Abstract
When damage to left hemisphere perisylvian regions unexpectedly does not result in aphasia, it is often assumed that the individual must be right lateralized for language. However, this supposition is rarely confirmed, and there are two other explanations that should be considered. First, many individuals demonstrate some degree of bilateral representation for language, raising the possibility that the contributions of the undamaged right hemisphere could be sufficient to sustain normal language function following left hemisphere damage. Second, there is considerable individual variability in the precise localization of critical language regions, raising the possibility that a localized lesion could entirely avoid a left-lateralized language network, leaving language function intact. We used functional neuroimaging to determine the reason for a lack of aphasia in a left-handed gentleman who sustained a left temporal lobe hemorrhage. We found unequivocal evidence for right lateralization of language function to explain his lack of aphasia. Although the alternative explanations were not observed in our case, we discuss their potential to underlie a lack of aphasia in other individuals. This report highlights questions that still remain about the various ways language can be organized in the brain, and the implications of damage to different regions in different scenarios.
Keywords: aphasia, fMRI, right-lateralized language, bilateral language, atypical language organization
Introduction
When left hemisphere perisylvian brain regions are damaged, individuals almost always experience aphasia. On the rare occasions when a patient presents with intact language function after substantial left perisylvian damage, clinicians often assume that the individual’s language network must be localized to the right hemisphere, especially if the individual is left-handed. But there are at least two other potential explanations that should be considered. First, many individuals demonstrate some degree of bilateral language representation, raising the possibility that in some cases, the contribution of the undamaged right hemisphere regions involved in language processing might be sufficient to sustain normal language function in the face of left hemisphere damage. Second, there is considerable variability between individuals in the precise localization of language regions within the left hemisphere, suggesting that in some cases, localized damage could spare an individual’s specific language network, leaving their language function intact.
The most common assumption—that a lack of aphasia following left hemisphere damage can be explained by a language network situated in the right hemisphere—is surely true in many cases (e.g., Hund-Georgiadis et al., 2001). Even in Broca’s (1865) seminal paper establishing that language is typically localized to the left hemisphere, Broca recognized that this was true of most but not all people, and that a minority of individuals likely had right hemisphere language. In support of this view, he described a case with a congenitally malformed left hemisphere who had nevertheless developed normal language, which he argued could only be explained if language had developed in the intact right hemisphere. Broca believed that right hemisphere language was akin to left-handedness, in the sense that a minority of individuals would rely on the right hemisphere for functions that are more commonly supported by the left hemisphere. Some researchers, most conspicuously Bramwell (1899), who introduced the notion of “crossed aphasia”, have promoted the view that right hemisphere language is the norm in left-handers. However, Broca had explicitly rejected the notion that there was a direct correspondence between individuals who are left-handed and those whose language function is localized to the right hemisphere. Subsequent work has supported Broca’s position: the great majority of left-handers still have left hemisphere language (Carey & Johnstone, 2014; Mazoyer et al., 2014; Penfield and Roberts, 1959; Szaflarski et al., 2002). It is true, though, that right hemisphere language is more frequently observed in left-handers than right-handers (Benson, 1985; Carey & Johnstone, 2014; Mazoyer et al., 2014; Springer et al., 1999; Szaflarski et al., 2002), suggesting that there may be ontogenetic factors shared between the two traits (Ocklenburg et al., 2014). In any case, it is clear that a minority of individuals do have right hemisphere language, and that this is a plausible explanation for lack of aphasia after left hemisphere damage.
Turning to the other two possible explanations for lack of aphasia after left hemisphere perisylvian damage, we will consider first the potential for bilateral language representation to account for this situation. Importantly, in individuals without typical left-hemisphere language localization, functional imaging studies have provided compelling evidence that bilateral activation for language processing is actually much more common than right-lateralized activation, in both right-handers (Mazoyer et al., 2014; Springer et al., 1999) and left-handers (Mazoyer et al., 2014; Szaflarski et al., 2002). Can such bilateral representations convey resilience to language deficits after unilateral damage? There are several lines of evidence suggesting that this might be the case. First, in a study designed specifically to address this question, Knecht and colleagues (2002) found that neurologically normal individuals with a higher degree of bilateral representation, in comparison to those with more lateralized networks, showed less disruption of language function when unilateral language network lesions were simulated using transcranial magnetic stimulation (TMS). Second, many studies using the Wada procedure have described cases with bilateral language in which one or more language functions were retained after anesthetization of either hemisphere, suggesting that both hemispheres were independently capable of supporting those functions (Kurthen et al., 1994; Rasmussen & Milner, 1977; Risse et al., 1997; see Bernal & Ardila, 2014 for recent review). Third, there is some evidence suggesting that non-right-handed individuals, who are more likely to have bilateral representations of language (Szaflarski et al., 2002), may experience aphasias that are less persistent than expected (Luria, 1947/1970). Taken together, it appears not unreasonable to consider the possibility that a bilateral language network could explain spared language function following unilateral left hemisphere damage.
We will now consider the second alternative explanation for a lack of aphasia after left hemisphere perisylvian damage, which is the possibility that the language network is localized to the left hemisphere, but that the specific lesion is situated in such a way that it avoids impacting regions that are critical for language. There is a certain amount of individual variability in the precise locations of perisylvian language regions (Penfield and Roberts, 1959; Fedorenko et al., 2012; Wilson, Yen, et al., 2018). Ojemann and colleagues (1989) have gone so far as to claim, based on cortical stimulation mapping, that language nodes are individually variable but discretely localized, and that substantial regions of perisylvian cortex between these critical nodes are not essential for language. This suggests that an isolated lesion that causes aphasia in one individual may leave another individual’s language function intact, if the lesion happens to spare their individual critical nodes. This could especially be the case for smaller lesions.
In clinical practice, when a patient presents with intact language function after left perisylvian damage, it is usually impossible to distinguish between the three possible explanations we have outlined. Indeed, determining the explanation is not necessarily a clinical priority, since it would be unlikely to have significant implications for patient management. However, even though the clinical implications may not be immediate, a better understanding of the different ways that the language network can be organized in different individuals, and the effects of damage in these various situations, will contribute to knowledge about the neurobiology of language and may have longer term clinical applications. In the current report we describe the case of a left-handed gentleman who presented with an unexpected absence of aphasia following a left temporal lobe hemorrhage. In particular, we had the opportunity to characterize the localization of his language network using functional magnetic resonance imaging (fMRI) with psychometrically validated language mapping paradigms.
Case study
Mr. B (not his real initial) was a 26-year-old, left-handed, native English-speaking veteran of the armed forces. He was a high-school graduate and was certified as an emergency medical technician (EMT), but he was not actively serving as an EMT at the time of study participation. Prior to his stroke, Mr. B was working as a receptionist, but following his stroke and throughout the duration of the present study, he was unemployed due to unresolved headaches and needing to serve as a caretaker for a family member. Mr. B’s only significant medical history prior to his stroke was that he had sustained multiple past concussions, including two where he had lost consciousness for brief periods of time. He additionally reported daily recreational use of marijuana and occasional use of other illicit drugs.
Mr. B reported to a local VA Medical Center in 2018 after sustaining a seizure. Computerized tomography (CT) revealed a substantial left temporal hemorrhage. The seizure lasted for approximately two minutes and reportedly involved Mr. B. being unresponsive with tense arms in a flexed position followed by shaking all over. Apart from these observations, no neurological abnormalities were observed by his girlfriend who was with him at the time of the seizure, and no neurological abnormalities, other than a headache, were revealed by neurological examination upon his arrival at the local VA Medical Center. Of particular note, no aphasia was observed by his girlfriend or on admission. He was immediately treated with 1g of Keppra and was prescribed 500mg of Keppra to be administered twice a day. Later that same day, he was transferred to Vanderbilt University Medical Center (VUMC).
Once at VUMC, Mr. B was in stable condition and had systolic blood pressure in the 90–100s. His acute left temporal hemorrhage was confirmed on both CT and MRI. Neurologic examination continued not to reveal any abnormalities. He was oriented to person, place and time and presented with no evidence of aphasia, slurred speech, facial droop, vision changes, numbness, weakness, or gait difficulty. Following stroke workup, the neurology service deemed the seizure to be secondary to the hemorrhage he had sustained. The etiology of hemorrhage was determined likely to be due to amphetamine use, which was confirmed by urine drug screening. Neither angiogram nor transthoracic echocardiogram revealed any abnormalities, and CT and MRI were both negative for any underlying lesion.
Acute language testing
Mr. B underwent acute language testing completed by our team as part of an ongoing study where any willing patient treated at VUMC who sustains a left hemisphere stroke is assessed for the presence of aphasia and evaluated for eligibility to participate in a longitudinal study of recovery from aphasia after stroke. This study was approved by the institutional review board at Vanderbilt University, and all study procedures were performed in accordance with the Declaration of Helsinki. We approached Mr. B on his second day at VUMC, two days after his stroke. At this time, Mr. B consented to participate, and we administered the Quick Aphasia Battery (QAB; Wilson, Eriksson, et al., 2018) at his bedside.
The QAB has been described in detail elsewhere (Wilson, Eriksson, et al., 2018), but briefly, it is a reliable and validated language assessment specifically designed to be used for the purpose of characterizing language function in a short period of time in a research context. Eight summary measures are derived, which taken together provide a profile of language function and identify strengths and weaknesses across the major domains of language.
Mr. B exhibited performance within normal limits across all domains assessed with the QAB. Mr. B’s summary measures are reported in Table 1. Note that all summary measures are out of a total of 10 points. Mr. B’s summary scores ranged from 9.6 to 10. His overall QAB score was 9.9, which was well above the previously established cut off score of 8.9 used to diagnose the presence of aphasia. The only item on which Mr. B made an error was a sentence comprehension item, which he subsequently self-corrected without prompting.
Table 1.
Acute and subacute language performance.
| Assessment | Acute | Subacute |
|---|---|---|
| QAB Single word comprehension (10) | 10.0 | 9.8 |
| QAB Sentence comprehension (10) | 9.6 | 10.0 |
| QAB Word finding (10) | 10.0 | 8.3 |
| QAB Grammatical construction (10) | 10.0 | 10.0 |
| QAB Speech motor programming (10) | 10.0 | 10.0 |
| QAB Repetition (10) | 10.0 | 10.0 |
| QAB Reading (10) | 10.0 | 10.0 |
| QAB Overall (10) | 9.9 | 9.7 |
| QAB Writing (extended form) (10) | 10.0 | |
| QAB Written word comprehension (extended form) (10) | 10.0 | |
| PPT (14) | 14 | |
| Communication questions from BOSS | 0 (no impairment) | |
| WAB-R Spontaneous speech (20) | 20.0 | |
| WAB-R Auditory verbal comprehension (10) | 10.0 | |
| WAB-R Repetition (10) | 9.8 | |
| WAB-R Naming and word finding (10) | 9.0 | |
| WAB-R Aphasia Quotient (100) | 97.6 | |
QAB = Quick Aphasia Battery (Wilson, Eriksson, et al., 2018); PPT = Pyramids and Palm Trees (Breining et al., 2015); BOSS = Burden of Stroke Scale (Doyle et al., 2003); WAB-R = Western Aphasia Battery-Revised (Kertesz, 2006). Numbers in parentheses represent total points possible for each measure. Acute testing was completed 2 days post onset. Subacute testing was completed 55 days post onset (QAB, PPT, BOSS questions) and 72 days post onset (WAB-R).
One of the sections of the QAB requires the speech-language pathologist scoring the evaluation to subjectively rate the participant’s spontaneous speech on several different aspects of language (i.e., phrase length, speech rate, anomia, agrammatism, empty speech, etc.) on a scale of zero (severely impaired) to four (within normal limits). Mr. B was rated at ceiling on all scored aspects of language during his spontaneous language sample.
Subacute language testing
To inform the present case study, Mr. B came back to the laboratory in the months following his stroke to undergo further language testing to confirm the lack of aphasia observed in the acute setting. The following batteries were administered 55 days post-onset: (1) an extended version of the QAB (Wilson, Eriksson, et al., 2018); (2) the short-form of Pyramids and Palm Trees (PPT; Breining et al., 2015); and (3) ten questions related to communication and communication distress from the Burden of Stroke Scale (BOSS; Doyle et al., 2003). Additionally, the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006) was administered 72 days post onset. Each of the batteries is briefly described below along with Mr. B’s performance on each battery.
An extended version of the previously described QAB (Wilson, Eriksson, et al., 2018) was administered to assess overall language function. This extended version is used in our laboratory for subacute and chronic visits when time is not so limited, as in the acute setting. The extended version contains double the number of single word and sentence comprehension items, to increase reliability, and adds two additional subtests to assess performance on single word reading comprehension and writing.
Mr. B’s summary measures on the extended version of the QAB are reported in Table 1. He performed similarly on the subacute administration of the QAB as he did on the acute administration. His overall QAB score was 9.7, again demonstrating language functioning within normal limits. Mr. B’s few errors were on a single word comprehension item, which he subsequently self-corrected without prompting, and in word finding. Mr. B’s spontaneous language sample was again rated at ceiling across all scored aspects. A selection from his speech sample is transcribed in Table 2.
Table 2.
Subacute speech sample.
| I don’t really remember much. |
| Um (..) I just remember, like, people in my room. |
| <I> I laid down... |
| Like, before getting there, I laid down to take a nap. |
| And then (.) I remember waking up. |
| And there’s, like, people in my room. |
| And I was just kind of confused. |
| And (.) um then I don’t really remember much after that. |
| And then I just remember, like, being in the hospital. |
| And then being, like, sleepy. |
| And going and in and out of, like, the CAT scan and other rooms and stuff. |
| But... |
| That’s about it. |
| Just, like, (.) vague stuff, I guess. |
The short form of the PPT (Breining et al., 2015) was administered to assess nonlinguistic semantic knowledge, because our functional imaging paradigm involves a semantic task. Mr. B performed at ceiling on this measure (Table 1).
Slightly modified versions of ten questions from the BOSS (Doyle et al., 2003) were administered to assess Mr. B’s perspective of his communication and communication distress. He rated himself as having no difficulty with any aspect of language functioning, and consequently no distress related to language function (Table 1).
The WAB-R (Kertesz, 2006) was administered to confirm Mr. B’s lack of aphasia. Mr. B. received an AQ of 97.6, which is indicative of performance within normal limits (Table 1).
Subacute neuropsychological testing
Mr. B completed a brief neuropsychological assessment to measure memory, executive function, verbal fluency, processing speed, and mood. This assessment was administered for completeness of the case study and given Mr. B’s history of repeated concussions.
The Rey Auditory Verbal Learning Test (Schmidt, 1996) was administered to assess verbal episodic learning and memory. Performance on recall of the 15-item word list was within normal limits after the fifth presentation (13/15) and the 30-min delay (11/15). The Digit Span and Letter-Number Sequencing subtests from the Wechsler Adult Intelligence Scale-IV (WAIS) (Wechsler, 2008) were administered to assess working memory. Age-corrected scaled scores on Digit Span and Letter-Number Sequencing were 9 and 8, respectively, which are in the average range.
The Symbol Search and Coding subtests from the WAIS-IV were administered to assess processing speed. Age-corrected scaled scores on Symbol Search and Coding were 9 and 8, respectively, which are in the average range. The Trail Making Test (Reitan & Wolfson, 1985) was administered as a measure of executive functioning. The scaled score on Trails B was 8, which is low average.
The Controlled Oral Word Association Test in the Multilingual Aphasia Examination (Benton, Hamsher, & Sivan, 1994) was administered as a measure of verbal fluency. Mr. B generated all the words he could think of for 1 minute for each of three letters. Across all letters, his score was 45 which is within normal range for his age and education. Finally, Mr. B completed the Beck Depression Inventory-II (Beck, 1996) scoring a 20 which, consistent with his self-report, suggests moderate depression.
In summary, we found Mr. B’s neuropsychological functioning to be within the average range, despite his history of concussions. Further, there was no evidence for any neuropsychological deficits following his left temporal hemorrhage.
Neuroimaging methods
Acquisition of neuroimaging data
Mr. B was scanned 55 days following his stroke on a Philips Achieva 3T scanner with a 32-channel head coil at the Vanderbilt University Institute of Imaging Science. To characterize Mr. B’s language network, two functional runs of T2*-weighted BOLD echo planar images were collected with the following parameters: 200 volumes + 4 initial volumes discarded; 35 axial slices in interleaved order; slice thickness = 3.0 mm with .5 mm gap; field of view = 220 × 220 mm; matrix = 96 × 96; repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle = 75°; SENSE factor = 2; voxel size = 2.3 × 2.3 × 3.5 mm. All visual stimuli for the functional runs were projected onto a screen at the end of the bore, which Mr. B viewed through a mirror mounted to the head coil.
T1-weighted structural images (voxel size = 1.0 × 0.8 × 0.8 mm) and T2-weighted FLAIR images (voxel size =0.6 × 0.6 × 2.0 mm) were acquired for anatomical reference and lesion delineation, and coplanar T2-weighted images were acquired to aid coregistration.
Functional paradigms
In the two functional runs, Mr. B completed two language mapping paradigms. First, he performed an adaptive semantic matching paradigm (Wilson, Yen, et al., 2018), and second, he performed an adaptive rhyme judgment paradigm (Yen et al., 2019). These paradigms were chosen for their demonstrated ability, when used in conjunction, to yield a map of regions important for both semantic and phonological aspects of language processing (Wilson et al., 2019). Both adaptive language mapping paradigms used an AB block design. In each paradigm, there were a total of twenty blocks, with the language and control condition alternating every twenty seconds. Total scan time for each paradigm was 400 seconds (6:40).
The adaptive semantic matching paradigm (Wilson, Yen, et al., 2018) contrasted a semantic decision condition with a perceptual decision condition and has demonstrated reliable and valid identification of lateralized frontal and temporal core language regions. During the semantic decision condition, Mr. B viewed two words, presented one above the other, and was asked to indicate whether a semantic relationship existed between the two words. If a semantic relationship existed, he pressed a single button situated in his left hand, and if no relationship existed, he did nothing. During the perceptual decision condition, Mr. B viewed two false font strings, presented one above the other, and was asked to indicate whether the two strings were identical. If they were identical, he pressed a single button situated in his left hand, and if the strings differed in any way, he did nothing.
The adaptive rhyme judgment paradigm (Yen et al., 2019) contrasted a rhyme decision condition with the same perceptual condition as above, and has demonstrated reliable and valid identification of lateralized regions important for phonological encoding (i.e. supramarginal gyrus and ventral precentral gyrus). Critically, these regions are not activated by the semantic paradigm, making this paradigm a complementary addition to the adaptive semantic decision paradigm described above. The rhyme judgment paradigm followed the same framework as the semantic decision paradigm, but instead of making a decision about whether two words share a semantic relationship in the language condition, Mr. B was asked to indicate whether two pseudowords, presented one above the other, rhymed. If so, he pressed the button, and if not, he did nothing. The perceptual control condition was the same as in the semantic paradigm.
Both paradigms involved an adaptive staircase procedure to adjust stimuli in both speed and complexity to Mr. B’s current performance level. The details of this procedure have been described previously (Wilson, Yen, et al., 2018; Yen et al., 2019), but briefly, we used a 2-down-1-up staircase with weighted step sizes (up twice as large as down) that theoretically converges at just over 80% accuracy (García-Perez, 1998). This procedure aims to keep tasks engaging yet feasible for any participant, regardless of ability level.
Mr. B was trained on both paradigms until he could perform them comfortably, prior to entering the scanner.
Analysis of neuroimaging data
Neuroimaging data were processed using standard methods exactly as described in Wilson, Yen, et al. (2018). Each language condition was compared to its control condition using the general linear model, and a relative thresholding approach was applied (Gross & Binder, 2014) such that voxels with the highest 5% of t statistics for each contrast were considered active, subject to a cluster extent threshold of 2000 mm3. Lateralization indices (LIs) were calculated based on the whole brain, except for the cerebellum.
Neuroimaging results
Structural imaging findings
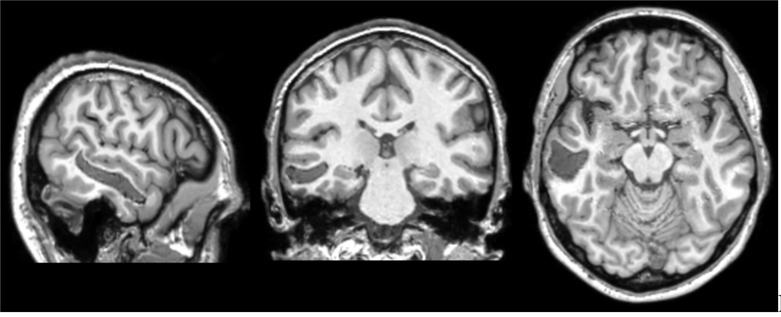
The hemorrhage that Mr. B sustained was clearly visible on T1 structural imaging, and was located in the left middle temporal gyrus (Figure 1). The lesion destroyed almost all of the white matter underlying this gyrus, from the posterior temporal region to the tip of the anterior temporal lobe. The volume of the lesion was 9206 mm3.
Figure 1.
Structural T1 scan. From left to right: left sagittal view, coronal view, axial view. Left is left.
Behavioral performance on functional paradigms
In the adaptive semantic matching paradigm, Mr. B was presented with 76 trials in each condition. In the language condition, he was correct on 60 out of 76 trials (78.9% accuracy) and in the perceptual control condition, he was correct on 65 of the 76 trials (85.5% accuracy). His reaction times (on correct trials only) for the two conditions were 1461 ± 429 ms and 2022 ± 378 ms, respectively.
In the adaptive rhyme judgment paradigm, Mr. B was presented with 72 trials in each condition. In the language condition, he was correct on 59 out of 72 trials (81.9% accuracy) and in the perceptual control condition, he was correct on 57 of the 72 trials (79.2% accuracy). His reaction times (on correct trials only) for the two conditions were 1603 ± 371 ms and 2035 ± 264 ms, respectively.
Consistent with his lack of aphasia, Mr. B’s accuracy and reaction times on language tasks did not differ from neurologically normal individuals studied previously (Yen et al., 2019) (semantic accuracy: t = –1.13, p = 0.28; semantic reaction time: t = –0.63, p = 0.54; rhyme accuracy: t = +1.22, p = 0.63; rhyme reaction time: t = –0.27, p = 0.79; Crawford-Howell t-tests, with years of education as covariate).
Functional imaging findings
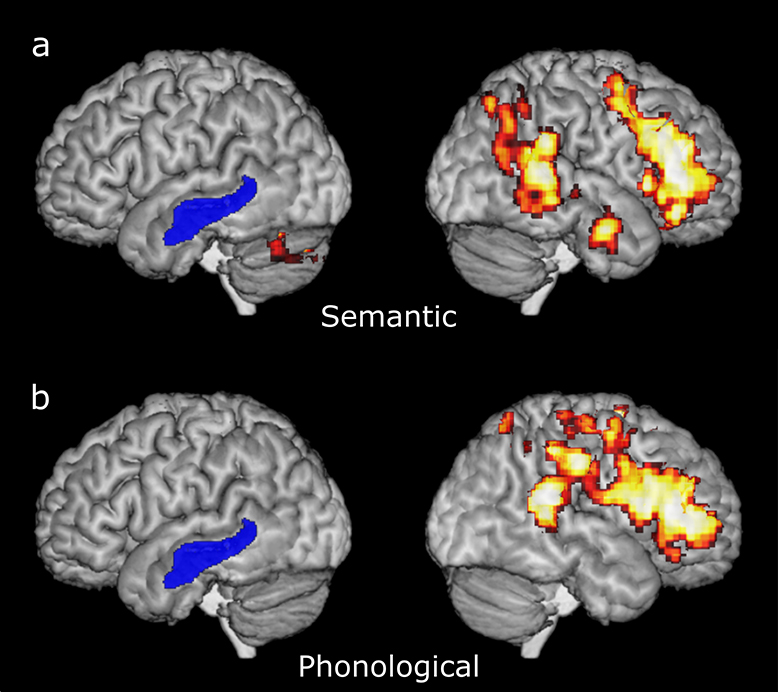
In the adaptive semantic matching paradigm, the contrast between the semantic condition and the perceptual control condition activated exclusively right hemisphere regions, with the exception of the left cerebellum (Figure 2a). The regions activated included the right inferior frontal gyrus (pars orbitalis, pars triangularis, and pars opercularis), extending into the right middle frontal gyrus and the right dorsal precentral sulcus, the right posterior superior temporal gyrus and posterior superior temporal sulcus, extending into the right angular gyrus, a right mid-anterior temporal region within the middle temporal gyrus, and a region in the left cerebellum.
Figure 2.
Language activation maps derived from the adaptive semantic matching paradigm (2a) and the adaptive rhyme judgment paradigm (2b). Voxels with the highest 5% of t statistics for each contrast between the language and control conditions were plotted, subject to a minimum cluster volume of 2000 mm 3. Color scale ranges from 5% to 0%. Left is left. Blue represents lesion.
In the adaptive rhyme judgment paradigm that targets phonological encoding, the contrast between the rhyme judgment condition and the perceptual control condition also activated exclusively right hemisphere regions (Figure 2b). The regions activated included the right inferior frontal gyrus (pars orbitalis, pars triangularis, and pars opercularis), extending into the right middle frontal gyrus, the right ventral precentral sulcus and the right ventral precentral gyrus, the right posterior superior temporal gyrus and the posterior superior temporal sulcus, and the right supramarginal gyrus, extending into the right intraparietal sulcus.
The exclusive right hemisphere lateralization observed for both paradigms entailed that Mr. B’s lateralization index (LI) for each paradigms was exactly –1. In contrast, none of the 16 neurologically normal individuals reported in Yen et al. (2019) had right hemisphere language; for the semantic matching paradigm, their mean LI was 0.83 ± 0.30 (t = 5.93, p < 0.0001, Crawford-Howell t-test), and for the rhyme judgment paradigm, their mean LI was 0.81 ± 0.32 (t = 5.50, p < 0.0001, Crawford-Howell t-test).
Discussion
At the outset, we outlined three plausible explanations for a lack of aphasia following left perisylvian damage. In the single patient we studied, Mr. B, we found unequivocal evidence that right hemisphere lateralization of language function was the reason why no aphasia was observed after his left temporal hemorrhage.
Two distinct functional paradigms—a semantic matching paradigm and a rhyme judgment paradigm—both revealed only right hemisphere activations for language processing, with the exception of activation in the left cerebellum in the semantic paradigm. Both paradigms activated right frontal and right temporal core language regions. Each paradigm also revealed additional regions specific to the linguistic domain targeted: the semantic paradigm activated the right anterior temporal lobe and right angular gyrus, while the phonological paradigm activated the right supramarginal gyrus and right ventral precentral gyrus. The nature of these observed patterns, and the differential activation patterns between them, are highly consistent with what has been observed in the left hemisphere in neurologically normal participants when the same paradigms have been used (see Figure 8 in Wilson, Yen, et al., 2018 and Figure 2a in Yen et al., 2019). This corroborates previous work demonstrating that when right-lateralization for language is observed, it typically mirrors the organization more commonly seen in the left hemisphere (Chang et al., 2011; Duffau et al., 2008). The activation of the left cerebellum observed in the semantic paradigm was also expected based on the same principle, since cerebellar involvement in language processing has been shown to be contralateral to the dominant hemisphere (Jansen et al., 2005). The mirror image nature of Mr. B’s language network clearly indicates premorbid right hemisphere language, rather than a process of functional plasticity (Wilson, Yen, et al., 2018).
We stated above that right hemisphere lateralization of language function is the default assumption of clinicians when aphasia does not follow from left hemisphere damage, and indeed that is what we observed. But we now consider in more detail the plausibility of the two other possible explanations we outlined. First, could bilateral language representation account for lack of aphasia after unilateral damage? As noted above, bilateral representation is the most common distribution of language function when left-lateralization is not observed, observed more frequently than right-lateralization (Mazoyer et al., 2014; Springer et al., 1999; Szaflarski et al., 2002). In a particularly compelling imaging study that used a psychometrically sound language mapping paradigm, Szaflarski and colleagues (2002) studied a group of 50 left-handers. They found that 39 out of the 50 participants (78%) had left lateralized language and just 4 (8%) had right lateralized language, while 7 participants (14%) showed bilateral activation for language processing.
But, does bilateral activation on functional imaging imply resilience to unilateral damage? Not necessarily. For bilateral representation to provide protection against aphasia following unilateral left hemisphere perisylvian damage, the spared parts of the bilateral network must be able to successfully process language without continued support from the damaged areas. The most straightforward way this could occur would be for each hemisphere to be completely independently capable of processing language. However, evidence from the Wada procedure suggests that this form of bilaterality, where each hemisphere is equipped with a fully capable and functionally redundant network, is actually extremely rare (Loring et al., 1990; Kurthen et al., 1994; Risse et al., 1997; see Bernal & Ardila, 2014 for recent review). In a study that specifically aimed to characterize the nature of bilaterality using the Wada procedure in a large cohort of individuals with epilepsy, Risse and colleagues (1997) found that while 39 out of 368 of their cases (10.6%) were classified as having bilateral language, only 2 out of the 39 (5% of bilateral cases; 0.5% of the whole sample) showed complete preservation of language function when each respective hemisphere was anesthetized. They found that in most patients classified as having bilateral language, the two hemispheres differed greatly in their capacity for language function, such that one hemisphere (more often the left) was clearly dominant, and was able to process language largely independently, while the other hemisphere had only a restricted capacity in a subset of language domains.
This suggests that in most cases of bilateral language, there is still a dominant hemisphere, usually the left hemisphere. If the dominant hemisphere is damaged, the sub-dominant hemisphere would not be equipped to sustain normal language processing, without continued input from the dominant hemisphere. Thus, it may not have been so surprising after all that bilateral representation did not underlie Mr. B’s lack of aphasia—for, if his left hemisphere was his dominant hemisphere, we likely would have still expected aphasia, and if his right hemisphere was his dominant hemisphere, the reason for his lack of aphasia would be due to the relative dominance of the right hemisphere, rather than being bilateral per se.
It is important to note that the Wada procedure can only provide information about the capabilities of the sub-dominant hemisphere when the dominant hemisphere is completely absent. We do not yet know the full potential of a bilateral network which sustains only partial damage to the dominant hemisphere. As discussed in the introduction, there are data to suggest that following partial damage to the dominant hemisphere, a bilateral network may aid in lessening significance of initial deficits (Knecht et al., 2002) or in persistence of initial deficits (Luria, 1947/1970). Crinion and Price (2005) provided further evidence using fMRI to suggest that individuals with more premorbid right hemisphere engagement may recover better from aphasia than their peers with less premorbid right hemisphere engagement. Taken together, it appears well supported that having at least some degree of bilateral representation, consisting of the spared regions of the dominant hemisphere and the undamaged sub-dominant hemisphere, may lessen the functional significance of partial unilateral damage. Could there be an extreme example of this in which a bilateral network would prevent aphasia altogether following a lesion to the dominant hemisphere? We think this remains a possibility, but clearly Mr. B turned out not to be such a case.
We will now consider the plausibility of the second possible alternative explanation for Mr. B’s lack of aphasia: that he might have had left hemisphere language, yet his lesion might have been localized in such a way to avoid his individually specific regions that were critical for language. Given the extent of individual variability in intrahemispheric language organization that has been documented (Penfield and Roberts, 1959; Ojemann et al., 1989; Wilson, Yen, et al., 2018), and given the focal nature of Mr. B’s lesion, we thought this explanation was worthy of consideration. It is important to note that most left temporo-parietal regions thought to be critical for language were spared in Mr. B, including the superior temporal gyrus and sulcus, the angular gyrus, and the supramarginal gyrus; moreover, his left frontal lobe was of course unaffected. However, if we take a closer look at the anatomy of Mr. B’s lesion, we think it is quite unlikely that such a lesion could spare language function entirely. As can be seen in Figure 1, the lesion destroyed essentially all of the white matter underlying the middle temporal gyrus. These white matter connections, which project to multiple frontal, temporal, and parietal language regions, have been argued to constitute a critical hub of the whole language network (Turken & Dronkers, 2011). In particular, Mr. B’s lesion could be expected to completely disconnect the ventral bank of the STS, where several higher order language sites are localized (Wilson, Bautista, et al., 2018), from the remainder of the language network. Even given the possibility of individual variability, the anterior-posterior extent of the lesion would seem to imply that had Mr. B’s language network been left-lateralized, aphasia would have been inescapable. We think it remains a possibility that a small left hemisphere lesion could avoid the critical regions of a left lateralized network, if it were situated in a way that critical cortical and subcortical regions are spared, though in Mr. B this was not the case.
In sum, we had the opportunity to document using fMRI a case where right lateralization was the underlying explanation for a lack of aphasia following left hemisphere perisylvian damage. We only know of one other case where fMRI was used to uncover the explanation for a lack of aphasia following left hemisphere damage, and that case also demonstrated evidence for right hemisphere language (Hund-Georgiadis et al., 2001). Although the possible alternative explanations we considered were not borne out in either of these cases, we have argued that they should still be considered when evaluating other cases. We hope that our case study has highlighted the questions that remain about the various ways that language can be organized in the brain, and the implications of damage to different regions in different scenarios.
Acknowledgements:
We thank our participant for taking part in this study, Howard Kirshner for facilitating patient recruitment, Caitlin Onuscheck for assistance with scoring, Melodie Yen for providing control data, Michael de Riesthal for helpful discussions, the staff of Vanderbilt University Institute of Imaging Science for technical support, and an anonymous reviewer for helpful feedback.
Disclosure of interest: This research was supported in part by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders) under Grant number R01 DC031270.
References
- Beck A (1996). Beck Depression Inventory-II. San Antonio: The Psychological Corporation. [Google Scholar]
- Benson DF (1985). Language in the left hemisphere. In Benson DF & Zaidel E (Eds.), The dual brain (pp. 193–203). Guilford Press. [Google Scholar]
- Benton A, Hamsher K, & Sivan A (1994). Multilingual Aphasia Examination (3rd edition). Florida: Psychological Assessment Resources, Inc. [Google Scholar]
- Bernal B, & Ardila A (2014). Bilateral representation of language: A critical review and analysis of some unusual cases. Journal of Neurolinguistics, 28, 63–80. [Google Scholar]
- Bramwell B (1899). On “crossed” aphasia and the factors which go to determine whether the “leading” or “driving” speech-centres shall be located in the left or the right hemisphere of the brain:, with notes of a case of “crossed” aphasia (aphasia with right sided hemiplegia) in a left-handed man. The Lancet, 153(3953), 1473–1479. [Google Scholar]
- Breining BL, Lala T, Cuitiño MM, Manes F, Peristeri E, Tsapkini K, Faria AV, & Hillis AE (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29(4), 488–505. [Google Scholar]
- Broca P (1865). Sur le siège de la faculté du langage articulé. Bulletins de la Société d’Anthropologie de Paris, 6, 377–393. [Google Scholar]
- Carey DP, & Johnstone LT (2014). Quantifying cerebral asymmetries for language in dextrals and adextrals with random-effects meta analysis. Front. Psychol 5,1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Wang DD, Perry DW, Barbaro NM, & Berger MS (2011). Homotopic organization of essential language sites in right and bilateral cerebral hemispheric dominance: Clinical article. Journal of Neurosurgery, 114(4), 893–902. [DOI] [PubMed] [Google Scholar]
- Crinion J, & Price CJ (2005). Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain: A Journal of Neurology, 128(Pt 12), 2858–2871. [DOI] [PubMed] [Google Scholar]
- Doyle P, McNeil M, Hula W, & Mikolic J (2003). The Burden of Stroke Scale (BOSS): Validating patient-reported communication difficulty and associated psychological distress in stroke survivors. Aphasiology, 17(3), 291–304. [Google Scholar]
- Duffau H, Leroy M, & Gatignol P (2008). Cortico-subcortical organization of language networks in the right hemisphere: An electrostimulation study in left-handers. Neuropsychologia, 46(14), 3197–3209. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2012). Language-selective and domain-general regions lie side by side within Broca’s area. Current Biology, 22(21), 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross WL, & Binder JR (2014). Alternative thresholding methods for fMRI data optimized for surgical planning. NeuroImage, 84, 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund-Georgiadis M, Zysset S, Weih K, Guthke T, & von Cramon DY (2001). Crossed nonaphasia in a dextral with left hemispheric lesions. Stroke, 32(11), 2703–2707. [PubMed] [Google Scholar]
- Jansen A, Flöel A, Randenborgh JV, Konrad C, Rotte M, Förster A-F, Deppe M, & Knecht S (2005). Crossed cerebro–cerebellar language dominance. Human Brain Mapping, 24(3), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (1982) Western Aphasia Battery. New York: Grune and Stratton. [Google Scholar]
- Kertesz A (2006). Western Aphasia Battery-Revised. The Psychological Corporation. [Google Scholar]
- Knecht S, Flöel A, Dräger B, Breitenstein C, Sommer J, Henningsen H, Ringelstein EB, & Pascual-Leone A (2002). Degree of language lateralization determines susceptibility to unilateral brain lesions. Nature Neuroscience, 5(7), 695–699. [DOI] [PubMed] [Google Scholar]
- Kurthen M, Helmstaedter C, Linke DB, Hufnagel A, Elger CE, & Schramm J (1994). Quantitative and qualitative evaluation of patterns of cerebral language dominance: An amobarbital study. Brain and Language, 46(4), 536–564. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, Gallagher BB, & King DW (1990). Cerebral language lateralization: Evidence from intracarotid amobarbital testing. Neuropsychologia, 28(8), 831–838. [DOI] [PubMed] [Google Scholar]
- Luria AR (1970). Traumatic aphasia: Its syndromes, psychology and treatment (D. Bowden, Trans.). Mouton & Co., Printers. (Original work published 1947) [Google Scholar]
- Mazoyer B, Zago L, Jobard G, Crivello F, Joliot M, Perchey G, Mellet E, Petit L, & Tzourio-Mazoyer N (2014). Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLOS ONE, 9(6), e101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S, Beste C, Arning L, Peterburs J, & Güntürkün O (2014). The ontogenesis of language lateralization and its relation to handedness. Neuroscience & Biobehavioral Reviews, 43, 191–198. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, & Berger M (1989). Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71(3), 316–326. [DOI] [PubMed] [Google Scholar]
- Penfield W, & Roberts L (1959). Speech and brain-mechanisms. Princeton University Press. [Google Scholar]
- Reitan R, & Wolfson D (1985). The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press. [Google Scholar]
- Rasmussen T, & Milner B (1977). The role of early left-brain injury in determining lateralization of cerebral speech functions. Annals of the New York Academy of Sciences, 299, 355–369. [DOI] [PubMed] [Google Scholar]
- Risse GL, Gates JR, & Fangman MC (1997). A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain and Cognition, 33(1), 118–132. [DOI] [PubMed] [Google Scholar]
- Schmidt M (1996) Rey Auditory and Verbal Learning Test. A handbook. Los Angeles: Western Psychological Association [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, Brewer CC, Perry HM, Morris GL, & Mueller WM (1999). Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain, 122(11), 2033–2046. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, & Hammeke TA (2002). Language lateralization in left-handed and ambidextrous people: FMRI data. Neurology, 59(2), 238–244. [DOI] [PubMed] [Google Scholar]
- Turken AU, & Dronkers NF (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale-Fourth Edition. Technical and interpretive manual. San Antonio, TX: Pearson. [Google Scholar]
- Wilson SM, Bautista A, & McCarron A (2018). Convergence of spoken and written language processing in the superior temporal sulcus. NeuroImage, 171, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Eriksson DK, Schneck SM, & Lucanie JM (2018). A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PloS One, 13(2), e0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Yen M, & Eriksson DK (2018). An adaptive semantic matching paradigm for reliable and valid language mapping in individuals with aphasia. Human Brain Mapping, 39(8), 3285–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Eriksson DK, Yen M, Demarco AT, Schneck SM, & Lucanie JM (2019). Language mapping in aphasia. Journal of Speech, Language & Hearing Research, 62(11), 3937–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, DeMarco AT, & Wilson SM (2019). Adaptive paradigms for mapping phonological regions in individual participants. NeuroImage, 189, 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]