Abstract
Background:
Few studies have examined how Δ9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, impacts brain reward circuitry in humans. In this study, we examined if an acute dose of THC altered resting state functional connectivity between the striatum and prefrontal cortex among healthy young adults with limited cannabis use.
Methods:
Participants received THC (n=24) or placebo (n=22) in a double-blind, randomized, between-subject design. Participants completed self-report measures of euphoria and drug-liking throughout the visit. Approximately 120 minutes after drug administration, participants completed an 8-minute resting state functional MRI (rs-fMRI) scan. We utilized seed-based connectivity of the striatum (bilateral putamen, caudate, and NAcc seeds) to the frontal cortex.
Results:
Individuals who received THC demonstrated greater rs-fMRI connectivity between the right NAcc and regions of the medial prefrontal cortex (mPFC) (p-values<0.05, corrected) and higher subjective euphoria ratings p=.03) compared to compared to individuals who received placebo. Higher ratings of euphoria were related to greater right NAcc-dorsal mPFC (dmPFC) connectivity for the THC group (p=.03), but not for the placebo group (p=.98).
Conclusions:
This is one of the first studies to examine rs-fMRI connectivity in healthy young non-users after THC administration. We found individuals receiving THC show greater rs-fMRI connectivity between the NAcc and mPFC, regions implicated in reward, compared to individuals receiving placebo. In addition, individuals receiving THC reported higher subjective euphoria ratings, which were positively associated with NAcc-dmPFC connectivity. Overall, our findings suggest THC may produce subjective and neural reward responses that contribute to the rewarding, reinforcing properties of cannabis.
Keywords: Cannabis, FMRI, Marijuana, Resting State, Reward, THC
1. Introduction
As cannabis becomes increasingly available in the U.S. there is concern about its negative health, mental health, and psychosocial outcomes (Brook et al., 2012; Crane et al., 2013; Degenhardt et al., 2003; Fergusson and Boden, 2008; Meier et al., 2012). To minimize the adverse impact of the increased use, it is critical to examine how different components of cannabis affect brain circuitry to understand what contributes to continued cannabis use, to the development of problematic cannabis use, and to identify treatment and prevention targets. The main psychoactive component of cannabis, Δ9-Tetrahydrocannabinol (THC), is linked to the rewarding and addictive properties of cannabis (see Bloomfield et al., 2016). The potency of cannabis (i.e., amount of THC) has steadily increased since the 1970s. Currently, THC potency is estimated to be 15-20% in street cannabis (Chandra et al., 2019; Sevigny, 2013). Cannabis with high THC potency has been shown to produce higher subjective reward response (Cooper and Haney, 2008; Niesink and van Laar, 2013), which may make it more addictive. Indeed, individuals who experience greater subjective reward response to a drug, especially drug-induced euphoria, are more likely to use that drug again, continue drug use, and develop a Substance Use Disorder (SUD) (Davidson et al., 1993; de Wit and Phillips, 2012; de Wit et al., 1986; Haertzen et al., 1983; King et al., 2014; Lambert et al., 2006; Quinn and Fromme, 2011; Rush et al., 2001). Therefore, to understand the rewarding, reinforcing properties of cannabis that contribute to continued cannabis use and problematic cannabis use, it is important to study THC and how THC affects brain reward circuitry.
Accumulating evidence suggests that THC activates frontostriatal reward circuitry, including the striatum and medial prefrontal cortex (mPFC) (see Bloomfield et al., 2016; Weinstein et al., 2016). THC binds to cannabinoid receptors in the brain, which are densely concentrated in the striatum, a key region in brain reward circuitry (see Bloomfield et al., 2016). Indeed, the endogenous cannabinoid system seems to increase dopamine transmission in the striatum (Fernandez-Ruiz et al., 2010), which is known to play an important role in the rewarding effect of drugs, the development of want or desire for the drug, and the development of drug-seeking habits (see Koob and Le Moal, 1997; Koob and Volkow, 2016). Animal studies have shown that acute THC administration increased dopaminergic cell firing and increased dopamine synthesis and release in the ventral striatum (i.e., nucleus accumbens (NAcc)) (Chen et al., 1990; French, 1997; Sperlagh et al., 2009; Tanda et al., 1997). In human studies using positron emission tomography (PET) imaging, THC administration seems to modestly increase striatal dopamine transmission among healthy volunteers (see Weinstein et al., 2016). Therefore, THC seems to activate frontostriatal brain reward regions, which may contribute to its pleasurable and rewarding effects.
To date, only a handful of studies have examined how THC impacts rs-fMRI functional connectivity of brain reward regions. Understanding how THC may alter rs-fMRI connectivity of brain reward regions provides valuable insights about how THC impacts the intrinsic circuitry underlying frontrostriatal brain reward regions. In animal and human studies of healthy adults, increased dopaminergic activity in reward circuitry was associated with increased frontostriatal rs-fMRI functional connectivity (Cole et al., 2013a; Cole et al., 2013b; Febo et al., 2017; Weafer et al., 2020), although see (Ramaekers et al., 2013). Growing evidence indicates THC increases dopaminergic activity and activates frontostriatal brain reward circuitry, but it is not clear how the connections between frontostriatal brain regions are altered during acute THC intoxication. One study found that an acute dose of THC (10mg oral) did not significantly alter frontostriatal rs-fMRI connectivity compared to placebo among 16 healthy male participants using dorsal striatal (i.e., putamen and caudate) seeds; however, THC levels in blood plasma during the rs-fMRI scan were low suggesting that THC rs-fMRI data in this study should not be interpreted (Grimm et al., 2018). Another study found that an acute dose of THC (450 micrograms/kg inhaled) reduced rs-fMRI connectivity between the NAcc and the prefrontal cortex, limbic lobe, striatum and thalamus (in a similar manner to an acute dose of cocaine) among 122 occasional and regular cannabis and cocaine users (Ramaekers et al., 2016). Two recent studies from the same group also found reduced rs-fMRI connectivity between the NAcc and the frontal cortex. Specifically, the first study found that among 10 occasional cannabis users receiving an acute dose of THC (300 micrograms/kg inhaled) showed reduced rs-fMRI between the NAcc and the frontal and limbic lobes, while 10 occasional cannabis users receiving an acute dose of THC that was divided over time (3 successive doses of 100 μg/kg inhaled, separated by 30 min) did not demonstrate any significant differences in rs-fMRI compared to placebo (Mason et al., 2019). The second study found that among 12 occasional cannabis users receiving an acute dose of THC (300 micrograms/kg inhaled) showed reduced rs-fMRI between the NAcc and the frontal cortex, limbic, parietal, and occipital lobes, while 12 chronic cannabis users did not demonstrate any changes in rs-fMRI (Mason et al., in press). More studies are needed to better understand how THC may impact frontostriatal resting state connectivity, especially among individuals with a limited history of cannabis use in order to better understand how initial experiences with THC may alter brain reward connectivity.
As such, the current study was derived from secondary analysis of study data presented in Gorka et al., 2016, to examine if an acute dose of THC altered rs-fMRI connectivity between striatal seeds (i.e., putamen, caudate, and NAcc) and the prefrontal cortex among healthy young adults. The sample was comprised of healthy young adults, who have a minimal history of cannabis use (or other drug use) given that chronic cannabis use is associated with structural and functional changes in frontolimbic and frontostriatal regions (see Bloomfield et al., 2016; Weinstein et al., 2016). Studying healthy young adults allows us to better understand how initial responses to THC may impact frontostriatal rs-fMRI connectivity, which could contribute to continued cannabis use. In addition, as an exploratory aim, we were interested in understanding how frontostriatal rs-fMRI connectivity was related to subjective measures of drug reward during THC intoxication, a known risk factor for SUD (de Wit and Phillips, 2012; Haertzen et al., 1983). An oral dose of Marinol (dronabinol) was used to specifically study the effects of THC instead of smoked cannabis, given Marinol’s well-known pharmokinetic and pharmodynamic properities and the fact that Marinol does not contain other cannabinoids that may also effect frontolimbic and frontostriatal regions, which are factors that are less controlled and are more variable when using smoked cannabis. Although reductions in frontostriatal connectivity in response to THC administration have been found by a few studies in occasional and regular cannabis users (Mason et al., in press; Mason et al., 2019; Ramaekers et al., 2016), we hypothesized that healthy non-cannabis users receiving THC (vs. placebo) would show greater rs-fMRI connectivity between the NAcc and the mPFC based on studies finding THC increased frontostriatal dopaminergic-related activity and that increased frontostriatal dopaminergic-related activity (in response to other drugs) is related to increased frontostriatal rs-fMRI functional connectivity in healthy animal and human samples (Cole et al., 2013a; Cole et al., 2013b; Febo et al., 2017; Weafer et al., 2020). We also hypothesized that individuals with greater NAcc-mPFC functional connectivity after receiving THC would report greater subjective reward response to THC.
2. Materials and Methods
2.1. Participants
The current study was derived from secondary analysis of study data presented in Gorka et al., 2016, which examined how THC affects frontolimbic activation and functional connectivity during a cognitive reappraisal task. Participants were healthy right-handed young adults recruited from the local community through online and printed advertisements. Eligibility was assessed using an in-person interview that involved comprehensive medical and psychiatric screening (using a research version of the Structured Clinical Interview for DSM-IV disorders; (First et al., 2002). Inclusion criteria included age 21-45, at least a high school education, English fluency, no lifetime DSM-IV Axis I psychiatric disorders (including substance use disorders), and no serious medical or neurological conditions. Participants were also required to have minimal history of cannabis use (≤10 lifetime exposures) to limit prior THC exposure. No participant reported that they had used cannabis within the past 30 days and all participants were required to test negative on a urine toxicology screen at the time of the study. No participant was a daily tobacco smoker. Exclusion criteria included night shift work, left-handedness, pregnancy or lactation (women), individuals with a contraindication for MRI scanning (i.e., claustrophobia, pacemaker, heart valves, or body mass too large for the scanner bore), and current or past allergic or adverse reaction or known sensitivity to cannabinoid-like substances (Dronabinol/Marijuana/Cannabis/THC, cannabinoid oil, sesame oil, gelatin, glycerin, and titanium dioxide.). The final sample included 46 individuals who completed a rs-fMRI scan during the drug administration visit; four participants (placebo n=1, THC n=3) were excluded due to excessive motion during fMRI scans (i.e., >2-mm displacement in any one direction). Participant characteristics of the final sample are presented in Table 1.
Table 1.
Participant Characteristics
| THC (N = 24) |
Placebo (N = 22) |
|
|---|---|---|
| Age | 25.95 (5.63) | 24.00 (4.01) |
| Gender (% Female) | 58% | 64% |
| Years of Education | 15.75 (1.42) | 16.32 (1.94) |
| Ethnicity (% Hispanic)* | 42% | 9% |
| Race | ||
| % Caucasian | 37% | 50% |
| % More than 1 Race or Unknown | 27% | 5% |
| % African-American | 9% | 5% |
| % Asian | 18% | 36% |
| % American Indian/Alaskan Native | 9% | 0% |
| % Native Hawaiian/Pacific Islander | 0% | 4% |
| Past Month Substance Use | ||
| Cannabis use frequency | 0.00 (0.00) | 0.00 (0.00) |
| Alcoholic drinks/week | 1.20 (0.99) | 1.02 (0.74) |
| Caffeine drinks/day | 1.15 (1.11) | 1.13 (0.81) |
| % Used tobacco (averaged cigarettes/day for users) | 17% (n=4; 0.40 ± 0.00) | 5% (n=1; 0.87 ± 1.10) |
| Lifetime Substance Use | ||
| Number of times used cannabis [range] | 1.54 (0.51) [1-2] | 1.73 (0.55) [1-3] |
| Number of times used barbiturates [range] | 0.00 (0.00) [0] | 0.00 (0.00) [0] |
| Number of times used stimulants (prescribed or recreational use) [range] | 0.00 (0.00) [0] | 0.00 (0.00) [0] |
| Number of times used opiates (recreational use) [range] | 0.00 (0.00) [0] | 0.00 (0.00) [0] |
| Number of times used hallucinogens [range] | 0.00 (0.00) [0] | 0.10 (0.31) [0-1] |
| Number of times used inhalants [range] | 0.00 (0.00) [0] | 0.00 (0.00) [0] |
Note. All values are means and standard deviations unless otherwise noted;
p< .05
2.2. Experimental Protocol
Data collection took place at the University of Illinois at Chicago (UIC). The protocol was approved by the UIC Institutional Review Board for human participants. All participants provided written informed consent and were compensated for their participation. Eligible participants completed a screening visit during which they provided informed consent and received instructions regarding drug and alcohol use before scanning sessions; they were required to abstain from drugs and alcohol for 24h prior to visits, as verified by breath and urine samples. At the screening visit, participants were informed that they may receive a capsule containing either placebo or dronabinol, a cannabinoid/marijuana-like drug in the study. After screening, participants were randomized to receive THC (n=24) or placebo (n=22) in a double-blind, between-subject design as previously described (Gorka et al., 2016). Participants then attended a separate drug administration visit during which resting state fMRI data was captured. Participants arrived to the drug administration visit at 10:00am and provided breath and urine samples before they completed subjective measures. Then around 10:10am, approximately 120 min before scanning, participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (Marinol; 7.5 mg; Solvay Pharmaceuticals, Marietta, GA) or dextrose alone (PBO). Plasma levels of THC and the subjective effects of THC have been shown to peak around 120 minutes after drug administration of Marinol (see Curran et al., 2002; Phan et al., 2008). The dose of 7.5 mg was chosen because it has been used in other pharmaco-fMRI studies (e.g., Phan et al., 2008) and is the lowest effective dose that has been found to be behaviorally active and increase subjective drug ratings without producing adverse effects or pronounced subjective or cardiovascular effects (Ballard and de Wit, 2011; Curran et al., 2002; Rabinak et al., 2012). Participants completed subjective reports every half hour or hour for about a 4-hour period. At the end of the drug administration visit, participants were asked, “Based on the effects of the capsule you were given, what do you think it was?” and 77% of participants who received placebo reported that they thought they received placebo, while 54% of participants who received THC reported that they thought they received a cannabinoid. Participants left the visit shortly after 2:10pm.
2.3. Subjective Measures
Addiction Research Center Inventory (ARCI; Martin et al., 1971).
To assess subjective euphoric response to drug, participants completed the ARCI throughout the drug administration visit, including at baseline (0 minutes; prior to drug administration), 90 minutes (prior to the fMRI scan), and 180 minutes (after the fMRI scan). The Morphine–Benzedrine Group (MBG, euphoric effects) scale was used, as this has been shown to represent the positive, rewarding effects of drugs (e.g., de Wit et al., 1986; Fischman and Foltin, 1991). The peak change difference score for ARCI-MBG (peak score (average of 90 minute and 180 minute assessments) minus baseline (0 minutes) score; Mayo and de Wit, 2015) was used for analyses.
Drug Effects Questionnaire (DEQ; Johanson and Uhlenhuth, 1980).
To assess subjective liking of drug response, participants completed the DEQ throughout the drug administration visit, including at baseline (0 minutes; prior to drug administration), 90 minutes (prior to the fMRI scan), and 180 minutes (after the fMRI scan). As part of the DEQ, participants were asked to rate their response to the question “Do you like the effects you are feeling now (rated from “dislike”=−4 to “like very much”=4)?”. Participants were instructed to choose “0” for neutral if they were not feeling any effects at all. The peak change difference score for DEQ-Like (peak score (average of 90 minute and 180 minute assessments) minus baseline (0 minutes) score; Mayo and de Wit, 2015) was used for analyses. For descriptive purposes, participants were also asked to rate their responses to the questions, “Do you feel any drug effects?” and “Do you feel high right now” (rated from “not at all”=0 to “very strongly”=4). Approximately 70% of participants receiving THC reported feeling the effect of the drug (DEQ-Feel scale) and 67% of participants receiving THC reported feeling high (DEQ-High scale).
2.4. fMRI Data Acquisition and Preprocessing
An eyes-open rs-fMRI scan was collected over 8 minutes on a 3T GE magnetic resonance scanner at the UIC Center for Magnetic Resonance Research. Participants were asked to look at a fixation cross on the screen and let their mind wander. Functional images were acquired using a gradient-echo echo-planar images (2s TR, 25ms TE, 82° flip, 64x64 matrix, 200mm FOV, 3mm slice thickness, 0mm gap, with 44 axial slices).
Motion outliers were identified based on framewise displacement (FD; >0.5 mm) using FSL’s motion outlier tool and then censored from analyses. In addition, imaging data were inspected and individuals with >2mm displacement in any direction were not included in the analysis. The remaining subjects met criteria for high quality and scan stability. There were no significant group differences in peak movement (PBO: M= 0.30 mm, SD= 0.23 mm; THC: M= 0.25 mm, SD= 0.18 mm), mean movement, or variability (p-values>.05). Preprocessing of fMRI data was conducted using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuro-Science, London, UK). Images were spatially realigned, slice-time corrected, warped to Montreal Neurological Institute (MNI) space using the participant’s mean functional image, resampled to 2 mm3 voxels, and smoothed (8 mm3 kernel). The effects of nuisance covariates (time-series predictors for global signal, white matter, cerebrospinal fluid and movement parameters including the first derivative), obtained during realignment to account for motion-related effects in blood-oxygen level dependent (BOLD) signal, were regressed from the data following the implemented anatomical component-based noise correction method (aCompCor; Behzadi et al., 2007). The data were then bandpass filtered to 0.01–0.09 Hz.
2.5. Statistical Analysis
Analyses were performed using SPM8 and SPSS 24.0 (IBM). Connectivity analyses were performed using the functional connectivity (CONN) toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012; www.nitrc.org/projects/conn) for statistical parametric mapping software (SPM8: Wellcome Trust Centre for Neuroimaging, London, UK).
2.5.1. Group differences in functional connectivity
Seed-based connectivity analyses were performed by calculating the temporal correlation between BOLD signals from bilateral striatal (putamen, caudate, and NAcc) seeds to all other voxels in the brain. The striatal seeds of interest (SOIs), except the NAcc, were anatomically defined via the AAL atlas and created using MARINA (http://www.bion.de/Marina.html, Walter et al., 2003; Figure 1). The NAcc SOI was anatomically predefined based on prior PET studies (Martinez et al., 2005; Martinez et al., 2003; Figure 1). Visual inspection, using the Check Reg tool in SPM, showed the NAcc ROI was nearly identical to the AAL3 NAcc anatomical ROI. First level correlation maps were created for all subjects. The participant maps of the correlations between the seed regions and all voxels of the brain were then entered into a second-level group analysis, using a two (group: PBO, THC) by one (rs-fMRI) analysis of variance (ANOVA). The contrast of interest was the main effect of group (THC versus PBO).
Figure 1.
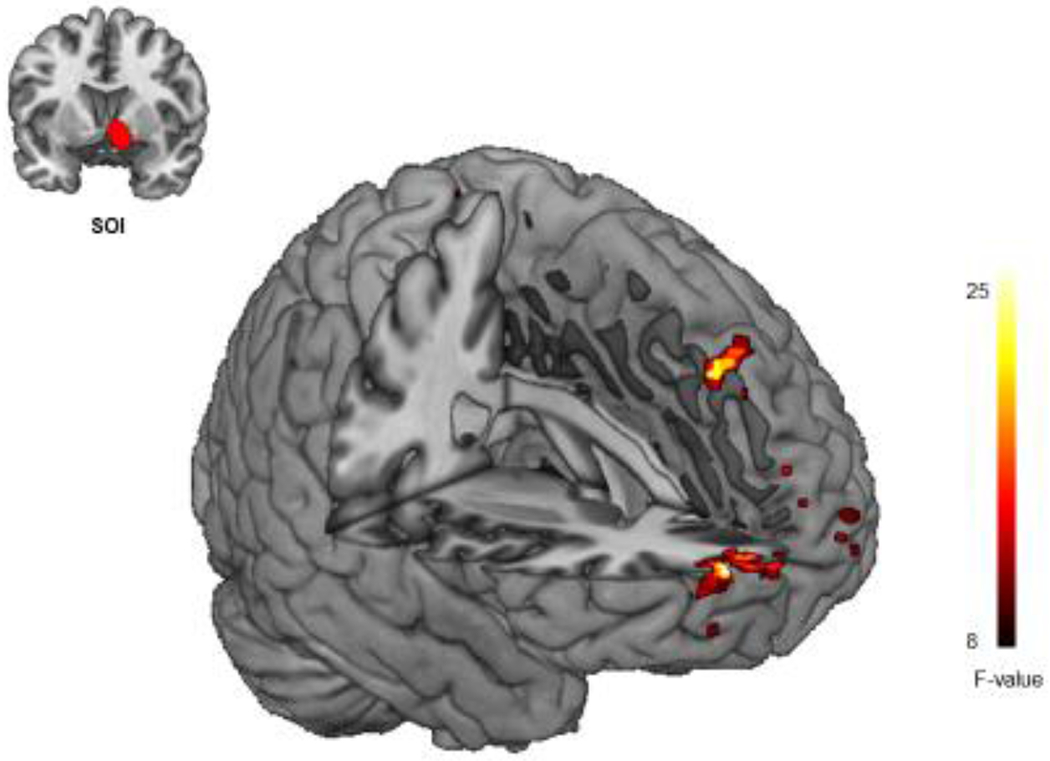
Group Differences in Resting State Functional Connectivity. Individuals who received THC demonstrated increased resting state fMRI (rs-fMRI) connectivity between the right NAcc and the right mPFC, the right dorsomedial prefrontal cortex (dmPFC) compared to individuals who received placebo (p< 0.05, corrected).
Given our a priori hypothesis regarding frontostriatal connectivity, neural activity from second-level models were considered significant if it exceeded correction of multiple comparisons across the entire frontal cortex and cingulate cortex. Due to concerns of high rates of false positives with lenient significance thresholds and following recent guidelines (Eklund et al., 2016; Woo et al., 2014), neural activity was considered significant if it exceeded correction of multiple comparisons within the frontal cortex and cingulate cortex (e.g., a gray matter mask [volume=54,426mm3]). Correction for multiple comparisons was performed using a spatial clustering operation in AFNI’s 3dClustSim utilizing the autocorrelation function (-acf) with 10,000 Monte Carlo simulations. Spatial autocorrelation was estimated from residuals from the individual-level GLMs. In line with current recommendations, the voxel threshold was set at p=.001; Cox et al., 2017). Significance at corrected α<0.05 and a voxel threshold of p<0.001 yielded a minimum cluster size of at least 55 contiguous voxels (volume=440mm3). In order to show the distribution of connectivity data in a scatterplot, we extracted parameter estimates/β-weights from a 10mm radius sphere surrounding the peak connectivity.
2.5.2. Group differences in subjective effects
Subjective ratings of euphoria and drug liking were assessed separately using two (group: PBO, THC) by one analysis of variance (ANOVA) tests.
2.5.3. Exploratory Aim: Relationship between functional connectivity and subjective effects
Pearson correlation analyses were used to examine if there were relationships between significant functional connectivity findings and subjective effects (ARCI-MBG and DEQ-Like).
3. Results
3.1. Group differences in functional connectivity
Individuals who received THC demonstrated increased functional connectivity between the right NAcc and the right mPFC and the right dorsomedial prefrontal cortex (dmPFC) compared to individuals who received PBO (Table 2; Figure 1). No results were significant for the putamen or caudate SOIs. There were no significant decreases in functional connectivity for any SOI. Of note, these results remained after controlling for past month tobacco user status.
Table 2.
Regions Demonstrating Increased Functional Connectivity in THC Group
| Region | Voxels (k) |
Z score | Peak Coordinates (MNI) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right NAcc seed | |||||
| Right mPFC | 70 | 4.22 | 20 | 68 | 18 |
| Right dmPFC | 59 | 4.18 | 20 | 26 | 46 |
Note. dmPFC= dorsomedial prefrontal cortex, MNI= Montreal Neurological Institute, and mPFC= medial prefrontal cortex.
3.2. Group differences in subjective effects
Individuals who received THC reported significantly higher ratings of euphoria (ARCI-MBG) (M= 0.88, SD= 2.26) compared to individuals who received PBO (M=−0.59, SD=2.29), F(1, 45)=4.77, p=.03. There were no significant differences between THC (M=−0.67, SD=1.63) and PBO (M=−0.19, SD=0.95) groups in ratings of drug liking (DEQ-Like), F(1, 43)=1.40, p=.24. The time-course of ARCI-MBG and DEQ-Like ratings are shown in Figure 2.
Figure 2.
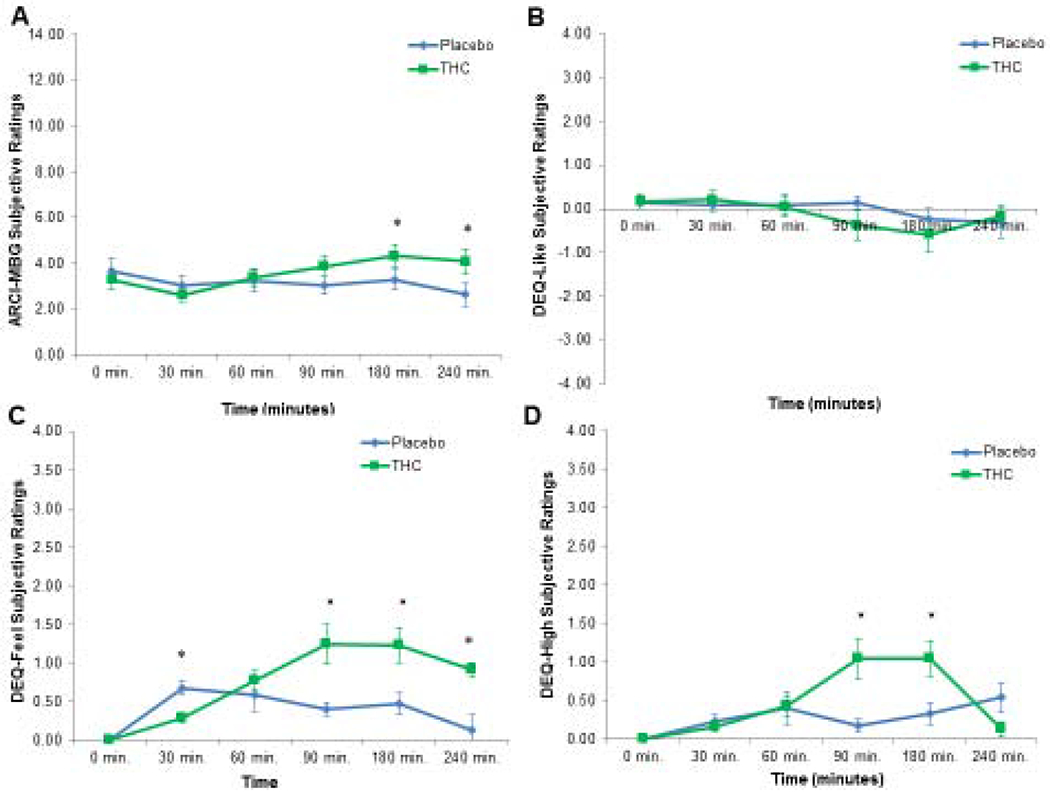
Group Differences in Subjective Drug Responses Over Time. Panel A shows ARCI-MBG scores for the placebo and THC groups over time (*, p< .05). Panel B shows DEQ-Like scores for the placebo and THC groups over time (*, p< .05). Panel C shows DEQ-Feel scores for the placebo and THC groups over time (*, p< .05). Panel D shows DEQ-High scores for the placebo and THC groups over time (*, p< .05).
For descriptive purposes, individuals who received THC reported significantly greater ratings of feeling the drug (M=1.14, SD=0.95) than individuals who received PBO (M=0.45, SD=0.55), F(1, 45)=8.23, p=.006. Individuals who received THC also reported significantly greater ratings of drug high (M=0.95, SD=0.99) than individuals who received PBO (M=0.26, SD=0.41), F(1, 45)=8.89, p=.005. The time-course of DEQ-Feel and DEQ-High ratings are shown in Figure 2.
3.3. Exploratory Aim: Relationship between functional connectivity and subjective effects
Higher ratings of euphoria were significantly related to greater right NAcc-dmPFC connectivity for the THC group (r=.44, p=.03), but not for the PBO group (r=.01, p=.98; see Figure 3). This finding did not survive correction for multiple comparisons using False Discovery Rate (FDR). No other significant relationships between subjective drug ratings (ARCI-MBG, DEQ-Like) and functional connectivity were found for either group (p >.05).
Figure 3.
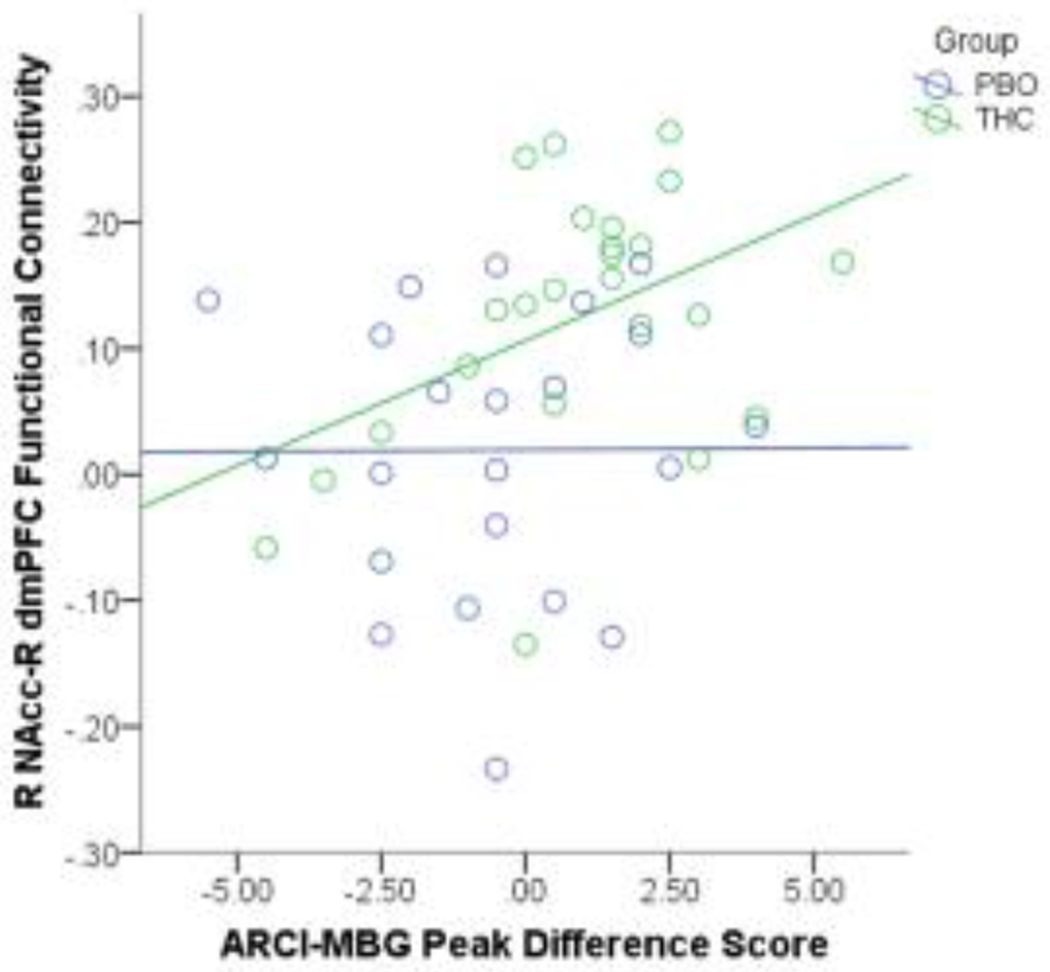
Frontostriatal Resting State Functional Connectivity is Associated with Subjective Ratings of Drug Reward. Greater right NAcc-dmPFC connectivity during THC intoxication was related to higher ratings of euphoria for the THC group, but the relationship of these measures was not significant among individuals in the placebo group.
4. Discussion
This is one of the first studies to examine rs-fMRI connectivity in healthy young adults with limited history of cannabis use after THC administration. We found that healthy individuals who received THC demonstrated greater rs-fMRI connectivity between frontostriatal regions implicated in reward including between the right NAcc and the right mPFC, as well as the right dorsomedial prefrontal cortex (dmPFC) compared to individuals who received placebo. No significant group differences were seen for the putamen or caudate seeds. No decreases in rs-fMRI were found. Additionally, individuals who received THC reported higher subjective euphoria ratings, but not subjective drug liking, compared to individuals who received placebo. Furthermore, in our exploratory aim, we found preliminary evidence that higher ratings of euphoria were related to greater right NAcc-dmPFC connectivity for the THC group, but not for the placebo group. Together, these findings indicate that THC may produce subjective and neural reward responses that may contribute to the rewarding, reinforcing properties of cannabis.
The finding that healthy young adults with a limited history of cannabis use receiving THC demonstrated greater rs-fMRI connectivity between frontostriatal regions implicated in reward than those receiving placebo provides novel information about how THC may affect frontostriatal reward circuitry in non-users. Previous studies among occasional and regular cannabis users have found reductions in frontostriatal connectivity (Mason et al., in press; Mason et al., 2019; Ramaekers et al., 2016). Given that history of cannabis use can impact frontostriatal functional connectivity (Mason et al., in press), it is possible that the discrepancy in findings is due to differences in cannabis use history among the samples. Specficially, the current study was comprised of healthy young adults who had used cannabis 10 times or less in their lifetime and had minimal other substance use, so the current findings may reflect how frontostriatal networks interact during initial exposure to THC. On the other hand, the previous studies had samples of occasional and regular cannabis users and one of the studies, Ramaekers et al., 2016, recruited occasional and regular cannabis and cocaine users. So, the previous findings may reflect frontostriatal network connectivity during THC intoxication among individuals who have already experienced drug-related alterations in brain reward cicruity from previous use. Indeed, it is well established that as drug use progresses and a Substance Use Disorder (SUD) develops, drug-related neuroadaptations occur such that brain reward circuitry becomes hypoactive, or less sensitive, to rewards (Volkow et al., 2016). It is possible that these neuroadaptions begin to occur even before an individual develops a SUD, impacting functional connectivity of brain reward circuitry. Taken together, our findings provide novel evidence that initial exposure to THC may increase connectivity between the ventral striatum and prefrontal regions including the mPFC and dmPFC among healthy young adults with a limited cannabis use history. The mPFC and dmPFC are regions within frontostriatal circuitry involved in reward processing, decision making, and cognitive flexibility and have known connections to the NAcc (Haber and Knutson, 2010; Rouault et al., 2019). Our findings of greater rs-fMRI connectivity between the NAcc and these regions among individuals who received THC (compared to those who received placebo) indicate that THC may increase the intrinsic connectivity of these reward-related regions, even at a relatively low dose (7.5 mg). Future studies are needed to better understand how cannabis use history and other substance use history may differently impact frontostriatal connectivity during acute THC intoxication.
In addition, we found novel, preliminary evidence for a relationship between rs-fMRI frontostriatal connectivity and individuals’ subjective euphoria ratings among individuals who received THC. First, we found that overall, participants who received THC reported higher subjective euphoria ratings, but not subjective drug liking compared to individuals who received placebo. Previous findings for how THC affects subjective ratings of euphoria and drug liking are mixed, with some reporting increased subjective ratings of drug liking and drug reward to THC (Hart et al., 2005; McDonald et al., 2003) and others reporting decreased subjective ratings of drug liking and drug reward to THC (Wardle et al., 2015), indicating there may be individual differences in how rewarding individuals find THC. Indeed, in the current study we found large individual differences in ratings of drug reward (see Figures 2 & 3). In general, participants in both groups reported relatively neutral drug liking (see Figure 2). Second, in exploratory analyses we found that these individual differences in subjective euphoria ratings to THC were related to the strength of rs-fMRI frontostriatal connectivity. To our knowledge this is the first study to find a relationship between rs-fMRI connectivity and subjective response to THC. Specifically, we found that individuals with higher ratings of euphoria demonstrated greater right NAcc-dmPFC connectivity among those who received THC, but this positive association was not significant among those who received placebo. However, it is important to note that although this relationship had a medium effect size, it did not survive correction for multiple comparisons, highlighting the preliminary nature of this finding and the need for replication. Despite the preliminary nature of this finding, it is interesting that increased connectivity between the NAcc and the dmPFC, but not a more ventral mPFC node, was related to higher euphoria ratings to THC. As mentioned above, the dmPFC is involved in reward processing, decision making, and cognitive flexibility and has known connections to the NAcc (Haber and Knutson, 2010; Rouault et al., 2019). Therefore, individuals with greater NAcc-dmPFC connectivity after receiving THC may experience greater THC-induced euphoria and it is possible that these individuals are more likely to use cannabis again.
It is important to note the limitations of the current study. First, the study is a between-subject design. Although THC and placebo groups were matched on participant characteristics, it is possible that the groups differed on measures not included in the current study that may have influenced the results. Future studies using within-subject designs with larger sample sizes are needed to replicate the findings. Second, the participants were healthy young adults who had minimal previous exposure to cannabis (≤10 lifetime exposures). Although this allowed us to better understand how initial responses to THC may impact frontostriatal rs-fMRI connectivity, which could contribute to continued cannabis use, this limits the generalizability of the findings. It is not clear that the findings would be generalizable to individuals who are at high risk, such as those with a family history of drug use, or to individuals with a history of more lifetime cannabis use. Third, we found that functional connectivity was related to ratings of euphoria, but not ratings of drug liking. In previous studies, the ARCI-MBG and DEQ-Like are only modestly correlated after administration of other substances (i.e., alcohol, amphetamine, nicotine; (Morean et al., 2013), suggesting that they are capturing difference subjective experiences. In the current study, these measures were not significantly correlated (r= −.10, p=.54). Overall, participants in both groups reported neutral/no effects of the drug for the DEQ-Like scale. Therefore, the lack of correlation between the ARCI-MBG and DEQ-Like scales may be due to a restricted range of drug liking ratings. More research is needed to better understand how THC impacts euphoria and liking ratings in healthy individuals and in cannabis users. Fourth, we tested only one dose of THC and only one mode of drug delivery (i.e., oral ingested pill). Examining different doses and different modes of THC delivery (e.g., smoked, oral ingested pill, edible) would provide a more complete picture of the pharmacological profile. Fifth, physiological measures (e.g., respiratory rate, heart rate, vascular changes) were not measured in the study. THC may alter these physiological measures, which may have influenced the BOLD response among individuals given THC in the study. Sixth, we did not collect pharmacokinetic measures to support the effectiveness of the THC manipulation. Seventh, we did not measure drug expectancies, which are known to impact subjective drug response (e.g., Kirk et al., 1998) and may also impact neural activity. Eighth, we did not measure caffeine or tobacco use on the day of the drug administration visit. Although groups did not differ on recent caffeine or tobacco use (and results did not change controlling for past month tobacco user status), it is possible that groups may have differed on caffeine and/or tobacco use on the day of the drug administration visit, which may have affected the results. Lastly, our study is cross-sectional and we did not measure substance use over time. Future and ongoing studies are needed to replicate the current study in larger, longitudinal samples.
5. Conclusions
Overall, our findings provide novel evidence that healthy, non-cannabis users who take THC experience greater subjective and neural reward responses compared to those who take placebo, which may contribute to the rewarding, reinforcing properties of cannabis. Specifically, we found that individuals who received THC demonstrated greater frontostriatal rs-fMRI connectivity and higher subjective euphoria ratings compared to individuals who received placebo. In addition, we found preliminary evidence that greater frontostriatal rs-fMRI connectivity is related to higher subjective ratings of euphoria among individuals who received THC compared to individuals who received placebo. These findings extend our understanding of how THC impacts the brain, especially frontostriatal brain reward circuitry, among healthy adults with limited lifetime cannabis use and provide preliminary evidence that individual differences in subjective euphoria ratings to THC are related to the strength of rs-fMRI frontostriatal connectivity, which may represent a profile of risk for continued cannabis use.
Highlights.
It is not clear how Δ9-Tetrahydrocannabinol (THC) impacts brain reward circuitry
We examined how THC alters resting state functional connectivity in non-users
THC increased frontostriatal rs-fMRI connectivity and subjective euphoria ratings
Higher euphoria ratings to THC were related to greater frontostriatal connectivity
These findings extend our understanding of how THC impacts the brain
Acknowledgments
Role of Funding Source
This publication was funded by the National Institute of Mental Health (NIMH) (R21MH093917, PI: KLP) and supported through the National Institutes of Health (NIH) through the Michigan Institute of Clinical and Health Research and the UIC Center for Clinical and Translational Science (CCTS) (UL1TR002003). NAC was supported by NIMH (T32MH067631, PI: Rasenick) and the National Institute on Drug Abuse (NIDA; K23DA048132, PI: NAC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMH or NIH. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The current study was presented at the 2018 American College of Neuropsychopharmacology meeting: Crane, N.A. & Phan, K.L. (December, 2018). Δ9-Tetrahydrocannabinol Enhances Fronto-Striatal Resting State Functional Connectivity. The 57th annual meeting of the American College of Neuropsychopharmacology, Hollywood, FL, December 9-13.
Conflict of Interest
None
References
- Ballard ME, de Wit H, 2011. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacology, biochemistry, and behavior 97, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MA, Ashok AH, Volkow ND, Howes OD, 2016. The effects of Delta9-tetrahydrocannabinol on the dopamine system. Nature 539, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Lee JY, Brown EN, Finch SJ, 2012. Comorbid trajectories of tobacco and marijuana use as related to psychological outcomes. Substance abuse : official publication of the Association for Medical Education and Research in Substance Abuse 33, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA, 2019. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci 269, 5–15. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL, 1990. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol 190, 259–262. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NY, Both S, van Gerven JM, Rombouts SA, 2013a. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. Neuroimage 78, 59–67. [DOI] [PubMed] [Google Scholar]
- Cole DM, Oei NY, Soeter RP, Both S, van Gerven JM, Rombouts SA, Beckmann CF, 2013b. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb Cortex 23, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2008. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addiction biology 13, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R, 2013. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology review 23, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J, 2002. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 164, 61–70. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S, 1993. Variability in subjective responses to cocaine: initial experiences of college students. Addictive behaviors 18, 445–453. [DOI] [PubMed] [Google Scholar]
- de Wit H, Phillips TJ, 2012. Do initial responses to drugs predict future use or abuse? Neuroscience and biobehavioral reviews 36, 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE, 1986. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug and alcohol dependence 16, 341–360. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M, 2003. Exploring the association between cannabis use and depression. Addiction 98, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Blum K, Badgaiyan RD, Perez PD, Colon-Perez LM, Thanos PK, Ferris CF, Kulkarni P, Giordano J, Baron D, Gold MS, 2017. Enhanced functional connectivity and volume between cognitive and reward centers of naive rodent brain produced by pro-dopaminergic agent KB220Z. PLoS One 12, e0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, 2008. Cannabis use and later life outcomes. Addiction 103, 969–976; discussion 977-968. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hernandez M, Ramos JA, 2010. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16, e72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams J, 2002. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Research Version, Administration Booklet. [Google Scholar]
- Fischman MW, Foltin RW, 1991. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86, 1563–1570. [DOI] [PubMed] [Google Scholar]
- French ED, 1997. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neuroscience letters 226, 159–162. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Lyons M, Mori S, Angstadt M, Rabinak CA, 2016. Cannabinoid Modulation of Frontolimbic Activation and Connectivity During Volitional Regulation of Negative Affect. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41, 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm O, Loffler M, Kamping S, Hartmann A, Rohleder C, Leweke M, Flor H, 2018. Probing the endocannabinoid system in healthy volunteers: Cannabidiol alters frontostriatal resting-state connectivity. Eur Neuropsychopharmacol 28, 841–849. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, Miyasato K, 1983. Reinforcements from the first drug experience can predict later drug habits and/or addiction: results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimulants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug and alcohol dependence 11, 147–165. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW, 2005. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology 181, 237–243. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH, 1980. Drug preference and mood in humans: d-amphetamine. Psychopharmacology 71, 275–279. [DOI] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D, 2014. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biological psychiatry 75, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JM, Doty P, De Wit H, 1998. Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacology, biochemistry, and behavior 59, 287–293. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S, 2006. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction 101, 713–725. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR, 1971. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12, 245–258. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A, 2005. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological psychiatry 58, 779–786. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M, 2003. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23, 285–300. [DOI] [PubMed] [Google Scholar]
- Mason NL, Theunissen EL, Hutten N, Tse DHY, Toennes SW, Jansen JFA, Stiers P, Ramaekers JG, in press. Reduced responsiveness of the reward system is associated with tolerance to cannabis impairment in chronic users. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason NL, Theunissen EL, Hutten N, Tse DHY, Toennes SW, Stiers P, Ramaekers JG, 2019. Cannabis induced increase in striatal glutamate associated with loss of functional corticostriatal connectivity. Eur Neuropsychopharmacol 29, 247–256. [DOI] [PubMed] [Google Scholar]
- Mayo LM, de Wit H, 2015. Acquisition of responses to a methamphetamine-associated cue in healthy humans: self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology 40, 1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H, 2003. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28, 1356–1365. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE, 2012. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America 109, E2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS, 2013. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology 227, 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJ, van Laar MW, 2013. Does Cannabidiol Protect Against Adverse Psychological Effects of THC? Frontiers in psychiatry 4, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H, 2008. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28, 2313–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K, 2011. Subjective response to alcohol challenge: a quantitative review. Alcoholism, clinical and experimental research 35, 1759–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Sripada CS, Angstadt M, de Wit H, Phan KL, 2012. Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J Neural Transm (Vienna) 119, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Evers EA, Theunissen EL, Kuypers KP, Goulas A, Stiers P, 2013. Methylphenidate reduces functional connectivity of nucleus accumbens in brain reward circuit. Psychopharmacology 229, 219–226. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, van Wel JH, Spronk D, Franke B, Kenis G, Toennes SW, Kuypers KP, Theunissen EL, Stiers P, Verkes RJ, 2016. Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav 10, 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault M, Drugowitsch J, Koechlin E, 2019. Prefrontal mechanisms combining rewards and beliefs in human decision-making. Nat Commun 10, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW, 2001. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. Journal of clinical psychopharmacology 21, 273–286. [DOI] [PubMed] [Google Scholar]
- Sevigny EL, 2013. Is today’s marijuana more potent simply because it’s fresher? Drug Test Anal 5, 62–67. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Windisch K, Ando RD, Sylvester Vizi E, 2009. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int 54, 452–457. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G, 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D, 2003. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. The 9th International Conference on Functional Mapping of the Human Brain, New York, New York. [Google Scholar]
- Wardle MC, Marcus BA, de Wit H, 2015. A Preliminary Investigation of Individual Differences in Subjective Responses to D-Amphetamine, Alcohol, and Delta-9-Tetrahydrocannabinol Using a Within-Subjects Randomized Trial. PLoS One 10, e0140501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Van Hedger K, Keedy SK, Nwaokolo N, de Wit H, 2020. Methamphetamine acutely alters frontostriatal resting state functional connectivity in healthy young adults. Addiction biology 25, e12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A, Livny A, Weizman A, 2016. Brain Imaging Studies on the Cognitive, Pharmacological and Neurobiological Effects of Cannabis in Humans: Evidence from Studies of Adult Users. Curr Pharm Des 22, 6366–6379. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD, 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]


