Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the presence of autoantibodies against various self-antigens. Systemic inflammation affects the skin, joints, central nervous system and, in particular, kidneys through so-called lupus nephritis (LN).1 The exact pathogenesis of systemic lupus erythematosus remains unclear. However, a growing body of evidence suggests that B-cells, a source of autoantibodies and effector T-cells, contribute to the pathogenesis of SLE by secreting cytokines. T-cell/B-cell interaction via their costimulation is crucial for an orchestrated immune response. This interaction is modulated by IL-21-producing T-follicular helper (Tfh) cells, which are located mainly in germinal centers. Tfh cells are required to help B-cells secrete high-affinity IgG antibodies.2
The delicate balance between regulatory and effector cells is important for immune tolerance. Specialized cell subsets, such as regulatory T-cells (Tregs), defined as CD4+CD25highCD127dimFoxP3+ T-cells, and the more recently discovered regulatory B-cells (Bregs), can regulate the T-cell and B-cell immune response and hyperactivity. Bregs are widely defined as CD19+CD24highCD38highIL-10+ B-cells, but a unique phenotype is lacking. In addition to immunoregulation by cell–cell interactions and the ligation of costimulatory molecules, microRNAs (miRNAs) with immunomodulatory properties were described in a remarkable finding. miRNAs, which are single-stranded RNAs of ~22 nucleotides, are potent negative regulators of gene activity. Previous studies have demonstrated that miRNAs can regulate various immune cells, particularly T-cells. Thus, the role of miRNAs as potential biomarkers in several autoimmune diseases, including rheumatoid arthritis, psoriasis, and multiple sclerosis, was tested. Interestingly, few studies had investigated the role of miR-142-3p/5p in systemic lupus erythematosus before the present elegant study by Ding et al.3 was published.
The authors demonstrated in a previous study that miR-142-3p/5p could reduce CD84, IL10, and SAP protein levels in the T-cells of SLE patients via its interaction with the 3′-UTR of the target mRNA.4 In contrast, overexpression of miR-142-3p/5p reduced IgG production and significantly decreased the levels of CD40L, ICOS, IL-4, IL-10, and IL-21, which are crucial for T-cell activation. In the context of these data, miR-142-3p/5p expression was analyzed in CD4+ T-cells from SLE patients, which were exposed ex vivo to mycophenolic acid (MPA).5 Interestingly, this frequently used immunomodulating drug in clinical practice upregulated miR-142-3p/5p in CD4+ T-cells.
MPA was suggested to increase H4 acetylation of a putative regulatory region of miR-142e. A more recent study in patients with granulomatosis with polyangiitis (GPA) demonstrated that miR-142-3p overexpression can also decrease Treg function in patients with GPA. This impaired Treg function might be explained by the ADCY9-dependent downregulation of cAMP, a pivotal axis that is prominent in the suppressive function of Tregs.6 Thus, the upregulation and inhibition of the miR-142-3p pathway can affect the regulatory and effector cell compartments, respectively.
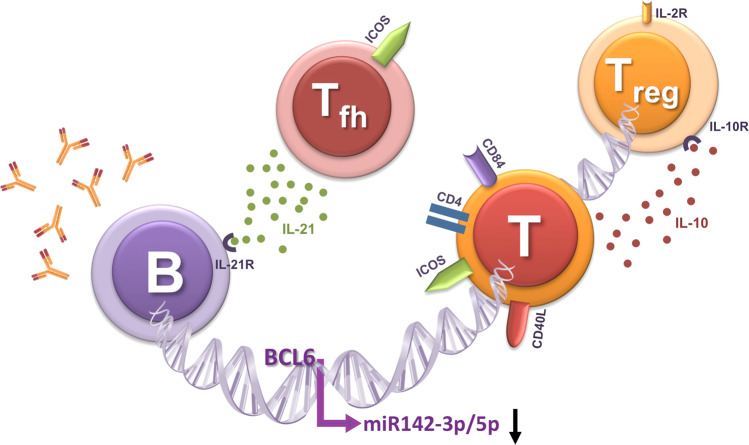
This study in the present issue is sound and unravels a new mechanism by which miR-142-3p/5p functions. Ding et al. used a sophisticated experimental approach to prove the hypothesis that the altered protein expression of B cell lymphoma 6 (BCL-6), which potentially binds to the miR-142-3p/5p promotor region, suppresses miR-142-3p/5p expression in the CD4+ T-cells of SLE patients (Fig. 1). It was demonstrated before that the two known isoforms of MIR142 exhibit different expression patterns. Whereas miR142p3 is preferentially expressed by effector T-cells, Tregs express high levels of miR142p5.7–9 This pattern is closely related to the differential impact of the miR142 isoforms on cellular cAMP levels. miR142p5 mainly inhibits the hydrolysis of cAMP via suppression of the enzyme phosphodiesterase-3b (PDE3b) and thereby upregulates cellular cAMP levels.8 In contrast, miR142p3 attenuates cAMP generation by repressing adenyl cyclase 9 (AC9), an enzyme that enhances cAMP generation.9 Thus, miR142p3 decreases the levels of intracellular cAMP in effector T-cells, promoting effector activity.
Fig. 1.
This figure illustrates the interaction between B-cells and T-cells. Follicular T-helper (Tfh) cells and regulatory T-cells (Tregs) can promote and inhibit immune response, respectively. The B cell lymphoma 6 (BCL-6) protein potentially binds to the miR-142-3p/5p promoter region and suppresses miR-142-3p/5p expression, which in turn enhances the expression of costimulatory molecules (CD40L, ICOS, and CD84) and cytokines (IL-4, IL-10, and IL-21) by T-cells, increases IgG production by B cells, and modulates the suppressive function of Tregs
Tregs make use of a suppressive mechanism known as metabolic disruption. During metabolic disruption, cAMP is transferred from Tregs to target cells, inducing suppression and anergy. Therefore, miR142p5 is critical for Treg function as high cellular cAMP levels are required for proper suppressive activity. A specialized Treg subset, the so-called regulatory follicular T-helper (rTfh) cells, was previously shown to regulate Tfh cell and B-cell activity.10 A hallmark of this subset of cells is their coexpression of BCL-6 and FoxP3. Ding et al. did not further differentiate between rTfh and Tfh cells. However, it would be interesting to know whether the impact of BCL-6 on miR142 depends on the cell subset i.e., whether the BCL-6-mediated regulation of miR142 is different in regulatory versus effector Tfh cells. Indeed, Dekkema et al. recently reported that in patients with autoimmune vasculitis, Tregs harbor higher levels of miR142p3, while miR142p3 levels in effector T-cells were similar in patients compared to healthy controls.6 The authors demonstrated that the transfection of healthy Tregs with miR142p3 inhibited their suppressive function. In addition to the lineage-specific regulatory effect of BCL-6 on miR142, it will be important to further elucidate whether specific isoforms of miR142 are dysregulated in SLE in future studies. These data would provide important insights into whether disturbances are dependent on T-cell lineage.
References
- 1.Dolff S, Witzke O, Wilde B. Th17 cells in renal inflammation and autoimmunity. Autoimmun. Rev. 2019;18:129–136. doi: 10.1016/j.autrev.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Dolff S, et al. Increase in IL-21 producing T-cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2011;13:R157. doi: 10.1186/ar3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding S., et al. BCL-6 suppresses miR-142-3p/5p expression in SLE CD4(+) T cells by modulating histone methylation and acetylation of the miR-142 promoter. Cell. Mol. Immunol.10.1038/s41423-019-0268-3 (2019). [DOI] [PMC free article] [PubMed]
- 4.Ding S, et al. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum. 2012;64:2953–2963. doi: 10.1002/art.34505. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, et al. Mycophenolic acid upregulates miR-142-3P/5P and miR-146a in lupus CD4+T cells. Lupus. 2015;24:935–942. doi: 10.1177/0961203315570685. [DOI] [PubMed] [Google Scholar]
- 6.Dekkema GJ, et al. Increased miR-142-3p expression might explain reduced regulatory T cell function in granulomatosis with polyangiitis. Front. Immunol. 2019;10:2170. doi: 10.3389/fimmu.2019.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchen S, et al. Regulation of microRNA expression and abundance during lymphopoiesis. Immunity. 2010;32:828–839. doi: 10.1016/j.immuni.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anandagoda N, et al. microRNA-142-mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance. J. Clin. Invest. 2019;129:1257–1271. doi: 10.1172/JCI124725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang B, et al. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10:180–185. doi: 10.1038/embor.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement RL, et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat. Immunol. 2019;20:1360–1371. doi: 10.1038/s41590-019-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]