Abstract
Objectives:
Mucoepidermoid carcinoma (MEC) is the most common type of salivary gland malignancy. Advanced or high-grade MECs are refractory to chemotherapy, often leading to tumor recurrence/metastasis and abysmal ~35% 5-year survival. Causal links have been established between Epithelial Growth Factor Receptor (EGFR) activation and poor outcome. Herein we investigated the therapeutic efficacy of EGFR inhibition against MEC using in vitro pre-clinical models.
Materials and Methods:
Five human MEC cell lines were used in cell viability, cytotoxicity, apoptosis, cell cycle, 2D-clonogenicity, and 3D-spheroid formation assays following treatment with Erlotinib (EGFR inhibitor), SAHA (Histone Deacetylase inhibitor; HDAC) and CUDC-101 (dual EGFR-HDAC inhibitor). Effects on MEC cancer stem cells were evaluated using flow cytometry. Gene expression and pathway regulation were evaluated via qPCR and Western blot, respectively.
Results:
MEC cells enter a quiescent, non-proliferative yet rapidly reversible drug tolerant state upon EGFR inhibition. Despite robust suppression of MEC cell proliferation, no discernable apoptosis is detected. Combination of EGFR and HDAC inhibitors exhibits synergistic effects, exerting ~5-fold more potent cell cytotoxicity compared to HDAC or EGFR monotherapy. CUDC-101, a single molecule with dual EGFR-HDAC inhibitor moieties, exerts irreversible and potent cytotoxic activity against MEC cells and blunts MEC cancer stem-cell tumorigenicity.
Conclusion:
MEC cells are intrinsically tolerant to EGFR inhibition. Combining EGFR and HDAC inhibitors exerts synergistic and potent cytotoxic effects, suggesting that EGFR inhibitors still hold significant promise against MEC. Future studies are needed to assess the applicability and efficacy of dual EGFR-HDAC inhibitors for the clinical management of MEC.
Keywords: Head and Neck Cancer, Salivary Cancer, Mucoepidermoid Carcinoma, Drug Tolerance, Drug Synergy, EGFR, HDAC, CUDC-101
INTRODUCTION
Mucoepidermoid carcinoma (MEC) is the most common salivary gland malignancy but remains understudied[1]. The majority of salivary MEC patients present with indolent low-grade disease and exhibit promising prognosis (90% 5yr-survival). However, some patients develop aggressive high-grade tumors that frequently recur and metastasize, leading to dismal outcomes (35% 5yr-survival)[1-3]. While most tumors are effectively treated with surgery and postoperative-radiation therapy, these treatments are associated with significant patient co-morbidities such as facial paralysis or xerostomia, among others[4,5]. Thus, there is a need for identifying novel treatments such as targeted therapies that lack the complications associated with conventional chemoradiation approaches. However, the poor overall understanding of MEC pathobiology has led to a dearth of targeted therapies to date[3,6].
At the molecular level, salivary MEC is defined by the t(11;19) chromosomal translocation which is estimated to occur in up to ~90% of all cases[7]. This translocation generates the oncogenic transcriptional co-activator gene fusion CRTC1-MAML2 (C1/M2). Chen et al. demonstrated that C1/M2 can, through its interactions with the transcription factor CREB, aberrantly upregulate the transcription of the Epithelial Growth Factor Receptor (EGFR) ligand Amphiregulin (AREG), thus creating a therapeutic vulnerability to EGFR inhibitors (EGFRi) in MEC[8]. Moreover, several retrospective clinical studies identified significant correlations between EGFR amplification/ERK hyperactivation and poor patient outcomes in MEC patients[4,8-13]. These studies support the notion that therapies targeting EGFR signaling may provide therapeutic benefit and warrant further pre-clinical investigation. However, EGFR monotherapy has not shown significant efficacy in MEC clinical trials thus far[14-16] (Supplementary Table 2). Notably, we and others have demonstrated that acquired resistance to EGFR inhibition is commonplace across many cancers[17-20], via acquisition of ‘drug-desensitizing’ mutations, activation of complementary signaling pathways[21,22], or due to drug-induced selection of pre-existing drug resistant tumor cell subpopulations[23].
Previous studies in MEC have suggested that histone deacetylase inhibitors (HDACi), such as SAHA, could be used as sensitizing agents against MEC cells when deployed in combination with chemotherapy[24] or NFκB[25] inhibition. Herein, we report for the first time that salivary MEC cells are intrinsically tolerant to EGFRi monotherapies and exhibit no cytotoxicity in response to EGFR inhibition. Furthermore, we demonstrate that SAHA can be used as a true ‘sensitizing’ agent in MEC cells, wherein combinatorial EGFRi + HDACi exhibits synergistic interactions against MEC cells. Lastly, we show that the single agent dual EGFR-HDAC inhibitor, CUDC-101, is effective at combatting this tolerance by potently suppressing MEC cell growth and proliferation and inducing rapid and robust cell death.
MATERIALS AND METHODS
Cell lines and drug reagents
Human MEC cell lines UM-HMC-1 (CVCL_Y473) UM-HMC-3A (CVCL_Y471), and UM-HMC-3B (CVCL_Y472) were provided by the Nör Lab (University of Michigan, Ann Arbor, MI). Unless otherwise specified, all UM-HMC lines were cultured in DMEM (Gibco, cat. #: 11965-118) media supplemented with 100 mM HEPES (Corning, cat. #: 25-060-Cl), 1 mM Sodium pyruvate (Gibco, cat. #: 11360070), 1x GlutaMAX (Gibco, cat. #: 35050061), 1xPSG (Gibco, cat. #: 10378-016), 10% FBS (Atlanta Biologicals, Cat. #S11550), 20 ng/mL EGF (Sigma-Aldrich, Cat. #E9644), 400 ng/mL Hydrocortisone (Sigma-Aldrich, Cat. #H0888) and 5 μg/mL Insulin (Sigma-Aldrich, Cat. #I6634) as previously described[26]. NCI-H3118 (CVCL_A464) and NCI-H292 (CVCL_0455) MEC lines were obtained from ATCC and cultured in DMEM (Gibco, cat. #: 11965-118) media supplemented with 100 mM HEPES (Corning, cat. #: 25-060-Cl), 1 mM Sodium pyruvate (Gibco, cat. #: 11360070), 1x GlutaMAX (Gibco, cat. #: 35050061), 1xPSG (Gibco, cat. #: 10378-016), 10% FBS (Atlanta Biologicals, Cat. #S11550) previously described[8]. Cells were dissociated using TrypLE (Gibco, cat. #: 12604013) and passaged every 2-3 days. A fresh vial of cells was thawed after every 15-20 passages in culture. All drugs were purchased from Sigma-Aldrich, CUDC-101 (cat. #: EPS003), SAHA (cat. #: SML0061) and Erlotinib (cat. #: SML2156). All drugs were dissolved in DMSO (Sigma-Aldrich cat. #: 472301) to make 5 mM working stocks.
Western blots
UM-HMC cells (UM-HMC-1= 400,000/well, UM-HMC-3A= 750,000/well or UM-HMC-3B= 600,000/well) were seeded in a 6 well plate. 24 h post seeding, wells were washed twice with 1xDPBS (Gibco, cat. #: 14190136) and 2 mL of UM-HMC media (see above) containing vehicle or drug at 1% DMSO final, was added. 24 h post drug addition, cells were lysed in RIPA lysis buffer [150 mM sodium chloride, 1.0% NP-40, 1.0% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 25 mM Tris, pH 7.6, 20 mM NaF, 1 MM EDTA, supplemented with protease inhibitors (cOmplete, EDTA-free Protease Inhibitor Cocktail - Roche, cat. #: 04693132001) and phosphatase inhibitors (phosphoSTOP, - Roche, cat. #: 10917400)]. Protein concentrations were determined (using Pierce BCA Protein Assay Kit, cat. #: 23225) and equal amounts of total protein were resolved by SDS-PAGE gel electrophoresis. All western blots for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
Antibodies
All western blotting antibodies were diluted in TBST buffer with 5% bovine serum albumin (for antibodies targeting phosphorylated proteins) or 5% non-fat milk (for all other antibodies). The following antibodies were used for western blotting, diluted as recommended by the manufacturer; Total EGFR (Cell Signaling Technologies, cat. #: 4267), p-EGFR (Y1068) (Cell Signaling Technologies, cat. #: 2234), Total AKT (Cell Signaling Technologies, cat. #: 4685), p-AKT (S473) (Cell Signaling Technologies, cat. #: 4060), Total ERK1/2 (Cell Signaling Technologies, cat. #: 4695), p-ERK1/2 (T202/Y204) (Cell Signaling Technologies, cat. #: 4370), Total Histone H4 (Cell Signaling Technologies, cat. #: 13919), H4K12ac (Cell Signaling Technologies, cat. #: 13944) and Tubulin (Sigma Aldrich, cat. #: T5168).
qPCR
RNA was isolated using the NucleoSpin RNA kit (Macherey-Nagel, #740955) according to the manufacturer’s protocol. RNA was eluted in RNase-free water and quantified using a Cytation 5 plate multi-mode reader spectrophotometer. cDNA was synthesized using the iScript cDNA Synthesis Kit (BioRad cat, #: 170-8891). Quantitative RT-PCR was performed using FastStart Universal SYBR Green Master (Roche cat, #: 04913850001), using 2 μL of cDNA (20 ng/μL) per reaction. qPCR primers used were as follows:
RPL23 sense: 5’-TGATGGCCACAGTCAAGAAA-3’ + anti-sense: 5’-ACACGCCATCTTTTCTACGG-3’.
AREG sense: 5’-AGGAGAAGCTGAGGAACGAAA-3’ + anti-sense: 5’-CACTGGAAAGAGGACCGACTC-3’.
TBP sense: 5’-CGGCTGTTTAACTTCGCTTC-3’ + anti-sense: 5’-CACACGCCAGAAACAGTGA-3’.
CRTC1-MAML2 (C1/M2) fusion sense: 5’- ATGGCGACTTCGAACAA-3’ + anti-sense: 5’-GGGTCGCTTGCTGTTGGC-3’
Viability curves (Cellular ATP levels)
MEC cells (H292= 8,000/well, H3118=12,000/well, UM-HMC-1=8000/well, UM-HMC-3A=12,000/well or UM-HMC-3B=12,000/well) were seeded in a volume of 100 μL media in a 96 well clear bottom white opaque walled plate (Corning cat. #: 3917). 24 h post seeding, 100 μL of 2x drug/media was added per well, 1% DMSO final. 72 h post drug addition, the amount of ATP in each well (a pseudo measurement for ‘viable’ cells) was measured using ATPlite Luminescence Assay (PerkinElmer, cat#: 6016949) kit, according to the manufacturer’s protocol. Briefly, the plates were removed from the cell culture incubator and equilibrated to room temperature for 30 min in the dark. Then, media was aspirated and 100 μL of reconstituted ATPlite 1-step reagent was added per well. Plates were shaken for 2 min in the plate reader (425 cpm, 3 mm orbit, Cytation 5 plate multi-mode reader, BioTek), shielded from light and incubated at room temperature for an additional 5 min. ATP levels were quantified by measuring total luminescent light output using a Cytation5™ microplate reader (BioTek Instruments, Inc). All assays were performed in biologic triplicate, with each experiment containing a minimum of two technical replicates for each condition. IC50 was calculated using a non-linear curve fit (log[agonist] vs response, four parameter-variable slope) in Prism (GraphPad, San Diego, CA, USA). The 1% DMSO control condition was defined as 100% viability All drug titration and cell viability experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
Cytotoxicity curves
UM-HMC-3A cells (12,000/well) were seeded in a volume of 100 μL media/well in a 96 well clear bottom white opaque walled plate (Corning cat. #: 3917). 24 h post seeding, 100 μL of 2x drug/media was added per well, 1% DMSO final. 72 h post drug addition, the amount of cell death per well was quantified using the CellTox (Promega, cat#: G8741) kit, according to the manufacturer’s protocol. Briefly, 50 μL of CellTox reagent (diluted 1:200 in the provided assay buffer) was added per well. Plate was shielded from light and incubated at room temperature for 30 min. CellTox fluorescence intensities were quantified using a Cytation5™ microplate reader (BioTek Instruments, Inc). Excitation wavelengths were set at 485 nm ± 20 nm bandwidth and emission wavelengths were set to 510 nm ± 20 nm bandwidth. Wells were imaged using Cytation5™ microplate reader (BioTek Instruments, Inc) using a 4x objective. Images were stitched using the Gen 2.09 software (BioTek Instruments, Inc), utilizing default parameters. All assays were performed in biologic triplicate, with each experiment containing a minimum of two technical replicates for each condition. IC50 was calculated using a non-linear curve fit (log[agonist] vs response, four parameter-variable slope) in Prism. All cytotoxicity experiments for Erlotinib and CUDC-101 were performed concurrently.
Apoptosis
400,000 UM-HMC 3A cells/well were seeded in a 6 well plate. 24 h post seeding, wells were washed twice with 1xDPBS (Gibco, cat. #: 14190136) and 2 mL of media containing vehicle or drug at 1% DMSO final, was added. Cells were trypsinized (Gibco TrypLE, cat. #: 12604013) at the indicated time point, spun down and resuspended in 1mL of 1xDPBS supplemented with 1x GlutaMAX (Gibco, cat. #: 35050-061) and 10% Fetal Bovine Serum (Atlanta Biologicals, cat. #: S11550). Apoptotic/Dead cells were visualized using the CellEvent Caspase 3/7 flow cytometry kit (ThermoFisher cat. #: C10427), following the manufacturer’s protocol. Briefly, 1 μL of the Caspase 3/7 detection reagent was added per 1 mL of cells, gently vortexed, and incubated for 25 min at 37°C. After 25 min, 1 μL of SYTOX 7AADvanced dead cells stain was added per 1 mL of cells, gently vortexed, and incubated for 5 min at 37°C. Cells were analyzed using flow cytometry (Accuri BD C6). An aliquot of unstained cells was saved as unstained controls that were used for gating cut-offs. All assays were performed in biologic triplicate. All apoptosis experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
Cell cycle
400,000 UM-HMC 3A cells/well were seeded in a 6 well plate. 24 h post seeding, wells were washed twice with 1xDPBS (Gibco, cat. #: 14190136) and 2 mL of media containing vehicle or drug at 1% DMSO final, was added. Cells were trypsinized (Gibco TrypLE, cat. #: 12604013) at the indicated time point, spun down and resuspended in 1 mL of ice-cold 1xDPBS supplemented with 1x GlutaMAX (Gibco, cat. #: 35050-061) and 10% Fetal Bovine Serum (Atlanta Biologicals, cat. #: S11550). Cells were fixed by slowly adding them to 10 mL of ice-cold 70% ethanol under constant vortexing. Cells were stored at −20C for 1 week prior to analysis. Cells were washed 3x in 5 mL 1x DPBS (ice-cold) to wash out the ethanol. Cells were permeabilized, RNA degraded and DNA stained by incubating the cells in a 0.1% Triton X-100 + RNAse (200 μg/mL) + propidium iodide (20 μg/mL) DNA staining buffer for 30 min at 37 C. Total cellular DNA content was analyzed on a benchtop flow cytometer (BD Accuri C6). An aliquot of unstained cells was saved as unstained controls that were used for gating cut-offs. All assays were performed in biologic duplicate. All cell cycle experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
EGFR mutational status
RNA was extracted from MEC cell lines (Macherey-Nagel RNA extraction kit, cat, #: 740955), following the manufacturer’s protocol. 2.5 μg of RNA was converted to cDNA using the Superscript IV Reverse Transcriptase kit (ThermoFisher, cat. #: 18091050). 125 ng of cDNA was used to PCR amplify regions spanning exons 15-25 of EGFR. PCR was conducted in two sets. Set I: EGFR exons 15-20 (predicted amplicon size= 619 bp), sense: GGCAGGAGTCATGGGAGAAA + anti-sense: GACATAGTCCAGGAGGCA GC Set II: EGFR exons 18-25 (predicted amplicon size= 970 bp), sense: GCTCCCAACCAAGCTCTCTT + anti-sense: GCTGTGGGATGAGGTACTC G. 1% DMSO (final) was added to the PCR reaction. PCR was performed using PfuUltra high fidelity DNA polymerase (Agilent, cat. #: 600380) with the following cycling conditions; 95° for 3 min followed by 30 cycles of amplification at 90°C for 30 s, 58°C for 45 s and 72°C for 1 min, followed by a final 5 min extension step at 72°C. PCR amplicons were run in a 1% agarose gel and the appropriate bands were excised and purified (Macherey-Nagel PCR clean up kit, cat. #: 740609). DNA amplicon concentrations and quality were quantified using a spectrophotometer (DeNovix, cat. #: DS-11). Amplicons were Sanger sequenced (Eton Bioscience, Inc) using the same primers as were used for PCR amplification. Sequenced reads were aligned to EGFR (NM_005228.5) using the NCBI Nucleotide BLAST alignment tool. Heterozygous mutations were identified by analyzing the sequencing chromatograms (overlapping peaks at ~half the intensity each as compared to the surrounding peak heights).
Erlotinib resistance experiment
UM-HMC cells (UM-HMC-1= 400,000/well, UM-HMC-3A= 750,000/well or UM-HMC-3B= 600,000/well) were seeded in a 6 well plate. 24 h post seeding, media was replaced with 3 mL of fresh media containing 25 μM Erlotinib, at 1% DMSO final. 72 h post drug addition, media was aspirated, and wells were washed thrice with 3 mL of 1xDPBS. Cells were trypsinized using TrypLE and cell densities were quantified using an automated cell counter (BioRad cat. #: TC20). Cells were re-seeded in a 24 well plate; 20,000 UM-HMC-1, 50,000 UM-HMC-3A or 25,000 UM-HMC-3B cells/well. Untreated UM-HMC cells were seeded on the same plate at identical densities to serve as untreated controls. 6 h post seeding, upon cell adhesion to the plate, the plate was placed into an IncuCyte ZOOM live-cell analysis machine (Essen BioScience cat. #: 2013A) for growth curve analysis. The IncuCyte was housed within a humidified cell culture incubator (37°C, 5% CO2) to enable longitudinal imaging. 4 representative fields of view per well were imaged every 2 h using a 10x objective (Essen BioScience cat. #: 4464), over a period of 120 h. Cell confluency was quantified using the IncuCyte ZOOM analysis software, utilizing default parameters for generating cell masks for analyzing changes in cell densities/confluency over time. All assays were performed in biologic triplicate, with each experiment containing a minimum of two technical replicates for each condition.
Serum starvation
MEC cells were seeded in a 6 well plate in full serum media, 24 h post seeding wells were washed with 1xDPBS and 3 mL of 0.2% Serum Starvation media was added per well (DMEM + 0.2% FBS + 1x HEPES + 1x PSG + 1x NaPyr + 1x GlutaMAX). At the indicated time points, wells were washed with 1xDPBS and lysed in 150 μL of RIPA buffer. Lysates were processed for western blotting as described above (See METHODS: Western Blotting). All assays were performed in biologic duplicate.
2D Clonogenic assay
600 UM-HMC 3A cells/well were seeded in a 24 well plate (Corning cat, #: CLS3527). ~48 h post cell seeding, media was aspirated and replaced with 1 mL media containing the appropriate drug at the indicated concentrations; DMSO was maintained at 1% final across all conditions. 1 week colony formation: 7 days post drug addition, wells were washed with 1 mL 1xDPBS and cells were fixed using 10% buffered formalin (Fisher scientific cat. #: SF99-4). After a 5 min incubation with 10% formalin, wells were washed thrice with tap water, and cell colonies were stained using a 0.05% crystal violet solution; 30 min incubation at room temperature. Crystal violet solution was aspirated, and wells were washed thrice with tap water. Plates were dried overnight, and wells were imaged on a Cytation5™ microplate reader (BioTek Instruments, Inc), using a 4x objective and images were stitched using the Gen 2.09 software (BioTek Instruments, Inc), utilizing default parameters. Cell colonies were counted manually using ImageJ. Cell colonies were defined as clusters of ≥50 cells. The amount of crystal violet staining per well was quantified by extracting the crystal violet dye, 30 min incubation with 1 mL of 1% SDS solution at room temperature and quantifying crystal violet dye absorbance at 570 nm. 1 week-WASH-1 week outgrowth: 7 days post drug addition, wells were washed twice with 1 mL 1xDPBS. 1 mL of fresh media (without any drug or vehicle) was added per well, plates were placed back into a cell culture incubator for an additional 7 days, following which the cells were processed as described above. All assays were performed in biologic duplicate, with each experiment containing a minimum of two technical replicates for each condition. All colony formation experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
3D spheroid assay (Matrigel sandwich culture)
100 μL of a 25% Matrigel (Fisher, cat#: CB4023A) solution diluted in cold 1xDPBS was added per well of a 48 well plate. Plate was centrifuged in a pre-chilled centrifuge (Thermo Scientific, cat#: Sorvall LYNX 4000) for 10 min at 2000g to ensure an even distribution of Matrigel across the well surface. After, the plate was placed in a cell culture incubator for 1 h to ensure complete polymerization of the Matrigel ‘bed’. Upon polymerization, excess 1xDPBS was gently aspirated from the wells without disrupting the Matrigel ‘bed’. 2000 UM-HMC 3A cells (suspended in 100 μL of media) were gently added to each well. The plate was centrifuged for 5 min at 2000g to ensure attachment/embedding of cells into the Matrigel ‘bed’. Plates were returned to a cell culture incubator for 1 h to ensure cell adhesion to the Matrigel. Excess media was carefully aspirated to minimize disruption of the Matrigel ‘bed’-cell layer. 50 μL of undiluted Matrigel was gently added to each well and the plate was placed in a cell culture incubator for 1 h to ensure complete polymerization of the Matrigel ‘cover’. 500 μL of media (containing either drug or vehicle, at 1% DMSO final) was added per well. Wells were imaged every 24 h using Cytation5™ microplate reader (BioTek Instruments, Inc) equipped with a 4x objective. Images were stitched using the Gen 2.09 software (BioTek Instruments, Inc) utilizing default parameters. Tumor spheroid areas were measured manually using the ROI area measurement function in ImageJ. All assays were performed in biologic triplicate and a minimum of 50 individual tumor spheroids were quantified for each condition in each experiment. All 3D tumor spheroid formation experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
3D spheroid assay (Ultra-low adhesion plates)
UM-HMC-3A cells were seeded on ultra-low attachment 6-well plates (Corning, New York, NY, USA) and left to grow for three days under minimal disturbance. Spheres were cultured in DMEM-High glucose (Hyclone Laboratories Inc., Logan, UT, USA) supplemented with 10% Fetal Bovine Serum (FBS, Thermo Scientific, Waltham, MA, USA), 1% antibiotics (Invitrogen, Carlsbad, CA, USA), 1% L-glutamine (Invitrogen, Carlsbad, CA, USA), 20 ng/ml epidermal growth factor (PeproTech, Rocky Hill, NJ, USA), 400 ng/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA) and 5μg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA). Cells were maintained in a 5% CO2-humidified incubator at 37°C. 50 nM CUDC-101, SAHA, or Erlotinib was applied to seeded tumor cells and maintained for 3 days. Spheres were classified and quantified as previously reported [27,28] using an Axiocan ERc5s color Camera (Zeiss, Munich, Germany) attached to a Nikon Eclipse Ts2 (Melville, NY, USA). All assays were performed in biologic triplicate, with each experiment containing a minimum of two technical replicates for each condition. All ultra low-adhesion 3D tumor spheroid formation experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
Flow cytometry for Cancer Stem Cell quantification
UM-HMC-3A cells were resuspended and counted using a Countess II FL automatic cell counter (Invitrogen, Carlsbad, CA, USA). The combination of ALDHbright plus CD44high was used to identify mucoepidermoid carcinoma cancer stem cells as previously reported[27]. The Aldefluor kit (StemCell Technologies, Durham, NC, USA) was used according to the manufacturer’s instructions to identify cells with high ALDH1 enzymatic activity. UM-HMC-3A cells were suspended with activated Aldefluor substrate (BODIPY amino acetate) or negative control (dimethylamino benzaldehyde, a specific ALDH inhibitor) for up to 45 minutes at 37°C. Then, cells were washed and suspended with anti-CD44/APC conjugated antibody (BD Biosciences, Mountain View, CA, USA) and incubated for 25 min in shaking rotor at 4°C. All samples were analyzed using a flow cytometer Accuri C6 (BD Biosciences, USA) equipped with two excitation lasers: a solid blue state (488 nm) and a diode red (640nm). All assays were performed in sextuplicate. All CSC flow cytometry experiments for the various drugs (Erlotinib, SAHA and CUDC-101) were performed concurrently.
Quantification and Statistical Analysis
All statistical tests were executed using GraphPad Prism software or the statistical software R (version 3.1.2). Differences between variables were assessed by 2-tailed Student’s t test or 2-way ANOVA multiple comparisons test with either Sidak or Dunnett post hoc tests, where appropriate. Data are expressed as mean ± SEM, P values less than 0.05 were considered statistically significant ns = p > 0.05, *= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001).
RESULTS
MEC cells exhibit reversible drug tolerance to EGFR monotherapy treatment
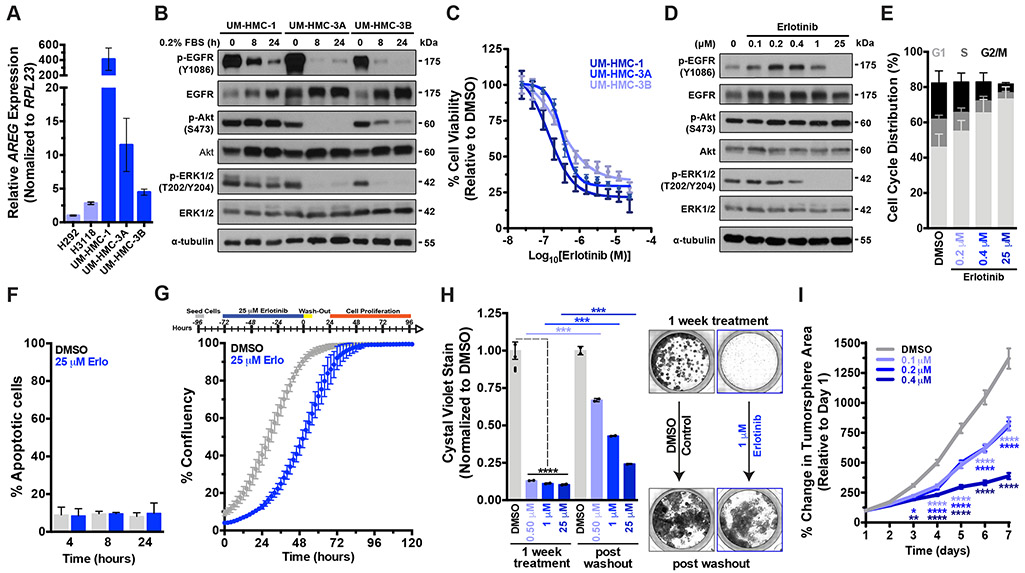
C1/M2 fusion positive MEC cells (NCI-H292 and NCI-H3118 cell lines) have been shown to sustain autocrine EGFR signaling by upregulating the EGFR ligand AREG, which is required for cell growth and survival[8]. To assess whether aberrant AREG expression is a general hallmark of C1/M2 fusion positive MEC cells, we examined three additional fusion positive MEC cell lines recently generated: UM-HMC-1, UM-HMC-3A, and UM-HMC-3B[26]. Quantification of AREG mRNA expression revealed that the UM-HMC lines express significantly higher (p<0.001) AREG levels compared to the H292 and H3118 cell lines[8] (Fig. 1A), suggesting that UM-HMC cell lines may be more sensitive to EGFR inhibition than their counterparts[29]. Serum starvation experiments confirmed that not only do MEC cells maintain autocrine pEGFR/pERK/pAkt signaling but that their ability to do so directly correlates with their relative baseline AREG expression levels (Fig. 1B). The importance of EGFR signaling to MEC cell proliferation, maintenance and survival was further underscored by the observation that MEC cells robustly and rapidly upregulated total EGFR receptor levels upon serum starvation, supporting the notion that MEC cells are addicted to constitutive EGFR signaling for proliferation and survival (Fig. 1B).
Figure 1. Continuous EGFR activation is required for proliferation but is not necessary for MEC cell survival.
A) Real-time qPCR was performed to quantify relative AREG mRNA expression in five C1/M2 fusion positive MEC cell lines (n = 3, mean ± SEM) under normal culture conditions.
B) Western blot analysis of EGFR signaling activation status as assessed by p-EGFR (Y1086) and downstream p-AKT (S473) and p-ERK1/2 (T202/Y204) levels under serum starvation.
C) Dose-response curves were performed using concentrations ranging from 25 nM to 25 μM and cell viability was assayed 72 hours post drug addition (mean ± SEM).
D) Erlotinib treatment of UM-HMC-3A cells causes a dose dependent decrease in p-EGFR and p-ERK1/2 See Supplementary Fig. 3B for full blots.
E) Erlotinib treatment causes robust and dose dependent cell cycle arrest in UM-HMC-3A cells (n = 3, mean ± SEM). G1 phase- DMSO vs 0.2 μM (**), vs 0.4 μM (****), vs 25 μM (****). S phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (**), vs 25 μM (***). G2/M phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (**), vs 25 μM (****). p values were determined by 2way ANOVA multiple comparisons test (Sidak); ns = p > 0.05, *= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001.
F) UM-HMC-3A cells were treated with 25 μM Erlotinib and activated caspase 3/7 levels measured (n = 3, mean ± SEM). 8 hr- DMSO vs Erlotinib, and 24 hr- DMSO vs Erlotinib, all not significant (p>0.05). P values were determined using Student’s t-test, comparing DMSO vs Erlotinib for each time point. Also see Supplementary Fig. 1A.
G) top, Schematic for drug withdrawal experiment. Erlotinib-treated MEC cells rapidly proliferate upon drug withdrawal (n = 2, mean ± SEM). Also see Supplementary Fig. 1B-C.
H) left, 2D clonogenic growth capacity of UM-HMC-3A cells (n = 2, mean ± SEM). Cells were treated with drug and then allowed to form colonies for 7 days, at which point cells were fixed and stained with crystal violet and colonies quantified. For the drug withdrawal condition, 7 Days post drug addition, drug was washed out and cells were allowed to outgrow for an additional 7 days and colonies quantified. P values were determined by 2-way ANOVA multiple comparisons test (Sidak); ***= p ≤ 0.001, ****= p ≤ 0.0001. Also see Supplementary Fig. 1D. right, Representative images of the 2D colony formation assays.
I) Quantification of 3D tumor spheroid growth, ≥100 individual UM-HMC-3A cell spheroids was monitored daily over a period of 7 days per experiment (mean ± SEM). P values were determined by 2way ANOVA multiple comparisons test (Dunnett); *= p ≤ 0.05, **= p ≤ 0.01, ****= p ≤ 0.0001. Also see Supplementary Fig. 1E
Cancer-associated EGFR ‘hot-spot’ mutations are widespread and have varied effects on tumor cell responses to various EGFR inhibitors[17-19]. Despite frequent EGFR amplification and overexpression in MEC (particularly in high grade MEC tumors)4,9–13, EGFR mutations are rare[30-32]. To confirm that the MEC cell lines used in this study were reflective of MEC tumors, we first sought to establish the EGFR mutational landscape of these MEC cell lines by performing targeted sequencing of EGFR exons 15-25. While several mutations were identified in EGFR exons 15, 16, 17, 20 and 23, all of these mutations were synonymous in nature and thus are predicted to have no effect on EGFR protein sequence, activation or drug sensitivity (Supplementary Table S1). Having established that these MEC cell lines do not have EGFR activating or drug de-sensitizing mutations and are thus not drug resistant, we next tested the potency of the EGFR inhibitor Erlotinib towards inhibiting MEC cell proliferation, maintenance and survival. Dose-response curves revealed that Erlotinib potently affects cell viability with IC50s within clinically relevant[33], low nanomolar ranges (Fig. 1C and Table 1). We next confirmed that Erlotinib administration leads to dose-dependent suppression of ERK kinase activation, but not Akt activation, indicating that EGFR preferentially signals through the EGFR-ERK axis in MEC (Fig. 1D). Interestingly, Erlotinib induced a dose-dependent increase in total EGFR levels, which may be a compensatory mechanism, lending further support to the importance of constitutive EGFR activation in MEC.
Table 1. Calculated IC50 of Drug Inhibitors on the Viability of Salivary MEC Cell Lines.
Table summarizing all calculated IC50s of the various drugs across the five C1/M2 fusion positive MEC cell lines used in this study.
| Cancer Cell Line | aErlotinib | aSAHA | aErlotinib + SAHA | aCUDC-101 |
|---|---|---|---|---|
| NCI-H292 | bND | 5193 | ND | 1526 |
| UM-HMC-1 | 135 | 810 | ND | 220 |
| UM-HMC-3A | 328 | 1410 | 280 | 150 |
| UM-HMC-3B | 272 | 1890 | ND | 200 |
IC50nM
Not Determined
Next, we evaluated Erlotinib’s effects on MEC cell proliferation and found that Erlotinib potently induced cell cycle arrest, leading to an accumulation of MEC cells in the G1-phase (Fig. 1E). However, while Erlotinib promoted strong dose-dependent suppression of MEC cell proliferation, it appeared to have no effect on MEC cell survival. Specifically, the administration of nearly 100x IC50 concentration of Erlotinib (25 μM) caused no appreciable cell death compared to DMSO only vehicle controls, as measured by caspase-3/7 activation (Fig. 1F). Moreover, even prolonged administration failed to promote cell death, and cells exposed to this extended Erlotinib treatment (72 hr) continued to display normal adherent morphologies in culture (Supplementary Fig. 1A). We next performed a drug withdrawal experiment to determine whether Erlotinib-treated MEC cells had 1) undergone irreversible cell cycle arrest and lost their proliferative capacity or 2) had entered a quiescent yet viable, reversible drug-tolerant state. Cells were treated with 25 μM Erlotinib for 72 hours followed by a drug washout, and subsequent cell outgrowth was monitored (Fig. 1G). Surprisingly, MEC cells rapidly resumed proliferation upon drug washout and proliferation rates were nearly identical to vehicle-treated cells, albeit with a longer initial lag period, despite having undergone a 72 hour treatment with 25 μM Erlotinib (Fig. 1G and Supplementary Fig. 1B-C). To assess whether EGFRi impaired the tumorigenic potential of MEC cells to grow as clonogenic colonies ex vivo, cells were plated at low density and colony formation monitored. As expected, based on the cell cycle experiments (Fig. 1G), we observed a significant reduction in 2D colony formation upon Erlotinib treatment (Fig. 1H and Supplementary Fig. 1D). However, subsequent drug withdrawal led to robust colony outgrowth, confirming that MEC cells are intrinsically tolerant to EGFR inhibition.
Mounting evidence has demonstrated that the presence of cancer stem cells (CSCs) in tumors promotes drug tolerant[21] or resistant phenotypes[23] and that salivary MEC tumors harbor such CSCs[27,34]. To test the effects of EGFR inhibition on blunting salivary MEC CSCs, we quantified the effects of Erlotinib on 3D tumor spheroid formation (embedded in a Matrigel sandwich) and found that sustained EGFRi treatment (7 days) drove MEC cells to enter a quiescent state. Notably, modest but significant reductions in spheroid growth were observed when treating MEC cells with ~IC50 concentrations of Erlotinib suggesting that MEC CSCs may promote EGFRi drug tolerance (Fig. 1I and Supplementary Fig. 1E). Collectively, these results demonstrate that EGFR inhibition induces potent cytostatic effects that blunt MEC cell growth but exhibits no discernable cytotoxicity.
Combination EGFR and HDAC inhibition synergistically overcomes EGFR tolerance in MEC
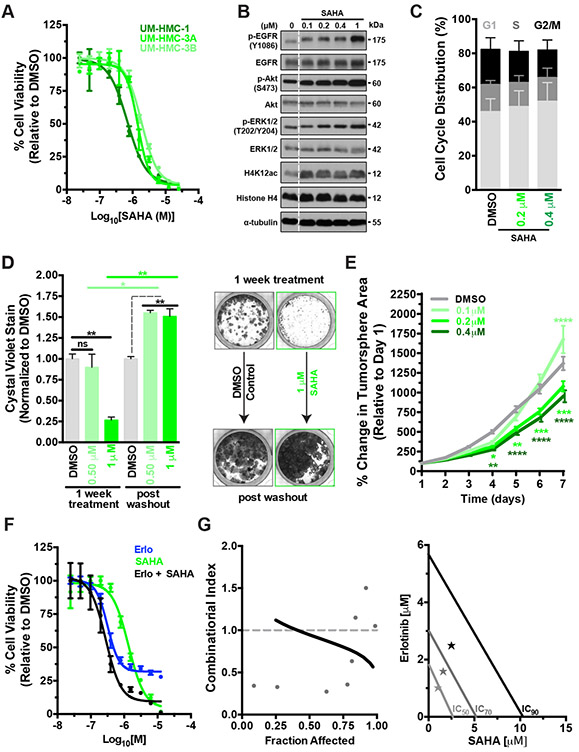
Two recent studies demonstrated that HDAC inhibition using the drug SAHA can sensitize MEC cells to combinatorial therapies when applied along with either Cisplatin[24] (chemotherapy agent) or Emetine[35] (non-specific NFκB inhibitor). Considering these findings, we tested if the combination of SAHA + Erlotinib could overcome the EGFRi tolerance observed in MEC. As observed in other recent studies, dose-response curves confirmed that the pan-HDAC inhibitor Vorinostat/SAHA reduced cell viability (Fig. 2A), however comparing IC50s reveals that SAHA requires concentrations several fold higher than that observed with a more ‘targeted’ drug such as Erlotinib (Table 1). Increasing doses of SAHA lead to a dose-depedant accumulation of acetylated histones as expected (Fig. 2B and Supplementary Fig. 3B), however proliferation/cell cycle was only minimally affected (Fig. 2C). Consequently, SAHA only had modest effects on blunting 2D colony formation (Fig. 2D and Supplementary Fig. 2A) and any apparent ability to reduce colony formation potential was lost upon drug withdrawal. Unexpectedly, drug withdrawal caused dramatic MEC cell outgrowth beyond vehicle-only conditions at all concentrations of SAHA tested (Fig. 2D). Similarly, SAHA had minimal effects on blunting 3D tumor spheroid growth and instead significantly enhanced (p<0.0001) spheroid growth at low doses (100 nM) (Fig. 2E). These observations suggest that treating MEC cells with epigenetic drugs like SAHA may reprogram the chromatin landscape to rewire transcriptional networks that enhance tumor initiation capacity (Fig. 2D and 2E). This enhanced MEC cell proliferation could be explained, in part, by the observed hyperactivation of EGFR-ERK signaling in cells treated with increasing doses of SAHA (Fig. 2B). Upregulating the levels of pEGFR/pERK/pAKT may represent a potential drug resistance mechanism that sustains the survival and proliferation of MEC cells when challenged with HDACi. Thus, we next tested whether combining SAHA and Erlotinib can overcome the limitations associated with each individual monotherapy. Dose-response curves generated using equimolar concentrations of SAHA and Erlotinib revealed that this combinatorial treatment strategy was ~5-fold more potent compared to SAHA alone and potently cytotoxic compared to the purely cytostatic effects of Erlotinib mono-therapy (Fig 2Fand Table 1). Chou-Talalay[36] analysis confirmed that simultaneous HDAC and EGFR inhibition exhibit synergistic interactions against MEC cells, strongly suggesting that when deployed in combination with EGFRi, SAHA can act as a potent sensitizing agent. Thus, combinatorial HDAC + EGFR inhibition in MEC could represent a viable therapeutic strategy for effectively overcoming the limitations associated with each individual monotherapy.
Figure 2. The HDAC inhibitor SAHA displays potent yet transient cytotoxicity in MEC cells but synergizes with EGFR inhibition.
A) Dose-response curves were performed using concentrations ranging from 25 nM to 25 μM and cell viability was assayed 72 hours post drug addition (mean ± SEM).
B) SAHA treatment of UM-HMC-3A leads to a dose dependent accumulation of acetylated-histones and causes upregulation of p-EGFR and p-ERK.
C) UM-HMC-3A cells were treated with SAHA for 24 hours and cell cycle profiles evaluated (n = 3, mean ± SEM). G1 phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (ns). S phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (ns). G2/M phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (ns). P values were determined by 2way ANOVA multiple comparisons test (Sidak); ns = p > 0.05.
D) 2D colony formation capacity of UM-HMC-3A cells (n = 2, mean ± SEM). Cells were treated with drug and then allowed to form colonies for 7 days, at which point cells were fixed and stained with crystal violet and colonies quantified. For the drug withdrawal condition, 7 Days post drug addition, drug was washed out and cells were allowed to outgrow for an additional 7 days and colonies quantified. P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ns = p > 0.05, *= p ≤ 0.05, **= p ≤ 0.01. Also see Supplementary Fig. 2A. right, Representative images of the 2D colony formation assays.
E) SAHA has minimal effects on MEC cell 3D tumor spheroid growth. ≥100 individual UM-HMC-3A cell spheroids were monitored daily over a period of 7 days per experiment (mean ± SEM). P values were determined by 2way ANOVA multiple comparisons test (Dunnett); *= p ≤ 0.05, **= p ≤ 0.01, ****= p ≤ 0.0001. Also see Supplementary Fig. 1E.
F) Dose-response curves were performed using combinatorial equimolar SAHA + Erlotinib [1:1] treatment ranging from 12.5 nM to 12.5 μM, cell viability was assayed 72 hours post drug addition (n = 2, mean ± SEM). center, Synergism curves generated using CompuSyn software analysis tool (Chou-Talay method). Data for Erlotinib, SAHA and SAHA+Erlotinib from Figures 1B, 2A and 2C were used for modeling synergism, respectively. A combinatorial index of <1 represents synergistic interactions.
G) Data from Figure 2C, center graphed as an Isobologram plot. SAHA+Erlotinib exhibits robust synergism relative to clinically-relevant IC70 and IC90 efficacies.
The single agent, dual HDAC-EGFR inhibitor, CUDC-101 exerts potent cytotoxic activity against MEC cells.
Although therapies targeting EGFR have shown great clinical promise across diverse cancer types, acquired drug resistance remains a significant clinical challenge. We and others have demonstrated that EGFR deletion/mutations (e.g. exon19 del/L858R or T790M), gene amplification, activation of compensatory kinase receptors (e.g. u-PAR[22] or IGF-1R[21]), and hyperactivation of downstream signaling factors (e.g. ERK or RAS) frequently underly the emergence of EGFRi drug resistance[17-20]. Depending on the mechanism driving resistance, newer generation EGFR inhibitors (e.g. Osimertinib/Cetuximab) or those targeting key downstream pathway(s) have been deployed. In this regard, CUDC-101, a dual EGFR-HDAC inhibitor (Supplementary Fig. 3A) has shown great promise against a wide variety of cancers in vitro and in vivo, including robust antiproliferative and proapoptotic activities towards tumor cells with acquired drug resistance to EGFRi[37,38]. More importantly, CUDC-101 exhibits greater efficacy against >39 different cell lines across 16 types of human cancers compared with strategies employing equimolar combination treatments of EGFR (Erlotinib) and HDAC (SAHA) inhibitors.
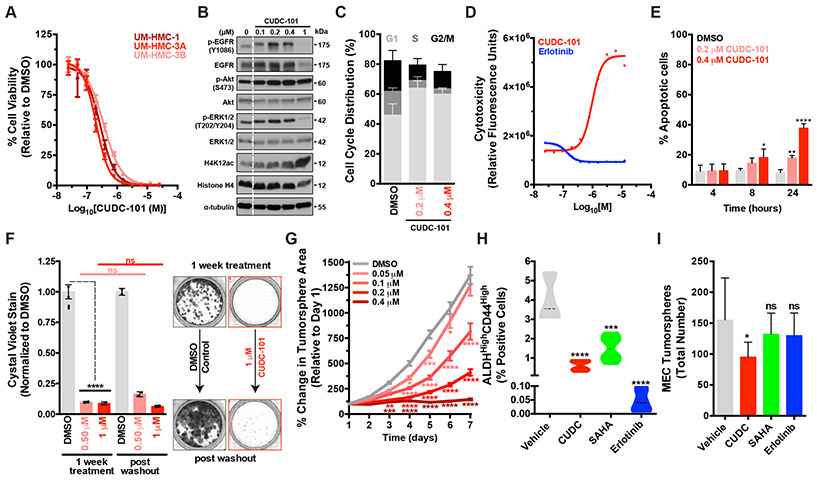
To assess the efficacy of CUDC-101 against MEC cells, we performed dose-response curves and confirmed that CUDC-101 potently suppressed cell viability and growth with IC50s 4-to 10-fold lower than SAHA and displayed potent dose dependent cytotoxicity compared to Erlotinib, which displays cytostatic effects only (Fig. 3A, Supplementary Fig. 2B and Table 1). As expected, treating cells with increasing doses of CUDC-101 led to a concomitant increase in histone acetylation (both histone H3K9ac and H4K12ac) accompanied by decreased pEGFR and pERK (Fig. 3B and Supplementary Fig. 3B). Intriguingly, CUDC-101 was more effective than its molecular constituents alone (i.e. EGFR and SAHA) at reducing p-EGFR levels and at promoting the accumulation of acetylated histones (Supplementary Fig. 3B, comparing 1 μM drug conditions). Similar to Erlotinib, CUDC-101 effectively blunted cell proliferation and arrested cells in the G1-phase of the cell cycle (Fig. 3C). In contrast to Erlotinib’s cytostatic effects, however, CUDC-101 exhibited potent dose and time-dependent cytotoxicity (Fig. 3D and Supplementary Fig. 3C) and led to a significant (p<0.0001) increase in apoptosis, as measured by caspase 3/7 activation (Fig. 3E). Next, we examined the effects of CUDC-101 on tumorigenicity. Unlike EGFR or SAHA monotherapy, CUDC-101 caused potent and irreversible inhibition of 2D colony formation (Fig. 3F) and 3D tumor spheroid growth (Fig. 3G). These data suggest that a single agent- dual EGFR-HDAC inhibitor, CUDC-101, can have long-lasting therapeutic benefits following subsequent drug withdrawal.
Figure 3. The multi-target EGFR-HDAC inhibitor CUDC-101 exerts robust and irreversible cytotoxicity in MEC cells and blunts CSC-mediated tumorigenic capacity.
A) Dose-response curves were performed using concentrations ranging from 25 nM to 25 μM and cell viability was assayed 72 hours post drug addition (mean ± SEM).
B) CUDC-101 treatment of UM-HMC-3A leads to a dose dependent accumulation of acetylated-histones and downregulation of p-EGFR and p-ERK.
C) UM-HMC-3A cells were treated with CUDC-101 for 24 hours and cell cycle profiles were evaluated (n = 3, mean ± SEM). G1 phase- DMSO vs 0.2 μM (**), vs 0.4 μM (****), vs 25 μM (****). S phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (**), vs 25 μM (***). G2/M phase- DMSO vs 0.2 μM (ns), vs 0.4 μM (**), vs 25 μM (****). p values were determined by 2-way ANOVA multiple comparisons test (Sidak); ns = p > 0.05, *= p ≤ 0.05, **= p ≤ 0.01, ***= p ≤ 0.001, ****= p ≤ 0.0001.
D) Dose-response curves were performed using the indicated drugs at concentrations ranging from 25 nM to 25 μM and cell toxicity/death was assayed 72 hours post drug addition (mean ± SEM). Also see Supplementary Fig. 1A and 2C.
E) UM-HMC-3A cells were treated with CUDC-101 for 24 hours and assayed for apoptosis. Experiment is representative of biologic triplicate (mean ± SEM). P values were determined using Student’s t-test, comparing DMSO vs Erlotinib for each timepoint. Also see Supplementary Fig. 2C.
F) 2D colony formation capacity of UM-HMC-3A cells and (n = 2, mean ± SEM). Cells were treated with drug and then allowed to form colonies for 7 days, at which point cells were fixed and stained with crystal violet and colonies were quantified. For the drug withdrawal condition, 7 Days post drug addition, drug was washed out and cells were allowed to outgrow for an additional 7 days and colonies quantified. P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ns = p > 0.05, *= p ≤ 0.05, **= p ≤ 0.01. Also see Supplementary Fig. 3D. right, Representative images of the 2D colony formation assays.
G) 3D tumor spheroid growth, ≥100 individual UM-HMC-3A cell spheroids were monitored daily over a period of 7 days per experiment (mean ± SEM). P values were determined by 2-way ANOVA multiple comparisons test (Dunnett); *= p ≤ 0.05, **= p ≤ 0.01, ****= p ≤ 0.0001. Also see Supplementary Fig. 1E.
H) MEC cells were treated with sub-IC50 doses of each respective drug (50 nM) for 7 days and flow cytometry was performed to quantify the ALDHhighCD44high CSC population.
I) MEC tumorsphere formation capacity was assessed using ultra-low adhesion conditions and measured 7 days post seeding.
CUDC-101 blunts MEC cancer stem cell tumorigenicity.
Recent evidence has suggested that MEC tumors harbor a prototypical cancer stem cell (CSC) population, defined as ALDHhighCD44high, that exhibits enhanced self-renewing capacity[27,34] and is suspected to drive drug resistance in MEC. To elucidate the effects of CUDC-101 on MEC CSCs, we specifically analyzed the self-renewing ALDHhighCD44high CSC sub-population. Surprisingly, flow cytometric analysis revealed that all three drugs significantly depleted (p<0.0001) ALDHhighCD44high CSCs despite being treated with sub-lethal (<IC50; 50 nM) doses of each drug (Fig. 3H). To evaluate the effects of depleting the ALDHhighCD44high CSCs on tumorigenicity, we analyzed tumorsphere formation in ultra-low attachment conditions, which have previously been shown to selectively enrich MEC CSCs[27,28]. Remarkably, even though Erlotinib treatment caused the greatest depletion of the presumed MEC CSC population (Fig. 3H), it had no discernible effect on tumorsphere initiation capacity (Fig. 3I). In contrast, CUDC-101 treatment caused a significant decrease (p<.05) in total tumorsphere numbers (Fig. 3I). Collectively, these data indicate that CUDC-101, unlike Erlotinib and SAHA, is effective at functionally depleting the self-renewing CSCs present within MEC tumors.
DISCUSSION
While MECs were first described as early as 1895[39] and formally acknowledged as a distinct salivary gland tumor type in 1945[40], this cancer remains understudied and there are no targeted therapeutics available. While most low grade MEC tumors can be treated effectively with surgical resection, patients with high grade or recurrent/metastatic tumors lack effective intervention options often resulting in a dismal prognosis (~35% 5-year survival rate)[1,3,6]. Retrospective clinical studies have linked activation of the EGFR-ERK signaling axis to poor outcomes in MEC patients[8,9,11-13]. Moreover, a recent molecular study identified a mechanistic link between the key oncogene fusion driver CRTC1-MAML2 (C1/M2) and aberrant expression of the EGFR ligand AREG[8]. Chen and colleagues showed that AREG is significantly elevated in MEC cell lines and human tumor samples where it promotes autocrine growth factor signaling and that targeting EGFR using the monoclonal antibody Cetuximab retards tumor growth in xenograft models[8]. Owing to the low MEC incidence rate (1 to 5 cases per 100,000 people)[1], MEC-only clinical trials are challenging to conduct due to limited patient enrollment. Despite these challenges, multiple clinical studies have indeed been performed that include MEC cases[14,15,41] (Supplementary Table S2), and to the best of our knowledge the allure of targeting EGFR as a MEC therapy has not yielded a single clinical trial with promising results exploiting this potential vulnerability.
Our data indicate that, though EGFR inhibition exerts robust cytostatic effects, it displays no appreciable cytotoxicity against MEC cells, strongly undermining its therapeutic potential. Rather, our findings reveal that EGFR inhibition causes MEC cells to enter a non-proliferative, quiescent state and that these dormant cells rapidly proliferate upon subsequent drug withdrawal. Similarly, we found that using inhibitors against targets downstream of EGFR signaling (e.g. PI3Ki, data not shown) also exert cytostatic but not cytotoxic effects. Interestingly, administration of EGFRi alone appears to achieve a staggering drop in the CD44/ALDH-positive sub-population of MEC CSCs, reducing it to a nearly undetectable level. However, our data also suggests that the apparent loss of the CD44/ALDH-positive CSC subpopulation may be driven by a transcriptional switch that leads to decreased ALDH expression thereby ‘masking’ the drug tolerant CSCs (Supplementary Fig. 4A). Although the exact mechanism of this transcriptional switch is currently unclear, we hypothesize based on our results that either: 1) Erlotinib-treated CSCs retain enhanced tumorigenic potential and spheroid formation capabilities, despite losing ALDH expression, or 2) MEC cells contain a currently unknown CSC sub-population that is unaffected by EGFR inhibition. Both possibilities should be carefully examined in future investigations into MEC tumor biology and CSC sub-populations. Additionally, we found that treatment of C1/M2-positive MEC cells with CUDC-101, in contrast to Erlotinib monotherapy, led to a robust reduction in expression of the primary oncogene, C1/M2 (Supplementary Fig. 4B). This suggests that the observed effects of CUDC-101 (potent cytotoxicity) and Erlotinib (cytostatic only) in C1/M2-positive MEC could be driven by their differential effects on the primary oncogene[42-44]. Thus, contrary to previous studies suggesting that EGFR represents an actionable target in MEC patients, our data strongly suggest that single agent EGFR monotherapies might not be clinically viable therapeutic strategies against MEC.
Previous studies have shown that SAHA can effectively deplete CSCs in MEC cell lines when administered as a monotherapy[24]. Interestingly, herein we discovered that the administration of HDAC inhibitors as a monotherapy is not a viable strategy against MEC cells due to the rapid and robust outgrowth of MEC cells upon drug withdrawal. Previous studies have shown that that HDAC inhibitors could serve as sensitizing agents against MEC when deployed in combination with additional therapeutic agents such as chemotherapies (cisplatin[24]) or NFkB targeting (via a non-specific inhibitor Emetine[35]). These combinatorial approaches have shown additive drug efficacies and appear to be effective at both debulking tumors and eradicating the highly tumorigenic, slow-cycling MEC CSC subpopulation[24,25]. Herein we identify robust synergy between EGFR (Erlotinib) and HDAC (SAHA) inhibitors in MEC and show that combinatorial HDACi + EGFRi could hold clinical promise in MEC cases. This synergism with HDACi has been observed in other solid tumors and appears to be linked to transcriptional rewiring caused by histone H3K27 hyperacetylation at gene promoters and/or enhancers[45]. Chemical engineering efforts have recently yielded novel molecular designs that enable one to drug multiple targets with one compound. In contrast to combination therapies of two or more drugs, multi-target agents have several advantages, including more predictable pharmacokinetics and lower probabilities of drug interactions[46]. The dual EGFR-HDAC inhibitor CUDC-101 is one such molecule that has shown great promise against >39 different cell lines across 16 types of human cancers both in vitro and in vivo[37,38]. Furthermore, CUDC-101 has demonstrated robust potency against tumors that are resistant to traditional EGFR mono-therapy and appears to be well tolerated in clinical trials (Phase I/Ib)[47]. Our findings strongly support the application of CUDC-101 as a multi-target monotherapy for treating MEC patients. Further, recent evidence demonstrate the robust tumor-specific benefits of combinatorial therapies[48,49] and highlight the need for future investigations into additional combinatorial drug treatments beyond HDACi + EGFRi for MEC. A caveat of our current study is that all experiments were performed in established MEC cancer cell lines. While these MEC cancer cell lines faithfully recapitulate primary MEC tumor histopathology when implanted in vivo[26], additional studies should be performed in more clinically-relevant model systems, such as patient derived xenografts and/or genetically engineered mouse models of MEC, that more closely resemble primary tumors[34]. Lastly, while our results strongly demonstrate the potent cytotoxic effects of CUDC-101 against MEC cells, the precise molecular mechanism underlying the observed EGFRi + HDACi synergism in MEC remains unknown and should be further explored in future investigations.
CONCLUSION
There remains a poor overall understanding of MEC pathobiology which has led to an undeniable lack of targeted therapeutics. Herein, we show that MECs are intrinsically tolerant (not resistant) to EGFR inhibition and provide direct evidence for the emergence of a reversible drug tolerant phenotype in MEC cells upon Erlotinib administration. Our data offer a potential explanation for the failure of past clinical trials that have focused solely on targeting EGFR signaling using monotherapies in salivary gland cancers such as MEC. Identification of the EGFRi-tolerant phenotype combined with our data demonstrating that a dual EGFR-HDAC inhibitor (CUDC-101) is effective at overcoming this drug tolerant phenotype suggests that the use of EGFRi in combination therapies still holds significant promise for salivary gland carcinomas and more specifically for MEC. Future studies should be aimed at defining the molecular mechanisms governing EGFRi drug tolerance in MEC cells and the applicability of additional combinatorial therapies against aggressive, poorly differentiated MEC tumors.
Supplementary Material
Supplementary Figure 2. Constant HDAC inhibition drives a dose dependent reduction in MEC cell tumorigenicity.
A) SAHA treatment leads to a dose dependent reduction of tumor initiation capacity, as measured by 2D colony formation. UM-HMC-3A cells were sparsely seeded and treated with SAHA at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2Way ANOVA multiple comparisons test (Sidak); ns= p > 0.05, *= p ≤ 0.05, ***= p ≤ 0.001.
B) Dose-response curve comparison of single agents to combinatorial SAHA and Erlotinib [1:1] treatment and CUDC-101 in UM-HMC 3A cells. Cell viability was assayed 72 hours post dug addition (n = 2 biological replicates, mean ± SEM).
Supplementary Figure 3. Combinatorial EGFR + HDAC inhibition exerts robust cytotoxic efficacy against MEC cells.
A) CUDC-101 is composed of EGFR + HDAC inhibitor moieties. Illustration showing the CUDC-101 molecular structure compared to Erlotinib and SAHA.
B) Full western blots for UM-HMC-3A cells treated with varying concentrations of Erlotinib, SAHA or CUDC-101. This blot was cropped to generate the individual drug treatment blots shown in Figures 1B, 2A and 3A.
C) CUDC-101 exerts robust and dose-dependent cytotoxic effects on MEC cells. UM-HMC-3A cells were treated with the indicated doses of CUDC-101 for 24 hours. Wells were stained with CellTox and imaged (488 nm/520 nm). Cells stained green represent dead/dying cells with compromised plasma membranes. Representative images are shown (n = 2 biological replicates, mean ± SEM). Also see Figure 3B, right.
D) CUDC-101 causes irreversible ablation of tumorigenic potential as measured by 2D colony formation. A single cell solution of UM-HMC-3A cells was seeded and treated with CUDC-101 at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ****= p ≤ 0.0001.
Supplementary Figure 4. Erlotinib monotherapy reduces ADLH expression in MEC cells but has no effect on C1/M2 fusion oncogene expression.
A) ALDH1A1 mRNA expression is significantly reduced upon Erlotinib treatment of MEC cells (24 hours post drug addition). RPL23 was used as an internal relative control. (n=3 biologic replicates, mean ± SEM **= p < 0.01).
B) Erlotinib treatment has no effect on C1/M2 fusion expression whereas CUDC-101 significantly reduces C1/M2 fusion expression in MEC cells. TATA-binding protein expression was affected by both Erlotinib and CUDC-101 treatment and was thus used as a positive control. RPL23 was used as an internal relative control (n=3 biologic replicates, mean ± SEM). **= p < 0.01, ***= p < 0.001, ****= p ≤ 0.0001.
Supplementary Table 1. MEC cell lines do not contain any activating or drug desensitizing mutations in EGFR. Targeted EGFR transcript sequencing revealed several mutations spread across exons 15 through 25, however all mutations were found to be synonymous in nature and thus should have no effects on EGFR activation, function, or drug sensitivity.
Supplementary Table 2. List of reported clinical trials involving MEC (complied from clinicaltrials.gov). Complied list of clinical trials involving MEC patients. Trials involving inhibitors targeting EGFR, known EGFR-downstream targets or HDACs are highlighted.
Supplementary Figure 1. Constitutive EGFR activation is required for proliferation but is not necessary for MEC cell survival.
A) Erlotinib fails to exert any cytotoxic effect on MEC cells. UM-HMC-3A cells were treated with the indicated doses of Erlotinib for 24 hours. Wells were stained with CellTox and imaged (488 nm/520 nm). Cells stained green represent dead/dying cells with compromised plasma membranes. Relative cell death was calculated as CellTox signal intensities relative to the DMSO condition (n = 2 biological replicates, mean ± SEM). Also see Fig. 3B, right.
B) MEC cells enter a quiescent, viable and reversible state upon Erlotinib treatment and rapidly proliferate upon drug withdrawal. Cells were treated as described in Fig. 1C, top. Cell proliferation was quantified as relative confluency over a period of 5 days post drug withdrawal. Representative experiment results are shown averaging four independent fields of view for each condition (n = 2 biological replicates, mean ± SEM).
C) Representative images for Erlotinib-induced MEC cell dormancy and subsequent drug withdrawal mediated-cell outgrowth from Supplementary Fig. 1B-C.
D) Erlotinib treatment robustly inhibits tumor initiation capacity, as measured by 2D colony formation. UM-HMC-3A cells were sparsely seeded and treated with Erlotinib at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ****= p ≤ 0.0001.
E) Matrigel sandwich culture for determining tumor initiation capacity in a 3D environment (tumor spheroid growth). A single-cell suspension of 2,000 UM-HMC-3A cells was embedded in a Matrigel sandwich culture and spheroids were imaged daily for 7 days. Drug was added to all components of the culture system, at the indicated doses, on the day of embedding. Changes in each individual tumor spheroid volume was calculated in ImageJ, using the area measurement tool. Relative growth for each individual spheroid was quantified as relative to that at Day 1.
Highlights.
Salivary MEC cells are tolerant to targeted EGFR inhibition
The drug tolerant state is rapidly reversible, and cells resume proliferation upon drug Withdrawal
The combination of EGFR and HDAC inhibitors synergizes to produce potent cytolytic effects
CUDC-101, a dual EGFR-HDAC inhibitor, displays irreversible growth arrest and cytotoxicity that dramatically reduce MEC cancer stem cell tumorigenicity
ACKNOWLEDGEMENTS
The authors thank Buddy Weissman, Andrew Waters, Eric Bankaitis and members of the Amelio Lab for helpful discussions, suggestions, and/ or scientific review of this manuscript. This work was supported by a NIH/NIDCR F31-DE027282 training grant (to A.M. Musicant), an Academy of Athens’ Scholarship in Medicine (to J. Tasoulas), a NIH/NCI R21-CA234979 and a V Scholar Grant V2018-009 (to P. Liu), University Cancer Research Fund (UCRF; to A.L. Amelio), UNC Lineberger Tier 3 Developmental Award (to A.L. Amelio), and by a NIH/NCI Howard Temin Pathway to Independence Award in Cancer Research R00-CA157954 (to A.L. Amelio). This work was also supported in part by NCI Comprehensive Cancer Center Grant P30-CA016806 awarded to the UNC Lineberger Comprehensive Cancer Center.
Footnotes
DECLARATION OF COMPETING INTERESTS
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].El-Naggar AKCJ, Grandis JR, Takata TSP. WHO Classification of Head and Neck Tumours. 4th Editio. IARC Press; 2016. [Google Scholar]
- [2].Jacob PK, McCoy JM. Diagnostic Imaging of Salivary Gland Pathology. 2nd Edition ed.-Chapter 2. Wiley-Blackwell; 2015. [Google Scholar]
- [3].Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol 2009;3:69–77. 10.1007/s12105-009-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Becker C, Pfeiffer J, Lange K DK. Health-related quality of life in patients with major salivary gland carcinoma. Eur Arch Otorhinolaryngol 2018;275:997–1003. 10.1007/s00405-018-4914-7. [DOI] [PubMed] [Google Scholar]
- [5].Wax MKTY. Quality of Life after Salivary Gland Surgery. Adv Otorhinolaryngol 2016;78:189–97. 10.1159/000442140. [DOI] [PubMed] [Google Scholar]
- [6].Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol 2006;13:293–307. 10.1097/01.pap.0000213058.74509.d3. [DOI] [PubMed] [Google Scholar]
- [7].O’Neill ID. t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol 2009;45:2–9. 10.1016/j.oraloncology.2008.03.012. [DOI] [PubMed] [Google Scholar]
- [8].Chen Z, Chen J, Gu Y, Hu C, Li J-L, Lin S, et al. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene 2013:1–9. 10.1038/onc.2013.348. [DOI] [PubMed] [Google Scholar]
- [9].Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A, et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer 2010;103:510–6. 10.1038/sj.bjc.6605788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Neill ID. Gefitinib as targeted therapy for mucoepidermoid carcinoma of the lung: Possible significance of CRTC1-MAML2 oncogene. Lung Cancer 2009;64:129–30. 10.1016/j.lungcan.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [11].Handra-Luca A, Bilal H, Bertrand JC, Fouret P. Extra-cellular signal-regulated ERK-1/ERK-2 pathway activation in human salivary gland mucoepidermoid carcinoma: Association to aggressive tumor behavior and tumor cell proliferation. Am J Pathol 2003;163:957–67. 10.1016/S0002-9440(10)63455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakano T, Yamamoto H, Hashimoto K, Tamiya S, Shiratsuchi H, Nakashima T, Nishiyama K-i, Higaki YKS& OY. HER2 and EGFR gene copy number alterations are predominant in high-grade MECs 2013. [DOI] [PubMed] [Google Scholar]
- [13].Shinomiya H, Ito Y, Kubo M, Yonezawa K, Otsuki N, Iwae S, et al. Expression of amphiregulin in mucoepidermoid carcinoma of the major salivary glands: a molecular and clinicopathological study. Hum Pathol 2016;57:37–44. 10.1016/j.humpath.2016.06.016. [DOI] [PubMed] [Google Scholar]
- [14].Jakob John A., MD, PhD, 1 2, Kies Merrill S., MD, 3 Glisson Bonnie S., MD, 3 Kupferman Michael E., MD, 4 Liu Diane D., MS, 5 Lee J. Jack, PhD, 5 El-Naggar Adel K., MD, PhD, 6 Gonzalez–Angulo Ana M., MD, 7 George R Blumenschein M Jr. Phase II study of gefitinib in patients with advanced salivary gland cancers John. Head Neck 2015;36:1391. 10.1002/HED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Agulnik M, Cohen EWE, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non-adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol 2007;25:3978–84. 10.1200/JCO.2007.11.8612. [DOI] [PubMed] [Google Scholar]
- [16].Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol 2009;45:574–8. 10.1016/j.oraloncology.2008.07.010. [DOI] [PubMed] [Google Scholar]
- [17].Byeon HK, Ku M, Yang J. Beyond EGFR inhibition: multilateral combat strategies to stop the progression of head and neck cancer. Exp Mol Med 2019;51. 10.1038/s12276-018-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389–400. 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cohen RB. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC). Cancer Treat Rev 2014;40:567–77. 10.1016/j.ctrv.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [20].Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol 2018;12. 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rajbhandari N, Lin W chi, Wehde BL, Triplett AA, Wagner KU. Autocrine IGF1 Signaling Mediates Pancreatic Tumor Cell Dormancy in the Absence of Oncogenic Drivers. Cell Rep 2017;18:2243–55. 10.1016/j.celrep.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uzawa K, Amelio AL, Kasamatsu A, Saito T, Kita A, Fukamachi M, et al. Resveratrol Targets Urokinase-Type Plasminogen Activator Receptor Expression to Overcome Cetuximab-Resistance in Oral Squamous Cell Carcinoma. Sci Rep 2019;9:1–8. 10.1038/S41598-019-48717-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell 2010;141:69–80. 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guimarães DM, Almeida LO, Martins MD, Warner KA, Silva ARS, Vargas PA, et al. Sensitizing mucoepidermoid carcinomas to chemotherapy by targeted disruption of cancer stem cells. Oncotarget 2016;5. 10.18632/oncotarget.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wagner VP, Martins MAT, Martins MD, Warner KA, Webber LP, Squarize CH, et al. Overcoming adaptive resistance in mucoepidermoid carcinoma through inhibition of the IKK-β/IκBα/NFκB axis. Oncotarget 2016;7:73032–44. 10.18632/oncotarget.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Warner KA, Adams A, Bernardi L, Nor C, Finkel KA, Zhang Z, et al. Characterization of tumorigenic cell lines from the recurrence and lymph node metastasis of a human salivary mucoepidermoid carcinoma. Oral Oncol 2013;49:1059–66. 10.1016/j.oraloncology.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adams A, Warner K, Pearson AT, Zhang Z, Kim HS, Mochizuki D, et al. ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget 2015;6:26633–50. 10.18632/oncotarget.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Almeida LO, Guimarães DM, Squarize CH, Castilho RM. Profiling the behavior of distinct populations of head and neck cancer stem cells. Cancers (Basel) 2016;8:1–14. 10.3390/cancers8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res 2008;14:6963–73. 10.1158/1078-0432.CCR-08-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dahse R, Driemel O, Schwarz S, Dahse J, Kromeyer-Hauschild K, Berndt A, et al. Epidermal growth factor receptor kinase domain mutations are rare in salivary gland carcinomas. Br J Cancer 2009;100. 10.1038/sj.bjc.6604875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Macarenco RS, Uphoff TS, Gilmer HF, Jenkins RB, Thibodeau SN, Lewis JE, et al. Salivary gland-type lung carcinomas: An EGFR immunohistochemical, molecular genetic, and mutational analysis study. Mod Pathol 2008;21. 10.1038/modpathol.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang H, Tan M, Bishop JA, Jones S, Sausen M, Ha PK, et al. Whole-Exome Sequencing of Salivary Gland Mucoepidermoid Carcinoma. Clin Cancer Res 2016:clincanres.0720.2016. 10.1158/1078-0432.CCR-16-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liston DR, Davis M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin Cancer Res 2017;23:3489–98. 10.1158/1078-0432.CCR-16-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Keysar SB, Eagles JR, Miller B, Jackson BC, Chowdhury FN, Reisinger J, et al. Salivary gland cancer patient-derived xenografts enable characterization of cancer stem cells and new gene events associated with tumor progression. Clin Cancer Res 2018;24:2935–43. 10.1158/1078-0432.CCR-17-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wagner VP, Martins MD, Martins MAT, Almeida LO, Warner KA, Nör JE, et al. Targeting histone deacetylase and NFκB signaling as a novel therapy for Mucoepidermoid Carcinomas. Sci Rep 2018;8. 10.1038/s41598-018-20345-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chou TC. Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res 2010;70:440–6. 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- [37].Lai CJ, Bao R, Tao X, Wang J, Atoyan R, Qu H, et al. CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res 2010;70:3647–56. 10.1158/0008-5472.CAN-09-3360. [DOI] [PubMed] [Google Scholar]
- [38].Wang J, Pursell NW, Samson MES, Atoyan R, Ma AW, Selmi A, et al. Potential advantages of CUDC-101, a multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion. Mol Cancer Ther 2013;12:925–36. 10.1158/1535-7163.MCT-12-1045. [DOI] [PubMed] [Google Scholar]
- [39].R. V. Ueber endotheliale Geschwülste, zugleich ein Beitrag zu den Speicheldrüsen- und Gaumentumoren. Dtsch Zeitschrift Für Chir 1895;41:1–180. [Google Scholar]
- [40].Stewart Frank W., Foote WFB Frank W.. Mucoepidermoid tumors of salivary glands. Cancer 1945;122. . [DOI] [Google Scholar]
- [41].Chintakuntlawar AV, Okuno SH, Price KA. Systemic therapy for recurrent or metastatic salivary gland malignancies. Cancers Head Neck 2016;1:1–9. 10.1186/s41199-016-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 2003;33:208–13. 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- [43].Coxon A, Rozenblum E, Park YS, Joshi N, Tsurutani J, Dennis PA, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB regulated genes. Cancer Res 2005;65:7137–44. 10.1158/0008-5472.CAN-05-1125. [DOI] [PubMed] [Google Scholar]
- [44].Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, et al. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J 2005;24:2391–402. 10.1038/sj.emboj.7600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang Y, Chen SY, Colborne S, Lambert G, Shin CY, Dos Santos N, et al. Histone deacetylase inhibitors synergize with catalytic inhibitors of EZH2 to exhibit antitumor activity in small cell carcinoma of the ovary, hypercalcemic type. Mol Cancer Ther 2018; 17:2767–79. 10.1158/1535-7163.MCT-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Talevi A Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front Pharmacol 2015;6:1–7. 10.3389/fphar.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Galloway TJ, Wirth LJ, Colevas AD, Gilbert J, Bauman JE, Saba NF, et al. A phase I study of CUDC-101, a multitarget inhibitor of HDACs, EGFR, and HER2, in combination with chemoradiation in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015;21:1566–73. 10.1158/1078-0432.CCR-14-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Palmer AC, Chidley C, Sorger PK. A curative combination cancer therapy achieves high fractional cell killing through low cross resistance and drug Additivity. Elife 2019;8:1–36. 10.7554/eLife.50036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palmer AC, Sorger PK. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017;171:1678–1691.e13. 10.1016/j.cell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. Constant HDAC inhibition drives a dose dependent reduction in MEC cell tumorigenicity.
A) SAHA treatment leads to a dose dependent reduction of tumor initiation capacity, as measured by 2D colony formation. UM-HMC-3A cells were sparsely seeded and treated with SAHA at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2Way ANOVA multiple comparisons test (Sidak); ns= p > 0.05, *= p ≤ 0.05, ***= p ≤ 0.001.
B) Dose-response curve comparison of single agents to combinatorial SAHA and Erlotinib [1:1] treatment and CUDC-101 in UM-HMC 3A cells. Cell viability was assayed 72 hours post dug addition (n = 2 biological replicates, mean ± SEM).
Supplementary Figure 3. Combinatorial EGFR + HDAC inhibition exerts robust cytotoxic efficacy against MEC cells.
A) CUDC-101 is composed of EGFR + HDAC inhibitor moieties. Illustration showing the CUDC-101 molecular structure compared to Erlotinib and SAHA.
B) Full western blots for UM-HMC-3A cells treated with varying concentrations of Erlotinib, SAHA or CUDC-101. This blot was cropped to generate the individual drug treatment blots shown in Figures 1B, 2A and 3A.
C) CUDC-101 exerts robust and dose-dependent cytotoxic effects on MEC cells. UM-HMC-3A cells were treated with the indicated doses of CUDC-101 for 24 hours. Wells were stained with CellTox and imaged (488 nm/520 nm). Cells stained green represent dead/dying cells with compromised plasma membranes. Representative images are shown (n = 2 biological replicates, mean ± SEM). Also see Figure 3B, right.
D) CUDC-101 causes irreversible ablation of tumorigenic potential as measured by 2D colony formation. A single cell solution of UM-HMC-3A cells was seeded and treated with CUDC-101 at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ****= p ≤ 0.0001.
Supplementary Figure 4. Erlotinib monotherapy reduces ADLH expression in MEC cells but has no effect on C1/M2 fusion oncogene expression.
A) ALDH1A1 mRNA expression is significantly reduced upon Erlotinib treatment of MEC cells (24 hours post drug addition). RPL23 was used as an internal relative control. (n=3 biologic replicates, mean ± SEM **= p < 0.01).
B) Erlotinib treatment has no effect on C1/M2 fusion expression whereas CUDC-101 significantly reduces C1/M2 fusion expression in MEC cells. TATA-binding protein expression was affected by both Erlotinib and CUDC-101 treatment and was thus used as a positive control. RPL23 was used as an internal relative control (n=3 biologic replicates, mean ± SEM). **= p < 0.01, ***= p < 0.001, ****= p ≤ 0.0001.
Supplementary Table 1. MEC cell lines do not contain any activating or drug desensitizing mutations in EGFR. Targeted EGFR transcript sequencing revealed several mutations spread across exons 15 through 25, however all mutations were found to be synonymous in nature and thus should have no effects on EGFR activation, function, or drug sensitivity.
Supplementary Table 2. List of reported clinical trials involving MEC (complied from clinicaltrials.gov). Complied list of clinical trials involving MEC patients. Trials involving inhibitors targeting EGFR, known EGFR-downstream targets or HDACs are highlighted.
Supplementary Figure 1. Constitutive EGFR activation is required for proliferation but is not necessary for MEC cell survival.
A) Erlotinib fails to exert any cytotoxic effect on MEC cells. UM-HMC-3A cells were treated with the indicated doses of Erlotinib for 24 hours. Wells were stained with CellTox and imaged (488 nm/520 nm). Cells stained green represent dead/dying cells with compromised plasma membranes. Relative cell death was calculated as CellTox signal intensities relative to the DMSO condition (n = 2 biological replicates, mean ± SEM). Also see Fig. 3B, right.
B) MEC cells enter a quiescent, viable and reversible state upon Erlotinib treatment and rapidly proliferate upon drug withdrawal. Cells were treated as described in Fig. 1C, top. Cell proliferation was quantified as relative confluency over a period of 5 days post drug withdrawal. Representative experiment results are shown averaging four independent fields of view for each condition (n = 2 biological replicates, mean ± SEM).
C) Representative images for Erlotinib-induced MEC cell dormancy and subsequent drug withdrawal mediated-cell outgrowth from Supplementary Fig. 1B-C.
D) Erlotinib treatment robustly inhibits tumor initiation capacity, as measured by 2D colony formation. UM-HMC-3A cells were sparsely seeded and treated with Erlotinib at the indicated doses. 2D colony formation capacity (with a colony defined as a cluster of ≥50 cells) was evaluated 7 days post drug addition (n = 2 biological replicates, mean ± SEM). P values were determined by 2-Way ANOVA multiple comparisons test (Sidak); ****= p ≤ 0.0001.
E) Matrigel sandwich culture for determining tumor initiation capacity in a 3D environment (tumor spheroid growth). A single-cell suspension of 2,000 UM-HMC-3A cells was embedded in a Matrigel sandwich culture and spheroids were imaged daily for 7 days. Drug was added to all components of the culture system, at the indicated doses, on the day of embedding. Changes in each individual tumor spheroid volume was calculated in ImageJ, using the area measurement tool. Relative growth for each individual spheroid was quantified as relative to that at Day 1.