In a study published in Cellular & Molecular Immunology, we described a novel mechanism by which IL-33 drives hepatic fibrosis through the MAPK pathway. The study demonstrated that hepatocyte-derived IL-33 is elevated upon bile duct ligation (BDL)-induced liver injury and fibrogenesis and activates hepatic stellate cells in a JNK/ERK/p38-dependent manner.1 As a member of the IL-1 family, IL-33 promotes CD4+ T helper 2 (Th2) cytokines but suppresses Th1 cytokines via IL-4, IL-6, and IL-10 production. Th cells are central to the adaptive immune response activation and suppression of B cells and cytotoxic T cells by releasing different types of cytokines in tissues. These regulations are essential for immune homeostasis and suppression of excessive response against self-antigens to prevent autoimmunity. Liver transplantation surgery is a complex procedure accompanied by graft or major organ ischemia-reperfusion, life-threatening hemorrhage, and homeostasis instability. Recipients with viral hepatitis, alcoholic hepatitis, or hepatic carcinoma could have underlying liver inflammation, and increased secretion of cytokines may result in graft rejection. This commentary will focus on the crucial role of IL-33/ST2 signaling in liver transplantation and highlight the potential of intervening in ST2/IL-33 signaling as a treatment option.
Previous reports suggested that IL-33 is consistently expressed by multiple organs and cell types in mice and humans, particularly in the nuclei of fibroblasts, epithelial cells, high endothelial venules, and hepatocytes.2 Using immunofluorescence, we also demonstrated that IL-33 is consistently expressed in albumin-labeled hepatocytes other than CK-19-positive cholangiocytes or GFAP-positive hepatic stellate cells during BDL-associated liver damage. Upon tissue damage, IL-33 is immediately released into the extracellular space and acts as an alarmin during acute tissue injury. Membrane-bound ST2 activates the MyD88/NF-κB signaling pathway, contributing to Th2, regulatory T cell (Treg), and innate lymphoid cell type 2 functions, which regulate late immune responses and enhance or suppress inflammatory reactions (Fig. 1).
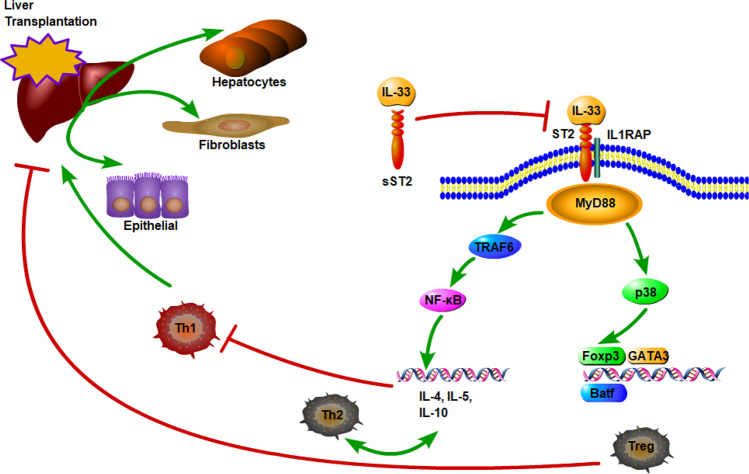
Fig. 1.
Diagram representing the probable mechanisms of IL-33/ST2 signaling in liver transplantation. Constitutively expressed IL-33 is released upon liver damage and binds to either the ST2/IL-1 receptor accessory protein (IL-1RAP) heterodimer, recruiting MyD88 to its intracellular domain, or the sST2 decoy receptor, which does not signal. Through NF-κB and Foxp3 activation, IL-33 enhances Th2-associated cytokines and promotes Treg differentiation, which induces alloimmune suppression and graft tolerance
Liver ischemia-reperfusion is an inevitable process in organ procurement and transplantation, in which the blood supply is excluded and reconstructed, and free oxygen radicals and proinflammatory cytokines are produced, resulting in a magnified inflammatory response in grafts and recipients. Initial research showed that IL-33 protein expression increases within 4 h of reperfusion and remains elevated for up to 8 h. Recombinant IL-33 reduces hepatocyte cell death as a result of increased NF-κB, p38, cyclin D1 activation, and Bcl-2 expression in hepatocytes.3 Sakai et al. also demonstrated that IL‐33 may have direct protective effects on hepatocytes, since ST2 is detected only in hepatocytes but not in the specialized liver macrophages, Kupffer’s cells. Núñez et al. showed that upregulated IL-33 predicts diminished liver damage in a steatosis-associated liver I/R model.4 Yazdani et al. found that liver ischemia and reperfusion injury cause the formation of neutrophil extracellular traps (NETs) that contribute to tissue damage in liver surgery. Liver sinusoidal endothelial cells release IL-33, which potentially promotes NET formation during liver I/R injury, exacerbating inflammatory cascades and inflammation.5 The discordance of these findings may result from the use of different schedules of IL-33 administration. Alternately, pretreatment with IL-33 may stimulate ST2-positive innate immune cells, which might eliminate further immune responses and liver damage. Thus, to understand the comprehensive function of IL-33, more investigations focusing on the shift in the immune microenvironment are required.
IL-33 was initially introduced as an important cytokine that induces Th2 cell differentiation. We did not observe diminished liver infiltration of Th2 cells in ST2-deficient mice. We hypothesize that liver infiltrating cells were harvested only 1 day after BDL. If inflammation persists, the hepatic microenvironment may differentiate T helper cells.
During transplantation, Th1 cells secrete proinflammatory cytokines such as IL-2, TNF, and IFN-γ that activate cytotoxic T lymphocytes and macrophages, facilitating graft destruction in several ways. Facilitation includes upregulating major histocompatibility complex-II, inducing allograft-specific cytotoxicity, inducing alloantibody class switching to complement-fixing IgG2a, and producing chemokines in the graft.6 Thus, the Th1-polarizing cytokine IL-2 is widely used as a therapeutic target to prevent solid organ rejection (FK506). Th2 cells that produce IL-4, IL-5, and IL-10 are implicated in suppressing Th1 differentiation and inducing graft tolerance.7 In 2018, Ferhat et al. showed that pretreatment with IL-33 was therapeutic for hepatic I/R injury, possibly via activation of invariant NKT (iNKT) cells. This pretreatment contributes to a shift from the initial proinflammatory (pro-Th1) profile of iNKT cells into their pro-Th2 resolving profile.8 Using 81 living donor liver transplant recipients, Lee et al. demonstrated a significant decrease in IFN-γ/IL-6, IFN-γ/IL-10, TNF-α/IL-6, and TNF-α/IL-10 ratios at 1 h after reperfusion, suggesting a shift in the Th1/Th2 cytokine ratio toward Th2.7 In 2019, Park et al. clarified the effect of ischemia time on recipient immune responses by analyzing serum IFN-γ, IL-6, IL-10, IL-2, and IL-17 in 171 LDLT recipients. At 1 h after reperfusion, IFN-γ/IL-6 and IFN-γ/IL-10 ratios were significantly increased when the warm-ischemia time was longer than 22 min, indicating upregulated Th1 cell activities with increasing warm-ischemia times.9 Both of these studies had limitations. First, all cytokines were measured by ELISA on samples collected from peripheral blood rather than tissue outflow. Second, Th cell shifting during transplantation procedures should be demonstrated by flow cytometry instead of by circulating cytokines. Therefore, the direct effect of the Th1/Th2 immune balance on liver transplantation is unknown. The impact of IL-33-mediated signals on transplantation rejection/tolerance requires further investigation.
The role of IL-33 in solid organ transplantation was initially examined in a mouse heart transplantation model. Yin et al. demonstrated that Th1-polarized CD4 T cells do not express the IL-33 membrane receptor ST2L. Moreover, IL-33 enhances expression of the Th2-associated cytokines IL-5 and IL-13 but not IFN-γ in Th cells. Treatment of recipient mice with recombinant IL-33 results in the improvement of allograft survival (>20 days) when compared with that of control groups (all <9 days).10 In an MHC class II-mismatched chronic heart rejection model, Brunner and colleagues found that exogenous IL-33 administration significantly prolonged allograft survival, which was associated with decreased IL-17A and increased Th2 (IL-10, IL-13, and IL-5) cytokine production. In addition, flow cytometry showed facilitation of Treg and CD11bhi Gr1int myeloid-derived suppressor cells in peripheral myeloid and lymphoid cells.11 Similar results were observed by Turnquist et al., emphasizing the immunoregulatory property of IL-33-induced Tregs.12
Mechanistically, Tregs are a subset of CD4+ T cells that are required for normal immune homeostasis and impair antitumor immune responses. Tregs are associated with transplantation tolerance in multiple ways.13 Matta et al. found that IL-33-expanded Tregs express ST2 and classical markers associated with the Treg phenotype and suppressor function.14 Yang et al. showed that ST2-expressing Tregs eliminate allogeneic hematopoietic cell transplantation-related graft-versus-host disease (GVHD) through RORγt expression.15 Recently, using a genome-wide DNA methylation landscape, Delacher et al. identified that ST2+ Tregs expand from donor thymus-derived Tregs, specifically in the liver, with stepwise acquisition of chromatin accessibility and reprogramming toward the nonlymphoid-tissue Treg phenotype via activation of the transcription factor Batf.16 Further studies suggest that the administration of recombinant IL-33 substantially increases ST2+ Tregs by 13-fold in the liver in a p38-Foxp3-dependent manner, which protects mice against GVHD.13 Cottagiri et al. suggested that IL-33 reduces anesthetic hepatitis via upregulation of Foxp3+CD4+CD25+ T cells in an ST2-independent manner.17 Therefore, further studies are required to investigate the role of IL-33 in human liver disease.
Serum soluble ST2 (sST2) is a decoy receptor of IL-33 signaling that is increased in epithelial cells and cardiac myocytes. Accumulating evidence from both experimental models and clinical assessments supports the hypothesis that elevated sST2 predicts rejection of small bowel and cardiac transplantation. Patients who have high concentrations of sST2 also have increased likelihood of treatment-resistant GVHD.18,19 More importantly, Zhang et al. found that the sST2/mST2 ratio in the small and large intestine significantly increases during hematopoietic cell transplantation-induced GVHD. Using a neutralizing monoclonal antibody to systemically inhibit sST2, the protective function of IL-33-responding cells was found to be predominantly enhanced in the peritransplant period.20
Therefore, although IL-33/ST2 signaling is important in regulating the immune response, more insights are required into IL-33/ST2-related cytokine profiles, Treg differentiation, and Th cell shifting. Modulation of IL-33/ST2 bioactivity might be used as a therapeutic strategy in liver transplantation.
Acknowledgements
This work was suppo rted by grants from the National Natural Science Foundation of China (81972675 to Z.T. and 81930086 to B.S.).
Competing interests
The authors declare no competing interests.
References
- 1.Tan Z, et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell Mol. Immunol. 2018;15:388–398. doi: 10.1038/cmi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Sakai N, et al. Interleukin-33 is hepatoprotective during liver ischemia/reperfusion in mice. Hepatology. 2012;56:1468–1478. doi: 10.1002/hep.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunez KG, et al. Interleukin-33/cyclin D1 imbalance in severe liver steatosis predicts susceptibility to ischemia reperfusion injury. PLoS ONE. 2019;14:e0216242. doi: 10.1371/journal.pone.0216242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdani, H. O. et al. IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation. J. Hepatol. 2017. In press. [DOI] [PMC free article] [PubMed]
- 6.Dallman MJ. Cytokines and transplantation: Th1/Th2 regulation of the immune response to solid organ transplants in the adult. Curr. Opin. Immunol. 1995;7:632–638. doi: 10.1016/0952-7915(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 7.Lee HM, et al. Changes in the ratio of T helper 1 to T helper 2 signature cytokines in patients undergoing living donor liver transplantation surgery: a prospective controlled study. Transplant. Proc. 2018;50:3621–3625. doi: 10.1016/j.transproceed.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 8.Ferhat MH, et al. The impact of invariant NKT cells in sterile inflammation: the possible contribution of the alarmin/cytokine IL-33. Front. Immunol. 2018;9:2308. doi: 10.3389/fimmu.2018.02308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CS, et al. Ischemic time of graft liver forces Th1-to-Th2 activity toward Th1 activity in patients who underwent living donor liver transplantation. Eur. Cytokine Netw. 2019;30:23–28. doi: 10.1684/ecn.2019.0422. [DOI] [PubMed] [Google Scholar]
- 10.Yin H, et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2 -type immune deviation. Transplantation. 2010;89:1189–1197. doi: 10.1097/TP.0b013e3181d720af. [DOI] [PubMed] [Google Scholar]
- 11.Brunner SM, et al. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl. Int. 2011;24:1027–1039. doi: 10.1111/j.1432-2277.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- 12.Turnquist HR, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J. Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai C, et al. Recombinant IL-33 prolongs leflunomide-mediated graft survival by reducing IFN-gamma and expanding CD4(+)Foxp3(+) T cells in concordant heart transplantation. Lab Investig. 2016;96:820–829. doi: 10.1038/labinvest.2016.54. [DOI] [PubMed] [Google Scholar]
- 14.Matta BM, Turnquist HR. Expansion of regulatory T cells in vitro and in vivo by IL-33. Methods Mol. Biol. 2016;1371:29–41. doi: 10.1007/978-1-4939-3139-2_3. [DOI] [PubMed] [Google Scholar]
- 15.Yang, J. et al. Rorc restrains the potency of ST2+ regulatory T cells in ameliorating intestinal graft-versus-host disease. JCI Insight. 4, 2019. In press. [DOI] [PMC free article] [PubMed]
- 16.Delacher M, et al. Precursors for nonlymphoid-tissue treg cells reside in secondary lymphoid organs and are programmed by the transcription factor BATF. Immunity. 2020;12:295–312. doi: 10.1016/j.immuni.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cottagiri M, et al. In drug-induced, immune-mediated hepatitis, interleukin-33 reduces hepatitis and improves survival independently and as a consequence of FoxP3+ T-cell activity. Cell Mol. Immunol. 2019;16:706–17. doi: 10.1038/s41423-018-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual-Figal DA, et al. Soluble ST2 is a marker for acute cardiac allograft rejection. Ann. Thorac. Surg. 2011;92:2118–2124. doi: 10.1016/j.athoracsur.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Mathews LR, et al. Elevated ST2 distinguishes incidences of pediatric heart and small bowel transplant rejection. Am. J. Transplant. 2016;16:938–950. doi: 10.1111/ajt.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci. Transl. Med. 2015;7:308ra160. doi: 10.1126/scitranslmed.aab0166. [DOI] [PMC free article] [PubMed] [Google Scholar]