect of CO monitor brand nor the interaction of adjusted cutoffs by monitor brand had a significant association with cessation effect size, ps > 0.44.
4. DISCUSSION
Biochemical verification of smoking status is crucial for the rigorous evaluation of cessation. Complicating such measurement is the variation in cutoffs used to classify participants as abstinent or not. Focusing on one commonly used bioverification method—expired-air CO—we used meta-analysis techniques to examine how the likelihood of being classified as abstinent varied across a range of cutoffs among randomized smoking cessation trials. Overall, we identified a significant amount of heterogeneity in the likelihood of being classified as abstinent across all studies. Importantly, CO cutoff was found to significantly affect cessation classification. As expected, studies using higher CO cutoffs to determine smoking status were more likely to classify participants as abstinent than those using lower cutoffs. The likelihood of being classified as abstinent increased with higher cutoffs, with an 18% rise in classification with each 1 ppm increase above 3 ppm—the lowest cutoff used among included studies.
Studies using cutoffs at the higher end of measurement may incorrectly classify nonabstinent smokers as being abstinent, leading to cessation outcomes that may not be indicative of real world patterns and quit success. On the other hand, cessation levels within studies using lower cutoffs would seem relatively underwhelming compared to those using higher cutoffs, which has implications on further evaluation of those strategies or implementation and adoption into the treatment of tobacco use disorder. The absolute proportion quit reported in a study likely influences future work and clinician adoption, without the consideration of how abstinence was determined.
Comparing between categorical CO cutoffs, we found that studies using the highest cutoffs 9–10 ppm were 261% more likely to classify participants as quit than those using cutoffs 3–4 ppm, consistent with earlier studies that found a similar pattern of cessation classification when comparing between low and high CO cutoffs within their respective samples (Brose et al., 2013; Cropsey et al., 2008). However, we also observed significant differences between the middle cutoff categories (i.e., 5–6 and 7–8 ppm) versus the highest 9–10 ppm category—contrary to Brose et al. (2013). It is important to note that each of these earlier studies examined how adjusting the CO cutoff affected quit proportions within their respective samples, whereas the current project compared between studies. It is unclear whether the current findings would generalize to within-study comparisons. Additional research is needed to confirm our findings by examining within-study differences in cessation classification across a range of CO cutoffs.
The likelihood of being classified as quit did not vary between the two most commonly used CO monitor brands Vitalograph and Bedfont. While contrary to earlier research documenting differences in measurement between these monitor brands (Karelitz et al., 2017), the current null findings could be due to within-brand model-level idiosyncrasies. The Vitalograph BreathCO model has been available since 1999 (Vitalograph USA, 1999), whereas Bedfont has released 13 different models since 2000 (Covita, 2020). A recent study by Tuck et al. (2020) found significant variation in CO measurement between different models of Bedfont CO monitors, supporting the notion that model-level variance among Bedfont monitors may have hindered our ability to detect brand-level effects. Under reporting of CO monitor model information prevented further probing for such an effect in the current project. Future work should examine the impact of model-level variance on CO outcomes and conclusions.
Results should be interpreted in consideration of the study limitations. It is possible that not all potentially eligible studies were identified in our literature search, given the vast amount of smoking cessation research in the literature. While we made efforts to identify additional studies outside of the literature search (i.e., SRNT listserv request), our meta-analysis may have unintentionally missed otherwise eligible studies. Excluding studies that recruited fewer than 50 participants may have prevented inclusion of otherwise eligible studies. This decision was made to prevent publication bias, the risk of which can increase when smaller studies are included in meta-analyses (Nüesch et al., 2010). We did not assess the influence of intervention type on cessation effect size, a variable that could have contributed to unexplained heterogeneity. Stated earlier, our aim was to assess how the CO cutoff used affected cessation effect sizes, not to gauge treatment or intervention effectiveness. Additional research is needed to determine whether CO cutoffs affect cessation outcomes within and between intervention types.
Findings from the current meta-analysis can inform design and reporting of findings from future smoking cessation studies. Overall, the current findings suggest that use of cutoffs ≥ 9 ppm can lead to significantly more participants being classified as quit compared to cutoffs ≤ 8 ppm. Researchers choosing to use CO cutoffs greater than the SRNT-recommended 5–6 ppm (Benowitz et al., 2020) in their cessation studies should also report outcomes when lower cutoffs are applied to allow for cross-study comparisons. Almost 60% of included studies did not report any information on the CO monitor used to confirm abstinence, consistent with an earlier literature search of smoking studies (Karelitz et al., 2017). Given that quit status is typically the primary dependent variable examined in smoking cessation research, it is important to identify how this variable was assessed, including information on the brand and model of the CO monitor. As shown here, the CO cutoff used has a large impact on abstinence classification and absolute rates of abstinence reported in smoking cessation publications. Consistent reporting of this CO monitor information—as earlier recommended (Benowitz et al., 2020; Karelitz et al., 2017)—would allow future research to explore variation in cessation outcomes due to CO monitor brand and model.
In conclusion, greater transparency is needed in the reporting of findings from smoking cessation research. We have shown that using a CO cutoff above the SRNT recommended 5–6 ppm (Benowitz et al., 2020) results in a greater likelihood of classifying participants as abstinent, potentially leading to artificially inflated estimates of abstinence. Therefore, reporting abstinence outcomes using a range of cutoffs (i.e., 6 ppm, 8 ppm, and 10 ppm) would provide transparency in results, allow for cross-study comparisons, and better inform decisions regarding novel treatments or strategies for tobacco use disorder. Additionally, studies relying on expired-air CO to determine smoking status should report the brand and model of the CO monitor used. It is standard practice to provide the names and citations for measures used to quantify dependent variables in smoking cessation research (e.g., withdrawal, craving, self-efficacy, etc.); providing the brand and model of the CO monitor should not be the exception to this rule.
Supplementary Material
Highlights.
Bioverification of smoking abstinence is critical for any study assessing cessation
Likelihood of quit classification increased 18% with each 1 ppm increase over 3 ppm
More transparency is needed in reporting of smoking cessation research
Cessation outcomes need to be reported across several CO cutoff levels
Details of the CO monitor brand and model need to be reported in cessation studies
Acknowledgements
The authors wish to thank Elizabeth Bradley, Elizabeth Chapman, Violet Fratzke, Jenny Nankoua, Benjamin Laprise, and Peter Leahy at the Medical University of South Carolina, who assisted in data coding and extraction. The authors would also like to thank the researchers who responded to our email requesting additional information about the CO monitor used in their studies.
Role of Funding Source
This project was supported by National Institutes of Health grants (T32CA186873 [PI: Yuan]; R37CA237245 [PI: McClure]; K01DA043413 [PI: Pacek]; R01DA046096-01A1 [PI: Cropsey]; R01DA044112-01A1 [PI: Cropsey]; R01DA039678-01A1 [PI: Cropsey]; U01AA020802 [PI: Cropsey]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
NOTE: * indicates that a study was included in the meta-analysis
- Abdullah AS, Hedley AJ, Chan SSC, Lam T-H, 2013. A randomized controlled trial of two different lengths of nicotine replacement therapy for smoking cessation. BioMed Res. Int. 2013, 961751. 10.1155/2013/961751*
- Abrantes AM, Bloom EL, Strong DR, Riebe D, Marcus BH, Desaulniers J, Fokas K, Brown RA, 2014. A preliminary randomized controlled trial of a behavioral exercise intervention for smoking cessation. Nicotine Tob. Res. 16, 1094–1103. 10.1093/ntr/ntu036*
- Andrews JO, Mueller M, Dooley M, Newman SD, Magwood GS, Tingen MS, 2016. Effect of a smoking cessation intervention for women in subsidized neighborhoods: A randomized controlled trial. Prev. Med. 90, 170–176. 10.1016/j.ypmed.2016.07.008*
- Ashare RL, Thompson M, Serrano K, Leone F, Metzger D, Frank I, Gross R, Hole A, Mounzer K, Collman RG, Wileyto EP, Schnoll R, 2019. Placebo-controlled randomized clinical trial testing the efficacy and safety of varenicline for smokers with HIV. Drug Alcohol Depend. 200, 26–33. 10.1016/j.drugalcdep.2019.03.011*
- Aveyard P, Lindson N, Tearne S, Adams R, Ahmed K, Alekna R, Banting M, Healy M, Khan S, Rai G, Wood C, Anderson EC, Ataya-Williams A, Attwood A, Easey K, Fluharty M, Freuler T, Hurse M, Khouja J, Lacey L, Munafò M, Lycett D, McEwen A, Coleman T, Dickinson A, Lewis S, Orton S, Perdue J, Randall C, Anderson R, Bisal N, Hajek P, Homsey C, McRobbie HJ, Myers-Smith K, Phillips A, Przulj D, Li J, Coyle D, Coyle K, Pokhrel S, 2018. Nicotine preloading for smoking cessation: the Preloading RCT. Health Technol. Assess. Winch. Engl. 22, 1–84. 10.3310/hta22410*
- Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, Fiore MC, 2016. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial. JAMA 315, 371–379. 10.1001/jama.2015.19284*
- Begg CB, Mazumdar M, 1994. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50, 1088–1101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, Piper ME, 2020. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res. 22, 1086–1097. 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL Iii, P.J., Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W, 2002. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 4, 149–159. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- Berlin I, Grangé G, Jacob N, Tanguy M-L, 2014. Nicotine patches in pregnant smokers:randomised, placebo controlled, multicentre trial of efficacy. BMJ 348, g1622. 10.1136/bmj.g1622*
- Bloom EL, Ramsey SE, Abrantes AM, Hunt L, Wing RR, Kahler CW, Molino J, Brown RA, 2020. A Pilot Randomized Controlled Trial of Distress Tolerance Treatment for Weight Concern in Smoking Cessation Among Women. Nicotine Tob. Res. ntaa026. 10.1093/ntr/ntaa026*
- Bock BC, Papandonatos GD, de Dios MA, Abrams DB, Azam MM, Fagan M, Sweeney PJ, Stein MD, Niaura R, 2014. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine Tob. Res. 16, 413–422. 10.1093/ntr/ntt166*
- Bohadana A, Freier-Dror Y, Peles V, Babai P, Izbicki G, 2020. Extending varenicline preloading to 6 weeks facilitates smoking cessation: A single-site, randomised controlled trial. EClinicalMedicine 19, 100228. 10.1016/j.eclinm.2019.11.021*
- Bolliger CT, Issa JS, Posadas-Valay R, Safwat T, Abreu P, Correia EA, Park PW, Chopra P, 2011. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 33, 465–477. 10.1016/j.clinthera.2011.04.013*
- Borenstein M, Hedges L, Higgins J, Rothstein H, 2013. Comprehensive Meta-Analysis. Biostat, Englewood, NJ. [Google Scholar]
- Borenstein M, Rothstein H, Cohen J, 2001. Comprehensive Meta-analysis Manual. [Google Scholar]
- Brody AL, Mukhin AG, Mamoun MS, Luu T, Neary M, Liang L, Shieh J, Sugar CA, Rose JE, Mandelkern MA, 2014. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry 71, 797–805. 10.1001/jamapsychiatry.2014.138*
- Brose LS, Tombor I, Shahab L, West R, 2013. The effect of reducing the threshold for carbon monoxide validation of smoking abstinence - Evidence from the English Stop Smoking Services. Addict. Behav. 38, 2529–2531. 10.1016/j.addbeh.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Strong DR, Niaura R, Kahler CW, Miller IW, Price LH, 2014. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine Tob. Res. 16, 197–207. 10.1093/ntr/ntt134*
- Brown RA, Palm Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Zvolensky MJ, Hayes SC, 2018. A randomized controlled trial of distress tolerance treatment for smoking cessation. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 32, 389–400. 10.1037/adb0000372*
- Brown RA, Reed KMP, Bloom EL, Minami H, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV, Hayes SC, 2013. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob. Res. 15, 2005–2015. 10.1093/ntr/ntt093*
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N, 2013.Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet Lond. Engl. 382, 1629–1637. 10.1016/S0140-6736(13)61842-5*
- Caldwell BO, Adamson SJ, Crane J, 2014. Combination rapid-acting nicotine mouth spray and nicotine patch therapy in smoking cessation. Nicotine Tob. Res. 16, 1356–1364. 10.1093/ntr/ntu084*
- Caldwell BO, Crane J, 2016. Combination Nicotine Metered Dose Inhaler and Nicotine Patch for Smoking Cessation: A Randomized Controlled Trial. Nicotine Tob. Res. 18, 1944–1951. 10.1093/ntr/ntw093*
- Caponnetto P, Cibella F, Mancuso S, Campagna D, Arcidiacono G, Polosa R, 2011. Effect of a nicotine-free inhalator as part of a smoking-cessation programme. Eur. Respir. J. 38, 1005–1011. 10.1183/09031936.00109610*
- Chen H-K, Lan T-H, Wu B-J, 2013. A double-blind randomized clinical trial of different doses of transdermal nicotine patch for smoking reduction and cessation in long-term hospitalized schizophrenic patients. Eur. Arch. Psychiatry Clin. Neurosci. 263, 75–82. 10.1007/s00406-012-0338-3*
- Chengappa KNR, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, Levine MD, George TP, 2014. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 75, 765–772. 10.4088/JCP.13m08756*
- Cheung YTD, Wang MP, Li HCW, Kwong A, Lai V, Chan SSC, Lam T-H, 2017. Effectiveness of a small cash incentive on abstinence and use of cessation aids for adult smokers: A randomized controlled trial. Addict. Behav. 66, 17–25. 10.1016/j.addbeh.2016.11.006*
- Cinciripini PM, Robinson JD, Karam-Hage M, Minnix JA, Lam C, Versace F, Brown VL, Engelmann JM, Wetter DW, 2013. Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 70, 522–533. 10.1001/jamapsychiatry.2013.678*
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, Lewis S, Smoking, Nicotine, and Pregnancy (SNAP) Trial Team, 2012. A randomized trial of nicotine-replacement therapy patches in pregnancy. N. Engl. J. Med. 366, 808–818. 10.1056/NEJMoa1109582*
- Cooney JL, Cooper S, Grant C, Sevarino K, Krishnan-Sarin S, Gutierrez IA, Cooney NL, 2017. A Randomized Trial of Contingency Management for Smoking Cessation During Intensive Outpatient Alcohol Treatment. J. Subst. Abuse Treat. 72, 89–96. 10.1016/j.jsat.2016.07.002*
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, Grainge MJ, Taggar J, Essex H, Parrott S, Dickinson A, Whitemore R, Coleman T, Smoking, Nicotine and Pregnancy Trial Team, 2014. The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy--clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol. Assess. Winch. Engl. 18, 1–128. 10.3310/hta18540*
- Covita, 2020. The Smokerlyzer range [WWW Document]. URL https://www.covita.net/the-smokerlyzer-range/#30-year-heritage (accessed 10.16.20).
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, 2006. Expired carbon monoxide levels in self-reported smokers and nonsmokers in prison. Nicotine Tob. Res. 8, 653–659. 10.1080/14622200600789684 [DOI] [PubMed] [Google Scholar]
- Cropsey K, Eldridge G, Weaver M, Villalobos G, Stitzer M, Best A, 2008. Smoking Cessation Intervention for Female Prisoners: Addressing an Urgent Public Health Need. Am. J. Public Health 98, 1894–1901. 10.2105/AJPH.2007.128207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Clark CB, Zhang X, Hendricks PS, Jardin BF, Lahti AC, 2015. Race and Medication Adherence Moderate Cessation Outcomes in Criminal Justice Smokers. Am. J. Prev. Med. 49, 335–344. 10.1016/j.amepre.2015.03.014*
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS, 2014. How Low Should You Go? Determining the Optimal Cutoff for Exhaled Carbon Monoxide to Confirm Smoking Abstinence When Using Cotinine as Reference. Nicotine Tob. Res. 16, 1348–1355. 10.1093/ntr/ntu085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, 2013. Internet-based contingency management to promote smoking cessation: A randomized controlled study: INTERNET-BASED CM FOR SMOKING CESSATION. J. Appl. Behav. Anal. 46, 750–764. 10.1002/jaba.89*
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ, 2017. Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial: Nationwide CM for smoking cessation. Addiction 112, 875–883. 10.1111/add.13715*
- Dennis PA, Kimbrel NA, Dedert EA, Beckham JC, Dennis MF, Calhoun PS, 2016. Supplemental nicotine preloading for smoking cessation in posttraumatic stress disorder: Results from a randomized controlled trial. Addict. Behav. 59, 24–29. 10.1016/j.addbeh.2016.03.004*
- Deveci SE, Deveci F, Açik Y, Ozan AT, 2004. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir. Med. 98, 551–556. 10.1016/j.rmed.2003.11.018 [DOI] [PubMed] [Google Scholar]
- Dezee KJ, Wink JS, Cowan CM, 2013. Internet versus in-person counseling for patients taking varenicline for smoking cessation. Mil. Med. 178, 401–405. 10.7205/MILMED-D-12-00272*
- Dogar O, Zahid R, Mansoor S, Kanaan M, Ahluwalia JS, Jawad M, Siddiqi K, 2018. Varenicline versus placebo for waterpipe smoking cessation: a double-blind randomized controlled trial. Addict. Abingdon Engl. 113, 2290–2299. 10.1111/add.14430*
- Duval S, 2005. The trim and fill method, in: Rothstein H, Sutton AJ, Borenstein M (Eds.), Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. John Wiley & Sons, Oxford, pp. 127–144. [Google Scholar]
- Duval S, Tweedie R, 2000. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 56, 455–463. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Hatsukami DK, Croghan IT, Schroeder DR, Allen SS, Hays JT, Hurt RD, 2014. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA 311, 155–163. 10.1001/jama.2013.283185*
- Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, Treadow J, Yu C-R, Dutro MP, Park PW, 2015. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA 313, 687–694. 10.1001/jama.2015.280*
- Ebbert JO, Severson HH, Danaher BG, Benowitz NL, Schroeder DR, 2016. Nicotine Metabolite Ratio Is Associated With Lozenge Use But Not Quitting in Smokeless Tobacco Users. Nicotine Tob. Res. 18, 366–370. 10.1093/ntr/ntv102*
- Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, Sarrafzadegan N, Sharma S, Lauzon C, Yadav R, Pilote L, ZESCA Investigators, 2013. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 61, 524–532. 10.1016/j.jacc.2012.08.1030*
- Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, Cassavar D, Dion D, Haught H, Mehta SR, Baril J-F, Lambert C, Madan M, Abramson BL, Dehghani P, EVITA Investigators, 2016. Varenicline for Smoking Cessation in Hospitalized Patients With Acute Coronary Syndrome. Circulation 133, 21–30. 10.1161/CIRCULATIONAHA.115.019634*
- Ellerbeck EF, Nollen N, Hutcheson TD, Phadnis M, Fitzgerald SA, Vacek J, Sharpe MR, Salzman GA, Richter KP, 2018. Effect of Long-term Nicotine Replacement Therapy vs Standard Smoking Cessation for Smokers With Chronic Lung Disease: A Randomized Clinical Trial. JAMA Netw. Open 1, e181843. 10.1001/jamanetworkopen.2018.1843*
- El-Mohandes AAE, Windsor R, Tan S, Perry DC, Gantz MG, Kiely M, 2013. A randomized clinical trial of trans-dermal nicotine replacement in pregnant African-American smokers. Matern. Child Health J. 17, 897–906. 10.1007/s10995-012-1069-9*
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, Achtyes ED, Ayer D, Schoenfeld DA, 2014. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA 311, 145–154. 10.1001/jama.2013.285113*
- Fagerstrom K, Rutqvist LE, Hughes JR, 2012. Snus as a smoking cessation aid: a randomized placebo-controlled trial. Nicotine Tob. Res. 14, 306–312. 10.1093/ntr/ntr214*
- Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, Boyd KA, McEwen A, Coleman T, 2012. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ 344, e1696. 10.1136/bmj.e1696*
- Foa EB, Asnaani A, Rosenfield D, Zandberg LJ, Gariti P, Imms P, 2017. Concurrent varenicline and prolonged exposure for patients with nicotine dependence and PTSD: A randomized controlled trial. J. Consult. Clin. Psychol. 85, 862–872. 10.1037/ccp0000213*
- Foulds J, Veldheer S, Hrabovsky S, Yingst J, Sciamanna C, Chen G, Maccani JZJ, Berg A, 2015. The effect of motivational lung age feedback on short-term quit rates in smokers seeking intensive group treatment: A randomized controlled pilot study. Drug Alcohol Depend. 153, 271–277. 10.1016/j.drugalcdep.2015.05.007*
- Fouz-Rosón N, Montemayor-Rubio T, Almadana-Pacheco V, Montserrat-García S, Gómez-Bastero AP, Romero-Muñoz C, Polo-Padillo J, 2017. Effect of 0.5 mg versus 1 mg varenicline for smoking cessation: a randomized controlled trial. Addiction 112, 1610–1619. 10.1111/add.13855*
- Garcia-Portilla MP, Garcia-Alvarez L, Sarramea F, Galvan G, Diaz-Mesa E, Bobes-Bascaran T, Al-Halabi S, Elizagarate E, Iglesias C, Saiz Martínez PA, Bobes J, 2016. It is feasible and effective to help patients with severe mental disorders to quit smoking: An ecological pragmatic clinical trial with transdermal nicotine patches and varenicline. Schizophr. Res. 176, 272–280. 10.1016/j.schres.2016.05.011*
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM, 2011. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behav. Ther. 42, 700–715. 10.1016/j.beth.2011.03.002*
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng L-J, McRae TD, Treadow J, 2014. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: a randomized, placebo-controlled trial. Clin. Pharmacol. Ther. 96, 390–396. 10.1038/clpt.2014.124*
- Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M, 2009. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res. 11, 12–24. 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
- Gray KM, McClure EA, Baker NL, Hartwell KJ, Carpenter MJ, Saladin ME, 2015. An exploratory short-term double-blind randomized trial of varenicline versus nicotine patch for smoking cessation in women. Addiction 110, 1027–1034. 10.1111/add.12895*
- Hajek P, McRobbie H, Myers Smith K, Phillips A, Cornwall D, Dhanji A-R, 2015. Increasing varenicline dose in smokers who do not respond to the standard dosage: a randomized clinical trial. JAMA Intern. Med. 175, 266–271. 10.1001/jamainternmed.2014.6916*
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji A-R, 2011. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch. Intern. Med. 171, 770–777. 10.1001/archinternmed.2011.138*
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ, 2019. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 380, 629–637. 10.1056/NEJMoa1808779*
- Hajek P, Smith KM, Dhanji A-R, McRobbie H, 2013. Is a Combination of Varenicline and Nicotine Patch More Effective in Helping Smokers Quit Than Varenicline Alone? A Randomised Controlled Trial. BMC Med. 11, 140. 10.1186/1741-7015-11-140*
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, Carmella SG, al’Absi M, Joseph AM, Allen SS, 2013. Reduced nicotine content cigarettes and nicotine patch . Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 22, 1015–1024. 10.1158/1055-9965.EPI-12-1439*
- Higgins JPT, Thompson SG, 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Hoogsteder PHJ, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP, 2014. Efficacy of the nicotine vaccine 3’-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction 109, 1252–1259. 10.1111/add.12573*
- Javors MA, Hatch JP, Lamb RJ, 2005. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction 100, 159–167. 10.1111/j.1360-0443.2004.00957.x [DOI] [PubMed] [Google Scholar]
- Jennings C, Kotseva K, De Bacquer D, Hoes A, de Velasco J, Brusaferro S, Mead A, Jones J, Tonstad S, Wood D, EUROACTION PLUS Study Group, 2014. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. Eur. Heart J. 35, 1411–1420. 10.1093/eurheartj/ehu051*
- Kahler CW, Cioe PA, Tzilos GK, Spillane NS, Leggio L, Ramsey SE, Brown RA, O’Malley SS, 2017. A Double-Blind Randomized Placebo-Controlled Trial of Oral Naltrexone for Heavy-Drinking Smokers Seeking Smoking Cessation Treatment. Alcohol. Clin. Exp. Res. 41, 1201–1211. 10.1111/acer.13396*
- Kalman D, Herz L, Monti P, Kahler CW, Mooney M, Rodrigues S, O’Connor K, 2011. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double-blind, placebo-controlled study. Drug Alcohol Depend. 118, 111–118. 10.1016/j.drugalcdep.2011.03.005*
- Karelitz JL, Michael VC, Perkins KA, 2017. Analysis of Agreement between Expired-Air Carbon Monoxide Monitors. J. Smok. Cessat. 12, 105–112. 10.1017/jsc.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, Kaprio J, Lerman C, Park PW, 2012. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 37, 641–650. 10.1038/npp.2011.232*
- Koegelenberg CFN, Noor F, Bateman ED, van Zyl-Smit RN, Bruning A, O’Brien JA, Smith C, Abdool-Gaffar MS, Emanuel S, Esterhuizen TM, Irusen EM, 2014. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 312, 155–161. 10.1001/jama.2014.7195*
- Laude JR, Bailey SR, Crew E, Varady A, Lembke A, McFall D, Jeon A, Killen D, Killen JD, David SP, 2017. Extended treatment for cigarette smoking cessation: a randomized control trial. Addiction 112, 1451–1459. 10.1111/add.13806*
- Lerman C, Schnoll RA, Hawk LW, Cinciripini P, George TP, Wileyto EP, Swan GE, Benowitz NL, Heitjan DF, Tyndale RF, PGRN-PNAT Research Group, 2015. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 3, 131–138. 10.1016/S2213-2600(14)70294-2*
- Leung MKW, Bai D, Yip BHK, Fong MY, Lai PMH, Lai P, Lai ISY, Lam ZHW, Leung ATF, To DKY, Wong MT, Wong TK, Chao DVK, 2019. Combined nicotine patch with gum versus nicotine patch alone in smoking cessation in Hong Kong primary care clinics: a randomised controlled trial. BMC Public Health 19, 1302. 10.1186/s12889-019-7634-z*
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, Marcus MD, 2010. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch. Intern. Med. 170, 543–550. 10.1001/archinternmed.2010.33*
- Lindson-Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P, 2016. Gradual Versus Abrupt Smoking Cessation: A Randomized, Controlled Noninferiority Trial. Ann. Intern. Med. 164, 585–592. 10.7326/M14-2805*
- Lipsey MW, Wilson DB, 2001. Practical meta-analysis, Applied social research methods series. Sage Publications, Thousand Oaks, Calif. [Google Scholar]
- Littlewood RA, Claus ED, Wilcox CE, Mickey J, Arenella PB, Bryan AD, Hutchison KE, 2017. Moderators of smoking cessation outcomes in a randomized-controlled trial of varenicline versus placebo. Psychopharmacology (Berl.) 234, 3417–3429. 10.1007/s00213-017-4721-7*
- Loughead J, Falcone M, Wileyto EP, Albelda B, Audrain-McGovern J, Cao W, Kurtz MM, Gur RC, Lerman C, 2016. Can brain games help smokers quit?: Results of a randomized clinical trial. Drug Alcohol Depend. 168, 112–118. 10.1016/j.drugalcdep.2016.08.621*
- Low ECT, Ong MCC, Tan M, 2004. Breath carbon monoxide as an indication of smoking habit in the military setting. Singapore Med. J. 45, 578–582. [PubMed] [Google Scholar]
- MacLaren DJ, Conigrave KM, Robertson JA, Ivers RG, Eades S, Clough AR, 2010. Using breath carbon monoxide to validate self-reported tobacco smoking in remote Australian Indigenous communities. Popul. Health Metr. 8, 2. 10.1186/1478-7954-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Aveyard P, Munafò MR, Prevost AT, Hollands GJ, Armstrong D, Sutton S, Hill C, Johnstone E, Kinmonth AL, 2012. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PloS One 7, e35249. 10.1371/journal.pone.0035249*
- Mercié P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, Duvivier C, Reynes J, Wirth N, Moinot L, Bénard A, Zucman D, Duval X, Molina J-M, Spire B, Fagard C, Chêne G, ANRS 144 Inter-ACTIV study group, 2018. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV 5, e126–e135. 10.1016/S2352-3018(18)30002-X*
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP, 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 6, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Schmitz JM, Allen S, Grabowski J, Pentel P, Oliver A, Hatsukami DK, 2016. Bupropion and naltrexone for smoking cessation: A double-blind randomized placebo-controlled clinical trial. Clin. Pharmacol. Ther. 100, 344–352. 10.1002/cpt.402*
- Murphy CM, MacKillop J, Martin RA, Tidey JW, Colby SM, Rohsenow DJ, 2017. Effects of varenicline versus transdermal nicotine replacement therapy on cigarette demand on quit day in individuals with substance use disorders. Psychopharmacology (Berl.) 234, 2443–2452. 10.1007/s00213-017-4635-4*
- Murray RL, Leonardi-Bee J, Marsh J, Jayes L, Li J, Parrott S, Britton J, 2013. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ 347, f4004. 10.1136/bmj.f4004*
- Nahvi S, Ning Y, Segal KS, Richter KP, Arnsten JH, 2014. Varenicline efficacy and safety among methadone maintained smokers: a randomized placebo-controlled trial. Addiction 109, 1554–1563. 10.1111/add.12631*
- Nanovskaya TN, Oncken C, Fokina VM, Feinn RS, Clark SM, West H, Jain SK, Ahmed MS, Hankins GDV, 2017. Bupropion sustained release for pregnant smokers: a randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 216, 420.e1–420.e9. 10.1016/j.ajog.2016.11.1036*
- Nelson PR, Chen P, Battista DR, Pillitteri JL, Shiffman S, 2019. Randomized Trial to Compare Smoking Cessation Rates of Snus, With and Without Smokeless Tobacco Health-Related Information, and a Nicotine Lozenge. Nicotine Tob. Res. 21, 88–94. 10.1093/ntr/nty011*
- Nikolakopoulou A, Mavridis D, Salanti G, 2014. Demystifying fixed and random effects meta-analysis. Evid. Based Ment. Health 17, 53–57. 10.1136/eb-2014-101795 [DOI] [PubMed] [Google Scholar]
- Nollen NL, Cox LS, Nazir N, Ellerbeck EF, Owen A, Pankey S, Thompson N, Ahluwalia JS, 2011. A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine Tob. Res. 13, 868–873. 10.1093/ntr/ntr063*
- Nüesch E, Trelle S, Reichenbach S, Rutjes AWS, Tschannen B, Altman DG, Egger M, Jüni P, 2010. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 341, c3515. 10.1136/bmj.c3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Goldade K, Whembolua G-L, Thomas JL, Eischen S, Sewali B, Guo H, Connett JE, Grant J, Ahluwalia JS, Resnicow K, Owen G, Gelberg L, Des Jarlais D, 2013. Motivational interviewing to enhance nicotine patch treatment for smoking cessation among homeless smokers: a randomized controlled trial. Addiction 108, 1136–1144. 10.1111/add.12140*
- Oncken C, Arias AJ, Feinn R, Litt M, Covault J, Sofuoglu M, Kranzler HR, 2014. Topiramate for smoking cessation: a randomized, placebo-controlled pilot study. Nicotine Tob. Res. 16, 288–296. 10.1093/ntr/ntt141*
- Oncken C, Dornelas EA, Kuo C-L, Sankey HZ, Kranzler HR, Mead EL, Thurlow SD, 2019. Randomized trial of nicotine inhaler for pregnant smokers. Am. J. Obstet. Gynecol. MFM 1, 10–18. 10.1016/j.ajogmf.2019.03.006*
- Perkins KA, Karelitz JL, Jao NC, 2013. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine Tob. Res. 15, 978–982. 10.1093/ntr/nts205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, Mermelstein R, Fraser D, Fiore MC, Baker TB, 2018. A Randomized Controlled Trial of an Optimized Smoking Treatment Delivered in Primary Care. Ann. Behav. Med. Publ. Soc. Behav. Med. 52, 854–864. 10.1093/abm/kax059*
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh W-Y, Bolt DM, Kim S-Y, Kaye JT, Hefner KR, Baker TB, 2011. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl.) 216, 569–578. 10.1007/s00213-011-2250-3*
- Politis A, Ioannidis V, Gourgoulianis KI, Daniil Z, Hatzoglou C, 2018. Effects of varenicline therapy in combination with advanced behavioral support on smoking cessation and quality of life in inpatients with acute exacerbation of COPD, bronchial asthma, or community-acquired pneumonia: A prospective, open-label, preference-based, 52-week, follow-up trial. Chron. Respir. Dis. 15, 146–156. 10.1177/1479972317740128*
- Pozzi P, Munarini E, Bravi F, Rossi M, La Vecchia C, Boffi R, Pastorino U, 2015. A combined smoking cessation intervention within a lung cancer screening trial: a pilot observational study. Tumori 101, 306–311. 10.5301/tj.5000282*
- Prapavessis H, De Jesus S, Fitzgeorge L, Faulkner G, Maddison R, Batten S, 2016. Exercise to Enhance Smoking Cessation: the Getting Physical on Cigarette Randomized Control Trial. Ann. Behav. Med. Publ. Soc. Behav. Med. 50, 358–369. 10.1007/s12160-015-9761-9*
- Qualtrics, 2020.. Qualtrics, Provo, UT. [Google Scholar]
- Rajaee S, Holder T, Indes JE, Muhs B, Sarac T, Sumpio B, Toll BA, Ochoa Chaar CI, 2019. A Pilot Study of a Standardized Smoking Cessation Intervention for Patients with Vascular Disease. Ann. Vasc. Surg. 61, 91–99.e3. 10.1016/j.avsg.2019.06.017*
- Ramon JM, Morchon S, Baena A, Masuet-Aumatell C, 2014. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med. 12, 172. 10.1186/s12916-014-0172-8*
- Ramon JM, Nerin I, Comino A, Pinet C, Abella F, Carreras JM, Banque M, Baena A, Morchon S, Jimenez-Muro A, Marqueta A, Vilarasau A, Bullon R, Masuet-Aumatell C, 2013. A multicentre randomized trial of combined individual and telephone counselling for smoking cessation. Prev. Med. 57, 183–188. 10.1016/j.ypmed.2013.05.014*
- Rash CJ, Petry NM, Alessi SM, 2018. A randomized trial of contingency management for smoking cessation in the homeless. Psychol. Addict. Behav. 32, 141–148. 10.1037/adb0000350*
- Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, St Aubin LB, Russ C, Flexible Quit Date Study Group, 2012. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob. Res. 14, 343–350. 10.1093/ntr/ntr220*
- Richmond R, Indig D, Butler T, Wilhelm K, Archer V, Wodak A, 2013. A randomized controlled trial of a smoking cessation intervention conducted among prisoners. Addiction 108, 966–974. 10.1111/add.12084*
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S, 2010. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 121, 221–229. 10.1161/CIRCULATIONAHA.109.869008*
- Rohsenow DJ, Martin RA, Tidey JW, Colby SM, Monti PM, 2017a. Treating Smokers in Substance Treatment With Contingent Vouchers, Nicotine Replacement and Brief Advice Adapted for Sobriety Settings. J. Subst. Abuse Treat. 72, 72–79. 10.1016/j.jsat.2016.08.012*
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Sirota AD, Swift RM, Monti PM, 2015. Contingent Vouchers and Motivational Interviewing for Cigarette Smokers in Residential Substance Abuse Treatment. J. Subst. Abuse Treat. 55, 29–38. 10.1016/j.jsat.2015.02.010*
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, Monti PM, 2017b. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms: Varenicline for smoking in SUD. Addiction 112, 1808–1820. 10.1111/add.13861*
- Rose JE, Behm FM, 2014. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. Am. J. Psychiatry 171, 1199–1205. 10.1176/appi.ajp.2014.13050595*
- Rose JE, Behm FM, 2013. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am. J. Psychiatry 170, 860–867. 10.1176/appi.ajp.2013.12070919*
- Rungruanghiranya S, Ekpanyaskul C, Sakulisariyaporn C, Watcharanat P, Akkalakulawas K, 2012. Efficacy of fresh lime for smoking cessation. J. Med. Assoc. Thail. Chotmaihet Thangphaet 95 Suppl 12, S76–82.*
- Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao NC, Paul Wileyto E, Carroll AJ, Kalhan R, Patel J, Langer C, Lubitz SF, Hitsman B, 2019. A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: Efficacy, safety, and adherence. Psychooncology. 28, 561–569. 10.1002/pon.4978*
- Schnoll RA, Cappella J, Lerman C, Pinto A, Patterson F, Wileyto EP, Bigman C, Leone F, 2011. A novel recruitment message to increase enrollment into a smoking cessation treatment program: preliminary results from a randomized trial. Health Commun. 26, 735–742. 10.1080/10410236.2011.566829*
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Wileyto EP, Hitsman B, 2015. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern. Med. 175, 504–511. 10.1001/jamainternmed.2014.8313*
- Schnoll RA, Martinez E, Tatum KL, Weber DM, Kuzla N, Glass M, Ridge JA, Langer C, Miyamoto C, Wileyto EP, Leone F, 2010. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes Control 21, 811–820. 10.1007/s10552-010-9507-8*
- Schuster RM, Pachas GN, Stoeckel L, Cather C, Nadal M, Mischoulon D, Schoenfeld DA, Zhang H, Ulysse C, Dodds EB, Sobolewski S, Hudziak V, Hanly A, Fava M, Evins AE, 2018. Phase IIb Trial of an α7 Nicotinic Receptor Partial Agonist With and Without Nicotine Patch for Withdrawal-Associated Cognitive Deficits and Tobacco Abstinence. J. Clin. Psychopharmacol. 38, 307–316. 10.1097/JCP.0000000000000919*
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, Thomson H, 2013. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann. Intern. Med. 158, 667–675. 10.7326/0003-4819-158-9-201305070-00006*
- Smits JAJ, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Powers MB, Frierson GM, Otto MW, Hopkins LB, Brown RA, Baird SO, 2016. The Efficacy of Vigorous-Intensity Exercise as an Aid to Smoking Cessation in Adults With High Anxiety Sensitivity: A Randomized Controlled Trial. Psychosom. Med. 78, 354–364. 10.1097/PSY.0000000000000264*
- Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd-Richardson EE, de Dios MA, Morrow KM, Makgoeng SB, Tashima KT, Niaura RS, 2015. Outcomes of a Tailored Intervention for Cigarette Smoking Cessation Among Latinos Living With HIV/AIDS. Nicotine Tob. Res. 17, 975–982. 10.1093/ntr/ntv014*
- Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, O’Gara C, McRobbie H, Humphrey K, Ali R, Strang J, Sutherland G, 2013. Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 108, 2193–2201. 10.1111/add.12304*
- Stein MD, Caviness CM, Kurth ME, Audet D, Olson J, Anderson BJ, 2013. Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial. Drug Alcohol Depend. 133, 486–493. 10.1016/j.drugalcdep.2013.07.005*
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL, 2011. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addict. Behav. 36, 1127–1132. 10.1016/j.addbeh.2011.07.002*
- Sun H, Guo S, Chen D, Yang F, Zou Y, Di X, Cao Y, Kosten T, Lu L, Zhang XY, 2012. Association of functional COMT Val108/Met polymorphism with smoking cessation in a nicotine replacement therapy. J. Neural Transm. 119, 1491–1498. 10.1007/s00702-012-0841-8*
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC, 2011. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 139, 591–599. 10.1378/chest.10-0865*
- Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A, 2012. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur. Respir. J. 40, 548–554. 10.1183/09031936.00155811*
- Tseng T-Y, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, Urbina A, Cleland CM, Shelley D, 2017. Combining Text Messaging and Telephone Counseling to Increase Varenicline Adherence and Smoking Abstinence Among Cigarette Smokers Living with HIV: A Randomized Controlled Study. AIDS Behav. 21, 1964–1974. 10.1007/s10461-016-1538-z*
- Tuck BM, Karelitz JL, Tomko RL, Dahne J, Cato P, McClure EA, 2020. Mobile, remote, and individual-focused: Comparing breath carbon monoxide readings and abstinence between smartphone-enabled and stand-alone monitors. Nicotine Tob. Res. 10.1093/ntr/ntaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD, 2016. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 14, 80. 10.1186/s12916-016-0626-2*
- van Rossem C, Spigt M, Viechtbauer W, Lucas AEM, van Schayck OCP, Kotz D, 2017. Effectiveness of intensive practice nurse counselling versus brief general practitioner advice, both combined with varenicline, for smoking cessation: a randomized pragmatic trial in primary care. Addiction 112, 2237–2247. 10.1111/add.13927*
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, Waters AJ, Li Y, Nguyen NTT, Cao Y, Tindle HA, Fine M, Safranek LV, Wetter DW, 2016. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. J. Consult. Clin. Psychol. 84, 824–838. 10.1037/ccp0000117*
- Vilardaga R, Rizo J, Palenski P, Mannelli P, Oliver JA, McClernon FJ, 2019. Pilot Randomized Controlled Trial of a Novel Smoking Cessation App Designed for Individuals with Co-Occurring Tobacco Dependence and Serious Mental Illness. Nicotine Tob. Res. ntz202. 10.1093/ntr/ntz202*
- Vitalograph USA, 1999. BreathCO [WWW Document]. URL https://web.archive.org/web/19990911120539/http://www.vitalograph.com/BreathCO.htm (accessed 10.16.20).
- Ward KD, Asfar T, Al Ali R, Rastam S, Weg MWV, Eissenberg T, Maziak W, 2013. Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction 108, 394–403. 10.1111/j.1360-0443.2012.04048.x*
- Webb Hooper M, Antoni MH, Okuyemi K, Dietz NA, Resnicow K, 2017. Randomized Controlled Trial of Group-Based Culturally Specific Cognitive Behavioral Therapy Among African American Smokers. Nicotine Tob. Res. 19, 333–341. 10.1093/ntr/ntw181*
- West R, May S, McEwen A, McRobbie H, Hajek P, Vangeli E, 2010. A randomised trial of glucose tablets to aid smoking cessation. Psychopharmacology (Berl.) 207, 631–635. 10.1007/s00213-009-1692-3*
- Westergaard CG, Porsbjerg C, Backer V, 2015. The effect of Varenicline on smoking cessation in a group of young asthma patients. Respir. Med. 109, 1416–1422. 10.1016/j.rmed.2015.07.017*
- Williams JH, Jones TE, 2012. Smoking cessation post-discharge following nicotine replacement therapy use during an inpatient admission. Intern. Med. J. 42, 154–159. 10.1111/j.1445-5994.2011.02442.x*
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, George TP, 2012. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J. Clin. Psychiatry 73, 654–660. 10.4088/JCP.11m07522*
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.