Abstract
Background:
Preclinical rodent studies have demonstrated reduced cocaine taking after administration of glucagon-like peptide 1 (GLP-1) analogues. We investigated effects of a GLP-1 analogue (exenatide) on behavioral and subjective effects of cocaine in individuals with cocaine use disorder (CUD).
Methods:
Non-treatment-seeking CUD subjects underwent two human laboratory cocaine self-administration test sessions following an acute 3-hour pre-treatment with exenatide (5 mcg; subcutaneously) or placebo. Primary outcomes consisted of infusions of cocaine and visual analog scale self-ratings of euphoria and wanting cocaine. Secondary outcomes consisted of pertinent hormone levels (GLP-1, insulin, and amylin).
Results:
Thirteen individuals completed the study. Acute pretreatment with exenatide versus placebo did not change cocaine infusions (8.5 ± 1.2 vs. 9.1 ± 1.2; p=0.39), self-reported euphoria (4.4 ± 0.8 vs. 4.1 ± 0.8; p=0.21), or wanting of cocaine (5.6 ± 0.9 vs. 5.4 ± 0.9; p=0.46). Exenatide vs. placebo reduced levels of GLP-1 (p = 0.03) and insulin (p = 0.02). Self-administered cocaine also reduced levels of GLP-1 (p < 0.0001), insulin (p < 0.0001), and amylin (p < 0.0001).
Conclusions:
We did not find evidence that low dose exenatide alters cocaine self-administration or the subjective effects of cocaine in people with CUD. Limitations such as single acute rather than chronic pre-treatment, as well as evaluation of only one dose, preclude drawing firm conclusions about the efficacy of exenatide. Exenatide and cocaine independently reduced levels of GLP-1 and insulin, while cocaine also reduced levels of amylin.
Keywords: cocaine use disorder, substance-related disorders, addictive behaviors, GLP-1, exenatide, cocaine self-administration
1. Introduction
According to the National Survey on Drug Use and Health, there are 2.2 million people in North America who regularly use cocaine and 1 million individuals with cocaine use disorder (CUD) (SAMHSA, 2018). The lack of effective FDA-approved medications for CUD despite nearly three decades of research on primarily monoaminergic agents suggests the need for new approaches and examination of novel medication targets (Amato et al., 2011; Shorter and Kosten, 2011). Recent insights into the neurobiology of substance use disorders suggest that peripheral metabolic factors that act centrally like glucagon-like peptide 1 (GLP-1) are important mediators of drug reward (Hernandez and Schmidt, 2019; Kenny, 2011; Volkow et al., 2013).
GLP-1 is an incretin hormone secreted from the intestinal L-cells in response to food ingestion (Creutzfeldt, 1979; Dupre et al., 1973). This hormone lowers glucose level by stimulating insulin release from pancreatic β-cells in a glucose-depended fashion (Hayes et al., 2014; Kreymann et al., 1987; Müller et al., 2019), slows gastric emptying, and promotes satiation with food intake. Thus, GLP-1 analogs were developed to improve glycemic control in patients with type-2 diabetes mellitus (T2DM) and were subsequently found to increase satiety in patients with obesity. Exendin-4 shares considerable sequence homology with GLP-1 (Chakraborti, 2010). Exenatide (Byetta®) is a synthetic form of Exendin-4 (van Bloemendaal et al., 2014), currently FDA approved for T2DM.
Clinical trials of exenatide for hyperglycemia/insulin resistance found unexpected “side effect” of exenatide, weight loss (Amori et al., 2007; DeFronzo et al., 2005; van Bloemendaal et al., 2014). Reductions in body mass index (BMI) were found to be independent of effects on glucose (Dushay et al., 2012; Kelly et al., 2013).
In addition to “peripheral” actions mediated by vagal projections to the brain, GLP-1 is synthesized in the brain (primarily by neurons of the nucleus tractus solitarius; NTS) where it functions as a neuromodulator (Hayes et al., 2014; Holst, 2007; van Bloemendaal et al., 2014). GLP-1 receptors are expressed in multiple nuclei, including the NTS, hypothalamus, ventral tegmental area and nucleus accumbens, regions with important roles in energy homeostasis, appetite regulation, and food and drug reward (Baggio and Drucker, 2014; Chambers et al., 2003).
1.1. GLP-1 Receptor Agonism: A Pharmacotherapeutic Strategy for Cocaine Use Disorder?
Recent preclinical research suggests a potentially important role for GLP-1 in drug-mediated behaviors. For example, an emerging literature indicates that exendin–4 attenuates cocaine-induced condition place preference (CPP), cocaine self-administration, and cocaine-primed as well as cue-primed reinstatement of drug seeking during abstinence (Egecioglu et al., 2013; Erreger et al., 2012; Graham et al., 2013; Harasta et al., 2015; Hernandez et al., 2018; Hernandez et al., 2019; Schmidt et al., 2016; Sorensen et al., 2015). Interestingly, doses of exendin-4 have been identified in cocaine-experienced rats that selectively reduce drug-taking and drug-seeking and do not produce adverse feeding and malaise-like effects (i.e., nausea-like behavior in rodents as measured by their pica response) (Kanoski et al., 2012), notable side effects of GLP-1 receptor agonists in humans and rodents (Hernandez et al., 2018). In addition to preclinical studies showing the effects of GLP-1 receptor agonists on cocaine-mediated behaviors, there are both human (Bouhlal et al., 2017) and rodent (You et al., 2019) studies showing changes in the expression of different metabolic factors, including GLP-1, in response to cocaine administration.
Taken together, these findings support a rationale for human studies designed to examine whether the behavioral and/or subjective effects of cocaine are altered by exenatide in individuals with CUD. We hypothesized that exenatide would attenuate cocaine taking and the subjective effects of cocaine. We also explored how cocaine, in the presence or absence of exenatide, influenced plasma levels of peripheral metabolic hormones of relevance to food intake and glycemic control, including GLP-1, insulin, and amylin.
2. Material and methods
2.1. Participants
CUD participants were recruited through online and newspaper advertisements and word-of-mouth referrals. After an initial telephone and in-person screening, eligible individuals were admitted to the Clinical Neuroscience Research Unit (CNRU) at the Connecticut Mental Health Center (CMHC). To participate, non-treatment-seeking individuals needed to be in good health as assessed by physical examination, electrocardiogram, and laboratory tests. Other inclusion criteria were: 30 to 55 years old, self-reported use of intravenous or smoked (crack/freebase) cocaine in excess of quantities used in the current study, detection of urine benzoylecgonine upon screening, and meeting DSM-5 criteria for moderate to severe CUD.
Candidates were ineligible if they had less than a year of CUD, a lifetime history of a primary DSM-5 major psychiatric diagnosis unrelated to cocaine use (as ascertained with the Structured Clinical Interview for the DSM-5 (First et al., 2015)), or a history of significant medical or neurological illnesses, a fasting glucose level <70 mg/dl, current use of psychotropic and/or potentially psychoactive medications, known hypersensitivity to exenatide, a history of or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, and, for females, a positive serum hCG pregnancy test or breastfeeding.
Study was registered on ClinicalTrials.gov and all study-related procedures were approved by the Yale University Human Investigation Committee (HIC) and performed in accordance with the Declaration of Helsinki. Participants enrolled provided voluntary written informed consent.
2.2. Study Design
The study was conducted between December, 2014 and July, 2018. Non-treatment-seeking participants were admitted to the CNRU, an elective 12-bed, locked inpatient clinical research unit, for the duration of their study involvement. Cocaine self-administration sessions were conducted on the Yale Center for Clinical Investigation Hospital Research Unit (HRU), located at Yale-New Haven Hospital (YNHH). The study examined effects of acute administration of exenatide vs. placebo on behavioral (self-administration) and subjective (self-reported euphoria/“high” and desire/“wanting”) effects of cocaine, using a randomized, double-blind, crossover, within-subject design. Participants were paid for participation.
2.3. Experimental Sessions
All participants partook in a cocaine training session prior to cocaine sessions (Figure 1A). Cocaine administration was conducted under the auspices of Investigational New Drug (IND) # 59,121 to the senior author. On the HRU, procedures on experimental training and cocaine sessions were conducted as described previously (Angarita et al., 2010; Matuskey et al., 2012), with the addition of a Yellow Spring Instrument (YSI) glucose analyzer to obtain measures of plasma glucose every 10 minutes.
Figure 1. Study Design, Training, and Cocaine Sessions.
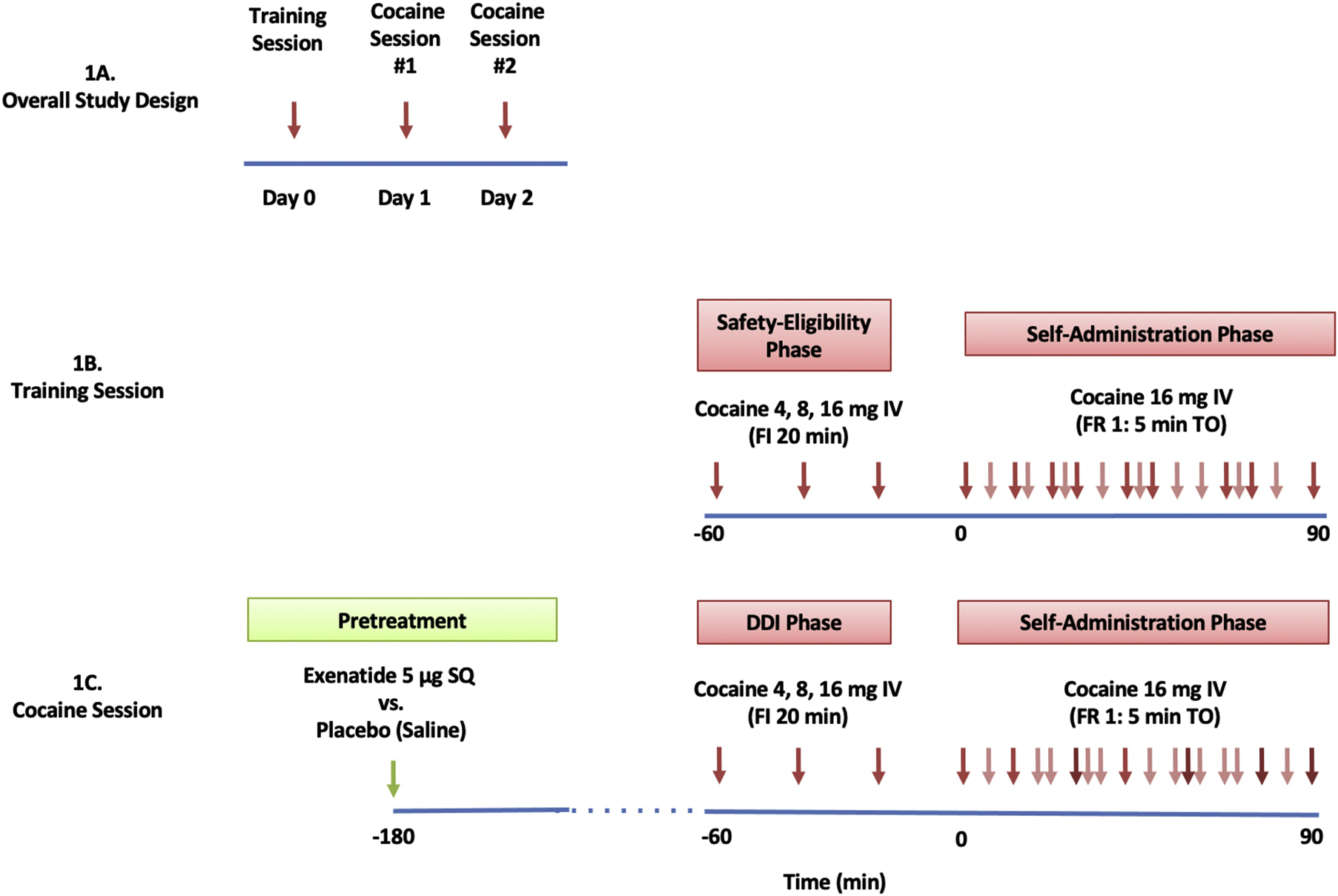
1A: Subjects participated in 3 experimental, human laboratory, sessions. Sessions were separated by > 36 hours.
1B: The training session consisted of a safety-eligibility and a self-administration phase. The safety-eligibility phase consisted of a 60-minute, fixed-order, fixed-interval (FI; 20 min), ascending-dose regimen of three, sequential, intravenous (IV) cocaine boluses (4, 8, and 16 mg/70 kg). The self-administration phase consisted of a 90-minute period of self-regulated (“binge”), IV cocaine administration (16 mg/70kg/infusion) under a fixed-ratio 1, 5-min time-out (FR1:5minTO) schedule.
1C: Cocaine sessions were identical to the training session with the exception that they were preceded by pre-treatment with exenatide or placebo. As a result, the safety eligibility phase is called the Drug-Drug Interaction (DDI) phase.
Training Session:
The cocaine training session was identical to cocaine sessions (Figure 1B) with the exception of exenatide/placebo pre-treatment. The purpose of this session was twofold: 1) to familiarize subjects with experimental study procedures; and, 2) to establish subjects’ tolerability of intravenous cocaine at study doses.
2.3.1. Exenatide / Placebo Administration.
An IND was not requested for exenatide as the intention of the study was not to report to the FDA a clinical study in support of a new indication for exenatide nor to support a significant change in the advertising of exenatide. Participants received a single subcutaneous injection of exenatide (5 μg, 0.02 ml) or placebo (saline, 0.02 ml) 3 hours before cocaine self-administration, on the mornings of the experimental cocaine sessions (Figure 1C). To minimize risks of hypoglycemia, subjects were allowed to drink clear liquids with sugar 2 hours before cocaine administration.
2.3.2. Safety Eligibility and Drug-Drug Interaction (DDI) Phase.
The first phase of each experimental session consisted of a 60-minute period of three sequential nurse-initiated (via PCA pump) single-bolus injections of intravenous cocaine administered every 20 minutes as a fixed-order, ascending dose regimen of 4, 8, and 16 mg/70 kg. This phase was called the safety eligibility phase on the training day as it minimized chances of unsafe cardiovascular responses by establishing the subject’s ability to tolerate cocaine doses (Matuskey et al., 2012). On experimental cocaine sessions, it consisted of a drug-by-drug interaction phase (DDI) focused on potential interactions between exenatide (or placebo) and cocaine. The DDI phase began approximately two hours after study medication.
2.3.3. Self-Administration Phase.
The DDI phase was immediately followed by a 90-minute period of cocaine self-administration, during which intravenous cocaine (16 mg/70kg/bolus) was available to participants under a fixed-ratio 1:5-minute time-out (FR1:5 Min TO) schedule (i.e., a press of the PCA pump button produced a single cocaine infusion, except during ensuing 5-minute factory-set lockout periods) (Figure 1C). Participants were able to receive up to a total of 18 cocaine doses (16 mg/70kg each dose) per each self-administration phase.
2.4. Measures
2.4.1. Primary Outcomes
Behavioral outcomes consisted of number of “infusions” (button presses associated with a cocaine injection) and subjective effects using computerized VAS self-ratings (i.e., euphoria or “high” and craving/desire for cocaine or“wanting cocaine”).
2.4.2. Secondary Outcomes
2.4.2.1. Hormonal:
Blood samples for hormones (GLP-1, insulin, and amylin) were obtained at baseline (prior to cocaine administration or time 1), after the first fixed intravenous bolus of cocaine during DDI phase (time 2), and at the end of the cocaine self-administration session (time 3). Peptide levels were analyzed with commercially available ELISA kits from MiliporeSigma.
2.4.3. Safety Measures
Laboratory measures of pancreatic and renal function (amylase, lipase, creatinine) were collected before and after study procedures. Plasma glucose levels were measured every 10 minutes during cocaine sessions. Clinician and patient versions of the UKU Side Effect Rating Scale (Lindstrom et al., 2001; Lingjaerde et al., 1987) were administered daily.
2.5. Data Analysis
Analyses were performed with SAS, version 9.4 (Cary, NC). Behavioral and subjective effects were analyzed using linear mixed models with drug (exenatide, placebo) as a within-subjects factor. Hormone levels were analyzed using mixed models with drug (exenatide, placebo) and time (baseline, post bolus, post cocaine-self-administration) included as within-subjects factors. The drug-by-time interaction effect was also modeled. Effects of sequence were tested but not significant and dropped for parsimony. In the above mixed models, correlations of repeated measures within individuals were modeled using structured variance-covariance and/or random subject effects. The best-fitting model was selected based on information criteria.
3. Results
3.1. Demographics
Thirty-eight individuals were screened in person (Figure 2). Of these, 16 were found eligible and 13 completed study procedures (Table 1). There were missing data for one subject for hormone levels. Some hormone levels were below standard curves (too low to be detected).
Figure 2. CONSORT Flow Diagram.
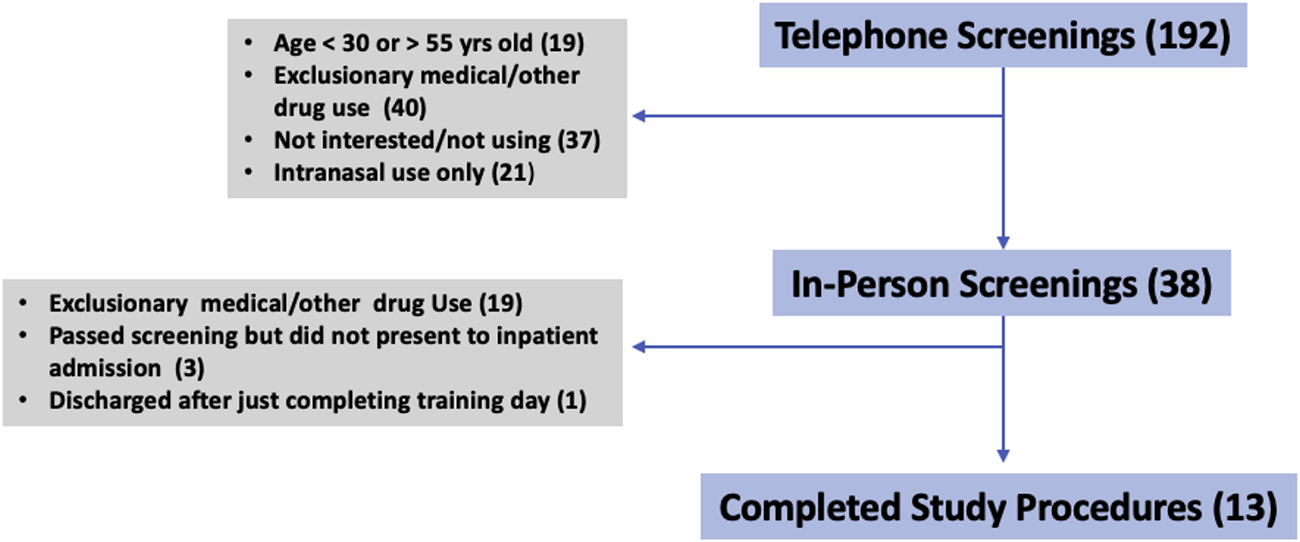
Flow diagram of the progress from telephone screening to completion of study procedures
Table 1.
Demographics
| Number of Participants | 13 |
| Age (mean ± SD) | 45 ± 7 |
| Sex (Female: Male) | 1:12 |
| Race (AA:WH:W) | 10:1:2 |
| Years of education (mean ± SD) | 12 ± 1 |
| Body mass index (mean ± SD) | 28 ± 4 |
| Lifetime years of cocaine use (mean ± SD) | 22 ± 10 |
| Dollar amount of money spent per day of cocaine use (mean ± SD) | 98 ± 51 |
| Days of cocaine use per month (mean ± SD) | 19 ± 9 |
Demographic features of subjects who completed the study
AA = African American; WH = White Hispanic; W = White
3.2. Behavioral Outcomes
Pre-treatment with exenatide (8.5 ± 1.2) did not change numbers of infusions in comparison to pre-treatment with placebo (9.1 ± 1.2) (F(1, 12)=0.76, p=0.39) (Figure 3).
Figure 3. Behavioral Effects.
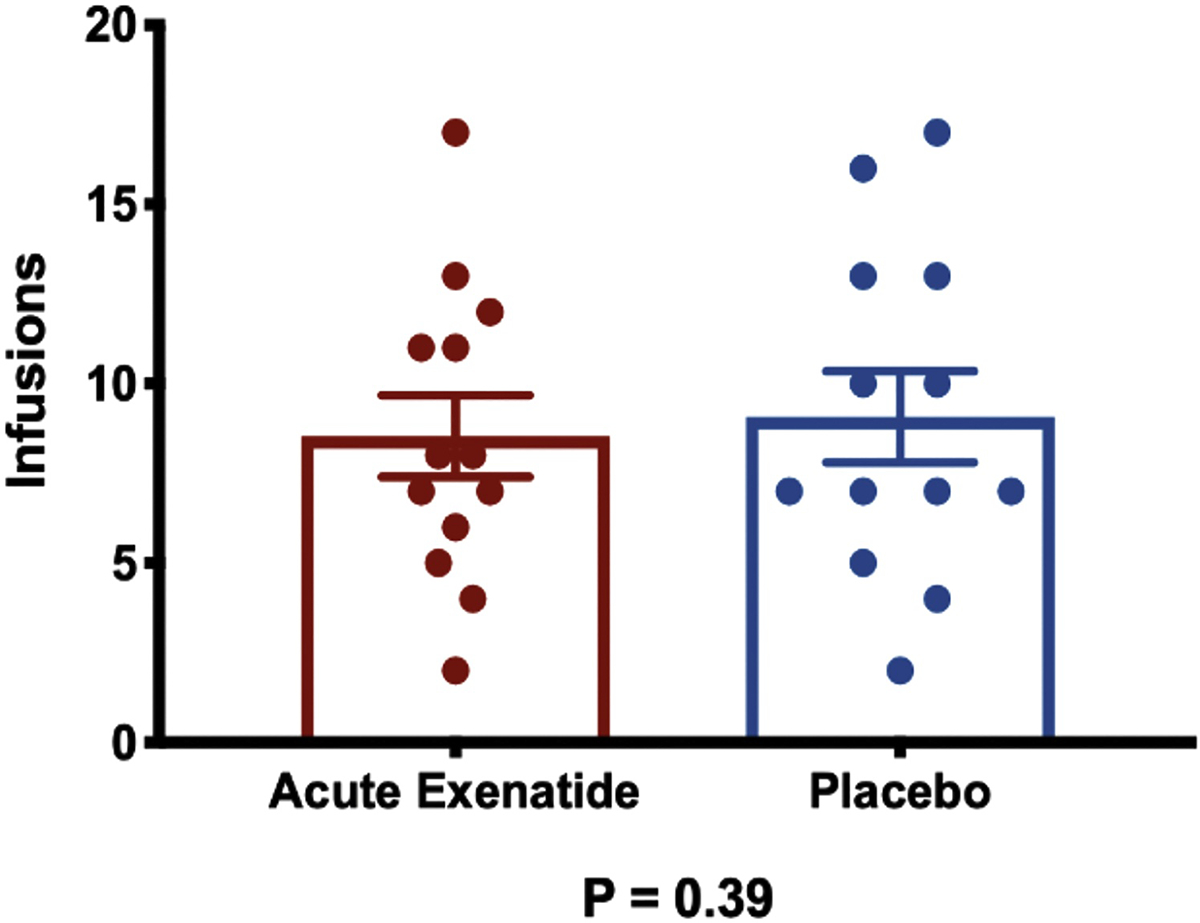
Number of infusions after acute pre-treatment with exenatide vs. placebo. Error bars represent standard errors of the mean.
3.3. Subjective Outcomes
Exenatide did not change primary subjective outcomes of cocaine-induced subjective effects of euphoria/“high” (4.4 ± 0.8 vs. 4.0 ± 0.8; F(1, 12)=1.73, p=0.21) (Figure 4A) nor wanting cocaine (5.5 ± 0.9 vs. 5.4 ± 0.9; F(1, 12)=0.58, p=0.46), compared to placebo (Figure 4B).
Figure 4. Subjective Effects.
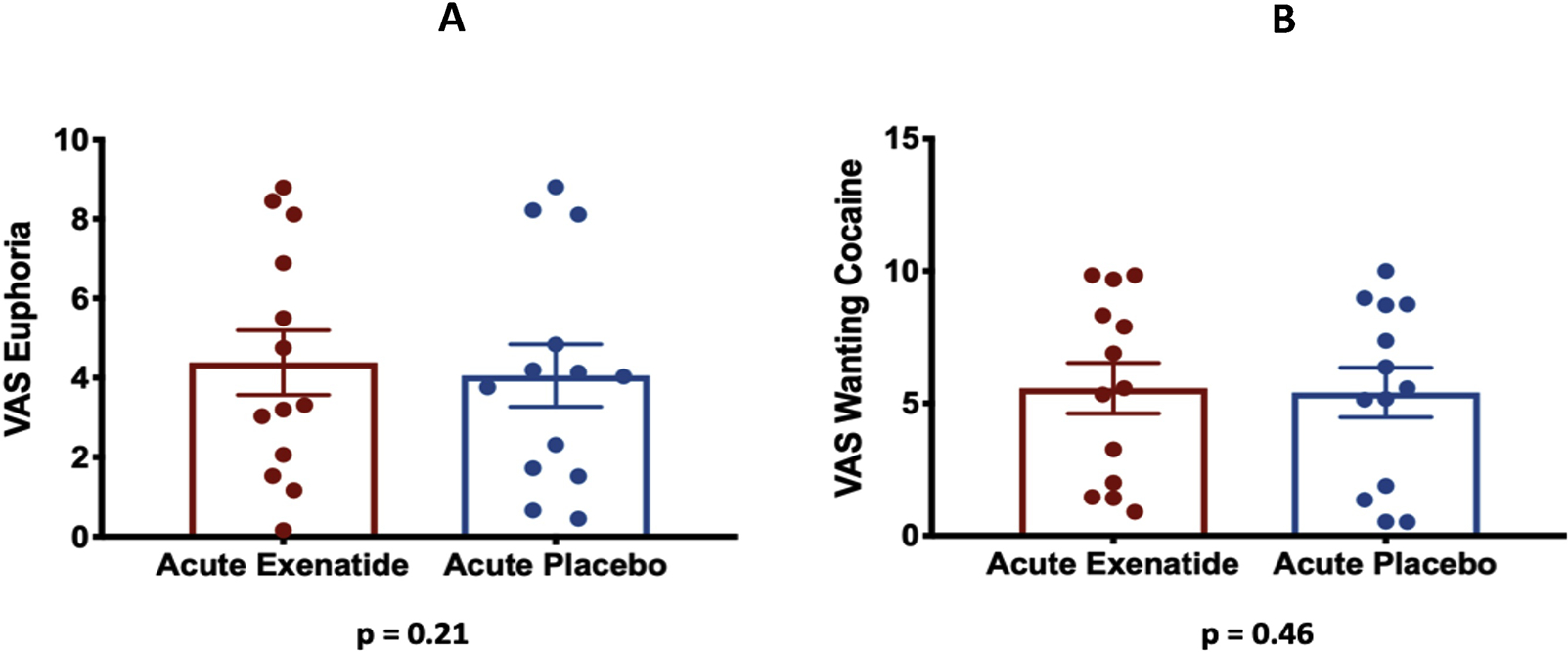
4A: Self-reported VAS scores for euphoria after acute pre-treatment with exenatide vs. placebo. Error bars represent standard errors of the mean.
4B: Self-reported VAS scores for wanting cocaine after acute pre-treatment with exenatide vs. placebo. Error bars represent standard errors of the mean.
3.4. Hormones
Pre-treatment with exenatide had an effect on levels of GLP-1 (F(1, 55)=4.65, p=0.03) and insulin (F(1, 55)=5.69, p=0.02), but not amylin (F(1, 50)=1.17, p=0.28). Both GLP-1 and insulin were lower following exenatide during cocaine self-administration (GLP-1=22.6±3.3 pg/ml; insulin=9.5±1.4 uIU/ml) as compared to placebo during cocaine self-administration (GLP-1=26.6±4.3; insulin=13.8±2.1 uIU/ml). Main effects of time indicated overall decreases related to cocaine administration for GLP-1 (F(2, 55)=18.43, p<0.0001), insulin (F(2, 55)=14.91, p<0.0001), and amylin (F(2, 50)=14.82, p<0.0001) levels. No exenatide-by-time interactions were observed (Supplementary Table 1; Supplementary Figure 1).
3.5. Safety Measures
There were no serious adverse events. Pre-treatment with exenatide (92.1 ± 2.3 mg/dl) did not change glucose levels in comparison to placebo (92 ± 1.8 mg/dl) (F(1, 12)=0.01, p=0.92) (Supplementary Figure 2). The lowest glucose measurement after exenatide was 61 mg/dl (which normalized to 73 mg/dl after 10 minutes), and the lowest glucose measurement after placebo was 74 mg/dl. Exenatide did not produce hypoglycemia in any subject during cocaine sessions.
4. Discussion
The current study is the first to examine effects of a GLP-1 receptor agonist on the behavioral and subjective effects of cocaine in people with CUD. This is also the first human study to examine effects of exenatide and cocaine self-administration on GLP-1, insulin, and amylin levels. In contrast to preclinical studies in rodents, exenatide administered at a clinically relevant (anti-diabetic) dose did not alter cocaine-related behaviors (infusions) or subjective effects (euphoria and wanting cocaine) in people with CUD. While this small pilot study suggests that exenatide has no efficacy in reducing cocaine self-administration in humans, there are multiple limitations that should be considered when interpreting these findings and designing follow-up studies.
The main limitations include using a single low dose of exenatide instead of higher doses and/or subchronic treatment, as higher doses could have led to the potential significant effects and aditionally subchronic treatment may have achieved stable exenatide levels more likely to impact cocaine effects. A second main limitation is the cocaine administration paradigm (i.e., having a drug-drug interaction [DDI] phase before self-administration may not entirely resemble how humans consume cocaine in a natural environment; only one dose of cocaine 16mg/70 kg was studied; and there was no access to “placebo cocaine” similar to preclinical studies examining behavior in response to a vehicle in addition to active cocaine). A third limitation involves the limited generalizability of results as the sample was composed of mainly Black men who heavily used cocaine and who were not seeking treatment. For instance; in other substance use disorders, such as nicotine dependence, motivation to quit has been found to be associated with successful attempts (Caponnetto and Polosa, 2008) and treatment efficacy (Ashare et al., 2012; Hughes et al., 2011). It is possible that the efficacy of GLP-1 receptor agonists in humans with CUD depends on whether individuals are seeking treatment.
A fourth limitation includes inter-species differences between preclinical and clinical data (i.e. levels of medication attained or environmental complexities between preclinical and clinical models (Regier et al., 2020; Roberts et al., 2007).
The half-life of exenatide is 2.4 hours and time to peak plasma concentration is 2.1 hours. Since behavioral testing occurred 3 hours after exenatide infusion, future studies should consider higher doses, subchronic pre-treatment and/or alternative GLP-1 receptor agonists with longer half-lives as a means to fully interrogate the potential of GLP-1 pharmacotherapies for CUD. Notably, once weekly GLP-1 analogues, such as semaglutide or dulaglutide, may be more suitable in terms of adherence in patients with substance use disorders.
It is also not clear whether these results may generalize to other GLP-1 receptor agonists, which are known to have different pharmacokinetic profiles, molecular structures, and central actions. Clinical trials using GLP-1 receptor agonists in T2DM and obesity have shown differences in efficacy (Madsbad, 2016). There are currently ongoing studies of GLP-1 receptor agonists for other substance use disorders (Antonsen et al., 2018; Yammine et al., 2018), with some trials using repeated administration of the 5 microgram dose of immediate-release exenatide for heavy drinking and liraglutide at 3 mg per day for opioid use disorder and tobacco use disorder. Results from these trials will help ascertain to what extent different doses, formulations and time courses of administration of GLP-1 receptor agonists may prove beneficial in helping people with substance use disorders including CUD.
Exenatide and cocaine each reduced plasma levels of both GLP-1 and insulin, and cocaine also reduced amylin levels. These results are consistent with clinical studies that showed that one to three doses of experimenter-administered intravenous injections of cocaine reduced both GLP-1 and insulin levels (Bouhlal et al., 2017; Rott et al., 2008), with trend reductions observed for amylin (Bouhlal et al., 2017). Interestingly, reductions of GLP-1, insulin, and amylin following cocaine administration are in contrast to the effects of cocaine on appetite for food (i.e., these three hormones are anorexigenic and cocaine suppresses appetite) (Camilleri, 2015; Ersche et al., 2013; Suzuki et al., 2010). Although this exploratory study showed reductions of the anorexigenic hormones GLP-1, insulin, and amylin after cocaine self-administration (Camilleri, 2015; Suzuki et al., 2010), it is still unknown whether cocaine self-administration affects other hormones such as ghrelin, which stimulates appetite for food and drugs (Bouhlal et al., 2017).
To the best of our knowledge, this is the first clinical study in cocaine-experienced subjects showing lower GLP-1 and insulin levels after administration of exenatide during cocaine-self administration. GLP-1 agents increase insulin secretion in a glucose-dependent fasion. Baseline values of both insulin (Supplementary Figure 1) and glucose (Supplemental Figure 2) likely reflect the preemptive intake of sugar containig liquids (to minimize risk of hypoglycemia), preceding cocaine administration. Glucose increased in response to cocaine self-administration on both the placebo and exenatide day (Supplemental Figure 1), potentially due to the known effect of cocaine to increase cortisol levels (not measured) (Heesch et al., 1995). As we did not see a glucose-lowering effect of the exenatide, the dose may have been too low to show a significant effect, or perhaps cocaine effects on cortisol had a stronger influence. However; other studies of appetitive behaviors showed reduction in weight independent of hypoglycemic effects (Dushay et al., 2012; Kelly et al., 2013). Since glucose levels did not differ between the two groups, a difference in terms of insulin levels between the two arms would not be expected. Therefore, it is difficult to conclude with certaintly that the observed differences in insulin levels are a reflection of cocaine or exenatide, or an interplay of several factors (e.g., intake of different amounts of sugar containing fluids preceding exenatide vs. placebo or different endogenous levels of GLP-1). The extent to which these and other possible mechanisms are operating concomitantly in subjects with CUD warrants more investigation.
In conclusion, while we found no evidence that a single low dose of exenatide influences cocaine self-administration or cocaine-induced subjective effects in the current model, multiple study limitations preclude us from drawing firm conclusions on the efficacy of GLP-1 in CUD treatment. With regard to metabolic factors, cocaine significantly lowered levels of GLP-1, insulin, and amylin, suggesting that these satiety signals may be physiologicaly relevant for CUD.
Supplementary Material
Highlights.
The effects of a GLP-1 agonist (exenatide) on behavioral and subjective effects of cocaine were examined
All subjects participated in a self-regulated cocaine self-administration paradigm
Exenatide had no effect on administration and subjective effects of cocaine
Both exenatide and cocaine decreased levels of GLP-1 and insulin
Acknowledgements:
We thank staff from the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center as well as Hospital Research Unit of Yale New Haven Hospital. We also thank Dr. Matthew Hayes who served as a scientific consultant.
Funding: The main source of support was NIDA grant R21DA040914 (GAA). Other sources were the NIH grant RO1DA037897 (HDS), and NIH grant R21DA0457792 (HDS).
Conflict of Interest: The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised RiverMend Health, Opiant Pharmaceuticals, Idorsia, the Addiction Policy Forum and AXA; has received research support from the Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to addictive disorders or other health topics; has consulted for or advised law offices and gambling entities on issues related to addictive disorders and behaviors; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. AMJ is consultant for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim; research support American Diabetes Association, Eli Lilly, Novo Nordisk, and NIH/NIDDK. HDS has received research support, not used in the current studies, from an investigator-initiated sponsored proposal from Novo Nordisk.
Footnotes
Disclosures: All authors have read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, Davoli M, 2011. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev(12), CD003352. [DOI] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG, 2007. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama 298(2), 194–206. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Pittman B, Gueorguieva R, Kalayasiri R, Lynch WJ, Sughondhabirom A, Morgan PT, Malison RT, 2010. Regulation of cocaine self-administration in humans: lack of evidence for loading and maintenance phases. Pharmacology, biochemistry, and behavior 95(1), 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsen KK, Klausen MK, Brunchmann AS, le Dous N, Jensen ME, Miskowiak KW, Fisher PM, Thomsen GK, Rindom H, Fahmy TP, Vollstaedt-Klein S, Benveniste H, Volkow ND, Becker U, Ekstrom C, Knudsen GM, Vilsboll T, Fink-Jensen A, 2018. Does glucagon-like peptide-1 (GLP-1) receptor agonist stimulation reduce alcohol intake in patients with alcohol dependence: study protocol of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open 8(7), e019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA, 2012. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol 26(10), 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ, 2014. Glucagon-like peptide-1 receptors in the brain: controlling food intake and body weight. The Journal of clinical investigation 124(10), 4223–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlal S, Ellefsen KN, Sheskier MB, Singley E, Pirard S, Gorelick DA, Huestis MA, Leggio L, 2017. Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses. Drug and alcohol dependence 180, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, 2015. Peripheral mechanisms in appetite regulation. Gastroenterology 148(6), 1219–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Polosa R, 2008. Common predictors of smoking cessation in clinical practice. Respir Med 102(8), 1182–1192. [DOI] [PubMed] [Google Scholar]
- Chakraborti CK, 2010. Exenatide: a new promising antidiabetic agent. Indian journal of pharmaceutical sciences 72(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN, 2003. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160(6), 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W, 1979. The incretin concept today. Diabetologia 16(2), 75–85. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD, 2005. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes care 28(5), 1092–1100. [DOI] [PubMed] [Google Scholar]
- Dupre J, Ross SA, Watson D, Brown JC, 1973. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of clinical endocrinology and metabolism 37(5), 826–828. [DOI] [PubMed] [Google Scholar]
- Dushay J, Gao C, Gopalakrishnan GS, Crawley M, Mitten EK, Wilker E, Mullington J, Maratos-Flier E, 2012. Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes. Diabetes care 35(1), 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PloS one 8(7), e69010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A, 2012. Exendin-4 decreases amphetamine-induced locomotor activity. Physiology & behavior 106(4), 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Stochl J, Woodward JM, Fletcher PC, 2013. The skinny on cocaine: insights into eating behavior and body weight in cocaine-dependent men. Appetite 71, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Spitzer R, 2015. Structured Clinical Interview for DSM-5, Research Version. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD, 2013. GLP-1 analog attenuates cocaine reward. Molecular psychiatry 18(9), 961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M, 2015. Septal Glucagon-Like Peptide 1 Receptor Expression Determines Suppression of Cocaine-Induced Behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 40(8), 1969–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC, 2014. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annual review of nutrition 34, 237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Keffer JH, Snyder RW 2nd, Risser RC, Eichhorn EJ, 1995. Effects of cocaine on cortisol secretion in humans. The American journal of the medical sciences 310(2), 61–64. [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD, 2018. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(10), 2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’Donovan B, Ortinski PI, Schmidt HD, 2019. Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addiction biology 24(2), 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Schmidt HD, 2019. Central GLP-1 receptors: Novel molecular targets for cocaine use disorder. Physiology & behavior 206, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ, 2007. The physiology of glucagon-like peptide 1. Physiological reviews 87(4), 1409–1439. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO, 2011. Efficacy of varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res 13(10), 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR, 2012. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62(5–6), 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ, 2013. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA pediatrics 167(4), 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, 2011. Common cellular and molecular mechanisms in obesity and drug addiction. Nature reviews. Neuroscience 12(11), 638–651. [DOI] [PubMed] [Google Scholar]
- Kreymann B, Williams G, Ghatei MA, Bloom SR, 1987. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2(8571), 1300–1304. [DOI] [PubMed] [Google Scholar]
- Lindstrom E, Lewander T, Malm U, Malt UF, Lublin H, Ahlfors UG, 2001. Patient-rated versus clinician-rated side effects of drug treatment in schizophrenia. Clinical validation of a self-rating version of the UKU Side Effect Rating Scale (UKU-SERS-Pat). Nordic journal of psychiatry 55 Suppl 44, 5–69. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K, 1987. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta psychiatrica Scandinavica. Supplementum 334, 1–100. [DOI] [PubMed] [Google Scholar]
- Madsbad S, 2016. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes, obesity & metabolism 18(4), 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Chen JI, Wanyiri J, Nadim H, Jatlow P, Gueorguieva R, Potenza MN, Morgan PT, Bhagwagar Z, Malison RT, 2012. A single-day paradigm of self-regulated human cocaine administration. Pharmacology, biochemistry, and behavior 103(1), 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D’Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, Holst JJ, Langhans W, Meier JJ, Nauck MA, Perez-Tilve D, Pocai A, Reimann F, Sandoval DA, Schwartz TW, Seeley RJ, Stemmer K, Tang-Christensen M, Woods SC, DiMarchi RD, Tschöp MH, 2019. Glucagon-like peptide 1 (GLP-1). Mol Metab 30, 72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier PS, Kampman KM, Childress AR, 2020. Clinical Trials for Stimulant Use Disorders: Addressing Heterogeneities That May Undermine Treatment Outcomes. Handb Exp Pharmacol. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y, 2007. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry 31(8), 1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott D, Langleben DD, Elman I, 2008. Cocaine decreases plasma insulin concentrations in non-diabetic subjects: a randomized double-blind study. Diabet Med 25(4), 510–511. [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2018. Substance Abuse and Mental Health Services Administration.(2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data. [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, 2016. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41(7), 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Kosten TR, 2011. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med 9, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A, 2015. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiology & behavior 149, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR, 2010. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J 57(5), 359–372. [DOI] [PubMed] [Google Scholar]
- van Bloemendaal L, RG IJ, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M, 2014. GLP-1 Receptor Activation Modulates Appetite- and Reward-Related Brain Areas in Humans. Diabetes 63(12), 4186–4196. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD, 2013. The addictive dimensionality of obesity. Biological psychiatry 73(9), 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yammine L, Kosten TR, Cinciripini PM, Green CE, Meininger JC, Minnix JA, Newton TF, 2018. Exenatide once weekly for smoking cessation: study protocol for a randomized clinical trial. Medicine (Baltimore) 97(2), e9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Gardner EL, Wise RA, 2019. Cocaine and cocaine expectancy increase growth hormone, ghrelin, GLP-1, IGF-1, adiponectin, and corticosterone while decreasing leptin, insulin, GIP, and prolactin. Pharmacology, biochemistry, and behavior 176, 53–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


