Abstract
Background:
High-volume systemic-to-pulmonary ductus arteriosus shunts in premature infants are associated with adverse neonatal outcomes. The role of an atrial communication (AC) in modulating effects of a presumed hemodynamically significant patent ductus arteriosus (hsPDA) is poorly studied. The objective of this study was to characterize the relationship between early AC and echocardiography indices of PDA shunt volume and clinical neonatal outcomes.
Methods:
We performed a retrospective review of preterm infants (born <32 weeks’ gestation) with echocardiography in the first postnatal week. We divided the cohort into four groups based on presence of a presumed hsPDA (≥1.5 mm vs. <1.5 mm) and AC size (≤1 mm vs. >1 mm) then compared echocardiography measures of PDA shunt volume. We also compared clinical outcomes, including chronic lung disease (CLD) and intraventricular hemorrhage (IVH) between all 4 groups.
Results:
A total of 199 preterm infants (mean birthweight and gestational age of 928 ± 632 grams and 26.6 ± 1.5 weeks, respectively) were identified; 159 infants had PDA ≥1.5 mm, of whom 52 had AC ≤1 mm and 107 had AC >1 mm. The remaining 40 infants had PDA <1.5 mm, of whom 23 had AC ≤1 mm and 17 had AC >1 mm. Infants with PDA ≥1.5 mm and AC >1 mm had higher pulmonary vein D wave velocity (p<0.05), higher left ventricular output (p<0.005), higher PDA score (p<0.001), and increased rate of reversed diastolic flow in the descending aorta (p<0.001), celiac artery (p<0.001), and middle cerebral artery (p<0.001) than infants with either PDA <1.5mm or PDA ≥1.5 mm and AC ≤1 mm. There was no difference in incidence of IVH, but infants with PDA ≥1.5 mm and AC >1 mm had higher risk of composite outcome of CLD or death prior to hospital discharge (p<0.05).
Conclusion:
Echocardiography evidence of AC >1 mm in patients with a PDA ≥1.5 mm, during the first postnatal week, may be a marker of a more pathologic hsPDA in premature infants. Future investigations should evaluate if early identification and treatment of patients with both high-volume PDA and larger atrial level communications may help mitigate adverse outcomes, such as CLD or death, in this high-risk patient population.
Keywords: patent ductus arteriosus, atrial septal defect, shunt volume, chronic lung disease, hemodynamics, prematurity, echocardiography
Introduction:
A patent ductus arteriosus (PDA) is a common diagnosis in the neonatal intensive care unit (NICU). The physiologic and clinical consequences of a hemodynamically significant (hs)PDA are attributable to the magnitude of the shunt and its impact on the systemic and pulmonary circulations, myocardial function, and antenatal and perinatal characteristics. Although there is compelling evidence of association between PDA and common neonatal morbidities, including intraventricular hemorrhage (IVH), necrotizing enterocolitis, chronic lung disease (CLD), and death, clinical trials of treatment have failed to show meaningful impact on outcomes.1–6 There is increased recognition that, in the setting of declining pulmonary vascular resistance, myocardial adaptive response and loading conditions in the systemic circulation during the transitional period influence ductal shunt volume. Therefore, identification of additional risk factors may shed light on the impact of a hsPDA on clinical outcomes.
The modulator role of the trans-atrial shunt on heart function and cardiac output among patients with PDA is poorly studied and may provide insight regarding its effect on the development of IVH and CLD. Specifically, the impact of PDA on cerebral blood flow (CBF) has been studied with conflicting findings.6, 7 Preliminary evidence suggests that a period of escalating left ventricular output (LVO), hence CBF, precedes the development of IVH in some babies.8 Some commentators have hypothesized that the presence of a large atrial communication (AC) may alter the magnitude of the increase in LVO seen with a hsPDA, thereby reducing risk of secondary reperfusion injury. In addition, augmentation of pulmonary blood flow through combined atrial and ductal level shunting may contribute to lung injury either directly or indirectly through increased need for respiratory support.
Accordingly, in this study, we evaluated the relationship between size of early AC on echocardiography indices of PDA shunt volume and neonatal outcomes. We hypothesized a priori that infants with presumed hsPDA (≥1.5 mm) and AC >1 mm, as compared to those with presumed hsPDA and small or no AC or those with presumed non-significant PDA (<1.5 mm), would demonstrate a different echocardiography phenotype, with a specific (but not limited) emphasis on signs of left heart volume loading.
Material and Methods:
We conducted a retrospective cohort study of premature infants admitted to the NICU at one of three tertiary care centers (Sunnybrook Health Sciences Centre or The Hospital for Sick Children in Toronto, Canada and The Rotunda Hospital in Dublin, Ireland) between June 2011 and October 2016. Infants were included if they satisfied the following criteria: (i) gestational age at birth less than 32 weeks; (ii) at least one comprehensive Targeted Neonatal Echocardiogram (TnECHO) was performed (one echocardiogram analyzed per patient); (iii) PDA shunt, when present, characterized by an exclusive or predominately left-to-right shunt determined by pulse wave Doppler;9 (iv) earliest PDA treatment naïve echocardiography assessment between postnatal day 1–7 (closest to postnatal day 2) analyzed; (v) exclusive left-to-right atrial shunt determined by pulse wave Doppler when present. Infants were excluded if there was a concurrent diagnosis of structural congenital heart disease, other major congenital anomalies, or suspected or proven genetic or chromosomal disorder. This study, including waiver of informed consent, was approved by each institutions’ respective research ethics board.
Group Classification:
Infants were divided into four groups depending on PDA size and size of AC; Group 1 consisted of those infants with a PDA diameter of 1.5 mm or larger and an AC greater than 1 mm; Group 2 consisted of infants with a PDA diameter of 1.5 mm or larger and an AC less than or equal to 1 mm in size or no visible AC or shunt; Group 3 consisted of infants with PDA diameter less than 1.5 mm or no measurable PDA and an AC greater than 1 mm; Group 4 included infants with a PDA diameter less than 1.5 mm or no measurable PDA and an AC less than or equal to 1 mm or no visible AC or shunt.
TnECHO Evaluation:
TnECHO is a non-invasive modality that is increasingly recognized as a tool to assist in the hemodynamic management of critically ill infants in the NICU.10, 11 The request for hemodynamic consultation in the first week of life was at the discretion of the attending neonatologist. All evaluations were performed by hemodynamic consultants, neonatal intensivists who have completed a formal hemodynamic fellowship program; specifically, individuals receive comprehensive training in image acquisition and study interpretation according to the 2011 published guidelines for TnECHO use and are immersed in comprehensive cardiovascular physiology education.12 Currently, hemodynamic fellows undergo one full year of dedicated training where they complete between 400–600 comprehensive TnECHO evaluations. All TnECHO evaluations are standardized within/across centers, include between 80–100 images, and in the hands of highly skilled operators take 15–20 minutes to complete. Each evaluation is archived to the echocardiography server and formally reported. Hemodynamic rounds are conducted daily where each TnECHO evaluation is presented to hemodynamic consultants for interpretation and the formulation of a diagnostic interpretation / medical recommendation within the context of the unique clinical scenario. Echocardiography imaging for this study was performed according to a standardized PDA protocol. (Table 1) All patients had comprehensive PDA evaluation in accordance with ASE recommendations for targeted neonatal echocardiography.12 If this was the first echocardiogram for the patient, imaging was obtained to screen for congenital heart disease and the study was reviewed within 6 hours by a cardiologist who was a pediatric echocardiography specialist.
Table 1.
Targeted Neonatal Echocardiography PDA protocol
| Morphology |
|
| Functional |
|
LV strain analysis was not assessed in this retrospective study, but can be included in LV functional assessment PDA: patent ductus arteriosus, IAA: interrupted aortic arch, PA: pulmonary artery, LPA: left pulmonary artery, RPA: right pulmonary artery, RVOTO: right ventricular outflow tract obstruction, LVOTO: left ventricular outflow tract obstruction, CHD: congenital heart disease, LV: left ventricle, LVEDD: left ventricular end diastolic dimension, LA: left atrium, Ao: aortic, ASD: atrial septal defect, MPA: main pulmonary artery, VTI: velocity time integral, FS: fractional shortening, EF: ejection fraction, IVRT: isovolumic relaxation time, LVO: left ventricular output, SMA: superior mesenteric artery, SVC: superior vena cava
The echocardiograms of each infant were reviewed to identify infants with PDA within the first postnatal week. Presence of PDA was determined using a high parasternal ductal view with color Doppler. Once identified, the size of the PDA was measured in 2D at the narrowest point. If multiple complete echocardiograms were obtained for an infant, the earliest treatment naïve echocardiogram in the first seven postnatal days, closest to postnatal day 2, was analyzed. Images were re-measured offline by a trained operator who was blinded to the patient’s clinical information.
Markers of PDA shunt volume:
Echocardiography markers of PDA high volume shunt that were evaluated in this study included PDA size, mitral valve E wave to A wave ratio, isovolumic relaxation time (IVRT), pulmonary vein D wave velocity, LVO calculated as π(aortic annulus radius)2 x velocity time integral (VTI) x heart rate/weight in kg, ratio of left atrium to aorta size (LA: Ao), left ventricular end diastolic diameter (LVEDD), left ventricular ejection fraction by Simpson’s biplane method, and diastolic flow pattern in the descending aorta (Figure 1), celiac artery, and middle cerebral artery. (Appendix 1) The surrogate consequences of volume loading on the heart and systemic hypoperfusion associated with PDA shunt were assessed and categorized according to a PDA score. The Iowa PDA score is an adaptation of the clinical markers noted to be most consistent with hemodynamically significant PDA in previous work.13, 14 The score is used clinically at University of Iowa to provide enhanced objectivity to the determination of hemodynamic significance of a PDA shunt. Elements of the PDA score included mitral valve E wave velocity, IVRT, pulmonary vein D wave velocity, LA: Ao, LVO, and presence of diastolic flow reversal in the descending aorta, celiac artery, or middle cerebral artery. All measurements were obtained according to standardized techniques based on an average of 3–5 beats (Appendix 1). Patients were given a score of 0 to 2 for each element added to [size of PDA in mm ÷ weight of the patient at the time of TnECHO] with a score of ≥ 6 signifying a presumed hsPDA. (Appendix 2)
Figure 1.
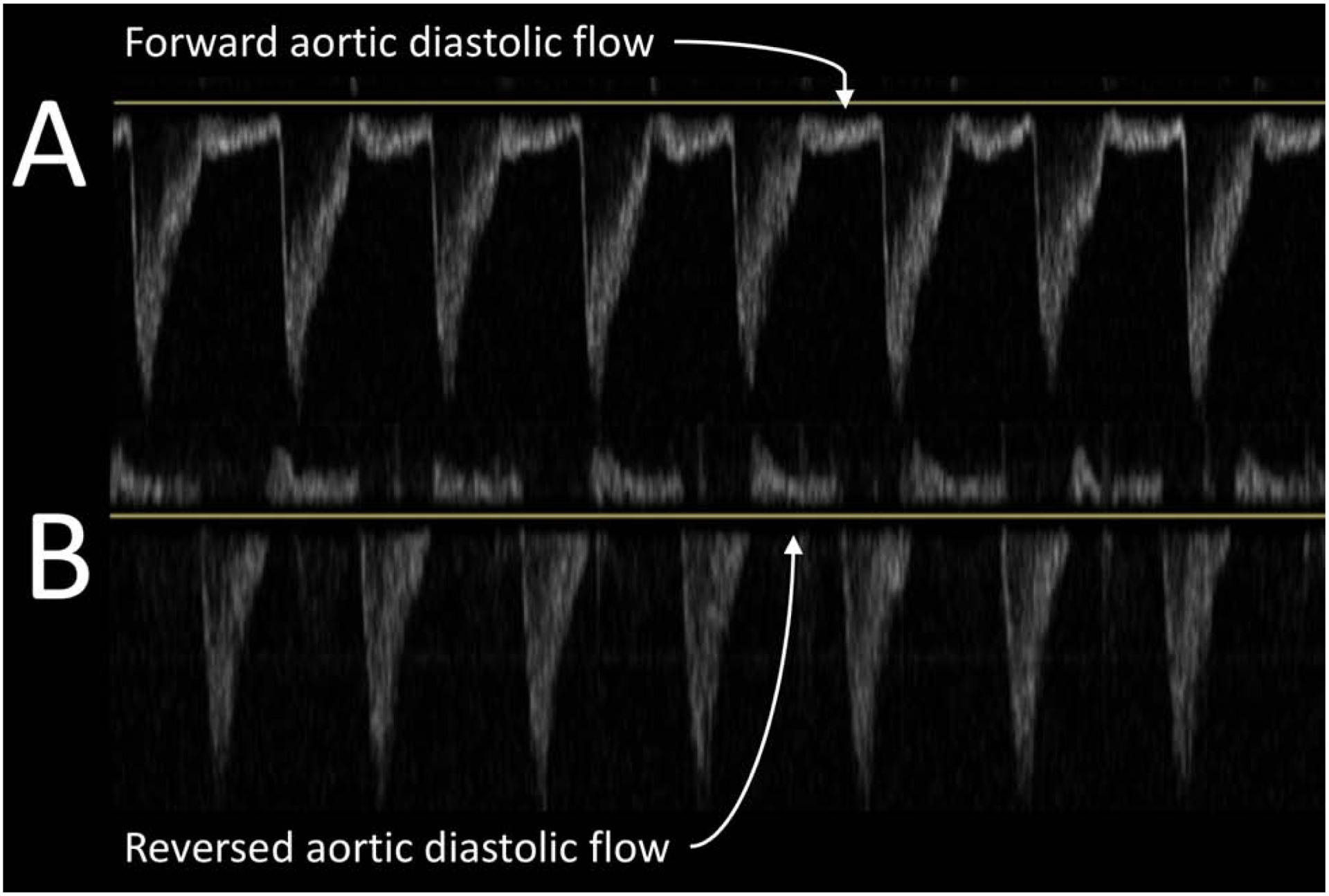
Doppler imaging of descending aorta
A represents normal flow in diastole. B represents reversal of diastolic flow. Reversal of flow may be due to hemodynamically significant PDA.
Description of atrial communication:
The flow across the AC was identified using color Doppler imaging from a subcostal long-axis atrial view (Figure 2) with rotation of the probe in order to exclude SVC and/or IVC flow which could mimic atrial level shunt. Once identified, the size of the communication was measured in 2D and classified as ≤1 mm or >1 mm. We decided by consensus, a priori, that due to the size and immaturity of the premature patients in this cohort, a width of 1 mm was an appropriate cut-off for determination of a trivial communication more likely to produce a negligible shunt and unlikely to have a large physiologic impact. The ascertainment of group assignment based on atrial shunt size was obtained by a trained operator who remained blind to other echocardiography markers and clinical data.
Figure 2.
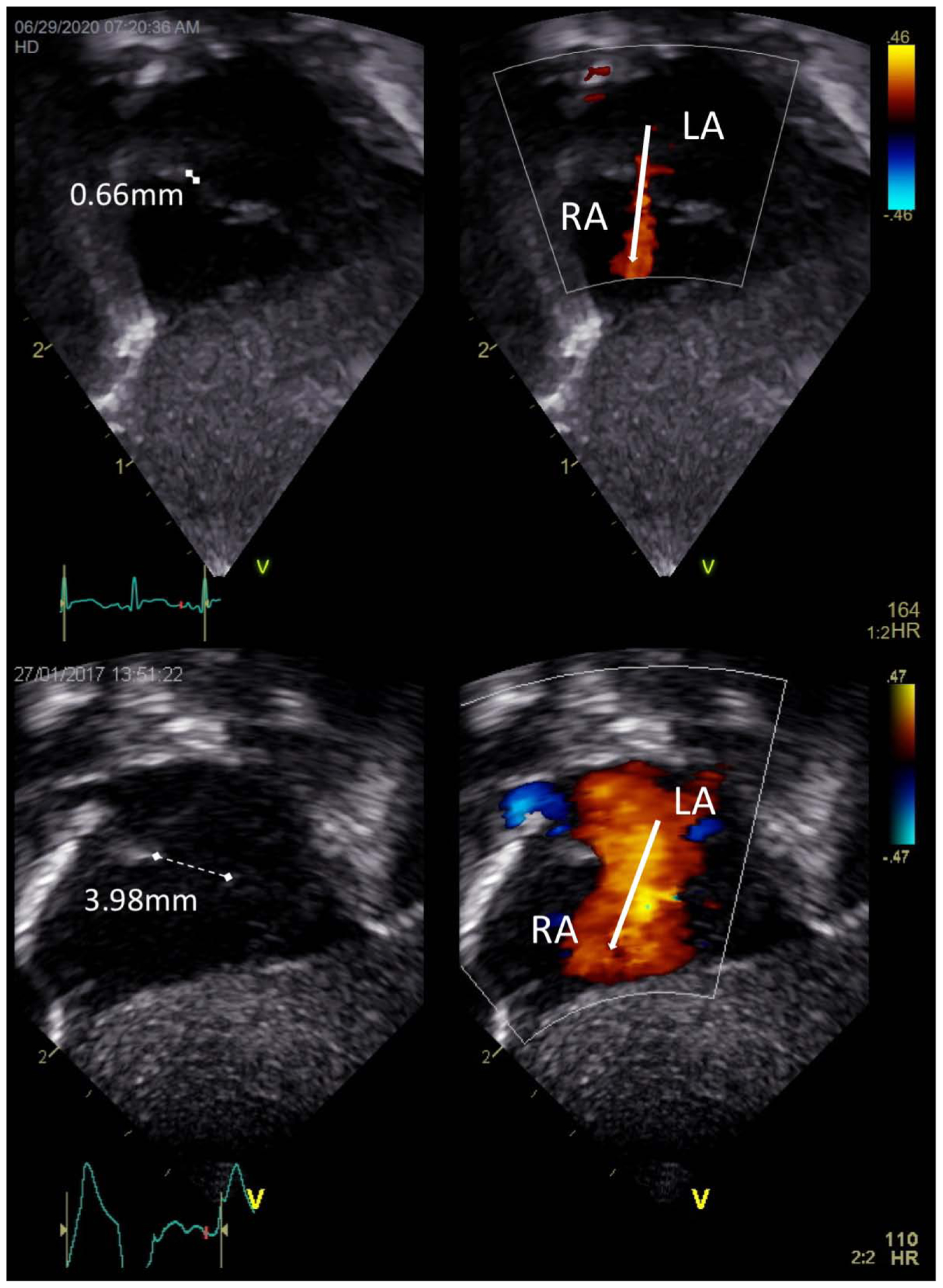
TnECHO images of ≤1 mm and >1 mm left- to-right atrial communications
Top images represent atrial communication ≤1 mm [measured 0.66 mm] and Bottom images represent atrial communication >1 mm [measured 3.98 mm] in size with white arrows depicting direction of flow from left atrium (LA) to right atrium (RA)
Data Sources and Collection:
The hospital records of all eligible infants were reviewed to abstract antenatal, neonatal, and outcome data. Details of respiratory support provided at time of echocardiography were collected, including need for continuous positive airway pressure, conventional mechanical ventilation, or high frequency oscillatory ventilation. Data on postnatal morbidities were collected until death or discharge from the NICU. These data included pulmonary hemorrhage [acute discharge of bloody fluid from the upper respiratory tract or endotracheal tube with increased oxygen requirement from baseline], intraventricular hemorrhage [grades I-IV Papile classification as described previously],15 periventricular leukomalacia [areas of cystic lesions as described in published guidelines],16 and CLD [defined as oxygen need at 36 weeks postmenstrual age].17 Use of prophylactic indomethacin was restricted to one center. Data on number of patients who received prophylactic indomethacin was collected. Data on receipt of medical or surgical treatment of the PDA were also collected. Medical treatment of PDA with indomethacin, ibuprofen, or acetaminophen were recorded. If surgical ligation was performed, age at surgery was documented.
Outcomes:
The primary outcome was a difference in the markers of ductal shunt including markers of left heart volume loading and systemic steal. (Appendix 1) The secondary clinical outcome was diagnosis of IVH with routine cranial ultrasound prior to hospital discharge. Per each hospitals’ local protocol, initial cranial ultrasounds were most often obtained between days 7–10 of age. Cases of IVH were analyzed as all cases (IVH or not) and those considered severe (Grades III or IV) vs Grades I and II.15 Other clinical outcomes included presence of pulmonary hemorrhage, need for treatment of PDA (medical or surgical), periventricular leukomalacia, need for respiratory support at 28 days of age, CLD defined as the need for oxygen at 36 weeks corrected age, death, and the composite outcome of CLD or death prior to discharge.
Statistical Analysis:
As there are no prior studies of AC effects in the transitional period, we report a sample size of convenience which included all patients with TnECHO during the first postnatal week over a 5-year period. Statistical analyses were performed using SPSS (IBM, Version 23, Armonk, NY). Descriptive statistical methods were performed to characterize the patient population (mean and SD or median and IQR). Univariate analysis was performed to compare demographics, shunt characteristics, and neonatal outcomes between the two groups. Data were analyzed using Pearson Chi-Square test, Student’s t-test, and Mann Whitney U test as appropriate. Binary logistic regression was performed to evaluate the effect of AC size and either PDA diameter or PDA score on the composite outcome of death or CLD. A priori, we chose to include gestational age and need for high frequency ventilation in the model to account for level of prematurity and amount of respiratory support necessary in the setting of a PDA. We performed reliability testing on group ascertainment based on atrial shunt size. A total of 26 patients were selected (approximating the same ratio of AC ≤1 mm to >1 mm as the study population) and classified independently by two operators (REG and DRR). Cohen’s kappa coefficient was used to estimate inter- and intra-rater reliability. Kappa values between 0 and 0.4 are considered slight to fair agreement, kappa 0.41–0.6 moderate agreement, kappa 0.61–0.8 substantial agreement, and kappa 0.81–1.0 almost perfect agreement.
Results:
A total of 208 preterm infants were identified who underwent TnECHO during the first 7 postnatal days (range of 30 hours to postnatal day 7). Of these, 199 infants with a mean birthweight and gestational age of 928 ± 632 grams and 26.6 ± 1.5 weeks, respectively, met inclusion criteria. (Figure 3) The 4 groups consisted of 107 infants with PDA ≥1.5 mm and AC>1 mm; 52 infants with PDA ≥ 1.5 mm and AC ≤1 mm; 23 infants with PDA <1.5 mm and AC ≤1 mm; and 17 infants with PDA <1.5 mm and AC >1 mm. Clinical and echocardiography characteristics of all groups can be found in Tables 2 and 3, respectively.
Figure 3.
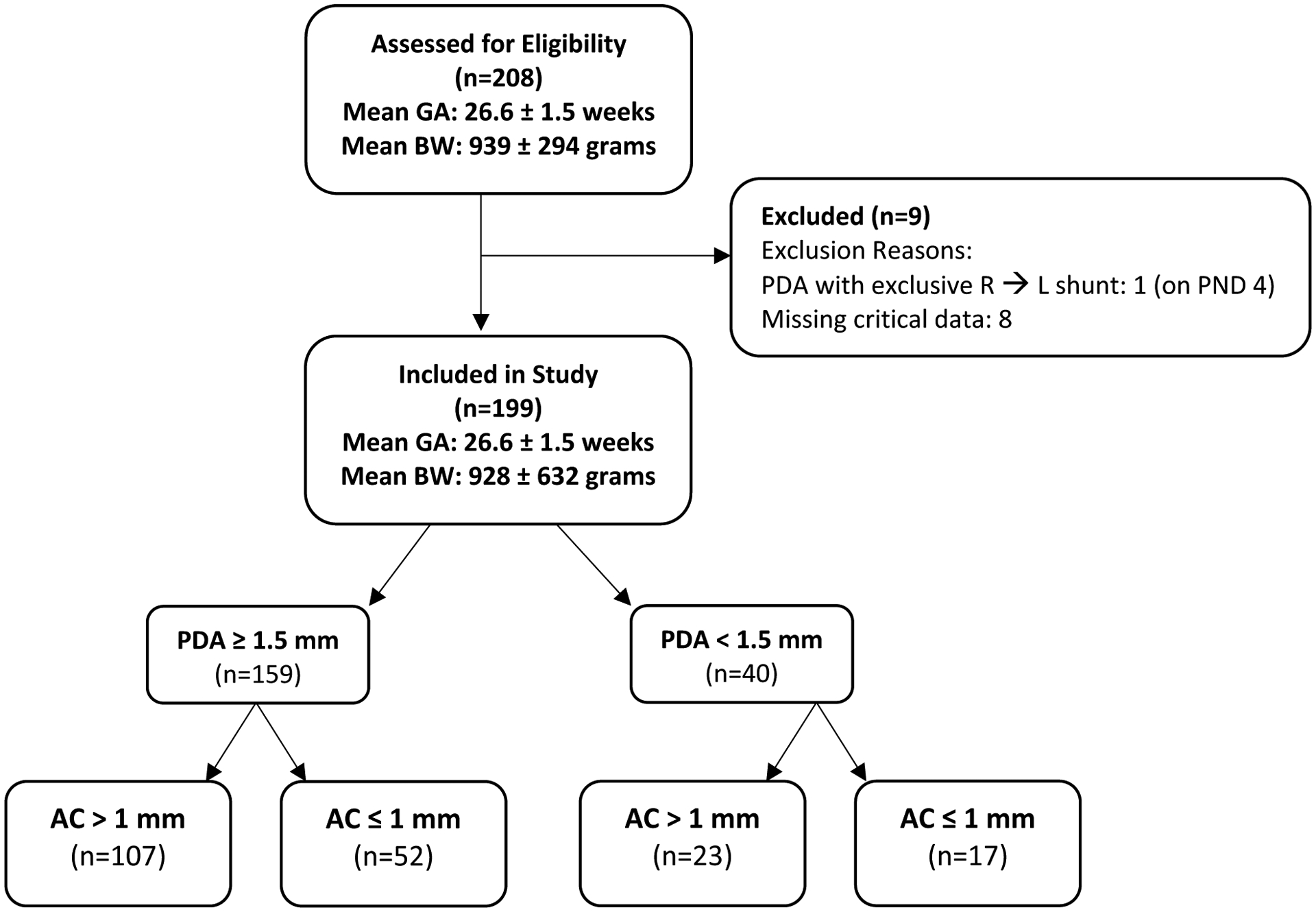
Diagram of Included and Excluded Patients
Abbreviations: AC: atrial communication; BW: birth weight; PDA: patent ductus arteriosus; PND: postnatal day; R→L: right-to-left
Table 2.
Clinical characteristics of neonates with PDA and ≤1 mm and >1 mm atrial communication
| Characteristic | PDA≥1.5mm, AC >1mm (n=107) |
PDA≥1.5mm, AC≤1mm (n=52) |
PDA<1.5mm, AC≤1mm (n=23) |
PDA<1.5mm, AC>1mm (n= 17) |
p-value |
|---|---|---|---|---|---|
| Gestational age, weeks* | 26.5 ± 1.5 | 26.6 ± 1.5 | 27.0 ± 1.5 | 26.5 ± 1.9 | ns |
| Birth weight, grams* | 909± 241 | 944 ± 275 | 966 ± 243 | 860 ± 259 | ns |
| Apgar 5-min, median [IQR] | 8 [6, 9] | 8 [7, 9] | 8 [7, 9] | 7 [6, 8] | ns |
| Age at TnECHO, (d), median[IQR] | 2 [2, 3] | 2 [2, 3] | 2 [2, 3.5] | 2 [2, 3.5] | ns |
| - High frequency oscillator ventilation | 27 (25) | 9 (17) | 1 (4)#^ | 3 (17) | |
| Iowa PDA score*† | 9.8 ± 3.4+ | 8.7 ± 3.7+ | 1.6 ± 1.4 | 1.5 ± 1 | <0.001 |
| Prophylactic Indomethacin | 12 (11) | 6 (12) | 2 (9) | 0 (0) | ns |
| Any treatment for PDA | 60 (57) | 27 (54) | 5 (22)#^ | 3 (18)#^ | 0.002 |
| Pharmacological treatment of PDA | 50 (47) | 24 (48) | 5 (22)#^ | 3 (18)#^ | 0.03 |
| Surgical ligation of PDA | 8 (8) | 3 (6) | 0 | 0 | ns |
| Pulmonary hemorrhage | 12 (11) | 7 (14) | 2 (9) | 1 (7) | ns |
| Intraventricular hemorrhage (IVH) | 48 (45) | 25 (48) | 12 (52) | 8 (47) | ns |
| Grade III/IV IVH | 24 (22) | 11 (21) | 3 (13) | 3 (18) | ns |
| Periventricular leukomalacia | 7 (7) | 3 (6) | 0 | 0 | ns |
| Ventilation at 28 days | 81 (76)+ | 35(67) | 13 (57) | 7 (41) | 0.04 |
| Chronic lung disease in survivors | 50 (56) | 17 (36) | 7 (33) | 3 (21)# | 0.04 |
| Mortality | 17 (16) | 5 (10) | 1 (4) | 3 (18) | ns |
| Composite of death or chronic lung disease | 67 (63)+ | 22 (42) | 8 (35) | 6 (35) | 0.017 |
mean, standard deviation, otherwise frequency (percent) unless otherwise noted.
Iowa PDA score = [# total points] + [PDA diameter ÷ weight at echo]
Different from all other groups;
Different from PDA ≥1.5 mm and AC>1mm group;
Different from PDA ≥1.5 mm and AC <1 mm group
AC: atrial communication, TnECHO: Targeted neonatal echocardiogram, CPAP: continuous positive airway pressure, (d): days, PDA: patent ductus arteriosus, IVH: intraventricular hemorrhage
Table 3.
Echocardiography markers of hemodynamic significance of PDA in neonates with atrial communication ≤1 mm and >1 mm in size
| Marker | PDA≥1.5mm, AC >1mm (n=107) |
PDA≥1.5mm, AC≤1mm (n=52) |
PDA<1.5mm, AC>1mm (n=23) |
PDA<1.5mm, AC<1mm (n= 17) |
p-value |
|---|---|---|---|---|---|
| PDA size (mm)*ǂ | 2.7 ± 0.5 | 2.5 ± 0.6 | 1.1 ± 0.2#^ | 1.2 ± 0.3#^ | <0.001 |
| Mitral valve E:A* | 0.9 ± 0.2 | 0.9 ± 0.3 | 0.8 ± 0.4 | 0.77 ± 0.2 | ns |
| IVRT (msec)* | 47 ± 13 | 43 ± 9 | 50 ± 11 | 53 ± 12 | ns |
| Pulmonary vein D wave (m/s)* | 0.43 ± 0.14+ | 0.36 ± 0.16 | 0.34 ± 0.1 | 0.34 ± 0.1 | 0.01 |
| Left Ventricular Output (ml/min/kg)* | 237 ± 78+ | 209 ± 74+ | 177 ± 60 | 177 ± 70 | 0.001 |
| LA: Ao ratio, median [IQR] | 1.61 [1.3, 1.8] | 1.52 [1.3, 1.7] | 1.27 [1.1, 1.4]#^ | 1.35 [1.3, 1.4]#^ | <0.001 |
| LVEDD (mm), median [IQR] | 12.7 [11, 14] | 12.3 [11, 14] | 10.4 [9, 12]#^ | 10.8 [10, 12]#^ | 0.001 |
| Ejection Fraction* | 63 ± 7 | 64 ± 8 | 57+ ± 7 | 62 ± 9 | 0.02 |
| Reversed descending aorta diastolic flow | 84 (80)+ | 30 (62)+ | 0 | 0 | <0.001 |
| Reversed celiac artery diastolic flow | 36 (37)+ | 4 (13)+ | 0 | 0 | <0.001 |
| Reversed middle cerebral artery diastolic flow | 38 (42)+ | 6 (21)+ | 0 | 0 | <0.001 |
Mean, standard deviation, otherwise frequency (percent) unless otherwise noted.
Different from all other groups;
Different from PDA ≥1.5 mm and AC>1mm group;
Different from PDA ≥1.5 mm and AC ≤1 mm group;
Includes only infants with PDA (n=12/23 and 10/17 had no PDA in the PDA <1.5 mm, AC >1 mm and PDA <1.5 mm, AC ≤1 mm groups, respectively)
AC: atrial communication, PDA: patent ductus arteriosus, IVRT: isovolumic relaxation time, LVEDD: left ventricular end diastolic dimension
Echocardiography characteristics of PDA shunt:
Infants with presumed hsPDA and AC >1 mm had higher pulmonary vein D wave velocity (p<0.05), LVO (p<0.005), and PDA score (p<0.001) than infants with either presumed hsPDA and AC ≤1 mm or PDA <1.5 mm regardless of AC size. This group was also found to have significantly higher LA: Ao and LVEDD than both groups with PDA <1.5 mm. Infants with presumed hsPDA and AC ≤1 mm had higher LVO (p<0.01) than both groups with PDA <1.5 mm. In addition, both groups with presumed hsPDA were more likely to have reversed diastolic flow in their descending aorta (p<0.001), celiac artery (p<0.001), and middle cerebral artery (p<0.001) as compared to all other groups.
Clinical Outcomes:
There was no difference in the incidence of either total or severe IVH between groups. There was also no difference in rate of pulmonary hemorrhage, need for surgical treatment of PDA, periventricular leukomalacia, or death between the four groups. Infants with a presumed hsPDA and AC>1 mm, however, had a higher PDA score (p<0.001), rate of ventilator need at 28 days (p<0.05), and risk of the composite outcome of CLD or death prior to hospital discharge (p<0.05) than all other groups. Of the 103 infants that had either CLD or death prior to hospital discharge, 67 (63%) had presumed hsPDA and AC >1 mm, 22 (42%) had presumed hsPDA and AC ≤1 mm, 8 (35%) had PDA <1.5 mm and AC ≤1 mm, and 6 (35%) had PDA <1.5 mm and AC >1 mm. Infants with a presumed hsPDA and AC>1 mm also had higher incidence of CLD than the PDA <1.5 mm and AC >1 mm group. Both groups with presumed hsPDA had higher incidence of any treatment (p<0.005) and medical treatment (p<0.05) of PDA with a lower frequency of CPAP for respiratory support at the time of TnECHO assessment (p<0.01) than both groups with PDA <1.5 mm. Additionally, the PDA ≥1.5 mm and AC ≤1 mm group had a higher PDA score than both groups with PDA <1.5 mm. For the composite of CLD or death, AC >1 mm (p<0.05), gestational age (p<0.001), PDA diameter (p<0.005), and PDA score (p<0.01) were found to be independently associated. (Table 4)
Table 4.
Multivariable Logistic Regression to Predict the Composite Outcome of CLD or Death utilizing PDA diameter (model 1) or PDA score (model 2)
| Variable | Model 1 OR [95% CI] p-value |
Model 2 OR [95% CI] p-value |
|---|---|---|
| Atrial communication ≥ 1 mm | 2.4 [1.2, 4.8] p=0.013 |
2.2 [1.1, 4.4] p=0.024 |
| Gestational age | 0.49 [0.37, 0.63] P<0.001 |
0.51 [0.41, 0.66] p<0.001 |
| High frequency ventilation | 1.1 [0.47, 2.7] p=ns |
1.1 [0.47, 2.7] p=ns |
| PDA diameter | 1.7 [1.2, 2.4] p=0.002 |
|
| PDA Score | 1.2 [1.03, 1.2] p=0.005 |
CLD: chronic lung disease; OR: odds ratio; CI: confidence interval; PDA: patent ductus arteriosus
Measures of Reliability:
Our team has previously published interobserver measurements of reliability for all echocardiography markers of PDA shunt velocity.13, 18 For atrial communication measurement, specific to this study, interrater reliability kappa (95% CI) was 0.83 (0.61–1) and intra-rater reliability kappa was 1.0 (1.0–1.0).
Discussion:
In this exploratory study of a cohort of premature infants with PDA during the first postnatal week, we showed that infants with presumed hsPDA and AC >1 mm had increased pulmonary vein D wave velocity, increased LVO, and reversed diastolic flow in the descending aorta, celiac artery, and middle cerebral artery. These data suggest that AC >1 mm in the first week of life may be a surrogate marker of hsPDA rather than a modulator of shunt volume and its consequences to left heart volume loading. In addition, the presence of AC >1 mm, in the setting of presumed hsPDA, was associated with an increased risk of ventilator requirement at 28 days and the composite outcome of death or CLD but not associated with a decreased incidence of IVH.
In this cohort, the most striking association was that of AC >1 mm to other echocardiography signs of hsPDA, including reversed diastolic flow in systemic vessels. There is a suggestion in published literature that the presence of a large atrial shunt would offload the left heart leading to pseudo-normalisation of the echocardiography features of left heart pressure/volume loading (including LVO), although the evidence is weak.19 This study, however, shows that infants with presumed hsPDA and AC >1 mm have higher pulmonary vein D wave velocity, higher LVO, and higher PDA score. The increase in pulmonary vein D wave velocity could be attributed to the increase of pulmonary blood flow due to combined left-to-right shunts at both the atrial and ductal levels. The association with increased LVO is less intuitive, as previous studies suggested that the magnitude of the increase in LVO would be less as more blood may traverse the atrial septum if right ventricular compliance was low.20 We speculate that the presence of AC >1 mm is a marker of higher-volume PDA shunts consistent with higher PDA scores; specifically, as the transductal shunt increases, pulmonary venous return and left atrial preload increases to the extent that, although the magnitude of the atrial shunt may rise if RV compliance also falls, there is a concurrent greater increase in LVO. Earlier identification of PDA/AC shunting is likely to underestimate echocardiography signs of hemodynamic significance during the transitional period when PVR may be higher. However, in patients with early signs of hemodynamic significance, identification could be clinically relevant as length of exposure is considered important in risk for future morbidities. Also, since the TnECHO was requested for clinical symptoms, a large number of infants identified on day 2 with hsPDA were treated for PDA; therefore, we elected to evaluate echocardiography studies in treatment naïve patients. The findings of this study suggest that the presence of AC >1 mm within the first few postnatal days can, and should, lead us to evaluate for and consider the presence of hsPDA. Perhaps, when this sign is present, closer attention should be paid to other clinical signs and symptoms and echocardiography markers of a presumed hsPDA in order to determine the infant’s risk of future morbidity (CLD) and mortality from prolonged exposure to a hemodynamically significant PDA.
Previous studies of atrial shunt have shown an increase in CLD in patients both with or without a hemodynamically significant PDA21 and increases in right ventricular output more than LVO in those with large atrial shunts.22 In our cohort of patients LVO was also increased, however it is important to recognize that right ventricular output may not be an accurate reflection of true pulmonary blood flow as it does not take into account the magnitude of the PDA shunt. Closure of atrial septal defects has been shown to result in rapid improvement in pulmonary hemodynamics23 in addition to longer-term improvement in flow-related pulmonary hypertension and CLD, although these data are from older infants.23, 24 These data are consistent with the findings of Liebowitz et al., who showed a decrease in the rate of CLD with early (within 7 days of age) constriction of the PDA.25 Evidence from animal experimental models demonstrate increased Qp, due to augmented PDA and atrial level shunt flow, which contributes to flow-related impaired alveologenesis and modulates the incidence of both CLD and pulmonary hypertension later in life.26 We speculate that these effects may be compounded by further exposure of the immature pulmonary vasculature to hyperoxemic blood leading to vascular remodeling. This consideration needs further investigation.
Previous studies have evaluated the utility of echocardiography in identifying markers of hsPDA.27, 28 These studies highlight the importance of evaluation of pulmonary blood flow in determining shunt volume and its effects. One marker that is most often utilized is that of ductal diameter. Though it has been shown that ductal size measurement is weakly correlated to shunt volume, unindexed ductal diameter showed the best correlation13 and the absolute diameter chosen for this study (≥1.5 mm) is consistent with hemodynamically significant PDA.29, 30 It is important to note that the degree of intra- and extra- cardiac shunting is influenced by other factors including, but not limited to, ventricular compliance, pulmonary vascular resistance, and intrathoracic pressure. We did not identify significant differences in surrogate indices of LV diastolic dysfunction between groups. Studies evaluating systemic perfusion have shown an association between abnormal diastolic flow31 or high shunt volume32 and hypoperfusion. The finding of reversed diastolic flow in the infants with presumed hsPDA and AC >1 mm in this cohort likely indicates the presence of hypoperfusion from high PDA shunt volume.
We were particularly interested in the relationship of atrial level shunting on the likelihood of IVH. It has been shown that decreased CBF is a risk factor for IVH.33 In addition, a significant increase in CBF precedes IVH8 leading us to believe that an ischemia-reperfusion insult may be an inciting etiological factor. Early PDA is thought to contribute to lower SVC flow, a surrogate for CBF,34 however, after 24 hours, CBF increases even in the most premature infants.8 Our data show no decrease in IVH in patients with AC >1 mm; contrary to our a priori hypothesis, we noted LVO to be higher in patients with larger AC, suggesting it to be a sign of higher PDA shunt volume. This suggests that the relationship between PDA and IVH is more complex and likely to be influenced by co-existing hypercarbia, hypoxia, impaired autoregulation, use of vasoactive medications, and changes in mean airway pressure which may all contribute to alterations in CBF. The inter-relationship of putative neuro-hemodynamic contributors to IVH requires prospective evaluation using multimodal monitoring.
Clinical Relevance:
The role of TnECHO and scope of hemodynamic consultations, performed by neonatologists who have completed a dedicated year of additional training in advanced echocardiography methods and cardiovascular physiology, in the NICU in general is increasing. The subspecialty of neonatology is broad and integration of cardiovascular physiology in daily practice is variable. With increased ability to observe physiologic changes in premature infants, using a non-invasive tool which provides comprehensive and dynamic hemodynamic appraisal, the potential for improvement of patient-directed therapies is great. Access to TnECHO evaluations, performed by hemodynamic consultants, has the potential to provide additional information to clinicians caring for critically ill premature infants which may provide novel physiologic insights or confirm a priori assumptions. The most recent American Academy of Pediatrics statement on the approach to PDA in preterm infants states that treatment of PDA in the first 2 weeks of life does not change outcomes.35 The findings of our study suggest that there may be subpopulations of patients with pathologic shunts in the early transitional period who require prospective investigation. Use of comprehensive and standardized echocardiography protocols which enable increased precision in the identification of patients at greatest risk of PDA attributable morbidity (e.g. recognizing the presence of an AC >1 mm in a patient with other features of hsPDA), a more targeted approach to use of medical therapy, and enhanced longitudinal appraisal of response to treatment can help mitigate outcomes associated with long-term exposure to large volume PDA shunt.
Limitations:
There are limitations to this multi-center, retrospective review. A trained operator remeasured the echocardiograms and though they were blinded to the clinical information, they were not blind to study design and hypothesis. Nevertheless, all measurements were obtained according to a standard framework and the conclusions are at discordance with our a priori hypothesis. Though each of the sites used a similar protocol for echocardiography, some images and measurements were missing resulting in patients being excluded. In addition, there may be error in the estimation of atrial communication size, which is a three-dimensional structure, from two-dimensional imaging planes as well as the possibility of inaccurate measurements. Our reliability testing, however, demonstrates that the ascertainment of groups was reliable. There are also limitations of point in time evaluation of PDA and atrial communications which are dynamic lesions. The Iowa PDA score, adapted from our previous work in appraising magnitude of hemodynamic significance and used in routine clinical practice at our center, has not been formally validated. Concurrent timing of cranial ultrasound and TnECHO was not regimented with a protocol which inhibits our ability to ensure IVH was not already present at the time of TnECHO. We were also limited by the amount of clinical information documented in the medical chart. In addition, we did not attempt to collect all potentially relevant data points including hematocrit, mean airway pressure, or more advanced imaging techniques of LV functional assessment such as strain analysis. We used a sample size of convenience which resulted in a disproportionate number of patients with >1 mm vs ≤1 mm atrial communication for presumed hsPDA and a small number of patients with PDA <1.5 mm; ideally, we would have had equal numbers in all 4 groups for analysis. Finally, the low rate of IVH may limit the ability to accurately appraise this association.
Conclusions:
In summary, our results show that echocardiography evidence of an atrial communication >1 mm during the first few postnatal days is suggestive of the presence of a hemodynamically significant PDA and perhaps, higher volume shunt. Future investigation should evaluate if early identification and treatment of patients with both high-volume PDA and large atrial level communications may help mitigate adverse outcomes, such as CLD or death, in this high-risk patient population.
Highlights:
Relationship of atrial communication, in first postnatal week in premature infants, to magnitude of patent ductus arteriosus (PDA) shunt is poorly studied
Infants with larger atrial communication had more echocardiography markers of high-volume PDA shunt
Infants with presumed hemodynamically significant PDA and larger atrial communication during the first postnatal week had higher risk of either death or chronic lung disease
Larger early atrial communication may be suggestive of higher volume PDA shunts in premature infants
Funding:
D.R.R is supported by the National Institutes of Health (1K23HLI130522) which was not involved in the study.
Abbreviations:
- AC
atrial communication
- CBF
cerebral blood flow
- CLD
chronic lung disease
- hsPDA
hemodynamically significant PDA
- IVH
intraventricular hemorrhage
- IVRT
isovolumic relaxation time
- LA: Ao
left atrium to aorta ratio
- LVEDD
left ventricular end diastolic diameter
- LVO
left ventricular output
- NICU
neonatal intensive care unit
- PDA
patent ductus arteriosus
- TnECHO
Targeted Neonatal Echocardiogram
- VTI
velocity time integral
Appendix 1: Echocardiography Markers Evaluated to Determine Volume of PDA Shunt
| Marker | View(s) | Mode | Detail |
|---|---|---|---|
| PDA size | Suprasternal ductal cut with color and 2D | 2D | Measured at narrowest diameter via sweep from aorta to pulmonary artery |
| Mitral valve E:A wave ratio | Apical 4-chamber view | 2D PW | Measured at base of open mitral valve leaflets |
| IVRT | Apical 5-chamber view with color | Color PW | Measured in left ventricle where both inflow and outflow are apparent |
| Pulmonary vein D wave velocity | Apical 4-chamber view with color narrowed to pulmonary vein inflow to left atrium | Color PW | Measured within the mouth of the pulmonary vein prior to its entry into the left atrium |
| Aortic VTI for LVO calculation | Apical 5-chamber view | PW | Measured in line with outflow tract at the level of the aortic leaflets |
| LA: Ao | Parasternal long axis | M-mode | Measured in end systole with well aligned/“opened up” aortic root |
| LVEDD | Parasternal long axis | M-mode | Measured in end-diastole |
| Ejection Fraction | Apical 4- and 2-chamber views | 2D | Measured in end-diastole and end-systole via Simpsons biplane |
| Diastolic flow in descending aorta | Suprasternal long axis aortic view | Color PW | Measured at the level of the diaphragm |
| Diastolic flow in celiac artery | Subcostal sagittal view | Color PW | Measured with slight angulation to bring celiac artery into view |
| Diastolic flow in MCA | Cranial axial view | Color PW | Measured in line with flow |
PDA: patent ductus arteriosus; PW: pulse wave Doppler; IVRT: isovolumic relaxation time; VTI: velocity time integral; LVO: left ventricular output; LA: Ao: ratio of left atrium to aorta; LVEDD: left ventricular end diastolic diameter; MCA: middle cerebral artery
Appendix 2: Echocardiography Markers Evaluated to Determine Iowa PDA Score
| Marker | 0 points | 1 point | 2 points |
|---|---|---|---|
| Mitral valve E wave velocity (cm/s) | <45 | 45–80 | >80 |
| IVRT (ms) | >50 | 30–50 | <30 |
| PV D wave velocity (cm/s) | <30 | 30–50 | >50 |
| LA: Ao | <1.3 | 1.3–2.2 | >2.2 |
| LVO (ml/min/kg) | <250 | 250–430 | >430 |
| Diastolic flow in descending aorta AND/OR celiac/middle cerebral artery | Forward | Reversed |
Total score = [# total points] + [PDA diameter ÷ weight at echo]
PDA: patent ductus arteriosus; IVRT: isovolumic relaxation time; PV: pulmonary vein; LA: Ao: ratio of left atrium to aorta; LVO: left ventricular output
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
References:
- 1.Hamrick SEG, and Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125(5):1020–30. [DOI] [PubMed] [Google Scholar]
- 2.Kluckow M, and Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. 2000;137(1):68–72. [DOI] [PubMed] [Google Scholar]
- 3.Evans N, and Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75(3):F183–F6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose CL, and Laughon MM. Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F498–F502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schena F, Francescato G, Cappelleri A, Picciolli I, Mayer A, Mosca F, et al. Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J Pediatr. 2015;166(6):1488–92. [DOI] [PubMed] [Google Scholar]
- 6.Shortland DB, Gibson NA, Levene MI, Archer LNJ, Evans DH, and Shaw DE. Patent ductus arteriosus and cerebral circulation in preterm infants. Dev Med Child Neurol. 1990;32(5):386–93. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Snider A, Katz S, Peabody J, and Brady J. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J Pediatr. 1982;101(4):587–93. [DOI] [PubMed] [Google Scholar]
- 8.Noori S, McCoy M, Anderson MP, Ramji F, and Seri I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J Pediatr. 2014;164(2):264–70.e3. [DOI] [PubMed] [Google Scholar]
- 9.van Laere D, van Overmeire B, Gupta S, El-Khuffash A, Savoia M, McNamara PJ, et al. Application of neonatologist performed echocardiography in the assessment of a patent ductus arteriosus. Pediatr Res. 2018;84(1):46–56.30072803 [Google Scholar]
- 10.Sehgal A, and McNamara PJ. Does point-of-care functional echocardiography enhance cardiovascular care in the NICU? J Perinatol. 2008;28(11):729–35. [DOI] [PubMed] [Google Scholar]
- 11.El-Khuffash A, Herbozo C, Jain A, Lapointe A, and McNamara PJ. Targeted neonatal echocardiography (TnECHO) service in a Canadian neonatal intensive care unit: a 4-year experience. J Perinatol. 2013;33(9):687–90. [DOI] [PubMed] [Google Scholar]
- 12.Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: Practice guidelines and recommendations for training: Writing group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr. 2011;24(10):1057–78. [DOI] [PubMed] [Google Scholar]
- 13.de Freitas Martins F, Ibarra Rios D, F. Resende MH, Javed H, Weisz D, Jain A, et al. Relationship of patent ductus arteriosus size to echocardiographic markers of shunt volume. J Pediatr. 2018;202:50–5.e3. [DOI] [PubMed] [Google Scholar]
- 14.Martins FF, Bassani DG, Rios DI, Resende MHF, Weisz D, Jain A, Lopes JMA, McNamara PJ. Relationship of Patent Ductus Arteriosus Echocardiographic Markers With Descending Aorta Diastolic Flow. J Ultrasound Med. 2020. October 12. doi: 10.1002/jum.15528. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Papile LA, Burstein J, Burstein R, and Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–34. [DOI] [PubMed] [Google Scholar]
- 16.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: Neuroimaging of the neonate. Neurology. 2002;58(12):1726–38. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–60. [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Mohamed A, Kavanagh B, Shah PS, Kuipers BCW, El-Khuffash A, et al. Cardiopulmonary adaptation during first day of life in human neonates. J Pediatr. 2018;200:50–7.e2. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal A, and McNamara PJ. The ductus arteriosus: A refined approach! Semin Perinatol. 2012;36(2):105–13. [DOI] [PubMed] [Google Scholar]
- 20.Evans N, and Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74(2):F88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans N, and Iyer P. Incompetence of the foramen ovale in preterm infants supported by mechanical ventilation. J Pediatr. 1994;125(5, Part 1):786–92. [DOI] [PubMed] [Google Scholar]
- 22.Evans N, and Iyer P. Assessment of ductus arteriosus shunt in preterm infants supported by mechanical ventilation: Effect of interatrial shunting. J Pediatr. 1994;125(5, Part 1):778–85. [DOI] [PubMed] [Google Scholar]
- 23.Lim DS, and Matherne GP. Percutaneous device closure of atrial septal defect in a premature infant with rapid improvement in pulmonary status. Pediatrics. 2007;119(2):398–400. [DOI] [PubMed] [Google Scholar]
- 24.Thomas VC, Vincent R, Raviele A, Diehl H, Qian H, and Kim D. Transcatheter closure of secundum atrial septal defect in infants less than 12 months of age improves symptoms of chronic lung disease. Congenit Heart Dis. 2012;7(3):204–11. [DOI] [PubMed] [Google Scholar]
- 25.Liebowitz M, and Clyman RI. Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: Effects on neonatal outcomes. J Pediatr. 2017;187:119–26.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCurnin D, Seidner S, Chang L-Y, Waleh N, Ikegami M, Petershack J, et al. Ibuprofen-induced patent ductus arteriosus closure: Physiologic, histologic, and biochemical effects on the premature lung. Pediatrics. 2008;121(5):945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehgal A, and McNamara PJ. Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus? Eur J Pediatr. 2009;168(8):907–14. [DOI] [PubMed] [Google Scholar]
- 28.El Hajjar M, Vaksmann G, Rakza T, Kongolo G, and Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F419–F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluckow M, and Evans N. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J Pediatr. 1995;127(5):774–9. [DOI] [PubMed] [Google Scholar]
- 30.D’Amato G, Errico G, Franco C, Brunetti G, Petrillo F, Faienza MF, et al. Ductal size indexed to weight and body surface area correlates with morbidities in preterm infants ≤32 weeks. J Matern Fetal Neonatal Med. 2019;Epub ahead of print:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Groves AM, Kuschel CA, Knight DB, and Skinner JR. Does retrograde diastolic flow in the descending aorta signify impaired systemic perfusion in preterm infants? Pediatr Res. 2008;63(1):89–94. [DOI] [PubMed] [Google Scholar]
- 32.Broadhouse KM, Price AN, Durighel G, Cox DJ, Finnemore AE, Edwards AD, et al. Assessment of PDA shunt and systemic blood flow in newborns using cardiac MRI. NMR Biomed. 2013;26(9):1135–41. [DOI] [PubMed] [Google Scholar]
- 33.Meek JH, Tyszczuk L, Elwell CE, and Wyatt JS. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F15–F8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kluckow M, and Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82(3):F188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benitz WE. Patent ductus arteriosus in preterm infants. Pediatrics. 2016;137(1):e20153730. [DOI] [PubMed] [Google Scholar]


