Abstract
Candida albicans has been detected in root carious lesions. The current study aimed to explore the action of this fungal species on the microbial ecology and the pathogenesis of root caries. Here, by analyzing C. albicans in supragingival dental plaque collected from root carious lesions and sound root surfaces of root-caries subjects as well as caries-free individuals, we observed significantly increased colonization of C. albicans in root carious lesions. Further in vitro and animal studies showed that C. albicans colonization increased the cariogenicity of oral biofilm by altering its microbial ecology, leading to a polymicrobial biofilm with enhanced acidogenicity, and consequently exacerbated tooth demineralization and carious lesion severity. More importantly, we demonstrated that the cariogenicity-promoting activity of C. albicans was dependent on PHR2. Deletion of PHR2 restored microbial equilibrium and led to a less cariogenic biofilm as demonstrated by in vitro artificial caries model or in vivo root-caries rat model. Our data indicate the critical role of C. albicans infection in the occurrence of root caries. PHR2 is the major factor that determines the ecological impact and caries-promoting activity of C. albicans in a mixed microbial consortium.
Subject terms: Biofilms, Bacteria, Fungi
Introduction
Dental caries, also known as tooth decay, is one of the most common oral diseases that cause increasing socioeconomic burden to the humans. According to the reports from Global Burden of Diseases, Injuries, and Risk Factors Study 2016, dental caries ranked number one in prevalence and number two in incidence of the ten causes with highest prevalence/incidence [1]. Root caries is a subtype of dental caries that mainly affects elder populations. The prevalence of root caries in the elderly reaches 25–100% [2]. Root caries occurs in areas where the root surface is exposed to the oral environment. As a clinical subtype of dental caries, root caries is a polymicrobial infectious disease associated with the enrichment of acidogenic/aciduric species (e.g., Streptococcus mutans) and depletion of less aciduric commensal residents (e.g., Streptococcus sanguinis) in the microbial biofilm [3]. The “competitive exclusion” of S. mutans and S. sanguinis has been observed in both in vivo and in vitro studies [4–6]. In addition, a positive correlation between the risk of dental caries and S. mutans/S. sanguinis has been observed [7, 8], further suggesting the critical role of microbial disequilibrium in the course of dental caries. Consistently, a recent study has reported microbial dysbiosis in root-caries lesions [9], further supporting the role of microbial ecology in the development of root caries. Another study on the microbial metatranscriptome in caries revealed that S. sanguinis was metabolically active in 3 types of caries lesions (i.e., noncavitated, open dentin, and hidden caries), while S. mutans was active only in noncavitated and open dentin caries [10], suggesting the role of oxygen availability in the interspecies interactions between these two bacteria.
Candida albicans is a commensal fungal species commonly colonizing the human mucosal surfaces. The oral carriage of C. albicans in healthy individuals is 18.5–40.9% based on cultivation [11–13]. Carriage rates are usually higher in individuals with compromised immunity, such as human immunodeficiency virus-positive individuals, diabetes patients, and infants and elder populations [11, 12, 14–16]. Hyphal formation and the ability to respond to extreme environmental pH levels are essential for C. albicans pathogenicity [17–19]. In addition, C. albicans can robustly interact with other oral microorganisms and impose significant impact on the virulence of polymicrobial biofilms. The microbial interaction and cross-kingdom feeding between C. albicans and oral bacteria such as Streptococcus, Actinomyces, and Fusobacterium species have been suggested being closely associated with the pathogenesis of oral infectious diseases for years [20–24]. Cross-kingdom interactions between C. albicans and Streptococcus oralis enhance biofilm virulence on mucosal surfaces [25–27]. In addition, a positive correlation between C. albicans prevalence/carriage and the severity of early childhood caries (ECC) has been noted [28–30], and C. albicans is associated with an increased abundance of plaque S. mutans in severe ECC [31, 32]. Findings by Falsetta et al. demonstrated that interactions between C. albicans and S. mutans not only increased bacterial–fungal carriage in the plaque but also enhanced the biofilm virulence by increasing the severity of carious lesions in a rodent model [33]. More importantly, C. albicans has been isolated from root caries lesions [34–36], and observed in carious dentin/dentine tubules [37]. Genes involved in metabolic activity, sugar transport, stress tolerance, invasion and pH regulation are upregulated in C. albicans colonized in root carious lesion as compared to C. albicans in sound root surface [38], further underscoring the potential role of C. albicans in the development of root caries.
Although the association of C. albicans with root caries has been suggested, the exact action of this fungal species on the microbial ecology and the pathogenesis of root caries is yet to be investigated. In addition, key factors that determine the successful colonization of C. albicans and the subsequent cross-kingdom interactions with other microorganisms in the polymicrobial biofilms are not clear. Here, we hypothesize that C. albicans may affect the microbial ecology and increase the cariogenicity of oral biofilms, and thus promote the development of root caries. Specifically, we found increased colonization of C. albicans in root carious lesions. C. albicans colonization imposed significant ecological impact on oral microbiota, leading to a polymicrobial biofilm with enhanced cariogenicity. PHR2 was identified as the major factor that determined the ecological impact and caries-promoting activity of C. albicans, which provided a promising target for the control of root caries.
Materials and methods
Clinical sample analyses
To investigate the association between C. albicans and root caries occurrence/microbial composition, we enrolled seventy volunteers aged 55–85 years old (35 root-caries subjects and 35 caries-free subjects) in this study. The study protocol was reviewed and approved by the Institution Review Board of West China Hospital of Stomatology, Sichuan University (WCHSIRB-D-2016-100). Written informed consent was obtained from all participants in the study. None of the volunteers had taken or received daily medicine, antibiotic treatment, hormone treatment or oral treatment within 3 months prior to the study. Dental plaque was collected from root carious lesions of root-caries molars and sound root surfaces of molars lacking carious lesions of root-caries patients, as well as the sound root surfaces of all molars of caries-free subjects.
Sample size was estimated using the following assumption: type I error equals 5%; type II error equals 10%; percentage of people infected oral C. albicans equals 30%; percentage of root-caries subjects infected oral C. albicans equals 72%. Based on these assumptions and using PASS 13.0 statistical software, the minimum required sample size in the root-caries subjects or caries-free controls was 30, so we included 35 subjects per group in our study.
Pooled saliva was additionally collected from ten caries-free healthy subjects without C. albicans infection (confirmed by PCR) for the establishment of saliva-derived biofilm. Each subject was instructed to spit 5 ml of saliva directly into a sterile collection tube. Saliva samples were then pooled together and centrifuged at 2600 × g for 10 min to spin down large debris and eukaryotic cells. The supernatant was referred to as pooled saliva and used for the establishment of saliva-derived biofilm throughout this study. Part of the collected saliva was further filtered at 0.22 μm filters, and the resulting cell-free saliva was used to coat enamel blocks and glass coverslips prior to growing the in vitro biofilms. See Supplementary file for details.
Genomic DNA of dental plaque samples were isolated using QIAamp DNA micro kit (Qiagen, Valencia, CA, USA) with additional lysozyme (3 mg/ml, 1.5 h) treatment. The DNA quality was evaluated with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The detection rate of C. albicans was determined by PCR. S. mutans, S. sanguinis, C. albicans counts were quantified using qPCR. The quantification of specific microbe was further normalized by the total DNA amount of each clinical sample. See Supplementary file for details (Supplementary Tables S1 and 2).
In vitro biofilm model
S. mutans UA159, S. sanguinis ATCC10556, and C. albicans SC5314 were commercially obtained from the American Type Culture Collection, and the mutants of C. albicans used in this study were listed in Supplementary Table S3. The in vitro saliva-derived biofilm and three-species biofilm was incubated in SHI medium at 37 °C under anaerobic conditions for 24 h [39, 40]. For saliva-derived biofilm, 30 μl of pooled saliva and 2 × 102 cell/ml C. albicans or its mutants were inoculated into 1500 μl SHI medium. For three-species biofilm, inoculum for the experiment was adjusted to 2 × 106 CFU/ml of S. mutans, 2 × 107 CFU/ml of S. sanguinis and 2 × 104 cell/ml of C. albicans or its mutants (bacterial inoculum with similar ratio to which was detected in clinical samples).
After 24 h incubation, the biofilms were dispersed by sonication, and a dilution series of cell suspensions were prepared and spread on selective culture plates. The total colony-forming unit (CFU) of viable S. mutans and S. sanguinis was counted on Mitis Salivarius Agar plate (MSA, Hopebio, Qingdao, China) after 48 h anaerobic incubation at 37 °C, and the CFU of viable S. mutans was counted on the MSA plate supplemented with 0.2 U/ml bacitracin and 20% sucrose. C. albicans or its mutants were selectively cultured with CHROMagar Candida plate (Difco Laboratories) aerobically at 35 °C for 48 h before CFU counting. Genomic DNA of biofilms was also isolated using QIAamp DNA micro kit (Qiagen) with additional lysozyme (3 mg/ml, 1.5 h) treatment. Total RNA of yeast was isolated using Yeast RNAiso Kit (TaKaRa, Shiga, Japan). Total bacterial RNA from saliva-derived biofilm was isolated using TriZol Reagent (Invitrogen, Carlsbad, CA, USA) [41]. The DNA and RNA qualities were evaluated with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). The amount of each microbe and the expression levels of PHR1, PHR2, CRZ1, CRZ2, RIM101, RIM8, UME6, TPK1, TPK2, CPH1, CPH2, ldh, arcA, and ureC were determined by qPCR. See Supplementary file for details (Supplementary Tables S2, 4, and 5).
16S rRNA sequencing and analyses
16s rRNA sequencing was performed to investigate the ecological impact of C. albicans or its PHR1/PHR2 deletion mutants on saliva-derived biofilm. PCR amplification of the nearly full-length bacterial 16S rRNA genes was performed using the forward primer (5′-AGAGTTTGATCMTGGCTCAG-3′) and reversed primer (5′-ACCTTGTTACGACTT-3′). A unique 12-mer tag for each DNA sample was added to the 5′-end of both primers to pool multiple samples together. The amplicons were sequenced by the PacBio Sequel platform (Shanghai Personal Biotechnology Co., Ltd, Shanghai, China). An average of 5000 reads per sample was generated from the amplicon library. Sequence data analyses were mainly performed using QIIME (v1.8.0) and R packages (v 3.2.0), including quality control of raw data, taxonomic annotation according to NCBI database. The data were further analyzed as follows: (1) Shannon diversity index was calculated among groups. (2) The weighted UniFrac principal coordinates analysis (PCoA) and dissimilarity tests including Adonis and ANOSIM were used to examine the community differences between groups. (3) Co-occurrence analysis was performed by calculating Spearman’s rank correlations between predominant taxa. Correlations with ρ > 0.6 and P < 0.01 were constructed into an association network and imported into Cytoscape software (http://www.cytoscape.org/) for visualization [42]. (4) The relative abundance difference of bacterial taxa between groups was compared by Kruskal–Wallis test at both the genus and species levels. (5) Microbial functions were predicted by Phylogenetic investigation of communities by reconstruction of unobserved states based on the 16S rRNA gene sequence data [43].
In vitro three-species biofilm imaging and structural analyses
To visualize the composition and structure of the biofilm, C. albicans or its mutants was stained with Calcofluor White (Sigma-Aldrich, St. Louis, MO, USA) [44], and fluorescent in situ hybridization (FISH) was performed as described previously to label S. mutans and S. sanguinis with species-specific probes (Supplementary Table S6) [45]. The microbe cells and extracellular polysaccharides (EPS) were labeled with SYTO 9 (Molecular Probes, Invitrogen) and Alexa Fluor 647-labeled dextran conjugate (Molecular Probes, Life Technologies, Grand Island, NY, USA) respectively [46]. Biofilm images were captured and analyzed with a laser scanning confocal microscope equipped with Fluoview SV1000 imaging software (Olympus FV1000, Tokyo, Japan). The population of microbes and EPS were analyzed based on integral optical density (IOD) with Image pro plus 6.0 (Media Cybernetics). See Supplementary file for details.
In vitro artificial caries model
To evaluate the demineralization-promoting capability of C. albicans and its mutants on saliva-derived biofilm, the in vitro artificial caries model was established by seeding saliva-derived biofilm with/without C. albicans on human enamel blocks in SHI medium for 5 days and refreshed 1 ml medium every 24 h. The pH values of the spent media were measured with a pH meter (Thermo Fisher Scientific). The NH3 level in the medium was determined with an ammonia assay kit (Jiancheng, Bioengineering institute, Nanjing, China) at the end of a 5-day period. After 5-day incubation, the enamel blocks were prepared for transversal microradiography (TMR) analysis [47]. See Supplementary file for details.
In vivo root-caries rat model
A root-caries rat model was used to further investigate the role of C. albicans and its mutants in the pathogenesis of root caries. The animal protocol was reviewed and approved by the animal research committee of West China School of Stomatology, Sichuan University (WCHSIRB-D-2017-113). The rat model was established as previously described with modifications (Supplementary Fig. S1) [48]. Male specific-pathogen-free Sprague-Dawley rats (aged 17 days and weighing 60 ± 5 g) were purchased from Dashuo Inc. (Chengdu, China), and were provided with cariogenic diet. Rats (n = 5 per group) were either infected with C. albicans or S. mutans/S. sanguinis, S. mutans/S. sanguinis/C. albicans, S. mutans/S. sanguinis/phr2Δ/Δ, S. mutans/S. sanguinis/phr1Δ/Δ with simple randomization. Rats without infection were also included as control. The rat mouths were infected by respective microbe with the same ratio as they were in the three-species biofilm as stated above. Gingivectomies were performed for all rats on day 10 after initial infection. Animals were sacrificed 6 weeks after initial infection. The dental plaque was collected for qPCR quantification, and the jaws were collected for root-caries scoring using Doff’s system [49]. The determination of the root-caries score was blinded by codification of the jaws. See Supplementary file for details.
We estimated the sample size considering the variation and means of the samples calculated from the preliminary experiment. The type I error equals 5%, type II error equals 10%. Based on these assumptions and using PASS 13.0 statistical software, the minimum required sample size in each group was 5.
Statistical analyses
Each in vitro experiment was repeated three times. Statistical analysis of data, other than those obtained from microbiome sequencing, was performed with SPSS (v 16.0). Detection rates of C. albicans and quantitative analyses of microbial composition in the fluorescence-labeled three-species biofilm were analyzed with chi-squared test. Data obtained from clinical samples, including quantification of C. albicans, S. mutans, S. sanguinis and S. mutans/S. sanguinis ratio were analyzed with Kruskal–Wallis test followed by post hoc Dunn’s test due to asymmetrical distributions, and results are presented as median with interquartile range. Linear correlation and regression were also calculated between the level of C. albicans and S. mutans or S. mutans/S. sanguinis ratio in clinical samples. After test for homogeneity of variance, other data obtained from two independent groups were analyzed with t-test, and multiple group comparisons were performed by one-way analysis of variance test followed by Tukey’s test to compare means of each group, and Dunnett t-test to compare the means of all other groups with the control group, and results are presented as mean ± standard deviation. Data were considered significantly different if the two-tailed P value was < 0.05. Sample sizes for each experiment are detailed in the figure legends.
Results
Root carious lesions harbor increased amount of C. albicans and S. mutans
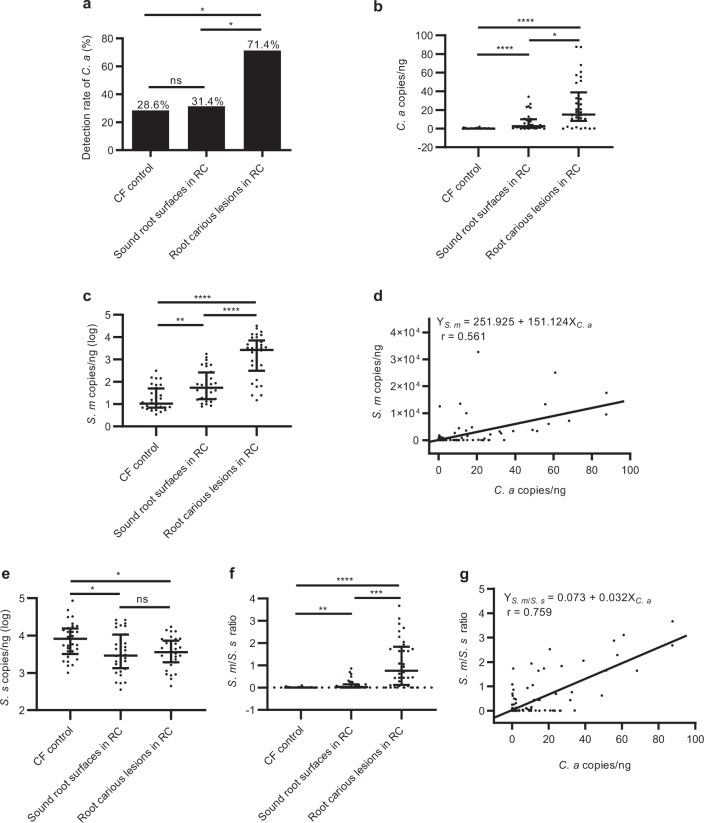
The microbial composition of supragingival dental plaque collected from root carious lesions was compared with that of sound root surfaces of root-caries subjects as well as caries-free controls. We found that C. albicans colonized more frequently at root carious lesions rather than at sound root surfaces of the same patients with root caries and the caries-free individuals (71.4%, 31.4%, and 28.6%, P < 0.05; Fig. 1a). Further quantification by qPCR showed that C. albicans was significantly enriched at root carious lesions [15.015 (27.386) copies/ng] compared to the sound root surfaces of root-caries patients [2.644 (8.738) copies/ng, P < 0.05] and the caries-free controls [0.135 (0.150) copies/ng, P < 0.0001; Fig. 1b]. In parallel, the root-caries lesions also showed an increased carriage of S. mutans [2.697 × 103 (6.284 × 103) copies/ng] as compared to the sound root surfaces of root-caries patients [5.419 × 101 (2.412 × 102) copies/ng, P < 0.0001] and the caries-free controls [1.060 × 101 (4.296 × 101) copies/ng, P < 0.0001; Fig. 1c]. A positive correlation was observed between the carriage of C. albicans and S. mutans (r = 0.561, P < 0.01; Fig. 1d). Conversely, a decreased amount of commensal S. sanguinis was observed in the root-caries lesions as compared to the caries-free controls (Fig. 1e). We further calculated the ratio of S. mutans/S. sanguinis, which was significantly higher in root carious lesions than that of the caries-free group (P < 0.0001), as well as that obtained from the sound root surfaces of root-caries patients (P < 0.001; Fig. 1f). More importantly, the S. mutans/S. sanguinis ratio showed a better positive correlation with the carriage of C. albicans detected at the same sites (r = 0.759, P < 0.01; Fig. 1g).
Fig. 1. Root carious lesions harbor increased amount of C. albicans and S. mutans.
a The detection rate of C. albicans by PCR in the root carious lesions, as well as on the sound root surfaces of root-caries patients and caries-free individuals. b, c qPCR quantification of the amount of C. albicans and S. mutans in the collected clinical samples. d Linear correlation and regression between the amount of C. albicans and S. mutans in the supragingival plaque of all recruited subjects. e qPCR quantification of the amount of S. sanguinis in the collected clinical samples. f The calculated S. mutans/S. sanguinis ratio of the corresponding samples. g Linear correlation and regression between the amount of C. albicans and S. mutans/S. sanguinis ratio in the supragingival plaque of all recruited subjects. The results are presented as median with interquartile range (a–c, e, f: n = 35 per group; d, g: n = 105; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant). RC root-caries group; CF caries-free group; S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
C. albicans alters the microbial ecology of oral biofilm
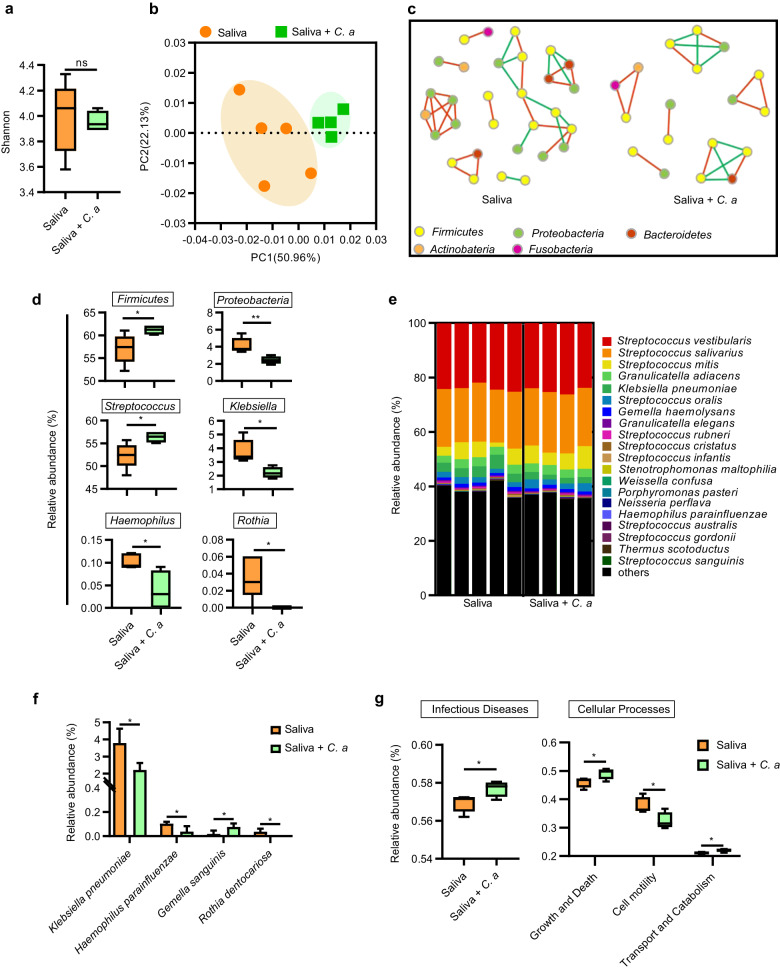
To investigate the ecological impact of C. albicans on the polymicrobial oral biofilm, we inoculated C. albicans in a saliva-derived biofilm. Data obtained from full-length 16S rRNA sequencing showed that C. albicans colonization imposed significant ecological impact on the oral biofilms. The α-diversity of oral biofilm was unaltered in the presence of C. albicans (Fig. 2a). However, the weighted PCoA based on Bray–Curtis distance (Fig. 2b) and dissimilarity tests including Adonis and ANOSIM showed distinct microbial clusters with/without C. albicans (ANOSIM: R = 0.5562, P = 0.019; Adonis: R2 = 0.34198, P = 0.012; Table 1). We also constructed co-occurrence ecological networks at the species level to better delineate how saliva-derived biofilm assemble and whether C. albicans colonization made significant impact on the oral microbial community network topology. Specifically, co-culture with C. albicans resulted in a salivary microbiome network of fewer nodes and links, and the negative relationship between species was slightly reduced (Fig. 2c).
Fig. 2. C. albicans alters the microbial ecology of oral biofilms.
a The Shannon indices and b the PCoA analysis on the polymicrobial oral biofilm in the absence/presence of C. albicans. c Network inferences of microbial relationships in biofilms. Each node represents an OTU, and each edge represents a significant pairwise association. Red lines indicate positive relationships while green lines represent negative relationships. d The relative abundance of the significantly different phyla and genera in the saliva-derived biofilm with/without C. albicans. e Relative abundance of the 20 most predominant oral bacteria at species level and f relative abundance of the significantly different species in the oral biofilm with/without C. albicans. g Prediction of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of oral biofilm in the absence/presence of C. albicans. The results are presented as mean ± standard deviation (n = 4 or 5 per group; *P < 0.05; **P < 0.01; ns not significant). S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
Table 1.
Dissimilarity tests (Adonis and ANOSIM) for the biofilm microbiota.
| ANOSIM | Adonis | |||
|---|---|---|---|---|
| R | P | R2 | P | |
| Over all | 0.2963 | 0.008 | 0.31515 | 0.003 |
| Saliva VS Saliva + C. albicans | 0.5562 | 0.019 | 0.34198 | 0.012 |
| Saliva VS Saliva + phr2Δ/Δ | −0.136 | 0.914 | 0.05396 | 0.968 |
| Saliva VS Saliva + phr1Δ/Δ | 0.352 | 0.036 | 0.25467 | 0.016 |
At phylum level, C. albicans significantly increased the growth of Firmicutes and decreased the growth of Proteobacteria (Fig. 2d). At genus level, C. albicans significantly improved the growth of Streptococcus and decreased the growth of Klebsiella, Haemophilus, and Rothia (Fig. 2d). Consistently, at species level, C. albicans significantly decreased the abundance of Klebsiella pneumoniae, Haemophilus parainfluenza, and Rothia dentocariosa and increased the abundance of Gemella sanguinis (Fig. 2e, f). Furthermore, the saliva-derived biofilm when co-culture with C. albicans, showed increased representation of predicted Kyoto Encyclopedia of Genes and Genomes pathways involved in human infectious disease and cellular processes including cell growth and death as well as transport and catabolism, and cell motility-associated pathway was decreased (Fig. 2g). All these data indicate that C. albicans could impose significant ecological impact on the oral microbiome.
C. albicans robustly interacts with oral streptococci in the polymicrobial biofilm
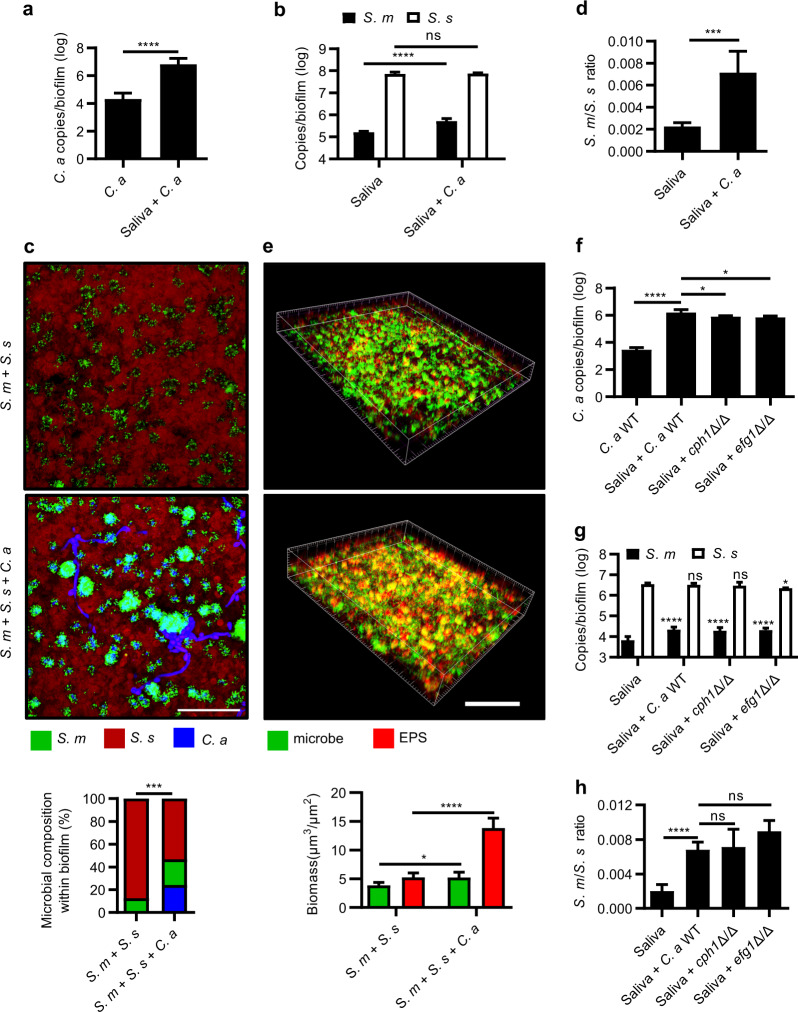
As the sequencing data showed an increased abundance of Streptococcus in the presence of C. albicans, we further quantified the carriage of C. albicans and S. mutans/S. sanguinis in the oral biofilms. Co-culture of C. albicans with oral biofilms synergistically promoted the growth of both C. albicans and S. mutans, while the growth of S. sanguinis was unchanged as demonstrated in either saliva-derived biofilms (Fig. 3a, b) or three-species biofilms by FISH, qPCR quantification (Fig. 3c and Supplementary Fig. S2a, b) as well as viable CFU counting (Supplementary Fig. S2c, d). Accordingly, the ratio of S. mutans/S. sanguinis was increased in the presence of C. albicans (Fig. 3d, Supplementary Fig. S2e, f). In addition, the biofilm generated more EPS in the presence of C. albicans as shown by laser scanning confocal microscopy (Fig. 3e). EFG1- and CPH1-deficient mutants of C. albicans were constructed to further investigate if the candidal–streptococcal interactions in the polymicrobial biofilm were hyphae-dependent. Deletion of either EFG1 or CPH1 did not affect the carriage of S. mutans, however, C. albicans and S. sanguinis in the polymicrobial biofilm were unchanged or slightly decreased as compared to the WT strain (Fig. 3f, g and Supplementary Fig. S2g–j). In parallel, the S. mutans/ S. sanguinis ratio was unchanged by the deletion of EFG1 and CPH1 compared with the biofilm co-cultured with the C. albicans WT strain (Fig. 3h and Supplementary Fig. S2k, l).
Fig. 3. C. albicans robustly interacts with oral streptococci in the polymicrobial biofilm.
a, b qPCR quantification of the amount of C. albicans, S. mutans and S. sanguinis in the saliva-derived biofilm. c Representative images of fluorescence-labelled S. mutans (green), S. sanguinis (red), and C. albicans (blue) within three-species biofilm and quantitative analyses of microbial composition. Scale bar, 60 μm. d The S. mutans/S. sanguinis ratio in the saliva-derived biofilm quantified by qPCR. e Representative confocal images of double fluorescence-labelled three-species biofilm. Bacteria and C. albicans were labelled green, EPS was labelled red, and quantitative analyses of microbe/EPS within biofilm. Scale bar, 100 μm. f–h qPCR quantification of C. albicans or its EFG1/CPH1 deletion mutants, S. mutans and S. sanguinis in the saliva-derived biofilm, and the corresponding S. mutants/S. sanguinis ratio. The control group of (g) was saliva-derived biofilm. The results are presented as mean ± standard deviation (n = 5 per group; *P < 0.05; ***P < 0.001; ****P < 0.0001; ns not significant). S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
C. albicans enhances the cariogenicity of oral biofilm and promotes the development of root caries
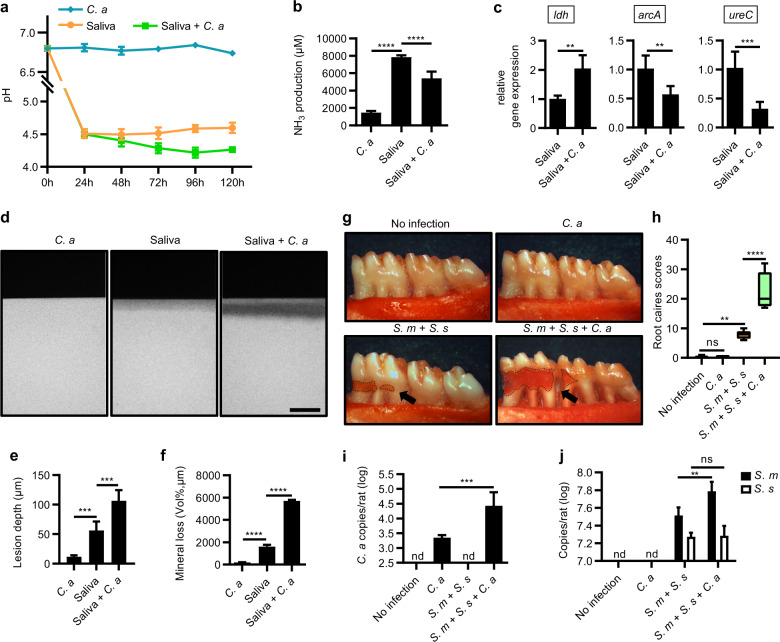
We further investigated if the altered microbial ecology in the presence of C. albicans could lead to increased cariogenicity of oral biofilm. C. albicans alone was not acidogenic in our culture conditions. However, it significantly promoted the acidogenicity of the saliva-derived biofilm as reflected by a lower supernatant pH along with decreased ammonia production relative to the C. albicans-free biofilm (Fig. 4a, b). Consistently, C. albicans significantly upregulated acid production-associated gene ldh, and downregulated ammonia production-associated genes arcA and ureC of the oral biofilm (Fig. 4c). In addition, data obtained from TMR showed that inoculation of C. albicans significantly increased the demineralized lesion depth and mineral loss of tooth hard tissue caused by the saliva-derived biofilm in the in vitro artificial caries model (Fig. 4d–f).
Fig. 4. C. albicans enhances the cariogenicity of oral biofilm and promotes the development of root caries.
a Dynamic pH values of the spent media of saliva-derived biofilm in the absence/presence of C. albicans. b NH3 production by the saliva-derived biofilm on the 5th day after inoculation. c Relative expression of ldh, arcA, and ureC of the 24 h saliva-derived biofilm. d Representative transversal microradiography (TMR) images of human enamel blocks demineralized by saliva-derived biofilm in the absence/presence of C. albicans. Scale bar, 100 μm. e, f Quantitative analyses of the lesion depth and mineral loss of the demineralized human enamel blocks by TMR. g Representative photographs of mandibular molars from gingivectomized rats infected with S. mutans, S. sanguinis, and/or C. albicans for 6 weeks. Black arrows indicated root carious lesions. h Root-caries scores according to Doff’s system. i, j Levels of C. albicans, S. mutans, and S. sanguinis in in vivo root-caries rat model quantified by qPCR. Data are presented as mean ± standard deviation (n = 5 per group; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant; nd not detectable). S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
We employed a root-caries rat model to validate the role of C. albicans in the pathogenesis of root caries in vivo. Rats co-infected with C. albicans, S. mutans, and S. sanguinis showed increased microbial carriage in the dental plaque and an exacerbated development of root caries. Specifically, infection with C. albicans alone caused nearly no root caries, while co-infection with C. albicans/S. mutans/S. sanguinis caused significantly more severe root caries compared to the rats infected with S. mutans/S. sanguinis (Fig. 4g, h). A significant increased carriage of C. albicans (16.4-fold) was observed in rats co-infected with C. albicans/S. mutans/S. sanguinis as compared to the group only infected with C. albicans (Fig. 4i). In parallel, the C. albicans/S. mutans/S. sanguinis co-infection group showed increased colonization of S. mutans as compared to the S. mutans/S. sanguinis infected group (Fig. 4j). In addition, the S. mutans/S. sanguinis ratio was also increased in the presence of C. albicans as compared to the group only infected with S. mutans/S. sanguinis (Supplementary Fig. S3).
PHR2 is the major contributor to the successful colonization of C. albicans in the polymicrobial biofilm under cariogenic condition
Since response to extreme environmental pH is critical for the pathogenicity of C. albicans, the pH-related genes of C. albicans were further investigated in the polymicrobial biofilms. PHR2 gene expression was significantly upregulated among the pH-relative genes of C. albicans in the three-species biofilms under cariogenic conditions (Fig. 5a). Deletion of PHR2 significantly suppressed the candidal–streptococcal interactions. Deletion of PHR2 significantly reduced the growth of C. albicans in the polymicrobial biofilms as quantified by qPCR and FISH (Fig. 5b–d). In addition, S. mutans/S. sanguinis ratio, as well as the growth of S. mutans and S. sanguinis in the S. mutans/S. sanguinis/phr2Δ/Δ three-species biofilm was comparable to those in the S. mutans/S. sanguinis dual species biofilm (Fig. 5c–e and Supplementary Fig. S4a). Deletion of other pH-relative genes such as PHR1, CRZ1, CRZ2, RIM101, RIM8, UME6, TPK1, TPK2, CPH1, and CPH2 also showed a similar trend, but to a lesser extent, regarding the growth of C. albicans and streptococcal composition in the three-species biofilms (Fig. 5b, e and Supplementary Fig. S4a). Consistent data regarding the growth of each species in the three-species biofilm were observed by CFU counting (Supplementary Fig. S4b–d).
Fig. 5. PHR2 contributes to the success colonization of C. albicans in the polymicrobial biofilm under cariogenic condition.
a The pH-relative genes expression of C. albicans in the S. mutans/S. sanguinis/C. albicans three-species biofilm. The control group was C. albicans-only biofilm. b qPCR quantification of C. albicans and its mutants in the three-species biofilm. The control group was C. albicans-only biofilm. c Representative images of fluorescence-labelled S. mutans (green), S. sanguinis (red), and C. albicans or its PHR1/PHR2 mutants (blue) within three-species biofilm. Scale bar, 60 μm. d Quantitative analyses of microbial composition in the fluorescence-labelled three-species biofilm based on IOD. e S. mutans/S. sanguinis ratio in the three-species biofilm quantified by qPCR. The control group was S. mutans/S. sanguinis two-species biofilm. f PCoA analysis of the polymicrobial oral biofilm in the absence/presence of C. albicans or its PHR1/PHR2 deletion mutant. g Relative abundance of the 20 most predominant oral bacteria in the absence/presence of C. albicans at genus level. h The relative abundance of Streptococcus in saliva-derived biofilm in the absence/presence of C. albicans or its mutants. i Quantification of S. mutans and S. sanguinis in oral biofilm by qPCR. The control group was saliva-derived biofilm. Data are presented as mean ± standard deviation (a, b, e: n = 3 per group; c, d, i: n = 5 per group; f–h: n = 5 or 4 per group; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant). S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
Consistent with data obtained from three-species biofilm, PHR2 gene expression was also significantly upregulated by 13.8-fold when C. albicans was co-cultured with saliva-derived biofilm, while PHR1 gene expression was unchanged (Supplementary Fig. S4e). Of note, although deletion of PHR2 did not affect the growth of C. albicans when grown alone, the growth of phr2Δ/Δ was significantly reduced when co-cultured with saliva-derived biofilm as compared to its WT strain (Supplementary Fig. S4f). The impact of C. albicans on the microbial ecology of oral biofilm was also PHR2-dependent. Both PCoA (Fig. 5f) and dissimilarity tests (ANOSIM: R = −0.1360, P = 0.914; Adonis: R2 = 0.05396, P = 0.968; Table 1) showed no significant differences in the microbial community structure in oral biofilm with/without phr2Δ/Δ. phr2Δ/Δ also showed little impact on the microbial composition of the oral biofilm, particularly the relative abundance of Streptococcus (Fig. 5g, h and Supplementary Fig. S4g). More importantly, the abundance of S. mutans, as well as the S. mutans/S. sanguinis ratio, was unchanged when the saliva-derived biofilms were grown with phr2Δ/Δ, as compared with the biofilm free of C. albicans (Fig. 5i and Supplementary Fig. S4h). However, phr1Δ/Δ showed similar synergistic interactions with streptococci in the saliva-derived biofilm as compared to its WT strain (Fig. 5f–i and Supplementary Fig. S4f–h).
PHR2 deletion deprives C. albicans of the cariogenicity-promoting ability on oral biofilm
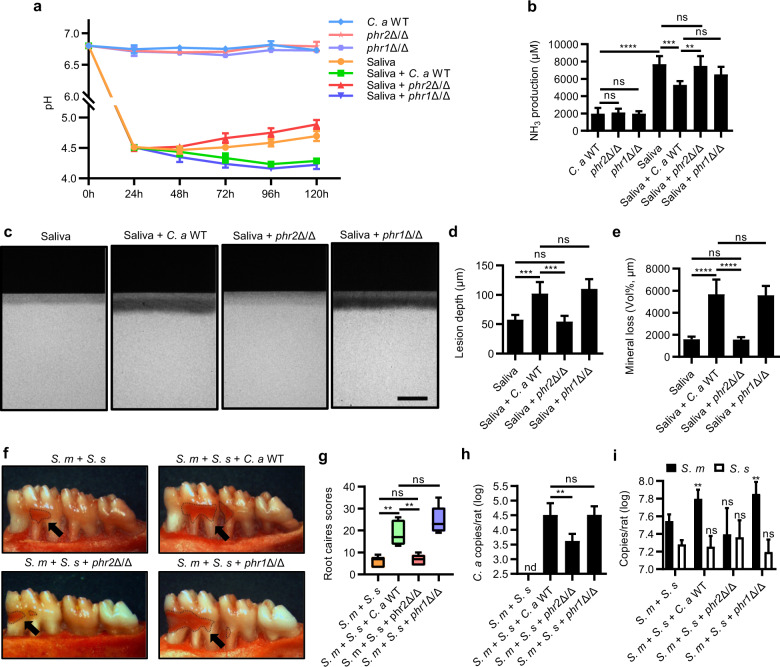
To further investigate whether the cariogenicity-promoting ability of C. albicans on oral biofilm is dependent of PHR2 or not, an in vitro artificial caries model and a root-caries rat model were further used. When the PHR2 was deleted, the cariogenicity-promoting effects of C. albicans was abolished, as reflected by the unchanged supernatant pH, ammonia production as well as ldh, arcA, or ureC gene expression of saliva-derived biofilms with/without phr2Δ/Δ (Fig. 6a, b and Supplementary Fig. S5). Consequently, the saliva-derived biofilm, when co-cultured with phr2Δ/Δ, induced less demineralization on the tooth hard tissue as compared to the biofilm co-cultured with WT C. albicans (Fig. 6c–e). Consistently, the synergistic interactions between C. albicans and streptococci on the development of root caries in vivo were also abolished when PHR2 gene was deleted (Fig. 6f–i and Supplementary Fig. S6). Conversely, deletion of PHR1 did not alter the cariogenicity-promoting effect of C. albicans on oral biofilms (Fig. 6a–i, and Supplementary Figs. S5 and S6).
Fig. 6. PHR2 deletion deprives C. albicans of the cariogenicity-promoting ability on oral biofilm.
a Dynamic pH values of the spent media of saliva-derived biofilm in the absence/presence of C. albicans or its PHR1/PHR2 deletion mutants. b NH3 production by the saliva-derived biofilm on the 5th day after inoculation. c Representative TMR images of human enamel blocks demineralized by saliva-derived biofilm in the absence/presence of C. albicans. Scale bar, 100 μm. d, e Quantitative analyses of the lesion depth and mineral loss of the demineralized human enamel blocks by TMR. f Representative photographs of mandibular molars from gingivectomized rats infected with S. mutans, S. sanguinis, and/or C. albicans/its mutants for 6 weeks. Black arrows indicated root carious lesions. g Root-caries scores according to Doff’s system. h, i Amount of C. albicans/its mutants and S. mutans/S. sanguinis in the root-caries rat model quantified by qPCR. The control group of (i) was rats infected with S. mutans and S. sanguinis. Data are presented as mean ± standard deviation (n = 5 per group; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns not significant; nd not detectable). S. s S. sanguinis; S. m S. mutans; C. a C. albicans.
Discussion
Dental caries is a result of microbial dysbiosis characterized by the enrichment of acidogenic pathogens and depletion of alkali-generating commensal microbes within the plaque biofilm [3, 50]. Root caries is a subtype of dental caries that specifically affects the exposed root of tooth, and a significant microbial dysbiosis in the root carious lesions has also been reported [9]. However, factors that drive the microbial alteration which predisposes an individual to root caries are still unclear. We found increased carriage of C. albicans in the root-caries lesions, which was positively correlated with increased number of S. mutans as well as S. mutans/S. sanguinis ratio. Further validation with in vitro polymicrobial biofilm models demonstrated that C. albicans colonization caused significant microbial dysbiosis characterized with distinct microbial composition and structure as compared to the biofilm in the absence of C. albicans. These findings are in agreement with previous studies showing that C. albicans influenced the microbial composition of saliva biofilms [51, 52]. In addition, recent study by Delaney et al. also showed that candida species had a subtle impact on the bacterial microbiome of denture patients and significantly influenced specific genera [53]. Our data showed that C. albicans enriched Streptococcus in the polymicrobial biofilms, particularly increased the ratio of S. mutans/S. sanguinis, leading to a more cariogenic biofilm that promoted the development of root caries.
Previous studies showed that C. albicans could robustly interact with oral streptococci but with distinct mechanisms. Cell-cell adhesion mediates C. albicans/Mitis Group Streptococci interactions [23, 24, 33, 54–58], while EPS plays a critical role in the C. albicans/S. mutans interactions [24, 33, 54, 55, 57–59] and also contributes to the antifungal resistance of C. albicans in the mixed biofilm [60]. Deletion of genes responsible for hyphae formation of C. albicans neither reduces microbial carriage nor inhibits cariogenicity of C. albicans/S. mutans mixed biofilm [57]. Consistently, the current study also showed that deletion of EFG1 and CPH1 (key hyphae-associated morphological regulators) [61] did not reduce the number of fungal cells in the polymicrobial biofilm under cariogenic condition as compared to the wild-type C. albicans. This is likely due to the fact that the robust production of extracellular α-glucans by S. mutans via glycosyltransferases (GTFs) under sugar-rich condition plays a major role in mediating the coaggregation and biofilm formation of C. albicans, which in turn promotes the growth and GTFs expression of S. mutans by secreting polysaccharides, quorum sensing molecules as well as metabolic cross-feedings [62–66]. The filamentation-independent synergistic interactions between C. albicans and S. mutans under cariogenic condition ultimately lead to a biofilm with enriched C. albicans and acidogenic S. mutans, and the latter competes the growth of S. sanguinis and counters the co-adherence of C. albicans/S. sanguinis, leading to an unchanged amount of S. sanguinis in the mixed microbial consortium. Deletion of EFG1 and CPH1 can compromise the co-adherence of C. albicans/S. sanguinis but still promote the accumulation of S. mutans in the polymicrobial biofilm, and this likely explains the reduced amount of S. sanguinis observed in the presence of EFG1 and CPH1 mutants.
Although the deletion of hyphae-related morphological regulators of C. albicans such as EFG1 and CPH1 did not affect its co-existence and synergistic interactions with S. mutans in the polymicrobial biofilm, the current study found that deletion of pH-relative genes, particularly PHR2 which is associated with acid adaptation of C. albicans, significantly reduced the number of fungal cells in the cariogenic biofilm. PHR1 and PHR2 are functional homologs regulated by extracellular pH through the Rim 101-dependent pathway in opposite ways. PHR1 is expressed at pH values of 5.5 or higher, whereas PHR2 has the reverse expression pattern [67–69]. They are critical factors that determine environment adaptation of C. albicans under differed pH conditions [69–71]. The current study demonstrated that PHR2, rather than PHR1 and other pH-relative genes, was significantly upregulated when C. albicans was co-cultured with oral biofilms under cariogenic conditions. More importantly, deletion of PHR2 partially restored the microbial ecology of the polymicrobial biofilm, resulting in a biofilm with decreased acidogenicity, compromised demineralizing capability and reduced cariogenicity as recapitulated by the root-caries rat model. It is noteworthy that deletion of PHR2 gene only abolished the cariogenicity-promoting effects of C. albicans on the biofilms, but the innate cariogenicity of oral biofilms under cariogenic conditions still existed. These findings suggest that the robust interactions between C. albicans with oral streptococci under cariogenic conditions make the traditional hyphae-related virulence genes not the optimal target for the reduction of biofilm cariogenicity at root-caries lesions. However, PHR2 that adapts the co-existence of C. albicans with oral streptococci under acidic/cariogenic conditions provides a potential target for the disruption of candidal–streptococcal interactions and their synergistic pathogenicity in the development of root caries.
Taken together, our data demonstrated the critical role of C. albicans infection in development of root caries. C. albicans colonization caused significant microbial dysbiosis characterized with an increased abundance of acidogenic/aciduric S. mutans, leading to a more cariogenic biofilm that promoted the development of root caries. PHR2 is the major factor that determines the successful colonization and ecological impact of C. albicans in a mixed microbial consortium under cariogenic conditions, and thus may represent a promising target for the control of root caries with candidal involvement. Further study is warranted to investigate the molecular target(s) and signaling pathways via which C. albicans binds to and interacts with oral bacteria and consequently elevates the virulence of polymicrobial oral biofilm.
Supplementary information
Acknowledgements
We greatly thank Professors Williama A. Fonzi, Dominique Sanglard, Aaron P. Mitchell, David Kadosh, Joachim F. Ernst, Gerald R. Fink, Haoping Liu and Lo Hsiu-Jung for kindly providing fungal strains. Vivian Chen is a recipient of a 2017–2018 Fulbright Research grant to China. This work was supported by the National Natural Science Foundation of China (81771099 to XX, 81870754 to XZ, 81600858 to BR, 31720103901 to Lixin Zhang), the “111” Project of China (B18022 to Lixin Zhang), a research grant from the Department of Science & Technology Sichuan Province (2018SZ0121 to XX), and the Fundamental Research Funds for the Central Universities (22221818014S to Lixin Zhang).
Author contributions
Conceptualization: QD, BR, L. Zhang, XZ, and XX; methodology: QD, BR, L. Zhang, XZ, and XX; formal analysis: QD, JH, QG, and L. Zheng; investigation: QD, JH, XP, QG; resources: XP, QG, L. Zheng; data curation: JL, HD; writing original draft: QD, BR, XZ, XX; writing, review, and editing: VC, L. Zhang, XZ, XX; visualization: QD, BR, XX; supervision: L. Zhang, XZ; funding acquisition: BR, XZ, XX.
Data availability
Microbiome sequencing data have been deposited in public database Sequence Read Archive (http://www.ncbi.nlm.nih.gov/Traces/sra) with accession no. PRJNA613946. All data sets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qian Du, Biao Ren
Contributor Information
Lixin Zhang, Email: zhanglixin@im.ac.cn.
Xuedong Zhou, Email: zhouxd@scu.edu.cn.
Xin Xu, Email: xin.xu@scu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41396-020-00823-8) contains supplementary material, which is available to authorized users.
References
- 1.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Olivera Carrilho MR. Root caries: from prevalence to therapy. Karger Medical and Scientific Publishers: Basel, 2017.
- 3.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Prim. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 4.Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–203.. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68:4018–23.. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikx FH, van der Hoeven JS, Plasschaert AJ, Konig KG. Establishment and symbiosis of Actinomyces viscosus, Streptococcus sanguis and Streptococcus mutans in germ-free Osborne-Mendel rats. Caries Res. 1976;10:123–32.. doi: 10.1159/000260196. [DOI] [PubMed] [Google Scholar]
- 7.Mitrakul K, Vongsawan K, Sriutai A, Thosathan W. Association between S. mutans and S. sanguinis in severe early childhood caries and caries-free children a quantitative real-time PCR analysis. J Clin Pediatr Dent. 2016;40:281–9. doi: 10.17796/1053-4628-40.4.281. [DOI] [PubMed] [Google Scholar]
- 8.Loesche W, Rowan J, Straffon L, Loos P. Association of Streptococcus mutans with human dental decay. Infect Immun. 1975;11:1252–60.. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Qin B, Du M, Zhong H, Xu Q, Li Y, et al. Extensive description and comparison of human supra-gingival microbiome in root caries and health. PLoS One. 2015;10:e0117064. doi: 10.1371/journal.pone.0117064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon-Soro A, Guillen-Navarro M, Mira A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J Oral Microbiol. 2014;6:25443. doi: 10.3402/jom.v6.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babatzia A, Papaioannou W, Stavropoulou A, Pandis N, Kanaka-Gantenbein C, Papagiannoulis L, et al. Clinical and microbial oral health status in children and adolescents with type 1 diabetes mellitus. Int Dent J. 2020;70:136–44.. doi: 10.1111/idj.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zomorodian K, Kavoosi F, Pishdad GR, Mehriar P, Ebrahimi H, Bandegani A, et al. Prevalence of oral Candida colonization in patients with diabetes mellitus. J Mycol Med. 2016;26:103–10.. doi: 10.1016/j.mycmed.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Mun M, Yap T, Alnuaimi AD, Adams GG, McCullough MJ. Oral candidal carriage in asymptomatic patients. Aust Dent J. 2016;61:190–5. doi: 10.1111/adj.12335. [DOI] [PubMed] [Google Scholar]
- 14.Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: a disease of opportunity. J Fungi. 2020;6:15. [DOI] [PMC free article] [PubMed]
- 15.Diaz PI, Hong BY, Dupuy AK, Strausbaugh LD. Mining the oral mycobiome: methods, components, and meaning. Virulence. 2017;8:313–23.. doi: 10.1080/21505594.2016.1252015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shay K, Truhlar MR, Renner RP. Oropharyngeal candidosis in the older patient. J Am Geriatr Soc. 1997;45:863–70.. doi: 10.1111/j.1532-5415.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 17.Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–8. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35.. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 19.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71.. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 20.Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8. doi: 10.1111/j.1574-6968.2009.01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamford CV, d’Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009;77:3696–704.. doi: 10.1128/IAI.00438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886. doi: 10.1371/journal.ppat.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–32.. doi: 10.1128/IAI.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo H, Andes DR, Krysan DJ. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018;14:e1007342. doi: 10.1371/journal.ppat.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, et al. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014;16:214–31.. doi: 10.1111/cmi.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Sobue T, Bertolini M, Thompson A, Dongari-Bagtzoglou A. Streptococcus oralis and Candida albicans synergistically activate mu-calpain to degrade E-cadherin from oral epithelial junctions. J Infect Dis. 2016;214:925–34.. doi: 10.1093/infdis/jiw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Sobue T, Bertolini M, Thompson A, Vickerman M, Nobile CJ, et al. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence. 2017;8:1602–17.. doi: 10.1080/21505594.2017.1326438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozano Moraga CP, Rodriguez Martinez GA, Lefimil Puente CA, Morales Bozo IC, Urzua, Orellana BR. Prevalence of Candida albicans and carriage of Candida non-albicans in the saliva of preschool children, according to their caries status. Acta Odontol Scand. 2017;75:30–5. doi: 10.1080/00016357.2016.1244560. [DOI] [PubMed] [Google Scholar]
- 29.Wu N, Lin J, Wu L, Zhao J. Distribution of Candida albicans in the oral cavity of children aged 3-5 years of Uygur and Han nationality and their genotype in caries-active groups. Genet Mol Res. 2015;14:748–57.. doi: 10.4238/2015.January.30.18. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Moon Y, Li L, Rustchenko E, Wakabayashi H, Zhao X, et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLoS One. 2016;11:e0164242. doi: 10.1371/journal.pone.0164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao J, Grier A, Faustoferri RC, Alzoubi S, Gill AL, Feng C, et al. Association between oral candida and bacteriome in children with severe ECC. J Dent Res. 2018;97:1468–76.. doi: 10.1177/0022034518790941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, et al. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 2018;52:102–12.. doi: 10.1159/000481833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–81.. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beighton D, Lynch E. Relationships between yeasts and primary root-caries lesions. Gerodontology. 1993;10:105–8. doi: 10.1111/j.1741-2358.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 35.Shen S, Samaranayake LP, Yip HK. Coaggregation profiles of the microflora from root surface caries lesions. Arch Oral Biol. 2005;50:23–32. doi: 10.1016/j.archoralbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Zaremba ML, Stokowska W, Klimiuk A, Daniluk T, Rozkiewicz D, Cylwik-Rokicka D, et al. Microorganisms in root carious lesions in adults. Adv Med Sci. 2006;51 Suppl 1:237–40. [PubMed] [Google Scholar]
- 37.Dige I, Nyvad B. Candida species in intact in vivo biofilm from carious lesions. Arch Oral Biol. 2019;101:142–6. doi: 10.1016/j.archoralbio.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Ev LD, Dame-Teixeira N, Do T, Maltz M, Parolo CCF. The role of Candida albicans in root caries biofilms: an RNA-seq analysis. J Appl Oral Sci. 2020;28:e20190578. doi: 10.1590/1678-7757-2019-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edlund A, Yang Y, Hall AP, Guo L, Lux R, He X, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1:25. doi: 10.1186/2049-2618-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian Y, He X, Torralba M, Yooseph S, Nelson KE, Lux R, et al. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol. 2010;25:357–67.. doi: 10.1111/j.2041-1014.2010.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Ren B, Zhou X, Xu HH, Chen Y, Han Q, et al. Effect of antimicrobial denture base resin on multi-species biofilm formation. Int J Mol Sci. 2016;17:e1033. [DOI] [PMC free article] [PubMed]
- 42.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21.. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–8. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klug B, Rodler C, Koller M, Wimmer G, Kessler HH, Grube M, et al. Oral biofilm analysis of palatal expanders by fluorescence in-situ hybridization and confocal laser scanning microscopy. J Vis Exp. 2011;56:e2967–e. doi: 10.3791/2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, III, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du Q, Fu M, Zhou Y, Cao Y, Guo T, Zhou Z, et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Sci Rep. 2020;10:2961. doi: 10.1038/s41598-020-59733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Firestone AR, Graves CN, Feagin FF. The effects of different levels of dietary sucrose on root caries subsequent to gingivectomy in conventional rats infected with Actinomyces viscosus M-100. J Dent Res. 1988;67:1342–5. doi: 10.1177/00220345880670101901. [DOI] [PubMed] [Google Scholar]
- 49.Doff RS, Rosen S, App G. Root surface caries in the molar teeth of Rice rats. I. A method for quantitative scoring. J Dent Res. 1977;56:1013–6. doi: 10.1177/00220345770560080301. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Chen F, Huang Z, Ma L, Chen L, Pan Y, et al. Meeting report: a close look at oral biofilms and microbiomes. Int J Oral Sci. 2018;10:28. doi: 10.1038/s41368-018-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambooij JM, Hoogenkamp MA, Brandt BW, Janus MM, Krom BP. Fungal mitochondrial oxygen consumption induces the growth of strict anaerobic bacteria. Fungal Genet Biol. 2017;109:1–6. doi: 10.1016/j.fgb.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Janus MM, Crielaard W, Volgenant CM, van der Veen MH, Brandt BW, Krom BP. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9:1270613. doi: 10.1080/20002297.2016.1270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delaney C, O’Donnell LE, Kean R, Sherry L, Brown JL, Calvert G, et al. Interkingdom interactions on the denture surface: implications for oral hygiene. Biofilm. 2019;1:100002. doi: 10.1016/j.bioflm.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang G, Marsh G, Gao L, Waugh R, Koo H. Binding force dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res. 2015;94:1310–7. doi: 10.1177/0022034515592859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregoire S, Xiao J, Silva BB, Gonzalez I, Agidi PS, Klein MI, et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl Environ Microbiol. 2011;77:6357–67.. doi: 10.1128/AEM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13:e1006407. doi: 10.1371/journal.ppat.1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D, Koo H. Spatial design of polymicrobial oral biofilm in its native disease state. J Dent Res. 2020;99:597–603. doi: 10.1177/0022034520909313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–42.. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim D, Liu Y, Benhamou RI, Sanchez H, Simon-Soro A, Li Y, et al. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018;12:1427–42.. doi: 10.1038/s41396-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38.. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D, Sengupta A, Niepa TH, Lee BH, Weljie A, Freitas-Blanco VS, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7:41332. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sztajer H, Szafranski SP, Tomasch J, Reck M, Nimtz M, Rohde M, et al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014;8:2256–71.. doi: 10.1038/ismej.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi DCP, Gleason JE, Sanchez H, Schatzman SS, Culbertson EM, Johnson CJ, et al. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017;13:e1006763. doi: 10.1371/journal.ppat.1006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellepola K, Truong T, Liu Y, Lin Q, Lim TK, Lee YM, et al. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans–Candida albicans mixed-species biofilms. Infect Immun. 2019;87:e00339–19. [DOI] [PMC free article] [PubMed]
- 66.Khoury ZH, Vila T, Puthran TR, Sultan AS, Montelongo-Jauregui D, Melo MAS, et al. The role of Candida albicans secreted polysaccharides in augmenting Streptococcus mutans adherence and mixed biofilm formation: in vitro and in vivo studies. Front Microbiol. 2020;11:307. doi: 10.3389/fmicb.2020.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baek YU, Martin SJ, Davis DA. Evidence for novel pH-dependent regulation of Candida albicans Rim101, a direct transcriptional repressor of the cell wall beta-glycosidase Phr2. Eukaryot Cell. 2006;5:1550–9. doi: 10.1128/EC.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muhlschlegel FA, Fonzi WA. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol. 1997;17:5960–7. doi: 10.1128/mcb.17.10.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saporito-Irwin SM, Birse CE, Sypherd PS, Fonzi WA. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–13.. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bernardis F, Muhlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–25.. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calderon J, Zavrel M, Ragni E, Fonzi WA, Rupp S, Popolo L. PHR1, a pH-regulated gene of Candida albicans encoding a glucan-remodelling enzyme, is required for adhesion and invasion. Microbiology. 2010;156:2484–94.. doi: 10.1099/mic.0.038000-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microbiome sequencing data have been deposited in public database Sequence Read Archive (http://www.ncbi.nlm.nih.gov/Traces/sra) with accession no. PRJNA613946. All data sets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.