Abstract
Background
To evaluate the impact of injection frequency on yearly visual outcomes of patients treated with intravitreal aflibercept for neovascular age-related macular degeneration (nAMD) over a period of 5 years in a tertiary ophthalmic centre.
Design
Single centre, retrospective cohort study.
Participants
Consecutive treatment-naive nAMD patients initiated on aflibercept injections 5 years ago.
Methods
The Moorfields OpenEyes database was searched for consecutive patients who were initiated on intravitreal aflibercept for nAMD in 2013–14 and the visual acuity (VA) in Early Diabetic Retinopathy Study (ETDRS) letters and injection records per year were recorded for a period of 5 years. Analyses of the whole cohort and a sub-sample of 5-year completers were done. The cohort was further grouped into Group A (on continuous treatment), Group B (early cessation of treatment) and Group C (interrupted treatment) to evaluate the relation between treatment frequency and visual outcomes.
Main outcome measures
The primary end point was change in VA at 5 years; secondary outcomes included proportion of eyes that gained or maintained VA, number of injections received and the effect of treatment frequency.
Results
Data were collected on 468 patients (512 eyes). Sixty-six percent of the patients completed 5-year follow-up. The mean age of the whole cohort was 79.5 ± 8.5 years and the mean baseline VA was 58.3 ± 15.4 letters. Amongst the completers, final VA change was −2.9 (SD 23.4) ETDRS letters and the cumulative number of injections over 5 years was 24.2 (10.6). Group A had three letter gain and received significantly higher cumulative number of injections over 5 years than Group B and C (31.8, 14.6 and 18.4 respectively, p = 0.001). After adjusting for age and baseline VA, on average, final VA was +8.0 letters higher in the ≥20 injections group than the <20 group (p = 0.001).
Conclusions
Aflibercept therapy results in sustained good visual outcome over 5 years in neovascular AMD eyes when early and persistent treatment is given.
Subject terms: Macular degeneration, Vision disorders
Introduction
Neovascular age-related macular degeneration (nAMD) is a common cause of visual impairment in patients’ ≥55 years. It is characterised by the presence of choroidal neovascularisation that may leak or bleed and if left untreated, results in visual loss of ~10 Early Treatment Diabetic Retinopathy Study (ETDRS) letters by the end of 1 year [1]. Anti-vascular endothelial growth factor (anti-VEGF) therapy is the standard of care for this condition. Aflibercept (Eylea; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, and Bayer AG, Leverkusen, Germany) is a fusion protein that inhibits VEGF-A, VEGF-B isoforms and placental growth factor and has demonstrated a long half-life and high affinity for VEGF. Aflibercept received marketing authorisation in the European Union on 21st November 2012 following which the National Institute for Health and Care Excellence in England and Wales recommended its use for nAMD. Since then, it has been widely used for this condition in the National Health Service (NHS).
At approximately the same time, there was an increased global awareness that a more stringent fixed or treat and extend dosing than a pro-re-nata (PRN) regimen is essential to sustain initial visual acuity (VA) gains with any anti-VEGF agents. Therefore, in the UK, the VIEW 1 and 2 fixed dosing in the first year was widely adopted as the protocol for treatment of nAMD in the first year and resulted in good visual outcomes [2]. However, reports from the second year onwards highlighted the need for more proactive dosing to sustain initial VA gains [3, 4]. Despite a mandated protocol for treating nAMD, not all patients are treated as per protocol in real-life and clinicians tend to individualise regimens to meet patient requirements. Moreover, by 5 years, treatment may have been stopped in a proportion of patients due to disease stability or futility and the outcomes of these patients also need to be considered in such cohort studies. Many patients also fail to attend appointments as duration of follow-up increases due to the inability to keep to the gruelling follow-up regimens due to logistic or health reasons. Unlike many other parts of the world, the cost of therapy is not a barrier as treatment is free at the point of care in the NHS. In order to understand the impact of these gaps in daily routine practice, there is a need to evaluate the long-term visual outcome of aflibercept therapy for treatment-naive nAMD.
The objective of this study was to evaluate the 5-year VA outcome of patients with treatment-naive nAMD initiated on aflibercept since its approval for use in Moorfields Eye Hospital.
Methods
Study design
This cohort study was designed to evaluate the VA outcomes and aflibercept injection frequency over 5 years in a protocol guided routine clinical practice. The study was approved by the clinical effectiveness department of Moorfields Eye Hospital (CA18/MR/15-141). The study setting is Moorfields Eye Hospital that provides retinal services across multiple centres in London, UK. The service delivery across all centres follows the same clinical guidelines and policies and patient records are electronically maintained.
Study cohort
Data were collected from a mandated electronic set of records maintained for each patient at each visit. All treatment-naive patients initiated on aflibercept from 1 January 2013 to 30 September 2014 were included in the study. The initial diagnosis of nAMD was confirmed using spectral domain optical coherence tomography (SDOCT) and fluorescein angiography. VA and SDOCT testing was performed at every visit in dedicated clinics for intravitreal injection service.
The records collected and analysed included VA at treatment initiation, VA at 12, 24, 36, 48 and 60 months and the mean number of injections per year. A window of ±1 month was allowed for each annual visit. Patients were excluded from participation if they received any other anti-angiogenic agents at any time point before or after initiation of aflibercept therapy.
Treatment protocol
The intravitreal aflibercept treatment protocol recommends three loading doses followed by 8-weekly fixed dosing until week 40 when the patients could be transferred to a treat and extend (T&E) regimen. The T&E regimen allowed visits to be extended at 2 weekly intervals and reduced by 2 weeks in case of reactivation [4]. When patients are injected at 12 weekly intervals for 3 consecutive visits without any evidence of reactivation, they could be referred to a stable AMD retinal clinic where they are monitored at 8–12 weekly intervals with VA and SDOCT measurements. If they show any signs of activation, the patients are reinitiated on a T&E regimen. Some receive PRN dosing whilst others a combination of T&E followed by PRN dosing according to clinician discretion. Stability was defined as three 12 weekly dosing showing no change in macular anatomy or VA. Some patients could be moved to 16 weekly T&E regimen if deemed appropriate by the clinician. Patients were discharged based on clinician discretion and these included patients with poor potential for visual improvement due to irreversible macular changes and those who have showed a complete resolution of the disease and achieved maximum visual potential.
Data variables
VA measurements were recorded in ETDRS letters at 4 m using patient’s habitual corrections where available supplemented with use of pinhole. VA of counting fingers or worse was given a value of 0 letters. The VA at treatment initiation and yearly intervals until 5 years were recorded for all patients. In a few instances (<5%), where VA scores were recorded in different notations, they were converted to ETDRS letters using a standardised conversion table. Baseline injection visit was defined as the visit of first injection. Maintenance of VA was defined as change in VA from +4 to −4 letters from baseline and gain in VA as gain of ≥5 letters. For non-completers, VA were recorded until the last clinic visit. Dates of administration of the injections by eye were collected to assess the frequency of aflibercept injections per year.
Statistical analysis
The analysis was done for (a) the whole cohort defined as every patient initiated on intravitreal aflibercept therapy in 2013 and (b) the completers defined as the sub-sample who completed 5-year follow-up. For missed visits, an average VA score was calculated using the observations before and after the missed visits. No observations were carried forward in patients lost to follow-up. The last visit was considered as 60 ± 3 months. All statistical tests were two-sided (α < 0.05). Continuous variables were described by mean ± standard deviation. A patient-level analysis was done for age, sex, first or second eye involvement and patients who received bilateral injections on the same day. An eye-level analysis was also conducted to assess VA per visit, dates of injections and monitoring visits. The association between final VA to the frequency of aflibercept injections was calculated using the Kruskal–Wallis and Dunnes test. While comparing the different VA categories with each other and the frequency of injections received, P value was calculated from post-hoc Dunnes test (Bonferroni adjusted by multiplying p value from each test by (m) total number of pairwise comparisons). The eyes of an individual were considered independent given the pathophysiology of nAMD. The 5-year completers were divided into three subgroups. Group A—eyes that continued to receive treatment throughout 5 years, Group B—eyes that stopped receiving treatment and stabilised requiring no further treatment until completion of 5 years and Group C—eyes that stopped receiving treatment for a minimum of 12 months and were re-initiated on treatment. The comparison among the groups was done using one-way analysis of variance. All analyses were performed with the statistical software SPSS (SPSS, USA).
Results
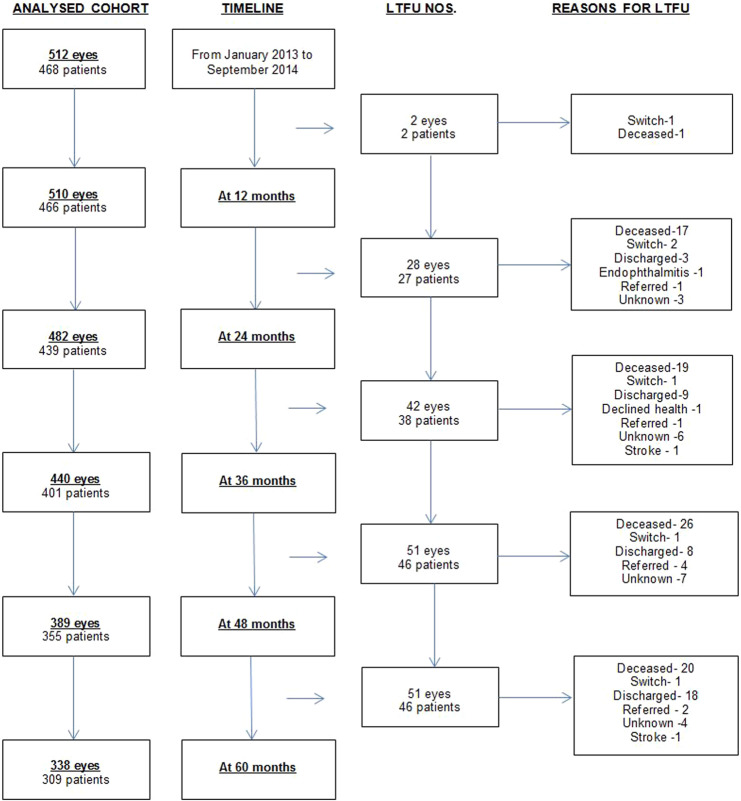
A total of 512 eyes of 468 patients were initiated on aflibercept therapy for nAMD and constituted the whole cohort, 338 eyes of 309 (66%) patients had completed 5-year follow-up and were included within the completers cohort. Figure 1 shows the flow of patients in the study period, the number of patients excluded from analysis and the reasons for exclusion, if provided.
Fig. 1. Flowchart of Patient cohort with Timeline.
Analysed cohort represents the number of eyes analysed and number of patients excluded (with reasons mentioned) at each timepoint.
Baseline characteristics
Mean age of the whole cohort was 79.5 ± 8.5 years and 54% of the patients were female. Mean baseline VA for the study eyes was 58.3 ± 15.4 letters and fellow eyes was 57.3 ± 27.6 letters. Proportion of study eyes with baseline VA ≥ 70, 54–69, 37–53 and <37 letters was 29.1%, 39%, 18.2% and 13.1%, respectively. Forty-four (9.4%) patients received injections in both eyes during the study period. Twenty-six (59%) of the 44 patients were initiated on bilateral injections on the same day.
VA and injection outcomes
VA outcomes in the whole cohort and the completers are shown in Table 1. There was no statistically significant difference between the 5-year completers and the whole cohort with regards to the mean changes in VA from baseline, from preceding year, cumulative number of injections and injections administered per year until last follow-up. Maximum VA gain was seen in the first year (5.6 letters in the whole cohort versus 6.3 in the completers). Gain in VA from baseline was maintained until the third year following which the VA dropped below the baseline with the final VA change in the completers at 5 years being −2.9 letters. By the end of fifth year, 42.9% eyes gained VA and 17.9% maintained VA (±4 letters). Among the 338 eyes that completed 5-year follow-up, 180 eyes (53.3%) were in Group A, 120 eyes (35.5%) in Group B and 38 eyes (11.2%) in Group C. Proportion of patients for whom treatment was stopped and never re-initiated were 0%, 3.3%, 15%, 6.8% and 10.4% at 1, 2, 3, 4 and 5 years, respectively.
Table 1.
Visual outcomes of whole cohort and the completers.
| Baseline | First year | Second year | Third year | Fourth year | Fifth year | |
|---|---|---|---|---|---|---|
| Whole cohort (N) eyes | N = 512 | N = 510 | N = 482 | N = 440 | N = 389 | N = 338 |
| Completers (n) eyes | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 |
| Visual acuity, mean (SD) | ||||||
| Whole cohort | 57.1 (14.7) | 63.0 (16.6) | 60.5 (19.5) | 57.7 (21.7) | 56.0 (23.4) | 55.0 (24.0) |
| Completers | 58.1 (14.0) | 64.5 (16.2) | 62.1 (19.3) | 59.8 (20.8) | 57.0 (23.0) | 55.0 (24.0) |
| P value | 0.32 | 0.19 | 0.24 | 0.17 | 0.56 | N/A |
| Change in VA from baseline, mean (SD) | ||||||
| Whole cohort | N/A | 5.6 (15.4) | 3.0 (18.3) | 0.3 (20.5) | −1.9 (22.7) | −2.9 (23.4) |
| Completers | N/A | 6.3 (14.3) | 3.9 (18.3) | 1.7 (19.9) | −1.2 (22.3) | −2.9 (23.4) |
| P value | N/A | 0.5 | 0.48 | 0.33 | 0.67 | N/A |
| Change in VA from preceding year, mean (SD) | ||||||
| Whole cohort | N/A | 5.6 (15.4) | −2.7 (12.3) | −2.96 (13.1) | −3.3 (12.3) | −1.9 (14.3) |
| Completers | N/A | 6.3 (14.3) | −2.4 (11.8) | −2.2 (12.5) | −2.9 (12.9) | −1.9 (14.3) |
| P value | N/A | 0.5 | 0.72 | 0.41 | 0.67 | N/A |
| Five-year completers (n = 338) | ||||||
| Eyes with ≥5 letter gain in VA from baseline, n (%) | N/A | 205 (60.1) | 182 (53.8) | 174 (51.5) | 162 (48) | 145 (42.9) |
| Eyes with ≥5 letter loss in VA from baseline, n (%) | N/A | 60 (17.8) | 80 (23.7) | 96 (28.4) | 123 (36.4) | 133 (39.3) |
| Eyes with VA change +4 to −4 letters from baseline, n (%) | N/A | 73 (21.6) | 76 (22.5) | 68 (20.1) | 53 (15.7) | 60 (17.8) |
| Eyes that avoided moderate visual loss (loss of 15 letters or less), n (%) | N/A | 319 (94.4) | 293 (86.7) | 289 (85.5) | 271 (80.2) | 255 (75.4) |
| Proportion of eyes with ≥70 letters, n (%) | 109 (32.2) | 184 (54.4) | 170 (50.3) | 150 (44.4) | 144 (42.6) | 135 (39.9) |
| Proportion of eyes with 54–69 letters, n (%) | 132 (39.0) | 86 (25.4) | 86 (25.4) | 97 (28.7) | 83 (24.6) | 85 (25.1) |
| Proportion of eyes with 37–53 letters, n (%) | 58 (17.2) | 34 (10.1) | 32 (9.5) | 39 (11.5) | 39 (11.5) | 45 (13.3) |
| Proportion of eyes with <37 letters, n (%) | 39 (11.5) | 34 (10.1) | 50 (14.8) | 52 (15.4) | 72 (21.3) | 73 (21.6) |
Injection frequency
Table 2 shows the decreasing frequency of injections received annually over 5 years. Of the 512 eyes included in the study, 1 eye received 3 injections or less in the first year of treatment. Proportion of eyes that received ≤3 injections in year 2, 3, 4 and 5 years were 23.7%, 39.1%, 45.9% and 53.6%, respectively. Proportion of eyes that received ≤4 injections in year 2, 3, 4 and 5 years are 39.9%, 48.8%, 54.1% and 63.6%, respectively. Those that received ≥ 5 injections in year 2, 3, 4 and 5 were 60.0%, 51.2%, 50.0% and 36.4% eyes.
Table 2.
Number of injections received by the whole cohort and the completers.
| Baseline | First year | Second year | Third year | Fourth year | Fifth year | |
|---|---|---|---|---|---|---|
| Whole cohort (N) eyes | N = 512 | N = 510 | N = 482 | N = 440 | N = 389 | N = 338 |
| Completers (n) eyes | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 | n = 338 |
| Number of injections per year, mean (SD) | ||||||
| Whole cohort | N/A | 7.8 (1.3) | 4.9 (2.7) | 3.8 (3.1) | 3.5 (3.1) | 3.2 (3.2) |
| Completers | N/A | 7.9 (1.3) | 5.2 (2.7) | 4.1 (3.1) | 3.7 (3.2) | 3.2 (3.2) |
| P value | N/A | 0.27 | 0.12 | 0.18 | 0.39 | N/A |
| Cumulative no. of injections at end of each year, mean (SD) | ||||||
| Whole cohort | N/A | 7.8 (1.3) | 12.7 (3.4) | 17.3 (5.8) | 20.5 (8.2) | 24.2 (10.6) |
| Completers | N/A | 7.9 (1.3) | 13.2 (3.4) | 16.7 (5.7) | 21.0 (8.2) | 24.2 (10.6) |
| P value | N/A | 0.27 | 0.04 | 0.15 | 0.41 | N/A |
Correlation between visual outcomes and injections received
Table 3 shows that the decline in mean number of injections was associated with worsening final VA across all years. Significantly more injections were given in eyes with good final VA (≥70 letters) compared with those with poor final VA (<37 letters) and this was observed in each year from 2 to 5 years. By assessing association between total injections and adjusted change in VA from baseline to year 5, every additional injection resulted in a 0.6 letter gain in VA (p < 0.001). When adjusted for age and baseline VA, there was a statistically significant association between total number of injections given and change in VA. On an average, the adjusted mean difference in final VA in the group who received ≥20 injections compared with the group <20 injections was +8.04 letters (p = 0.001). Change in VA from baseline of those who received ≤3 injections in year 2, 3, 4 and 5 years was 1.2, −2.0, −7.5 and –6.5 letters respectively while the change in VA observed in eyes that received ≤4 injections was 1.7, −0.4, −5.3 and −6.4 letters, respectively. In contrast, eyes that received ≥5 injections had a mean change in VA of 5.4, 4.1, 3.52 and 3.76 letters in year 2, 3 4 and 5.
Table 3.
Correlation between injection frequency, baseline VA and final visual outcomes.
| N = 338 | Number of injections/year, mean (SD) | |||||
|---|---|---|---|---|---|---|
| Final VA at 5 years | First year | Second year | Third year | Fourth year | Fifth year | Cumulative injections at 5 years |
| ≥70 letters | 8.1 (1.2) | 5.4 (2.4) | 4.6 (3.1) | 4.0 (3.2) | 3.8 (3.5) | 26.0 (10.9) |
| 54–69 | 7.9 (1.5) | 5.9 (2.7) | 4.6 (3.1) | 5.1 (2.8) | 4.3 (2.9) | 27.8 (10.8) |
| 37–53 | 7.7 (1.3) | 4.2 (2.6) | 4.0 (2.5) | 3.7 (3.1) | 2.2 (2.5) | 21.9 (8.2) |
| <37 letters | 7.8 (1.2) | 4.1 (2.9) | 2.1 (2.6) | 1.4 (2.1) | 1.5 (2.4) | 17.9 (7.6) |
| P value (K–W)a | 0.3 | 0.0013 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| P value (K–W)b | 0.1 | 0.0007 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| P value (D–T)c | ||||||
| Comparing <37 with 37–53 | 0.7 | 0.2 | 0.3 | 0.04 | 0.4 | 0.07 |
| Comparing <37 with 54–69 | 0.3 | 0.0006 | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Comparing <37 with ≥70 | 0.3 | 0.003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Comparing 37–53 with 54– 69 | 1 | 0.5 | 0.2 | 0.006 | 0.0004 | 0.01 |
| Comparing 37–53 with ≥70 | 1 | 1 | 0.2 | 0.2 | 0.02 | 0.1 |
| Comparing 54–69 with ≥70 | 1 | 1 | 1 | 1 | 0.3 | 0.6 |
K–W Kruskal–Wallis, D-T Dunnes test.
aP value generated from Kruskal–Wallis test with ties assessing whether number of injections per year differed based on final VA groups.
bP value generated from Kruskal–Wallis comparing ≥70 with <37 letters VA [n = 208].
cP value from post-hoc Dunnes test (Bonferroni adjusted by multiplying p value from each test by (m) total number of pairwise comparisons; m = 6).
Statistically significant p-values are in italic.
Subgroup analysis
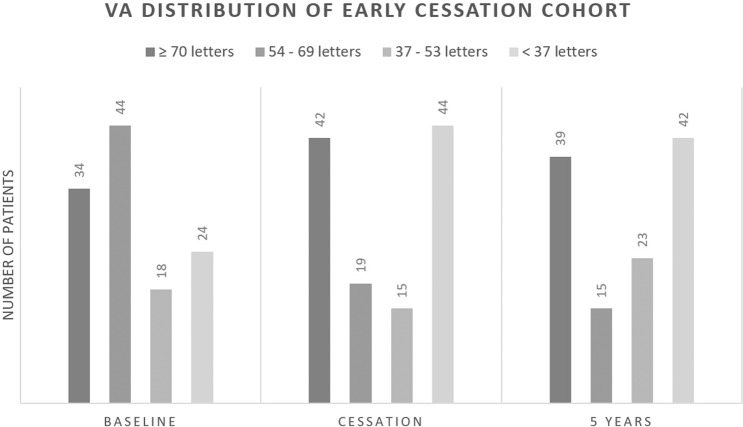
Change in VA from baseline was significantly different among the three groups with the maximum letter loss seen in Group B (−11.2 ± 25.8), followed by Group C (−3.8 ± 26.8) (p < 0.05). Eyes with continued treatment (Group A) throughout 5 years sustained a 3.2 ± 18.8 letter gain at the end of 5 years and received significantly higher cumulative number of injections. Group B received less than half number of injections as compared with Group A (14.6 ± 5.5 injections versus 31.8 ± 7.7 injections, respectively) (p < 0.05). Group C received a mean of 18.4 ± 4.6 injections. Figure 2 shows the distribution of patients in each VA category in the Group B patients to show that the decrease in VA was mainly while the patients were on treatment.
Fig. 2. Visual acuity change among eyes in Group B (early cessation group).
The proportion of patients (shown on X axis) in each VA category are plotted at 3 specific timepoints, baseline, at cessation of treatment and at 5 years (shown on Y axis).
Lost to follow-up data
Number of patients lost to follow-up per year and reasons for treatment discontinuation is shown in Fig. 1. Total of 159 patients were excluded from analysis, of which 6 patients switched to another anti-VEGF agent, and 1 developed visual loss due to endophthalmitis. Among the patients who were lost to follow-up, the mean change in VA was −2.9 (±22.2) at last follow-up with 32.2% having a VA of ≥70 letters at last follow-up. Mean change in VA defined as mean of final recorded VA minus baseline VA of patients lost to follow up per year was 3.5, 8.3, 0.8, −8.3 and −6.8 letters. The proportion of eyes with a VA of ≥70 at last follow-up in each year was 0, 42.8, 40.4, 17.6 and 35.3% and the proportion of eyes with a VA of <37 letters at last follow-up visit in each year was 0, 17.8, 19.0, 31.3 and 35.3%.
Discussion
This study provides data on the 5-year outcome of aflibercept therapy for nAMD in a health system where the treatment costs are borne by the NHS. Therefore, the outcomes are not influenced by challenges in reimbursement. The results of the study showed that on average, 80% of patients with treatment naive nAMD who underwent aflibercept injections either gained VA or lost <4 letters from baseline over 5 years. These results reinforce the likelihood of maintaining good VA in these patients when continuously monitored and treated on a T&E treatment protocol [3]. The study brought up a few points.
First, the mean baseline VA of our cohort of 58.1 letters is better than those recorded in previous clinical trials and real-life data indicating that our referral pathway is efficient [5–9].
Second, in the first year, 89% of eyes received a minimum of seven injections and the outcome of our study results are similar to clinical trials and other UK real-world studies [10–12]. These results are similar to the real-life evidence provided by the RAINBOW study that evaluated aflibercept outcomes in nAMD in the French Health System and reported eyes that received irregular dosing with no loading phase, gained 5.5 letters (6.0 injections) whilst those who received loading dose gained +6.8 letters (6.6 injections)[12]. In our study, only 2 patients were lost to follow-up in the first year of therapy. These findings show that in real-life, it is possible to deliver outcomes that mirror clinical trials in first year of treatment when the dosing regimen is rigorously implemented.
Third, we evaluated the outcomes from year 2 to 5. The change in VA over 5 years was loss of 2.9 letters. Although one could argue that the results may be confounded by the ceiling effect of the baseline VA or due to the onset of structural changes in the macula caused by atrophy, fibrosis and/or outer retinal tubulations, we evaluated the relation of VA outcomes to injection frequency because this is a modifiable factor that could improve outcomes. Over 5 years, our cohort received a mean of 24 injections. Previous real-life reports have highlighted the need for more rigorous re-treatment protocols after the first year to sustain the initial VA gains [13]. In our cohort, the VA gain in the first year of +6.3 letters dropped to +3.9 in second and +1.7 letters in the third year despite a recommended T&E protocol. The number of injections in the second and third years were 5.2 and 4.1 and the cumulative injection count was 16.7 over 3 years. In the fourth and fifth years, the mean VA declined to below the baseline. In the second year, 23.7% of the eyes in this study received ≤3 injections. In contrast, the proportion of patients receiving ≤3 in week 52–96 in the VIEW 1 and 2 studies was 48%. Mean number of injections received in the second year was higher in our study when compared with the 96 week results of the VIEW studies (5.9 versus 4.2, respectively)[14]. The VA gain from baseline among the eyes receiving ≤3 injections in the second year of study was only +1.2 letters against the +3.9 letter gain in the rest of the cohort receiving a mean of 5.9 injections. By 5 years, the mean visual loss was significantly higher at −6.5 letters in the ≤3 injection cohort versus −2.9 letters in the rest of the eyes. A similar pattern of poorer visual outcomes for patients who received ≤4 injections was observed when compared with those who received ≥5 injections. These results suggest that even in a T&E regimen, quarterly capped dosing may be necessary to ensure sustained improved VA. In addition, our study shows that on average, a minimum of five injections are required in the second to fifth year within a strictly adhered T&E protocol to retain the VA gains in the first year.
Our study also showed a gain of 0.6 letters for each additional injection from baseline and >20 injections over 5 years resulted in a visual gain of 8 letters when adjusted for baseline VA and age. Despite significant evidence that a reactive approach using PRN regime results in fewer injections and inferior visual outcomes for patients over long term follow up, this study shows that decreased injection frequency in a T&E regimen also results in poor VA outcomes [15–18]. Therefore, despite a paradigm shift from PRN to a T&E regimen, the study results emphasise the need for a proactive approach of an average of ≥5 injections each year up to 5 years to sustain initial VA gains and reduce the “efficacy-gap” seen between clinical trial results and real-world data.
The injection frequency in other long-term studies on anti-VEGF in nAMD show that although natural disease progression is inevitable, frequency of injections needs to be sustained on a strict protocol to attain maximal visual potential for our patients [19, 20].
There is significant interest to decrease treatment burden by increasing dosing intervals with new trial designs attempting to extend injection frequency to 16 weeks under a T&E protocol. The HAWK and HARRIER trials compared brolucizumab, a single-chain antibody fragment that inhibits VEGF-A, with aflibercept to treat neovascular AMD. Brolucizumab was non-inferior to aflibercept in VA at week 48, and more than 50% of brolucizumab treated eyes were maintained on 12 weekly dosing intervals through week 48. Due to the trial design, it is challenging to decipher the visual outcomes of those who were maintained on more frequent dosing compared with 12 weekly dosing [19, 20]. A recent report on recommendations for aflibercept T&E pathway by UK experts also recommends extension up to 12 weeks from fifth injection. Following that if the patient has not lost ≥1 line vision and has a dry OCT then the panel recommends that extension can be tried up to a maximum of 16 weeks [21]. Our study results caution against such extensions. To deliver five injections a year to maintain VA over 5 years, an ideal treatment interval is on average 10 weeks if disease activity does not mandate a shorter frequency.
We observed that apart from the number of injections and the regime followed the continuity of treatment also contributed to the final visual results. The subgroup analysis indicated that best visual outcomes were noted in the group that received injections without interruption throughout the course of follow-up. The worse visual results were seen in eyes that stopped treatment early and at any time due to any reason but still in follow-up. The VA gains corresponded to the number of injections with Group A receiving almost double injections than Group B. Keeping in mind the chronicity of the disease and the fact that the natural history of the disease continues its course, these results further re-inforce the substantial role of sustained anti-VEGF therapy and monitoring in patients with nAMD.
The limitation of our study is the retrospective nature and that we have not evaluated the anatomical outcomes of these patients. There is evidence that subretinal fluid may in fact be exuding from choroidal neovascularisation that is nurturing the outer retina and so it may be appropriate to defer treatment in these cases. However, currently, it is difficult to decipher the protective element of choroidal neovascularisation and future analysis on the fluid status of these eyes may possibly provide a solution to this conundrum. Additionally, even though the protocol mandates T&E for all the patients, the study shows that it is challenging to adhere to a T&E protocol over 5 years and that clinician discretion plays a more significant role with increasing duration of therapy. Nevertheless, our study shows that we do need to continue on an aggressive treatment protocol to maintain VA outcomes through to 5 years.
Our study also has multiple other strengths: it is the largest study on long-term assessment of aflibercept in nAMD eyes over 5 years where patients in all retinal services within a single institution follow the same treatment protocol in the real-world setting. All eyes received treatment in the same centre allowing for same recommendations for starting treatment, same methodology for VA measurement, injection protocols and management strategy. All eyes were treatment-naive thereby excluding the confounding effect of any other treatments/switches. We also attempted to minimise potential for bias by reporting data on consecutively collected patients.
In conclusion, aflibercept therapy administered over 5 years offers good visual outcomes in nAMD eyes. However, persistent, proactive and early treatment is necessary to achieve and maintain good VA over 5 years.
Summary
What was known before
Aflibercept therapy administered over long-term offers good visual outcomes in neovascular AMD eyes.
What this study adds
We emphasise the need for a proactive approach of an average of five or more injections each year up to 5 years to sustain initial VA gains and reduce the “efficacy-gap” seen between clinical trial results and real-world data.
To deliver five injections a year to maintain visual acuity over 5 years, an ideal treatment interval is on average 10 weeks if disease activity does not mandate a shorter frequency.
The worse visual results are seen in eyes that stop treatment early due to any reason.
Acknowledgements
I would like to thank the Moorfields Medical Retina Group for their contribution and collation of patients. The research was supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
Compliance with ethical standards
Conflict of interest
SS has received research grants, attended advisory board meetings and received honorarium from Bayer, Allergan, Novartis, Roche, Boehringer Ingleheim, Heidelberg Engineering, Optos, Apellis, Ophtea, Oxurion.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosenfeld PJ. Bevacizumab versus ranibizumab for AMD. N. Engl J Med. 2011;364:1966–7. doi: 10.1056/NEJMe1103334. [DOI] [PubMed] [Google Scholar]
- 2.Talks JS, Lotery AJ, Ghanchi F, Sivaprasad S, Johnston RL, Patel N, et al. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology. 2016;123:337–43.. doi: 10.1016/j.ophtha.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Eleftheriadou M, Gemenetzi M, Lukic M, Sivaprasad S, Hykin PG, Hamilton RD, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: evidence from a clinical setting. Ophthalmol Ther. 2018;7:361–8. doi: 10.1007/s40123-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eleftheriadou M, Vazquez-Alfageme C, Citu CM, Crosby-Nwaobi R, Sivaprasad S, Hykin P, et al. Long-term outcomes of aflibercept treatment for neovascular age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2017;174:160–8. doi: 10.1016/j.ajo.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa K, Oishi A, Hata M, Miyake M, Ooto S, Yamashiro K, et al. Four-year outcome of aflibercept for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci Rep. 2019;9:3620. doi: 10.1038/s41598-019-39995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser PK, Singer M, Tolentino M, Vitti R, Erickson K, Saroj N, et al. Long-term safety and visual outcome of intravitreal aflibercept in neovascular age-related macular degeneration: VIEW 1 extension study. Ophthalmol Retina. 2017;1:304–13.. doi: 10.1016/j.oret.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Framme C, Eter N, Hamacher T, Hasanbasic Z, Jochmann C, Johnson KT, et al. Aflibercept for patients with neovascular age-related macular degeneration in routine clinical practice in germany: twelve-month outcomes of PERSEUS. Ophthalmol Retina. 2018;2:539–49.. doi: 10.1016/j.oret.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Budzinskaya MV, Plyukhova AA, Toropygin SG. [Modern view on the treatment of wet-AMD patients]. Vestn Oftalmol. 2019;135:107–15. [DOI] [PubMed]
- 9.Ohji M, Okada AA, Takahashi K, Kobayashi M, Terano Y, editors. Two different treat and extend dosing regimens of intravitreal aflibercept for wAMD in Japanese patients: 52 week results of the ALTAIR study. Presentation at the 17th European Society of Retina Specialists (EURETINA) Congress; Barcelona: Euretina Meeting; 2017.
- 10.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.DeCroos FC, Reed D, Adam MK, Salz D, Gupta OP, Ho AC, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.. doi: 10.1016/j.ajo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Weber M, Velasque L, Coscas F, Faure C, Aubry I, Cohen SY. Effectiveness and safety of intravitreal aflibercept in patients with wet age-related macular degeneration treated in routine clinical practices across France: 12-month outcomes of the RAINBOW study. BMJ Open Ophthalmol. 2019;4:e000109. doi: 10.1136/bmjophth-2017-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almuhtaseb H, Johnston RL, Talks JS, Lotery AJ. Second-year visual acuity outcomes of nAMD patients treated with aflibercept: data analysis from the UK Aflibercept Users Group. Eye. 2017;31:1582–8. doi: 10.1038/eye.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Lanzetta P, Loewenstein A. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73.. doi: 10.1007/s00417-017-3647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1418–31. doi: 10.1097/IAE.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 17.Oubraham H, Cohen SY, Samimi S, Marotte D, Bouzaher I, Bonicel P, et al. Inject and extend dosing versus dosing as needed: a comparative retrospective study of ranibizumab in exudative age-related macular degeneration. Retina. 2011;31:26–30. doi: 10.1097/IAE.0b013e3181de5609. [DOI] [PubMed] [Google Scholar]
- 18.Lee AY, Lee CS, Egan CA, Bailey C, Johnston RL, Natha S, et al. UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol. 2017;101:1683–8. doi: 10.1136/bjophthalmol-2016-309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124:1296–304.. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 20.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Ross AH, Downey L, Devonport H, Gale RP, Kotagiri A, Mahmood S, et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye (Lond). 2020. 10.1038/s41433-019-0747-x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]