Abstract
Introduction:
Fibrostenosis is a hallmark of Crohn’s disease (CD), remains a challenge in today’s clinical management of inflammatory bowel disease patients and represents a key event in the disease course necessitating improved preventative strategies and a multidisciplinary approach to diagnosis and management. With the advent of anti-fibrotic therapies and well-defined clinical endpoints for stricturing CD, there is promise to impact the natural history of disease.
Areas covered:
This review summarizes current evidence in the natural history of stricturing Crohn’s disease, discusses management approaches as well as future perspectives on intestinal fibrosis.
Expert opinion:
Currently, there are no specific therapies to prevent progression to fibrosis or to treat it after it becomes clinically apparent. In addition to the international effort by the Stenosis Therapy and Anti-Fibrotic Research (STAR) consortium to standardize definitions and propose endpoints in the management of stricturing CD, further research to improve our understanding of mechanisms of intestinal fibrosis will help pave the way for the development of future anti-fibrotic therapies.
Keywords: Anti-fibrotic, stricture, stenosis, fibrosis, enterography, endoscopic balloon dilation, strictureplasty, surgery, inflammatory bowel disease, Crohn’s disease
1. Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder of the gut, characterized by transmural inflammation and variable asymmetric, segmental involvement along the entire gastrointestinal tract. Stricturing disease is a known complication of CD and is likely a result of chronic relapsing and remitting inflammation[1]. Up to approximately 20% of patients present with stricturing complications at diagnosis and more than half of patients develop clinically apparent strictures in their lifetime[2,3]. Strictures are a main cause of hospitalization and surgery in patients with Crohn’s disease[4]. Although several treatment options are currently used in the management of strictures, there are currently no anti-fibrotic therapies to address fibrosis directly and potentially alter the CD natural history[5].
In this review, we summarize the current pathophysiology of stricturing CD, describe the natural history of stricture formation and its complications, and detail the methods for detection, as well as the treatment strategies. Finally, we review the concept of prevention of stricturing CD, and end with an outlook into the future of therapies and advances in the realm of fibrostenotic CD.
2. Epidemiology and natural history
Stricture formation in Crohn’s disease with the associated bowel obstruction and/or penetrating disease leads to substantial morbidity in patients and is a common occurrence. Approximately 20% of patients present with complicated CD at diagnosis, including 10.8% with a stricturing phenotype[6]. In a population-based cohort study in Olmsted County, the cumulative incidence of stricturing or penetrating disease, as defined by the Montreal classification, was 22% (95% CI, 17.2–26.5) within 1 year of diagnosis[7]. Approximately 15% and 21% of patients with CD were found to progress to a stricturing phenotype by 10 and 20 years, respectively[7]. In a more recent European population-based inception cohort (the Epi-IBD cohort), 29% of patients presented with complicated CD at diagnosis, including 21% with a stricturing phenotype. Among patients with non-stricturing non-penetrating disease at diagnosis, rates of progression to complicated disease were found to be similar to previous cohorts, with ~ 10% of patients progressing to stricturing disease at the end of the 5-year follow-up[8].
Further, it is highly likely that studies using the hierarchical Montreal or Vienna classification underestimate the incidence of stenosis in CD. In these classification paradigms, internal penetrating disease is recorded separately, irrespective of an underlying stricture. In the vast majority of cases, internal penetrating disease is associated with a stricture[9]. A retrospective study found patients with internal fistulas were 5.7 times more likely to have an underlying stricture than patients without fistulas[10]. In a study examining the histopathology of surgical resection specimens, 96.3% of fistulas were associated with a stricture[11]. Although fistulizing disease is commonly thought to occur due to progression of stricturing disease, there are no prospective studies to support this notion. These data lead to the estimate that over the lifetime of patients with CD, more than half develop a clinically apparent stricturing phenotype[12].
The most common site of strictures along the gastrointestinal tract is the small bowel. However, strictures can occur anywhere along the intestinal tract and generally follow the segmental location of inflammation[13]. Colonic strictures deserve special consideration given the increased risk of dysplasia compared with small bowel strictures[14]. A colonic stricture in a patient with CD carries a colorectal cancer risk of 3.6% at 5 years and 4.9% at 10 years[15]. Even with negative endoscopic biopsies and brush findings, 3.5% of colonic strictures in patients with inflammatory bowel disease (IBD) may harbor dysplasia or malignancy on histopathologic evaluation after surgical resection[14]. Given the increased risk of malignancy as well as the paucity of data evaluating outcomes of medical therapy in patients with colonic strictures, earlier referral to surgery should be considered, especially in the setting of ongoing medically refractory inflammation or lack of response to endoscopic approaches such as endoscopic balloon dilation[16].
Unfortunately, the rate of progression of CD to a stricturing phenotype has only been minimally altered by the therapies currently available to treat the disease[17–19]. Most theorize that this is partly due to the existence of damaged tissue by the time of CD diagnosis, but also due to possible non-inflammatory pathways that may not be reversible with medical therapy[9].
Strictures are an important indication for surgery. Together with fistulas and abscesses, stricturing complications may account for 40–70% of surgeries across the first 10 years of CD diagnosis[20]. Unfortunately, postoperative recurrence of strictures is common, especially at the ileocolonic anastomotic site[21]. The 10-year risk of re-operative management after initial resection for CD is estimated to be around 35%[22].
3. Pathophysiology
Although not yet fully understood, the pathogenesis of stricturing CD involves an intricate interplay of both inflammatory and non-inflammatory pathways in the development of fibrostenosis[1,7]. Over the past 15 years, there has been a paradigm shift in the understanding of the extent of stricture reversibility, as well as the existence of fibrotic pathways outside of inflammatory pathways[1,23]. Chronic inflammation, nevertheless, remains a key contributor to intestinal fibrosis, as shown by both its impact on fibrosis expression patterns, and on the evidence of fibrosis reduction through anti-inflammatory molecules, at least in vitro and in experimental animal models[24–26].
Fibrosis in the digestive tract, similar to other organs, involves the aberrant deposition of collagen-rich extracellular matrix (ECM), which is largely driven by activated mesenchymal cells. It also involves smooth muscle hyperplasia and hypertrophy[27], which together with an increase in the number of myofibroblasts[28], contribute to the luminal narrowing that ultimately culminates in intestinal obstruction[3]. Different pathways may drive fibrogenesis, including pro-fibrotic molecules and signaling pathways, such as transforming growth factor beta (TGF-β), tyrosine kinases, interleukin (IL)-11[29], IL-17[30], IL-34[31], reactive oxygen species (ROS) and peroxisome proliferator activator receptors[23], among other factors. These so called “profibrotic” molecules can directly activate a vast array of fibroblasts, myofibroblasts and smooth muscle cells, and lead to transient or permanent expansion of these mesenchymal cells in damaged tissue[20]. Interestingly, animal models of inflammation-induced fibrogenesis suggest that levels of fibrotic mediators may remain elevated despite the resolution of inflammation, offering one explanation for inflammation-independent pathways to fibrosis[32,33]. Other proposed mechanisms include an increased ECM stiffness and changes in ECM turnover. The first is a property held by a pathologically altered ECM, as can be found in CD, where physical tissue properties alone can be a strong mesenchymal cell activator[34]. The latter relates to an imbalance of down-regulated matrix degrading enzymes, such as matrix metalloproteinases (MMP), and up-regulated inhibitors of matrix metalloproteinases, such as tissue inhibitors of MMPs (TIMPs). This is believed to lead to a net positive ECM deposition[1,35,36]. Unique to the intestine, the gut microbiota likely has an impact on fibrogenesis. The bacterial protein flagellin was found to be a critical driver of the fibrogenic response of intestinal mesenchymal cells[37]. Supporting this concept, it was also shown that intestinal fibrosis cannot develop in animals lacking a microflora[1].
4. Clinical features and diagnosis
Although symptoms of obstruction, such as nausea, vomiting, post-prandial abdominal pain, distention and dietary restrictions may suggest the presence of a stricture, they are not highly correlated with strictures on imaging or endoscopy[38]. In addition, there is no correlation between the presence of obstructive symptoms and the severity of small bowel strictures[38]. This suggests that symptoms alone are not accurate enough to diagnose strictures and further testing is therefore required to diagnose stricturing CD[38].
The CrOhN’s disease anti-fibrotic STRICTure therapies (CONSTRICT) group, an international group of IBD experts, has proposed endoscopic and radiologic definitions of strictures in order to standardize diagnostic criteria[38]. According to the CONSTRICT criteria, stricturing on endoscopy refers to the “inability to pass an adult colonoscope through the narrowed area without prior endoscopic dilation with a reasonable amount of pressure applied”[38]. The presence of stenosis is also included in both validated CD endoscopic scores, the Simple Endoscopic Score for Crohn’s Disease (SES-CD) and the Crohn’s Disease Endoscopic Index of Severity (CDEIS)[39,40]. This item, however, comprises the least reliable one within each score[41] and therefore is not the optimal approach to stricture diagnosis. In addition, endoscopy is not able to assess inflammation and/or fibrosis in a transmural fashion.
Cross-sectional imaging is important as it helps better define the characteristics of a stricture and allows full thickness evaluation of the bowel wall. It also assesses the extramural components of the stricture including the mesentery and concomitant penetrating disease[42]. Imaging modalities used for the diagnosis of strictures include abdominal ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI)[42–46]. Several studies have assessed their accuracy in identifying a stricture[42]. While the sensitivity and specificity are high for all imaging modalities (sensitivity/specificity for stricture diagnosis in each of ultrasound elastography, CT enterography/enteroclysis, MR enterography, hybrid positron emission tomography with CT/MR were 88–100/0–100%, 92.3–100/38–100%, 75–100/91–96%, 85%/not reported, respectively), MR enterography remains the preferred diagnostic method because the stricture and its components can be more fully characterized using multiple, different sequences without and with contrast enhancement and without ionizing radiation[42].
Despite high reliability of detecting a stricture with imaging, a recent systematic review found significant heterogeneity in definitions of strictures[42]. The CONSTRICT group therefore proposed a consensus-based definition of stenosis on imaging in an effort to standardize diagnostic criteria[38]. For clinical purposes outside of clinical trials, a combination of at least 2 of the following features for a diagnosis is required: localized luminal narrowing (> 50% luminal narrowing), bowel wall thickening and pre-stenotic dilation (in most cases > 3 cm). For clinical trials, all 3 features should be present[38].
Imaging may also assist in assessing the extent of inflammation and fibrosis within strictures, which could guide management. Anti-inflammatory therapy can be considered in the setting of an inflammatory component, whereas a purely fibrotic stricture is unlikely to respond to anti-inflammatory therapy and would be better managed with EBD or surgery[1,47]. However, these features often coexist, making it difficult to accurately distinguish inflammatory from fibrotic stricture[1,42,47,48]. In fact, a recent study found that despite a pre-operative diagnosis of purely fibrotic strictures, histologic assessment of surgical specimens revealed a predominant component of chronic inflammation in all strictures[49]. Equally, other resection studies found a strong correlation between inflammation and fibrosis with purely fibrotic strictures being exceedingly rare or non-existent[50,51]. This led to the conclusion of the CONSTRICT consensus group that current imaging approaches are able to detect inflammation with high accuracy, but are not able to distinguish inflammation from fibrosis[38].
In one investigation, delayed contrast enhancement on MR differentiated between mild to moderate and severe fibrosis deposition with good sensitivity and specificity, independently of the degree of inflammation[52]. However, delayed enhancement did not allow the distinction of mild from moderate or moderate from severe fibrosis, all of which are important for clinical decision making and for a clinical trial endpoint. New imaging techniques such as diffusion-weighted imaging, magnetization transfer MRI, MR with dynamic contrast enhancement, shear-wave and strain-wave ultrasound elastography or artificial intelligence may help better quantify the degree of fibrosis but are still under evaluation and not yet used in routine clinical practice[9,48]. Ideally, imaging would be able to identify even pre-fibrotic characteristics. Many groups are now employing radiomics and artificial intelligence to imaging in an effort to determine if any pre- or early fibrotic characteristics are present. It may be that those findings are present in the mesenteric fat rather than the bowel wall itself.
Given the poor estimation of the extent of fibrosis by imaging, histopathology of intestinal resection specimens should be the standard for fibrosis quantification and is critical to develop novel imaging approaches, as it serves as the gold-standard for these studies. A recent systematic review performed by the STAR consortium detected a large heterogeneity across proposed histopathologic scoring systems and none of them was tested for reliability or validated based on modern index methodology[53]. The development of a reproducible and validated histopathologic scoring system is greatly needed and for this reason the STAR consortium is currently developing a novel histopathologic score for stricturing CD.
Importantly, strictures are not diagnostic of inflammatory bowel disease and occur in other conditions, some of which can coexist with CD. These include diverticular disease, malignancy, radiation enteritis, nonsteroidal anti-inflammatory drugs (NSAID) enteropathy and cryptogenic multifocal ulcerous stenosing enteritis[54]. A detailed clinical assessment, in addition to imaging and histopathology, can help exclude other conditions. Biopsies have a limited role in diagnosing fibrosis but may be helpful in ruling out an underlying malignancy. However, dysplasia may still be missed[9].
5. Treatment
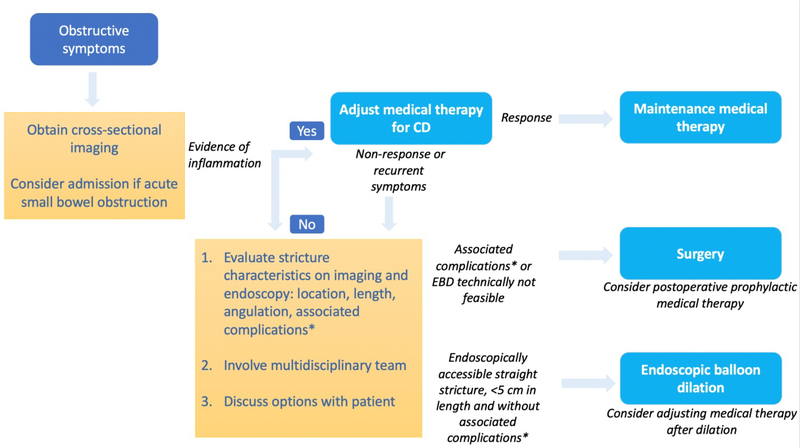
The management of stricturing CD, similar to its diagnosis, may be complex and requires the collaborative efforts of gastroenterologists, radiologists and colorectal surgeons. Several modalities are used in the management of stricturing CD and are described below (Figure 1).
Figure 1.
Algorithm for the management of symptomatic small bowel strictures in Crohn’s disease. CD: Crohn’s disease. EBD: endoscopic balloon dilation. *abscess, fistula, malignancy
5.1. Acute small bowel obstruction
In most cases, acute small bowel obstruction requires hospitalization. Complications (free perforation, abscess, fistulizing disease or malignancy) should be excluded by rapid evaluation with a physical examination and especially cross-sectional imaging (in the acute setting this is most often a CT enterography)[16]. GI decompression with a naso-gastric tube, hydration and electrolyte replacement are the mainstay of initial management, followed by close monitoring of the clinical status, as well as C-reactive protein and abdominal X-rays[9,16]. Although corticosteroid therapy is generally used in this setting, evidence is limited. In a small case series of 26 patients with CD, a majority of patients developed recurrence of obstructive symptoms within 2 years[55]. Adjustment of anti-inflammatory therapy, endoscopic interventions, surgery or a combination thereof are often required. In a recent retrospective cohort of patients with CD presenting with acute small bowel obstruction, 22.5% required surgery within 6 months. Factors associated with surgery within 6 months included female gender, BMI <25, the presence of penetrating disease, length of affected segment and bowel wall enhancement on CT scan[56].
5.2. Medical Approach
5.2.1. Immunomodulators
Azathioprine and mesalamine have been compared in a randomized controlled study of 72 patients with sub-occlusive ileal CD. Patients received either azathioprine or mesalamine after initially responding to 3 days of intravenous hydrocortisone therapy. Azathioprine was associated with significantly lower rates of hospitalization (61% vs 83.3%; P = 0.03) and surgery (25% vs 56%, respectively; P = 0.01) during follow-up[57]. In addition, azathioprine was associated with significantly lower rates of recurrent sub-occlusion and longer occlusion-free time intervals in a post-hoc analysis[58]. Although methotrexate is used in the treatment of Crohn’s disease[59,60], this therapy has not been evaluated in the setting of stricturing CD.
5.2.2. Biologic agents
5.2.2.1. Anti-TNFs:
Anti-tumor necrosis factor (anti-TNF) agents in the treatment of stricturing CD in both prospective and retrospective cohorts are effective and safe[61–65]. However, these observational studies had no controls.[66] Contrary to prior belief[67,68], these agents do not appear to cause further stricturing as a response to rapid healing[61–65,69].
Adalimumab was evaluated in a single arm, multicenter prospective observational cohort study of 97 patients with small bowel stricturing CD (the CREOLE study)[61]. At 24 weeks, 64% were still on therapy without having received steroids or undergone endoscopic balloon dilation or surgery. Continued adalimumab treatment was successfully maintained in 29% of patients at 4 years, with about half of all patients requiring surgery during this time frame. This study also developed a predictive risk score for adalimumab efficacy, in which a score of at least 4 points was associated with 88% probability of treatment success at 24 weeks[61]. Factors independently associated with treatment success were obstructive symptoms for less than 5 weeks, the use of immunosuppressants at the time of adalimumab initiation, a CD obstructive score >4, stricture length less than 12cm, marked enhancement on delayed phase, pre-stenotic bowel diameter of 18 to 29mm, and the absence of fistulizing disease[61].
A recent multicenter retrospective study evaluated the efficacy of early anti-TNFs in 262 biologic-naïve patients with a newly diagnosed CD-associated stricture. At 1 year, 73% had steroid-free drug persistence, with no hospitalization, endoscopic therapy, surgery or any change in therapy. However, after a median follow-up of 40 months, drug persistence had decreased to 26%, and 32% had undergone surgery[70]. Interestingly, starting anti-TNF within 18 months after the diagnosis of stricturing disease was associated with higher effectiveness[70]. Although this study demonstrates that a proportion of patients respond to anti-TNFs and that early treatment may be beneficial, it does highlight the need for other therapeutic alternatives for stricturing disease.
7.2.2.2. Vedolizumab and ustekinumab:
Although other biologics such as vedolizumab and ustekinumab are safe and effective in Crohn’s disease[71,72], there are no data on their effect on strictures. Interestingly, deep remission in a patient with stricturing CD receiving combination vedolizumab, ustekinumab and azathioprine has been described[73]. Strictures or fistulas were present in 118/212 (55.7%) of patients receiving vedolizumab for moderate to severe CD in the US VICTORY consortium[74]. In this cohort, two out of the 212 patients underwent resection for small bowel strictures at 12 months of therapy. Unfortunately, in this series, the effect of vedolizumab on stricture formation was not separated analyzed.
Overall, in a recent systematic review evaluating treatment outcomes in stricturing CD, a pooled rate of 28.3% (95% CI: 18.2%−41.3%) for surgical resection was observed over a median follow-up of 23 months in patients receiving any systemic medical therapy[66].
5.3. Endoscopic Approach
5.3.1. Endoscopic balloon dilation
5.3.1.1. Patient selection
Endoscopic balloon dilation (EBD) can be performed in the setting of an endoscopically accessible, non-angulated and short stricture (<5 cm long)[75]. There should be no contraindications including complications such as penetrating disease, abscess or malignancy[16]. The endoscopist should also evaluate for contraindications for an endoscopic procedure[1]. EBD can be used in both naïve and anastomotic strictures and can be performed in the upper, mid and lower gastrointestinal tract[75,76]. Although all endoscopically reachable strictures may be theoretically dilated, certain locations are associated with worse outcomes[75]. For example, duodenal strictures are associated with a 5-times increased hazard for shorter time to surgery compared with strictures in the jejunum, ileum or colon[77]. Colonic strictures can be dilated, but surgery should be strongly considered given the increased risk of dysplasia or malignancy[14].
5.3.1.2. Technique
EBD is typically done using a through-the-scope balloon and can be accomplished either through an antegrade or retrograde approach[16]. Although a higher maximal caliber of dilation was associated with an increased likelihood of technical success in a pooled analysis, this did not translate into increased clinical efficacy[75]. Before dilation, biopsies of the stricture should be performed to exclude the rare malignancy[47].
5.3.1.3. Outcomes
A pooled analysis of 33 retrospective studies, including 1463 patients with a total of 3212 EBDs found a technical success rate of 89.1%, and an immediate clinical efficacy rate of 80.8%. Technical success was defined as the ability to pass a colonoscope through the non-traversable stricture after dilation or correct stent placement[75]. The rate of re-dilation and surgery at 24 months was 73.5% and 42.9%, respectively. Factors associated with better short-term outcomes are EBD technical success, stricture length <5 cm, and the absence of ulcers[47].
5.3.1.4. Complications
In the above-mentioned pooled analysis, the rate of complications was 3–4% and included bleeding, hospitalization, infection or perforation[75,78]. Repeat dilations, naïve vs anastomotic strictures, and presence of active inflammation at the site of the stricture were not associated with increased risk of complications[47,75,79].
5.3.2. Other endoscopic therapies
5.3.2.1. Intralesional therapy
Other endoscopic modalities include intralesional corticosteroids or infliximab injection. The only randomized controlled trial evaluating intralesional therapy was terminated early due to a trend toward re-dilation in the intralesional steroid injection group[80]. A recent systematic review of this technique showed no impact on outcomes[66]. Thus, this modality is not currently recommended[81].
5.3.2.2. Stents
Based on limited data, stent placement in strictures in CD has some efficacy[82,83]. Unfortunately, this technique is associated with a high rate of complications, including stent migration, fistula formation or perforation[81,83]. This technique is therefore not currently recommended[81], but novel approaches such as removable, biodegradable or custom-made stents may change this recommendation.
5.3.2.3. Stricturotomy
Needle-knife therapy, including stricturotomy and radial incision and cutting, has been evaluated in the setting of CD-associated strictures in non-controlled settings and case series. Although available data show promise[84–86], further data are needed to understand its potential benefit, its long-term efficacy and in particular its potential risks compared with conventional methods.
5.4. Surgical Approach
5.4.1. Indications
Surgical management is indicated for symptomatic stricturing disease that is refractory to or not amenable to medical or endoscopic therapy, as well as in cases associated with suspected or confirmed malignancy or penetrating disease, especially with complex fistulae[87]. Stricture length ≥ 5 cm was also found to be associated with an increased need for surgery in a pooled data analysis of EBD outcomes[75]. In this study, every 1 cm increase in stricture length led to an increased hazard for surgery by 8% [75]. Additionally, surgical resection at time of diagnosis or early during the disease course may lead to longer clinical remission periods, decreased long-term surgery rates and decreased need for steroids and biologic therapies during follow-up[88–90]. Ultimately, surgical intervention depends upon the disease and stricture pattern, patient preference, whether there are associated complications, such as abscess, phlegmon or internal penetrating disease and an interdisciplinary team discussion[9].
5.4.2. Surgical techniques
If feasible, a laparoscopic approach is favored over laparotomy due to faster recovery, fewer adhesions and comparable rates of surgical recurrence[47,91]. Using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, a recent study evaluating surgical outcomes in stricturing disease found laparoscopy to be associated with fewer complications and a shorter hospital stay[92].
Segmental resection and strictureplasty are the mainstays of surgical treatment of stricturing disease[93]. Bypass surgery is another consideration for select upper gastrointestinal strictures, though in general, is not the preferred approach.[94] Segmental resection involves the resection of the affected segment typically followed by the construction of an end-to-end, end-to-side or side-to-side anastomosis[87]. The optimal type of anastomosis has long been debated in the surgical literature and is generally guided by surgeon preference[87]. A recent meta-analysis found the Kono-S (antimesenteric functional end-to-end handsewn) anastomosis sparing the mesentery to be associated with a lower incidence of both endoscopic and surgical recurrence, as well as a decreased anastomotic leak rate[95].
Strictureplasty, on the other hand, may be preferred in the setting of diffuse small bowel stricturing disease, rapidly recurring disease or if there are concerns with short bowel syndrome[87]. This intervention should also be considered in the case of multiple well-spaced out strictures that would necessitate an extensive bowel resection[81]. This technique should not be performed in cases where penetrating or malignant disease is suspected, or in those cases where multiple strictures are adjacent to one another and would be better off addressed by resection [87]. Prior to stricturoplasty, stricture biopsy should be performed in order to rule out malignancy [87].
Strictureplasty is a safe and effective technique.[96] In a meta-analysis of 1,112 patients who underwent 3,259 strictureplasties, the recurrence rate at 5 years was found to be 28%[96]. Interestingly, 90% of patients experienced recurrence at a non-strictureplasty site. This observation has been seen in other studies using radiologic follow-up of patients after strictureplasty, showing regression of the disease in the strictureplasty sites even in patients were not exposed to medical therapy[97–99]. The reason for the disease improvement at strictureplasty sites remains unclear. Resolution of fecal stasis has been proposed as a possible contributing factor[97]. Strictureplasty methods vary largely based on stricture length and their unique technical challenges; initial methods were adapted from techniques for pyloroplasty[100]. The 2 most common methods include the Heineke–Mikulicz and the Finney technique, for short (<10 cm) and intermediate-length strictures (10–20 cm), respectively[87]. Other less frequently used methods include the “non-conventional” isoperistaltic Michelassi, d’Hoore or Michelassi II techniques for long strictures (>25 cm) or repeated juxtaposed areas of continuous disease [87]. Although strictures located in the ileocecal region are traditionally referred for surgical resection, ileocolonic strictureplasties are emerging as a possible alternative for extensive disease[97]. The latter technique may in fact serve as a novel human model to study stricture regression, as it is amendable to endoscopic evaluation and sampling.
Strictureplasty is typically not recommended with CD colonic strictures, as they carry a higher risk of underlying or future malignancy, and these are more commonly managed with segmental resection [87].
6. Prevention and reversibility of fibrosis
Patient risk stratification close to diagnosis in naïve, non-complicated CD would be ideal to determine which patients may or may not progress to stricturing CD. This would guide clinical decision making (e.g. combination therapy, how often to monitor, and additionally the design of future clinical trials). The TREAT registry and the ACCENT I trial found that disease duration, disease severity, ileal location and new corticosteroid use were associated with an increased risk of progression to stenotic CD[69]. The pediatric RISK inception cohort of 913 patients were followed close to diagnosis and later analysed for clinical factors, genotype, serology, and ileal gene expression signatures. 9% developed complications during follow up. A validated risk model was able to predict complicated CD at diagnosis with an area under the receiver operator curve (ROC) of 0.72[18]. Interestingly, certain fecal bacterial strains identified at diagnosis were linked to the later development of fibrostenosis. Although other predictors have been evaluated, including clinical, serological, genetic and epigenetic biomarkers, these have not been shown to be specific for the development of stricturing disease. None of the available markers has been validated or can be recommended for clinical practice at this time [1].
6.1. Potential targets for anti-fibrotics
Given the current inability to predict strictures, reversing fibrosis in already existing strictures would therefore be an important goal in CD management. Reversibility of fibrosis has in fact been documented in other organs, such as the lung, heart, skin or kidney[3]. In CD, as discussed above, studies have shown disease regression at strictureplasty sites[97,98], suggesting possible fibrogenesis reversal in this setting[1].
Some of the mechanisms involved in the process of fibrosis reversal in other organs are being investigated in the intestine[5]. Tranilast inhibits transforming growth factor-β (TGF-β), which is involved in fibrogenesis through activation of mesenchymal cells[1]. This molecule has shown anti-fibrotic properties in skin, cardiac and pulmonary tissue[101]. In CD, it was evaluated in a small prospective study of patients with asymptomatic strictures and was found to be associated with lower rates of developing symptoms over a median follow-up of 2 years compared with controls[102]. Pirfenidone, one of only two anti-fibrotic agents approved for the treatment of idiopathic pulmonary fibrosis (IPF), has been found to inhibit fibroblast proliferation and MMP-3 production in patients with CD[103]. Pirfenidone is thought to act in part through reduction of TGF-b1-mediated fibrosis signaling[3,103]. Nintedanib is the other molecule approved for IPF treatment which may represent a potential antifibrotic target for intestinal fibrosis [3]. Interleukin-36 (IL-36) is thought to induce the expression of genes that mediate fibrogenesis[104]. Antibodies to interleukin-36 (IL-36) receptors were recently found to reduce fibrosis and inflammation in mice with chronic intestinal inflammation, suggesting a possible role for the treatment of intestinal fibrosis in IBD[104]. In addition, AMA0825, a Rho kinase inhibitor, was found to reverse and prevent intestinal fibrosis in animal models of chronic intestinal inflammation and fibrosis[105,106]. Interestingly, when combined with anti-TNF, AMA0825 prevented histopathologically documented fibrosis as well, suggesting a role for combination therapy with anti-inflammatory agents[106]. Several additional molecules have also been evaluated with promising results. A summary of potential anti-fibrotic targets can be found in table 1.
Table 1.
List of potential anti-fibrotic agents that have been tested in murine or human intestinal models.
| Molecules | Mechanism of action | Model system | Outcome relevant to the gastrointestinal tract | References |
|---|---|---|---|---|
| AMA0825 | Rho-associated protein kinase inhibitor | Murine intestinal fibrosis | Prevention and reversal of intestinal fibrosis | [106] |
| Tranilast | Reduction of TGF- β activity | Pilot study in human CD patients | Reduced rate of symptom occurrence in asymptomatic strictures | [102] |
| GED-0507–34 Levo | PPARγ Receptor agonist | Murine intestinal fibrosis | Prevention of intestinal fibrosis | [107] |
| Il-36R antibody | Interleukin 36 receptor inhibition | Primary human cells and murine intestinal fibrosis | Prevention and reversal of intestinal fibrosis and reduction in profibrotic gene signatures in human fibroblasts | [104] |
| Thalidomide | Regulates multiple inflammatory and fibrosis pathways | Murine intestinal fibrosis | Regulation and reversal of intestinal fibrosis | [108] |
| Andrographolide sulfonate | Inhibits activation of macrophages, suppresses Th1/Th17 response, and down-regulates MAPKs and NF-κB pathways | Murine intestinal fibrosis | Prevention of intestinal fibrosis | [109,110] |
| EW-7197 | Transforming growth factor-β type I receptor kinase inhibitor | Murine intestinal fibrosis | Prevention of intestinal fibrosis | [111] |
| TM5275 | PAI-1 inhibition | Murine intestinal fibrosis | Reversal of intestinal fibrosis | [112] |
| Pirfenidone | Inhibits cell proliferation and collagen I production | In vitro primary human intestinal fibroblasts. | Inhibition of fibroblast growth and suppression of collagen production | [113] |
| Mouse p40 peptide-based vaccines | Sustained Blockage of IL-12 and IL-23 | Murine intestinal fibrosis | Prevention and reduction of intestinal fibrosis | [114–116] |
| Wu-Mei-Wan, a classic traditional Chinese herb medicine | Inhibition of colon fibroblast activation | Murine intestinal fibrosis | Prevent intestinal fibrosis | [117] |
| ICG-001 | TGF-β/ WNT signaling inhibition | Intestinal fibroblasts | Inhibition of β-catenin and collagen I production | [118] |
| Melanin-concentrating hormone antibody | Melanin-concentrating hormone blockage | Murine intestinal fibrosis | Reduction of collagen production and reduction of fibrosis | [119] |
| Daikenchuto (Da-Jian-Zhong-Tang) | Activating myofibroblast transient receptor potential ankyrin 1 channel | Murine intestinal fibrosis | Prevention of intestinal fibrosis | [120] |
| Losartan | Downregulation of TGF-β1 expression | Murine intestinal fibrosis | Prevention of intestinal fibrosis | [121] |
| Triptolide (PG490) | Anti-inflammatory and immunomodulatory activities | Murine intestinal fibrosis | Prevention and reversal of intestinal fibrosis | [122] |
| BGB324 | AXL Receptor tyrosine kinase inhibitor | Human colonic fibroblasts, murine intestinal fibrosis, Human intestinal organoid culture, colon resections of patients with CD | Prevention and reversal of intestinal fibrosis | [123] |
CD: Crohn’s disease; TGF- β: Transforming growth factor beta; PPARγ: Peroxisome Proliferator Activated Receptor Gamma; MAPK: mitogen-activated protein kinase; NF-κB: Nuclear factor kappa B.
7. Expert Opinion
Stricturing complications are common in patients with CD. Despite advances in medical and endoscopic therapies, most CD patients eventually undergo surgery for complicated CD. Unfortunately, postoperative recurrence is common. The ultimate goal would therefore be to prevent the development of aberrant tissue repair, manifesting as intestinal fibrosis. Despite the availability of an increasing number of biologic therapies, the progression to stricturing complications has to date largely remained unchanged. Therefore, an important current objective would be to attempt to reverse already established fibrosis.
Increased knowledge of fibrosis pathophysiology will likely lead to the identification of novel anti-fibrotic targets. Alongside targeted molecular therapies, research is evolving in the space of regenerative medicine. This encompasses cell-based therapies, either using regulatory T cells, mesenchymal cells or amniotic epithelial cells, as well exosome-based approaches for the treatment of intestinal fibrosis. Although promising, implementation of these therapies would likely be challenging given issues surrounding the cost, logistics, delivery and possible risks that are yet to be clarified[124]. Another potential target may be the gut microbiota, which has been found to have an impact on fibrogenesis in CD[1,37]. A microbiome-based therapy could be topically delivered and may be gut selective.
Given the inability of our presently available therapies to prevent or reverse CD-associated strictures, there is an urgent need for the development of anti-fibrotic agents in CD. However, this requires a better understanding of intestinal fibrosis and faces several important challenges as outlined below.
The pathophysiology of CD-associated strictures needs to be further elucidated. It will be crucial to better understand predictors of stricturing disease among patients with CD, in order to allow risk-stratification and early identification of patients at risk for progression to specifically stricturing complications[77]. Another research priority will be the identification of biomarkers associated with CD fibrosis, which would ideally allow monitoring of response while on therapy [77]. Although a number of animal models are used in research, none of them fully recapitulates the pathogenesis leading to fibrosis in IBD. Linking pro-fibrotic pathways in the murine system to pathways in human disease will better utilize their translatability[1]. This will support mechanistically validating pathways of fibrogenesis and screen therapeutic agents prior to human trials. Recently, murine precision-cut intestinal slices were successfully used and allowed the assessment of several anti-fibrotic compounds[125]. Intestinal organoids are another promising model for representing in vivo physiology. Spironolactone was examined in this setting and was found to block the fibrogenic response of human intestinal organoids to TGF-β[126]. Both approaches could emerge as alternate preclinical screening models for anti-fibrotic therapies.
As anti-fibrotic therapies are being developed, attention should be paid to developing gut-selective drugs, in an attempt to minimize systemic side-effects and potential detrimental effects on wound healing elsewhere in the body. However, this may be challenging to achieve as there is currently no specific intestinal anti-fibrotic target and existing gut selective delivery systems may not achieve transmural penetration [77].
Ultimately, the development of anti-fibrotic molecules requires standardization of diagnostic criteria and outcomes prior to proceeding with clinical trials. The STAR consortium, an international group of experts, is leading a global initiative to define important endpoints in CD fibrosis, paving the way for future clinical trials. Such endpoints include patient-reported outcome measures (PROs), radiologic and histopathologic indices, some of which are currently being validated in prospective studies[9]. The first clinical trial with an anti-fibrotic stricture therapy is set to start in 2021, which will further accelerate interest in this field of large unmet clinical need and ultimately benefit patient care.
Article Highlights.
More than half of patients with Crohn’s disease (CD) develop clinically apparent strictures with subsequent intestinal obstruction or penetrating disease. This has remained largely unchanged despite advances in medical therapy
Both inflammation-dependent and inflammation-independent mechanisms may drive fibrogenesis in CD
A stricture is defined radiologically by the presence of at least 2 out of the 3 following criteria: localized luminal narrowing (>50% luminal narrowing), bowel wall thickening and pre-stenotic dilation (generally > 3 cm in diameter).
Endoscopic balloon dilation is an option for short, non-angulated strictures which are accessible endoscopically and not associated with penetrating disease or malignancy
Bowel resection and strictureplasty are the mainstays of surgical treatment of stricturing disease
Colon strictures deserve special attention given an increased risk of dysplasia compared with small bowel strictures. Earlier referral to surgery should be considered
Acknowledgments
Funding
This paper was supported by the Helmsley Charitable Trust through the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium and National Institutes of Health [K08DK110415 & R01DK123233] to F.Rieder.
Declaration of Interests
F Rieder is on the advisory board or consultant for Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene, CDISC, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Janssen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Theravance, Thetis, UCB. B L Cohen receives the following financial support: advisory boards and consultant for Abbvie, Celgene-Bristol Myers Squibb, Pfizer, Sublimity Therapeutics, TARGET RWE; CME Companies: Cornerstones, Vindico; speaking: Abbvie. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Abbreviations
- Anti-TNF
anti-tumor necrosis factor
- CDEIS
Crohn’s Disease Endoscopic Index of Severity
- CONSTRICT
CrOhN’s disease anti-fibrotic STRICTure therapies
- CD
Crohn’s Disease
- CT
computed tomography
- EBD
Endoscopic balloon dilation
- ECM
extracellular matrix
- IBD
inflammatory bowel disease
- IPF
idiopathic pulmonary fibrosis
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinases
- MRI
Magnetic resonance imaging
- NF-κB
Nuclear factor kappa B
- NSAIDs
nonsteroidal anti-inflammatory drugs
- NSQIP
National Surgical Quality Improvement Program
- PPARγ
Peroxisome Proliferator Activated Receptor Gamma
- PROs
patient-reported outcome measures
- ROC
receiver operator curve
- ROS
reactive oxygen species
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
- STAR consortium
The Stenosis Therapy and Anti-Fibrotic Research (STAR) consortium
- TGF-B
Transforming growth factor beta
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Rieder F, Fiocchi C, Rogler G. Mechanisms, Management, and Treatment of Fibrosis in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017. February;152(2):340–350 e6.**Thorough review of fibrosis in IBD, with a detailed section on pathophysiology
- 2.Rieder F Managing Intestinal Fibrosis in Patients With Inflammatory Bowel Disease. Gastroenterology & hepatology. 2018. February;14(2):120–122. [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo JH, Holubar S, Rieder F. Fibrostenotic strictures in Crohn’s disease. Intest Res. 2020;0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golovics PA, Lakatos L, Mandel MD, et al. Prevalence and predictors of hospitalization in Crohn’s disease in a prospective population-based inception cohort from 2000–2012. World Journal of Gastroenterology: WJG. 2015;21(23):7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettenworth D, Rieder F. Reversibility of Stricturing Crohn’s Disease-Fact or Fiction? Inflammatory bowel diseases. 2016;22(1):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001. December;49(6):777–82.**Landmark paper describing the course of disease behavior in patients with Crohn’s disease
- 7.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010. October;139(4):1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019. March;68(3):423–433. [DOI] [PubMed] [Google Scholar]
- 9.El Ouali S, Click B, Holubar SD, et al. Natural history, diagnosis and treatment approach to fibrostenosing Crohn’s disease. United European Gastroenterol J. 2020. April;8(3):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurgens M, Brand S, Laubender RP, et al. The presence of fistulas and NOD2 homozygosity strongly predict intestinal stenosis in Crohn’s disease independent of the IL23R genotype. J Gastroenterol. 2010. July;45(7):721–31. [DOI] [PubMed] [Google Scholar]
- 11.Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn’s disease. Virchows Arch. 2000. September;437(3):293–7. [DOI] [PubMed] [Google Scholar]
- 12.Munkholm P, Langholz E, Davidsen M, et al. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995. July;30(7):699–706. [DOI] [PubMed] [Google Scholar]
- 13.Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985. June;88(6):1818–25. [DOI] [PubMed] [Google Scholar]
- 14.Fumery M, Pineton de Chambrun G, Stefanescu C, et al. Detection of Dysplasia or Cancer in 3.5% of Patients With Inflammatory Bowel Disease and Colonic Strictures. Clin Gastroenterol Hepatol. 2015. October;13(10):1770–5. [DOI] [PubMed] [Google Scholar]
- 15.Lovasz BD, Lakatos L, Golovics PA, et al. Risk of colorectal cancer in Crohn’s disease patients with colonic involvement and stenosing disease in a population-based cohort from Hungary. J Gastrointestin Liver Dis. 2013. September;22(3):265–8. [PubMed] [Google Scholar]
- 16.Lu C, Holubar SD, Rieder F. How I Approach the Management of Stricturing Crohn’s Disease. Am J Gastroenterol. 2019. August;114(8):1181–1184. [DOI] [PubMed] [Google Scholar]
- 17.Jeuring SF, van den Heuvel TR, Liu LY, et al. Improvements in the Long-Term Outcome of Crohn’s Disease Over the Past Two Decades and the Relation to Changes in Medical Management: Results from the Population-Based IBDSL Cohort. Am J Gastroenterol. 2017. February;112(2):325–336. [DOI] [PubMed] [Google Scholar]
- 18.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet. 2017. April 29;389(10080):1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarev M, Ullman T, Schraut WH, et al. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010. May;16(5):830–5. [DOI] [PubMed] [Google Scholar]
- 20.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013. July;62(7):1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990. October;99(4):956–63. [DOI] [PubMed] [Google Scholar]
- 22.Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014. November;109(11):1739–48. [DOI] [PubMed] [Google Scholar]
- 23.Zhao JF, Ling FM, Li JR, et al. Role of non-inflammatory factors in intestinal fibrosis. J Dig Dis. 2020. June;21(6):315–318. [DOI] [PubMed] [Google Scholar]
- 24.Sadler T, Bhasin JM, Xu Y, et al. Genome-wide analysis of DNA methylation and gene expression defines molecular characteristics of Crohn’s disease-associated fibrosis. Clin Epigenetics. 2016;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008. November 1;178(9):948–55. [DOI] [PubMed] [Google Scholar]
- 26.Horton MR, Santopietro V, Mathew L, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med. 2012. September 18;157(6):398–406. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Lu C, Hirota C, et al. Smooth Muscle Hyperplasia/Hypertrophy is the Most Prominent Histological Change in Crohn’s Fibrostenosing Bowel Strictures: A Semiquantitative Analysis by Using a Novel Histological Grading Scheme. J Crohns Colitis. 2017. January;11(1):92–104. [DOI] [PubMed] [Google Scholar]
- 28.Zidar N, Langner C, Jerala M, et al. Pathology of Fibrosis in Crohn’s Disease-Contribution to Understanding Its Pathogenesis. Front Med (Lausanne). 2020;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim WW, Ng B, Widjaja A, et al. Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice. PLoS One. 2020;15(1):e0227505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latella G, Viscido A. Controversial Contribution of Th17/IL-17 Toward the Immune Response in Intestinal Fibrosis. Dig Dis Sci. 2020. May;65(5):1299–1306. [DOI] [PubMed] [Google Scholar]
- 31.Franze E, Dinallo V, Laudisi F, et al. Interleukin-34 Stimulates Gut Fibroblasts to Produce Collagen Synthesis. J Crohns Colitis. 2020. April 9. [DOI] [PubMed] [Google Scholar]
- 32.Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol. 2007. November 15;179(10):6988–7000. [DOI] [PubMed] [Google Scholar]
- 33.Rieder F, Kessler S, Sans M, et al. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012. October;303(7):G786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson LA, Rodansky ES, Sauder KL, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis. 2013. April;19(5):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ura H, Obara T, Yokota K, et al. Effects of transforming growth factor-beta released from gastric carcinoma cells on the contraction of collagen-matrix gels containing fibroblasts. Cancer Res. 1991. July 1;51(13):3550–4. [PubMed] [Google Scholar]
- 36.Johnson LA, Rodansky ES, Haak AJ, et al. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014. January;20(1):154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S, Dejanovic D, Yao P, et al. Selective deletion of MyD88 signaling in alpha-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol. 2020. July;13(4):665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther. 2018. August;48(3):347–357.**Important paper proposing definitions and outcomes in stricturing Crohn’s disease according to an expert consensus
- 39.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989. July;30(7):983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004. October;60(4):505–12. [DOI] [PubMed] [Google Scholar]
- 41.Khanna R, Zou G, Stitt L, et al. Responsiveness of Endoscopic Indices of Disease Activity for Crohn’s Disease. Am J Gastroenterol. 2017. October;112(10):1584–1592. [DOI] [PubMed] [Google Scholar]
- 42.Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019. June;68(6):1115–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maconi G, Carsana L, Fociani P, et al. Small bowel stenosis in Crohn’s disease: clinical, biochemical and ultrasonographic evaluation of histological features. Aliment Pharmacol Ther. 2003. October 1;18(7):749–56. [DOI] [PubMed] [Google Scholar]
- 44.Vogel J, da Luz Moreira A, Baker M, et al. CT enterography for Crohn’s disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007. November;50(11):1761–9. [DOI] [PubMed] [Google Scholar]
- 45.Pous-Serrano S, Frasson M, Palasi Gimenez R, et al. Accuracy of magnetic resonance enterography in the preoperative assessment of patients with Crohn’s disease of the small bowel. Colorectal Dis. 2017. May;19(5):O126–O133. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Hakim A, Alexakis C, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015. January;30(1):86–91. [DOI] [PubMed] [Google Scholar]
- 47.Rieder F, Latella G, Magro F, et al. European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s Disease. J Crohns Colitis. 2016. August;10(8):873–85. [DOI] [PubMed] [Google Scholar]
- 48.Rimola J, Capozzi N. Differentiation of fibrotic and inflammatory component of Crohn’s disease-associated strictures. Intest Res. 2020. April;18(2):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shivaji UN, Evans M, Critchlow T, et al. Chronic inflammation and other changes are significant components of clinically fibrotic strictures in Crohn’s disease: a histological study of resected strictures clinically characterized as noninflamed. Eur J Gastroenterol Hepatol. 2020. July 3;Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 50.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012. May;18(5):849–56. [DOI] [PubMed] [Google Scholar]
- 51.Chiorean MV, Sandrasegaran K, Saxena R, et al. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007. November;102(11):2541–50. [DOI] [PubMed] [Google Scholar]
- 52.Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015. March;110(3):432–40. [DOI] [PubMed] [Google Scholar]
- 53.Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology Scoring Systems of Stenosis Associated With Small Bowel Crohn’s Disease: A Systematic Review. Gastroenterology. 2020. January;158(1):137–150.e1.* Recent comprehensive systematic review evaluating histopathologic indices of fibrosis in Crohn’s disease
- 54.Keuchel M, Kurniawan N, Baltes P. Small bowel ulcers: when is it not inflammatory bowel disease? Curr Opin Gastroenterol. 2019. May;35(3):213–222. [DOI] [PubMed] [Google Scholar]
- 55.Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol. 1983. June;5(3):211–5. [DOI] [PubMed] [Google Scholar]
- 56.Lowe SC, Ream J, Hudesman D, et al. A clinical and radiographic model to predict surgery for acute small bowel obstruction in Crohn’s disease. Abdominal Radiology. 2020. 2020/09/01;45(9):2663–2668. [DOI] [PubMed] [Google Scholar]
- 57.de Souza GS, Vidigal FM, Chebli LA, et al. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in sub-occlusive ileocecal Crohn’s disease patients. Med Sci Monit. 2013. August 30;19:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidigal FM, de Souza GS, Chebli LA, et al. Azathioprine is more effective than mesalazine at preventing recurrent bowel obstruction in patients with ileocecal Crohn’s disease. Med Sci Monit. 2014. November 5;20:2165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000. June 1;342(22):1627–32. [DOI] [PubMed] [Google Scholar]
- 60.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995. February 2;332(5):292–7. [DOI] [PubMed] [Google Scholar]
- 61.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018. January;67(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallotta N, Barberani F, Hassan NA, et al. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J Gastroenterol. 2008. March 28;14(12):1885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allocca M, Bonifacio C, Fiorino G, et al. Efficacy of tumour necrosis factor antagonists in stricturing Crohn’s disease: A tertiary center real-life experience. Dig Liver Dis. 2017. August;49(8):872–877. [DOI] [PubMed] [Google Scholar]
- 64.Pelletier AL, Kalisazan B, Wienckiewicz J, et al. Infliximab treatment for symptomatic Crohn’s disease strictures. Aliment Pharmacol Ther. 2009. February 1;29(3):279–85. [DOI] [PubMed] [Google Scholar]
- 65.Holtmann M, Wanitschke R, Helisch A, et al. [Anti-TNF antibodies in the treatment of inflammatory intestinal stenoses in Crohn’s disease]. Z Gastroenterol. 2003. January;41(1):11–7. [DOI] [PubMed] [Google Scholar]
- 66.Lu C, Baraty B, Lee Robertson H, et al. Systematic review: medical therapy for fibrostenosing Crohn’s disease. Aliment Pharmacol Ther. 2020. June;51(12):1233–1246.*Recent exhaustive systematic review evaluating medical and intralesional therapy for stricturing Crohn’s disease
- 67.Toy LS, Scherl EJ, Kornbluth A, et al. Complete bowel obstruction following initial response to infliximab therapy for crohn’s disease: A series of a newly described complication. Gastroenterology. 2000;118(4):A569. [Google Scholar]
- 68.Vasilopoulos S, Kugathasan S, Saeian K, et al. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease. The American Journal of Gastroenterology. 2000;95(9):2503–2503. [Google Scholar]
- 69.Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006. May;101(5):1030–8. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Lago I, Hoyo JD, Perez-Girbes A, et al. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn’s disease. United European Gastroenterol J. 2020. July 28;0(0):2050640620947579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013. August 22;369(8):699–710. [DOI] [PubMed] [Google Scholar]
- 72.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014. September;147(3):618–627 e3. [DOI] [PubMed] [Google Scholar]
- 73.Elmoursi A, Barrett TA, Perry C. Double Biologic Therapy for Refractory Stricturing Crohn’s Disease: A Successful Case of Deep Remission with Ustekinumab and Vedolizumab. Inflamm Bowel Dis. 2020. June 18;26(7):e62–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dulai PS, Singh S, Jiang X, et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016. August;111(8):1147–55. [DOI] [PubMed] [Google Scholar]
- 75.Bettenworth D, Gustavsson A, Atreja A, et al. A Pooled Analysis of Efficacy, Safety, and Long-term Outcome of Endoscopic Balloon Dilation Therapy for Patients with Stricturing Crohn’s Disease. Inflamm Bowel Dis. 2017. January;23(1):133–142.*Meta-analysis evaluating outcomes of endoscopic balloon dilation in stricturing Crohn’s disease
- 76.Bettenworth D, Mucke MM, Lopez R, et al. Efficacy of Endoscopic Dilation of Gastroduodenal Crohn’s Disease Strictures: A Systematic Review and Meta-Analysis of Individual Patient Data. Clin Gastroenterol Hepatol. 2019. November;17(12):2514–2522 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bettenworth D, Rieder F. Medical therapy of stricturing Crohn’s disease: what the gut can learn from other organs - a systematic review. Fibrogenesis & tissue repair. 2014;7(1):5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Navaneethan U, Lourdusamy V, Njei B, et al. Endoscopic balloon dilation in the management of strictures in Crohn’s disease: a systematic review and meta-analysis of non-randomized trials. Surg Endosc. 2016. December;30(12):5434–5443. [DOI] [PubMed] [Google Scholar]
- 79.Thienpont C, D’Hoore A, Vermeire S, et al. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut. 2010. March;59(3):320–4. [DOI] [PubMed] [Google Scholar]
- 80.East JE, Brooker JC, Rutter MD, et al. A pilot study of intrastricture steroid versus placebo injection after balloon dilatation of Crohn’s strictures. Clin Gastroenterol Hepatol. 2007. September;5(9):1065–9. [DOI] [PubMed] [Google Scholar]
- 81.Adamina M, Bonovas S, Raine T, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. Journal of Crohn’s and Colitis. 2019;14(2):155–168. [DOI] [PubMed] [Google Scholar]
- 82.Levine RA, Wasvary H, Kadro O. Endoprosthetic Management of Refractory Ileocolonic Anastomotic Strictures After Resection for Crohn’s Disease: Report of Nine-year Follow-up and Review of the Literature. Inflammatory Bowel Diseases. 2011;18(3):506–512. [DOI] [PubMed] [Google Scholar]
- 83.Attar A, Maunoury V, Vahedi K, et al. Safety and efficacy of extractible self-expandable metal stents in the treatment of Crohn’s disease intestinal strictures: a prospective pilot study. Inflamm Bowel Dis. 2012. October;18(10):1849–54. [DOI] [PubMed] [Google Scholar]
- 84.Bedogni G, Ricci E, Pedrazzoli C, et al. Endoscopic dilation of anastomotic colonic stenosis by different techniques: an alternative to surgery? Gastrointestinal Endoscopy. 1987;33(1):21–24. [DOI] [PubMed] [Google Scholar]
- 85.Lan N, Shen B. Endoscopic Stricturotomy with Needle Knife in the Treatment of Strictures from Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017. April;23(4):502–513. [DOI] [PubMed] [Google Scholar]
- 86.Moroi R, Shiga H, Kuroha M, et al. Endoscopic radial incision and cutting for Crohn’s Disease-associated intestinal stricture: a pilot study. Endosc Int Open. 2020;8(1):E81–E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strong S, Steele SR, Boutrous M, et al. Clinical Practice Guideline for the Surgical Management of Crohn’s Disease. Dis Colon Rectum. 2015. November;58(11):1021–36. [DOI] [PubMed] [Google Scholar]
- 88.Aratari A, Papi C, Leandro G, et al. Early versus late surgery for ileo-caecal Crohn’s disease. Aliment Pharmacol Ther. 2007. November 15;26(10):1303–12. [DOI] [PubMed] [Google Scholar]
- 89.Latella G, Cocco A, Angelucci E, et al. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis. 2009. April;41(4):269–76. [DOI] [PubMed] [Google Scholar]
- 90.Golovics PA, Lakatos L, Nagy A, et al. Is early limited surgery associated with a more benign disease course in Crohn’s disease? World J Gastroenterol. 2013. November 21;19(43):7701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel SV, Patel SV, Ramagopalan SV, et al. Laparoscopic surgery for Crohn’s disease: a meta-analysis of perioperative complications and long term outcomes compared with open surgery. BMC Surg. 2013. May 24;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaglani T, Davis CH, Bailey HR, et al. Trends and Outcomes for Minimally Invasive Surgery for Inflammatory Bowel Disease. J Surg Res. 2019. March;235:303–307. [DOI] [PubMed] [Google Scholar]
- 93.Strong SA. Strictureplasty in Complex Crohn’s Disease: Beyond the Basics. Clin Colon Rectal Surg. 2019. July;32(4):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee CHA, Rieder F, Holubar SD. Duodenojejunal Bypass and Strictureplasty for Diffuse Small Bowel Crohn’s Disease with a Step-by-Step Visual Guide. Crohn’s & Colitis 360. 2019;1(1). [Google Scholar]
- 95.Alshantti A, Hind D, Hancock L, et al. The role of Kono-S anastomosis and mesenteric resection in reducing recurrence after surgery for Crohn’s disease: a systematic review. Colorectal Disease. 2020. 2020/05/17;n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 96.Yamamoto T, Fazio VW, Tekkis PP. Safety and Efficacy of Strictureplasty for Crohn’s Disease: A Systematic Review and Meta-Analysi. Diseases of the Colon & Rectum. 2007;50(11):1968–1986. [DOI] [PubMed] [Google Scholar]
- 97.de Buck van Overstraeten A, Vermeire S, Vanbeckevoort D, et al. Modified Side-To-Side Isoperistaltic Strictureplasty over the Ileocaecal Valve: An Alternative to Ileocaecal Resection in Extensive Terminal Ileal Crohn’s Disease. J Crohns Colitis. 2016. April;10(4):437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maconi G, Sampietro GM, Cristaldi M, et al. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: a prospective study. Ann Surg. 2001. March;233(3):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fazio VW, Tjandra JJ, Lavery IC, et al. Long-term follow-up of strictureplasty in Crohn’s disease. Dis Colon Rectum. 1993. April;36(4):355–61. [DOI] [PubMed] [Google Scholar]
- 100.Mohan HM, Coffey JC. Surgical treatment of intestinal stricture in inflammatory bowel disease. J Dig Dis. 2020. June;21(6):355–359. [DOI] [PubMed] [Google Scholar]
- 101.Martin J, Kelly DJ, Mifsud SA, et al. Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-beta. Cardiovasc Res. 2005. February 15;65(3):694–701. [DOI] [PubMed] [Google Scholar]
- 102.Oshitani N, Yamagami H, Watanabe K, et al. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn’s disease. Gut. 2007. April;56(4):599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kadir S-I, Wenzel Kragstrup T, Dige A, et al. Pirfenidone inhibits the proliferation of fibroblasts from patients with active Crohn’s disease. Scandinavian Journal of Gastroenterology. 2016. 2016/11/01;51(11):1321–1325. [DOI] [PubMed] [Google Scholar]
- 104.Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156(4):1082–1097. e11. [DOI] [PubMed] [Google Scholar]
- 105.Rieder F ROCKing the Field of Intestinal Fibrosis or Between a ROCK and a Hard Place? Gastroenterology. 2017;153(4):895–897. [DOI] [PubMed] [Google Scholar]
- 106.Holvoet T, Devriese S, Castermans K, et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology. 2017;153(4):1054–1067. [DOI] [PubMed] [Google Scholar]
- 107.Speca S, Rousseaux C, Dubuquoy C, et al. Novel PPARγ Modulator GED-0507–34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflammatory bowel diseases. 2016;22(2):279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H, Xu H, Luo L, et al. Thalidomide prevented and ameliorated pathogenesis of Crohn’s disease in mice via regulation of inflammatory response and fibrosis. Frontiers in Pharmacology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao J, Cui J, Zhong H, et al. Andrographolide sulfonate ameliorates chronic colitis induced by TNBS in mice via decreasing inflammation and fibrosis. International Immunopharmacology. 2020. [DOI] [PubMed] [Google Scholar]
- 110.Liu W, Guo W, Guo L, et al. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol. 2014. June;20(2):337–45. [DOI] [PubMed] [Google Scholar]
- 111.Binabaj MM, Asgharzadeh F, Avan A, et al. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. Journal of Cellular Physiology. 2019. [DOI] [PubMed] [Google Scholar]
- 112.Imai J, Yahata T, Ichikawa H, et al. Inhibition of plasminogen activator inhibitor-1 attenuates against intestinal fibrosis in mice. Intestinal Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cui Y, Zhang M, Leng C, et al. Pirfenidone Inhibits Cell Proliferation and Collagen I Production of Primary Human Intestinal Fibroblasts. Cells. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guan Q, Ma Y, Hillman CL, et al. Development of recombinant vaccines against IL-12/IL-23 p40 and in vivo evaluation of their effects in the downregulation of intestinal inflammation in murine colitis. Vaccine. 2009;27(50):7096–104. doi: 10.1016/j.vaccine.2009.09.058. Epub 2009 Sep 26. [DOI] [PubMed] [Google Scholar]
- 115.Guan Q, Ma Y, Hillman CL, et al. Targeting IL-12/IL-23 by employing a p40 peptide-based vaccine ameliorates TNBS-induced acute and chronic murine colitis. Mol Med. 2011;17(7–8):646–56. doi: 10.2119/molmed.2010.00252. Epub 2011 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guan Q, Weiss CR, Wang S, et al. Reversing Ongoing Chronic Intestinal Inflammation and Fibrosis by Sustained Block of IL-12 and IL-23 Using a Vaccine in Mice. Inflammatory Bowel Diseases. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu F, Shao Q, Hu M, et al. Wu-Mei-Wan ameliorates chronic colitis-associated intestinal fibrosis through inhibiting fibroblast activation. Journal of Ethnopharmacology. 2020. [DOI] [PubMed] [Google Scholar]
- 118.Lewis A, Nijhuis A, Berti G, et al. P035 Pharmacological inhibition of the canonical WNT signalling pathway represents a potential novel therapy for fibrosis in Crohn’s disease. Journal of Crohn’s and Colitis. 2019;13(Supplement_1):S103–S104. [Google Scholar]
- 119.Ziogas DC, Gras-Miralles B, Mustafa S, et al. Anti-melanin-concentrating hormone treatment attenuates chronic experimental colitis and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2013;304(10):G876–G884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hiraishi K, Kurahara L-H, Sumiyoshi M, et al. Daikenchuto (Da-Jian-Zhong-Tang) ameliorates intestinal fibrosis by activating myofibroblast transient receptor potential ankyrin 1 channel. World journal of gastroenterology. 2018;24(35):4036–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wengrower D, Zanninelli G, Latella G, et al. Losartan reduces trinitrobenzene sulphonic acid-induced colorectal fibrosis in rats. Can J Gastroenterol. 2012;26(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tao Q, Wang B, Zheng Y, et al. Triptolide ameliorates colonic fibrosis in an experimental rat model. Mol Med Rep. 2015;12(2):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steiner CA, Rodansky ES, Johnson LA, et al. AXL Is a Potential Target for the Treatment of Intestinal Fibrosis. Inflammatory Bowel Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwab R, Lim R, Goldberg R. Resolving intestinal fibrosis through regenerative medicine. Current Opinion in Pharmacology. 2019. 2019/12/01/;49:90–94. [DOI] [PubMed] [Google Scholar]
- 125.Iswandana R, Pham BT, Suriguga S, et al. Murine Precision-cut Intestinal Slices as a Potential Screening Tool for Antifibrotic Drugs. Inflamm Bowel Dis. 2020. April 11;26(5):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodansky ES, Johnson LA, Huang S, et al. Intestinal organoids: a model of intestinal fibrosis for evaluating anti-fibrotic drugs. Exp Mol Pathol. 2015. June;98(3):346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]