Abstract
Switch/sucrose-non-fermentable (SWI/SNF) chromatin remodeling complexes are critical regulators of chromatin dynamics during transcription, DNA replication, and DNA repair. A recently identified SWI/SNF subcomplex termed GLTSCR1/1L-BAF (GBAF; or “non-canonical BAF”, ncBAF) uniquely contains bromodomain-containing protein BRD9 and glioma tumor suppressor candidate region 1 (GLTSCR1) or its paralog GLTSCR1-like (GLTSCR1L). Recent studies have identified a unique dependency on GBAF (ncBAF) complexes in synovial sarcoma and malignant rhabdoid tumors, both of which possess aberrations in canonical BAF (cBAF) and Polybromo-BAF (PBAF) complexes. Dependencies on GBAF in malignancies without SWI/SNF aberrations, however, are less defined. Here, we show that GBAF, particularly its BRD9 subunit, is required for the viability of prostate cancer cell lines in vitro and for optimal xenograft tumor growth in vivo. BRD9 interacts with androgen receptor (AR) and CCCTC-binding factor (CTCF), and modulates AR-dependent gene expression. The GBAF complex exhibits overlapping genome localization and transcriptional targets as bromodomain and extraterminal domain containing (BET) proteins, which are established AR-coregulators. Our results demonstrate that GBAF is critical for coordinating SWI/SNF – BET cooperation and uncover a new druggable target for AR-positive prostate cancers, including those resistant to androgen deprivation or antiandrogen therapies.
INTRODUCTION
Prostate cancer is the second leading cause of cancer-related deaths in men worldwide. Prostate cancer can initially be treated by surgery, radiotherapy, and androgen deprivation [1]. While these treatments are initially effective, patients eventually develop castration resistance. Currently, castration resistant prostate cancer (CRPC) is treated with androgen receptor (AR)-antagonists such as enzalutamide and apalutamide [2]; however, resistance to these drugs also eventually develops.
AR-mediated transcription is dependent on coregulatory proteins to recruit AR, increase chromatin accessibility for AR binding, and/or enhance AR transactivation [3]. Targeting the coregulatory proteins of AR is being explored as a novel therapy option to overcome resistance [3]. One such target is the set of bromodomain and extra terminal domain (BET) proteins, which directly interact with AR and are required for AR localization and target gene expression [4–6]. Similar to BET proteins, switch/sucrose nonfermentable (SWI/SNF) chromatin remodeler complexes play critical roles in AR-mediated transactivation [7–10]; however, therapeutic targeting of SWI/SNF has not been previously explored. Although 20% of malignancies have genetic alterations in SWI/SNF subunits [11,12], prostate cancer rarely has SWI/SNF mutations [13], which may imply a requirement for intact SWI/SNF complexes in prostate cancer. Recent studies identified BRG1, the ATPase subunit of SWI/SNF complexes, as an essential factor for survival of PTEN-null prostate cancer [14], and ARID1A-containing BAF complexes as a cofactor for ETS transcription factors, translocation of which are common genetic drivers in prostate cancer [15].
SWI/SNF complexes can be grouped into three subcomplexes of differing size. The most recently characterized SWI/SNF subcomplex is GLTSCR1/1L-BAF (GBAF), also called noncanonical BAF (ncBAF), which lacks the core BAF subunits ARID, BAF47, and BAF57, but includes unique subunits GLTSCR1/1L and BRD9 [16–22] (Fig. 1a). Although not essential for viability in most cell lines, BRD9 is required for a subset of leukemias [23,24], as well as SWI/SNF-perturbed synovial sarcoma [18,19] and rhabdoid tumors [19,20]. Core SWI/SNF subunits have been investigated in prostate cancer [7,8,10,25]; however, the specific role of GBAF in SWI/SNF-intact cancers has not been addressed. We demonstrate that the GBAF complex is critical for maintenance of prostate cancer cells in vitro and in vivo. Knockdown of non-redundant GBAF subunit BRD9 or its inhibition using BRD9 bromodomain ligands reduces AR target gene expression. BRD9 interacts with AR and BET proteins, specifically BRD2 and BRD4, and is required for AR binding at target genes. This study suggests GBAF acts in concert with BET proteins and AR to regulate CRPC progression.
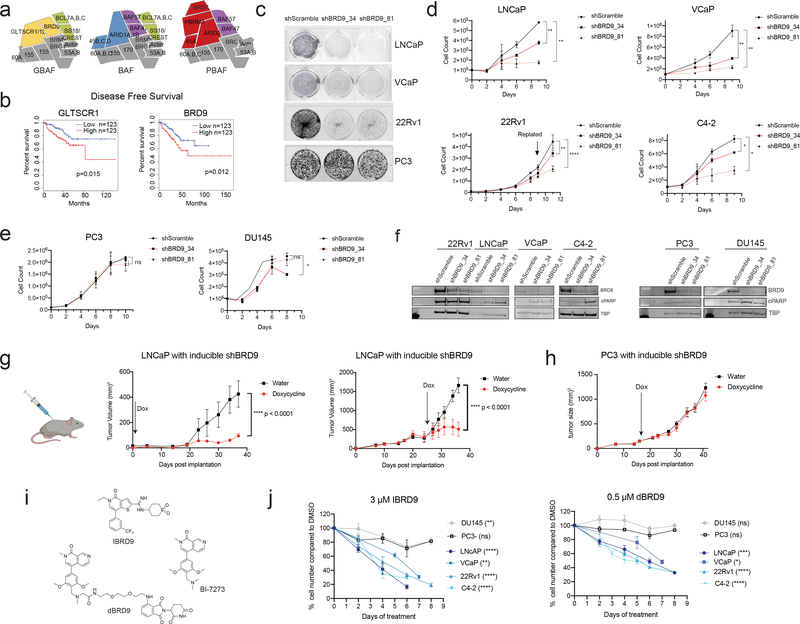
Fig. 1:
BRD9 in prostate cancer cell lines. a Subunit composition of three distinct SWI/SNF subcomplexes. b Kaplan-Meier curves for disease-free survival calculated using TCGA data for PRAD. c Crystal violet staining of prostate cancer cells after 12 days (12d) (LNCaP, VCaP, PC3) and 14d (22Rv1) with lentiviral-mediated shBRD9. d,e Growth curves of cells with doxycycline-inducible BRD9 knockdown. n=3 f Immunoblot of BRD9 and cleaved PARP (cPARP) from whole cell lysates following 6d of inducible BRD9 knockdown. TBP = TATA-box binding protein. g Mouse xenograft of LNCaP cells expressing doxycycline-inducible shBRD9. (left) Administration of doxycycline in drinking water immediately after injection of cells in nude mice. Tumors harvested 37d after injection. (right) Administration of doxycycline in drinking water after average tumor size reaches ~200 mm3 in NRG mice (26d post injection). Tumors harvested 36d after injection. Lines represents the average tumor size from control or knockdown groups (n = 7), error bars represent s.d. h Mouse xenograft of PC3 cells expressing doxycycline-inducible shBRD9. Administration of doxycycline in drinking water after average tumor size reaches ~150 mm3 in NRG mice (17d post injection). Tumors harvested 42d after cell injection. Lines represents the average tumor size from control or knockdown groups (n = 7), error bars represent s.d. i Chemical structures of BRD9 bromodomain inhibitors I-BRD9 and BI-7273, and BRD9 degrader dBRD9. j Cells plated with DMSO, 3 μM I-BRD9, or 0.5 μM dBRD9. Viable cell counts taken at designated time points are normalized to DMSO control. Data points represent two separate experiments each with 3–6 replicates. error bars represent s.d. Statistical significance for all growth curves determined using two-way ANOVA. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
MATERIALS AND METHODS
Cell Lines and Cell Culture
LNCaP (clone FGC) (RRID:CVCL_1379), VCaP (RRID:CVCL_2235), PC3 (RRID:CVCL_0035), RWPE-1 (RRID:CVCL_3791), HEK293T (RRID:CVCL_0063), DU145 (RRID:CVCL_0105), 22Rv1 (RRID:CVCL_1045) and C4–2 (RRID:CVCL_4782) cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). HEK293T and VCaP cells were cultured in DMEM media supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 g/ml streptomycin, and 2 mM L-alanyl-L-glutamine (Corning Glutagro™) and 1: 10000 plasmocin (Invivogen, San Diego, CA). LNCaP and 22Rv1 cells were cultured in RPMI 1640 and PC3 cells were cultured in F12K media with the same supplements as above. DU145 and C4–2 cells were cultured based on ATCC suggestions. RWPE-1 was cultured in Keratinocyte SFM (Gibco 17005–042, Thermo Scientific). All other media and supplements were obtained from Corning Mediatech, Inc. For androgen deprivation studies, charcoal-dextran stripped FBS was used at 10% with phenol red-free DMEM or RPMI 1640. All cultures were used up to passage number 20 and tested monthly for Mycoplasma contamination with MycoAlertTM Mycoplasma Detection Kit (Lonza, Switzerland).
Compounds
I-BRD9, JQ1, MDV3100 (enzalutamide) and MZ-1 were purchased from Cayman Chemicals (Ann Arbor, MI). dBRD9 was purchased from Tocris (Minneapolis, MN). Dihydrotestosterone was purchased from Millipore-Sigma (Burlington, MA).
Antibodies
Antibodies are listed in Supplementary Table 1.
Primers
Primers for qRTPCR, ChIP, and FAIRE are listed in Supplementary Table 2.
Statistics
Statistics were performed using GraphPad Prism 8. Statistical details are provided in figure legends and within the text.
Generation of stable cell lines
GLTSCR1L and GLTSCR1 knockout LNCaP cell lines were generated as previously reported [16]. BRD9-targeting shRNA sequences, shBRD9#34 (TRCN0000127634) and shBRD9#81 (TRCN0000131081) were cloned into pLKO.1-puro (Addgene plasmid ID #8453, RRID:Addgene_8453) for constitutive knockdown and tet-pLKO-puro (Addgene plasmid ID #21915, RRID:Addgene_21915) for inducible knockdown. MSCV-BRD9_N216A-PGK-Puro-IRES-GFP (Addgene plasmid # 75116) and MSCV-BRD9-PGK-Puro-IRES-GFP (Addgene plasmid # 75114) were gifts from Christopher Vakoc. GLTSCR1 was synthesized by Biomatik (Wilmington, DE). BRD9, BRD9-N216A, and GLTSCR1 were amplified with N-terminal (BRD9) or C-terminal (GLTSCR1) tags and subcloned into EcoRI-digested TetO-FUW-puro (Addgene plasmid #85747) using In-Fusion HD cloning kit (Takara, USA). Cells were assayed within a month following lentiviral transduction and selection.
Cell proliferation and survival assays
Cells (300,000/well in 6-well) were plated six days (6d) after transduction with lentivirus containing shBRD9. HEK293T and PC3 cells were plated at 20 000 cells/well. Cells were fixed in ice-cold methanol and stained with crystal violet to assess viability. For inhibitor treatments, cells (4000/well) were plated in 96-well plates and treated with varying concentrations of I-BRD9 for 4d in complete media. Alternatively, 300,000 cells/well were plated in 6-well plates and treated with inhibitors (3 μM I-BRD9 or 0.5 μM dBRD9), which were refreshed every 48 hours (48h). Percent viability was assessed using CellTiter-Glo® reagent (Promega, Madison, WI), or typan blue-based call counting, and calculated compared to DMSO-treated cells. For exogenous GLTSCR1L and BRD9 expression, 4000 cells were plated in 96-well plate with 200 ng/mL or 20 ng/mL doxycycline, respectively. Cells were treated with enzalutamide for 4–5d. Viability was measured using CellTiter-Glo® reagent.
RT-qPCR assays
RNA was extracted using TRIzol® (Ambion, Inc.) and cDNA was synthesized using Verso cDNA synthesis kit (Thermo Scientific) with 3:1 oligo dT: random hexamer oligonucleotides. Specific targets were amplified using PowerUp SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in Biorad CFX qPCR instrument. For I-BRD9 inhibition studies, 200,000–300,000 cells were plated into a 6-well and treated with various concentrations of the inhibitor for 4–5 days. For induction studies, cells were plated in charcoal-stripped serum (CSS) containing medium. Next day, the cells were pretreated with I-BRD9 for 24h before inducing AR target genes by switching to regular medium. RNA was harvested 7–8h and 24–30h post-induction. For GLTSCR1L or BRD9 expression studies, the cells were plated in CSS-containing medium and gene expression was induced with 200 ng/mL (for GLTSCR1L) or 20 ng/mL (for BRD9) doxycycline. 48h post-induction, the media was replaced with regular medium and RNA was harvested 8h or 24h later.
Immunoblotting
Whole cell lysates or nuclear lysates were quantified using BCA Assay (Pierce, Rockford, IL). Equal amounts of protein were electrophoresed on Novex 4–12% precast gel or 10% hand-cast gels; transferred onto 0.45μm PVDF membrane and blocked 1h in 5% BSA in TBST. After overnight incubation in primary antibodies, the membrane was incubated with infrared-dye labeled Licor goat anti-mouse or anti-rabbit antibodies. The images were acquired using Li-Cor® imaging system.
ChIP-qPCR and ChIP-seq
For ChIP-seq, eight million LNCaP cells were plated in 15 cm dishes. Two days later, the cells were treated with 10 μM I-BRD9 for 24h, and harvested. The plate was rinsed in PBS + 1 mM MgCl2 three times, crosslinked in 1% formaldehyde for 10 minutes at RT, and quenched with 125 mM Gly for 5 min. After extensive ice-cold PBS washes, the cell pellet was flash-frozen and stored at −80 until the time of processing. The pellet was sequentially extracted and washed in L1, L2 and L3 buffers with protease inhibitors [26]. After the final wash, the pellet was resuspended in sonication buffer 10 mM Tris pH 8.0; 1 mM EDTA; 0.1% SDS and sonicated using Branson SFX250 at 20% amplitude with 0.5s on and 1.5s off cycles for 8 minutes (2 minutes on). The immunoprecipitation was performed as previously reported [17]. DNA concentration and size was determined using Agilent Bioanalyzer and FluorNanodrop by Purdue Genomics Core Facility and libraries of samples with 200–500 bp average size were generated using Ovation Ultralow System V2 UDI according to manufacturer’s directions. Libraries were sequenced in NovaSeq 6000 platform (Novogene, Sacramento, CA). For ChIP-qPCR, experiments were performed above, or for androgen induction, cells were first cultured in CSS for 48h before treatment with DMSO, 1 μM JQ1 or 10 μM I-BRD9 for 18h and induction with regular FBS medium for 4h.
ChIP-Seq Analysis:
Quality Control and Mapping:
Sequencing was performed using Illumina technology to generate > 30 million paired-end reads for each sample. Two biological replicates were combined for each condition. Sequence data quality was determined using FastQC software [27] and quality based trimming and filtering (minimum quality score 30 and minimum read-length 20) were performed using TrimGalore [28]. Retained reads (>95%) were mapped to the human reference genome (hg19) using Bowtie2 [29] with a maximum of 1 mismatch. Overall mapping rate was >92% for all samples. Bowtie2 derived BAM files were further filtered to retain the reads with minimum MAPping Quality (MAPQ) 10. See Supplementary Table 3 for sample data.
Peak-calling, annotation and visualization:
Peak calling was performed using epic2 [30] for each Input-ChIP pair with MAPQ10 filtered BAM files and the default parameters (--falsediscovery-rate-cutoff 0.05, --binsize 200). Peak annotation was performed with R-package ChIPseeker [31]. Genome-wide heatmap of peak score distribution was generated using deepTools [32]. Motif analysis was performed using Homer [33]. Peak overlap and peak randomization were performed using bedtools Multiple Intersect and ShuffleBed [34].
RNA sequencing, RNA-seq analysis
All RNA samples with described treatments were harvested with Trizol reagent at indicated end points. For 3d BRD9 knockdown: After selection with puromycin, LNCaP cells expressing inducible scrambled shRNA or BRD9-targeting shRNA (shBRD9_81) were plated and treated with 2 μg/ml doxycycline for 3d. For 11d knockdown, LNCaP cells were transduced with lentiviruses encoding scrambled shRNA or BRD9 shRNA (shBRD9_81), selected for 8d with puromycin (2 μg/mL) and cultured for 3d. For I-BRD9 treatment, LNCaP cells were plated and treated with DMSO or 5 μM I-BRD9 for 3d. For androgen induction, LNCaP cells were cultured in charcoal-stripped serum (CSS) medium for 48h, treated with 10 μM enzalutamide, 5 μM I-BRD9, 500 nM dBRD9 or 500 nM JQ1 for 6h then switched to regular FBS-containing medium or fresh CSS medium for 18h with fresh compound. RNA-Seq libraries were generated and sequenced by Novogene and reads were mapped to the hg19 genome. Differentially expressed genes were calculated using edgeR using padj<0.5. Raw sequencing data for I-BET151 (0.5 μM I-BET151 for 48h; GEO GSE103907) and enzalutamide (10 μM ENZA for 48h; GEO GSE110903) treatment in LNCaP cells was analyzed using the same methods.
Mouse xenograft
All studies were approved by the Purdue Institutional Animal Care and Use Committee. Fourteen, five-week old male NCr nude mice (Taconic, Rensselaer, NY) were subcutaneously injected in the right flank with 3 million LNCaP tet-on shBRD9 cells in 200 μL PBS: Matrigel (Corning) (1:1) mixture. Following injection, the mice were randomized to receive regular drinking water (n=7) or 1.2 mg/mL doxycycline hyclate (Fagron, Inc.) with 2% sucrose in drinking water (n=7). The water was refreshed twice a week and tumor size measured using a caliper. The same cell line was injected into fourteen 12-week old male NRG mice (generated in-house) at the same density. After tumor size reached 200 mm3, the mice were randomized, and doxycycline treatment was initiated. PC3 tet-on shBRD9 injections were performed in the same way with NRG mice. After the tumor size reached 150 mm3, the mice were randomized, and doxycycline treatment initiated.
Immunohistochemistry
Formalin fixed and paraffin embedded sections of the subcutaneous tumors were deparaffinized, rehydrated and boiled in 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval. The sections were stained for Brd9 and Ki-67, followed by incubation with biotinylated secondary antibodies. Detection was performed with Vectastain Elite ABC kit (Vector Labs) and DAB reagent (Vector Labs). Sections were counterstained with hematoxylin.
Immunoprecipitation
Forty million LNCaP or VCaP cells were harvested by trypsinization following relevant treatments. The cells were washed in ice-cold PBS and lysed in 2 mL Buffer A (20 mM HEPES, pH 7.9, 25 mM KCl, 10% glycerol, 0.1% Nonidet P-40 with protease inhibitors) for 7 min on ice. Nuclei were pelleted at 600 x g for 10 minutes and resuspended in lysis buffer (20 mM HEPES, pH 7.5, 200 mM KOAc, 0.2% NP-40, 2 mM MgCl2) containing protease inhibitors and benzonase at 125 U/ml. For the IPs with compound treatment, DHT (10nM), enzalutamide (10 μM), JQ1 (2 μM) or I-BRD9 (10 μM) was spiked into the extraction and IP buffers. Nuclei were lysed at 27 °C for 30 minutes on a thermoshaker, and the lysate was cleared at 21,000 x g, for 30 minutes at 4 °C. Protein was quantitated using Pierce 660nm assay and lysate containing 200–300 μg was incubated for 3h with Protein A Dynabeads preconjugated to rabbit IgG, BRD4, BRG1, BAF155 or BRD9 antibodies. The beads were washed 3 times in wash buffer (20 mM HEPES pH 7.5, 100 mM KCl, 10% glycerol, 0.1% NP-40, protease inhibitors) or lysis buffer. Beads were resuspended in 1x LDS sample buffer and proteins were eluted by shaking at 90 °C for 10 minutes. Immunoprecipitates and inputs (5%) were loaded on 4–12% polyacrylamide gels and separated for immunoblotting.
Glycerol Gradient sedimentation analysis
Ten million LNCaP cells with inducible expression of V5-tagged wild-type BRD9, V5-tagged bromodomain-mutant BRD9 (BRD9-N216A), V5-tagged GLTSCR1, or FLAG-tagged GLTSCR1L were plated on a 15 cm culture dish and treated with doxycycline (1 μg/mL) for 2d. Cells were harvested, nuclear proteins were extracted and 0.5 mg extracts were resolved on 10–30% glycerol gradient according to the published protocol [16]. Twenty fractions of 0.5 mL were collected per sample and 30 μL of each fraction was separated on SDS-PAGE.
FAIRE-qPCR
500,000 cells LNCaP cells were cultured in charcoal-stripped serum (CSS) medium for 2d. Next, cells were treated in triplicate with 3 μM I-BRD9, 500 nM dBRD9 or 500 nM JQ1 for 18h in CSS medium followed by addition of 10 nM dihydrotestosterone (DHT) for an additional 4h. Cells were processed for FAIRE according to published protocol [35].
Sequencing Data
Processed and unprocessed RNA-Seq and ChIP-Seq Data has been deposited at GEO GSE146625. Published datasets utilized in this study are listed in Supplemental Table 4.
RESULTS
1. BRD9 knockdown reduces the viability of prostate cancer cells in vitro
Analysis of TCGA prostate cancer expression data, as well as a recent tissue microarray study [36], reveals that primary prostate tumors have elevated GLTSCR1 expression compared to normal prostate tissue (Supplementary Figure 1a). Higher GLTSCR1 and BRD9 expression correlates with decreased disease-free survival (Fig. 1b), and higher GLTSCR1 protein expression correlates with increased tumor invasion and metastasis [36]. Using CRISPR-Cas9, we knocked out GLTSCR1 and GLTSCR1L in the LNCaP prostate cancer cell line, and observed a mild decrease in cell growth (Supplementary Figure 1b). Our attempts to generate dual knockout LNCaP cell lines were unsuccessful, indicating that cells with complete loss of mutually-exclusive paralogous subunits GLTSCR1/1L cannot be recovered. Therefore, we used short hairpin RNA (shRNA) to knock down BRD9, a non-redundant subunit exclusive to GBAF complexes [16–20] (Supplementary Figure 1c). Two different shBRD9 constructs abrogated cell growth in AR-positive cell lines LNCaP, VCaP, and 22Rv1, but not AR-negative cell line PC3 (Fig. 1c). Similarly, doxycycline-inducible shBRD9 significantly decreased the growth rate of AR-positive cell lines LNCaP, VCaP, 22Rv1 and C4–2 (Fig. 1d), without affecting AR-negative prostate cancer cell lines PC3 and DU145 (Fig. 1e). In addition, shBRD9 in AR positive cells lines resulted in increased cleaved PARP (cPARP) levels, while AR-negative cells were minimally affected (Fig. 1f). Likewise, no growth effect was observed with shBRD9 knockdown in embryonic kidney cell line HEK293T (Supplementary Figure 1d), or immortalized normal prostate cell line RWPE-1 (Supplementary Figure 1e). The BRD9 dependency in AR-dependent cell lines is not related to differences in BRD9 expression levels, as there was no correlation between BRD9 levels and sensitivity to knockdown (Supplementary Figure 1f).
2. BRD9 knockdown reduces tumor growth in xenograft models
We next investigated the effect of BRD9 reduction in vivo using a xenograft mouse model. LNCaP cells expressing doxycycline-inducible shBRD9 were injected subcutaneously into the flanks of NCr nude mice. Doxycycline treatment immediately after injection resulted in significantly smaller tumors compared to control groups, in many cases preventing tumor development altogether (Fig. 1g). When tumors were first allowed to reach 200 mm3, doxycycline treatment slowed tumor growth (Fig. 1g), resulting in smaller tumor masses (Supplementary Figure 1g) with reduced BRD9 and Ki67 staining (Supplementary Figure 1h). In contrast, doxycycline-induced BRD9 knockdown in PC3 cells showed minimal effect on tumor growth (Fig. 1h, Supplementary Figure 1i).
3. BRD9 inhibition reduces the viability of prostate cancer cells in vitro
BRD9 contains a bromodomain that can be targeted by several selective inhibitors, such as I-BRD9 [37] or BI-7273 [38], as well as the PROTAC degrader, dBRD9 [39] (Fig. 1i). Five days of I-BRD9 treatment led to a dose-dependent reduction in cell viability in LNCaP, VCaP, 22Rv1, and C4–2, with IC50 values around 3 μM (Supplemental Figure 1j), similar to concentrations reported for inhibition of BRD9 target gene expression [37] and BRD9 chromatin binding [17]. We observed a reduction in RWPE-1 viability at concentrations above 5 μM, indicating potential off-target effects and limiting the usable concentration to 1–5 μM (Supplemental Figure 1j). We also tested PROTAC degrader dBRD9, which induces complete loss of BRD9 protein in prostate cell lines with no effect on BRD2 levels (Supplemental Figure 1k). When treated with 3 μM I-BRD9 or 0.5 μM dBRD9, the proliferation of AR-positive cell lines was significantly reduced, while AR-negative cell lines were not strongly affected (Fig 1j).
4. BRD9 knockdown has overlapping transcriptional effects with AR inhibition
To gain insight into the transcriptional effects of BRD9 knockdown, we performed RNA-seq in LNCaP cells with 3d of doxycycline-induced shBRD9. We identified 2461 genes differentially expressed >1.3-fold relative to untreated cells (padj<0.05) (Fig. 2a-left). Consistent with the viability defects, general processes like cell cycle, DNA replication, and protein metabolism were enriched in differentially expressed genes (DEGs) from shBRD9 (Fig. 2a-right). In addition, prostate cancer genes were enriched, prompting us to investigate whether BRD9 is involved in AR-dependent gene regulation. We compared DEGs from LNCaPs with shBRD9 to DEGs from LNCaPs treated with the anti-androgen enzalutamide (ENZA) [40]. We observed the largest overlap between genes decreased with shBRD9 and decreased with ENZA, and Gene Set Enrichment Analysis (GSEA) indicated significant enrichment only for genes decreased with ENZA (Fig 2b). DEGs from 11d of shBRD9 in LNCaP cells correlated strongly with DEGS from 3d of shBRD9, although with a larger amplitude of change (Supplementary Figure 2a). Notably, AR transcription was decreased with 11d but not 3d shBRD9 (Supplemental Figure 2a,b), implicating AR downregulation as a secondary effect of BRD9 knockdown that may contribute to long-term viability defects.
Fig. 2:
BRD9 regulates AR-dependent genes. a (Left)-Volcano plot of gene expression changes in LNCaP cells after 3d of doxycycline-inducible shBRD9. Differentially expressed genes (DEGs, padj<0.05, FC> 1,3) are depicted in red. (right)- the most highly enriched KEGG pathways for DEGS with shBRD9, listed with lowest p value at the top. b (left)- Venn diagram of DEG overlap from 3d with shBRD9 and 48h with 10 μM ENZA [40]. (Right)- GSEA with 3d shBRD9 RNA-Seq for sets of genes increased or decreased with 10 μM ENZA for 48h[40]. c Immunoblot of BRD9 immunoprecipitation from LNCaP nuclear lysates. LNCaP cells were cultured in charcoal-stripped serum (CSS)-containing medium and treated with 10 nM DHT for 18h after 6h pretreatment with DMSO or 10 μM enzalutamide. d qRT-PCR analysis of AR-target gene expression in prostate cancer cells expressing inducible shBRD9 for 3d. Data shown are representative of two independent experiments. Error bars represent s.d. n = 3. Statistical significance determined using unpaired t-test. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
We next tested whether AR and BRD9 proteins physically interact. LNCaP cells were cultured in charcoal-stripped serum (CSS)-containing medium for 48h to deplete androgens and then pretreated with ENZA or DMSO before induction with dihydrotestosterone (DHT). AR was enriched with BRD9 immunoprecipitation only with DHT treatment (Fig. 2c). Notably, the nuclear import of AR was enhanced by ligand binding, as evidenced in the input lanes of nuclear lysates (Fig. 2c). This might indicate that it is not the ligand binding per se but the subcellular localization of AR that determines its interaction with BRD9. When we added DHT and enzalutamide into nuclear extracts from cells grown in normal media, the immunoprecipitation of BRD9 enriched similar amounts of AR (Supplementary Figure 2c), indicating that BRD9 associates with nuclear AR, but that the association is not necessarily directly dependent on ligand binding.
We next tested whether AR target genes are affected by shBRD9 in other AR-positive cell lines. We utilized a series of prostate cancer cell lines with varying degrees of castration resistance. LNCaP has a mutation in the AR ligand binding domain that allows for activation by additional ligands [41], VCaP and 22Rv1 cells express both ligand-responsive and ligand-unresponsive copies of AR [42,43], and C4–2 cells are fully androgen-insensitive [44]. BRD9 knockdown across these lines uniformly resulted in decreased expression of several AR-target genes (Fig. 2d). Similar to LNCaP, longer shBRD9 treatment (10–15 d) in VCaP and 22Rv1 cells more robustly decreased AR target gene expression, but also moderately decreased AR and ARv7 mRNA and protein levels, complicating the interpretation (Supplementary Figure 2d, Supplementary Figure 2e).
5. BRD9 bromodomain inhibition has overlapping transcriptional effects with BRD9 knockdown and AR inhibition
We also performed RNA-seq from LNCaP cells treated with 3 μM I-BRD9 for 3d, identifying 4461 differentially expressed genes (fold change >1.3, padj <0.05) (Fig 3a). DEG overlap from shBRD9 and I-BRD9 treatments is largest for genes decreased in both conditions; however, both increased and decreased genes from shBRD9-treated cells are significantly enriched in I-BRD9-treated cells (Fig. 3b). There are discrepancies in gene regulation between shBRD9 and I-BRD9 due to 1) off-target effects for I-BRD9 or the shRNA construct, 2) different functional outputs from depletion of BRD9 compared to inhibition of the full GBAF complex, or 3) the timing of transcriptional effects from gene knockdown compared to inhibition. Despite these differences, I-BRD9 treatment also enriched for genes downregulated with ENZA (Fig. 3c), and overlaped with shBRD9 treatment in the regulation of the majority of these gene targets (Fig. 3d). We tested the effect of I-BRD9 on the expression of AR-regulated transcripts in LNCaP, VCaP, 22Rv2, and C4–2 cell lines and observed dose-dependent response to I-BRD9 following a similar trend as observed in Fig 2d with shBRD9 (Fig. 3e). The expression of CENPN and FKBP5, two AR-target genes expressed in the AR-negative RWPE-1 prostate epithelial cell line, are not affected by I-BRD9 (Supplementary Figure 2f).
Fig 3:
Transcriptional effects of I-BRD9 in LNCaP cells. a Volcano plot of DEGs in LNCaP cells after 3d treatment with 3 μM I-BRD9. DEGs (padj<0.05) are depicted in red. b (left) Venn diagram of DEG overlap from 3d I-BRD9 and 3d shBRD9 treated LNCaP cells, (right)- GSEA of I-BRD9 RNA-Seq using gene sets comprised of the top 500 increased or decreased DEGs with shBRD9. c (left) Venn diagram of DEG overlap from 3d I-BRD9 and 2d enzalutamide (10 μM ENZA)-treated LNCaP cells (Zhang et al., 2018). (right)- GSEA of I-BRD9 RNA-Seq with sets of genes increased or decreased with ENZA for 48h [40]. d Venn diagram of decreased DEGs from LNCaP cells treated with I-BRD9 for 3d, shBRD9 for 3d or ENZA for 2d [40]. e qRT-PCR analysis of prostate cancer cells treated with I-BRD9 for 4d. Data shown are representative of two independent experiments. Error bars represent s.d. n = 3. Statistical significance determined using unpaired t-test. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
6. GBAF associates with BET proteins BRD2 and BRD4
Previous evidence suggests that BET proteins and GBAF subunits GLTSCR1 and BRD9 physically interact [16,17,45–48]. Since BET family member BRD4 is well-established as an AR-coregulator required for AR-dependent prostate cancer [4,5], we first explored whether this interaction is preserved in prostate cancer cell lines. In LNCaP and VCaP cells, BRD9 immunoprecipitated BET proteins BRD2 and BRD4, but not BRD3 (Fig. 4a). Conversely, BRD4 immunoprecipitated BRD9, GLTSCR1/1L and BAF155, but not BAF47 (Fig. 4b). Since BAF47 is specific to cBAF and PBAF complexes, this observation suggests that SWI/SNF complexes interact with BRD4 through the GBAF subcomplex. Supporting this finding, degradation of BRD9 decreased the amount of BAF155 immunoprecipitated with BRD4, as well as the amount of BRD2 and BRD4 immunoprecipitated with BRG1 (Fig. 4b).
Fig. 4:
BRD9 associates with BRD2 and BRD4 in prostate cancer cells. Immunoblots of a BRD9 immunoprecipitation (IP) from LNCaP and VCaP nuclear extracts, b BRG1 and BRD4 IP from nuclear extracts of VCaP cells treated with 0.5 μM BRD9 degrader (dBRD9) for 24h, c BRD4 and BRG1 IP from nuclear extracts of LNCaP cells with sgGLTSCR1 or sgGLTSCR1L, d BRG1 IP from nuclear extracts of LNCaP cells expressing inducible FLAG-tagged GLTSCR1L or GLTSCR1 treated with doxycycline for 48h, e BRD9 and BRD4 IP from nuclear extracts of VCaP cells treated with 0.5 μM JQ1 or 10 μM I-BRD9 for 24h, f V5 IP from nuclear extracts of LNCaP cells expressing doxycycline-inducible V5-tagged wild type BRD9 (V5-BRD9) or bromodomain-mutant BRD9 (V5-BRD9 N216A) treated with doxycycline for 48h, and g VCaP cells cultured with 100 nM MZ-1 for 20h before BRD9 IP. Same blot as in Figure 8e stained for CTCF. h (Left) - Venn diagram of overlapping DEGs from LNCaP cells treated with shBRD9 for 3d (this study) or 0.5 μM I-BET151 for 2d [5]. (Right)- GSEA of I-BET151 RNA-Seq using gene sets comprised of the top 500 differentially expressed genes increased or decreased with shBRD9. i Overlapping DEGs decreased >1.3 fold with 48h 10 μM ENZA-treatment [40], 48h 0.5 μM I-BET151 treatment [5], and 72h shBRD9 (this study).
While BRD9 degradation decreased GLTSCR1 association with BRG1, it increased GLTSCR1L association (Fig. 4b), leading us to investigate their contribution to BET protein interaction. Similar to HEK293T cells [16] BRG1 immunoprecipitated similar amounts of BRD9 in LNCaP cells with knockout of GLTSCR1 or GLTSCR1L, indicating biochemical compensation within GBAF complexes (Fig. 4c). Overexpression of either paralog reduced the protein expression of the other paralog in lysates, as well as the amount of the other paralog associated with BRG1 (Fig. 4d). The knockout or overexpression of either paralog did not significantly change GBAF association with BET proteins or AR (Fig. 4c,d), indicating that GLTSCR1 and GLTSCR1L are redundant for BET protein association.
To determine the contribution of the bromodomains on the interaction between BRD9 and BET proteins, we performed reciprocal IPs in the presence of bromodomain inhibitors. The interaction between BRD9 and BRD4/BRD2 was reduced in the presence of either JQ1 or I-BRD9 (Fig. 4e). Additionally, expression of wild type BRD9 immunoprecipitated BRD2 and BRD4 while bromodomain-mutant BRD9 (BRD9-N216A) immunoprecipitated less BRD4 and BRD2 (Fig. 4f). These results indicate that bromodomains of both BRD9 and BET proteins, at least partially, contribute to the interaction between these proteins, similar to what has been observed in ES cells [17]. Since BRD9-N216A also displayed significantly reduced enrichment of AR (Fig. 4f), and BRD4 is a direct binding partner of AR [4], we used the BET protein degrader MZ-1 [49] to define whether the interaction between BRD9 and AR is mediated through BET proteins. We observed a substantial decrease in AR association with BRD9 after MZ-1 treatment, indicating that the association between BRD9 and AR is facilitated, at least in part, through BET proteins (Fig. 4g).
7. BRD9 and BET proteins have overlapping transcriptional targets
To define if BRD9 and BET proteins regulate similar sets of genes, we compared DEGs from shBRD9 and DEGs from LNCaP cells treated with IBET151, a BET bromodomain inhibitor similar to JQ1 [5]. We observed an overlap in DEGs between the two treatments, and DEGS with IBET151 were enriched for genes regulated by shBRD9 in both directions, although genes with decreased expression show the strongest overlap and enrichment (Fig. 4h). Previous studies [4,6] identified co-regulation of AR gene targets by BET proteins, which we confirmed by comparing DEGs from I-BET151 [5] and ENZA treated LNCaP cells [40], (Supplementary Figure 3). We further observed a large overlap in DEGS from all three conditions (Fig. 4i), potentially indicating that GBAF and BET proteins cooperate functionally to regulate at least a subset of AR target genes.
8. GBAF facilitates AR-target gene induction.
BET protein inhibition affects steady state AR-mediated transcription, as well as the induction of AR-target genes following androgen depletion (Supplementary Figure 4a). Therefore, we adapted a steroid starvation and replacement approach to monitor the effect of BRD9 inhibition on induction of AR-target genes after androgen stimulation (Fig. 5a). When grown in charcoal-stripped serum (CSS)-containing medium, LNCaP and VCaP cells display ~50% reduced proliferation and 22Rv1 cells display 25% reduced proliferation compared to FBS-containing medium (Supplementary Figure 4b), consistent with the fact that 22Rv1 cells contain one AR isoform lacking ligand binding domain. Similarly, androgen deprivation with CSS reduces the AR-target gene expression in LNCaP and VCaP cells while gene expression in 22Rv1 cells is less affected by androgen deprivation (Supplementary Figure 4c). Using this approach coupled to RNA sequencing, we found no DEGs with ENZA treatment in CSS conditions, validating that CSS successfully depleted androgens (Fig. 5b). Upon switching from CSS to regular FBS in control conditions, approximately 1846 genes were upregulated. Since FBS may contain other steroids that regulate gene expression, we designated the androgen-induced genes as the subset of FBS-induced genes that are downregulated when pretreated with ENZA (1135 genes) (Supplementary Figure 4d). Using this same approach, we found that treatment with JQ1, IBRD9 and dBRD9 all reduced the induction of several hundred androgen-induced genes, including known AR target genes KLK3, CENPN, FKBP5, KLK2, TMPRSS2, and MAF (Fig. 5c). We further employed RT-qPCR to verify the ability of I-BRD9, dBRD9, and BI-7273 to delay the induction of AR-target genes upon addition of regular FBS (Fig. 5d, Supplementary Figure 4f) or dihydrotestesterone (DHT) (Supplemental Figure 4e) in LNCaP and VCaP cells. Similarly, expression of exogenous mutant BRD9 (N216A) reduces AR target gene induction compared to expression of exogenous WT BRD9 (Supplementary Figure 4g).
Fig 5.
BRD9 regulation of androgen-induced gene expression. a Scheme for compound treatment (DMSO, 10 μM enzalutamide (ENZA), 5 μM IBRD9, 500 nM dBRD9, or 500 nM JQ1) in CSS medium (CSS) or FBS medium (FBS). b. Principle component analysis of LNCaP RNA-Seq samples. c Venn diagram of the overlap between androgen-induced genes (genes increased with FBS and decreased with ENZA-Supplementary Figure 4b) and genes decreased in I-BRD9, dBRD9 or JQ1 treated cells in FBS [e.g. the genes with lower transcription in induced-treated sets (FBS I-BRD9, FBS dBRD9, FBS JQ1) relative to induced control (FBS DMSO)]. d qRT-PCR of AR-target genes during androgen stimulation and 5 μM I-BRD9. e Immunoblot of BAF155 immunoprecipitation from nuclear lysates of LNCaP cells stably transduced with doxycycline inducible empty vector (Empty) or GLTSCR1L-FLAG. f Inducible GLTSCR1L-expressing LNCaP cells were grown in CSS containing medium and treated with doxycycline for 2d. Medium was switched to standard FBS containing medium (FBS) with doxycycline. Data shown are representative of two independent experiments. Error bars represent s.d. n = 3. Statistical significance determined using unpaired t-test. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
To investigate whether an increase in GBAF complexes is sufficient to drive AR-target gene expression, we overexpressed GLTSCR1L, which increases the overall stoichiometry of GBAF complexes. This is evident from increased BRD9 levels in lysates, an increase of BRD9 with BAF155 immunoprecipitations, and an increase in BAF155 levels in BRD9-containing glycerol gradient fractions (Fig. 5e, Supplementary Figure 4h). In contrast, overexpression of BRD9 increases BRD9 monomer levels with no increase in BAF155 levels in BRD9-containing glycerol gradient fractions (Supplementary Figure 4h), and no increase of GLTSCR1 protein levels in lysates (Supplementary Figure 4i). Overexpression of GLTSCR1L in LNCaP cells led to higher basal levels of some AR target genes in CSS conditions (Fig 5f, Supplementary Figure 4j), and significantly increased the expression of all tested AR target genes upon the switch to regular FBS compared to control cells (Fig. 5f). This implies that increasing the amount of GBAF can increase AR activity at target loci. ENZA treatment reduced the expression of AR targets in cells with GLTSCR1L overexpression, but TMPRSS2 maintained higher expression when compared to control cells (Supplementary Figure 4j). In addition, GLTSCR1L overexpression reduced growth inhibition by ENZA (Supplementary Figure 4k), raising the possibility that enhanced AR signaling by GLTSCR1L and enhanced cell survival under anti-androgen treatment could account for the clinical correlation between GBAF subunit expression and poorer prognosis in patients (Supplementary Figure 1a).
9. BRD9 and BRD4 have overlapping genomic binding sites
To identify the genomic targets of GBAF and BET proteins, we performed ChIP-sequencing for BRD9 and BRD4 in LNCaP cells. We identified 30,231 BRD9 peaks and 39,661 BRD4 peaks. The peaks are similarly distributed across genomic features, although a greater proportion of BRD4 peaks are at promoters and a greater proportion of BRD9 peaks are in distal intergenic regions (Fig. 6a). Metagene analysis indicated an enrichment of BRD4 ChIP signal at BRD9 peaks as well as an enrichment of BRD9 ChIP signal at BRD4 peaks (Fig. 6b). In addition, metagene analysis indicated almost complete reduction in ChIP signal with I-BRD9 treatment (Supplementary Figure 5a). Using a published LNCaP AR ChIP-Seq dataset GSM3223722 [15], we identified 7052 overlapping peaks between BRD4 and BRD9, and confirmed the high overlap between BRD4 and AR reported in VCaP cells [4,6] (Fig. 6c). In addition, we observed AR binding at a subset of BRD9/BRD4 co-bound sites (Fig. 6c), several of which are adjacent to AR target genes (Fig. 6d, Supplementary Figure 5b). Using ChIP-qPCR, we confirmed that BRD4 and BRD9 co-bind at BMPR1B, KLK3 (PSA), and TMPRSS2 enhancers. Importantly, this binding was reduced by I-BRD9 treatment (Fig. 6e). To test whether BRD9 localization depends on AR, we used ChIP-qPCR to probe BRD9 enrichment during androgen stimulation. Upon androgen stimulation, AR was increased at BMPR1B, KLK3 and TMPRSS2 binding sites, while BRD9 was simultaneously increased at a subset of the sites (Fig. 6f). Conversely, I-BRD9 treatment (as well as JQ1) reduced AR binding at all AR target sites upon androgen stimulation (Fig. 6g), suggesting that AR localization at these sites depends on BRD9, as it does for BET proteins [4].
Fig. 6:
BRD9 binds with BRD4 at AR-dependent genes. a Annotation of BRD9 and BRD4 ChIP-Seq peaks in LNCaP cells based on distance to gene coding regions in the hg19 genome. b Metagene analysis of BRD9 and BRD4 ChIP-Seq signal ± 5kb from center of BRD9 peaks (top) and BRD4 peaks (bottom). c (left)-Venn diagram of overlapping peaks from BRD9, BRD4, and AR (from [15]). ChIP-Seq in LNCaP cells. (right) Venn diagram of the overlap between the nearest gene defined for AR/BRD9/BRD4 shared peaks, and genes decreased with 48h of 10μM ENZA treatment [40]. d Genome browser tracks for BRD9 and BRD4 ChIP-Seq enrichment at the BMPR1B, KLK3, and TMPRSS2 loci. Peak sites from AR (GSM3223722) and BAF155 (GSM3223719) ChIP-Seq in LNCaP [15] depicted below. Regions analyzed with ChIP-qPCR are highlighted in boxes. e ChIP-qPCR of BRD9 and BRD4 enrichment at BMPR1B, KLK3, and TMPRSS2 enhancers after 24h DMSO or 10 μM I-BRD9 in LNCaP cells. f AR and BRD9 ChIP-qPCR for BMPR1B, KLK3 and TMPRSS2 sites during androgen stimulation with DMSO, 10 μM I-BRD9, and 1 μM JQ1 in LNCaP cells. g ChIP-qPCR of AR enrichment at BMPR1B, KLK3, and TMPRSS2 sites. Representative of two independent experiments with n=3 replicates. Error bars represent s.d. Statistical significance calculated using unpaired t-test. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
10. BRD9 localizes at AR-target genes sites marked with CTCF
The overlap between BRD9 and AR peaks is more modest than the overlap between BRD4 and AR peaks (Fig 6c), even though BRD9 binds at, and regulates, many of the same AR target genes (Fig 7a). To identify other cofactors that facilitate BRD9 function at AR target genes, we performed motif analysis for BRD9 peaks using HOMER [33]. Gratifyingly, the FOXA1/AR binding motif is enriched at BRD9 peaks; however, CTCF and related zinc finger motifs are more highly enriched (Fig 7b). Similar to other cell types [17,19,22], we confirmed a large overlap between CTCF and BRD9 peaks in LNCaP cells using publicly available CTCF ChIP-Seq ENCODE dataset GSE105648 (Fig. 7c). Since mass spectrometry experiments identified BRG1 as a CTCF interactor [50], and previous studies had mixed observations regarding direct association between BET proteins and CTCF [51,52], we next investigated whether BRD9 associates directly with CTCF in prostate cells. BRD2/BRD4 and CTCF both associated with wild type BRD9, and these associations were reduced with the bromodomain mutant BRD9 (Fig. 7d). Unlike AR, the BRD9-CTCF association was not affected by BET protein degradation (Fig 7e), providing evidence that GBAF association with CTCF is not mediated through BET proteins. At many AR target gene loci, BRD9 is localized at CTCF sites (Fig 7f) in addition to AR sites (Supplemental Figure 6a). CTCF binding at these sites was not affected by BRD9 inhibition, BET inhibition or androgen stimulation (Supplementary Figure 6b), indicating that BRD9 does not facilitate CTCF loading but performs some other function.
Fig. 7:
BRD9 binds with CTCF at AR-dependent and AR-independent genes. a Venn diagram of the overlap between the nearest gene defined for AR/BRD9/BRD4 shared peaks (Fig 7c), the nearest gene defined for BRD9/BRD4 shared peaks (Fig 7c), and transcripts decreased with 10 μM ENZA treatment [40]. b Motif analysis for BRD9 peaks performed using HOMER [33]. The number of BRD9 peaks containing the motif is listed in parentheses, followed by the calculated significance based on the number of background sites containing the motif. c Venn diagram of the overlap between CTCF (GSE105648) and BRD9 ChIP-seq peaks. d Immunoblot of IP of exogenous V5-tagged wild type BRD9 (BRD9-WT) and bromodomain-mutant BRD9 (BRD9-N216A) in LNCaP cells. Representative of at least 3 separate experiments e Immunoblot analysis of VCaP cells cultured with 100 nM MZ-1 for 20h before BRD9 IP. Same blot as in Fig. 5g. f (left)-Genome browser tracks for BRD9 and BRD4 ChIP-Seq enrichment at the CENPN locus. Also depicted are peaks from CTCF (GSE105648), AR (GSM3223722) and BAF155 (GSM3223719) ChIP-Seq in LNCaP (AR and BAF155 from [15]). Regions analyzed with FAIRE-qPCR are highlighted in boxes. (right)- FAIRE-qPCR in LNCaP cells with 48h CSS media followed by 18h with DMSO, 0.5 μM JQ1, 3 μM I-BRD9, or 0.5 dBRD9 and an additional 4h with ethanol or 10 nM DHT. FAIRE CT values are normalized to input DNA and plotted as the fold change compared to DMSO treated cells in CSS media. n=3. error bars represent s.d. Significance determined using unpaired t-test. * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001. g same as in f with RNA-Pol2 ChIA-PET associations from [54] at the bottom. h Venn diagram of the overlap between genes differentially regulated with 5 μM I-BRD9 and 0.5 μM dBRD9 in LNCaP cells cultured in CSS. From RNA-Seq performed in Fig. 6b. i same as in g. j Proposed model for the regulation of ligand-induced AR targeting and transactivation by GBAF complex and BET proteins. Created with BioRender.com.
A FAIRE-Seq study in LNCaP cells revealed that CTCF sites are the most enriched regions with increased DNA accessibility after stimulation with the androgen analog R1881 [53]. Using this dataset, we identified FAIRE peaks at BRD9-bound CTCF sites within the CENPN and KCNN2 locus (Fig 7f, Supplementary Figure 6c). We confirmed an increase in accessibility at these sites upon addition of DHT using FAIRE-qPCR and found that inhibition of BRD9 reduced this accessibility (Fig. 7f). At distal sites upstream of CENPN, we observed that BRD9 is co-bound with AR and BRD4 at a site not originally associated with CENPN using nearest gene annotation (Fig. 7f). This led us to hypothesize that BRD9/CTCF sites may physically associate with distal AR-bound enhancers to facilitate AR-mediated gene expression. Using a recent RNA-PolII Chia-PET [54] we visualized PolII-bound chromatin loops at the KLK locus, and identified extensive enrichment of BRD9 at PolII-bound loop regions marked with both AR and CTCF. This indicates that CTCF/BRD9 sites may serve to bridge AR-bound enhancers to AR-target genes. We further validated that BRD9 is required for increased accessibility at an enhancer region distal to KLK4 upon androgen stimulation (Fig 7g).
11. BRD9 localizes at CTCF sites near EZH2.
Prostate cancer cell lines display a global increase in enhancers per gene, and gene targets per enhancer, compared to RWPE-1 cells [54], particularly for top dysregulated genes. To determine whether BRD9 is associated with enhancer regulation at AR-independent genes in prostate cancer, we identified the subset of genes inhibited by I-BRD9 and dBRD9 in CSS media conditions. These include several genes important in prostate cancer progression, including EZH2 and E2F1 (Fig 7h). BRD9 is highly enriched at CTCF-bound enhancer regions of EZH2 identified by PolII-ChiaPET in LNCaP cells [54], and several of these peaks colocalize with FAIRE sites identified in androgen-starved LNCaP (FAIRE-CSS) [53] There was no increase in accessibility upon DHT addition at these sites using FAIRE-qPCR; however, these sites were dependent on BRD9 for accessibility in the absence of androgens (Fig. 7i). This indicates that long term viability effects resulting from a decrease in EZH2, potentially through regulating AR itself [55], could contribute to the long-term viability phenotype observed with BRD9 depletion (Fig 1).
DISCUSSION
Here we report that the BRD9 subunit of GBAF chromatin remodeling complex is required for the viability of androgen-sensitive and CRPC cell lines, but is relatively dispensable for viability in non-transformed or AR-negative prostate cancer cell lines. Our mechanistic studies establish that BRD9 inhibition or depletion suppresses AR-target gene induction, even in castration-resistant cell lines, suggesting that BRD9 is a potential target in therapy-resistant prostate cancers. In addition, increased abundance of GBAF enhances AR target expression in the absence of ligand stimulation, and promotes more robust induction of gene expression upon ligand introduction, providing mechanistic insight into clinical data suggesting that increased GBAF subunit expression contributes to the progression of CRPC.
Our evidence suggests that BRD9 function in AR-mediated gene expression is related to GBAF’s association with BET proteins. Our genome-wide and gene-specific binding data suggest co-enrichment of BRD9 and BRD4 at genomic sites, similar to ESCs [17]. Previous reports suggest this is through a physical interaction between GBAF-specific subunits and BRD4 [16,17,45–48], and in prostate cancers we find BRD9 interacts with BRD4 as well as BRD2. Both BET bromodomain inhibition and BRD9 bromodomain inhibition/mutation can reduce the association between these proteins, indicating that the association is at least partially bromodomain dependent. While both BET proteins and SWI/SNF subunits can be acetylated, further research is needed to define the cognate acyl-lysine moieties that enables bromodomain-mediated interaction. Further, we find that the AR-BRD9 association is likely mediated through the bromodomain-dependent interaction between BET proteins and AR [4,6], although additional biophysical or biochemical approaches will be required to determine whether a direct BRD9-AR interaction is possible.
We find that BRD9 and AR colocalize genome-wide and are co-dependent for binding at select AR-target genes, such as KLK3 (PSA), TMPRSS2 and BMPR1B. This mode of recruitment has been reported for other SWI/SNF-transcription factor partnerships such as GATA3-BAF for genes required for mesenchymal-epithelial-transition [56], or ERG-BAF for ERG-target genes [15]. Additionally, BRD9 inhibition, similar to BET inhibition [4,6], reduces AR binding at enhancers and promoters, raising the possibility that BRD9 and BET proteins cooperate in facilitating long-range interactions required for AR-mediated transcription. Supporting this possibility, we observe enrichment of CTCF sites in our BRD9 ChIP-Seq, at both AR-dependent and AR-independent genes. CTCF directs the cohesin complex to the boundaries of topologically associated domains (TADs) [57], and to dynamic loops required for cell-type specific gene transcription [57,58]. Prostate cancers in particular are characterized by a general increase in chromatin relaxation and CTCF-mediated looping [53,59], which is critical for AR-mediated transcription [60,61]. We find that CTCF binding is not affected by BET inhibitor, BRD9 inhibitor, or androgen stimulation, indicating that BET and GBAF are not required for establishing CTCF sites. While a functional role for BRD2 in maintaining loops at CTCF sites has been reported in other cell types [51,52], the connection to chromatin remodeling hasn’t been clear. In prostate cancer cells, CTCF sites display increased accessibility upon androgen stimulation [53], which we find can be blocked with BRD9 inhibitors. This indicates a potential role for GBAF in remodeling nucleosomes to facilitate cohesion progression [62], although more comprehensive research is needed to validate this possibility. Supporting a role for BRD9 in facilitating the formation of chromatin loops (Fig 7j), we find that BRD9 inhibition has the greatest effect on gene expression during androgen stimulation when the formation of new looping occurs [63]. In steady state, BRD9 inhibitors often take longer to show effects, which is in agreement with reports demonstrating that depletion of CTCF or cohesin mostly deregulates inducible gene expression with minimal effect on steady-state gene expression [64].
Supplementary Material
SIGNIFICANCE.
Advanced prostate cancers resistant to androgen receptor (AR) antagonists are still susceptible to non-toxic BRD9 inhibitors, making them a promising alternative for halting AR signaling in progressed disease.
ACKNOWLEDGEMENTS
This research was supported with a grant from the NIH (U01CA207532 for ECD) and the Purdue Center for Cancer Research Pilot Grant Program. A.A. was supported by a SIRG grant administered through the Purdue Center for Cancer Research, and the Bilsland Dissertation Fellowship from the Purdue Office of Interdisciplinary Graduate Programs. The authors gratefully acknowledge support from the Histology Core, the Genomics Core, Collaborative Core for Cancer Bioinformatics (C3B), and the Biological Evaluation Core from the Purdue Center for Cancer Research, NIH grant P30 CA023168. We thank Prof. Chang Deng Hu and his team (Purdue University) for fruitful discussions and resources, Prof. Brittany Allen-Peterson (Purdue University) for histology assistance, and all members of Dykhuizen Lab for valuable feedback.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Coutinho I, Day TK, Tilley WD, Selth LA. Androgen receptor signaling in castration-resistant prostate cancer: A lesson in persistence. Endocr Relat Cancer. 2016;23:T179–97. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Kumari S, Hu Q, Senapati D, Venkadakrishnan VB, Wang D, et al. A comprehensive analysis of coregulator recruitment, androgen receptor function and gene expression in prostate cancer. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welti J, Sharp A, Yuan W, Dolling D, Rodrigues DN, Figueiredo I, et al. Targeting Bromodomain and Extra-Terminal (BET) family proteins in Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res. 2018;24:3149–62. [DOI] [PubMed] [Google Scholar]
- 6.Faivre EJ, McDaniel KF, Albert DH, Mantena SR, Plotnik JP, Wilcox D, et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature. 2020;578:306–10. [DOI] [PubMed] [Google Scholar]
- 7.Hong CY, Suh JH, Kim K, Gong E-Y, Jeon SH, Ko M, et al. Modulation of Androgen Receptor Transactivation by the SWI3-Related Gene Product (SRG3) in Multiple Ways. Mol Cell Biol. 2005;25:4841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link KA, Balasubramaniam S, Sharma A, Comstock CES, Godoy-Tundidor S, Powers N, et al. Targeting the BAF57 SWI/SNF subunit in prostate cancer: A novel platform to control androgen receptor activity. Cancer Res. 2008;68:4551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin ML, Kim YW, Jeong KW. BAF53A regulates androgen receptor-mediated gene expression and proliferation in LNCaP cells. Biochem Biophys Res Commun. 2018;505:618–23. [DOI] [PubMed] [Google Scholar]
- 10.Van De Wijngaart DJ, Dubbink HJ, Molier M, De Vos C, Trapman J, Jenster G. Functional screening of FxxLF-like peptide motifs identifies SMARCD1/BAF60a as an androgen receptor cofactor that modulates TMPRSS2 expression. Mol Endocrinol. 2009;23:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shain AH, Pollack JR. The Spectrum of SWI/SNF Mutations, Ubiquitous in Human Cancers. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RS, Roberts CWM. Linking the SWI/SNF complex to prostate cancer. Nat Genet. 2013;45:1268–9. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Li N, Dong B, Guo W, Wei H, Chen Q, et al. Chromatin remodeling ATPase BRG1 and PTEN are synthetic lethal in prostate cancer. J Clin Invest. 2019;129:759–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval GJ, Pulice JL, Pakula H, Schenone M, Takeda DY, Pop M, et al. Binding of TMPRSS2-ERG to BAF Chromatin Remodeling Complexes Mediates Prostate Oncogenesis. Mol Cell. 2018;71:554–566.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpsoy A, Dykhuizen EC. Glioma tumor suppressor candidate region gene 1 (GLTSCR1) and its paralog GLTSCR1-like form SWI/SNF chromatin remodeling subcomplexes. J Biol Chem. 2018;293:3892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatchalian J, Malik S, Ho J, Lee DS, Kelso TWR, Shokhirev MN, et al. A non-canonical BRD9-containing BAF chromatin remodeling complex regulates naive pluripotency in mouse embryonic stem cells. Nat Commun. 2018;9:5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brien GL, Remillard D, Shi J, Hemming ML, Chabon J, Wynne K, et al. Targeted degradation of BRD9 reverses oncogenic gene expression in synovial sarcoma. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel BC, D’Avino AR, Cassel SH, Mashtalir N, McKenzie ZM, McBride MJ, et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat Cell Biol. 2018;20:1410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Wang S, Troisi EC, Howard TP, Haswell JR, Wolf BK, et al. BRD9 defines a SWI/SNF subcomplex and constitutes a specific vulnerability in malignant rhabdoid tumors. Nat Commun. 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashtalir N, D’Avino AR, Michel BC, Luo J, Pan J, Otto JE, et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell. 2018;175:1272–1288.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue D, Chew GL, Liu B, Michel BC, Pangallo J, D’Avino AR, et al. Spliceosomal disruption of the non-canonical BAF complex in cancer. Nature. 2019;574:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohmann AF, Martin LJ, Minder JL, Roe JS, Shi J, Steurer S, et al. Sensitivity and engineered resistance of myeloid leukemia cells to BRD9 inhibition. Nat Chem Biol. 2016;12:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Gaudio N, Di Costanzo A, Liu NQ, Conte L, Migliaccio A, Vermeulen M, et al. BRD9 binds cell type-specific chromatin regions regulating leukemic cell survival via STAT5 inhibition. Cell Death Dis. Springer US; 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun A, Tawfik O, Gayed B, Thrasher JB, Hoestje S, Li C, et al. Aberrant expression of SWI/SNF catalytic subunits BRG1/BRM is associated with tumor development and increased invasiveness in prostate cancers. Prostate. 2007;67:203–13. [DOI] [PubMed] [Google Scholar]
- 26.Kim TH, Dekker J. Preparation of cross-linked chromatin for chip. Cold Spring Harb Protoc. 2018;2018:311–3. [DOI] [PubMed] [Google Scholar]
- 27.Andrews S FastQC - A quality control tool for high throughput sequence data. Available from http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Internet]. 2010.
- 28.Krueger F T rim Galore!: A wrapper tool around Cutadapt and FastQC to consistently apply quality and adapter trimming to FastQ files. Available from https://www.bioinformatics.babraham.ac.uk/projects [Internet]. 2015.
- 29.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stovner EB, Sætrom P, Hancock J. Epic2 efficiently finds diffuse domains in ChIP-seq data. Bioinformatics. 2019;35:4392–3. [DOI] [PubMed] [Google Scholar]
- 31.Yu G, Wang LG, He QY. ChIP seeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–3. [DOI] [PubMed] [Google Scholar]
- 32.Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Mol Cell. 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinlan AR, Hall IM. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon JM, Giresi PG, Davis IJ, Lieb JD. Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA. Nat Protoc. 2012;7:256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Du T, Zhu D, Chen X, Lai Y, Wu W, et al. High levels of glioma tumor suppressor candidate region gene 1 predicts a poor prognosis for prostate cancer. Oncol Lett. 2018;16:6749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theodoulou NH, Bamborough P, Bannister AJ, Becher I, Bit RA, Che KH, et al. Discovery of I-BRD9, a Selective Cell Active Chemical Probe for Bromodomain Containing Protein 9 Inhibition. J Med Chem. 2016;59:1425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin LJ, Koegl M, Bader G, Cockcroft XL, Fedorov O, Fiegen D, et al. Structure-Based Design of an in Vivo Active Selective BRD9 Inhibitor. J Med Chem. American Chemical Society (ACS); 2016;59:4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remillard D, Buckley DL, Paulk J, Brien GL, Sonnett M, Seo HS, et al. Degradation of the BAF Complex Factor BRD9 by Heterobifunctional Ligands. Angew Chemie - Int Ed. 2017;56:5738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Pitchiaya S, Cieślik M, Niknafs YS, Tien JCY, Hosono Y, et al. Analysis of the androgen receptor-regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat Genet. 2018;50:814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GGJM, Jenster G, Trapman J, et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41:665–9. [DOI] [PubMed] [Google Scholar]
- 42.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehm SM, Schmidt LJ, Heemers HV., Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent Cancer Progression and Bone Metastasis in the LNCaP Model of Human Prostate Cancer. Cancer Res. 1994;54:2577–81. [PubMed] [Google Scholar]
- 45.Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, et al. The Brd4 Extraterminal Domain Confers Transcription Activation Independent of pTEFb by Recruiting Multiple Proteins, Including NSD3. Mol Cell Biol. 2011;31:2641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han F, Zhang L, Chen C, Wang Y, Zhang Y, Qian L, et al. GLTSCR1 Negatively Regulates BRD4-Dependent Transcription Elongation and Inhibits CRC Metastasis. Adv Sci. 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambert JP, Picaud S, Fujisawa T, Hou H, Savitsky P, Uusküla-Reimand L, et al. Interactome Rewiring Following Pharmacological Targeting of BET Bromodomains. Mol Cell. 2019;73:621–638.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schick S, Rendeiro AF, Runggatscher K, Ringler A, Boidol B, Hinkel M, et al. Systematic characterization of BAF mutations provides insights into intracomplex synthetic lethalities in human cancers. Nat Genet. 2019;51:1399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zengerle M, Chan KH, Ciulli A. Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem Biol. 2015;10:1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marino MM, Rega C, Russo R, Valletta M, Gentile MT, Esposito S, et al. Interactome mapping defines BRG1, a component of the SWI/SNF chromatin remodeling complex, as a new partner of the transcriptional regulator CTCF. J Biol Chem. 2019;294:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu SC, Gilgenast TG, Bartman CR, Edwards CR, Stonestrom AJ, Huang P, et al. The BET Protein BRD2 Cooperates with CTCF to Enforce Transcriptional and Architectural Boundaries. Mol Cell. 2017;66:102–116.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung KL, Zhang F, Jaganathan A, Sharma R, Zhang Q, Konuma T, et al. Distinct Roles of Brd2 and Brd4 in Potentiating the Transcriptional Program for Th17 Cell Differentiation. Mol Cell. 2017;65:1068–1080.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbanucci A, Barfeld SJ, Kytölä V, Itkonen HM, Coleman IM, Vodák D, et al. Androgen Receptor Deregulation Drives Bromodomain-Mediated Chromatin Alterations in Prostate Cancer. Cell Rep. 2017;19:2045–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramanand SG, Chen Y, Yuan J, Daescu K, Lambros MB, Houlahan KE, et al. The landscape of RNA polymerase II-associated chromatin interactions in prostate cancer. J Clin Invest. 2020;130:3987–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q, et al. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell Rep. 2018;25:2808–2820.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takaku M, Grimm SA, Shimbo T, Perera L, Menafra R, Stunnenberg HG, et al. GATA3-dependent cellular reprogramming requires activation-domain dependent recruitment of a chromatin remodeler. Genome Biol. 2016;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren G, Jin W, Cui K, Rodrigez J, Hu G, Zhang Z, et al. CTCF-Mediated Enhancer-Promoter Interaction Is a Critical Regulator of Cell-to-Cell Variation of Gene Expression. Mol Cell. 2017;67:1049–1058.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rustøen Braadland P, Urbanucci A. Chromatin reprogramming as an adaptation mechanism in advanced prostate cancer. Endocr Relat Cancer. 2018;26:R211–35. [DOI] [PubMed] [Google Scholar]
- 60.Khoury A, Achinger-Kawecka J, Bert SA, Smith GC, French HJ, Luu PL, et al. Constitutively bound CTCF sites maintain 3D chromatin architecture and long-range epigenetically regulated domains. Nat Commun. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taslim C, Chen Z, Huang K, Huang THM, Wang Q, Lin S. Integrated analysis identifies a class of androgen-responsive genes regulated by short combinatorial long-range mechanism facilitated by CTCF. Nucleic Acids Res. 2012;40:4754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lou Bailey M, Surovtsev I, Williams J, Yan H, Mochrie S, King M. Nucleosome-constrained loop extrusion model for the origin of topologically associating domains. bioRxiv doi.org/101101/20200229969683. 2020;1–27. [Google Scholar]
- 63.Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19:631–42. [DOI] [PubMed] [Google Scholar]
- 64.Cuartero S, Weiss FD, Dharmalingam G, Guo Y, Ing-Simmons E, Masella S, et al. Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat Immunol. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.