Abstract
Cisplatin, a potent chemotherapeutic drug, induces ototoxicity, which limits its clinical utility. Cisplatin-induced oxidative stress plays a causal role in cochlear apoptosis while the consequent nitrative stress leads to the nitration of LIM domain only-4 (LMO4), a transcriptional regulator, and decreases its cochlear expression levels. Here, we show a direct link between cochlear LMO4 and cisplatin-induced hearing loss by employing a Lmo4 conditional knockout mouse model (Lmo4lox/lox; Gfi1Cre/+). Hair cell-specific deletion of Lmo4 did not alter cochlear morphology or affect hearing thresholds and otoacoustic emissions, in the absence of apoptotic stimuli. Cisplatin treatment significantly elevated the auditory brainstem response thresholds of conditional knockouts, across all frequencies. Moreover, deletion of Lmo4 compromised the activation of STAT3, a downstream target that regulates anti-apoptotic machinery. Immunostaining indicated that the expression of phosphorylated STAT3 was significantly decreased while the expression of activated caspase 3 was significantly increased in Lmo4 deficient hair cells, post cisplatin treatment. These findings suggest an otoprotective role of LMO4 as cisplatin-induced decrease in cochlear LMO4 could compromise the LMO4/STAT3 cellular defense mechanism to induce ototoxicity.
Keywords: LMO4, STAT3, cisplatin, ototoxicity, cochlea, hearing loss
Introduction
Cisplatin, a first-generation platinum-based drug, is the backbone of combination therapies employed to treat cancers of the bladder, cervix, lung, ovary, head and neck, testicle, mesothelium, and other solid tumors. Cisplatin and its analogs are prescribed to 10–20% of all cancer patients (NCI report, 2014). However, ototoxicity remains a serious dose-limiting adverse effect of this highly effective anticancer drug. Cisplatin-induced hearing loss has been detected in 19% to 77% of patients, depending on the criteria used in different studies[1]. Cisplatin arrests cell division to prevent tumor growth and induces apoptosis to reduce tumor size. However, cisplatin-induced apoptosis is not restricted to tumor cells but, instead, extends to susceptible cells, such as cochlear outer hair cells and stria vascularis. Because cisplatin accumulates in the stria and is retained indefinitely[2] its ability to induce cochlear damage persists even after treatment. Therefore, ototoxicity is a major side-effect, which significantly affects the quality of life in cancer survivors and has devastating consequences in children as it affects their speech and language development, education, and social integration[3,4].
The mechanism responsible for cisplatin-induced ototoxicity involves oxidative stress and consequent apoptosis of cochlear hair cells[5,6]. Protein nitration is an important sequela of oxidative stress and cisplatin treatment leads to nitration of LIM Domain Only 4 (LMO4), a transcriptional regulator involved in cellular apoptosis. Decreased protein levels of cochlear LMO4 and its downstream target STAT3, a promoter of cell-survival[7–9], has been detected after cisplatin treatment. A significant and dose-dependent decrease in the protein levels of LMO4 has also been detected in the renal and neuronal cells, which are susceptible to toxic side-effects of cisplatin[10]. LMO4 plays an important role in the development of the inner ear[11] and repression of LMO4 has been reported to promote cellular apoptosis[12,13]. Recent studies provided evidence supporting a critical role of LMO4 in cisplatin ototoxicity as LMO4 overexpression prevented cisplatin-induced cytotoxicity[14] while CRISPR/Cas9-mediated knockout of Lmo4 enhanced cytotoxicity in auditory sensory epithelial cell cultures[15]. However, a causal link between cisplatin-induced changes in LMO4 levels and hearing loss was not established in these in vitro studies.
As a molecular adaptor for protein-protein interactions, LMO4 forms transcriptional complexes and regulates cell survival and cell death[16–19]. Particularly, it associates with IL-6 receptor glycoprotein 130 (GP130) and stabilizes this protein complex, which, in turn, enables the activation of JAK1, TYK2, and STAT3[16]. Cisplatin-induced decrease in LMO4 could affect the binding and/or activation of proteins in this protein complex. Analysis of the growth and migration of auditory sensory epithelial cells suggested that although LMO4 is not essential for cell survival under physiological conditions, it is required for defending the cells against adverse apoptotic stimuli[15], most likely, via the regulation of STAT3-mediated transcription of anti-apoptotic genes[9]. JAK/STAT signaling appears to play a crucial role in cisplatin-induced cochlear apoptosis because cisplatin treatment decreased the expression, phosphorylation and nuclear localization of STAT3[7,20,9], a downstream target of LMO4 and a mediator of cell survival[12]. A critical role of STAT proteins in cisplatin ototoxicity was also reported by Schmitt et al.,[21] and Kaur et al.,[22] as repression of STAT1, which is cross-regulated by STAT3[23,24], attenuated cisplatin-induced hearing loss. The objective of this study is to define the direct link between cochlear LMO4 and cisplatin-induced hearing loss by testing the hypothesis that deficiency of Lmo4 in hair cells exacerbates cisplatin-induced cochlear apoptosis and hearing loss by compromising STAT3-mediated anti-apoptotic machinery.
Materials and Methods
Generation of Lmo4 conditional knockout mice.
Lmo4 conditional knockouts (Lmo4lox/lox; Gfi1Cre/+) were generated by breeding Lmo4lox mice with tissue specific Gfi1Cre deleter mice (see Fig. 1A) to remove Lmo4 specifically in the hair cells. Heterozygous Lmo4lox and Gfi1Cre mice, derived from a mixed C57BL/6J and 129S6 background, were obtained from Dr. Lin Gan, University of Rochester, NY[11,25]. The heterozygous Lmo4lox mice were interbred to generate homozygous Lmo4lox mice, which was then mated with Gfi1Cre/+ mice to generate Lmo4lox/+; Gfi1Cre/+ mice. Then the Lmo4lox/+; Gfi1Cre/+ mice were mated with Lmo4lox/lox mice to generate the Lmo4 conditional knockouts. Both male and female mice were used in this study and littermates of Lmo4 conditional knockout mice were used as wild-type controls. All animal studies were approved by Wayne State University’s IACUC (animal protocol # 17–02-214) and were performed in accordance with the National Institutes of Health’s animal user guidelines.
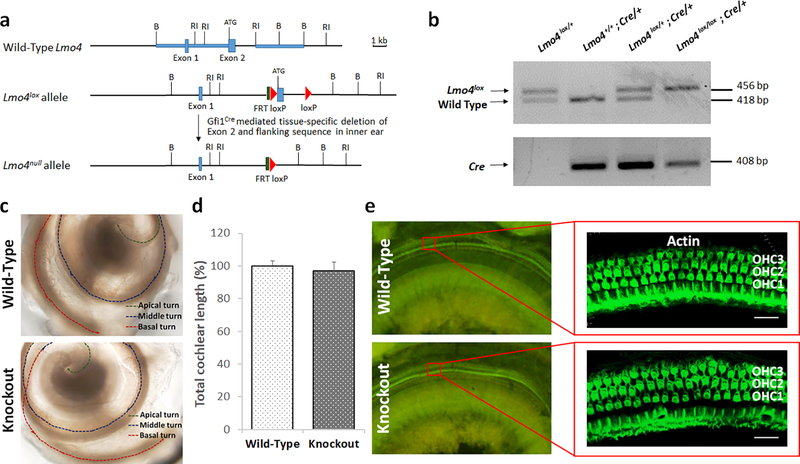
Fig. 1.
Lmo4 conditional knockout scheme and cochlear morphology of knockout mice. a) Genomic organization of wild-type Lmo4 gene, Lmo4lox allele, and Lmo4null allele are illustrated. In the Lmo4lox allele the loxP sites flank exon 2, which was cleaved by using Gfi1Cre to generate Lmo4null allele in the hair cells of Lmo4 conditional knockout mice. b) Targeted deletion of Lmo4 was verified by genomic PCR. c) Microscopic observation of cochlea dissected from 6-week old mice indicated that the appearance of Lmo4 conditional knockout mouse cochlea is similar to that of wild-type cochlea. Two and a half turns were observed in both groups. Images are representative of three replicates. d) Measurement of the length of the cochlea indicated that both the knockout and wild-type cochlea were of similar length. The results are expressed as mean ± standard deviation, n = 3. e) Visualization of hair cells stained with phalloidin indicated that the structure and organization of the outer hair cells (OHC) are similar in both the knockout and wild-type mice. Images are representative of three replicates. Scale bar = 20 μm.
Genotyping.
DNA extracted from tissue obtained by ear punch was used to genotype mice. Lmo4 null allele was identified using the following primers: 5’-TGAGACCTAGTGGTAGTG-3’ and 5’-CCTAGAATTCAAGTGCG-3’. PCR was performed in Step One RT-PCR system (Applied Biosystems, Foster City, CA), programmed to include initial denaturation at 94°C for 3 min followed by 35 cycles of 1 min denaturation at 94°C, 1 min annealing at 58°C, 1 min extension at 72°C, and a final extension at 72°C for 10 min. GfiCre was identified using the following primers: 5’-GCATTACCGGTCGATGCAACGAGTGATGAG-3’ and 5’-GAGTGAACGAACCTGGTCGAAATCGCGT-3’ and the amplification was done under the following condition: initial denaturation at 94°C for 3 min followed by 35 cycles of 1 min denaturation at 94°C, 1 min annealing at 69°C, 1 min extension at 72°C, and a final extension at 72°C for 10 min. The PCR products were separated on a 2% agarose gel and the genotype of mice were determined based on the size of detected bands. Accordingly, 456 bp band indicated the presence of Lmo4lox allele, 418 bp band indicated the presence of wild-type Lmo4, and 408 bp band indicated the presence of GfiCre (see Fig 1B).
Cisplatin treatment.
Six-week old Lmo4 conditional knockout mice or wild-type littermates that had normal hearing were treated with cisplatin. Veterinary-grade cisplatin (# NDC 68001–283-27, Blue Point Laboratories, Dublin, Leinster, Ireland) was administered at the dose of 3 mg/kg body weight for 5 days[26] by slow intraperitoneal infusion at the concentration of 1 mg/ml in sterile saline (0.9%). Controls were infused with an equal volume of saline. All animals were hydrated with 10 ml/kg of saline until they were euthanized on day 8.
Microdissection and immunohistochemistry.
Cochlea was dissected out in ice-cold phosphate-buffered saline (PBS) and immediately perfused with 10% phosphate-buffered formalin (4% formaldehyde) by slow injection via the round window. The cochlea was fixed in formalin and then decalcified with 100 mM EDTA for at least 3 days. Sensory epithelia were micro-dissected from the cochleae and the tissue was permeabilized and blocked in a solution containing PBS, 1% v/v Triton X-100, 2% w/v bovine serum albumin, and 10% v/v goat serum (# S26–100ML, Millipore Sigma, St. Louis, MO) for 1 h at room temperature. The tissue was then incubated overnight at 4°C with primary antibodies (mouse monoclonal anti-LMO4, # sc-293440, Santa Cruz Biotechnology Inc., Santa Cruz, CA; rabbit monoclonal anti-pSTAT3, # 9145S, Cell Signaling Technology, Danvers, MA; rabbit polyclonal anti-Caspase-3, # ab13847, Abcam, Cambridge, MA). The specificity of anti-LMO4 and anti-pSTAT3 was tested in previous studies by western blotting[10,9]. After three 5-min washes in PBS, tissue was incubated with Alexa Fluor 568 donkey anti-mouse (# A10037) or Alexa Fluor 647 goat anti-rabbit (# A21244) IgG secondary antibody (Invitrogen/Molecular Probes, Carlsbad, CA) in blocking solution at room temperature for 1 h. F-actin was labeled with fluorescein-conjugated phalloidin (# F432, Life Technologies, Carlsbad, CA). Stained specimens were mounted on slides with ProLong Gold antifade reagent containing DAPI nuclear stain ((# P36935, Invitrogen/Molecular Probes) and Carl Zeiss Laser Scanning Systems (Zeiss LSM 780, Jena, Germany) was used to capture the images. The intensity of the immune-staining was quantified by measuring the pixel values using ImageJ/Fiji software (version IJ 1.46r).
Auditory brainstem responses (ABR).
Hearing thresholds were measured after anesthetizing the animals with isoflurane (4% induction, 1.5% maintenance with 1 L/min O2). ABR was recorded using subcutaneous differential active needle electrodes with sound stimuli of 1-ms tone bursts (4, 8, 16, 24, or 32 kHz) generated using Tucker-Davis Technologies BioSigRZ software and TDT System3 hardware (TDT, Alachua, FL). The stimuli were presented to the external auditory meatus and the sound intensity varied in 5 dB intervals. Two hundred stimulus presentations, delivered at 21/s, was averaged to obtain the waveform of the brainstem response. The lowest intensity of stimulation at which a waveform with an identifiable peak was detected was considered as the hearing threshold. ABR amplitudes and latencies were measured for wave I generated by 8 and 16 kHz acoustic stimuli at 90 dB SPL. The difference in the voltage levels of the peak and the trough of the first wave was measured to determine the amplitude while the amount of time elapsed from the onset of the stimulus to the peak of the first wave was measured to determine the latency.
Distortion product otoacoustic emissions (DPOAE).
Otoacoustic emissions were measured after anesthetizing the animals with isoflurane (4% induction, 1.5% maintenance with 1 L/min O2). DPOAEs were elicited with two primary tones, f1 and f2 at an f2/f1 ratio of 1.2, holding L2 - L1 + 10 dB, for L1 levels from 80 to 20 dB SPL in 10-dB increments. TDT’s RZ6 system was used to generate the stimulus and Multi-Field Magnetic Speakers (TDT, Alachua, FL) were used to deliver f1 and f2. Frequency f2 varied from 4 to 32 kHz. Sound pressure levels was measured at the cubic difference frequency (2f1-f2) using a ER10B_ probe microphone (Etymotic Research, Inc., Elk Grove Village, IL) and hardware-software from Tucker-Davis Technologies. Distortion-product data was collected every 20.971 milliseconds and averaged 512 times. The noise floor was measured in a 100 kHz band surrounding 2f1-f2.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism 6 software (GraphPad, La Jolla, CA). ANOVA followed by Holm-Sidak’s multiple comparisons test was used to analyze ABR and DPOAE thresholds. Unpaired two-tailed t tests were used to analyze cochlear length, wave I amplitude, wave I latency, STAT3 expression, and caspase 3 expression. All results are expressed as mean ± standard deviation/standard error mean, and P value of < 0.05 was considered significant. Each replicate represent data derived from an individual animal.
Results
Hair cell-specific deletion of Lmo4 does not alter cochlear morphology.
To evaluate the suitability of the conditional knockout mouse model for investigating the critical role of Lmo4 in cisplatin ototoxicity, the impact of deletion of Lmo4 on the morphometric characteristics of the cochlea was assessed. Lmo4 conditional knockout mice with targeted deletion of Lmo4 in the hair cells were identified by PCR analysis of DNA extracted from pinna. The presence of Lmo4lox allele (456 bp band) as well as GfiCre allele (408 bp band) along with the absence of wild-type Lmo4 allele (418 bp band) indicated the hair cell specific deletion of Lmo4 (Fig 1a & b). Unlike the Lmo4 conditional knockouts generated using Foxg1Cre deleter mice, which have a shortened cochlea[25], the morphometric assessment of the knockouts generated using Gfi1Cre indicated that the typical two and a half turns were present in the cochlea (see Fig 1c). Moreover, the length of the cochlea was also similar to that of the wild-type controls (see Fig 1d). This suggested that the hair cell specific deletion of Lmo4 did not alter the general morphology of the cochlea. Confocal microscopy images of surface preparations of organ of Corti stained with fluorescein-conjugated phalloidin indicated that the structure and organization of the hair cells in the knockouts were not altered and appeared similar to that of wild-type controls (see Fig 1e).
Knockout of Lmo4 does not induce apoptosis or affect hearing thresholds under physiological conditions.
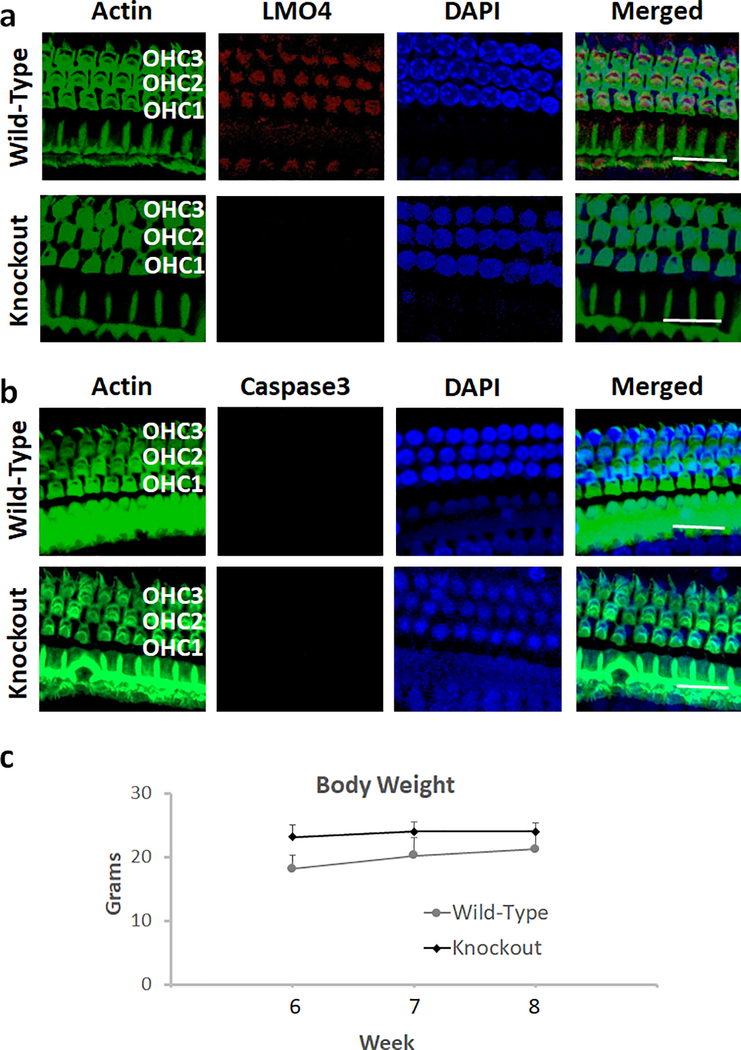
The deletion of Lmo4 in the hair cells of conditional knockout mice was verified by the absence of immuno-staining of hair cells in the surface preparations of organ of Corti that were immuno-reacted with anti-LMO4 (see Fig 2a). The effect of deletion of Lmo4 on cochlear apoptosis was analyzed by evaluating the expression of activated caspase 3 in Lmo4 conditional knockout mice. In the absence of apoptotic stimuli, the expression of activated caspase 3 in the knockouts was very low and was similar to that of the wild-type littermates. This suggested that the deficiency of Lmo4 did not induce the expression of activated caspase 3 in the hair cells under physiological conditions (see Fig 2b). Moreover, the weight gain of the knockouts was similar to that of the wild-type controls (see Fig 2c) although the body weight of the conditional knockout mice was slightly lower than that of the wild-type littermates.
Fig. 2.
Deletion of LMO4 and apoptosis in the hair cells of knockout mice. a) Immunolocalization with anti-LMO4 indicated the expression of LMO4 (red stain) in the outer hair cells of wild-type mice. However, the expression of LMO4 was not detected in the outer hair cells of knockout mice. Green indicates staining of actin with phalloidin while blue indicates staining of the nucleus with DAPI. Images are representative of three replicates. Scale bar = 20 μm. b) Immunolocalization with anti-Caspase 3 indicated that the expression of activated caspase 3 (red stain) in the outer hair cells of wild-type and knockout mice was very low. Green indicates staining of actin with phalloidin while blue indicates staining of the nucleus with DAPI. Images are representative of three replicates. Scale bar = 20 μm. c) Measurement of the body weight at 6, 7 and 8 weeks indicated that the weight gain of knockout mice was similar to that of the wild-type. The results are expressed as mean ± standard deviation, n = 6.
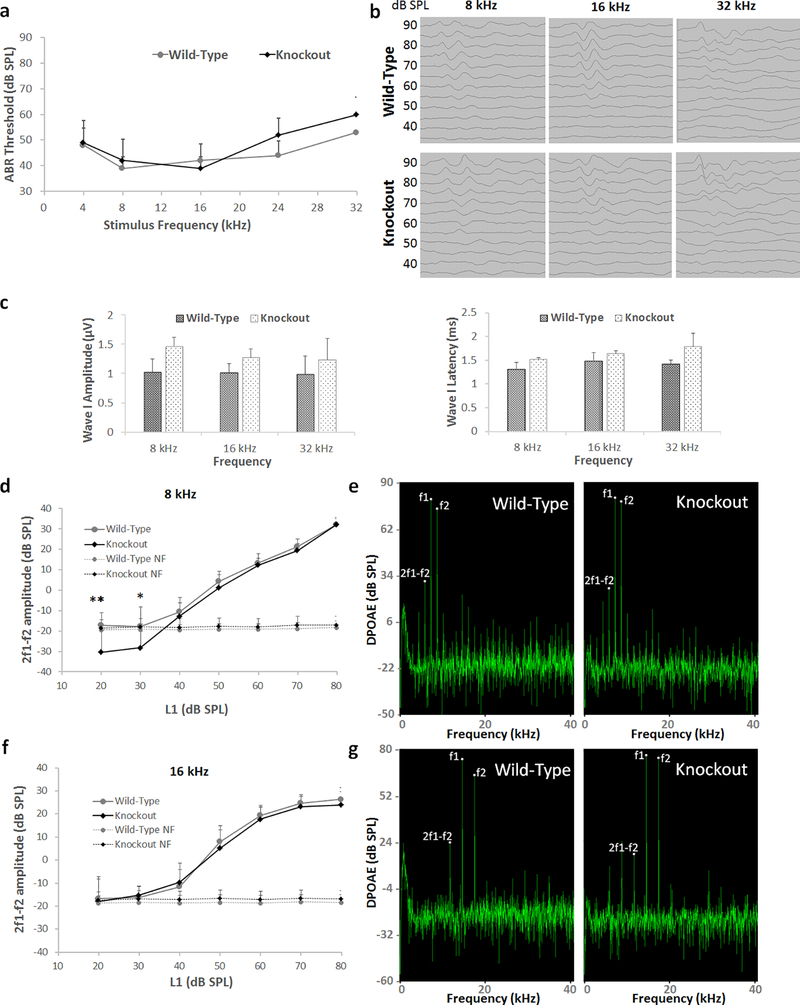
To assess the potential influence of Lmo4 deletion on auditory function, Auditory brainstem responses (ABR) and distortion product otoacoustic emissions (DPOAE) were analyzed in conditional knockouts. ABRs for pure-tone stimuli at 4, 8, 16, 24, and 32 kHz indicated that the hearing thresholds of 6-week old Lmo4 conditional knockout mice were at the same level as that of the wild-type littermates (see Fig 3a). The ABR thresholds ranged from 39 to 60 dB for knockouts and 39 to 53 dB for wild-type controls suggesting that although the thresholds were slightly elevated they were similar in both groups. The analysis of wave I amplitude and latency indicated that the signal transmission characteristics of the auditory nerve are similar in both the knockouts and the wild-type controls (see Fig 3b & c). DPOAEs elicited by 8 and 16 kHz f2 primary tones indicated that the distortion product amplitudes were largely similar in both knockouts and the wild-type controls (see Fig 3d–g). The 2f1-f2 amplitudes elicited by L1 levels from 20 to 80 dB SPL ranged from −18 to 32 dB for knockouts and −30 to 32 dB for wild-type controls for 8 kHz f2 primary tone and from −17 to 26 dB for knockouts and −18 to 24 dB for wild-type controls for 16 kHz f2 primary tone. Collectively, these results indicate that, in the absence of apoptotic stimuli, Lmo4 deficiency did not affect the auditory signal transmission as well as outer hair cell activity.
Fig. 3.
Auditory brainstem responses and distortion product otoacoustic emissions of Lmo4 conditional knockout mice. a) ABR thresholds of 6-week old mice, measured at 4, 8, 16, 24, and 32 kHz, indicated that the hearing thresholds of knockout mice are similar to that of wild-type mice across all the frequencies. The results are expressed as mean ± standard deviation, n = 5–6. b) Representative ABR waveform in response to stimuli at 8, 16, and 32 kHz is illustrated. c) Measurement of the amplitudes and latencies of wave I for 90 dB stimuli at 8, 16 and 32 kHz indicated that the ABR wave pattern is also similar in both knockout and wild-type mi,ce. The results are expressed as mean ± standard error mean, n = 6–12. d) DPOAE amplitudes of 6-week old mice, measured at 8 kHz, indicated that the otoacoustic emissions of knockout mice are largely similar to that of wild-type mice except for the DPOAEs recorded with 40 and 20 dB L1, which were below the noise floor. The results are expressed as mean ± standard deviation, n = 6–7 (*p<0.05, **p<0.01). e) Representative DPOAE waveform in response to stimuli at 8 kHz is illustrated. f) DPOAE amplitudes measured at 16 kHz also indicated that the otoacoustic emissions of knockout mice are similar to that of wild-type mice. The results are expressed as mean ± standard deviation, n = 6–7. g) Representative DPOAE waveform in response to stimuli at 16 kHz is illustrated.
Lmo4 deficiency enhances the susceptibility to cisplatin-induced hearing loss.
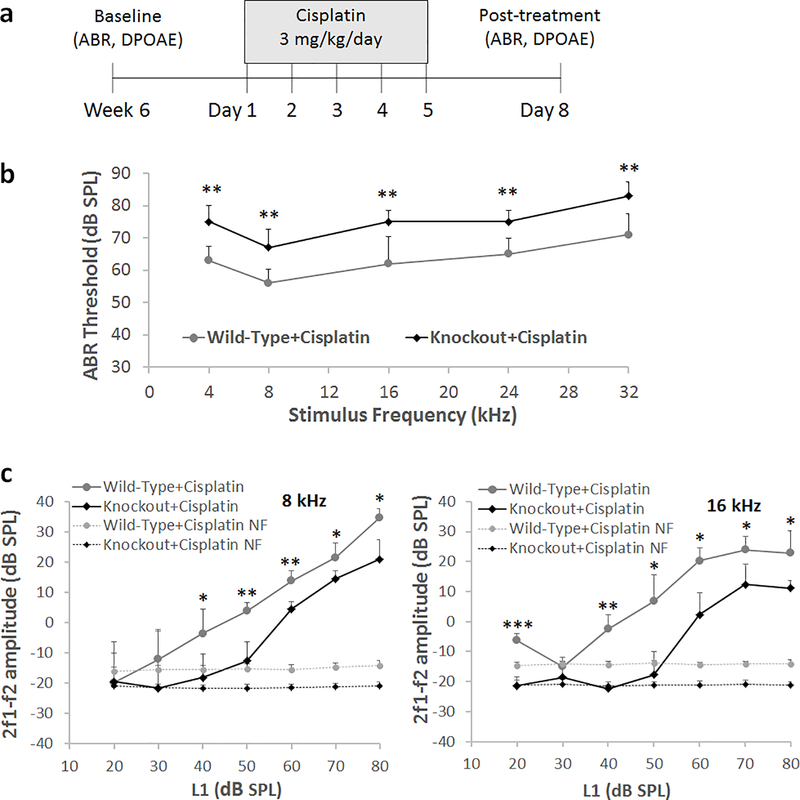
The Lmo4 conditional knockouts were treated with cisplatin to test the hypothesis that Lmo4 plays a protective role and absence of Lmo4 would enhance the vulnerability of the knockouts to cisplatin-induced ototoxicity. Both conditional knockouts and wild-type littermates were treated with 3 mg/kg dose of cisplatin daily for five consecutive days and hearing was assessed on the eighth day by recording ABRs (see Fig 4a). Cisplatin treatment increased the hearing thresholds in both wild-type and knockout mice, however, the shift in the ABR thresholds was significantly higher in the knockouts when compared to wild-type controls (see Fig 4b). The cisplatin-induced shift was 10–13 dB higher in the knockout mice. Moreover, the 2f1-f2 amplitudes elicited by 8 and 16 kHz f2 primary tones were significantly lower in cisplatin-treated knockouts when compared to cisplatin-treated wild-type controls (see Fig 4c). Together, these results suggest that the deficiency of Lmo4 in the hair cells enhances the susceptibility to cisplatin-induced hearing loss. These results suggest that the deficiency of Lmo4 in the hair cells enhances the susceptibility to cisplatin-induced hearing loss.
Fig. 4.
Cisplatin-induced hearing loss in Lmo4 conditional knockout mice. a) Schematic illustration of the experimental design to analyze cisplatin-induced hearing loss. b) Assessment of ABRs indicated that cisplatin treatment induced a significantly higher shift in the hearing thresholds of Lmo4 conditional knockout mice when compared to that of wild-type littermates that express LMO4 in hair cells. ABRs recorded from the left ear of 5 mice are illustrated. The results are expressed as mean ± standard deviation, (**p<0.01). c) Assessment of DPOAEs indicated that cisplatin treatment significantly decreased the DPOAE amplitudes in Lmo4 conditional knockout mice when compared to that of wild-type littermates that express LMO4 in hair cells. DPOAEs recorded from the left ear of 3 mice are illustrated. The results are expressed as mean ± standard deviation (*p<0.05, **p<0.01, ***p<0.001).
Lmo4 deficiency augments cisplatin-induced inactivation of STAT3 and cochlear apoptosis.
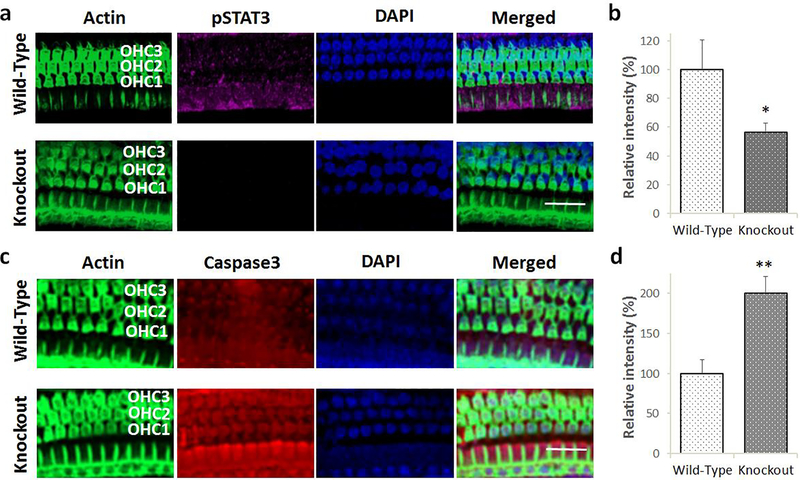
To assess the effect of deletion of Lmo4 on STAT3 mediated signaling, the expression of phosphorylated STAT3 was evaluated in Lmo4 conditional knockout mice after cisplatin treatment. (see Fig 5a & b). The expression of pSTAT3 was significantly lower in the hair cells of the knockouts when compared to wild-type littermates. This suggested that when challenged with an apoptotic stimulus such as cisplatin, the absence of Lmo4 compromises the activation of STAT3. This in turn is likely to affect the transcription of anti-apoptotic genes and facilitate cochlear apoptosis.
Fig. 5.
Effect of Lmo4 deletion on cisplatin-induced inactivation of STAT3 and apoptosis in the hair cells. a) Immunolocalization with anti-pSTAT3 indicated that cisplatin-induced decrease in the expression levels of phosphorylated STAT3 (magenta stain) in the knockout mice was greater than that observed in the wild-type littermates. Green indicates staining of actin with phalloidin while blue indicates staining of the nucleus with DAPI. Images are representative of five replicates. Scale bar = 20 μm. b) Quantification of the immunostaining indicated that the cisplatin-induced decrease in the expression of pSTAT3 in the knockouts was significantly lower than that of wild-type littermates. The results are expressed as mean ± standard error mean, n = 5 (*p = 0.0395). c) Immunolocalization with anti-Caspase 3 indicated that cisplatin treatment induced the expression of activated caspase 3 (red stain) in the outer hair cells of wild-type and knockout mice. However, cisplatin-induced increase in the expression levels of activated caspase 3 in the knockout mice was much higher than that observed in the wild-type littermates. Green indicates staining of actin with phalloidin while blue indicates staining of the nucleus with DAPI. Images are representative of five replicates. Scale bar = 20 μm. d) Quantification of the immunostaining indicated that the cisplatin-induced increase in the expression of activated caspase 3 in the knockouts was significantly higher than that of wild-type littermates. The results are expressed as mean ± standard error mean, n = 5 (**p = 0.0026).
The role of Lmo4 in regulating cisplatin-induced cochlear apoptosis was analyzed by measuring the expression of activated caspase 3 in Lmo4 conditional knockout mice (see Fig 5c & d). Cisplatin treatment increased the expression of activated caspase 3 in both the knockouts and wild-type mice. However, the cisplatin-induced increase in the expression of activated caspase 3 in knockouts was significantly higher than that of the wild-type littermates suggesting that the deficiency of Lmo4 amplifies the cisplatin-induced apoptosis in the hair cells.
Discussion
Among the cochlear cell death mechanisms reported to date[5,27,28,8,29–31], oxidative stress is considered to play a causal role in cisplatin ototoxicity; it activates the enzyme NOX3, which increases the production of superoxide radicals in the inner ear[32]. In addition, the activation of the iNOS pathway and the generation of nitric oxide have been detected in cisplatin ototoxicity[33,34]. Superoxide and nitric oxide radicals react to form peroxynitrite, which can lead to the nitration of susceptible proteins. In agreement, cisplatin treatment increased the nitration of cochlear proteins[35] and LMO4 was identified as the most abundantly nitrated cochlear protein[8]. Furthermore, cisplatin treatment decreased the levels of cochlear LMO4 as well as the phosphorylation of its downstream target STAT3[8,9]. These observations were consistent with reports that indicate protein nitration can modulate phosphorylation cascades, alter protein function, and facilitate proteolytic degradation of nitrated proteins[36–41]. Thus, previous studies implicate a critical role for LMO4 in cisplatin-induced ototoxicity as it can regulate the induction of cochlear apoptosis via JAK/STAT signaling.
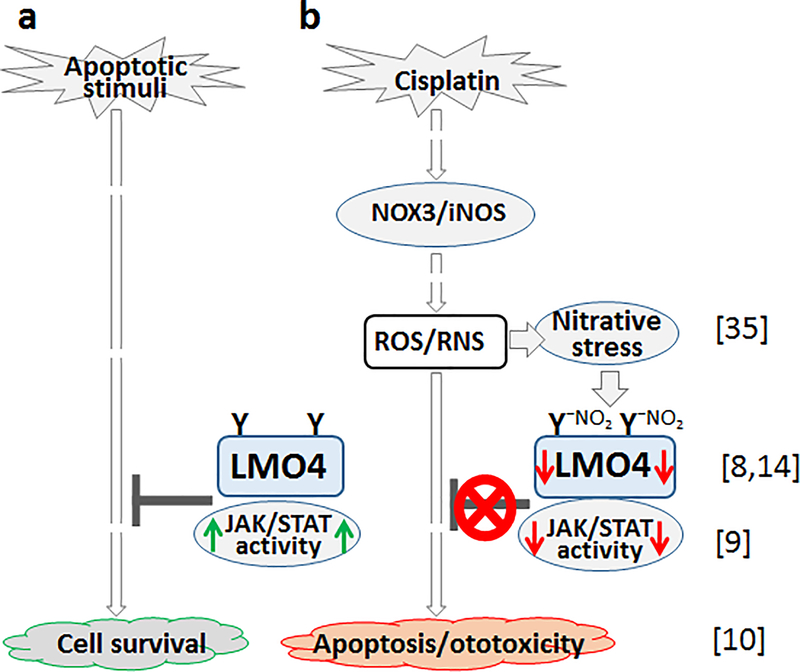
In this study, an Lmo4 conditional knockout mouse model was developed and employed to test the hypothesis that a decrease in the levels of cochlear LMO4 compromises STAT3-mediated anti-apoptotic machinery to facilitate ototoxicity in cisplatin-induced hearing loss (see Fig 6a & b). Lmo4 was deleted specifically in the hair cells, using Gfi1Cre, because the Lmo4 conditional knockouts generated previously, using Foxg1Cre, targeted the otocyst, which resulted in a shortened cochlea[25]. In the new model, targeted deletion of Lmo4 in hair cells did not alter the cochlear morphology, induce apoptosis in hair cells, or affect hearing under physiological conditions. This suggested that Lmo4 is a nonfactor for the survival and function of hair cells in the absence of apoptotic stimuli and that this conditional knockout mouse would be a good model to study the role of Lmo4 signaling in cisplatin ototoxicity.
Fig. 6.
Schematic of putative role of LMO4 in cisplatin ototoxicity. The schematic illustrates the collective findings of current as well as prior studies. a) Under physiological conditions LMO4 defends the cells from apoptotic stimuli via JAK/STAT mediated activation of anti-apoptotic genes thereby facilitating cell survival. b) Cisplatin treatment induces oxidative stress by activating NOX3, which eventually leads to the nitration of LMO4 and decreases its level in the cochlea. Because LMO4 acts as a scaffold for protein complexes (e.g., glycoprotein 130), the cisplatin-induced decrease in LMO4 probably destabilizes such protein complexes, which in turn compromises the STAT3-regulated anti-apoptotic machinery. The numbers in parenthesis refer to cited publications containing corresponding data that support the link between LMO4 and other signaling molecules/pathways represented in the schematic.
LMO4 acts as a scaffold for protein complexes and binds with several transcription factors and co-regulators that promote the transcription of anti-apoptotic genes[42,16,43,44,18,19]. Therefore, deficiency of LMO4 in the hair cells is expected to compromise the anti-apoptotic machinery and promote cell death when challenged with an apoptotic stimulus such as cisplatin. Conversely, interventions that prevent the decrease in cochlear LMO4 are likely to have therapeutic value because blocking cochlear apoptotic pathways that lead to cell death is a promising strategy for mitigating cisplatin ototoxicity[6]. Evaluation of cisplatin ototoxicity in an Lmo4 conditional knockout mouse model provides an opportunity to gain critical insights on the potential therapeutic value of this pathway in preventing cochlear apoptosis and hearing loss. The findings of this study indicate that deficiency of Lmo4 in the hair cells of mice significantly exacerbated the hearing loss induced by cisplatin treatment. In the conditional knockouts, the ABR thresholds were significantly elevated and DPOAE amplitudes were significantly depressed relative to wild-type littermates, after cisplatin treatment. This indicated that the absence of Lmo4 in the hair cells enhances the susceptibility to cisplatin-induced hearing loss suggesting an otoprotective role of LMO4.
STAT3, a major downstream target of LMO4, plays an important role in regulating the anti-apoptotic signaling in cisplatin ototoxicity[9]. The potential regulation of STAT3 activity by cisplatin-induced changes in LMO4 is supported by many studies. Generally, LMO4 binds to ESR1, which has a protective role in the auditory system[45], and represses its transactivation activities[44]. Because LMO4 negatively regulates ESR1, which can negatively modulate STAT3 by direct physical interaction[46], cisplatin-induced decrease in LMO4, as well as increase in ESR1[7], could inhibit STAT3 activity. LMO4 also mediates ATP signaling, which leads to the phosphorylation of STAT3[12]. Since cisplatin has been reported to decrease cochlear ATP[47] as well as LMO4 levels[8], it could eventually decrease STAT3 activity through this pathway[16]. Therefore, the absence of Lmo4 is likely to compromise the activation of STAT3 and exacerbate the toxic effects of cisplatin. Consistent with this notion, Lmo4 deficiency in the hair cells of conditional knockout mice significantly augmented the cisplatin-induced decrease in the phosphorylation of cochlear STAT3 and apoptotic responses in the organ of Corti. Relative to wild-type littermates, the expression of phosphorylated STAT3 was lower and the expression of activated caspase 3 was higher in the hair cells of conditional knockout mice after cisplatin-treatment. Together, these findings suggest that LMO4 is a critical regulator of cochlear anti-apoptotic responses and the deficiency of Lmo4 compromises its otoprotective signaling and thereby facilitates cochlear apoptosis in cisplatin-induced ototoxicity.
Overall, the findings of this study demonstrated a direct link between LMO4 protein levels and cisplatin-induced ototoxicity because hair cell-specific deletion of Lmo4 in mice enhanced their vulnerability to cisplatin-induced hearing loss. Though LMO4 is not essential for the survival of hair cells under optimal physiological conditions, it is required for defending the cells against adverse apoptotic stimuli via the regulation of STAT3-mediated anti-apoptotic machinery. Thus, the complexity of LMO4 signaling in cisplatin-induced hearing loss is characterized by its critical role as a protector against cellular apoptosis, which when compromised by cisplatin results in ototoxicity. This, in turn, implies that LMO4 signaling molecules, particularly, those that facilitate its degradation or those that mediate its anti-apoptotic signaling, are plausible targets for mitigating cisplatin-induced ototoxicity.
Acknowledgements
This work was supported by faculty startup grant to SJ from Wayne State University.
Funding This work was supported by faculty startup grant to SJ from Wayne State University and P30 Grant (P30 ES020957) to Center for Urban Responses to Environmental Stressors (CURES).
Footnotes
Conflicts of interest/Competing interests The authors declare no competing financial interests. In addition, the authors declare no conflicting non-financial interests.
Declarations:
Ethics approval This study was approved by the Institutional Animal Care and Use Committee of Wayne State University.
Consent to participate Not applicable
Consent for publication Not applicable
Availability of data and material All data and materials comply with field standards
Code availability Not applicable
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, Feldman DR, Pagliaro LC, Miller RC, Vaughn DJ, Einhorn LH, Cox NJ, Dolan ME, Platinum Study G (2014) Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst 106 (5). doi:10.1093/jnci/dju044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L, Cunningham LL (2017) Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun 8 (1):1654. doi:10.1038/s41467–017-01837–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA (2012) Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 30 (19):2408–2417. doi:10.1200/JCO.2011.39.1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O (2013) Understanding platinum-induced ototoxicity. Trends Pharmacol Sci 34 (8):458–469. doi:10.1016/j.tips.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V (2007) Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res 226 (1–2):157–167. doi:10.1016/j.heares.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 6.Ruhl D, Du TT, Wagner EL, Choi JH, Li S, Reed R, Kim K, Freeman M, Hashisaki G, Lukens JR, Shin JB (2019) Necroptosis and Apoptosis Contribute to Cisplatin and Aminoglycoside Ototoxicity. J Neurosci 39 (15):2951–2964. doi:10.1523/JNEUROSCI.1384–18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamesdaniel S (2014) Downstream targets of Lmo4 are modulated by cisplatin in the inner ear of Wistar rats. PLoS One 9 (12):e115263. doi:10.1371/journal.pone.0115263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamesdaniel S, Coling D, Hinduja S, Ding D, Li J, Cassidy L, Seigel GM, Qu J, Salvi R (2012) Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J Biol Chem 287 (22):18674–18686. doi:10.1074/jbc.M111.297960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosati R, Shahab M, Neumann WL, Jamesdaniel S (2019) Inhibition of protein nitration prevents cisplatin-induced inactivation of STAT3 and promotes anti-apoptotic signaling in organ of Corti cells. Exp Cell Res 381 (1):105–111. doi:10.1016/j.yexcr.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathinam R, Ghosh S, Neumann WL, Jamesdaniel S (2015) Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discov 1. doi:10.1038/cddiscovery.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng M, Luo XJ, Pan L, Yang H, Xie X, Liang G, Huang L, Hu F, Kiernan AE, Gan L (2014) LMO4 functions as a negative regulator of sensory organ formation in the mammalian cochlea. J Neurosci 34 (30):10072–10077. doi:10.1523/JNEUROSCI.0352–14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HH, Schock SC, Xu J, Safarpour F, Thompson CS, Stewart AF (2007) Extracellular ATP-dependent upregulation of the transcription cofactor LMO4 promotes neuron survival from hypoxia. Exp Cell Res 313 (14):3106–3116. doi:10.1016/j.yexcr.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 13.Tian Y, Wang N, Lu Z (2010) Repression of Lim only protein 4-activated transcription inhibits proliferation and induces apoptosis of normal mammary epithelial cells and breast cancer cells. Clin Exp Metastasis 27 (7):455–463. doi:10.1007/s10585–010-9332–1 [DOI] [PubMed] [Google Scholar]
- 14.Jamesdaniel S, Rathinam R, Neumann WL (2016) Targeting nitrative stress for attenuating cisplatin-induced downregulation of cochlear LIM domain only 4 and ototoxicity. Redox Biol 10:257–265. doi:10.1016/j.redox.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathinam R, Rosati R, Jamesdaniel S (2018) CRISPR/Cas9-mediated knockout of Lim-domain only four retards organ of Corti cell growth. J Cell Biochem 119 (4):3545–3553. doi:10.1002/jcb.26529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novotny-Diermayr V, Lin B, Gu L, Cao X (2005) Modulation of the interleukin-6 receptor subunit glycoprotein 130 complex and its signaling by LMO4 interaction. J Biol Chem 280 (13):12747–12757. doi:10.1074/jbc.M500175200 [DOI] [PubMed] [Google Scholar]
- 17.Schock SC, Xu J, Duquette PM, Qin Z, Lewandowski AJ, Rai PS, Thompson CS, Seifert EL, Harper ME, Chen HH (2008) Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-gamma requires a novel essential cofactor LMO4. J Neurosci 28 (47):12433–12444. doi:10.1523/JNEUROSCI.2897–08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setogawa T, Shinozaki-Yabana S, Masuda T, Matsuura K, Akiyama T (2006) The tumor suppressor LKB1 induces p21 expression in collaboration with LMO4, GATA-6, and Ldb1. Biochem Biophys Res Commun 343 (4):1186–1190. doi:10.1016/j.bbrc.2006.03.077 [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Lin KK, Lu Z, Lam KS, Newton R, Xu X, Yu Z, Gill GN, Andersen B (2007) The LIM-only factor LMO4 regulates expression of the BMP7 gene through an HDAC2-dependent mechanism, and controls cell proliferation and apoptosis of mammary epithelial cells. Oncogene 26 (44):6431–6441. doi:10.1038/sj.onc.1210465 [DOI] [PubMed] [Google Scholar]
- 20.Levano S, Bodmer D (2015) Loss of STAT1 protects hair cells from ototoxicity through modulation of STAT3, c-Jun, Akt, and autophagy factors. Cell Death Dis 6:e2019. doi:10.1038/cddis.2015.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt NC, Rubel EW, Nathanson NM (2009) Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J Neurosci 29 (12):3843–3851. doi:10.1523/JNEUROSCI.5842–08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V (2011) Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis 2:e180. doi:10.1038/cddis.2011.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephanou A, Latchman DS (2005) Opposing actions of STAT-1 and STAT-3. Growth Factors 23 (3):177–182. doi:10.1080/08977190500178745 [DOI] [PubMed] [Google Scholar]
- 24.Hu X, Ivashkiv LB (2009) Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31 (4):539–550. doi:10.1016/j.immuni.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng M, Pan L, Xie X, Gan L (2010) Requirement for Lmo4 in the vestibular morphogenesis of mouse inner ear. Dev Biol 338 (1):38–49. doi:10.1016/j.ydbio.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes AL, Hussain N, Pafford R, Parham K (2014) Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol Head Neck Surg 150 (1):115–120. doi:10.1177/0194599813511948 [DOI] [PubMed] [Google Scholar]
- 27.Berndtsson M, Hagg M, Panaretakis T, Havelka AM, Shoshan MC, Linder S (2007) Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer 120 (1):175–180. doi:10.1002/ijc.22132 [DOI] [PubMed] [Google Scholar]
- 28.More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM (2010) Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci 30 (28):9500–9509. doi:10.1523/JNEUROSCI.1544–10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC (2013) Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci 33 (10):4405–4414. doi:10.1523/JNEUROSCI.3940–12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur T, Borse V, Sheth S, Sheehan K, Ghosh S, Tupal S, Jajoo S, Mukherjea D, Rybak LP, Ramkumar V (2016) Adenosine A1 Receptor Protects Against Cisplatin Ototoxicity by Suppressing the NOX3/STAT1 Inflammatory Pathway in the Cochlea. J Neurosci 36 (14):3962–3977. doi:10.1523/JNEUROSCI.3111–15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HJ, Kim MJ, Rothenberger C, Kumar A, Sampson EM, Ding D, Han C, White K, Boyd K, Manohar S, Kim YH, Ticsa MS, Gomez AS, Caicedo I, Bose U, Linser PJ, Miyakawa T, Tanokura M, Foster TC, Salvi R, Someya S (2019) GSTA4 mediates reduction of cisplatin ototoxicity in female mice. Nat Commun 10 (1):4150. doi:10.1038/s41467–019-12073–0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH (2004) NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279 (44):46065–46072. doi:10.1074/jbc.M403046200 [DOI] [PubMed] [Google Scholar]
- 33.Li G, Liu W, Frenz D (2006) Cisplatin ototoxicity to the rat inner ear: a role for HMG1 and iNOS. Neurotoxicology 27 (1):22–30. doi:10.1016/j.neuro.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T (2002) Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res 22 (6C):4081–4085 [PubMed] [Google Scholar]
- 35.Jamesdaniel S, Ding D, Kermany MH, Davidson BA, Knight PR, 3rd, Salvi R, Coling DE (2008) Proteomic analysis of the balance between survival and cell death responses in cisplatin-mediated ototoxicity. J Proteome Res 7 (8):3516–3524. doi:10.1021/pr8002479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low IC, Loh T, Huang Y, Virshup DM, Pervaiz S (2014) Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood 124 (14):2223–2234. doi:10.1182/blood-2014–03-563296 [DOI] [PubMed] [Google Scholar]
- 37.Joshi MS, Mihm MJ, Cook AC, Schanbacher BL, Bauer JA (2015) Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. J Diabetes 7 (2):250–259. doi:10.1111/1753–0407.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, Basso M, Melero Fernandez de Mera RM, Sparrow NA, Calingasan NY, Kiaei M, Rhoads TW, Ma TC, Grumet M, Barnes S, Beal MF, Beckman JS, Mehl R, Estevez AG (2013) Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A 110 (12):E1102–1111. doi:10.1073/pnas.1215177110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venkatesan A, Uzasci L, Chen Z, Rajbhandari L, Anderson C, Lee MH, Bianchet MA, Cotter R, Song H, Nath A (2011) Impairment of adult hippocampal neural progenitor proliferation by methamphetamine: role for nitrotyrosination. Mol Brain 4:28. doi:10.1186/1756–6606-4–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curry-McCoy TV, Osna NA, Donohue TM, Jr. (2009) Modulation of lysozyme function and degradation after nitration with peroxynitrite. Biochim Biophys Acta 1790 (8):778–786. doi:10.1016/j.bbagen.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza JM, Choi I, Chen Q, Weisse M, Daikhin E, Yudkoff M, Obin M, Ara J, Horwitz J, Ischiropoulos H (2000) Proteolytic degradation of tyrosine nitrated proteins. Arch Biochem Biophys 380 (2):360–366. doi:10.1006/abbi.2000.1940 [DOI] [PubMed] [Google Scholar]
- 42.Sum EY, Peng B, Yu X, Chen J, Byrne J, Lindeman GJ, Visvader JE (2002) The LIM domain protein LMO4 interacts with the cofactor CtIP and the tumor suppressor BRCA1 and inhibits BRCA1 activity. J Biol Chem 277 (10):7849–7856. doi:10.1074/jbc.M110603200 [DOI] [PubMed] [Google Scholar]
- 43.Manetopoulos C, Hansson A, Karlsson J, Jonsson JI, Axelson H (2003) The LIM-only protein LMO4 modulates the transcriptional activity of HEN1. Biochem Biophys Res Commun 307 (4):891–899. doi:10.1016/s0006–291x(03)01298–1 [DOI] [PubMed] [Google Scholar]
- 44.Singh RR, Barnes CJ, Talukder AH, Fuqua SA, Kumar R (2005) Negative regulation of estrogen receptor alpha transactivation functions by LIM domain only 4 protein. Cancer Res 65 (22):10594–10601. doi:10.1158/0008–5472.CAN-05–2268 [DOI] [PubMed] [Google Scholar]
- 45.Charitidi K, Meltser I, Tahera Y, Canlon B (2009) Functional responses of estrogen receptors in the male and female auditory system. Hear Res 252 (1–2):71–78. doi:10.1016/j.heares.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A (2000) Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett 486 (2):143–148. doi:10.1016/s0014–5793(00)02296–1 [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Berrocal JR, Nevado J, Ramirez-Camacho R, Sanz R, Gonzalez-Garcia JA, Sanchez-Rodriguez C, Cantos B, Espana P, Verdaguer JM, Trinidad Cabezas A (2007) The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br J Pharmacol 152 (7):1012–1020. doi:10.1038/sj.bjp.0707405 [DOI] [PMC free article] [PubMed] [Google Scholar]