Abstract
Immune cells play essential roles in metabolic homeostasis and thus, undergo analogous changes in normal physiology (e.g., puberty and pregnancy) and in various metabolic and immune diseases. An essential component of this close relationship between the two is sex differences. Many autoimmune diseases, such as systemic lupus erythematous and multiple sclerosis, feature strikingly increased prevalence in females, whereas in contrast, infectious diseases, such as Ebola and Middle East Respiratory Syndrome, affect more men than women. Therefore, there are fundamental aspects of metabolic homeostasis and immune functions that are regulated differently in males and females. This can be observed in sex hormone-immune interaction where androgens, such as testosterone, have shown immunosuppressive effects whilst estrogen is on the opposite side of the spectrum with immunoenhancing facilitation of mechanisms. In addition, the two sexes exhibit significant differences in metabolic regulation, with estrous cycles in females known to induce variability in traits and more pronounced metabolic disease phenotype exhibited by males. It is likely that these differences underlie both the development of metabolic and autoimmune diseases and the response to current treatment options. Sexual dimorphism in immunometabolism has emerged to become an area of intense research, aiming to uncover sex-biased effector molecules in the various metabolic tissues and immune cell types, identify sex-biased cell-type-specific functions of common effector molecules, and understand whether the sex differences in metabolic and immune functions influence each other during autoimmune pathogenesis. In this review, we will summarize recent findings that address these critical questions of sexual dimorphism in immunometabolism as well as their translational implications for the clinical management of autoimmune diseases.
Keywords: sexual dimorphism, immunometabolism, autoimmune diseases, systemic lupus erythematous, multiple sclerosis, Sjögren’s syndrome
1. Introduction
Sexual dimorphism, or biological differences between male and female (the sexes of a species), can be noted throughout countless developmental, pathological, and physiological processes which humans go through1–3. Sex disparity in the manifestation of autoimmune disease represents one of the most remarkable and unexplained examples of the biological differences between men and women3–6. According to the American Autoimmune Related Diseases Association (AARDA), there are 80–100 different autoimmune diseases ranging from the rare disorders such as Asheron’s Syndrome to common disorders such as type 1 diabetes. Notably, rheumatic diseases including systemic lupus erythematosus (SLE, female: male 9:1) and Sjögren’s syndrome (SS, female: male 20-9:1) are chronic systemic autoimmune disorders that predominantly affect women. Other common autoimmune diseases have moderately skewed ratios between the sexes, i.e., multiple sclerosis (MS, female: male 2-3:1). It is important to note that there are few known autoimmune diseases that are exceptions - these diseases processes are ankylosing spondylitis (AS, male: female 2-3:1), type 1 diabetes (male: female 3:2), and psoriasis (male: female 2:1)4, 7, 8.
To better understand the sexually dimorphic basis of autoimmune etiology, sex as a biological variable has become, in the last decade, a standard of research design and analysis in vertebrate animal and human studies - backed by peer-review literature that the consideration of sex is critical to the interpretation, validation, and generalizability of research findings9, 10.Though mechanisms have been put forward in order to elucidate sex bias in immune processes, its molecular underpinnings and their translation into disease phenotype have yet to fully come to fruition3, 11–13.
One intriguing mechanism for sex-biased autoimmunity that emerged from recent study is sexual dimorphism in immunometabolism, which describes the changes in intracellular metabolic pathways in immune cells that alter their function. Fundamental metabolic pathways are essential for mammalian cells to produce energy, precursors for biosynthesis of macromolecules, and reducing power in redox regulations. There is a growing interest in the role of immunometabolism as a critical regulator of the fate and homeostatic function of immune cells. Changes in metabolic pathways within immune cells can be triggered by events of nutrient loss or anoxia, and by immune signals and regulation. Other than energy production and biosynthesis, distinct metabolic pathways can govern the phenotype and function of immune cell subtypes.
Systemic and cellular metabolism of specific immune cell populations highlight novel targets for immune-based therapies. Further understanding of sex differences in immunometabolic regulation will guide personalized medicine for immune-associated diseases. This review aims to highlight key discoveries and unanswered questions in sexual dimorphism in immunometabolism, paving the way for future studies that explore new prevention and treatment strategies for autoimmune diseases.
2. Sexual dimorphism in the immune system
Sex differences in autoimmune diseases can be incompletely elucidated by known differences in the immune system3, 21, 58–60. The following sections will outline observed sexual dimorphism in the immune system and their molecular basis.
2.1. Sexual dimorphism in innate and adaptive immunity
Sex differences in humans are exhibited by both the innate and adaptive immune systems. During an innate immune response, Toll-like receptors (TLRs) are able to sense bacterial and viral components and provoke the stimulation of the cell in order to eliminate the infection14, 15. In addition, TLRs are found to regulate development of dendritic cells (DCs) and initiate antigen-specific adaptive immune responses as they bridge the innate and adaptive immunity15. In the context of autoimmunity, TLR dysregulation is central to disease pathogenesis because when inappropriately activated by self-components, a sustained or exacerbated TLR stimulation can lead to an overproduction of proinflammatory mediators, resulting in sterile inflammation and autoimmunity15.
Importantly, TLR pathways exhibit sexual dimorphism. Souyris and collogues have shown sex-biased expression of genes from the TLR pathway, including increased expression of TLR7 in females compared to males in B cells and myeloid cells15, 16. It has been shown that peripheral blood lymphocytes (PBLs) from women produce higher amounts of IFN-α after stimulation by TLR7 and TLR9 ligands16, 17. Similarly, upon TLR7 stimulation, human female plasmacytoid dendritic cells (pDCs)18 produce higher amounts of IFN-α than their male counterpart, in addition to increased levels of IRF5 at basal state in females compared to males17, 19.
While peripheral blood mononuclear cells (PBMCs) from men produce less IFN-α after TLR7 stimulation, upon TLR9 stimulation, they produce higher levels of the anti-inflammatory cytokine IL-10 than their female counterparts20–22. Additionally, male neutrophils have higher levels of TLR4 and produce more TNF than female neutrophils both at basal state and after stimulation with LPS23, a TLR4 ligand15, 24. Consequently, the increased reactivity of male neutrophils to LPS and resultant increased secretion of proinflammatory cytokines justifies increased risk for septic shock in males25.
In addition to innate immunity, the human adaptive immune system shows strong evidence of sexual dimorphism15, 26. Varying differences are found with immune cell counts dependent on cell type. For examples, higher counts of the cluster of differentiation-4 (CD4+) T cells and increased CD4+/CD8+ ratio are found in females versus males15, 18, 27, 28. Differences in cell function are also observed as CD4+ T cells in females produce higher levels of IFN-γ, and proliferate quicker than CD4+ T cells from men22. Male activated CD4+ T cells have a greater tendency for IL-17α production versus females22, 29. Although B cell counts of the two sexes appear to be comparable, the concentration of serum immunoglobulins (Ig) differs between the sexes16, 30.
In summary, the two sexes exhibit significant differences in both innate and adaptive immunity, which is thought to underlie the observed sex bias in autoimmune diseases (Figure 1).
Figure 1: Summary of sexually dimorphic factors which contribute to sex bias in immune-associated diseases.
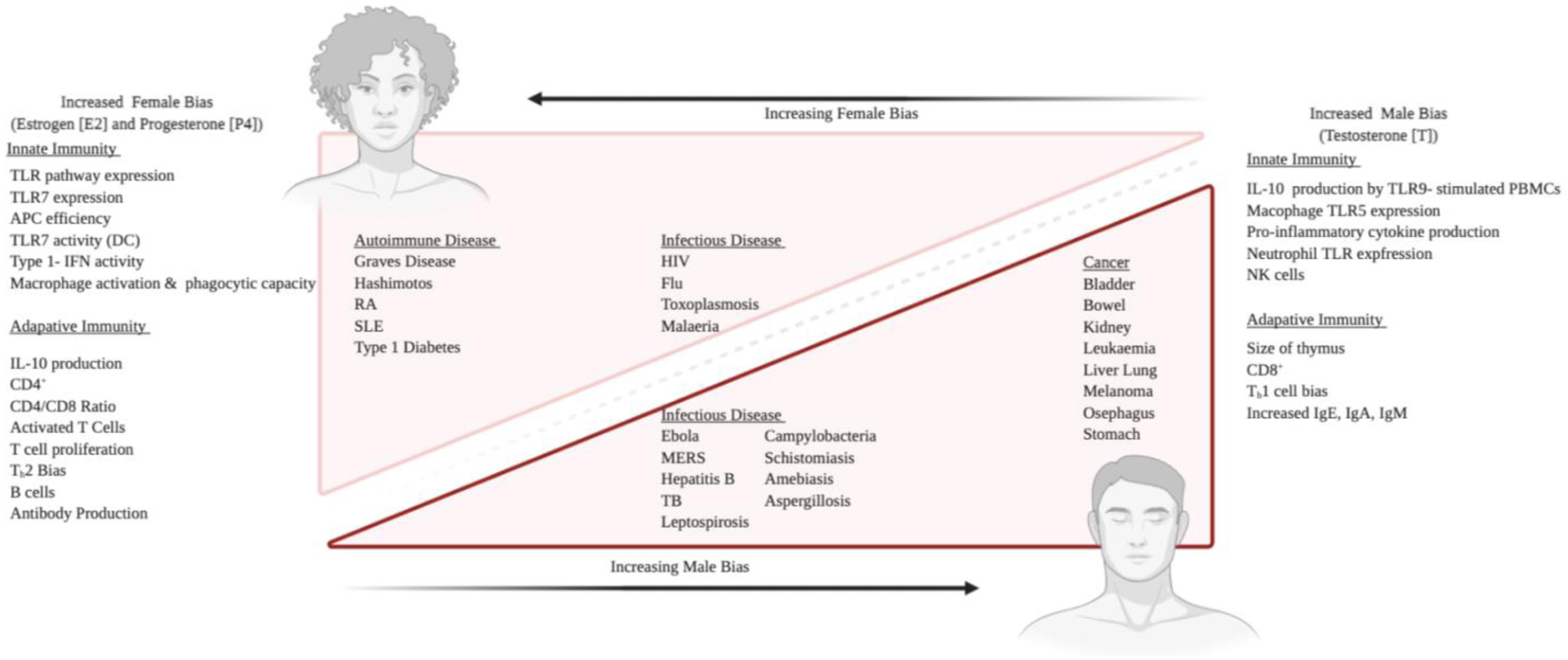
Females and males differ in regulation of both innate and adaptive immunity, including female-biased TLR7 expression, type I - IFN activity, CD4+ T cell count (left) and male-biased IL-10 production and CD8+ T cell count (right). The sexual dimorphism of immunological factors is consistent with the sex bias observed in shown disease processes, where incidence rates, prevalence, susceptibility to and even prognosis of single diagnoses are different for male and females in most cases.
2.2. Causes of sexual dimorphism in immunity
Numerous factors could hypothetically contribute to sex differences in immune cell functions, but several have stood out in the last few years - sex hormones9, 10, 12, sex chromosomes9, 10, 12, epigenetics12, 31–33, and environmental factors12, 32, 34. It is highly important to note that established sex differences in immune cell function change with age and are altered during puberty and pregnancy and parturition18, 33, 35. These changes are associated with lifespan milestones where hormone levels within the body are changed significantly and confirm that sex hormones as well as their regulators play a role in immune responses12. Androgens, such as testosterone (T), have shown immunosuppressive effects whilst estrogen is on the opposite side of the spectrum with immunoenhancing facilitation of mechanisms (Table 1)18, 36–38.
Table 1: Hormonal effects on immune processes11, 26–28, 42–50.
The table summarizes the influence of estrogen, prolactin and testosterone on different cell types of the immune system.
| Estrogen | Prolactin | Testosterone | |||
|---|---|---|---|---|---|
| Cell Type | Effect | Cell Type | Effect | Cell Type | Effect |
| B cell | Retarded B cell maturation Increased plasma cell and autoantibody producing cells Increased expression in CD22, SHP-1, and BCL-2 |
B cell | Increased induction of CD40 Decreased B cell receptor mediated activation threshold Increased IgM and IgG secretion Increased JAK2 expression via B cell autoreactivity. Increased STAT phosphorylation and upregulation Decreased B cell apoptosis related to increased BAFF production and BCL-2 expression. |
B Cell | Increased B cells and decreased IgM and lymphopoiesis |
| DCs | Retarded DC maturation Altered regulation of cytokine and chemokine expression (IL-6, IL-10, IL-12, IL-23, CCL2, and TGFβ |
DCs | Increased expression of CD80/86 via enhanced MHC-II Increased maturation of APCs |
DCs | Decreased MHC-2 and CD86 Decreased proinflammatory cytokines and TLR-mediated activation Increased anti-inflammatory cytokines |
| Macrophage | Altered chemotaxis and phagocytic activity Increased induction of IL-6 and TNFα |
Macrophage | Increased in TNFα, IFNγ, IL-1β, and IL-12 Increased secretion of MCP Controversial increase of IL-10 contingent upon concentration |
Macrophage | Decreased in TNFα, TLR4, as well as eosinophil mediated chemokines Increase in M2 and decreased MCP-1 |
| Neutrophils | Increased induction of TNFα, IL-1β, and IL-6 | Granulocytes | Increased regulation of IRF-1 and iNOS Increased activation of MAPK pathways via STAT1 |
Neutrophils | Increase in granulopoiesis and IL-10 and TGFβ concentrations Decrease in ROS and proinflammatory cytokines and chemokines |
| Th1 | Increased IFNγ expression Increase in Th1 bias |
NKCs | Increased secretion of IFNγ Increased proliferation and cytotoxic activity |
Th1 | Decrease in Th1 bias |
| Th2 | Decrease in Th2 bias | T cell | Increased adhesion of ECs by LFA-1 and VLA-4 | Th2 | Increase in Th2 bias |
| Treg | Increase in regulation of FOXP3 and CTLA-4 | T cell | Increased apoptosis and decreased proliferation | ||
| Mast cell | Increased IL-6 production |
Testosterone influences the immune system by altering T-helper 1 (Th1) response and the action of CD8+ cells whilst down-regulating natural killer (NK) cell response and production of TNFα22. Furthermore, testosterone is found to increase the production of anti-inflammatory cytokines such as IL-1020. Consistently, the presence of testosterone leads to higher production of Th1 by peripheral blood cells, signified by a higher Th1:Th2 ratio in men39. Further sexual dimorphic behavior was shown in immune cell subtypes in a humanized mouse model (DRAG mouse - HLA-DRA,HLA-DRB1*040140) of inflammation where exogenous supplementation of estradiol (E2) in castrated male mice led to an surge in autoimmunity by amplifying Major Histocompatibility Complex II (MHC2) expression and moderating B cell function (Table 1)39. The regulation of immune response of estrogen can be seen by the impairment of B cells and skewing of Th1 response1, 39 and has been confirmed in rheumatoid arthritis (RA) mouse model (DRAG mouse- HLA-DR4/DQ841). A summary of the effect of sex hormones on immune cells can be found in Table 1.
The X chromosome encodes the largest number of immune related genes9, and a large portion of these genes escape from X chromosome inactivation leading to female-biased expression12. The human males produce two types of sex chromosomes, X and Y. Hence, the gametes produced by them are also of two types- one bearing X chromosome and the other bearing the Y chromosome. Thus, human males are said to be heterogametic, and deleterious recessive alleles in X-linked genes (i.e., TLR7, FOXP3, CD4+, and IRAK1) are more likely to cause immune phenotypes in males than in females9, 10. TLR7 and IRAK1 proteins play critical roles in pathogen recognition and induction of a proinflammatory immune response, ensuing in type I IFN production and induction of the IFN inducible genes15, 16. The TLR7 gene escapes X inactivation, leading to gene dosage effects9 that may be relevant for the recognition of both viral and self-RNA-related antigens during autoimmune pathogenesis51–53. Additionally, the X chromosome contains a large amount of microRNAs associated with the immune system, further contributing to sex differences in metabolic and immune function12. Sex differences in immune response are suggestive that sex-specific treatments would be efficacious for clinical and acute care treatment within these population groups.
3. Metabolic regulation of the immune system and its sexual dimorphism
Immune and metabolic functions closely regulate each other at a systemic level, which suggests that crosstalk, as well as, cross-inhibition plays a role in the regulation of their sexually dimorphic functions12. Therefore, immunometabolism, the study of the multilayered interactions between immune and metabolic systems, has emerged as an exciting and important area of scientific investigation. It is expected that a better understanding of sex differences in immunometabolic regulation will help guide personalized, sex-specific treatment of autoimmune diseases.
3.1. Concept of immunometabolism
The immune system encompasses a heterogeneous populace of cells that are relatively quiescent in the steady state but share the ability to rapidly respond to infection and inflammation8. The ability to rapidly and effectively mount an inflammatory reaction requires considerable energy expense and is accompanied by metabolic changes. Metabolism consists of exceedingly interconnected, and complicated biochemical pathways within the human body11, 45, 54. The major metabolic pathways are: glycolysis, where glucose is oxidized in order to generate ATP, albeit in a relatively inefficient manner; citric acid cycle (CCA) cycle, a nexus for multiple nutrients inputs that is used for efficient ATP generation; the pentose phosphate pathway (PPP), allowing diversion of intermediates from glycolysis towards the production of nucleotide and amino acid precursors; fatty acid oxidation, allowing the conversion of fatty acids into downstream products for energy generation; fatty acid synthesis, generating lipids for cellular growth and proliferation; amino acid metabolic pathways, using amino acids for protein synthesis and signaling regulation28.
Cells use intricate mechanisms to sense levels of metabolites produced by these metabolic pathways and activate signaling pathways accordingly to maintain metabolic homeostasis. Of these mechanisms, one central metabolic regulator of immunity is the mechanistic target of rapamycin (mTOR) - AMP kinase (AMPK) pathway28, 51, 55, 56. mTOR is the catalytic subunit of mTOR complex (mTORC-) 1 and 2 which sense amino acids and growth factors and promote mRNA translation57. Additionally, mTORC1/2 signaling contributes to lipid synthesis and cell growth57. Intriguingly, in the immune system, mTOR signaling facilitates events critical for T cell and monocyte differentiation, suggesting immunometabolic crosstalk. Nutrient deprivation signals to AMP kinase, which promotes catabolism of free fatty acids (FFA) and inhibits mTOR activity, thus limiting immune cell activation57.
mTOR function is regulated by the protein kinase B (PKB/Akt), which is known to play a critical role in cell growth, metabolism, proliferation, and survival. PKB/Akt activation is controlled by a complex stepwise progression that involves phosphoinositide-3-kinase (PI3K)58–60. Stimulated receptors incite class 1A PI3Ks that triggers the activation of PI3K and conversion by its catalytic domain of phosphatidylinositol (3,4)-bisphosphate (PIP2) lipids to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). Subsequently, PKB/Akt binds to PIP3, permitting PDK1 to access and phosphorylate T308 in the activation loop, leading to partial activation of PKB/Akt58. Successively this activate mTOR-complex 1 (mTORC1) by phosphorylating and inhibiting tuberous sclerosis protein 2 (TSC2)59.
mTORC1 substrates are found to further phosphorylate ribosomal protein-S6 (RPS6), promoting protein synthesis and cellular proliferation58. Depletion of energy leads to inactivation of mTORC1, activation of AMPK, forkhead box transcription family-O (FOXO), and promotes constitution of mTORC2 that leads to phosphorylation of Akt58–60. Akt can also be activated without PI3K; which appears to be advantageous in situations like nutrition deprivation, where insulin/insulin growth factor signaling is not optimal59. An applied example of this can be seen when CD3/CD28 ligation activates CD4+ T cells, leading to signaling through PI3K/Akt/mTOR. PI3K/Akt/mTOR signaling subsequently leads to activation of glycolysis and mitochondrial oxidative phosphorylation (OXPHOS), resulting in CD4+ activation7–8, 19–23,60 (Table 2).
Table 2: Immunometabolic pathways in immune cells.
Components of the inflammatory response where ‘inducers, sensors, mediators, effectors, and outcomes’ are associated with specific metabolic processes. Herein, inducers of inflammation activate ‘mediator’ signaling, resulting in modulation of ‘effector’ metabolic pathways and leading to cellular outcomes such as activation, proliferation, and cytokine production.
| Cell Type | Inducers | Mediators | Effectors | Outcome | Reference |
|---|---|---|---|---|---|
| Activated CD4+ T Cell | CD3/CD28 | PI3K/Akt/mTOR ERK/MAPKc-MycHIF-1α | Glycolysis, Mitochondrial OXPHOS | Activation, Proliferation, Cytokine production | 7–8, 19–23 |
| Activated Dendritic Cell | PAMPs | PI3K/AktHIF-1α | Glycolysis | Presentation, Cytokine production | 7 |
| B Cell | PAMPs | PI3K/Akt | Glycolysis | Activation, Proliferation | 7, 20 |
| Memory CD8+ T Cell | IL-15 | AMPK | FAO | Survival, Quiescence | 7, 24–28 |
| Naive CD4+ T Cell | IL-7 | PI3K/Akt | Mitochondrial OXPHOS, FAO | Survival | 7–8, 22–23, 28–30 |
| Neutrophil | PAMPs, | HIF-1α | Glycolysis | ROS | 7 |
| Resting Dendritic Cell | Growth factors (GM-CSF, FLT3) | - | FAO | Growth, Survival Activation | 7, 31–32 |
In addition to CD4+ T cells, PI3K/Akt/mTOR/AMPK regulates immunometabolic functions in a variety of immune cells. A summary of immunometabolic pathways regulating immune cell function can be found in Table 2.
The direct regulation of immune processes by metabolism can further be observed within various immune cell types where they switch between distinctive metabolic pathways to respond to changes in a dynamic immune response57. For example, an inflammatory M1 macrophage uses the glycolysis pathway to support phagocytosis and inflammatory cytokine production, and utilizes the pentose phosphate pathway to support nucleotide and ROS production57. Another depiction of this specific pathway reliance can be observed in regulatory T cells when utilizing the CCA pathway instead of FFA oxidation because an suppressive function is needed versus the generation of Treg cells in response to tolerogenic stimuli57.
3.2. Sexual dimorphism in immunometabolism
While historically metabolism has been studied with the assumption that basic cellular machineries operate in the same way in males and females, it has been recently accepted that the two sexes exhibit significant differences in metabolic regulation. Estrous cycles in females are known to induce variability in traits, and males can exhibit more pronounced metabolic disease phenotype than females57.
Similarly, sex-biased regulation of immunometabolism is supported by the finding that the sex steroid regulator sex hormone-binding globulin (SHBG) regulates the tissue availability of sex steroids and influences E2 signaling in lymphocytes, which possibly underlies the female bias in multiple sclerosis61. At the intersection of immune and metabolic functions, SHBG also contributes to pathogenesis of metabolic diseases such as obesity and metabolic syndrome62.
In addition, in research of the immunometabolic alterations in diabetes, it was found that sex hormones regulate visceral adipose tissue mesenchymal stromal cells and their production of IL-33, which could account for differences in regulatory T cells (Tregs) in basal or obese state between males and females63, 64.
With the last decades seeing a growing interest in immunometabolism research, the recent recognition of sex differences being a fundamental feature of immunometabolism calls for attention from the scientific community. A better understanding of sexual dimorphism in immunometabolism will provide scientific basis to develop sex-based precision medicine for immune and metabolic diseases.
4. Metabolic alterations in autoimmune disease and immunometabolism as a fundamental mechanism for sexual dimorphism in autoimmunity
With mounting evidence supporting the metabolic regulation of immune functions, it is not surprising that metabolic alterations in autoimmune disease have been documented2, 7, 65–67. The findings highlighting major metabolic alterations in autoimmunity are summarized below.
4.1. Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease that is characterized by chronic inflammation2, 8, 68; often illustrated by the involvement of multiple organs and clinical displays of nephritis, vasculitis and pathogenic autoantibodies such as anti-double stranded DNA (dsDNA)69, 70. In addition to altered function of immune cells1, 11 including CD4+ T Cells26, 71, 72, dendritic cells (DC)73, macrophages8, 73–78, and neutrophils76, metabolic systems play an integral role in checkpoints that control immune cell fate and function28, 54, 79, 80. Therefore, it is crucial to examine the relationship between mitochondrial dysfunction, oxidative stress, and abnormal metabolism that involves glucose, lipid and amino acid metabolism of immune cells to understand the underlying pathogenic mechanisms of SLE13, 52, 81. Notably, metabolite intermediates that are produced within mitochondria have been found to serve as inflammatory signals (e.g., succinate in myeloid cells)21, 52. A sentential breakthrough by Frauwirth and colleagues82 highlighted the activation of CD28 by glycolysis in T cells, leading to a large push in researchers looking to elucidate the regulation of T cells by metabolic substrates11, 45, 54, 71, 83. It is now understood that resting T cells are influenced by mitochondrial oxidative phosphorylation (OXPHOS) and that antigen-mediated stimulation and acquisition of effector functions elicit a striking metabolic reprogramming, shown an upregulation of glucose use followed by the activation of mitochondria-independent glycolysis as the major source of building blocks necessary to cope with considerable proliferation as well as production of effector molecules13, 29, 83.
Glucose is a fundamental energy source for most cells and aids cellular proliferation, development and survival28, 72. It is known that activated T cells enhance glucose metabolism in order to meet requirements of cellular proliferation and differentiation. Subsequently, glucose deficiency leads to decreased levels of ATP and AMP-activated protein kinase (AMPK) activation84, which in the normal setting has a positive homeostatic effect on signaling pathways that compensate for cellular ATP. This can be shown in the activation of AMPK promoting GLUT4 transcription and translocation to promote glucose intake72. Conversely, AMPK negatively modulates key proteins in ATP-consuming reactions such as mTORC228, 51, 56, glycogen synthase, sterol regulatory element binding protein 1 (SREBP-1) and tuberous sclerosis 2 (TSC2), leading to inhibition of gluconeogenesis as well as glycogen, lipid, and protein synthesis72. It is important to note that GLUT1 overexpression in CD4+ T cells has an influence on Treg cell expansion, which has led to the concept that there is a difference in glucose metabolism for regulatory and effector T cells. GLUT1 is induced by HIF1α, which ultimately aids in Th17 differentiation22, 72, 85. Additionally, the increase in GLUT1 expression and glucose uptake occurs in a PI3K/Akt-dependent manner, allowing cells to maintain their mitochondrial potential and ATP homeostasis72. Correspondingly, in the absence of sufficient extrinsic signals, cell surface GLUT1 expression decreases, resulting in diminished glucose uptake, drop in mitochondrial membrane potential and ATP synthesis, and cell death72. Since this decline in viability occurs in the presence of appropriate glucose and oxygen, it suggests that growth factor signaling is indispensable for maintenance of metabolic homeostasis in naïve CD4+ T cells52, 72, 83.
Yet, the inhibition of AMPK and the downstream mTORC1 activation by Roquin-1 promotes a lupus-prone phenotype71. Roquin-1 blocks AMPK activation, allowing the function of mTORC1 and mTORC2, which are known to impact T helper follicular (Tfh) cell differentiation86. In addition, recent studies have found that retention of activated mTORC1 during asymmetric cell division in CD8+ T cells presents the daughter cell with effector functions, whereas the mTORC1-low daughter cell acquires memory properties. It is likely that a similar asymmetric distribution of mTORC1 exists between effector and memory CD4+ T cells55. Dysregulation of Tfh, CD8+ and CD4+ T cell differentiation altogether may underlie the lupus-prone phenotype induced by Roquin-1.
Similarly, mTORC1 activation was observed in CD4+ T cells from several strains of lupus-prone mice. Interestingly, treatment of these mice with 2-Deoxy-D-glucose (2-DG) and metformin normalized mTORC1 activation concomitant with disease reversal52.
Consistent with mouse model studies, mTORC1 activation has been demonstrated in CD4+ T cells of SLE patients and has been proposed to serve as a biomarker of autoimmune inflammation56, 72. Treatment with rapamycin, which inhibits mTOR and enhances Treg suppressive function, is effective in SLE patients and in lupus-prone New Zealand mixed (NZW/NZW F1) mice28, 51, 56, 72, 87. Therefore, targeting of mTORC, the critical player integrating environmental cues, nutrient levels and immune response output, is promising in treatment of SLE (Figure 2).
Figure 2: Potential metabolic pathways of intervention in autoimmune diseases.
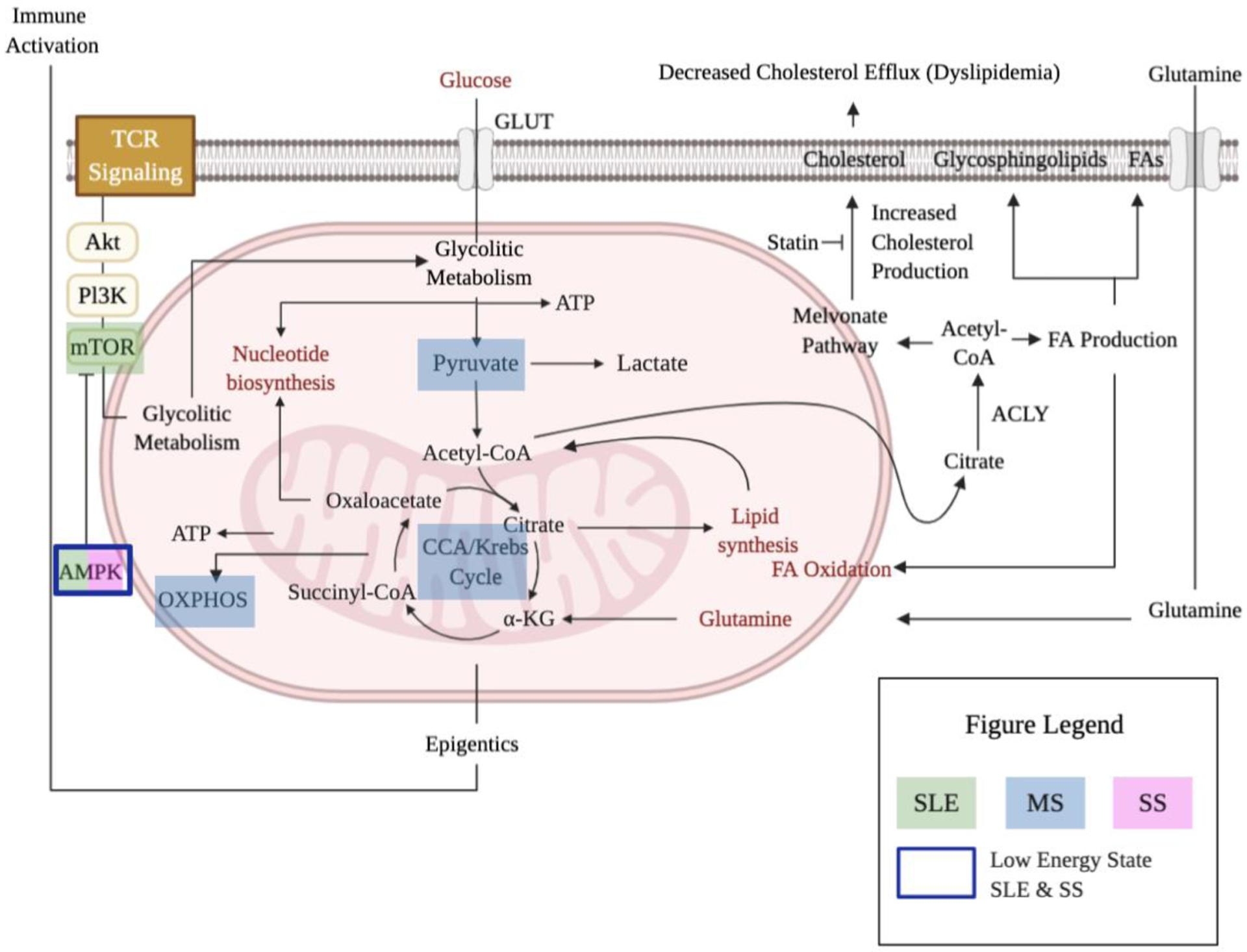
Schematic representing important metabolic targets in SLE treatment in efforts to correct the immunometabolic alterations, as AMPK and mTORC are mechanistically critical for SLE pathogenesis (green). In a similar fashion, pyruvate metabolism, OXPHOS, and CCA are highlighted as targets for MS (blue). Low energy level is associated with both SS (pink) and SLE which can be linked to the antiapoptotic effects of adiponectin mediated by phosphorylation of AMPK (blue box).
Although most research has been conducted on the influence of glucose metabolism in SLE T cells11, 28, 72, 81, glucose is also important to other immune cell types. It has been shown that B cells in the lupus-prone NZW/NZW F1 mice exhibit a highly glycolytic phenotype52. However, their mechanistic actions remain unsettled72.
4.2. Sjögren’s syndrome
Sjögren’s syndrome (SS) is a systemic autoimmune disease that is characterized by infiltration of lymphocytes into the exocrine glands, inflammation, tissue damage, and dysfunctional glandular secretion84, 88, 89. Destruction of the lacrimal and salivary glands, which typically occurs in patients with SS, results in ocular dryness (keratoconjunctivitis sicca) and oral dryness (xerostomia)84. Patients with SS often have extra-glandular complications such as non-erosive polyarthritis, arthralgias, vasculitis, and chronic fatigue84. Furthermore, patients with SS have an increased incidence of progression to various non-Hodgkin lymphomas, which may influence the rate of morbidities84, 89. The pathogenesis of SS is mediated by complex mechanisms involving infiltration by lymphocytes (mainly T and B cells) of target organs during a dysregulated adaptive immune response84, 88. In the T- and B-cell-containing ectopic lymphoid structures in the salivary and lacrimal glands, hyperactivated B cells produce autoantibodies, e.g., anti-SSA/Ro and -SSB/La, against small RNA molecules and rheumatoid factors84, 88, 89. Activation of B cells by follicular helper T (Tfh) cells is crucial for the clonal selection and affinity maturation71, 86.
Since metabolic aberrations of immune cells drive immune regulation in mammals, it was postulated that the “immune” phenotype of salivary gland epithelial cell (SGEC) undergoes similar control by their metabolism and may actively shape the autoimmune response in SS30, 84. SGEC are secretory cells with constant high energy demands and their metabolic machinery is expected to suit their lifestyle84. Disturbances of this process may be enforced by insufficient energy supply, endoplasmic reticulum (ER) stress or even chronic stress, leading to metabolic reprogramming and eventually immunogenic cell death characterized by the release of cellular autoantigens84. Differential adiponectin production by SGEC in SS indicates a low energy phenotype and the antiapoptotic effects of adiponectin mediated by phosphorylation of AMPK provide a robust paradigm for the interconnection between metabolism and immune functions of SGEC in the context of glandular lesion in SS84 (Figure 2).
4.3. Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS)43. The incursion of the brain by activated immune cells across the endothelial cells (ECs) of the blood brain barrier is due to the loss of immune self-tolerance43. MS is characterized by inflammation, demyelination, nonspecific reactive changes of glial cells, and neuronal loss. From a pathological perspective, the presence of perivascular lymphocytic infiltrates are indicative of the disease process, with consequent macrophage degradation of myelin sheaths that surround neurons43. The MS predominance ratio of female to male has increased within the last few decades from 2.3–3 – 5:1. This is suggestive that the presence of hormone receptors associated with immune cells and sex hormones (androgens, estrogens, progesterone, and prolactin) have a great influence on immune system function and disease progression38, 43, 47, 49, 87, 90.
Biochemical studies regarding MS have established the notion of defective pyruvate metabolism, in addition to increased sera concentrations of citric acid cycle (CCA) acid such as α-ketoglutarate (AKG)91 and citrate (Figure 2). AKG is one of the most important nitrogen transporters in metabolic pathways, produced by oxidative decarboxylation via isocitrate dehydrogenase as well as oxidative deamination of glutamate via glutamate dehydrogenase91. MS patients are found to have elevated serum and cerebrospinal fluid pyruvate levels, as well as antibodies that were reactive with triose phosphate isomerase and GAPDH and inhibit glycolytic activity of GAPDH42, 47. In regard to altered OXPHOS, there is a striking reduction of ATP synthase and increased activation of mitochondrial electron transport chain47. Therefore, correcting deficiencies in pyruvate metabolism is critical to managing MS clinically (Figure 2).
Reiterated from the glucose metabolism of SLE above, the metabolism of glucose in the setting of MS is the same. The foundation of glucose metabolism starts with glucose entering cells via GLUT transporters and being phosphorylated by hexokinase49. The product glucose 6-phosphate can be metabolized via glycolysis - producing pyruvate, ATP, and NADH, where pyruvate enters the mitochondria and is metabolized via the CCA cycle and OXPHOS13, 29, 83, 92. It is important to mention that pyruvate can also be reduced to lactate-by-lactate dehydrogenase and released into extracellular space via monocarboxylate transporters (MCTs). Additionally, glucose 6-phosphate can be taken through PPP or converted to glycogen via glycogenesis in astrocytes in the brain28. However, unlike the role of insulted glucose metabolism in SLE, the role of glucose metabolism in MS is still incompletely understood.
4.4. Immunometabolism as a fundamental mechanism for sexual dimorphism in autoimmunity
It has been discussed that maintaining metabolic homeostasis is critical in the prevention of autoimmunity. A bulk of metabolism, including energy balance - glucose and lipid metabolism, are regulated in a sexually dimorphic manner and successively influence the pathogenesis of autoimmune disease. However, the fundamental question of why sex differences in autoimmunity exist remains unanswered. To address this question, researchers such as Pagenkopf and colleagues3, 6 have focused on immunometabolic functions of transcriptional cofactors that provide an evolutionary validation for sexual dimorphism in autoimmunity. An example of this can be highlighted within the female-biased gene network that has been described in human skin that is associated significantly with the susceptibility to female-biased autoimmunity. An upstream regulator of this gene network, vestigial family member-3 (VGLL3), exhibits female-biased expression in healthy human skin and is further upregulated in autoimmune diseases including SLE, SS and systemic sclerosis6. In secondary studies from this group, their results demonstrated that energy deficiency is a critical trigger that upregulates VGLL3 and that female-biased expression of VGLL3 helps cells adapt to metabolic stress. Intriguingly, when placental mammals evolved, the need to feed a developing embryo posed significant challenge to metabolic pathways93. Therefore, the finding that VGLL3 helps non-placental tissue such as the skin adapt to energy stress provides an evolutionary rational for the selection of its increased expression in females. This study further identifies nutritional deficiency as a trigger that can turn this evolutionary strength into weakness by causing autoimmune pathogenesis, and highlights the importance of maintaining metabolic homeostasis in prevention of autoimmunity3.
5. Translational Implications
It can be inferred that immunometabolism in regard to autoimmune disease since its inception has primarily focused on glycolysis, the CCA cycle, OXPHOS, and free fatty acids (FFA) synthesis and oxidation. This is based on the findings of pathways associated with the energy needs of cell growth, membrane rigidity, cytokine production and proliferation. Seemingly translational immunometabolism is suggestive of a repositioning of metabolic drugs that exploit new targets.
Novel drugs which modulate metabolic processes have the potential to correct the aberrant immune responses and be used to treat autoimmune disease patients (Figure 2). Looking at SLE specifically, strategies targeting mTOR activation, including use of rapamycin, could be promising ways to diminish the disease severity in SLE patient populations28, 51, 55, 56.
Additionally, tuning of FFA pathways, including that seen in glucocorticoid (prednisone) treatment, has been directly linked to leptin reduction through inhibition of mTOR in SLE patient populations51, 56, 68. Also, the complex interaction among mitochondrial, and mTOR signaling pathways and their ability to control the chemotaxis of neutrophils suggest metabolic options to restore normal neutrophil functions in SLE51. Based on the finding that macrophage polarization follow distinct metabolic pathways, the translation of metabolic shifts to disease has gained importance, especially for diseases that clearly lean toward either phenotype (M1 vs M2)51. Notably, the influence of macrophage polarization has met with relative success clinically for ovarian carcinoma, showing that therapeutically targeting macrophage metabolism might be a viable option in the future for SLE and MS51.
Symptomatically, fatigue and low energy states are commonly reported amongst patients hindering with SLE and MS. Defining fatigue can be tricky, where varying definitions can be grouped according to type (i.e., subjective, physiological, and/or performance). Herein this review, we define fatigue as insufficient cellular capacity or system-wide energy to maintain the original level of activity and/or processing by using normal resources. Furthermore, physiological processes have been described to play a role in fatigue that include oxygen/nutrient supply and metabolism - which are exaggerated by inflammation. Effects contributing to fatigue are associated with enhanced inflammation and increased cytokine expression amongst others94. In addition, with nutritional deficiency as an autoimmune trigger, it is reasonable to assume that nutritional monitoring strategies can be employed to develop in order to prevent and/or treat autoimmune disorders.
This is not without considering the impact the biological sex has on personal immunity. Clear differences in male and female immunity contribute to variations in disease predisposition, severity, and drug responses (Figure 1). Additional co-factors that influence sex hormones, such as environment stress and toxin exposure, could also impact immunometabolic responses and autoimmune pathogenesis in a dynamic manner (i.e., change with age and events in life). One prominent example of sex-specific drug response is the impact of gender on immune checkpoint inhibitors-induced autoimmunity80. Using the example of MS, Golden and colleagues95 were able to elucidate the efficacious clinical benefit of discussing sex differences with treatment options of patients. Taking these observations to the laboratory bench allowed researchers to describe the mechanisms underlying sex differences, and to investigate therapeutics based on findings. Continually examining sex differences in the same “bedside to bench to bedside” fashion will bare endless novel therapeutics and treatment strategies following the identification of sex-specific disease drivers. In addition, sexual dimorphic studies will allow us to design sex-stratified treatment strategies that maximize efficiency and minimize side effects in both male and female patients.
6. Conclusions
There is a growing amount of academic literature on immunometabolism that provides novel insights into autoimmune pathogenesis. When adding in additional contributory factors such as sex and its biological implications the complexity of the topic grows. Notably, the biological sex effects the production, maturation, differentiation, metabolism and ultimately the functioning of cells, in both physiology and pathology of the immune system. Taken together the topics covered can shed light on how sex-specific metabolic reprogramming therapeutic can be implored to enhance outcomes in autoimmune diseases.
Highlights.
Susceptibility and progression of autoimmune diseases exhibit sex differences, which necessitates sex-specific prevention and treatment strategies
Fundamental aspects of immune functions and metabolic homeostasis are regulated differently in males and females, underlying sex differences in autoimmune diseases
Recent discoveries in the area of immunometabolism, the regulation of immune responses by metabolic processes, represent exciting opportunities to combat autoimmune diseases
Based on the metabolic regulation of immune cell functions, sex-specific metabolic reprogramming therapeutics can be implored as novel approaches to enhance outcomes in autoimmune diseases in a personalized manner
Acknowledgements
R.M. is funded by the Molecular and Environmental Toxicology T32 Training Grant (T32 ES007015). Y.L. is funded by the US National Institutes of Health (K01-AR073340) and Wisconsin Partnership Program New Investigator Award.
Abbreviations
- AAM
Alternatively activated macrophage
- 5-HT
5-Hydroxytryptamine (Serotonin)
- AARDA
American Autoimmune Related Diseases Association, Inc
- AKG
α-ketoglutarate
- AMPK
AMP-activated protein kinase
- APC
Antigen-presenting cell
- AS
Ankylosing spondylitis
- ATP
Adenosine triphosphate
- BCR
B cell receptor
- CAM
Classically activated macrophage
- CCA
Citric acid cycle / Krebs cycle
- CD
Cluster of differentiation
- CNS
Central nervous system
- COX5b
Cytochrome c oxidase subunit 5b
- cTfh
Circulating-Tfh
- dsDNA
Anti-double-stranded DNA
- E2
Estradiol
- EC
Endothelial cell
- ERK
Extracellular signal-regulated kinase
- FADH2
Fuel oxidative phosphorylation
- FAO
Fatty-acid oxidation
- FFA
Free fatty acid
- FLT3
Fms-related tyrosine kinase 3 ligand
- FOXO
Forkhead box transcription factors-O
- FOXP
Forkhead box protein-P
- GH
Growth hormone
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- HIF-1α
Hypoxia-inducible factor 1-alpha
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- Ig-
Immunoglobulin
- IL
Interleukin
- ILC
Innate lymphoid cells
- iNOS
Inducible nitric oxide synthase
- IRAK-1
Interleukin-1 receptor associated kinase
- IRF-1
Interferon regulatory factor 1
- IRS
Insulin receptor substrate
- LFA-1
Lymphocyte function-associated antigen 1
- MAPK
Mitogen-activated protein kinase
- MCL-2
Macrophage C-type lectin 2
- MCP
Monocyte chemoattractant protein
- MCP-1
Monocyte chemoattractant protein 1
- MCT
Monocarboxylate transporters
- MHC
Major Histocompatibility Complex (1/2)
- MS
Multiple sclerosis
- mTOR
Mechanistic target of rapamycin
- mTORc
Mechanistic target of rapamycin complex
- NADH
Nicotinamide adenine dinucleotide
- NK
Natural killer
- NO
Nitric oxide
- NOD
Nucleotide oligomerization domain
- OXPHOS
Oxidative phosphorylation
- P4
Progesterone
- PAMP(s)
Pathogen-associated molecular pattern
- PBL
Peripheral blood lymphocytes
- PDK-1
Phosphoinositide-dependent kinase 1
- PGC1β
Peroxisome proliferator-activated receptor beta
- PI3K
Phosphoinositide 3-kinase
- PIP2
Phosphatidylinositol (3,4)-bisphosphate
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- PKB/Akt
Protein kinase B
- PPP
Pentose phosphate pathway
- PRR(s)
Pattern recognition receptors
- PVM
Perivascular macrophage
- RA
Rheumatoid arthritis
- ROS
Reactive oxygen species
- RPS6
Ribosomal protein-S6
- SGEC
Salivary gland epithelial cell
- SHBG
Sex hormone-binding globulin
- SLE
Systemic lupus erythematosus
- SREBP-1
Sterol regulatory element binding protein 1
- SS
Sjögren’s syndrome
- STAT1
Signal transducer and activation of transcription factor 1
- T
Testosterone
- TB
Tuberculosis
- TCA
Tricarboxylic acid
- TCR
T cell receptor
- Tfh
T-follicular helper cells
- Th
T-helper (1/2)
- TLR
Toll-like receptors
- TSC2
Tuberous sclerosis 2
- VGLL3
Vestigial family member 3
- VLA-4
Very late antigen 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
No conflicting interests to declare.
References
- 1.Bhatia A, Sekhon HK, Kaur G. Sex hormones and immune dimorphism. ScientificWorldJournal. 2014;2014:159150. Epub 2014/12/06. doi: 10.1155/2014/159150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christou EAA, Banos A, Kosmara D, Bertsias GK, Boumpas DT. Sexual dimorphism in SLE: above and beyond sex hormones. Lupus. 2019;28(1):3–10. Epub 2018/12/07. doi: 10.1177/0961203318815768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagenkopf A, Liang Y. Immunometabolic function of the transcription cofactor VGLL3 provides an evolutionary rationale for sexual dimorphism in autoimmunity. FEBS Lett. 2020. Epub 2020/08/18. doi: 10.1002/1873-3468.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Y, Xu WD, Peng H, Pan HF, Ye DQ. SOCS signaling in autoimmune diseases: molecular mechanisms and therapeutic implications. Eur J Immunol. 2014;44(5):1265–75. Epub 2014/03/07. doi: 10.1002/eji.201344369. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Kahlenberg JM, Gudjonsson JE. A vestigial pathway for sex differences in immune regulation. Cell Mol Immunol. 2017;14(7):578–80. Epub 2017/05/23. doi: 10.1038/cmi.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagenkopf AC, Liang Y. A New Driver for Lupus Pathogenesis is conserved in Humans and Mice. Lupus (Los Angel). 2019;4(2). Epub 2020/02/08. doi: 10.35248/2684-1630.19.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostensson M, Monten C, Bacelis J, Gudjonsdottir AH, Adamovic S, Ek J, et al. A possible mechanism behind autoimmune disorders discovered by genome-wide linkage and association analysis in celiac disease. PLoS One. 2013;8(8):e70174. Epub 2013/08/13. doi: 10.1371/journal.pone.0070174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039. Epub 2016/06/17. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 9.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35(3):97–104. Epub 2013/11/19. doi: 10.1016/j.it.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.vom Steeg LG, Klein SL. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016;12(2):e1005374. Epub 2016/02/20. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freitag J, Berod L, Kamradt T, Sparwasser T. Immunometabolism and autoimmunity. Immunol Cell Biol. 2016;94(10):925–34. Epub 2016/08/27. doi: 10.1038/icb.2016.77. [DOI] [PubMed] [Google Scholar]
- 12.Mishra S, Bassi G, Xu YXZ. Sex Differences in Immunometabolism: An Unexplored Area. Methods Mol Biol. 2020;2184:265–71. Epub 2020/08/19. doi: 10.1007/978-1-0716-0802-9_18. [DOI] [PubMed] [Google Scholar]
- 13.Takeshima Y, Iwasaki Y, Fujio K, Yamamoto K. Metabolism as a key regulator in the pathogenesis of systemic lupus erythematosus. Semin Arthritis Rheum. 2019;48(6):1142–5. Epub 2019/05/06. doi: 10.1016/j.semarthrit.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Spachidou MP, Bourazopoulou E, Maratheftis CI, Kapsogeorgou EK, Moutsopoulos HM, Tzioufas AG, et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2007;147(3):497–503. Epub 2007/02/17. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37(1):20–36. Epub 2017/10/14. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- 16.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19). Epub 2018/01/28. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Ma SS, Zhang WY, Duan JP. Changes in peripheral blood inflammatory factors (TNF-α and IL-6) and intestinal flora in AIDS and HIV-positive individuals. J Zhejiang Univ Sci B. 2019;20(10):793–802. Epub 2019/09/07. doi: 10.1631/jzus.B1900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol. 2019;56(3):308–21. Epub 2017/10/01. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Panchanathan R, Rajavelu P, Duan X, Gould KA, Choubey D. Gender-dependent expression of murine Irf5 gene: implications for sex bias in autoimmunity. J Mol Cell Biol. 2010;2(5):284–90. Epub 2010/08/31. doi: 10.1093/jmcb/mjq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev. 2002;13(4–5):403–12. Epub 2002/09/11. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 21.Stojic-Vukanic Z, Kotur-Stevuljevic J, Nacka-Aleksic M, Kosec D, Vujnovic I, Pilipovic I, et al. Sex Bias in Pathogenesis of Autoimmune Neuroinflammation: Relevance for Dimethyl Fumarate Immunomodulatory/Anti-oxidant Action. Mol Neurobiol. 2018;55(5):3755–74. Epub 2017/05/24. doi: 10.1007/s12035-017-0595-2. [DOI] [PubMed] [Google Scholar]
- 22.Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine. 2015;72(2):146–53. Epub 2015/02/04. doi: 10.1016/j.cyto.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Millett CE, Phillips BE, Saunders EFH. The Sex-specific Effects of LPS on Depressive-like Behavior and Oxidative Stress in the Hippocampus of the Mouse. Neuroscience. 2019;399:77–88. Epub 2018/12/17. doi: 10.1016/j.neuroscience.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Nusbaum JS, Mirza I, Shum J, Freilich RW, Cohen RE, Pillinger MH, et al. Sex Differences in Systemic Lupus Erythematosus: Epidemiology, Clinical Considerations, and Disease Pathogenesis. Mayo Clin Proc. 2020;95(2):384–94. Epub 2020/02/08. doi: 10.1016/j.mayocp.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Li M, Liu L, Geng B. Muscarinic M1 and M2 receptor subtypes play opposite roles in LPS-induced septic shock. Pharmacol Rep. 2019;71(6):1108–14. Epub 2019/10/22. doi: 10.1016/j.pharep.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Schneider-Hohendorf T, Gorlich D, Savola P, Kelkka T, Mustjoki S, Gross CC, et al. Sex bias in MHC I-associated shaping of the adaptive immune system. Proc Natl Acad Sci U S A. 2018;115(9):2168–73. Epub 2018/02/15. doi: 10.1073/pnas.1716146115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold SM, Willing A, Leypoldt F, Paul F, Friese MA. Sex differences in autoimmune disorders of the central nervous system. Semin Immunopathol. 2019;41(2):177–88. Epub 2018/10/27. doi: 10.1007/s00281-018-0723-8. [DOI] [PubMed] [Google Scholar]
- 28.Stathopoulou C, Nikoleri D, Bertsias G. Immunometabolism: an overview and therapeutic prospects in autoimmune diseases. Immunotherapy. 2019;11(9):813–29. Epub 2019/05/24. doi: 10.2217/imt-2019-0002. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann U, Kahlfuss S, Yang J, Ivanova E, Koralov SB, Feske S. Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metab. 2019;29(5):1104–18.e6. Epub 2019/02/19. doi: 10.1016/j.cmet.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trzeciak M, Bagavant H, Papinska J, Deshmukh US. Immune Response Targeting Sjogren’s Syndrome Antigen Ro52 Suppresses Tear Production in Female Mice. Int J Mol Sci. 2018;19(10). Epub 2018/09/29. doi: 10.3390/ijms19102935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiaroni-Clarke RC, Munro JE, Ellis JA. Sex bias in paediatric autoimmune disease - Not just about sex hormones? J Autoimmun. 2016;69:12–23. Epub 2016/03/14. doi: 10.1016/j.jaut.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Edwards M, Dai R, Ahmed SA. Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production. Front Immunol. 2018;9:478. Epub 2018/04/18. doi: 10.3389/fimmu.2018.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacini G, Paolino S, Andreoli L, Tincani A, Gerosa M, Caporali R, et al. Epigenetics, pregnancy and autoimmune rheumatic diseases. Autoimmun Rev. 2020;19(12):102685. Epub 2020/10/30. doi: 10.1016/j.autrev.2020.102685. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MH, Erdei E. Comparative United States autoimmune disease rates for 2010–2016 by sex, geographic region, and race. Autoimmun Rev. 2020;19(1):102423. Epub 2019/11/17. doi: 10.1016/j.autrev.2019.102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun. 2012;38(2–3):J109–19. Epub 2011/11/15. doi: 10.1016/j.jaut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol Rev. 2016;96(1):55–97. Epub 2015/11/20. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–23. Epub 2013/02/26. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes M, Pauling JD, Armstrong-James L, Denton CP, Galdas P, Flurey C. Gender-related differences in systemic sclerosis. Autoimmun Rev. 2020;19(4):102494. Epub 2020/02/18. doi: 10.1016/j.autrev.2020.102494. [DOI] [PubMed] [Google Scholar]
- 39.Taneja V Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. Epub 2018/09/14. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One. 2012;7(4):e36095. Epub 2012/05/04. doi: 10.1371/journal.pone.0036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens M, Luckey D, Luthra H, David C, Taneja V. B cells influence sex specificity of arthritis via myeloid suppressors and chemokines in humanized mice. Clin Immunol. 2017;178:10–9. Epub 2015/06/10. doi: 10.1016/j.clim.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Adiele RC, Adiele CA. Metabolic defects in multiple sclerosis. Mitochondrion. 2019;44:7–14. Epub 2017/12/17. doi: 10.1016/j.mito.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Bhargava P, Fitzgerald KC, Calabresi PA, Mowry EM. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight. 2017;2(19). Epub 2017/10/06. doi: 10.1172/jci.insight.95302. [DOI] [PMC free article] [PubMed] [Google Scholar]; Biogen, grants from Novartis, grants from Teva, personal fees from Biogen, and personal fees from Vertex outside the submitted work. E.M. Mowry reports grants from Biogen, other support from UpToDate, nonfinancial support from Teva, other support from Sun Pharma, other support from Biogen, and grants from Sanofi Genzyme outside the submitted work.
- 44.Bizzarri C, Benevento D, Giannone G, Bongiovanni M, Anziano M, Patera IP, et al. Sexual dimorphism in growth and insulin-like growth factor-I in children with type 1 diabetes mellitus. Growth Horm IGF Res. 2014;24(6):256–9. Epub 2014/09/02. doi: 10.1016/j.ghir.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Gaber T, Strehl C, Buttgereit F. Metabolic regulation of inflammation. Nat Rev Rheumatol. 2017;13(5):267–79. Epub 2017/03/24. doi: 10.1038/nrrheum.2017.37. [DOI] [PubMed] [Google Scholar]
- 46.Li R, Sun X, Shu Y, Mao Z, Xiao L, Qiu W, et al. Sex differences in outcomes of disease-modifying treatments for multiple sclerosis: A systematic review. Mult Scler Relat Disord. 2017;12:23–8. Epub 2017/03/12. doi: 10.1016/j.msard.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Mathur D, López-Rodas G, Casanova B, Marti MB. Perturbed glucose metabolism: insights into multiple sclerosis pathogenesis. Front Neurol. 2014;5:250. Epub 2014/12/19. doi: 10.3389/fneur.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poddighe S, Murgia F, Lorefice L, Liggi S, Cocco E, Marrosu MG, et al. Metabolomic analysis identifies altered metabolic pathways in Multiple Sclerosis. Int J Biochem Cell Biol. 2017;93:148–55. Epub 2017/07/20. doi: 10.1016/j.biocel.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Sheikh MH, Henson SM, Loiola RA, Mercurio S, Colamatteo A, Maniscalco GT, et al. Immuno-metabolic impact of the multiple sclerosis patients’ sera on endothelial cells of the blood-brain barrier. J Neuroinflammation. 2020;17(1):153. Epub 2020/05/11. doi: 10.1186/s12974-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun. 2016;7:11350. Epub 2016/04/14. doi: 10.1038/ncomms11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang N, Perl A. Metabolism as a Target for Modulation in Autoimmune Diseases. Trends Immunol. 2018;39(7):562–76. Epub 2018/05/10. doi: 10.1016/j.it.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Sivakumar R, Titov AA, Choi SC, Morel L. Metabolic Factors that Contribute to Lupus Pathogenesis. Crit Rev Immunol. 2016;36(1):75–98. Epub 2016/08/03. doi: 10.1615/CritRevImmunol.2016017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syrett CM, Paneru B, Sandoval-Heglund D, Wang J, Banerjee S, Sindhava V, et al. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI Insight. 4(7). doi: 10.1172/jci.insight.126751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–34. Epub 2014/03/25. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, Waickman AT, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17(6):704–11. Epub 2016/04/12. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suto T, Karonitsch T. The immunobiology of mTOR in autoimmunity. J Autoimmun. 2020;110:102373. Epub 2019/12/14. doi: 10.1016/j.jaut.2019.102373. [DOI] [PubMed] [Google Scholar]
- 57.Mauvais-Jarvis F, Arnold AP, Reue K. A Guide for the Design of Pre-clinical Studies on Sex Differences in Metabolism. Cell Metab. 2017;25(6):1216–30. Epub 2017/06/08. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. Epub 2012/09/07. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vadlakonda L, Dash A, Pasupuleti M, Anil Kumar K, Reddanna P. The Paradox of Akt-mTOR Interactions. Front Oncol. 2013;3:165. Epub 2013/06/27. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:54. Epub 2020/04/09. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Balogh A, Karpati E, Schneider AE, Hetey S, Szilagyi A, Juhasz K, et al. Sex hormone-binding globulin provides a novel entry pathway for estradiol and influences subsequent signaling in lymphocytes via membrane receptor. Sci Rep. 2019;9(1):4. Epub 2019/01/11. doi: 10.1038/s41598-018-36882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29(5):749–60. Epub 2015/11/03. doi: 10.1016/j.beem.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, et al. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 2018;174(2):285–99.e12. Epub 2018/06/12. doi: 10.1016/j.cell.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16(3):276–85. Epub 2015/01/20. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- 65.Billi AC, Kahlenberg JM, Gudjonsson JE. Sex bias in autoimmunity. Curr Opin Rheumatol. 2019;31(1):53–61. Epub 2018/11/06. doi: 10.1097/bor.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK, et al. VGLL3-regulated gene network as a promoter of sex biased autoimmune diseases. Nat Immunol. 2017;18(2):152–60. doi: 10.1038/ni.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf SJ, Estadt SN, Gudjonsson JE, Kahlenberg JM. Human and Murine Evidence for Mechanisms Driving Autoimmune Photosensitivity. Front Immunol. 2018;9:2430. Epub 2018/11/09. doi: 10.3389/fimmu.2018.02430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu T, Xie C, Han J, Ye Y, Weiel J, Li Q, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS One. 2012;7(6):e37210. Epub 2012/06/23. doi: 10.1371/journal.pone.0037210. [DOI] [PMC free article] [PubMed] [Google Scholar]; alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials. Other than the above, all authors have declared that no competing interests exist.
- 69.Lewis MJ, McAndrew MB, Wheeler C, Workman N, Agashe P, Koopmann J, et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J Autoimmun. 2018;91:1–12. Epub 2018/03/27. doi: 10.1016/j.jaut.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Liang YC, Yao Y, Zhang RJ, Shao M, Sun XL, Shi GX, et al. [Role of circulating T follicular helper subsets and T follicular helper effector memory cells in systemic lupus erythematosus]. Zhonghua Yi Xue Za Zhi. 2019;99(3):164–8. Epub 2019/01/24. doi: 10.3760/cma.j.issn.0376-2491.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Crotty S T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. 2019;50(5):1132–48. Epub 2019/05/23. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmer CS, Hussain T, Duette G, Weller TJ, Ostrowski M, Sada-Ovalle I, et al. Regulators of Glucose Metabolism in CD4(+) and CD8(+) T Cells. Int Rev Immunol. 2016;35(6):477–88. Epub 2015/11/26. doi: 10.3109/08830185.2015.1082178. [DOI] [PubMed] [Google Scholar]
- 73.Doerner J, Chalmers SA, Friedman A, Putterman C. Fn14 deficiency protects lupus-prone mice from histological lupus erythematosus-like skin inflammation induced by ultraviolet light. Exp Dermatol. 2016;25(12):969–76. Epub 2016/06/16. doi: 10.1111/exd.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee TP, Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev. 2012;11(6–7):A422–9. Epub 2011/12/17. doi: 10.1016/j.autrev.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Palomar APD, Montolio A, Cegonino J, Dhanda SK, Lio CT, Bose T. The Innate Immune Cell Profile of the Cornea Predicts the Onset of Ocular Surface Inflammatory Disorders. J Clin Med. 2019;8(12). Epub 2019/12/08. doi: 10.3390/jcm8122110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomczynska M, Salata I, Saluk J. [The role of gender in the pathogenesis and development of autoimmune diseases]. Pol Merkur Lekarski. 2016;41(243):150–5. Epub 2016/10/19. [PubMed] [Google Scholar]
- 77.Wang K, Song F, Fernandez-Escobar A, Luo G, Wang JH, Sun Y. The Properties of Cytokines in Multiple Sclerosis: Pros and Cons. Am J Med Sci. 2018;356(6):552–60. Epub 2018/11/19. doi: 10.1016/j.amjms.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–12. Epub 2013/08/27. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh H, Khan AA, Dinner AR. Gene regulatory networks in the immune system. Trends Immunol. 2014;35(5):211–8. Epub 2014/04/29. doi: 10.1016/j.it.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Triggianese P, Novelli L, Galdiero MR, Chimenti MS, Conigliaro P, Perricone R, et al. Immune checkpoint inhibitors-induced autoimmunity: The impact of gender. Autoimmun Rev. 2020;19(8):102590. Epub 2020/06/21. doi: 10.1016/j.autrev.2020.102590. [DOI] [PubMed] [Google Scholar]
- 81.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17(1):29. Epub 2015/04/19. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–77. Epub 2002/07/18. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 83.He N, Fan W, Henriquez B, Yu RT, Atkins AR, Liddle C, et al. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proc Natl Acad Sci U S A. 2017;114(47):12542–7. Epub 2017/11/08. doi: 10.1073/pnas.1715363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katsiougiannis S, Tenta R, Skopouli FN. Autoimmune epithelitis (Sjögren’s syndrome); the impact of metabolic status of glandular epithelial cells on auto-immunogenicity. J Autoimmun. 2019;104:102335. Epub 2019/09/22. doi: 10.1016/j.jaut.2019.102335. [DOI] [PubMed] [Google Scholar]
- 85.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–97. Epub 2019/03/21. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 86.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42. Epub 2014/11/05. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Merrheim J, Villegas J, Van Wassenhove J, Khansa R, Berrih-Aknin S, le Panse R, et al. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun Rev. 2020;19(3):102468. Epub 2020/01/14. doi: 10.1016/j.autrev.2020.102468. [DOI] [PubMed] [Google Scholar]
- 88.Fernández-Ochoa Á, Borrás-Linares I, Quirantes-Piné R, Alarcón-Riquelme ME, Beretta L, Segura-Carretero A. Discovering new metabolite alterations in primary sjögren’s syndrome in urinary and plasma samples using an HPLC-ESI-QTOF-MS methodology. J Pharm Biomed Anal. 2020;179:112999. Epub 2019/11/30. doi: 10.1016/j.jpba.2019.112999. [DOI] [PubMed] [Google Scholar]
- 89.Hwang SH, Park JS, Yang S, Jung KA, Choi J, Kwok SK, et al. Metabolic abnormalities exacerbate Sjögren’s syndrome by and is associated with increased the population of interleukin-17-producing cells in NOD/ShiLtJ mice. J Transl Med. 2020;18(1):186. Epub 2020/05/07. doi: 10.1186/s12967-020-02343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Recalde G, Moreno-Sosa T, Yúdica F, Quintero CA, Sánchez MB, Jahn GA, et al. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmun Rev. 2018;17(5):504–12. Epub 2018/03/13. doi: 10.1016/j.autrev.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol Ther (Seoul). 2016;24(1):1–8. Epub 2016/01/14. doi: 10.4062/biomolther.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cronin SJF, Woolf CJ, Weiss G, Penninger JM. The Role of Iron Regulation in Immunometabolism and Immune-Related Disease. Front Mol Biosci. 2019;6:116. Epub 2019/12/12. doi: 10.3389/fmolb.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Della Torre S, Benedusi V, Fontana R, Maggi A. Energy metabolism and fertility: a balance preserved for female health. Nat Rev Endocrinol. 2014;10(1):13–23. Epub 2013/10/23. doi: 10.1038/nrendo.2013.203. [DOI] [PubMed] [Google Scholar]
- 94.Zielinski MR, Systrom DM, Rose NR. Fatigue, Sleep, and Autoimmune and Related Disorders. Front Immunol. 2019;10:1827. Epub 2019/08/27. doi: 10.3389/fimmu.2019.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golden LC, Voskuhl R. The importance of studying sex differences in disease: The example of multiple sclerosis. J Neurosci Res. 2017;95(1–2):633–43. Epub 2016/11/22. doi: 10.1002/jnr.23955. [DOI] [PMC free article] [PubMed] [Google Scholar]


