Abstract
In animal models of cancer, oncologic imaging has evolved from a simple assessment of tumor location and size to sophisticated multi-modality exploration of molecular, physiological, genetic, immunological and biochemical events at microscopic to macroscopic levels, performed non-invasively and sometimes in real time. We briefly review animal imaging technology and molecular imaging probes together with selected applications from recent literature. Fast and sensitive optical imaging is primarily used to track luciferase-expressing tumor cells, image molecular targets with fluorescent probes, and report on metabolic and physiological phenotypes using smart switchable luminescent probes. MicroPET/ SPECT have proven to be two of the most translational modalities for molecular and metabolic imaging of cancers: Immuno-PET is a promising and rapidly evolving area of imaging research. Sophisticated MRI techniques provide high-resolution images of small metastases, tumor inflammation, perfusion, oxygenation and acidity. Disseminated tumors to the bone and lung are easily detected by microCT, while ultrasound provides real-time visualization of tumor vasculature and perfusion. Recently available photoacoustic imaging provides real time evaluation of vascular patency, oxygenation, and nanoparticle distributions. New hybrid instruments such as PET-MRI promise more convenient combination of the capabilities of each modality, enabling enhanced research efficacy and throughput.
Recent technological developments in scanner design and advances in image reconstruction have secured the rapid application of noninvasive imaging for detection, characterization and monitoring of cancer etiology in a variety of animal models (1–3). Obvious advantages arise from the ability to study structure, metabolism and function of cancer cells and cancer supporting microenvironment longitudinally, without the need for necropsy. Indeed, imaging is non-invasive and repetitive studies are performed in the same animals, with each animal serving as its own control. Importantly, most imaging platforms can efficiently survey whole animals, opening new horizons for studying metastatic disease. Furthermore, many imaging technologies are intrinsically translational by applying identical imaging protocols, imaging tracers and image analysis to various species, thereby providing a bridge from laboratory animals to companion animals and ultimately to humans with the goal of easing the burden of human cancer (4–6). There are various imaging platforms, also referred to as imaging modalities, each based on a specific physical principle (Table 1A), allowing unique information/data to be generated. The primary reason for applying a multi-platform imaging approach to cancer research is to obtain comprehensive information from a cancer-bearing animal (Table 1B). The in-vivo cancer imaging modalities are highly complementary, providing a variety of quantitative biomarkers for cancer cell tracking, and assessing tumor dimensions, pathophysiology, metabolism and molecular composition (Table 1B, Figure 1), but each has specific advantages and weaknesses (6–8). In this review, we highlight the state-of-the-art applications of pre-clinical multi-modal multi-scale imaging and focus on the specific applicability to cancer research.
Table 1:
(A) physical principles of the main pre-clinical imaging modalities and their basic characteristics; (B) the ultimate guide for choosing a specific imaging platform in a cancer research study design.
| Table 1A Modality | Physical Principles | Whole Body/ Target Organ | Resolution Scale |
|---|---|---|---|
| MRI/ MRS | External magnetic field; nuclear spin; radio wave pulses (for magnetization of hydrogens in tissue water/ metabolites) | 4 – 6 cm FOV: brain, heart, liver, pancreas, muscle | 35 – 150 microns |
| microCT | 3-Dimensional X-ray beam absorption and scattering | Whole body/ lung, bone | 10 – 50 microns |
| Ultrasound (US) | Reflection of high-frequency sound waves | 2–4 cm FOV: heart, pelvic, liver, pancreas, OBGYN | 60 – 120 microns |
| Photoacoustic (PAI, MSOT) | Spectrally selective near infra-red light excitation of chromophores inducing sound waves providing tomographic images; notably oxy-deoxyhemoglobin, exogenous 800CW tagged agents and gold nanoparticles | Tomographic slices of whole mouse or larger animal to 4 cm depth; breast, thyroid | 150 microns; 100 ms |
| Optical: BLI and FLI | Light emitting chemical reaction, often enzyme facilitated, e.g., luciferin/luciferase; photo stimulated fluorescent chromophores | Whole body | mm- depth dependent |
| PET/SPECT | Decay of short-lived radioactive beta+ and photon emitters | Whole body | 1.0 – 1.8 mm |
| Table 1B: Tumor Etiology | Appropriate Imaging Modality to Assess Tumor Characteristics | Quantitative Imaging Biomarkers |
|---|---|---|
| Dimensions | CT, T1/T2-MRI, Ultrasound | Tumor volume, mm3 Tumor diameter, mm |
| Cellularity Proliferation | Diffusion-weighted MRI 18FLT-PET | Apparent diffusion coefficient (ADC) Standard uptake values (SUV) |
| Metastases | CT, MRI ⇨ BLI, PET | Number of lesions ⇨ qualitative |
| Vascularity/Oxygenation/ Hypoxia | MRA, DCE, CE-CT, PAI sO2-MSOT oxygen-enhanced MRI (BOLD/TOLD), 18F-MISO, 18F-FAZA PET | Exchange rate constants Ktrans, Ve ΔR2* maps, ΔR1, AUC, tBV HbO2; SO2MSOT; SUV |
| Metabolism/Tumor pH | PET, FLI 1H-MRSI, hyperpolarized 13C-MRSI, 31P-MRS, 19F-MRS pH: 31P-MRS, CEST-MRI | SUVs, Signal intensities (SIs) Metabolite concentrations, metabolite ratios, metabolite maps Intra-extracellular pH values and pH maps |
| Inflammation Redox Imaging | immunoPET, Iron Oxide NP T2-MRI, PFC 19F-MRI, EPR | SUVs, ΔT2 relaxation times SIs |
| Cellular Tracking | BLI, 19F-MRI, Iron Oxide T2-MRI, PET | SIs, SUVs |
| Molecular Targets | SPECT, PET, BLI and FLI imaging | SUVs, SIs ⇨ qualitative |
Figure 1:
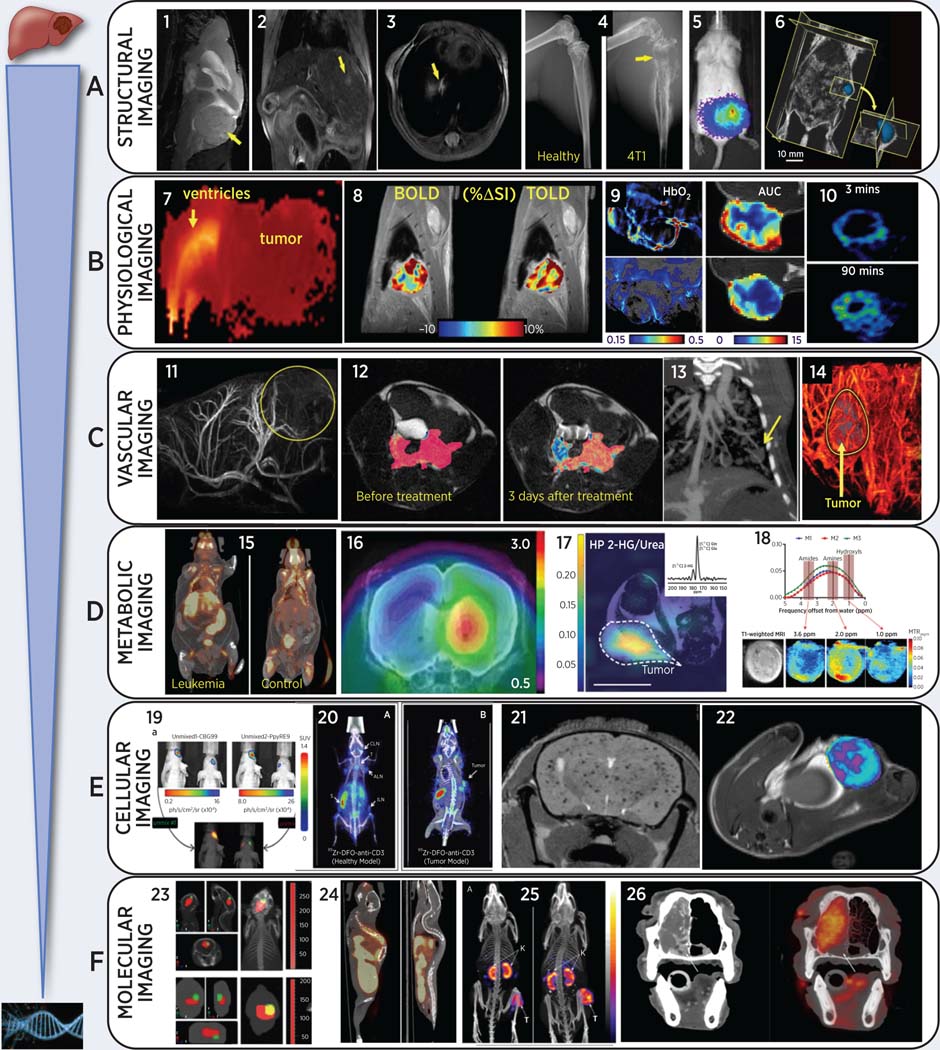
Representative multi-modality images of animal cancer models (from left to right):
(A) Anatomical cancer detection in mouse models: (1) T2-weighted MRI of pediatric cerebellar brain tumor (medulloblastoma) pdx; (2) gadolinium-enhanced T1-MRI of an orthotopic liver HCC; (3) proton-density MRI of a lung metastasis from breast cancer; (4) microCT of bone metastasis from engrafted breast cancer cells, adapted from (62); (5) BLI of multi-organ breast cancer metastases, adapted from (62); (6) 3D-ultrasound of pancreatic cancer in a genetically modified LSL-Kras mouse, adapted from (80).
(B) Physiology-based images in rodent cancer models: (7) high ADC (brain edema and ventricle hydrocephalus) and low ADC (highly proliferative medulloblastoma mouse pdx) from diffusion-weighted MRI; (8) blood and tissue oxygen level dependent (BOLD and TOLD) MRI in response to O2 gas breathing challenge in orthotopic human A549 lung tumor xenograft in nude rat, adapted from (23); (9) left: photoacoustic imaging of subcutaneous A549 human lung tumor growing in leg of nude rat showing endogenous HbO2 concentration before (upper) and 48 hrs after (lower) administration of vascular disrupting agent (VDA), based on multiple wavelengths (MSOT), while breathing O2 and right: corresponding DCE MRI showing area under the curve reflecting reduced perfusion after VDA (10) 18F-MISO (hypoxia tracer) PET in a syngeneic Dunning R3327-AT1 rat prostate tumor, adapted from (143)
(C) Imaging tumor vasculature in-vivo: (11) high-resolution magnetic resonance angiography (MRA) after gadolinium injection in an orthotopic rat isograft C6 glioma model; (12) DCE-MRI during gadolinium injection in mouse TRAMP model for prostate cancer, adapted from (33); (13) contrast-enhanced microCT of lung vasculature and small lung tumor using liposomal-iodinated contrast agent, adapted from (72); (14) US enhanced with microbubbles reveals high perfusion in the rim of a flank pancreatic cancer xenograft in a mouse.
(D) Imaging tumor metabolism non-invasively: (15) abnormal 18F-FDG uptake in spleen, liver, and lymph nodes in transgenic leukemic (left) vs. control mouse, adapted from (129); (16) increased GBM uptake of 18F-ethyltyrosine (18F-FET) without (left) and with bevacizumab treatment in an orthotopic U87 glioma mouse model, adapted from (138); (17) Representative heatmap of spectral data from a mouse with a mutant IDH1 tumor xenograft following injection of hyperpolarized [1-13C]-glutamine showing accumulation of 2-HG in the tumor region only, which was referenced and normalized to a 5 mM [1-13C] urea phantom. Dotted lines highlight the tumor, and the white line at the bottom represents 10 mm for scaling, adapted from (46); (18) In-vivo CEST-MRI of MDA-MB-231 breast tumor xenografts showing representative CEST MRI maps (top row, A), T1-weighted RARE MRI (bottom left, B), and MTRasym for three individual mice with orthotopic human MDA-MB-231 breast tumor xenografts, which were labeled M1 for mouse 1, M2 for mouse 2, and M3 for mouse 3. CEST shifts of amide, amine, and hydroxyl resonances are highlighted in C, adapted from (52);
(E) Cellular tracking using non-invasive imaging in mouse cancer models: (19) Dual reporter bioluminescence imaging using spectral unmixing algorithm. CBG99 cells were transplanted into the right striatum, PpyRE9 cells into the left striatum of the nude mouse on the right. A spectral unmixing algorithm was applied in order to select green light from CBG99 and red light from PpyRE9, adapted from (191); (20) immune-PET to image T-lymphocytes using 89Zr-anti-CD3 in normal and BBN975 bladder cancer tumor-bearing mice, adapted from (147); (21) T2-weighted brain MRI of ferumoxytol-labeled breast cancer cells after intra-cardiac injection, adapted from (35); (22) T2-weighted maps for macrophage imaging after ferumoxytol injection in inflamed mammary gland tumor mouse model, adapted from (37);
(F) Molecular imaging of tumor-specific molecules: (23) tracking fluorescent micelles (red signal) to bioluminescent brain tumors (green) in anatomical context (124); (24) whole-body 18F-estradiol (FES) PET/CT of estrogen receptor in ER positive and negative bone metastases in mouse models of breast cancer; (25) whole body SPECT/CT with 111In-MSH peptide (melanocyte stimulating hormone) to image melanocortin-1 receptor in mouse B16/F1 melanoma model, adapted from (154); (26) CT (left) and 18FDG-PET of nasal adenocarcinoma in a canine cancer patient (a 10-year old standard poodle), adapted from (187).
Magnetic Resonance Imaging and Spectroscopy (MRI/MRS):
MR physics is complicated, but offers extraordinary opportunities to manipulate tissue water signals based on relaxation mechanisms, chemical exchange, flow and diffusion to reveal diverse anatomical, physiological and cellular properties of cancer at high external magnetic fields. The most sensitive nucleus is the proton, notably in H2O.
Anatomical MRI:
Among all imaging modalities, MRI possesses the best soft tissue contrast, which may be enhanced still further using exogenous paramagnetic contrast agents. Excellent spatial resolution can reveal ultra-small cancer lesions (as small as 0.2 mm diameter with 9.4 T MRI), particularly in well-structured tissues such as the brain. MRI is the “gold-standard” for orthotopic brain tumors and brain metastases (Figure 1–A1, (9–13)), and is also widely applied for the detection of other soft tissue lesions including liver (Figure 1–A2) and lung metastases (Figure 1–A3).
Physiological MRI:
Beyond high-resolution anatomical MRI, tumor cellular density and edema are easily quantified using diffusion-weighted MRI, which is sensitive to restricted or enhanced diffusion of water molecules, respectively (Figure 1–B7) (2, 14, 15). Several recent publications reported increased apparent diffusion coefficients (ADCs) associated with treatment-induced necrosis (16–19). Tissue oxygenation may be examined using oxygen–sensitive MRI. Notably, apparent transverse relaxation rate (R2*) is sensitive to the concentration of deoxyhemoglobin, as exploited in blood oxygen level dependent (BOLD) contrast and forming the basis of fMRI to assess neurological activation (20). Meanwhile, so-called tissue oxygen level dependent (TOLD) MRI exploits the sensitivity of the spin-lattice relaxation rate, R1, to the paramagnetic oxygen molecule (O2) itself (Figure 1–B8) (21–27). Noting the importance of hypoxia in cancer development, aggressiveness and response to therapy, an oxygen gas breathing challenge has been shown to provide a simple effective theranostic: well oxygenated tissues show response to an oxygen-gas breathing challenge, whereas hypoxic tissue does not (28). This approach has been demonstrated to provide a prognostic imaging biomarker in rats with respect to stereotactic ablative radiation therapy (SABR) (24, 28) and is feasible in man (21, 29).
Vascular MRI:
The use of exogenous MR contrast agents, namely gadolinium chelates as T1- and iron oxide nanoparticles as T2-contrast, enables imaging of tumor angiogenesis and changes in tumor vascularity. Intravenous injection of gadolinium contrast agent allows direct visualization of tumor vasculature by magnetic resonance angiography (MRA, Figure 1–C11) (30) or the generation of tumor perfusion/ permeability Ktrans maps using dynamic-contrast enhanced (DCE)-MRI (Figure 1–C12) (31–33). The use of T2-contrast blood pool agents (based on ferumoxytol and other iron oxide nanoparticles) allows susceptibility-contrast imaging to assess tumor blood volume (32, 34).
Cellular and Receptor MRI:
The same iron oxide nanoparticles can be used for cell tracking. Breast cancer cells prelabelled with ferumoxytol in-vitro, could be detected in the brain by T2-MRI following IV injection (Figure 1–E21) (35). Meanwhile, injection of ferumoxytol itself leads to extensive uptake by macrophages, which has been observed as reduced T2-signal, revealing M1 (anti-tumor) or M2 (pre-tumor) activity (Figure 1–E22) (36–38). Some reports have explored the possibilities of using iron oxide- or gadolinium-based contrast for detecting cell receptors including HER2 or C2 imaging in mouse models of breast cancer and pre-cancerous renal inflammation (39–41). In mouse prostate cancer models, PSMA-receptors have been successfully imaged using targeted iron oxide nanoparticles by T2-MRI or a diamagnetic dextran-based CEST MRI agent (see below) (42–44). Receptor imaging with MRI poses unique challenges for signal amplification to deposit sufficient MRI contrast per receptor molecule for its detection.
Other nuclei and metabolic MRS:
Beyond proton MRI of tissue water, spectroscopic imaging can detect several endogenous metabolites that occur at sufficiently high concentrations, such as lactate, glutamine, glutamate, creatine, N-acetyl aspartate (NAA), gamma-aminobutyric acid (GABA), citrate, choline, and, most recently, 2-hydroxyglutarate (2HG) (45). The oncometabolite 2-HG accumulates in low-grade glioma, secondary glioblastoma, and acute myeloid leukemia, owing to mutations in the metabolic enzymes isocitrate dehydrogenase (IDH) 1 or 2. Mutant IDH1/2 aberrantly produces 2-HG (instead of ketoglutarate), which is detectable by 1H-MRS or 13C-MRSI following hyperpolarized [1-13C]-glutamine administration (Figure 1–D17) (46). For 13C-MRSI, the most developed hyperpolarized probe today is [1-13C]-pyruvate, which enables the detection of activated lactate dehydrogenase in tumors (47). Isotopically labeled substrates and metabolites are clearly seen against naturally low abundance endogenous signals (e.g., 100%-enriched isotopomers versus 1.1% naturally abundant 13C). Furthermore, hyperpolarization of 13C substrates can be achieved by various techniques, including dynamic hyperpolarization (48) or parahydrogen induced polarization (49) and leads to a significant boost in the naturally low 13C MRS signal. However, magnetization decays rapidly within minutes, necessitating fast 13C MR imaging techniques. It has been shown that hyperpolarized 13C-pyruvate/ lactate MRS(I) is superior to 18FDG-PET (another metabolic imaging technique, see below) in detecting treatment response to novel targeted therapies and radiation (50, 51). Another approach to amplify MRS signals uses chemical exchange saturation transfer (CEST) MRI, which detects the exchange of protons from hydroxyl, amine, and amide groups to tissue water through the transfer of signal loss, with repeated proton exchange enhancing the effective signal in endogenous (52) and exogenous compounds (53) (Figure 1–D18). Amide proton transfer (APT) contrast, which is the CEST signal from endogenous cellular proteins and peptides, differentiates viable glioma from radiation necrosis (54). The use of D-glucose administration as a contrast agent for noninvasive CEST detection of tumors has been termed glucoCEST, and offers cancer detection with glucose as a biodegradable, nontoxic contrast agent (55). CEST measurements of regional pH, based on the clinically approved X-ray contrast agent iopamidol, have been applied in kidney and lung cancer models (56, 57). Another important nucleus for cancer characterization by MRS is 31P for detection of phospholipid precursors, high energy phosphates and inorganic phosphate, which exhibits a pH-sensitive chemical shift in the physiological range (58), although it can be difficult to discriminate intra vs. extra cellular components. Meanwhile, 19F-MR agents can offer superior chemical shift response (59). 19F-MRI with perfluorocarbon agents has been used as an alternative to iron oxide T2-MRI (see above and Figure 1–E22) to detect tumor-associated macrophages with the benefit of no endogenous background signal (60). Perfluorocarbons exhibit very high gas solubility and can serve as molecular amplifiers, as exploited to assess tumor pO2 providing evidence for hypoxia, heterogeneity and differential regional response to interventions (28, 59, 61).
X-Ray Computed Tomography (microCT):
Micro-computed tomography (microCT) is a high-resolution 3-dimensional imaging technique; the physical principle of CT is based on scattering and absorption of x-rays by tissues based on their electron density. There are essentially three levels of attenuation yielding color-coded contrast in CT: air (black), soft tissue (grey shades) and bones (white).
Anatomical microCT:
Compared to MRI, CT is inferior in distinguishing soft tissues/ organs, but the major strength of microCT lies in supreme high-resolution (<50 microns) fast imaging of lungs and bones revealing cancer lesions. Since bones are the most common metastatic site for major cancers (including breast and prostate), several studies reported the use of high-resolution (10 μm) microCT for detecting engrafted breast cancer cells in the bone (Figure 1–A4) (62). Inhibition of the development of osteolytic bone lesions by zolendronic acid has been reported in MDA-MB-231 breast xenograft mice, also identifying IL-1 as one of the key players for metastatic development (63–65). Due to the inherent contrast between air and tissue structures and the resulting attenuation of the x-rays passing through tissue, microCT is particularly well suited for providing high quality anatomical information in the lung. With the development of pre-cancerous lung conditions, including inflammation (66), fibrosis (67), and emphysema (68), and their progression to lung tumors (69–71), tissue structure becomes dense and can easily be differentiated from both normal lung and airspace. The use of 3-dimensional analysis to quantify tumor number, size and progression is advantageous over traditional histology (69) or macro-dissection of the lung to isolate tumors (70).
Vascular microCT:
Gated respiratory-holding techniques, fast acquisition times and the introduction of novel metal nanoparticles, such as ExiTron, allow lung microvasculature to be easily visualized, simultaneously with lung tumor detection (Figure 1–C13) (72, 73). The low radiation dose of modern instruments makes longitudinal microCT possible without long-term harm to animals (74). Recently, contrast-enhanced microCT has been applied to visualization and mapping of tumor vasculature in brain tumor and neuroblastoma mouse models (75–77).
Ultrasound (US) uses high-frequency sound waves and captures the ultrasound energy reflected from interfaces in the body (“echoes”) that separate tissue with different acoustic impedances, where the acoustic impedance is the product of physical density and velocity of sound in the tissue. Typically, a cyst appears sonolucent, because it gives few if any echoes (being mostly water), while liver and spleen have solid homogenous echo texture due to medium level echoes from the fibrous interstitial tissues. High-intensity echoes (increased echogenicity) are caused by calcification, fat and air interface; however, they do not propagate through bone. Among real time modalities, US features the highest frame rate up to 20,000 fps, enabling US-guided animal procedures, such as orthotopic cell tumor injections and left ventricular infusion of cancer cells to generate models of metastasis while avoiding lung engraftment (78, 79).
Anatomical US:
Pancreatic cancer is one of the most challenging mouse models for preclinical imaging. US provides fast precise quantification of pancreatic tumor burden longitudinally and without contrast administration (Figure 1–A6) (80, 81).
Vascular US:
US is also an excellent technique to assess tumor vasculature, e.g., Doppler US measures the speed and direction of flowing blood and has revealed vascular response to anti-angiogenic and Notch therapies in an orthotopic renal cell carcinoma mouse model (82) as well as irradiated rat fibrosarcoma tumors (83). Considerable attention has been given to the development of US-specific nanoparticles and microbubbles, which may be used both for vascular imaging and as theranostic drug carriers. The latest include VEGFR2 targeted microbubbles (84), oxygen microbubbles (85, 86) and US-destructible microbubbles for better delivery of paclitaxel-loaded nanoparticles in pancreatic cancer models (87). Acoustic Angiography (AA) is another contrast enhanced ultrasound technique, which uses the super-harmonic signals from microbubbles to produce high-resolution maps of vasculature with exceptional contrast since tissue yields no signal. Furthermore, AA can provide quantitative measurements of vascular density, blood perfusion, and vessel morphology, helpful to evaluate response to anti-angiogenic therapy in cancer (82). Quantitative US (QUS, (88)) is obtained from B-mode images and raw radiofrequency data and has been used to examine treatment response. Attenuation coefficients (ATN) and backscatter coefficients (BSC) can be derived (89). On the other hand, ultrasound elastography can visualize and quantify tissue stiffness noninvasively (90). These data can be used as a potential biomarker to assess changes in the tumor microenvironment, particularly changes affecting the extra cellular matrix (ECM), which may affect treatment efficacy (91, 92).
Photoacoustic imaging (PAI) represents the newest addition to the commercial armamentarium for pre-clinical imaging studies and progressively experimental investigations in man (93, 94). PAI exploits spectrally selective pulsed laser excitation of chromophores generating local thermoelastic tissue expansion, which is detected based on the resultant ultrasound acoustic waves, analogous to lightning generating thunder. Application of multiple wavelengths allows spectral discrimination, which has been applied to endogenous molecules such as oxy- and deoxyhemoglobin (HbO2 and Hb) and melanin, and exogenous agents such as organic dyes, gold nanoparticles and genetically encoded proteins (95, 96). Indeed, spectral unmixing allows multiple materials to be detected simultaneously. The technology is particularly rapid, typically achieving single slice images in <100 ms, but usually images are acquired at multiple wavelengths, and signals may be averaged so that a typical acquisition time is 1–2 s. Gating may become relevant for assessing rapid changes in tissues subject to motion (97). Selection of an appropriate non-negative data reconstruction model is vital and choice of filters can enhance signal to noise (98, 99).
Various commercial instruments are optimized for in-vivo microscopic, mesoscopic, whole mouse tomographic and human applications, and may incorporate additional ultrasound excitation to enhance anatomical discrimination with typical spatial resolution approaching 100 μm at depths up to 5 cm.
The most effective application is assessment of tumor vasculature based on the ability to identify and quantify relative Hb and HbO2 (Figure 1–B9) with effective studies of antiangiogenic therapy (100), acute vascular disruption induced by combretastatin (101, 102) and potentially prognostic observations following tumor irradiation (103). It appears that response to an oxygen breathing challenge characterized as ΔSO2 is more closely related to perfusion and hypoxia than baseline static parameters (102), e.g., low CAIX expression correlated with higher ΔSO2MSOT. Blood volume and perfusion may be effectively examined using contrast agents such as indocyanine green (ICG) (102, 104) or the liposomal formulation Genhance (105). Small molecule dyes may be incorporated in targeted liposomal formulations or used to directly label antibodies for detection of tumors or revealing receptor expression (106). Gold nanoparticles (which could also be used in microCT) exhibit exceptionally high photoacoustic activity based on surface plasmon resonance and may be tuned to wavelengths in the range 600–1000 nm based on size and shape (96, 107). Additional innovations include “smart” activatable probes, e.g., sensitive to β-galactosidase activation (108) and genetically encoded proteins such as BphP1 (109). PAI essentially bridges two modalities to exploit spectrally selective optical excitation and robust spatial detection using ultrasound. It is very much an emerging technology.
Optical Imaging: Bioluminescence and Fluorescence (BLI and FLI):
Two decades after its invention, in-vivo optical imaging is now a well-established standard method to non-invasively monitor biological activity in mouse (and rat) research models. Optical imaging includes four molecular imaging modalities: BLI, FLI, chemiluminescence and Cherenkov imaging. The relatively low threshold of implementation, as well as the high sensitivity of in-vivo BLI, make this whole body, non-invasive imaging technique a go-to method in preclinical research (Figure 1–A5 & E19) (62, 110). Beyond tracking tumor growth and regression via constitutive firefly luciferase expression for drug efficacy determination, the toolbox for this molecular imaging technique has vastly expanded. Bioluminescent enzymes can be used to genetically tag cells, viruses, bacteria, gene therapy, and now also antibodies and their fragments (111). These enzymes such as firefly, renilla, gaussia and Nanoluc luciferases can be constitutively or inducibly expressed, and as such used for ratiometric imaging, gene expression studies, or dual labeling purposes (e.g. tracking T-cells infiltrating tumor) (12, 112–114) (Figure 1–E19). Split luciferases to evaluate protein-protein interaction, as well as split luciferin substrates to monitor apoptosis have been designed and are utilized to evaluate mechanism of action (115). Potential drawbacks of BLI are the need for cell transfection and delivery of reactive substrate. Luciferin effectively crosses barriers such as blood brain and placenta and its very delivery to tissue has been used to assess selective vascular destruction in tumors (101, 116). Bioluminescent resonance energy transfer (BRET) constructs such as Antares, which red shifts the lower wavelength Nanoluc luciferase for better in-vivo sensitivity, are also available (117). Chemiluminescent compounds, substrates and sensors are luminophores that emit red shifted light upon chemiexcitation have been reported for detection of H2O2, H2S, formaldehyde, beta-galactosidase and nitroreductase activity (118–121). Dr. Cherenkov received the Nobel Prize in 1958 for his discovery of the bluish hue of light emitted by decaying radioisotopes. This same light emission can be detected by screening mice injected with diagnostic radioisotopes such as 18FDG in an in-vivo optical imaging system, adopting the epithet of a poor man’s PET scanner (122) and may also be relevant for radiation dosimetry (123). FLI on the other hand features both genetically encoded fluorescent proteins (FPs) and fluorescent dyes. The powerful combination of BLI and FLI is exemplified by Zeng et al. (124) (Figure 1–F23), illustrating the tracking of fluorescent micelles to bioluminescent brain tumors. In comparison with BLI however, the contrast to noise is less with fluorescence due to non-specific autofluorescent noise originating from innate proteins in tissue. This issue is being combatted with the discovery of red-shifted FP’s for better in-vivo sensitivity, an initiative led by Nobel laureate Dr. Roger Tsien (125). A second window of opportunity for in-vivo FLI is currently being explored in the short wavelength infrared (SWIR) using ultra-bright near-infrared-IIb rare-earth nanoparticles. Here, tissue absorption and light scattering are significantly reduced (126) rendering higher resolution, higher depth penetration images. Crafty alternatives have also been invented in which fluorescent sensors are quenched until activated by an enzymatic reaction (e.g., cathepsin, matrix metalloprotease, neutrophil elastase, etc.) or in which fluorophores shift wavelength upon binding their target (127). A great advantage of fluorophores is that they are also readily detectable ex-vivo for histopathological evaluation. This is highly translational, and intrasurgical fluorescence imaging is actively being explored to both highlight tumor burden, and also improve tumor margin of resection (128). Preclinical optical cancer imaging begs for anatomical context, prompting co-registration with anatomical imaging modalities such as X-ray, microCT, MRI or the recently developed robotic ultrasound, which features inexpensive, exogenous contrast free 3D soft tissue resolution (78).
PET and SPECT:
Nuclear medicine images are produced by giving the animal short-lived radioactive isotopes and detecting their decay using a gamma camera (SPECT) or positron emission (PET) scanner, revealing spatial and temporal distribution of target-specific radiotracers and pharmaceuticals. An extensive array of radiopharmaceuticals, or molecular probes exist (based on 11C, 13N, 15O, 18F, 124I, 64Cu, 68Ga, 89Zr for PET and 123I, 99mTc, 201Ti, 111In for SPECT) to image diverse aspects of tumor physiology and biology. Data can reveal properties such as glucose metabolism, blood volume and flow, tissue uptake, receptor binding, and oxygen utilization. Since both modalities have relatively low spatial resolution, CT is usually added for an anatomical overlay of the biodistribution of the radio-labeled probe.
Metabolic PET:
18FDG-PET is the most established metabolic cancer imaging approach both pre-clinically and clinically. Most tumors have a highly glycolytic phenotype (the Warburg effect) providing the basis for increased uptake and accumulation of the radioactive glucose analogue 18FDG, as shown in various mouse models of leukemia, pancreatic, lung, colorectal, breast, prostate cancers (Figure 1–D15) (51, 129–132). Other tracers have recently been introduced to elucidate abnormal metabolic phenotypes, including, either 11C- or 18F-, acetate (mitochondrial metabolism) (133), choline (membrane phospholipids) (133, 134), amino acids in brain tumors (glutamine, tyrosine or methionine, Figure 1–D16) (135–138).
Physiological PET:
Several essential 18F-labeled tracers should be mentioned here as potential (although not entirely specific) markers for tumor cell proliferation (18F-fluorothymidine, 18FLT) and hypoxia (18F-fluoroazomycin arabinoside, 18F-FAZA, and 18F-fluoromisonidazole, 18F-MISO). Radioactive thymidine is readily incorporated into DNA synthesis, making an increased uptake of 18FLT visible on animal PET and correlating with increased ADC on diffusion weighted MRI, albeit exhibiting low specificity (139–142). 18F-MISO is trapped in hypoxic areas as compared with BOLD and TOLD MRI (Figure 1–B10) (143). While 18F-MISO has been tested for many years, its uptake selectivity is suboptimal and many other potential hypoxia imaging agents are under development and evaluation (e.g., 18F-FAZA shows more rapid background clearance (144, 145).
Cellular PET:
With the development of check-point inhibitor and immunotherapies, significant efforts have been dedicated to develop so-called “immunoPET”. Several T-lymphocyte targeting molecules were radiochemically labelled with long-lived radionuclides (such as 64Cu, 68Ga, 89Zr). Following intravenous injection intra-tumoral accumulation of T-lymphocytes has been non-invasively detected in response to check-point inhibitor treatment (Figure 1–E20) (146–148).
Molecular PET/SPECT:
Specific molecular targets have been visualized using PET- or SPECT-based peptides, antibodies and receptor-binding ligands. One of the most explored is hormone imaging, 18F-fluoroestradiol (18FES) PET, as used for pre-clinical and clinical imaging of ER+ breast and ovarian cancer (Figure 1–F24) (149–152). Recent examples of hormone imaging include PET of androgen receptor in rat brain (153). Several 111In/203Pb labelled peptides for SPECT (154) and 68Ga-MSH for PET (155) have been developed to target the melanocortin-1 receptor in melanoma mouse models (Figure 1–F25). A 203Pb/212Pb theranostic pair has been reported for PSMA-based α-particle targeted radiopharmaceutical therapy in advanced prostate cancer (156).
Other notable imaging technologies include MPI and ESR. Magnetic Particle Imaging (MPI) is an emerging imaging modality that involves iron oxide nanoparticles. Unlike MRI, MPI measures electronic moment of particles, which is more sensitive than measuring changes in proton relaxation by MRI. The detection is linear, sensitive (ng of iron per voxel) and has a high signal to background ratio. Using MPI of iron oxide particles, kinetics of accumulation of nanoparticles in rat tumors (157) and kinetics of drug release in mouse breast tumors (158) were studied. Further applications of MPI are dependent on improving the acquisition speed and resolution, as well as improving circulation and targeting properties of nanoparticles.
Electron paramagnetic resonance (EPR), also termed Electron spin resonance (ESR) has been a research tool for many years, but remains somewhat esoteric in cancer research, largely due to lack of available instrumentation. It directly detects free radicals but the extremely high frequencies tend to limit tissue penetration, though effective studies have been performed in mice and human teeth and tattoos (159). The most popular application has been based on imaging signal line width and relaxation mechanisms, which may be directly responsive to the presence of oxygen and hence pO2. Reporter agents may be injected directly into tumors (e.g., India ink or chars (160), or infused systemically (OX63- oxygen-measuring spin probe, coincidently the same material used to achieve hyperpolarization of 13C substrates for NMR) (161). Sensitivity to oxygen can be particularly high at very low, radiobiologically relevant pO2 values (0–15 Torr) and significant correlations have been observed between pO2 values and radiation response (50, 160–162). A significant drawback of EPR is the lack of integrated anatomical information, generally requiring that separate MRI be performed and co-registered.
Image-Guided Irradiation:
Radiation plays an important role in cancer therapy; radiation-based therapy has been applied to animal models for decades and recently has undergone significant improvement in terms of applying multi-modality imaging to guide radiation planning (163, 164). Radiation kills cancer cells by damaging DNA, either directly or indirectly through the creation of reactive oxygen species. Because radiation kills both cancer cells and healthy cells alike, various methods are used to increase the tumoricidal effects of radiation while minimizing damage to the surrounding normal tissue, including spatial modulation of the dose distribution to conform to a specific target region. While such conformal dose distributions allow for significant reductions in normal tissue toxicity, they also require onboard image guidance systems to ensure the tumor is in the correct location when the radiation beam is turned on. Modern animal irradiators incorporate multi-modal imaging detectors to precisely guide the radiation, combining the ability to deliver targeted radiation treatments using a 225 kVp, gantry-mounted x-ray tube with digital radiography, fluoroscopy, cone-beam computed tomography (CBCT), and bioluminescence imaging (BLI) (164, 165). Image-guided irradiation has been successfully applied even for small orthotopic head-and-neck and lung lesions in tumor-bearing mice (166, 167). The software also allows import of existing imaging data sets from other modalities such as MRI – which often plays a crucial role for irradiating intracranial brain tumor models (9, 163)
Image Analysis and Quantitative Biomarkers:
There is increasing interest in using imaging to develop non-invasive quantitative imaging biomarkers (surrogate endpoints) for cancer characterization. Indeed, most imaging read-outs are provided in both qualitative and quantitative form (Table 1B) (168). This is especially true for MRI, CT and ultrasound, due to their high spatial resolution to provide precise tumor dimensions as well as number of suspicious lesions/ metastases (169, 170). The well-established mathematical modeling algorithms for tracer kinetics allow quantification of tumor vasculature based on gadolinium, nanoparticle and microbubble uptake for MRI, CT and US, respectively (32, 34). The biomarkers include the exchange rate constants (Ktrans), which reflect the efflux rate of gadolinium contrast from blood plasma into the tissue/ tumor extravascular extracellular space (EES), the volume of contrast agent distribution Ve, or simply the area under enhancement curves after the administration of contrast (19, 171–174). Finally, physiological MRI provides established quantitative end-points in the form of apparent diffusion coefficients (ADC) from diffusion-weighted MRI: low ADC (0.5–0.8×10−3 mm2/sec) indicates densely cellular aggressive tumors, while treatment-induced necrosis results in increased ADC up to 1.2×10−3 mm2/sec, and radiation-induced edema’s ADC as high as 2.2 (17, 19). PET and SPECT tracer uptake is usually reported as standardized uptake values (SUVs) which includes normalization to injected dose and accounts for radionuclide decay (129, 130, 175). Several studies report ratios of signal intensities of the tumor-to-normal tissue (most often for brain tumors as tumor-to-brain ratios, TBR) (138, 174). Optical imaging (BLI and FLI) is rather semi-quantitative, but can provide signal intensities related to tumor volume or tissue perfusion (SIs) (11, 114), e.g., the change in light emission from a luciferase expressing tumors following an acute intervention such as a vascular disrupting agent provides an indication of vascular shutdown (101, 176, 177). Multimodality imaging ideally combines the advantages of each modality, while mitigating their deficiencies. Image registration is necessary when more than one imaging modality is used. Histology can often serve as the ground-truth for the validation of image-based biomarkers or new imaging modalities.
Identifying non-invasive biomarkers to be used clinically as surrogate endpoints is not only valuable, but also promising. The advent of machine learning and artificial intelligence in medical imaging has led to the field of radiomics (170, 178–181). Like genomics and other “-omics”, radiomics allows quantifiable characterization of image features that provide a means to identify image-based biomarker surrogates for response to cancer treatment. Cameron et al. report a radiomics method, MAPS, based on Morphology, Asymmetry, Physiology, and Size (MAPS) using multi-parametric MRI (182). Most radiomics data have been reported for multicenter human studies, since a large number of subjects needs to be enrolled – the number of experimental animals in a single imaging study often being a limiting factor. As quantitative imaging and radiomics lead to more image-based biomarkers, standardization and assessment of reproducibility are becoming important and will require a centralized image archive for multi-center preclinical studies.
Future Directions in Translational Imaging:
Imaging is highly translational by nature and murine models have contributed enormously to the development of oncologic imaging methodologies (183). However, the complex, heterogeneous tumor microenvironment observed in human cancer is challenging to model in an immunodeficient animal system, particularly in terms of immunotherapeutic strategies. Lack of optimal pre-clinical models for testing is likely responsible for the dismal success rate (5–8%) of cancer therapeutics developed in murine models to eventually obtain FDA approval for use in human patients (184). Dogs with naturally occurring cancers provide an alternative, complementary system for preclinical cancer research. The recent completion of the sequencing of the dog genome has shown that most of their 19,000 genes are orthologous or similar to humans (185). Companion animals live in our homes and are exposed to similar environmental and lifestyle influences. Their cancers grow slowly in an immunocompetent milieu, allowing for complex carcinogenesis, genomic instability and immune avoidance to develop. Their size is such that serial biologic sampling can be performed before, during and after therapy. These patients are imaged in human equipment, allowing for standardization of imaging protocols, improved spatial resolution for more accurate quantitative analysis and adequate quality assurance of biodistribution for novel imaging probes. Power Doppler ultrasound and contrast-enhanced ultrasound were used to demonstrate tumor vascular response to anti-vascular therapy in canine cancer patients non-invasive (186). There are several success stories to report today: 18FDG- and 18F-NaF PET/CT have been successfully used in canine cancer patients to detect head-and-neck cancer and bone involvement of the nasal cavity (Figure 1–F23) (187). An iodinated nanoparticle CT tracer initially developed and validated in a murine lung cancer model (described above (73)), has been successfully used in a CT study of companion dogs with spontaneous tumors (188). An anatomic and functional imaging probe for a novel immunotherapeutic was developed in dogs with spontaneous lymphoma (189). A recombinant oncolytic vesicular stomatitis virus that expresses a surface sodium-iodide symporter (NIS) protein and IFNβ was characterized. Based on clinical response to VSV-IFNβ-NIS therapy in dogs with T cell lymphoma, a phase I clinical trial in people has been started (NCT03017820) (189). In a follow up study, dogs administered VSV-IFNβ-NIS were evaluated to determine whether 18F-tetrafluoroborate radiopharmaceutical that binds to the cell surface NIS can be used to confirm successful viral gene replication (190). Veterinary patients with naturally occurring cancers may assist in the development of new molecular imaging probes, shorten the approval process of oncologic therapies and create a mutually beneficial bridge between the fields of veterinary and human oncology.
In summary, multi-modal oncologic imaging has become a cutting-edge necessity in pre-clinical (animal) cancer research. Understanding the physical principles of each modality is essential for applying the correct non-invasive imaging protocol to an animal-based study. Development of imaging probes for multimodal imaging technologies is also an important scientific and clinical goal. Each imaging modality brings specific insights into oncological questions and allows researchers to follow the biology dictating the choice of the optimal reporter and imaging modality to best characterize cancer phenotype (191). The future also holds a big promise for PET/MRI (similarly to existing PET/CT) combining two powerful molecular, physiological and structural techniques into one scanner. Finally, we anticipate the introduction of novel predictive models and deep learning algorithms (192) in the near future for managing sophisticated and complex image data sets in animal models of cancer.
Acknowledgments
This review is based on the 2019 Animal Imaging Workshop held at the University of Colorado Anschutz Medical Campus. The workshop was partly supported by the NIH Shared Instrumentation Grant Program (S10 OD023485, S10 OD023491, S10 OD027023, S10 OD018094), the University of Colorado and the University of Southwestern CCSG Cancer Center grants (P30 CA046934 and CA142543, respectively), the University of Colorado Clinical Translational Institute (UL1 TR002535), and the Nutritional and Obesity Research Center (P30 DK048520).
Abbreviations:
- ADC
apparent diffusion coefficient
- AUC
area under the curve
- BLI
bioluminescence imaging
- BOLD
blood oxygen level dependent
- CT
computed tomography
- CE-CT
contrast enhanced computed tomography
- DCE
dynamic contrast enhanced
- EPR
electron paramagnetic resonance
- ESR
electron spin resonance
- FLI
fluorescence imaging
- fMRI
functional MRI
- FOV
field of view
- HbO2
oxy-hemoglobin
- Ktrans
volume transfer constant
- MPI
magnetic particle imaging
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MSOT
multispectral optoacoustic tomography
- NP
nanoparticle
- PAI
photoacoustic imaging
- PDX
patient-derived xenograft
- PET
positron emission tomography
- ΔR1
change in longitudinal relaxation rate (R1=1/T1)
- ΔR2*
change in apparent transverse relaxation rate (R2=1/T2)
- SI
signal intensity
- SO2MSOT
hemoglobin oxygen saturation measured using MSOT
- SPECT
single photon emission computed tomography
- SUV
standard uptake value
- tBV
tumor blood volume
- TOLD
tissue oxygen level dependent
- US
ultrasound
- Ve
extra cellular- extra vascular space
Footnotes
The authors declare no potential conflicts of interest
References
- 1.de Jong M, Essers J, van Weerden WM. Imaging preclinical tumour models: improving translational power. Nature reviews Cancer. 2014;14(7):481–93. [DOI] [PubMed] [Google Scholar]
- 2.Jardim-Perassi BV, Huang S, Dominguez-Viqueira W, Poleszczuk J, Budzevich MM, Abdalah MA, et al. Multiparametric MRI and Coregistered Histology Identify Tumor Habitats in Breast Cancer Mouse Models. Cancer research. 2019;79(15):3952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissleder R, Schwaiger MC, Gambhir SS, Hricak H. Imaging approaches to optimize molecular therapies. Science translational medicine. 2016;8(355):355ps16. [DOI] [PubMed] [Google Scholar]
- 4.Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb Perspect Biol. 2010;2(12):a003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausner SH, Bold RJ, Cheuy LY, Chew HK, Daly ME, Davis RA, et al. Preclinical Development and First-in-Human Imaging of the Integrin alphavbeta6 with [(18)F]alphavbeta6-Binding Peptide in Metastatic Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(4):1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hormuth DA 2nd, Sorace AG, Virostko J, Abramson RG, Bhujwalla ZM, Enriquez-Navas P, et al. Translating preclinical MRI methods to clinical oncology. Journal of magnetic resonance imaging : JMRI. 2019;50(5):1377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wessels JT, Busse AC, Mahrt J, Dullin C, Grabbe E, Mueller GA. In vivo imaging in experimental preclinical tumor research--a review. Cytometry A. 2007;71(8):542–9. [DOI] [PubMed] [Google Scholar]
- 8.Mannheim JG, Kara F, Doorduin J, Fuchs K, Reischl G, Liang S, et al. Standardization of Small Animal Imaging-Current Status and Future Prospects. Mol Imaging Biol. 2018;20(5):716–31. [DOI] [PubMed] [Google Scholar]
- 9.Pierce AM, Witt DA, Donson AM, Gilani A, Sanford B, Sill M, et al. Establishment of patient-derived orthotopic xenograft model of 1q+ posterior fossa group A ependymoma. Neuro-oncology. 2019;21(12):1540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veo B, Danis E, Pierce A, Sola I, Wang D, Foreman NK, et al. Combined functional genomic and chemical screens identify SETD8 as a therapeutic target in MYC-driven medulloblastoma. JCI Insight. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green AL, DeSisto J, Flannery P, Lemma R, Knox A, Lemieux M, et al. BPTF regulates growth of adult and pediatric high-grade glioma through the MYC pathway. Oncogene. 2020;39(11):2305–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartorius CA, Hanna CT, Gril B, Cruz H, Serkova NJ, Huber KM, et al. Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene. 2016;35(22):2881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boult JKR, Apps JR, Holsken A, Hutchinson JC, Carreno G, Danielson LS, et al. Preclinical transgenic and patient-derived xenograft models recapitulate the radiological features of human adamantinomatous craniopharyngioma. Brain pathology. 2018;28(4):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boult JKR, Box G, Vinci M, Perryman L, Eccles SA, Jones C, et al. Evaluation of the Response of Intracranial Xenografts to VEGF Signaling Inhibition Using Multiparametric MRI. Neoplasia. 2017;19(9):684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deep G, Kumar R, Nambiar DK, Jain AK, Ramteke AM, Serkova NJ, et al. Silibinin inhibits hypoxia-induced HIF-1alpha-mediated signaling, angiogenesis and lipogenesis in prostate cancer cells: In vitro evidence and in vivo functional imaging and metabolomics. Molecular carcinogenesis. 2017;56(3):833–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heid I, Steiger K, Trajkovic-Arsic M, Settles M, Esswein MR, Erkan M, et al. Co-clinical Assessment of Tumor Cellularity in Pancreatic Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(6):1461–70. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Li J, Fu C, Kuhn B, Wang X. Chemotherapy response of pancreatic cancer by diffusion-weighted imaging (DWI) and intravoxel incoherent motion DWI (IVIM-DWI) in an orthotopic mouse model. MAGMA. 2019;32(4):501–9. [DOI] [PubMed] [Google Scholar]
- 18.Chung YH, Yu CF, Chiu SC, Chiu H, Hsu ST, Wu CR, et al. Diffusion-weighted MRI and (18)F-FDG PET correlation with immunity in early radiotherapy response in BNL hepatocellular carcinoma mouse model: timeline validation. European journal of nuclear medicine and molecular imaging. 2019;46(8):1733–44. [DOI] [PubMed] [Google Scholar]
- 19.Crowe W, Wang L, Zhang Z, Varagic J, Bourland JD, Chan MD, et al. MRI evaluation of the effects of whole brain radiotherapy on breast cancer brain metastasis. Int J Radiat Biol. 2019;95(3):338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gore JC, Li M, Gao Y, Wu TL, Schilling KG, Huang Y, et al. Functional MRI and resting state connectivity in white matter - a mini-review. Magn Reson Imaging. 2019;63:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor JPB, Robinson SP, Waterton JC. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. The British journal of radiology. 2019;92(1095):20180642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panek R, Welsh L, Baker LCJ, Schmidt MA, Wong KH, Riddell AM, et al. Noninvasive Imaging of Cycling Hypoxia in Head and Neck Cancer Using Intrinsic Susceptibility MRI. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(15):4233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Belzile O, Zhang Z, Wagner J, Ahn C, Richardson JA, et al. The effect of flow on blood oxygen level dependent (R(*) 2 ) MRI of orthotopic lung tumors. Magn Reson Med. 2019;81(6):3787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White DA, Zhang Z, Li L, Gerberich J, Stojadinovic S, Peschke P, et al. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer letters. 2016;380(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang DM, Arai TJ, Campbell JW 3rd, Gerberich JL, Zhou H, Mason RP Oxygen-sensitive MRI assessment of tumor response to hypoxic gas breathing challenge. NMR Biomed. 2019;32(7):e4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeman SC, Shui YB, Perez-Torres CJ, Engelbach JA, Ackerman JJ, Garbow JR. O2 -sensitive MRI distinguishes brain tumor versus radiation necrosis in murine models. Magn Reson Med. 2016;75(6):2442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colliez F, Gallez B, Jordan BF. Assessing Tumor Oxygenation for Predicting Outcome in Radiation Oncology: A Review of Studies Correlating Tumor Hypoxic Status and Outcome in the Preclinical and Clinical Settings. Front Oncol. 2017;7(10):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallac RR, Zhou H, Pidikiti R, Song K, Stojadinovic S, Zhao D, et al. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014;71(5):1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Hallac RR, Yuan Q, Ding Y, Zhang Z, Xie XJ, et al. Incorporating Oxygen-Enhanced MRI into Multi-Parametric Assessment of Human Prostate Cancer. Diagnostics (Basel). 2017;7(3):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doblas S, He T, Saunders D, Pearson J, Hoyle J, Smith N, et al. Glioma morphology and tumor-induced vascular alterations revealed in seven rodent glioma models by in vivo magnetic resonance imaging and angiography. Journal of magnetic resonance imaging : JMRI. 2010;32(2):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannan P, Kretzschmar WW, Winter H, Warren D, Bates R, Allen PD, et al. Functional Parameters Derived from Magnetic Resonance Imaging Reflect Vascular Morphology in Preclinical Tumors and in Human Liver Metastases. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(19):4694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson SP, Boult JKR, Vasudev NS, Reynolds AR. Monitoring the Vascular Response and Resistance to Sunitinib in Renal Cell Carcinoma In Vivo with Susceptibility Contrast MRI. Cancer research. 2017;77(15):4127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raina K, Ravichandran K, Rajamanickam S, Huber KM, Serkova NJ, Agarwal R. Inositol hexaphosphate inhibits tumor growth, vascularity, and metabolism in TRAMP mice: a multiparametric magnetic resonance study. Cancer prevention research. 2013;6(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zormpas-Petridis K, Jerome NP, Blackledge MD, Carceller F, Poon E, Clarke M, et al. MRI Imaging of the Hemodynamic Vasculature of Neuroblastoma Predicts Response to Antiangiogenic Treatment. Cancer research. 2019;79(11):2978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murrell DH, Zarghami N, Jensen MD, Dickson F, Chambers AF, Wong E, et al. MRI surveillance of cancer cell fate in a brain metastasis model after early radiotherapy. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2017;78(4):1506–12. [DOI] [PubMed] [Google Scholar]
- 36.Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(17):5695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serkova NJ. Nanoparticle-Based Magnetic Resonance Imaging on Tumor-Associated Macrophages and Inflammation. Front Immunol. 2017;8:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makela AV, Gaudet JM, Foster PJ. Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Scientific reports. 2017;7:42109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen TJ, Cheng TH, Chen CY, Hsu SC, Cheng TL, Liu GC, et al. Targeted Herceptin-dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14(2):253–60. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Ni Q, Xu C, Wan B, Geng Y, Zheng G, et al. Smart Bacterial Magnetic Nanoparticles for Tumor-Targeting Magnetic Resonance Imaging of HER2-Positive Breast Cancers. ACS applied materials & interfaces. 2019;11(4):3654–65. [DOI] [PubMed] [Google Scholar]
- 41.Serkova NJ, Renner B, Larsen BA, Stoldt CR, Hasebroock KM, Bradshaw-Pierce EL, et al. Renal inflammation: targeted iron oxide nanoparticles for molecular MR imaging in mice. Radiology. 2010;255(2):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse BW, Cowin GJ, Soekmadji C, Jovanovic L, Vasireddy RS, Ling MT, et al. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine (Lond). 2015;10(3):375–86. [DOI] [PubMed] [Google Scholar]
- 43.Ngen EJ, Benham Azad B, Boinapally S, Lisok A, Brummet M, Jacob D, et al. MRI Assessment of Prostate-Specific Membrane Antigen (PSMA) Targeting by a PSMA-Targeted Magnetic Nanoparticle: Potential for Image-Guided Therapy. Molecular pharmaceutics. 2019;16(5):2060–8. [DOI] [PubMed] [Google Scholar]
- 44.Liu G, Banerjee SR, Yang X, Yadav N, Lisok A, Jablonska A, et al. A dextran-based probe for the targeted magnetic resonance imaging of tumours expressing prostate-specific membrane antigen. Nat Biomed Eng. 2017;1:977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andronesi OC, Arrillaga-Romany IC, Ly KI, Bogner W, Ratai EM, Reitz K, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nature communications. 2018;9(1):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salamanca-Cardona L, Shah H, Poot AJ, Correa FM, Di Gialleonardo V, Lui H, et al. In Vivo Imaging of Glutamine Metabolism to the Oncometabolite 2-Hydroxyglutarate in IDH1/2 Mutant Tumors. Cell metabolism. 2017;26(6):830–41 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mignion L, Acciardo S, Gourgue F, Joudiou N, Caignet X, Goebbels RM, et al. Metabolic imaging using hyperpolarized pyruvate-lactate exchange assesses response or resistance to the EGFR inhibitor cetuximab in patient-derived HNSCC xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019. [DOI] [PubMed] [Google Scholar]
- 48.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer research. 2008;68(20):8607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavallari E, Carrera C, Sorge M, Bonne G, Muchir A, Aime S, et al. The (13)C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Scientific reports. 2018;8(1):8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto S, Kishimoto S, Saito K, Takakusagi Y, Munasinghe JP, Devasahayam N, et al. Metabolic and Physiologic Imaging Biomarkers of the Tumor Microenvironment Predict Treatment Outcome with Radiation or a Hypoxia-Activated Prodrug in Mice. Cancer research. 2018;78(14):3783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hesketh RL, Wang J, Wright AJ, Lewis DY, Denton AE, Grenfell R, et al. Magnetic Resonance Imaging Is More Sensitive Than PET for Detecting Treatment-Induced Cell Death-Dependent Changes in Glycolysis. Cancer research. 2019;79(14):3557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan KW, Jiang L, Cheng M, Wijnen JP, Liu G, Huang P, et al. CEST-MRI detects metabolite levels altered by breast cancer cell aggressiveness and chemotherapy response. NMR in biomedicine. 2016;29(6):806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Z, Li Y, Zhang J, Liu J, Chen C, van Zijl PC, et al. Molecular Imaging of Deoxycytidine Kinase Activity Using Deoxycytidine-Enhanced CEST MRI. Cancer research. 2019;79(10):2775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature medicine. 2011;17(1):130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sehgal AA, Li Y, Lal B, Yadav NN, Xu X, Xu J, et al. CEST MRI of 3-O-methyl-D-glucose uptake and accumulation in brain tumors. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2019;81(3):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindeman LR, Jones KM, High RA, Howison CM, Shubitz LF, Pagel MD. Differentiating lung cancer and infection based on measurements of extracellular pH with acidoCEST MRI. Scientific reports. 2019;9(1):13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longo DL, Dastru W, Digilio G, Keupp J, Langereis S, Lanzardo S, et al. Iopamidol as a responsive MRI-chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65(1):202–11. [DOI] [PubMed] [Google Scholar]
- 58.Krikken E, van der Kemp WJM, Khlebnikov V, van Dalen T, Los M, van Laarhoven HWM, et al. Contradiction between amide-CEST signal and pH in breast cancer explained with metabolic MRI. NMR in biomedicine. 2019;32(8):e4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu JX, Hallac RR, Chiguru S, Mason RP. New frontiers and developing applications in 19F NMR. Prog Nucl Magn Reson Spectrosc. 2013;70:25–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makela AV, Foster PJ. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2018;80(3):1138–47. [DOI] [PubMed] [Google Scholar]
- 61.Diepart C, Karroum O, Magat J, Feron O, Verrax J, Calderon PB, et al. Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer research. 2012;72(2):482–90. [DOI] [PubMed] [Google Scholar]
- 62.Previdi S, Abbadessa G, Dalo F, France DS, Broggini M. Breast cancer-derived bone metastasis can be effectively reduced through specific c-MET inhibitor tivantinib (ARQ 197) and shRNA c-MET knockdown. Molecular cancer therapeutics. 2012;11(1):214–23. [DOI] [PubMed] [Google Scholar]
- 63.Previdi S, Scolari F, Chila R, Ricci F, Abbadessa G, Broggini M. Combination of the c-Met inhibitor tivantinib and zoledronic acid prevents tumor bone engraftment and inhibits progression of established bone metastases in a breast xenograft model. PloS one. 2013;8(11):e79101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(11):2922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(46):75571–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu EK, Eliseeva S, Rahimi H, Schwarz EM, Georas SN. Restrictive lung disease in TNF-transgenic mice: correlation of pulmonary function testing and micro-CT imaging. Exp Lung Res. 2019;45(7):175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruscitti F, Ravanetti F, Essers J, Ridwan Y, Belenkov S, Vos W, et al. Longitudinal assessment of bleomycin-induced lung fibrosis by Micro-CT correlates with histological evaluation in mice. Multidiscip Respir Med. 2017;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaki M, Chubachi S, Kameyama N, Sato M, Haraguchi M, Miyazaki M, et al. Evaluation of cigarette smoke-induced emphysema in mice using quantitative micro-computed tomography. American journal of physiology Lung cellular and molecular physiology. 2015;308(10):L1039–45. [DOI] [PubMed] [Google Scholar]
- 69.Ramasamy K, Dwyer-Nield LD, Serkova NJ, Hasebroock KM, Tyagi A, Raina K, et al. Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(4):753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L, Xiao SM, Qiang JW, Li YA, Zhang Y. Early Lung Adenocarcinoma in Mice: Micro-Computed Tomography Manifestations and Correlation with Pathology. Translational oncology. 2017;10(3):311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hegab AE, Kameyama N, Kuroda A, Kagawa S, Yin Y, Ornitz D, et al. Using Micro-computed Tomography for the Assessment of Tumor Development and Follow-up of Response to Treatment in a Mouse Model of Lung Cancer. Journal of visualized experiments : JoVE. 2016(111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badea CT, Athreya KK, Espinosa G, Clark D, Ghafoori AP, Li Y, et al. Computed tomography imaging of primary lung cancer in mice using a liposomal-iodinated contrast agent. PloS one. 2012;7(4):e34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashton JR, Clark DP, Moding EJ, Ghaghada K, Kirsch DG, West JL, et al. Dual-energy micro-CT functional imaging of primary lung cancer in mice using gold and iodine nanoparticle contrast agents: a validation study. PloS one. 2014;9(2):e88129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clark DP, Badea CT. Micro-CT of rodents: state-of-the-art and future perspectives. Phys Med. 2014;30(6):619–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghaghada KB, Starosolski ZA, Lakoma A, Kaffes C, Agarwal S, Athreya KK, et al. Heterogeneous Uptake of Nanoparticles in Mouse Models of Pediatric High-Risk Neuroblastoma. PloS one. 2016;11(11):e0165877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starosolski Z, Villamizar CA, Rendon D, Paldino MJ, Milewicz DM, Ghaghada KB, et al. Ultra High-Resolution In vivo Computed Tomography Imaging of Mouse Cerebrovasculature Using a Long Circulating Blood Pool Contrast Agent. Scientific reports. 2015;5:10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Badea CT, Clark DP, Holbrook M, Srivastava M, Mowery Y, Ghaghada KB. Functional imaging of tumor vasculature using iodine and gadolinium-based nanoparticle contrast agents: a comparison of spectral micro-CT using energy integrating and photon counting detectors. Physics in medicine and biology. 2019;64(6):065007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Czernuszewicz TJ, Papadopoulou V, Rojas JD, Rajamahendiran RM, Perdomo J, Butler J, et al. A new preclinical ultrasound platform for widefield 3D imaging of rodents. The Review of scientific instruments. 2018;89(7):075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou H, Zhao D. Ultrasound imaging-guided intracardiac injection to develop a mouse model of breast cancer brain metastases followed by longitudinal MRI. Journal of visualized experiments : JoVE. 2014(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sastra SA, Olive KP. Quantification of murine pancreatic tumors by high-resolution ultrasound. Methods in molecular biology. 2013;980:249–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goetze RG, Buchholz SM, Patil S, Petzold G, Ellenrieder V, Hessmann E, et al. Utilizing High Resolution Ultrasound to Monitor Tumor Onset and Growth in Genetically Engineered Pancreatic Cancer Models. Journal of visualized experiments : JoVE. 2018(134). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rojas JD, Papadopoulou V, Czernuszewicz TJ, Rajamahendiran RM, Chytil A, Chiang YC, et al. Ultrasound Measurement of Vascular Density to Evaluate Response to Anti-Angiogenic Therapy in Renal Cell Carcinoma. IEEE transactions on bio-medical engineering. 2019;66(3):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasoji SK, Rivera JN, Gessner RC, Chang SX, Dayton PA. Early Assessment of Tumor Response to Radiation Therapy using High-Resolution Quantitative Microvascular Ultrasound Imaging. Theranostics. 2018;8(1):156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J, Wang H, Zhang H, Lutz AM, Tian L, Hristov D, et al. VEGFR2-Targeted Three-Dimensional Ultrasound Imaging Can Predict Responses to Antiangiogenic Therapy in Preclinical Models of Colon Cancer. Cancer research. 2016;76(14):4081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fix SM, Papadopoulou V, Velds H, Kasoji SK, Rivera JN, Borden MA, et al. Oxygen microbubbles improve radiotherapy tumor control in a rat fibrosarcoma model - A preliminary study. PloS one. 2018;13(4):e0195667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song KH, Trudeau T, Kar A, Borden MA, Gutierrez-Hartmann A. Ultrasound-mediated delivery of siESE complexed with microbubbles attenuates HER2+/− cell line proliferation and tumor growth in rodent models of breast cancer. Nanotheranostics. 2019;3(2):212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xing L, Shi Q, Zheng K, Shen M, Ma J, Li F, et al. Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19–9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer. Theranostics. 2016;6(10):1573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tran WT, Sannachi L, Papanicolau N, Tadayyon H, Al Mahrouki A, El Kaffas A, et al. Quantitative ultrasound imaging of therapy response in bladder cancer in vivo. Oncoscience. 2016;3(3–4):122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rowles JL 3rd, Han A, Miller RJ, Kelly JR, Applegate CC, Wallig MA, et al. Low fat but not soy protein isolate was an effective intervention to reduce nonalcoholic fatty liver disease progression in C57BL/6J mice: monitored by a novel quantitative ultrasound (QUS) method. Nutr Res. 2019;63:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elyas E, Papaevangelou E, Alles EJ, Erler JT, Cox TR, Robinson SP, et al. Correlation of Ultrasound Shear Wave Elastography with Pathological Analysis in a Xenografic Tumour Model. Scientific reports. 2017;7(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riegler J, Labyed Y, Rosenzweig S, Javinal V, Castiglioni A, Dominguez CX, et al. Tumor Elastography and Its Association with Collagen and the Tumor Microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(18):4455–67. [DOI] [PubMed] [Google Scholar]
- 92.Ahmed R, Ye J, Gerber SA, Linehan DC, Doyley MM. Preclinical Imaging Using Single Track Location Shear Wave Elastography: Monitoring the Progression of Murine Pancreatic Tumor Liver Metastasis In Vivo. IEEE transactions on medical imaging. 2020;39(7):2426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taruttis A, van Dam GM, Ntziachristos V. Mesoscopic and macroscopic optoacoustic imaging of cancer. Cancer research. 2015;75(8):1548–59. [DOI] [PubMed] [Google Scholar]
- 94.Zackrisson S, van de Ven S, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer research. 2014;74(4):979–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNally LR, Mezera M, Morgan DE, Frederick PJ, Yang ES, Eltoum IE, et al. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(14):3432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weber J, Beard PC, Bohndiek SE. Contrast agents for molecular photoacoustic imaging. Nat Methods. 2016;13(8):639–50. [DOI] [PubMed] [Google Scholar]
- 97.O’Kelly D, Zhou H, Mason RP. Tomographic breathing detection: a method to noninvasively assess in situ respiratory dynamics. Journal of biomedical optics. 2018;23(5):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cox B, Laufer JG, Arridge SR, Beard PC. Quantitative spectroscopic photoacoustic imaging: a review. Journal of biomedical optics. 2012;17(6):061202. [DOI] [PubMed] [Google Scholar]
- 99.O’Kelly D, Guo Y, Mason RP. Evaluating online filtering algorithms to enhance dynamic multispectral optoacoustic tomography. Photoacoustics. 2020;19:100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohndiek SE, Sasportas LS, Machtaler S, Jokerst JV, Hori S, Gambhir SS. Photoacoustic Tomography Detects Early Vessel Regression and Normalization During Ovarian Tumor Response to the Antiangiogenic Therapy Trebananib. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(12):1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dey S, Kumari S, Kalainayakan SP, Campbell J, 3rd, Ghosh P, Zhou H, et al. The vascular disrupting agent combretastatin A-4 phosphate causes prolonged elevation of proteins involved in heme flux and function in resistant tumor cells. Oncotarget. 2018;9(3):4090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tomaszewski MR, Gehrung M, Joseph J, Quiros-Gonzalez I, Disselhorst JA, Bohndiek SE. Oxygen-Enhanced and Dynamic Contrast-Enhanced Optoacoustic Tomography Provide Surrogate Biomarkers of Tumor Vascular Function, Hypoxia, and Necrosis. Cancer research. 2018;78(20):5980–91. [DOI] [PubMed] [Google Scholar]
- 103.Rich LJ, Miller A, Singh AK, Seshadri M. Photoacoustic Imaging as an Early Biomarker of Radio Therapeutic Efficacy in Head and Neck Cancer. Theranostics. 2018;8(8):2064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hupple CW, Morscher S, Burton NC, Pagel MD, McNally LR, Cardenas-Rodriguez J. A light-fluence-independent method for the quantitative analysis of dynamic contrast-enhanced multispectral optoacoustic tomography (DCE MSOT). Photoacoustics. 2018;10:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou HL, Campbell J, O’Kelly D, Gerberich J, Mason R. Exploring a fluorescent blood pool agent in photoacoustic imaging. Journal of Nuclear Medicine. 2016;57(supplement 2):1214.26985055 [Google Scholar]
- 106.Hudson SV, Huang JS, Yin W, Albeituni S, Rush J, Khanal A, et al. Targeted noninvasive imaging of EGFR-expressing orthotopic pancreatic cancer using multispectral optoacoustic tomography. Cancer research. 2014;74(21):6271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu D, Huang L, Jiang MS, Jiang H. Contrast agents for photoacoustic and thermoacoustic imaging: a review. Int J Mol Sci. 2014;15(12):23616–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L, Zemp RJ, Lungu G, Stoica G, Wang LV. Photoacoustic imaging of lacZ gene expression in vivo. Journal of biomedical optics. 2007;12(2):020504. [DOI] [PubMed] [Google Scholar]
- 109.Wang LV, Yao J. A practical guide to photoacoustic tomography in the life sciences. Nat Methods. 2016;13(8):627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan CM, Jing X, Pike LA, Zhou Q, Lim DJ, Sams SB, et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(13):3580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boute N, Lowe P, Berger S, Malissard M, Robert A, Tesar M. NanoLuc Luciferase - A Multifunctional Tool for High Throughput Antibody Screening. Front Pharmacol. 2016;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duong MT, Collinson-Pautz MR, Morschl E, Lu A, Szymanski SP, Zhang M, et al. Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches. Mol Ther Oncolytics. 2019;12:124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kleinovink JW, Mezzanotte L, Zambito G, Fransen MF, Cruz LJ, Verbeek JS, et al. A Dual-Color Bioluminescence Reporter Mouse for Simultaneous in vivo Imaging of T Cell Localization and Function. Front Immunol. 2018;9:3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Contreras-Zarate MJ, Ormond DR, Gillen AE, Hanna C, Day NL, Serkova NJ, et al. Development of Novel Patient-Derived Xenografts from Breast Cancer Brain Metastases. Frontiers in oncology. 2017;7:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Godinat A, Bazhin AA, Goun EA. Bioorthogonal chemistry in bioluminescence imaging. Drug Discov Today. 2018;23(9):1584–90. [DOI] [PubMed] [Google Scholar]
- 116.Winn BA, Devkota L, Kuch B, MacDonough MT, Strecker TE, Wang Y, et al. Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted toward Tumor-Associated Hypoxia. J Nat Prod. 2020;83(4):937–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chu J, Oh Y, Sens A, Ataie N, Dana H, Macklin JJ, et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nature biotechnology. 2016;34(7):760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hananya N, Shabat D. Recent Advances and Challenges in Luminescent Imaging: Bright Outlook for Chemiluminescence of Dioxetanes in Water. ACS Cent Sci. 2019;5(6):949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L, Mason RP. Imaging beta-galactosidase activity in human tumor xenografts and transgenic mice using a chemiluminescent substrate. PloS one. 2010;5(8):e12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.An W, Ryan LS, Reeves AG, Bruemmer KJ, Mouhaffel L, Gerberich JL, et al. A Chemiluminescent Probe for HNO Quantification and Real-Time Monitoring in Living Cells. Angew Chem Int Ed Engl. 2019;58(5):1361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao J, Lopez R, Thacker JM, Moon JY, Jiang C, Morris SN, et al. Chemiluminescent Probes for Imaging H2S in Living Animals. Chem Sci. 2015;6(3):1979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lewis MA, Kodibagkar VD, Oz OK, Mason RP. On the potential for molecular imaging with Cerenkov luminescence. Optics letters. 2010;35(23):3889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pogue BW, Wilson BC. Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine. Journal of biomedical optics. 2018;23(12):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zeng LJ, Zou LL, Yu HJ, He XY, Cao HQ, Zhang ZW, et al. Treatment of Malignant Brain Tumor by Tumor-Triggered Programmed Wormlike Micelles with Precise Targeting and Deep Penetration. Advanced functional materials. 2016;26(23):4201–12. [Google Scholar]
- 125.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnology. 2004;22(12):1567–72. [DOI] [PubMed] [Google Scholar]
- 126.Zhong Y, Ma Z, Zhu S, Yue J, Zhang M, Antaris AL, et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nature communications. 2017;8(1):737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ran C, Moore A. Spectral unmixing imaging of wavelength-responsive fluorescent probes: an application for the real-time report of amyloid Beta species in Alzheimer’s disease. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2012;14(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. The lancet oncology. 2019;20(7):e354–e67. [DOI] [PubMed] [Google Scholar]
- 129.Ye H, Adane B, Khan N, Alexeev E, Nusbacher N, Minhajuddin M, et al. Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells. Cancer cell. 2018;34(4):659–73 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, et al. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer cell. 2017;32(3):392. [DOI] [PubMed] [Google Scholar]
- 131.Schlaepfer IR, Glode LM, Hitz CA, Pac CT, Boyle KE, Maroni P, et al. Inhibition of Lipid Oxidation Increases Glucose Metabolism and Enhances 2-Deoxy-2-[(18)F]Fluoro-D-Glucose Uptake in Prostate Cancer Mouse Xenografts. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2015;17(4):529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McKinley ET, Bugaj JE, Zhao P, Guleryuz S, Mantis C, Gokhale PC, et al. 18FDG-PET predicts pharmacodynamic response to OSI-906, a dual IGF-1R/IR inhibitor, in preclinical mouse models of lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(10):3332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Verwer EE, Kavanagh TR, Mischler WJ, Feng Y, Takahashi K, Wang S, et al. [(18)F]Fluorocholine and [(18)F]Fluoroacetate PET as Imaging Biomarkers to Assess Phosphatidylcholine and Mitochondrial Metabolism in Preclinical Models of TSC and LAM. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(23):5925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Witney TH, Alam IS, Turton DR, Smith G, Carroll L, Brickute D, et al. Evaluation of deuterated 18F- and 11C-labeled choline analogs for cancer detection by positron emission tomography. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(4):1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Z, Zha Z, Li G, Lieberman BP, Choi SR, Ploessl K, et al. [(18)F](2S,4S)-4-(3-Fluoropropyl)glutamine as a tumor imaging agent. Molecular pharmaceutics. 2014;11(11):3852–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ploessl K, Wang L, Lieberman BP, Qu W, Kung HF. Comparative Evaluation of 18F-Labeled Glutamic Acid and Glutamine as Tumor Metabolic Imaging Agents. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(10):1616–24. [DOI] [PubMed] [Google Scholar]
- 137.Qu W, Oya S, Lieberman BP, Ploessl K, Wang L, Wise DR, et al. Preparation and characterization of L-[5–11C]-glutamine for metabolic imaging of tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(1):98–105. [DOI] [PubMed] [Google Scholar]
- 138.Holzgreve A, Brendel M, Gu S, Carlsen J, Mille E, Boning G, et al. Monitoring of Tumor Growth with [(18)F]-FET PET in a Mouse Model of Glioblastoma: SUV Measurements and Volumetric Approaches. Front Neurosci. 2016;10:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morelli MP, Tentler JJ, Kulikowski GN, Tan AC, Bradshaw-Pierce EL, Pitts TM, et al. Preclinical activity of the rational combination of selumetinib (AZD6244) in combination with vorinostat in KRAS-mutant colorectal cancer models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(4):1051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Honndorf VS, Schmidt H, Wiehr S, Wehrl HF, Quintanilla-Martinez L, Stahlschmidt A, et al. The Synergistic Effect of Selumetinib/Docetaxel Combination Therapy Monitored by [(18) F]FDG/[(18) F]FLT PET and Diffusion-Weighted Magnetic Resonance Imaging in a Colorectal Tumor Xenograft Model. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016;18(2):249–57. [DOI] [PubMed] [Google Scholar]
- 141.Zhang CC, Yan Z, Li W, Kuszpit K, Painter CL, Zhang Q, et al. [(18)F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(5):1303–12. [DOI] [PubMed] [Google Scholar]
- 142.Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539(7627):112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou H, Chiguru S, Hallac RR, Yang D, Hao G, Peschke P, et al. Examining correlations of oxygen sensitive MRI (BOLD/TOLD) with [(18)F]FMISO PET in rat prostate tumors. Am J Nucl Med Mol Imaging. 2019;9(2):156–67. [PMC free article] [PubMed] [Google Scholar]
- 144.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers [(1)(8)F]HX4, [(1)(8)F]FAZA, and [(1)(8)F]FMISO in a preclinical tumor model. International journal of radiation oncology, biology, physics. 2015;91(2):351–9. [DOI] [PubMed] [Google Scholar]
- 145.Melsens E, De Vlieghere E, Descamps B, Vanhove C, Kersemans K, De Vos F, et al. Hypoxia imaging with (18)F-FAZA PET/CT predicts radiotherapy response in esophageal adenocarcinoma xenografts. Radiation oncology. 2018;13(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Levi J, Lam T, Goth SR, Yaghoubi S, Bates J, Ren G, et al. Imaging of Activated T Cells as an Early Predictor of Immune Response to Anti-PD-1 Therapy. Cancer research. 2019;79(13):3455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Beckford Vera DR, Smith CC, Bixby LM, Glatt DM, Dunn SS, Saito R, et al. Immuno-PET imaging of tumor-infiltrating lymphocytes using zirconium-89 radiolabeled anti-CD3 antibody in immune-competent mice bearing syngeneic tumors. PloS one. 2018;13(3):e0193832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Srideshikan SM, Brooks J, Zuro D, Kumar B, Sanchez J, Echavarria Parra L, et al. ImmunoPET, [(64)Cu]Cu-DOTA-Anti-CD33 PET-CT, Imaging of an AML Xenograft Model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(24):7463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paquette M, Ouellet R, Archambault M, Croteau E, Lecomte R, Benard F . [18F]-fluoroestradiol quantitative PET imaging to differentiate ER+ and ERalpha-knockdown breast tumors in mice. Nuclear medicine and biology. 2012;39(1):57–64. [DOI] [PubMed] [Google Scholar]
- 150.Paquette M, Phoenix S, Ouellet R, Langlois R, van Lier JE, Turcotte EE, et al. Assessment of the novel estrogen receptor PET tracer 4-fluoro-11beta-methoxy-16alpha-[(18)F]fluoroestradiol (4FMFES) by PET imaging in a breast cancer murine model. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2013;15(5):625–32. [DOI] [PubMed] [Google Scholar]
- 151.Fowler AM, Chan SR, Sharp TL, Fettig NM, Zhou D, Dence CS, et al. Small-animal PET of steroid hormone receptors predicts tumor response to endocrine therapy using a preclinical model of breast cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(7):1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsujikawa T, Yoshida Y, Kiyono Y, Kurokawa T, Kudo T, Fujibayashi Y, et al. Functional oestrogen receptor alpha imaging in endometrial carcinoma using 16alpha-[(1)(8)F]fluoro-17beta-oestradiol PET. European journal of nuclear medicine and molecular imaging. 2011;38(1):37–45. [DOI] [PubMed] [Google Scholar]
- 153.Khayum MA, Doorduin J, Antunes IF, Kwizera C, Zijlma R, den Boer JA, et al. In vivo imaging of brain androgen receptors in rats: a [(18)F]FDHT PET study. Nuclear medicine and biology. 2015;42(6):561–9. [DOI] [PubMed] [Google Scholar]
- 154.Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, et al. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(5):823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang J, Xu J, Gonzalez R, Lindner T, Kratochwil C, Miao Y. (68)Ga-DOTA-GGNle-CycMSHhex targets the melanocortin-1 receptor for melanoma imaging. Science translational medicine. 2018;10(466). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Banerjee SR, Minn I, Kumar V, Josefsson A, Lisok A, Brummet M, et al. Preclinical Evaluation of 203/212Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. Journal of Nuclear Medicine. 2020;61(1):80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu EY, Bishop M, Zheng B, Ferguson RM, Khandhar AP, Kemp SJ, et al. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano letters. 2017;17(3):1648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu X, Li J, Peng P, Hosseini Nassab N, Smith BR. Quantitative Drug Release Monitoring in Tumors of Living Subjects by Magnetic Particle Imaging Nanocomposite. Nano letters. 2019;19(10):6725–33. [DOI] [PubMed] [Google Scholar]
- 159.Flood AB WV, Schreiber W, Williams BB, Gallez B, Swartz HM. . Guidance to Transfer ‘Bench-Ready’ Medical Technology into Usual Clinical Practice: Case Study - Sensors and Spectrometer Used in EPR Oximetry. . In: Thews O LJ, Harrison DK, editor. Oxygen Transport to Tissue Xl Advances in Experimental Medicine and Biology. 1072: Springer International Publishing Ag; 2018. p. 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goda F, O’Hara JA, Rhodes ES, Liu KJ, Dunn JF, Bacic G, et al. Changes of oxygen tension in experimental tumors after a single dose of X-ray irradiation. Cancer research. 1995;55(11):2249–52. [PubMed] [Google Scholar]
- 161.Epel B, Maggio MC, Barth ED, Miller RC, Pelizzari CA, Krzykawska-Serda M, et al. Oxygen-Guided Radiation Therapy. International journal of radiation oncology, biology, physics. 2019;103(4):977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jordan BF, Gregoire V, Demeure RJ, Sonveaux P, Feron O, O’Hara J, et al. Insulin increases the sensitivity of tumors to irradiation: involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer research. 2002;62(12):3555–61. [PubMed] [Google Scholar]
- 163.Chiu TD, Arai TJ, Campbell Iii J, Jiang SB, Mason RP, Stojadinovic S. MR-CBCT image-guided system for radiotherapy of orthotopic rat prostate tumors. PloS one. 2018;13(5):e0198065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghita M, Brown KH, Kelada OJ, Graves EE, Butterworth KT. Integrating Small Animal Irradiators withFunctional Imaging for Advanced Preclinical Radiotherapy Research. Cancers. 2019;11(2):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parodi K, Assmann W, Belka C, Bortfeldt J, Clevert DA, Dedes G, et al. Towards a novel small animal proton irradiation platform: the SIRMIO project. Acta oncologica. 2019;58(10):1470–5. [DOI] [PubMed] [Google Scholar]
- 166.Oweida AJ, Bhatia S, Van Court B, Darragh L, Serkova N, Karam SD. Intramucosal Inoculation of Squamous Cell Carcinoma Cells in Mice for Tumor Immune Profiling and Treatment Response Assessment. Journal of visualized experiments : JoVE. 2019(146). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bhatia S, Oweida A, Lennon S, Darragh LB, Milner D, Phan AV, et al. Inhibition of EphB4-Ephrin-B2 Signaling Reprograms the Tumor Immune Microenvironment in Head and Neck Cancers. Cancer research. 2019;79(10):2722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Keenan KE, Biller JR, Delfino JG, Boss MA, Does MD, Evelhoch JL, et al. Recommendations towards standards for quantitative MRI (qMRI) and outstanding needs. Journal of magnetic resonance imaging : JMRI. 2019;49(7):e26–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Turco S, Tardy I, Frinking P, Wijkstra H, Mischi M. Quantitative ultrasound molecular imaging by modeling the binding kinetics of targeted contrast agent. Physics in medicine and biology. 2017;62(6):2449–64. [DOI] [PubMed] [Google Scholar]
- 170.Hormuth DA 2nd, Jarrett AM, Lima E, McKenna MT, Fuentes DT, Yankeelov TE. Mechanism-Based Modeling of Tumor Growth and Treatment Response Constrained by Multiparametric Imaging Data. JCO Clin Cancer Inform. 2019;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pitman KE, Bakke KM, Kristian A, Malinen E. Ultra-early changes in vascular parameters from dynamic contrast enhanced MRI of breast cancer xenografts following systemic therapy with doxorubicin and liver X receptor agonist. Cancer imaging : the official publication of the International Cancer Imaging Society. 2019;19(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cao J, Pickup S, Clendenin C, Blouw B, Choi H, Kang D, et al. Dynamic Contrast-enhanced MRI Detects Responses to Stroma-directed Therapy in Mouse Models of Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2019;25(7):2314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, et al. Dual CTLA-4 and PD-L1 Blockade Inhibits Tumor Growth and Liver Metastasis in a Highly Aggressive Orthotopic Mouse Model of Colon Cancer. Neoplasia. 2019;21(9):932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Scott AJ, Arcaroli JJ, Bagby SM, Yahn R, Huber KM, Serkova NJ, et al. Cabozantinib Exhibits Potent Antitumor Activity in Colorectal Cancer Patient-Derived Tumor Xenograft Models via Autophagy and Signaling Mechanisms. Molecular cancer therapeutics. 2018;17(10):2112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dhar D, Raina K, Kant R, Wempe MF, Serkova NJ, Agarwal C, et al. Bitter melon juice-intake modulates glucose metabolism and lactate efflux in tumors in its efficacy against pancreatic cancer. Carcinogenesis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Niu H, Strecker TE, Gerberich JL, Campbell JW 3rd, Saha D, Mondal D, et al. Structure Guided Design, Synthesis, and Biological Evaluation of Novel Benzosuberene Analogues as Inhibitors of Tubulin Polymerization. Journal of medicinal chemistry. 2019;62(11):5594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bothwell KD, Folaron M, Seshadri M. Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers (Basel). 2016;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Theek B, Opacic T, Magnuska Z, Lammers T, Kiessling F. Radiomic analysis of contrast-enhanced ultrasound data. Scientific reports. 2018;8(1):11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moiseev A, Snopova L, Kuznetsov S, Buyanova N, Elagin V, Sirotkina M, et al. Pixel classification method in optical coherence tomography for tumor segmentation and its complementary usage with OCT microangiography. Journal of biophotonics. 2018;11(4):e201700072. [DOI] [PubMed] [Google Scholar]
- 180.Zinn PO, Singh SK, Kotrotsou A, Hassan I, Thomas G, Luedi MM, et al. A Coclinical Radiogenomic Validation Study: Conserved Magnetic Resonance Radiomic Appearance of Periostin-Expressing Glioblastoma in Patients and Xenograft Models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(24):6288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ellingson BM. On the promise of artificial intelligence for standardizing radiographic response assessment in gliomas. Neuro-oncology. 2019;21(11):1346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cameron A, Khalvati F, Haider MA, Wong A. MAPS: A Quantitative Radiomics Approach for Prostate Cancer Detection. IEEE transactions on bio-medical engineering. 2016;63(6):1145–56. [DOI] [PubMed] [Google Scholar]
- 183.Dragani TA, Castells A, Kulasingam V, Diamandis EP, Earl H, Iams WT, et al. Major milestones in translational oncology. BMC Med. 2016;14(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8. [PMC free article] [PubMed] [Google Scholar]
- 185.Tarone L, Barutello G, Iussich S, Giacobino D, Quaglino E, Buracco P, et al. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2019;68(11):1839–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abma E, Stock E, De Spiegelaere W, Van Brantegem L, Vanderperren K, Ni Y, et al. Power Doppler ultrasound and contrast-enhanced ultrasound demonstrate non-invasive tumour vascular response to anti-vascular therapy in canine cancer patients. Scientific Reports. 2019;9(1):9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Spriet M, Willcox JL, Culp WTN. Role of Positron Emission Tomography in Imaging of Non-neurologic Disorders of the Head, Neck, and Teeth in Veterinary Medicine. Front Vet Sci. 2019;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ghaghada KB, Sato AF, Starosolski ZA, Berg J, Vail DM. Computed Tomography Imaging of Solid Tumors Using a Liposomal-Iodine Contrast Agent in Companion Dogs with Naturally Occurring Cancer. PloS one. 2016;11(3):e0152718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Naik S, Galyon GD, Jenks NJ, Steele MB, Miller AC, Allstadt SD, et al. Comparative Oncology Evaluation of Intravenous Recombinant Oncolytic Vesicular Stomatitis Virus Therapy in Spontaneous Canine Cancer. Molecular cancer therapeutics. 2018;17(1):316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.LeBlanc AK. Cancer and comparative imaging. ILAR J. 2014;55(1):164–8. [DOI] [PubMed] [Google Scholar]
- 191.Mezzanotte L, Aswendt M, Tennstaedt A, Hoeben R, Hoehn M, Lowik C. Evaluating reporter genes of different luciferases for optimized in vivo bioluminescence imaging of transplanted neural stem cells in the brain. Contrast media & molecular imaging. 2013;8(6):505–13. [DOI] [PubMed] [Google Scholar]
- 192.Banzato T, Bernardini M, Cherubini GB, Zotti A. A methodological approach for deep learning to distinguish between meningiomas and gliomas on canine MR-images. BMC Vet Res. 2018;14(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]


