Abstract
Global opioid use and misuse remains high, despite efforts to decrease rates of prescribing and diversion. Chronic exposure to opioids, particularly during critical periods of development, can lead to long-lasting effects, including effects that may extend to future generations. Using a rodent model, we have demonstrated significant transgenerational effects of female adolescent morphine exposure, despite the absence of in utero drug exposure. While these effects have been observed in both sexes, effects on anxiety-like behavior were only observed in F1 females. The current study was designed to examine both inter- and transgenerational effects of adolescent morphine exposure on anxiety-like behavior. Female Sprague Dawley rats were administered increasing doses of morphine (5-25 mg/kg s.c.) or saline for 10 days during adolescence (PND30-39). Adult diestrous female offspring (MORF1 or SALF1) and grand offspring (F2) were tested for anxiety-like behavior using the elevated plus maze (EPM). F1 females cross-fostered to donor mothers were also examined. The results show that MORF1 and MORF2 females spend significantly more time on the open arms of the EPM compared to SALF1 controls, an effect that persisted in cross-fostered females. Additional studies demonstrate that this effect is estrous cycle dependent, as decreased anxiety-like behavior was observed in diestrus, while increased anxiety-like behavior was observed in estrus. These behavioral effects were not associated with any differences in circulating corticosterone either at baseline or following EPM testing. Thus, female adolescent morphine exposure alters the regulation of anxiety-like behavior in an estrous-dependent manner and this effect persists in the F2 generation.
Keywords: Elevated Plus Maze, Corticosterone, Estrous Cycle, Sex Differences, Opioids, Intergenerational
1. Introduction
Both the medical and non-medical use of opioids remains at historic levels, despite significant efforts to decrease drug diversion and reduce the number of opioid prescriptions [1–3]. Beyond the risk of addiction and overdose, the potential consequences of widespread opioid use on other health outcomes remain unknown [4]. And yet, when one considers the important modulatory role of endogenous opioids, chronic exposure to exogenous opioids could have long-lasting effects, particularly if exposure occurs during critical developmental windows. Animal models have proven effective in revealing which systems and/or processes are vulnerable to the effects of opioids during development. While numerous preclinical studies have revealed significant developmental effects of opioids during pre- and postnatal development [5, 6], fewer studies have examined effects during adolescence [7, 8]. This gap in our knowledge is unfortunate given the increased number of adolescent males and females prescribed opioids and the important modulatory role that endogenous opioids play during this developmental stage [9, 10].
In rodents, adolescent development includes the onset of puberty, an event that can be delayed by exposure to exogenous opioids [11, 12]. Moreover, in both adult males and females, endogenous opioids serve to inhibit luteinizing hormone (LH) secretion [13–15], suggesting a role for endogenous opioids in regulation of reproductive function. Based on these known effects of endogenous opioids, we previously hypothesized that in females, adolescent exposure to opioids leads to alterations in the development of the reproductive axis. To that end, we have conducted a number of studies examining the effects of female adolescent morphine exposure on various aspects of female reproduction [8, 16], including effects observed in the next generation [17, 18]. Interestingly, while adolescent morphine exposure did not impact fertility or fecundity, or disrupt the quality of maternal behavior, significant phenotypic effects were observed in both male and female offspring [19]. These effects included alterations in anxiety-like behavior that are specific to females; however, we have observed both increased [17] and decreased [20] anxiety-like behavior in the female offspring of mother’s who were exposed to morphine during adolescent development.
A number of factors may explain the divergent effects of adolescent morphine exposure on anxiety-like behavior in F1 females, including our use of two different dosing regimens and two different tasks. Our initial work, in which we reported increased anxiety-like behavior on the elevated plus maze (EPM) [17], was longer in duration (20 days) and used significantly higher doses (twice daily injections of increasing morphine doses from 2.5-50 mg/kg). In our more recent studies, we use a shorter dosing regimen (10 days) with lower overall exposure (once daily injections of increasing morphine doses from 5.0-25 mg/kg) and report decreased anxiety-like behavior, as determined using open field behavior [20]. This study, however, revealed that effects of maternal morphine exposure on F1 open field behavior was estrous cycle dependent, with decreased anxiety-like behavior in MORF1 females only observed in diestrous. Thus, it remains unclear whether the differences in anxiety-like behavior in F1 females are due to differences in the drug regimen received by their mother, the type of test used to measure anxiety-like behavior (EPM versus open field) or the influence of the estrous cycle. In addition, our prior studies on anxiety-like behavior only examined F1 animals, so whether any effects on anxiety-like behaviors persist beyond the first generation also remains unknown. Moreover, to what extent differences in the postnatal maternal environment play a role in these phenotypic variations has not been examined.
The current set of studies addresses these questions using our shorter, lower dose morphine regimen and determining effects on anxiety-like behavior as measured on the EPM during diestrous. Both F1 and F2 females were studied, as were F1 females reared by naïve donor mothers. In a separate group of F1 females, we determined whether effects on anxiety-like behavior using the EPM were estrous cycle dependent. Finally, to begin addressing potential mechanisms underlying the observed effects, we measured both basal and post-EPM corticosterone levels. Testing on the EPM is considered a psychological stressor capable of activating the HPA axis and resulting in significant release of corticosterone. A number of pre- and postnatal manipulations that lead to either increased, or decreased, anxiety-like behavior in offspring, also alter basal and/or stress-induced corticosterone release. These effects on corticosterone, however, do not always correlate with changes in anxiety-like behavior, and are often sex-specific [21–25]. For example, neonatal maternal separation increases anxiety-like behavior in both males and females but only increases stress-induced corticosterone secretion in males [21, 24]. We have previously reported significantly blunted morphine-induced corticosterone secretion in adult MORF1 and MORF2 offspring, an effect observed in males but not females [26]. Higher levels of cocaine-stimulated corticosterone, however, were observed in MORF1 females [ref]. Thus, whether alterations in activation of the HPA axis in response to EPM testing might play a role in the observed transgenerational effects on anxiety-like behavior will also be examined.
2. Materials and Methods
2.1. Animals and housing:
All animals were housed in standard acrylic laboratory cages (40 cm × 20 cm × 18 cm) at Cummings School for Veterinary Medicine at Tufts University. Animals were maintained on a 12-hour light/dark cycle with lights on at 7:00 am and all procedures were performed during the light phase. Food and water were available ad libitum, unless otherwise stated. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University and were carried out in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal distress and reduce the number of animals used.
2.2. Generation of F0, F1, and F2 animals:
For all experiments, post-natal day 23 (PND23) female Sprague-Dawley rats [Crl:CD(SD)BR] were purchased from Charles River Breeding Laboratories. All animals were housed 3-4 per cage. Beginning at PND30 females were injected (s.c.) once daily with morphine sulfate (MS) for a total of 10 days using an increasing dosing regimen with doses increased every other day (5, 5, 10, 10, 15, 15, 20, 20, 25, 25 mg/kg). Age-matched control animals received saline injections (s.c) with volumes adjusted to match those of drug-treated females as per our standard regimen. On PND 60-80 (3 to 6 weeks after their final injection), females were mated with drug-naïve colony males. Each male was placed with age-matched females (MORF0 and SALF0). Once an animal was visibly pregnant (approximately E16-E20) she was housed singly. On PND1 (day of birth = PND0) all litters were culled to ten pups (5 male, 5 female). The weight of the pups and the dam was recorded. All litters were weighed and weaned on PND21 and group housed with same-sex littermates (5/cage and then 2-3/cage based on body weight). Offspring of morphine-exposed females are designated MORF1. The current set of studies were only conducted in female offspring, as previous findings using this paradigm did not observe effects on anxiety-like behaviors in F1 males [17, 20]. To generate F2 animals (grandoffspring), naïve, adult, female F1 animals were mated with drug-naïve colony males in the same way as F0 animals. To generate donor-reared subjects, all F1 males and females were removed from their biological mothers and reared by naïve donor mothers beginning on PND1. In all studies offspring were culled to ten pups (5 male, 5 female) on PND1. All litters were weighed and weaned on PND21 and housed with same sex littermates. Offspring of saline controls are designated SALF1. F1 female animals used to generate F2 grandoffspring did not experience any behavioral manipulations. Grandoffspring are designated MORF2 and SALF2, respectively. All testing was conducted once F1 and F2 animals were at least 60 days of age. In all experiments, only 1-2 pups per litter were used in any experimental group to minimize the risk of litter effects. Offspring from 21 MORF0 litters and 24 SALF0 litters were used in these experiments.
2.3. Elevated Plus Maze (EPM):
To measure anxiety-like behavior rats were tested for 5 min on a fully automated EPM (Hamilton-Kinder; Poway, CA) consisting of two open arms (38 × 5 cm) and two closed arms (38 × 5 × 15 cm) with a central intersection (5 cm × 5 cm). The apparatus was elevated 75 cm above the floor. Ethanol (70%) was used to clean the apparatus between individual test sessions. All data were automatically collected and quantified using MotorMonitor® software (Hamilton-Kinder). Percent time spent in the open arms was used as the measure of anxiety-like behavior and basic movement measured throughout the apparatus was used to assess general activity.
2.4. Estrous Cycle Staging, Blood Collection and Corticosterone Measurement:
Estrous cycle stage was based on vaginal lavage which was conducted immediately after EPM testing. Dominant cell type was used to determine cycle stage (e.g. leukocytes for diestrous; nucleated and cornified for proestrous and estrous respectively). For a subset of subjects, blood samples were collected 5 minutes after EPM testing to determine corticosterone levels. Additional samples were collected from home cage controls (i.e. baseline) with sample collections from SALF1 and MORF1 occurring at the same time of day (all collected between 0900-1300h). For all blood collections, animals were briefly exposed to CO2 (<120 sec), decapitated, and trunk blood collected into heparinized tubes. For these subjects, vaginal lavage was performed immediately post-mortem. Blood was centrifuged and plasma was removed and frozen at −20 °C until assayed for corticosterone using radioimmunoassay. Coat-A-Count Rat Corticosterone solid-phase 125I radioimmunoassay was used for quantitative measurement of corticosterone in the subject plasma according to manufacturer’s directions (Siemens Medical Solutions Diagnostics).
2.5. Statistical Analysis:
EPM data were analyzed using t-tests comparing generational effects of F0 treatment. For studies comparing estrous cycle stage, a two-way ANOVA with F0 treatment and stage (diestrus or pro/estrous) was conducted. Corticosterone data were analyzed using two-way ANOVA with F0 treatment and time (i.e. home cage or EPM exposed) as the condition factors. F1 and F2 generations were analyzed separately as these data were collected at different times. Tukey’s tests were used for post hoc analysis and significance was defined as p < 0.05.
3. Results
As in our previous studies using this model, there were no differences in litter size, sex distribution, or body weights at birth or at weaning (all p’s>0.5). When tested on the EPM as adults, the percent of time that females spent exploring the open arms of the EPM was significantly affected by F0 treatment. As shown in Figure 1a, MORF1 females spent significantly more time (t[42] = 2.19; p<0.05) on the open arms then their SALF1 control counterparts. Similar effects were observed in F2 females (i.e. grandoffspring of adolescent exposed dams). As shown in figure 1b, MORF2 females also spent more time exploring the open arms of the EPM (t[46]=2.1; p<0.05). There were no significant effects on basic movement as measured by photobeam breaks in either group of F1 females (SALF1 = 845±37.5; MORF1 = 855± 20.9, t[42] = −0.24, p=0.8) or F2 (SALF2 = 889±30.6; MORF1 = 924± 27.9, t[46] = −0.85, p=0.4) supporting an effect on anxiety-like behavior rather than a general shift in locomotor activity. Similar effects were observed in MORF1 females reared by donor mothers. As shown in Figure 2, MORF1 females tended to spend more time in the open arm when compared to their SALF1 counterparts, although this effect failed to reach significance (t[23]= 1.69; p=0.054). Again, no effects on basic movement were observed (SALF1 = 692±37.6; MORF1 = 624.9±65.3, t[23] = −0.90, p=0.39). These findings suggest that changes in the postnatal environment are not the primary mechanism for transmission of the intergenerational effect of adolescent morphine exposure on anxiety-like behavior.
Figure 1.
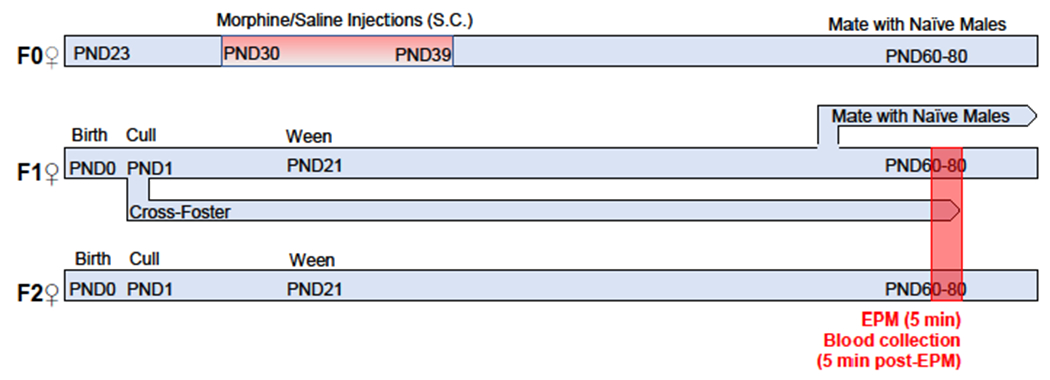
Experimental schematic depicting the generation of experimental subjects and the timing of data collection.
Figure 2.
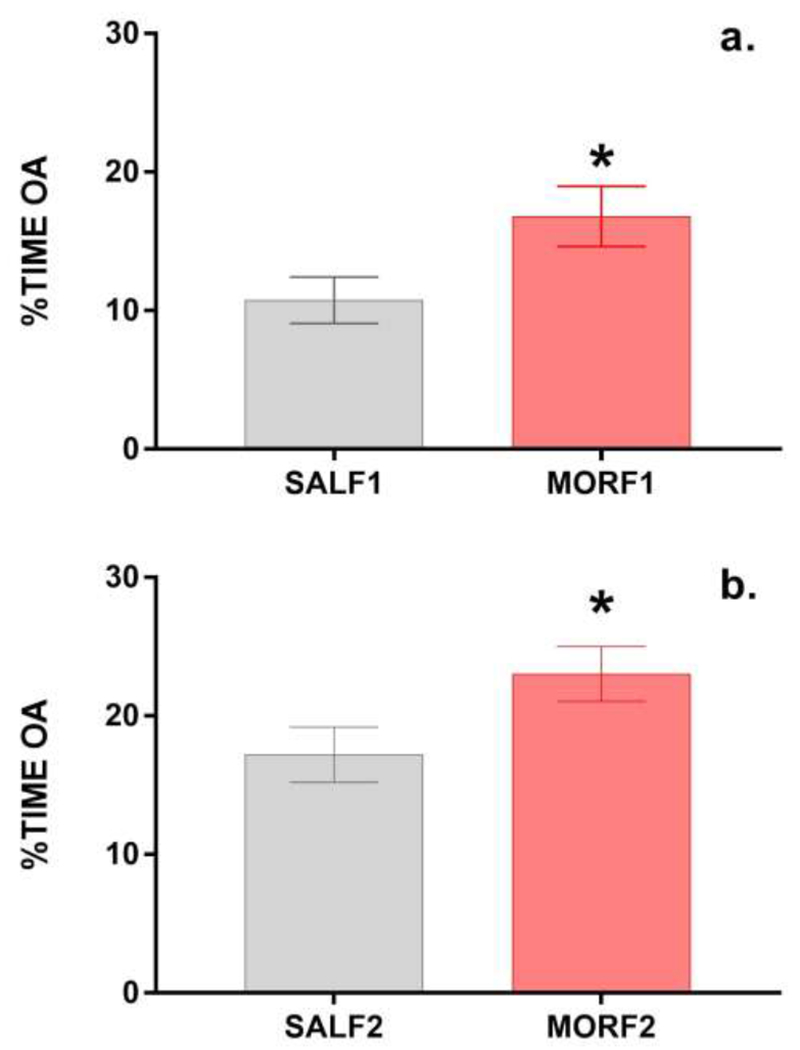
Mean±SEM percent of time spent on the open arms of the EPM in F1 (Panel a) and F2 (Panel b) females. SALF1 (N=22); MORF1 (N=22); SALF2 (N=22); MORF2 (N=26); *p<0.05.
While significant effects on anxiety-like behavior were observed across multiple cohorts of F1 and F2 females, EPM data displayed variability in both SAL and MOR groups. As previous studies have reported significant differences in anxiety-like behavior based on estrous cycle stage[27] and we have previously reported estrous-cycle dependent effects in F1 females using a different task[20], we conducted a follow-up study in a separate cohort of F1 females to determine whether the observed effects on the EPM were estrous-cycle dependent. While there were no main effects of F1 estrous cycle stage (F[1,36]=1.14;p=0.29) or F0 adolescent exposure (F[1,36]=0.02;p=0.87), there was a significant interaction (F[1,36]=12.52;p<0.01). As shown in Figure 3a, post-hoc analyses revealed that the direction of the effect of F0 adolescent exposure on F1 female anxiety-like behavior was cycle dependent with decreased anxiety-like behavior observed during diestrus and increased anxiety-like behavior observed during proestrus/estrus (both p’s <0.05). SALF1 females displayed the expected decrease in anxiety-like behavior during proestrus/estrus when compared to diestrus (p<0.01), while MORF1 females did not demonstrate this effect (p=0.18). Analysis of basic movement, however, suggest that the effect of estrous cycle on open arm exploration in SALF1 females could be influenced by cycle-dependent effects on overall activity. Thus, while there was no main effect of F0 adolescent exposure on F1 activity (F[1,36]=0.13;p=0.72), there was a significant main effect of F1 estrous cycle stage (F[1,36]=4.1;p<0.05) and a trend toward a significant interaction (F[1,36]=3.7;p=0.06). As shown in Figure 3b, increased activity was observed in SALF1 females in proestrous/estrous when compared to SALF1 diestrus females (p<0.05). No effect of estrous cycle stage on activity was observed in MORF1 females (p=0.99).
Figure 3.
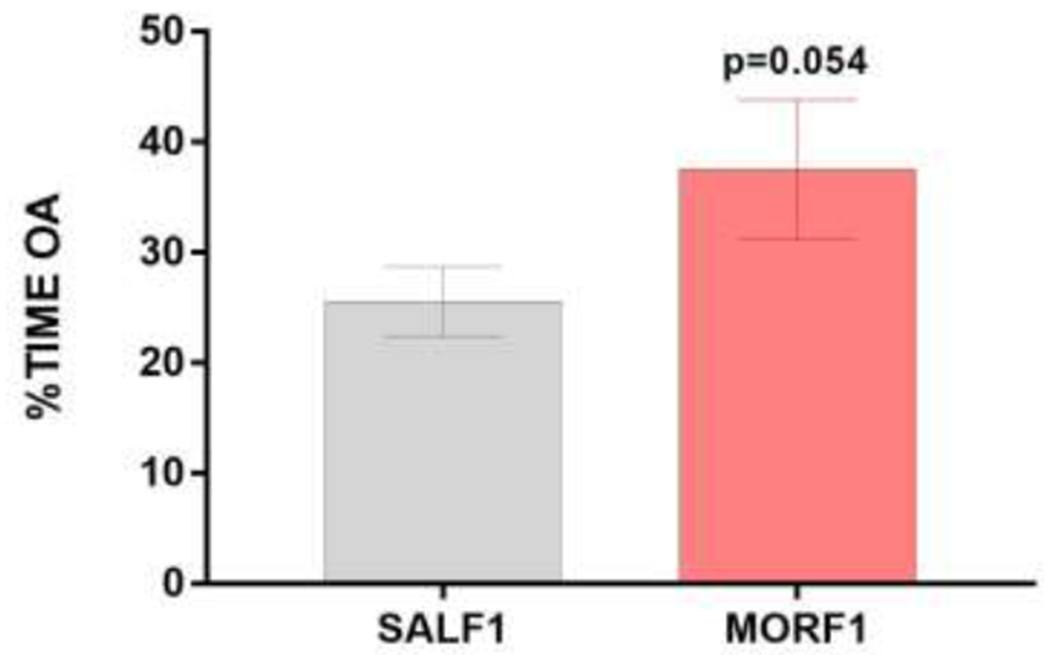
Mean±SEM percent of time spent on the open arms of the EPM in F1 females reared by naïve donor mothers. SALF1 (N=13) and MORF1 (N=12), p=0.054.
In addition to serving as a measure of anxiety-like behavior, EPM exposure is a well-validated psychological stressor. To determine whether F0 treatment influences the level of acute stress induced by exposure to the EPM in F1 offspring, corticosterone was measured either in home cage controls or after EPM testing (5 min) in SALF1 and MORF1 females. Data were collected in both diestrous (SALF1 N=14; MORF1 N=11) and proestrous/estrous (SALF1 N=13; MORF1 N=16) females; however, a three-way ANOVA including F0 treatment, F1 estrous cycle, and F1 testing condition revealed no significant main effect of F1 estrous cycle, nor any interaction between F0 treatment or F1 testing condition and estrous cycle (all p’s>0.3), thus data were collapsed across F1 estrous cycle stage. As shown in figure 4, no significant main effect of F0 treatment was observed (F[1,50]=0.004;p=0.95) nor was there a significant F0 treatment by F1 testing condition interaction (F[1,50]=0.31;p=0.58). There was, however, a significant main effect of F1 testing condition (F[1,50]=144.9;p<0.001) with increased corticosterone secretion observed in all F1 females following EPM testing.
Figure 4.
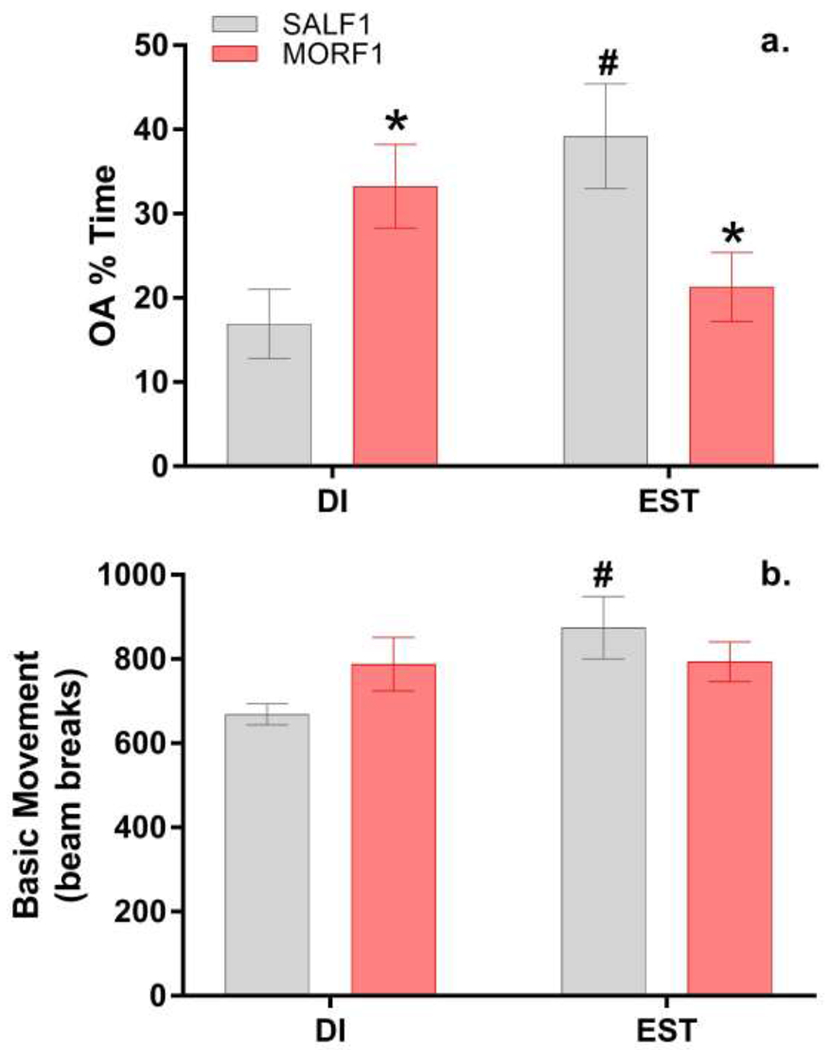
Mean±SEM percent of time spent on the open arms of the EPM (Panel a) and mean±SEM number of photobeam breaks activated by locomotion throughout the entire EPM (Panel b) during either diestrous (SALF1 N=12; MORF1 N=9) or estrous (SALF1 N=9; MORF1 N=10) females; *p<0.05 as compared to SALF1 within estrous cycle phase; #p<0.05 as compared to diestrous SALF1 females.
4. Discussion
The current set of studies demonstrate significant transgenerational effects of adolescent morphine exposure on anxiety-like behaviors in females. In the current set of studies, most females were examined during diestrus and across multiple independent cohorts of both F1 and F2 females. We observed a significant decrease in anxiety-like behavior, which aligns with our previous open field data in F1 females born to F0 dams exposed to morphine for 10 days during adolescence and extends these findings to the F2 generation. Thus, MORF2 females (offspring of MORF1 females mated to naïve males), also demonstrate decreased anxiety-like behavior during diestrous. To determine whether these effects are mediated by changes in postnatal environment experienced by F1 females, we conducted a separate study using donor females. Cross fostering both SALF1 and MORF1 to naïve, lactating dams did not reverse these effects. While the decreased percent time on the open arm only approached significance (p-0.054), these strongly suggest that postnatal maternal care and/or substances passed from the dam to the pups via milk after PND1, do not underlie the observed effects on EPM behavior. In addition, there were no differences in locomotor behavior, indicating a selective effect on systems regulating anxiety-like behavior as measured using the EPM.
These findings appear to suggest a less anxious phenotype. Indeed, using the same shorter morphine regimen as that used in the current study, we report decreased anxiety-like behavior in diestrus F1 females as measured on using open field [20]. Yet, we previously reported increased anxiety-like behavior in female offspring of F0 dams exposed to higher doses of morphine for a longer period during adolescence [17]. Those animals, however, were all tested during estrous. To determine whether similar EPM behaviors would be observed in estrous following the shorter F0 adolescent treatment regimen, the current study examined behavior on the EPM in a separate cohort of F1 females in either diestrus or estrus. Similar to previous observations, diestrus MORF1 females spent more time on the open arms of the EPM than diestrus SALF1 females while estrus MORF1 females spent less time on the open arm when compared to estrus SALF1 females. These data support the hypothesis that morphine exposure in F0 adolescent females alters anxiety-like behavior in their female offspring and that the direction of this effect is dependent upon the phase of the estrous cycle.
In contrast to MORF1 females, diestrus SALF1 females spent less time on the open arm of the EPM when compared to estrus SALF1 females, replicating previous studies in the literature [28]. The influence of estrous cycle on EPM behavior, however, is equivocal. Recent findings exploring interactions between sex, estrous cycle, lighting, and novelty in female rats, suggest that while estrous has been associated with reduced anxiety-like behavior on exploratory tasks, some of these effects are likely due to a general increase in activity [29]. Yet, another recent set of studies found that there was no relationship between measures of anxiety-like behavior and overall activity [30]. In the current study, estrus SALF1 females had higher overall levels of activity on the EPM when compared to diestrus SALF1 females, leaving open the possibility that cycle effects on anxiety-like behavior in SALF1 females were influenced by activity levels. It is important to note, however, that no such effects on activity were observed in MORF1 females; suggesting that in MORF1 females, cycle-mediated effects on anxiety-like behavior are not likely mediated by alterations in general activity.
One factor that can regulate EPM exploration is regulation of the HPA axis [31] with trait anxiety significantly influenced by stress reactivity [32]. For example, environmental enrichment, in both high and low anxiety rat strains, increases open arm exploration while decreasing stress-induced corticosterone [33]. And yet, other studies suggest that blunted stress-induced corticosterone is associated with high anxiety phenotypes [34]. Thus, the influence of the HPA axis on anxiety-like behavior is complex. To determine whether a shift in the HPA axis, either at baseline or following the acute stress of the EPM, plays a role in the shift in anxiety-like behavior observed in MORF1 females, we measured both home cage and post-EPM corticosterone. No differences between SALF1 and MORF1 were observed, nor were there any effects of the estrous cycle. The absence of differences in corticosterone levels between females in diestrus and estrus was not unexpected, as previous studies report significant effects of the estrous cycle on corticosterone are largely driven by increased levels in the afternoon of proestrus [35]. These findings, however, do suggest that dysregulation of the HPA axis is not the mechanism underlying the observed differences in anxiety-like behavior in F1 females at either phase of the estrous cycle.
The female specific nature of this transgenerational effect on anxiety-like behavior, estrous cycle dependence, and the apparent lack of baseline differences in the HPA axis points to an epigenetic effect on endocrine regulation of anxiety-like behavior. Whether the effects of the estrous cycle suggest a primarily organization or activation effect remains to be determined. Effects on anxiety-like behavior, however, have been associated with a number of perturbations during development ranging from maternal stress to high fat diet [36–38]. Significant alterations in the amygdala, as well as in other sexually dimorphic brain regions, are observed in such models and several demonstrate a similar increase in anxiety-like behavior in female offspring [39]. Interestingly, prenatal manipulations that impact the placenta have been shown to lead to a similar phenotype [40]. Thus, one potential mechanism underlying the transmission of these effects is an alteration in the development of the placenta that is triggered by adolescent exposure to morphine. While we have not examined placental function in either F0 or F1 females, we have observed significant alterations in gene expression in the medial basal hypothalamus in F0 females both pre- and postpartum [8]. Of note, this brain region plays an important role in regulating the HPA axis during pregnancy, which could have significant implications for the development of the placenta and the fetus [41]. Future studies will explore such potential mechanisms with an emphasis on changes in the placenta related to the maternal HPA axis.
From a broader, evolutionary perspective, to what extent these transgenerational effects impact overall fitness remains an important unanswered question. In females, increased exploratory behavior coincident with reduced fear is an important aspect of mating optimally timed to correspond with periods of peak fertility [42, 43]. And yet, this pattern appears to be reversed in MORF1 females. While we did not observed any effects on fertility rates when mating MORF1 females with naïve males to generate F2 subjects, mating behavior in the laboratory setting does not require the behavioral complexity necessary in a more naturalistic environment. Indeed, in the wild female rats often set the pace of mating and compete for the dominant male [44]. We have observed increased rough and tumble play in juvenile MORF1 females [45], but to what extent these alterations in social behavior affect sex behaviors or other aspects of reproductive fitness are unknown. Still, the nature of the current findings, demonstrating that F0 adolescent opioid exposure can impact endocrine regulation of critical approach/avoidance behaviors for two generations, suggest potentially far-reaching effects of opioids that extend beyond effects on the user.
5. Conclusions
The current set of studies provides evidence for a transgenerational effect of adolescent morphine exposure on anxiety-like behavior in female offspring. These effects are likely mediated by changes in the prenatal environment impacting developmental trajectories, as they persist even when offspring are reared by donor females. Moreover, these effects continue to be observed in F2 females indicating a shift that persists well beyond the effects of initial drug exposure. These effects due not appear to be due to a shift in stress responsiveness but they are significantly modulated by gonadal hormones. The mechanisms underlying transmission as well as the specific neural modifications mediating these effects remain to be determined.
Figure 5.
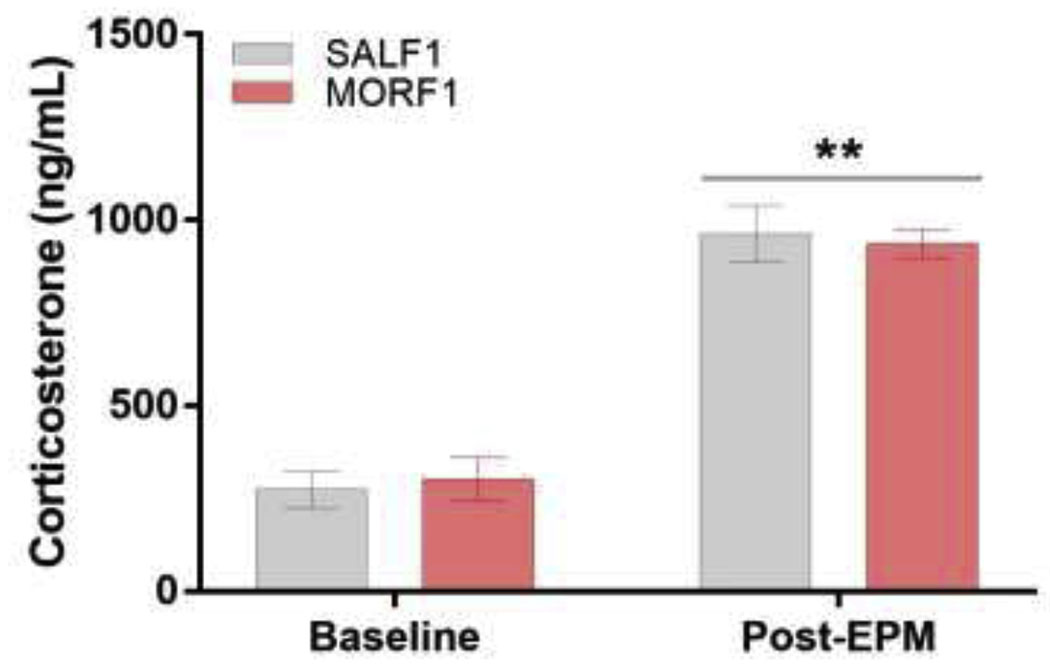
Mean±SEM levels of corticosterone (ng/ml) in plasma at baseline (SALF1 N=14; MORF1 N=14) and 5 minutes after EPM testing (SALF1 N=10; MORF1 N=12) in F1 females. **p<0.001 as compared to baseline collapsed across F0 treatment groups.
Highlights.
Morphine exposure in adolescent F0 female rats decreases anxiety-like behavior measured on the elevated plus maze (EPM) in F1 and F2 female offspring.
Effects on anxiety-like behavior in F1 females are maintained even when offspring are raised by donor mothers.
Effects on anxiety-like behavior in F1 females are estrous cycle dependent.
These effects are not associated with changes in basal or stress-induced corticosterone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ansari B, Tote KM, Rosenberg ES, Martin EG, A Rapid Review of the Impact of Systems-Level Policies and Interventions on Population-Level Outcomes Related to the Opioid Epidemic, United States and Canada, 2014-2018, Public Health Rep, 135 (2020) 100S–127S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Althoff KN, Leifheit KM, Park JN, Chandran A, Sherman SG, Opioid-related overdose mortality in the era of fentanyl: Monitoring a shifting epidemic by person, place, and time, Drug Alcohol Depend, 216 (2020) 108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Silver ER, Hur C, Gender differences in prescription opioid use and misuse: Implications for men’s health and the opioid epidemic, Prev Med, 131 (2020) 105946. [DOI] [PubMed] [Google Scholar]
- [4].Gilardi F, Augsburger M, Thomas A, Will Widespread Synthetic Opioid Consumption Induce Epigenetic Consequences in Future Generations?, Front Pharmacol, 9 (2018) 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Byrnes EM, Vassoler FM, Modeling prenatal opioid exposure in animals: Current findings and future directions, Front Neuroendocrinol, 51 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fodor A, Timar J, Zelena D, Behavioral effects of perinatal opioid exposure, Life Sci, 104 (2014) 1–8. [DOI] [PubMed] [Google Scholar]
- [7].Cicero TJ, Adams ML, Giordano A, Miller BT, O’Connor L, Nock B, Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring, J Pharmacol Exp Ther, 256 (1991) 1086–1093. [PubMed] [Google Scholar]
- [8].Byrnes EM, Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus, Brain Res, 1190 (2008) 186–192. [DOI] [PubMed] [Google Scholar]
- [9].Sizonenko PC, Physiology of puberty, J Endocrinol Invest, 12 (1989) 59–63. [PubMed] [Google Scholar]
- [10].Genazzani AR, Bernardi F, Monteleone P, Luisi S, Luisi M, Neuropeptides, neurotransmitters, neurosteroids, and the onset of puberty, Ann N Y Acad Sci, 900 (2000) 1–9. [DOI] [PubMed] [Google Scholar]
- [11].MacDonald MC, Wilkinson M, Occurrence of sexual maturation in chronic opiate-treated female rats, J Endocrinol, 129 (1991) 253–259. [DOI] [PubMed] [Google Scholar]
- [12].Cicero TJ, Meyer ER, Miller BT, Bell RD, Age-related differences in the sensitivity of serum luteinizing hormone to prototypic mu, kappa and delta opiate agonists and antagonists, J Pharmacol Exp Ther, 246 (1988) 14–20. [PubMed] [Google Scholar]
- [13].Cicero TJ, Schainker BA, Meyer ER, Endogenous opioids participate in the regulation of the hypothalamus-pituitary-luteinizing hormone axis and testosterone’s negative feedback control of luteinizing hormone, Endocrinology, 104 (1979) 1286–1291. [DOI] [PubMed] [Google Scholar]
- [14].Pfeiffer A, Herz A, Endocrine actions of opioids, Horm Metab Res, 16 (1984) 386–397. [DOI] [PubMed] [Google Scholar]
- [15].Cicero TJ, Schmoeker PF, Meyer ER, Miller BT, Bell RD, Cytron SM, Brown CC, Ontogeny of the opioid-mediated control of reproductive endocrinology in the male and female rat, J Pharmacol Exp Ther, 236 (1986) 627–633. [PubMed] [Google Scholar]
- [16].Byrnes EM, Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats, Pharmacol Biochem Behav, 80 (2005) 445–451. [DOI] [PubMed] [Google Scholar]
- [17].Byrnes EM, Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring, Psychopharmacology (Berl), 182 (2005) 537–544. [DOI] [PubMed] [Google Scholar]
- [18].Vassoler FM, Byrnes EM, Pierce RC, The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects, Neuropharmacology, 76 Pt B (2014) 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM, Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure, Neuropharmacology, 113 (2017) 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM, Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring, Behav Brain Res, 218 (2011) 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG, Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats, Pharmacol Biochem Behav, 73 (2002) 131–140. [DOI] [PubMed] [Google Scholar]
- [22].Durand M, Sarrieau A, Aguerre S, Mormede P, Chaouloff F, Differential effects of neonatal handling on anxiety, corticosterone response to stress, and hippocampal glucocorticoid and serotonin (5-HT)2A receptors in Lewis rats, Psychoneuroendocrinology, 23 (1998) 323–335. [DOI] [PubMed] [Google Scholar]
- [23].Harvey BH, Regenass W, Dreyer W, Moller M, Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: Role of corticosterone, oxytocin, and vasopressin, J Psychopharmacol, 33 (2019) 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brummelte S, Lieblich SE, Galea LA, Gestational and postpartum corticosterone exposure to the dam affects behavioral and endocrine outcome of the offspring in a sexually-dimorphic manner, Neuropharmacology, 62 (2012) 406–418. [DOI] [PubMed] [Google Scholar]
- [25].Simone JJ, Baumbach JL, McCormick CM, Sex-specific effects of CB1 receptor antagonism and stress in adolescence on anxiety, corticosterone concentrations, and contextual fear in adulthood in rats, Int J Dev Neurosci, 69 (2018) 119–131. [DOI] [PubMed] [Google Scholar]
- [26].Vassoler FM, Toorie AM, Byrnes EM, Transgenerational blunting of morphine-induced corticosterone secretion is associated with dysregulated gene expression in male offspring, Brain Res, 1679 (2018) 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, Estrous cycle influences the response of female rats in the elevated plus-maze test, Physiol Behav, 74 (2001) 435–440. [DOI] [PubMed] [Google Scholar]
- [28].ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS, Relevance of stress and female sex hormones for emotion and cognition, Cell Mol Neurobiol, 32 (2012) 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miller CK, Halbing AA, Patisaul HB, Meitzen J, Interactions of the estrous cycle, novelty, and light on female and male rat open field locomotor and anxiety-related behaviors, Physiol Behav, 228 (2020) 113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL, Sex differences in anxiety-like behaviors in rats, Physiol Behav, 211 (2019) 112670. [DOI] [PubMed] [Google Scholar]
- [31].Chekmareva NY, Umriukhin AE, Landgraf R, Sotnikov SV, Inborn vs. acquired anxiety in cross-breeding and cross-fostering HAB/LAB mice bred for extremes in anxiety-related behavior, Behav Neurosci, 133 (2019) 68–76. [DOI] [PubMed] [Google Scholar]
- [32].Jakovcevski M, Schachner M, Morellini F, Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety, Genes Brain Behav, 7 (2008) 235–243. [DOI] [PubMed] [Google Scholar]
- [33].Ravenelle R, Byrnes EM, Byrnes JJ, McInnis C, Park JH, Donaldson ST, Environmental enrichment effects on the neurobehavioral profile of selective outbred trait anxiety rats, Behav Brain Res, 252 (2013) 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hassan W, Gomes Vde C, Pinton S, Batista Teixeira da Rocha J, Landeira-Fernandez J, Association between oxidative stress and contextual fear conditioning in Carioca high- and low-conditioned freezing rats, Brain Res, 1512 (2013) 60–67. [DOI] [PubMed] [Google Scholar]
- [35].Atkinson HC, Waddell BJ, Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle, Endocrinology, 138 (1997) 3842–3848. [DOI] [PubMed] [Google Scholar]
- [36].Benmhammed H, El Hayek S, Berkik I, Elmostafi H, Bousalham R, Mesfioui A, Ouichou A, El Hessni A, Animal Models of Early-Life Adversity, Methods Mol Biol, 2011 (2019) 143–161. [DOI] [PubMed] [Google Scholar]
- [37].Fine R, Zhang J, Stevens HE, Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders, Mol Psychiatry, 19 (2014) 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sullivan EL, Nousen EK, Chamlou KA, Maternal high fat diet consumption during the perinatal period programs offspring behavior, Physiol Behav, 123 (2014) 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weinstock M, Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update, Stress, 14 (2011) 604–613. [DOI] [PubMed] [Google Scholar]
- [40].Shook LL, Kislal S, Edlow AG, Fetal brain and placental programming in maternal obesity: A review of human and animal model studies, Prenat Diagn, 40 (2020) 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Duthie L, Reynolds RM, Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes, Neuroendocrinology, 98 (2013) 106–115. [DOI] [PubMed] [Google Scholar]
- [42].Frye CA, Paris JJ, Infusions of bicuculline to the ventral tegmental area attenuates sexual, exploratory, and anti-anxiety behavior of proestrous rats, Pharmacol Biochem Behav, 93 (2009) 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Frye CA, Rhodes ME, Infusions of 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3alpha,5alpha-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats, J Neuroendocrinol, 18 (2006) 960–975. [DOI] [PubMed] [Google Scholar]
- [44].Mcclintock MK, Adler NT, Role of Female during Copulation in Wild and Domestic Norway Rats (Rattus-Norvegicus), Behaviour, 67 (1978) 67–96. [Google Scholar]
- [45].Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM, Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior, Front Psychiatry, 2 (2011) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]


