Abstract
Objectives:
To characterize primary care physicians’ (PCPs) attitudes and beliefs about people with opioid use disorder (OUD) and to understand the association between PCPs’ stigmatizing attitudes and their OUD treatment practices, beliefs about treatment effectiveness, and support for policies designed to improve access to OUD medications.
Methods:
We conducted a national postal survey of U.S. PCPs from January to August 2019. Survey items measured respondents’ attitudes, beliefs, and current treatment practices. Data were analyzed using descriptive statistics and logistic regression.
Results:
Of the original 1,000 PCPs in the survey sample, 668 were deemed eligible to participate in the study. The survey was completed by 361 PCPs for an adjusted response rate of 54%. PCPs reported high levels of stigmatizing attitudes. Less than 30% of PCPs reported that they were willing to have a person taking medication for OUD as a neighbor or marry into their family, even if that person was being treated with medication. Greater stigma was associated with an 11 percentage point lower likelihood that PCPs prescribed OUD medication and lower support for policies intended to increase access to OUD medication.
Conclusions:
Addressing OUD stigma among PCPs is a public health priority in addressing the ongoing opioid crisis.
Keywords: stigma, Opioid Use Disorder, primary care
1. Introduction
In 2019, at least 1.6 million people in the United States met diagnostic criteria for opioid use disorder (OUD) and nearly 450,000 people have died from an opioid-related overdose since 1999 (SAMHSA, 2020; WONDER, 2020). There is early evidence that opioid overdose deaths may be increasing in the context of the COVID-19 pandemic: one study found a 50% increase in emergency medical service responses to fatal opioid overdoses in March and April 2020 compared with January and February of the same year (Slavova et al., 2020). Disruptions to treatment and harm reduction service delivery, economic strain, and increased psychological distress due to COVID-19 are all potential drivers of increased opioid overdose deaths (Alexander et al., 2020; McGinty et al., 2020a; Volkow, 2020).
Increasing access to treatment for OUD with one of three Food and Drug Administration (FDA)-approved medications – methadone, buprenorphine, and extended-release injectable naltrexone – is fundamental to addressing the opioid crisis (NASEM, 2019). A 2019 report of the National Academies of Science, Engineering, and Medicine (NASEM) confirmed the effectiveness of all three medications in reducing opioid use, increasing retention in treatment, and reducing mortality (NASEM, 2019). Methadone and buprenorphine in particular have been shown to reduce mortality by up to 50% among people with OUD (NASEM, 2019). The NASEM report also concluded that, while it has traditionally been accepted that treatment should combine medication with behavioral counseling, current evidence supports the use of medication for treatment of OUD even when behavioral interventions are not available (NASEM, 2019).
In addition to playing an important role in the treatment of physical health comorbidities for individuals with OUD, primary care physicians (PCPs) play a key role in delivery of OUD medication both directly, through prescribing, and indirectly, through referring patients to providers who prescribe these FDA-approved OUD medications. Currently, two of the three medications, buprenorphine and extended-release injectable naltrexone, are approved for prescription in the primary care setting (NASEM, 2019). To prescribe buprenorphine for OUD, providers must apply and complete training for a waiver and only a small minority of PCPs are currently waivered (NASEM, 2019).
The NASEM report identifies barriers to the delivery of OUD medication including stigma toward individuals with OUD and toward the FDA-approved OUD treatment medications (e.g., the belief that use of medication is “replacing one drug with another”) (NASEM, 2019; Olsen and Sharfstein, 2–14). Link and Phelan (2001) define stigma as the convergence of several processes, including the distinguishing and labeling of difference, linking the labeled difference to stereotypes and unfavorable characteristics, separation of groups to “us” and “them”, status loss and discrimination, and the exercise of social, cultural, economic, or political power over the stigmatized group. Stigma can be inter-personal, systemic, or self-imposed through internalization of the stigmatized person themselves and can have important implications for multiple health outcomes over an individual’s life (Link and Phelan, 2001). Stigma is arguably a significant driver of the health inequities experienced by those with OUD (Link and Phelan, 2006; Hatzenbuehler et al., 2013).
Studies of PCPs’ attitudes toward individuals with OUD and substance use disorder more generally have found high rates of stigma (DeFlavio et al., 2015; Kennedy-Hendricks et al., 2016; van Boekel et al., 2015). Most recently, a 2016 national survey of 1,010 PCPs found that majorities of respondents believed addiction to prescription opioids was the fault of the individual, believed that people with opioid addiction were more dangerous than the general population, and supported employers’ denying employment to people experiencing addiction (Kennedy-Hendricks et al., 2016). These attitudes have important implications as provider stigma contributes to suboptimal clinical care for individuals with a substance use disorder and is associated with lower support for policies benefiting those individuals with addiction (DeFlavio et al., 2015; Kennedy-Hendricks et al., 2016; van Boekel et al., 2015).
Previous work by our team found negative attitudes among PCPs toward medications for OUD: one third of PCPs did not believe that treatment with OUD medication was more effective than treatment without medication, despite robust evidence to the contrary (McGinty et al., 2020b). Further, the majority of PCPs were opposed to policies designed to expand delivery of medication treatment for OUD in the primary care setting, including policies allowing PCPs to prescribe methadone for OUD and eliminating the buprenorphine waiver requirement, two restrictions that pose barriers to medication access (McGinty et al., 2020b).
In order to address provider stigma as a barrier to OUD medication, it is important to understand the association between stigma and PCPs’ OUD treatment practices and beliefs. No prior studies have explored how stigmatizing attitudes are associated with PCPs’ OUD treatment practices, beliefs about treatment effectiveness, and support for policies designed to improve access to OUD medications. We fielded a national survey of PCPs to fill these gaps in the literature.
2. Material and methods
2.1. Survey sample and administration
We randomly selected 1,000 PCPs from the American Medical Association (AMA) Physician Masterfile, which includes information on all physicians licensed to practice in the U.S., for participation in a postal survey (AMA, 2016). PCPs included those who identified as family, internal, or general medicine practitioners.
We fielded the survey following a modified Dillman method (De Leeuw et al., 2008). First, we sent a letter informing physicians of their selection for this survey in January 2019. We then mailed a survey questionnaire, $2 cash incentive, and stamped return envelope in February 2019 to all participants. We sent identical survey mailings to non-responders in five follow-up waves in March, April, June, July, and August 2019. A unique identification number assigned to each survey participant allowed for tracking of responses.
2.2. Response rate calculation
We calculated the response rate as the number of returned surveys divided by the number of eligible physicians. Eligible physicians included those actively practicing primary care at the location of record in the AMA Masterfile. Physicians were ineligible if they did not meet this criteria for any reason, including retirement, death, practicing in a specialty other than primary care, having an administrative position not involving direct patient care, or no longer practicing at the address on file. We determined ineligibility in one of two ways. First, if a survey recipient – either the physician to whom the survey was addressed or someone else at the practice location – notified the research team that they were ineligible, e.g., by calling the research team to inform us that the physician had retired or by returning the survey with a note flagging ineligibility. Second, following the final wave of the survey, we selected a random sample of 100 nonresponding physicians and conducted tracing through internet searches, email messages, and phone calls. If we were able to definitively determine that a physician did not meet eligibility criteria, we defined them as ineligible. We classified physicians who were definitively determined to be actively practicing primary care at the location on file and physicians whose eligibility status was unclear as eligible. We removed ineligible physicians from the sample and extrapolated the rate of ineligibility to the rest of the non-responders (AAPOR, 2011). In the random sample of 100 physicians for whom tracing was performed, 27% were determined to be ineligible. This ineligibility rate is comparable to other surveys using the AMA Masterfile sample frame (Rutkow et al., 2015).
2.3. Measures
The survey included 25 items in five modules: OUD attitudes and beliefs; OUD treatment and referral practices; belief in OUD treatment effectiveness; support for policies to expand access to OUD medications; and physician demographics. We measured OUD attitudes and beliefs with nine items. The first four items assessed desire for social distance, a measure of a respondents’ preferences for separation from the stigmatized group, using questions adapted from prior research evaluating stigma (Bogardus, 1933; Corrigan et al., 2001; Link et al., 1999). These items assessed PCPs’ willingness to have an individual with OUD marry into their family or as a neighbor; these two items were repeated twice, once referring to a “person with OUD” and once referring to a “person taking medication for OUD”. We measured responses to social distance items on 5-point Likert scales ranging from very willing to very unwilling. The first module also included five items measuring beliefs about the causes and treatability of OUD. We assessed PCPs’ agreement with beliefs that people with OUD have poor moral character or have only themselves to blame for their problem, that people who need medication to stop using opioids lack willpower, that OUD is a chronic medical condition, and that people with OUD can, with treatment, get well and return to productive lives. PCPs responded to cause and treatability items using 5-point agree/disagree Likert scales.
The second module consisted of one question assessing current OUD treatment practices. This question asked PCPs to select all responses that applied to their current treatment practices from the following options: provision of OUD counseling, prescription of buprenorphine, and prescription of injectable extended-release naltrexone, referral of patients to OUD counseling inside or outside of the PCP’s practice, referral to another clinician for prescription of buprenorphine or naltrexone, and referral to a methadone program.
The third module included three questions assessing PCPs’ perceptions of the effectiveness of each of the three FDA-approved OUD medications, with responses measured on 5-point agree/disagree Likert scales. The fourth module examined support for four policies intended to increase access to OUD treatment: requiring insurers to cover medication for OUD; increasing government spending on medication for OUD; allowing clinicians to prescribe methadone to treat OUD in primary care settings and eliminating the buprenorphine waiver requirement. We measured policy support using 5-point Likert scales ranging from strongly favor to strongly oppose. The final module measured patient demographic information and personal history with OUD defined as self, close friend, or family member. See Appendix A for complete survey item wording.
For analyses, we collapsed 5-point Likert scales into dichotomous measures indicating willingness (very willing + somewhat willing), agreement (strongly agree + somewhat agree), and support (strongly favor + somewhat favor). We generated a summary stigma measure based on the nine items in module one. Five-point Likert scale responses for these items were coded so that a higher score indicated greater stigma for all measures. A factor analysis showed high internal reliability (Cronbach’s alpha=0.83). We created a summary stigma measure by summing an individual’s responses across the nine measures and then dividing by nine, the number of items, to create a mean score (range: 1–5) with 1 indicating the lowest measure of stigma and 5 indicating the highest (Appendix B).
2.4. Statistical analysis
We assessed differences between responders and non-responders assessed using chi-squared tests. Non-responders were more likely than responders to be from the south (37.2% compared to 22.1%). All analyses used survey weights to account for this difference. There were no differences for other geographic regions, specialty (family, internal, or general medicine), age, sex, degree (MD/DO), or practice type by response status.
We generated descriptive statistics for measures of OUD attitudes and beliefs and current treatment practices. The proportions of respondents endorsing beliefs in treatment effectiveness or support for policies were reported previously in a prior study by our team (McGinty et al., 2020b). We used logistic regression to assess the associations between the summary stigma measure and OUD treatment practices, belief in treatment effectiveness, and policy support. To improve interpretability, we present adjusted average marginal effects (AME) rather than coefficients (i.e., log odds) or odds ratios. The AME indicates the percentage point change in the predicted probability of the outcome associated with a one-unit change in the summary stigma measure (range 1–5). All models included covariates for physician sex, age, region, highest degree (MD/DO), year of graduation from medical school, specialty, practice type, and personal experience with OUD. Analyses were performed using Stata version 15. This study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
3. Results
Of the original 1,000 PCPs in the survey sample, 668 were deemed eligible to participate in the study; 218 physicians or someone at their practice location notified us that they were ineligible. Of the remaining 782 physicians, 361 responded to the survey. We applied the extrapolated ineligibility rate of 27%, based on the tracing effort described above, to the remaining 421 non-responders and subtracted the estimated 114 ineligible physicians (.27 × 421 = 114) from the eligible sample, for a final sample of 668 eligible physicians. Thus, the response rate was 361 returned surveys divided by 668 eligible physicians, or 54%. We excluded 25 surveys with <50% of items completed from analyses, for an analytic sample of N=336. Respondent demographics closely paralleled national primary care physician population characteristics (Petterson et al., 2018) (Appendix C).
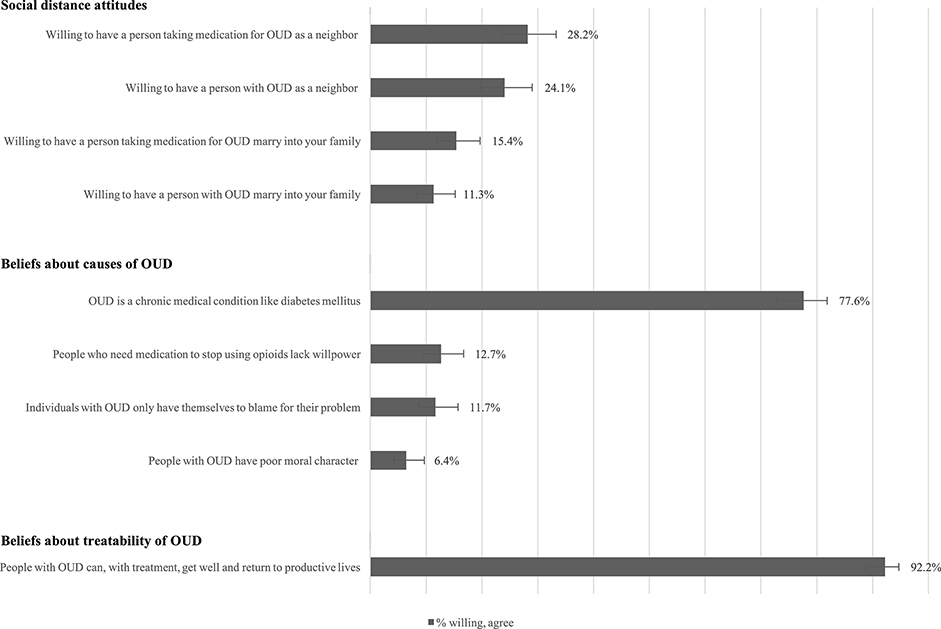
On measures of social distance, a minority of PCPs were willing to have a person with OUD (24%) or a person taking medication for OUD (28%) as a neighbor (Figure 1). A lower proportion were willing to have a person with OUD (11%) or a person taking medication for OUD (15%) marry into their family. Three-fourths of physicians believed that OUD was a chronic medical condition. Few physicians endorsed beliefs that people with OUD lack willpower for using medication (13%), have only themselves to blame (12%), or have poor moral character (6%). Most PCPs endorsed the belief that people with OUD could return to productive lives with treatment (92%).
Fig. 1.
Beliefs about causes of OUD.
Few PCPs currently provide any type of OUD treatment in their practice, with more reporting that they provide counseling (35%) than either prescribing buprenorphine (7%) or naltrexone (4%) (Table 1). Among PCPs who did not prescribe any medication themselves, 52% referred patients to another clinician who prescribed medication or to a methadone program. More PCPs referred to another clinician who prescribed buprenorphine (46%), than to a provider who prescribed naltrexone (22%) or a methadone program (18%). Among PCPs who did not provide counseling themselves, 80% referred patients to another clinician for OUD counseling. These referrals were primarily to clinicians outside of the PCP’s practice (68%).
Table 1.
Opioid use disorder (OUD) treatment practices among U.S. primary care physician survey respondents (N=336)
| Provision of OUD treatment | Percent of respondents (95% CI) |
|---|---|
| Percent of primary care physicians who report that they: | |
| Prescribe either buprenorphine or injectable extended-release naltrexone for OUD | 9.4 (6.7, 13.0) |
| Prescribe buprenorphine (also called Suboxone or Subutex) for OUD | 7.1 (4.8, 10.4) |
| Prescribe injectable extended-release naltrexone (also called Vivitrol) for OUD | 4.4 (2.7, 7.2) |
| Provide OUD counseling themselves | 35.3 (30.3, 40.7) |
| Referral to OUD treatment1 | |
| Percent of primary care physicians who report that they: | |
| Refer patients to another clinician who prescribes buprenorphine, injectable extended-release naltrexone, or methadone for OUD | 52.4 (46.7, 58.1) |
| Refer patients to another clinician who prescribes buprenorphine for OUD | 46.3 (40.7, 52.0) |
| Refer patients to another clinician who prescribes injectable extended-release naltrexone for OUD | 22.4 (18.0, 27.5) |
| Refer patients to an OUD methadone treatment program | 17.9 (13.9, 22.7) |
| Refer patients to OUD counseling | 79.7 (73.6, 84.6) |
| Refer patients to OUD counseling outside their practice | 67.6 (60.9, 73.6) |
| Refer patients to OUd counseling inside their practice | 20.8 (15.9, 26.8) |
Among primary care physician respondents who do not provide treatment themselves.
Higher scores on the summary stigma measure were associated with a decreased probability of PCPs providing any type of OUD medication themselves or providing referrals to OUD medication for patients with OUD (Table 2): each unit increase on the stigma scale was associated with 11 percentage point lower (AME=−0.11, 95% CI: −0.16, −0.07) probability of a provider providing OUD medication themselves and a 20 percentage point lower (AME=−0.20, 95% CI: −0.29, −0.11) probability of a provider providing a referral for OUD medication. Each unit increase in stigma was associated with a 10 percentage point lower (AME=−0.10, 95% CI: −0.17, −0.03) probability of referring patients to OUD counseling within the physicians’ own practice. See Appendix D for full model results.
Table 2.
Association between stigmatizing attitudes and opioid use disorder (OUD) treatment practices among U.S. primary care physicians1,2 (N=336)
| Dependent variables | Average marginal effect of stigma3 (95% CI) |
|---|---|
| Provision of OUD treatment | |
| Prescribe either buprenorphine or injectable extended-release naltrexone for OUD | −0.1%** (0.16, −0.07) |
| Prescribe buprenorphine (also called Suboxone or Subutex) for OUD | −0.08*** (0.12, −0.03) |
| Prescribe injectable extended-release naltrexone (also called Vivitrol) for OUD | −0.07*** (0.11, −0.03) |
| Provide OUD counseling themselves | −0.03 (−0.11, 0.05) |
| Referral to OUD treatment4 | |
| Refer patients to another clinician who prescries buprenorphine, injectable extended-release naltrexone, or methadone for OUD | −0.20*** (0.29, −0.11) |
| Refer patients to another clinician who prescribes buprenorphine for OUD | −0 17*** (0.26, −0.08) |
| Refer patients to another clinician who prescribes injectable extended-release naltrexone for OUD | −0.07* (0.14, 0.00) |
| Refer patients to an OUD methadone treatment program | −0.04 (−0.10, 0.03) |
| Refer patients to OUD counseling | −0.02 (−0.10, 0.06) |
| Refer patients to OUd counseling outside their practice | 0.03 (−0.07, 0.12) |
| Refer patients to OUD counseling inside their practice | −0.10* (0.17, −0.03) |
p<0.05
p<0.01
p<0.001
All models control for sex, age, region, highest degree (MD/DO), year of graduation from medical school, specialty, practice type, and personal experience (self, close friend, or family member) with OUD and are weighted for nonresponse.
Independent variable is a continuous summary stigma measure created using the mean of an individuals’ responses to the 12 stigma items (range: 1–5) with 1 indicating the lowest degree of stigma and 5 indicating the highest degree of stigma.
The average marginal effect indicates the percentage point change in the predicted probability of the outcome associated with a one-unit change in the stigma scale (range 1–5).
Among primary care physician respondents who do not provide treatment themselves.
Higher scores on the summary stigma measure were significantly associated with lower probability of believing in the effectiveness of any of the three OUD medications: each unit increase on the stigma scale was associated with a 19 percentage point lower (AME= −0.19, 95% CI: −0.26, −0.12) probability of endorsing the effectiveness of buprenorphine, 19 percentage point lower (AME= −0.19, 95% CI: −0.27, −0.12) probability of endorsing the effectiveness of methadone, and 12 percentage point lower (AME= −0.12, 95% CI: −0.20, −0.03) probability of endorsing the effectiveness of naltrexone (Table 3). Higher stigma was also associated with lower support for three of the four policies intended to increase access to OUD medication. Each unit increase in stigma was associated with a 22 percentage point lower (AME= −0.22, 95% CI: −0.28, −0.15) probability of supporting a policy requiring insurers to cover medication for OUD, a 21 percentage point lower (AME= −0.21, 95% CI: −0.29, −0.14) probability of supporting a policy allowing clinicians to prescribe methadone for OUD in primary care settings, and a 15 percentage point lower (AME= −0.15, 95% CI: −0.22, −0.09) probability of supporting a policy increasing government spending on OUD medication.
Table 3.
Association between stigmatizing attitudes and beliefs in opioid use disorder (OUD) treatment effectiveness and policy support among U.S. primary care physicians1,2 (N=336)
| Dependent variables | Average marginal effect of stigma3 (95% CI) |
|---|---|
| Belief in OUD treatment effectiveness | |
| Buprenorphine is an effective treatment for OUD | −0.19** (−0.26, −0.12) |
| Methadone is an effective treatment for OUD | −0.19*** (−0.27, −0.12) |
| Injectable extended-release naltrexone is an effective treatment for OUD | −0.12** (−0.20, −0.03) |
| Policy support | |
| Requiring insurers to cover medication for people with OUD | −0.22*** (−0.28, −0.15) |
| Increasing government spending on medication for OUD | −0.15*** (−0.22, −0.09) |
| Allowing clinicians to prescribe methadone to treat OUD in primary care settings | −0.21*** (−0.29, −0.14) |
| Eliminating the requirement to complete an additional 8-hour training and register with the federal government to prescribe buprenorphine (also called Suboxone or Subutex)? | −0.03 (−0.11, 0.06) |
p<0.05
p<0.01
p<0.001
All models control for sex, age, region, highest degree (MD/DO), year of graduation from medical school, specialty, practice type, and personal experience (self, close friend, or family member) with OUD and are weighted for nonresponse.
Independent variable is a continuous summary stigma measure created using the mean of an individuals’ responses to the 12 stigma items (range: 1–5) with 1 indicating the lowest degree of stigma and 5 indicating the highest degree of stigma.
The average marginal effect indicates the percentage point change in the predicted probability of the outcome associated with a one-unit change in the stigma scale (range 1–5).
4. Discussion
Consistent with current clinical understanding of OUD (McLellan et al., 2000 NASEM, 2019), the large majority of PCPs responding to the survey characterized OUD as a chronic condition, and less than 13% attributed of OUD to failings of the individual affected. At the same time, PCPs expressed a high desire for social distance from people with OUD. Stigmatizing attitudes were associated with lower likelihood of treating OUD with medication. This builds upon prior research suggesting that endorsing a biologic model of substance use disorder does not, by itself, eliminate stigma, and may instead increase perceptions of “otherness” of the person and immutability of the disease (Pescosolido et al., 2010; Phelan, 2005).
Study results provide strong support for the NASEM conclusion that stigmatizing attitudes are a barrier to the delivery of evidence-based treatment for OUD (NASEM, 2019). We found that higher levels of stigma were correlated with lower likelihood of prescribing medication to treat OUD or referring patients to other clinicians for OUD medication. PCPs’ stigmatizing attitudes toward people with OUD and reluctance to treat OUD with medication limits health system capacity to deliver guideline-concordant OUD treatment and may impact other aspects of care for individuals with OUD (e.g., treatment of physical comorbidities). Stigma may also impede OUD treatment seeking and engagement. A review of 28 studies found that healthcare providers’ stigmatizing attitudes toward people with substance use disorders were associated with poor quality treatment and low patient engagement in care (van Boekel et al., 2015).
Stigmatizing attitudes were also strongly correlated with lower support for policies to increase access to OUD treatment. Relative to PCPs with lower levels of stigma, PCPs with more stigmatizing attitudes were less likely to support policies that would increase government spending on OUD medication, require insurers to cover OUD medication, and allow methadone for OUD to be prescribed in primary care settings. Historically, physicians have had strong political influence through the AMA and their Political Action Committee (Peterson, 2001). Without support from PCPs (McGinty et al., 2020b), these policies that could expand access to medication treatment for OUD are less likely to be enacted. If enacted by policymakers without PCP support, policy implementation may face hurdles due to insufficient uptake among physicians.
Our findings suggest that addressing OUD stigma among PCPs is a public health priority. Stigma reduction interventions targeting PCPs could be delivered through continuing medical education, academic detailing, and/or communication campaigns. These efforts could also be incorporated in medical school or residency training. Recent efforts in stigma reduction interventions through medical school may already be showing some positive effects, as trainee physicians report significantly less stigmatizing attitudes toward OUD than attending physicians (Kennedy-Hendricks et al., 2020).
One relatively straightforward evidence-based strategy for addressing stigma is through promotion of person-first language. The use of terms such as “abuser” and “addict” perpetuate inaccurate beliefs that OUD is the fault of the individual and reduce health professionals’ inclination to respond therapeutically (Kelly and Westerhoff, 2010). When stigmatizing language is used in the healthcare setting, it affects the perceptions of other clinicians interacting with the patient and may impact clinical decision-making (Goddu et al., 2018). In recent years, some healthcare systems have adopted language pledges, for example, in which providers pledge to use non-stigmatizing, person-first language such as, “person with a substance use disorder” – in the medical record and when interacting with patients (BMC, 2020).
Evidence suggests that positive recovery stories and interactions with individuals in recovery may also be an effective component of stigma reduction initiatives targeting PCPs (Knaak et al., 2014; McGinty et al., 2015; McGinty and Barry, 2020). Many physicians have had challenging experiences with patients with OUD. These challenges may include difficulty helping patients successfully manage withdrawal symptoms and cravings and barriers to connecting them with effective OUD treatment and social services (Beetham et al., 2020; Lowenstein et al., 2019; Mojtabai et al., 2019). Seeing patients repeatedly relapse, a phenomenon exacerbated by ineffective non-medication OUD treatment approaches, may reinforce a sense that all OUD treatment is ineffective. Future research is needed to generate an evidence-base for primary care provider stigma reduction efforts and to understand the best message frames and messengers (e.g., other providers, patients, administrators) for this population (Entman, 1993).
While addressing provider stigma is important, it is only one pathway through which provision of evidence-based OUD treatment can be increased. Challenges related to PCP stigma are attributable, at least in part, to structural stigma – the societal and institutional policies that have led to under-resourced health and social service systems for people with OUD and other vulnerable groups. Stigma, in this sense, is self-perpetuating: stigmatizing attitudes among healthcare administrators and front-line providers contribute to structural stigma, and structural stigma reinforces stigmatizing attitudes by making it difficult for physicians and other providers to facilitate successful treatment and recovery. For individual-level interventions to be effective they ideally need to accompany system-level efforts that support physicians in treating OUD with medication and address the medical and social needs of people with OUD. These system-level interventions could include education for providers on the effectiveness of medication for OUD and inclusion of medication for OUD treatment in performance metrics.
Results of this study should be viewed in light of its limitations. This survey may have been subject to nonresponse and social desirability bias. Previous survey research has found that respondents who require higher incentives to return surveys have a lower likelihood of endorsing public health perspectives (Pollack et al., 2014; van Boekel et al., 2015). While we did not find differences in responses between early (waves 1–3) and late (waves 4–6) responders, we cannot completely eliminate the possibility of non-response bias. Additionally, responders may have under-reported stigmatizing attitudes toward individuals with OUD (Corrigan et al., 2015; van Boekel et al., 2015). In both cases, results likely underestimate the true extent of OUD stigma among PCPs in general. We attempted to minimize these risks by following established survey research methods, including survey weights to account for nonresponse, and assigning each survey a unique identification number. We also included language related to confidentiality in the survey cover letter.
5. Conclusions
PCPs’ stigmatizing attitudes were associated with lower likelihood of treating OUD with medication and lower support for policies designed to increase access to OUD medication. High levels of stigma among this critical part of our health care workforce could impede ongoing and future efforts to scale-up access to effective treatment of OUD with medication. OUD stigma reduction interventions for PCPs are critically needed to support delivery of effective OUD treatment and to ameliorate the ongoing opioid crisis.
Supplementary Material
Highlights.
National survey of U.S. primary care physicians
PCPs reported high levels of stigmatizing attitudes related to opioid use disorder
Stigma negatively associated with likelihood of prescribing OUD medications
Stigma negatively associated with support for increasing access to OUD medication
Acknowledgments
Role of Funding Source
Funding for this study was provided by a Johns Hopkins Frontier Award and the National Institutes of Health (T32 NIMH10943602). The funding sources had no role in the design and conduct of the study, analysis or interpretation of the data, and preparation or final approval of the manuscript prior to publication.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GC, Stoller KB, Haffajee RL, Saloner B, 2020. An epidemic in the midst of a pandemic: Opioid use disorder and COVID-19. Ann. Intern. Med 173 (1), 57–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American Association for Public Opinion Research (AAPOR), 2011. Standard definitions: Final dispositions of case codes and outcome rates for surveys 7th edition. https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ESOMAR_Standard-Definitions-Final-Dispositions-of-Case-Codes-and-Outcome-Rates-for-Surveys.pdf.
- American Medical Association (AMA), 2016. AMA physician masterfile. https://www.ama-assn.org/practice-management/masterfile/ama-physician-masterfile.
- Beetham T, Saloner B, Gaye M, et al. , 2020. Therapies offered at residential addiction treatment programs in the United States. JAMA J. Am. Med. Assoc 324 (8), 804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus ES, 1933. A social distance scale. Sociol. Soc. Res 17, 265–271. [Google Scholar]
- Boston Medical Center (BMC)., 2020. Reducing stigma: Why words about addiction matter. https://www.bmc.org/addiction/reducing-stigma.
- Corrigan PW, Green A, Lundin R, et al. , 2001. Familiarity with and social distance from people who have serious mental illness. Psychiatr. Serv. 52 (7), 953–958. [DOI] [PubMed] [Google Scholar]
- Corrigan PW, Bink AB, Fokuo JK, Schmidt A, 2015. The public stigma of mental illness means a difference between you and me. Psychiatry Res. 226 (1), 186–191. [DOI] [PubMed] [Google Scholar]
- DeFlavio JR, Rolin SA, Nordstrom BR, Kazal LA Jr., 2015. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural. Remote. Health. 15, 3019. [PubMed] [Google Scholar]
- De Leeuw ED, Hox JJ, Dillman DA, 2008. International handbook of survey methodology. Taylor & Francis Group/Lawrence Erlbaum Associates. [Google Scholar]
- Entman RM, 1993. Framing: Toward clarification of a fractured paradigm. J. Commun. 43 (4), 51. [Google Scholar]
- Goddu AP, O’Conor KJ, Lanzkron S, et al. , 2018. Do words matter? Stigmatizing language and the transmission of bias in the medical record. J. Gen. Intern. Med. 33 (5), 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Phelan JC, Link BG, 2013. Stigma as a fundamental cause of population health inequalities. Am. J. Public. Health. 103 (5), 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Westerhoff CM, 2010. Does it matter how we refer to individuals with substance-related conditions? A randomized study of two commonly used terms. Int. J. Drug. Policy. 21 (3), 202–207. [DOI] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, Busch SH, McGinty EE, et al. , 2016. Primary care physicians’ perspectives on the prescription opioid epidemic. Drug. Alcohol. Depend. 165, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy-Hendricks A, Barry CL, Stone E, et al. , 2020. Comparing perspectives on medication treatment for opioid use disorder between national samples of primary care trainee physicians and attending physicians. Drug. Alcohol. Depend 216, 108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaak S, Modgill G, & Patten SB, 2014. Key ingredients of anti-stigma programs for health care providers: a data synthesis of evaluative studies. Can. J. Psychiatry 59 (1_suppl), 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, Phelan JC, 2001. Conceptualizing stigma. Annu. Rev. Sociol 27 (1), 363–385. [Google Scholar]
- Link BG, Phelan JC 2006. Stigma and its public health implications. Lancet. 367 (9509), 528–529. [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan JC, Bresnahan M, et al. , 1999. Public conceptions of mental illness: Labels, causes, dangerousness, and social distance. Am. J. Public. Health 89 (9), 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein M, Kilaru A, Perrone J, et al. , 2019. Barriers and facilitators for emergency department initiation of buprenorphine: A physician survey. Am. J. Emerg. Med 37 (9), 1787–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty EE, Barry CL, 2020. Stigma reduction to combat the addiction crisis - Developing an evidence base. N. Engl. J. Med. 382 (14), 1291–1292. [DOI] [PubMed] [Google Scholar]
- McGinty EE, Goldman HH, Pescosolido B, Barry CL, 2015. Portraying mental illness and drug addiction as treatable health conditions: Effects of a randomized experiment on stigma and discrimination. Soc. Sci. Med. 126, 73–85. [DOI] [PubMed] [Google Scholar]
- McGinty EE, Presskreischer R, Han H, Barry CL, 2020a. Psychological distress and loneliness reported by US adults in 2018 and April 2020. JAMA J. Am. Med. Assoc. 324 (1), 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty EE, Stone EM, Kennedy-Hendricks A, et al. , 2020b. Medication for opioid use disorder: A national survey of primary care physicians. Ann Intern Med. 173 (2), 160–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD, 2000. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA J. Am. Med. Assoc. 284 (13), 1689–1695. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Mauro C, Wall MM, et al. , 2019. Medication treatment for opioid use disorders in substance use treatment facilities. Health. Aff. 38 (1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM), 2019. Medications for opioid use disorder save lives. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Olsen Y, Sharfstein JM, 2014. Confronting the stigma of opioid use disorder—and its treatment. JAMA J. Am. Med. Assoc. 311 (14), 1393–1394. [DOI] [PubMed] [Google Scholar]
- Pescosolido BA, Martin JK, Long JS, et al. , 2010. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. Am. J. Psychiatry. 167 (11), 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MA, 2001. From trust to political power: interest groups, public choice, and health care. J. Health. Polit. Policy. Law. 26 (5), 1145–1163. [DOI] [PubMed] [Google Scholar]
- Petterson S, McNellis R, Klink K, et al. , 2018. The state of primary care in the United States: A chartbook of facts and statistics. https://www.graham-center.org/content/dam/rgc/documents/publications-reports/reports/PrimaryCareChartbook.pdf
- Phelan JC, 2005. Geneticization of deviant behavior and consequences for stigma: The case of mental illness. J. Health Soc. Behav. 46 (4), 307–322. [DOI] [PubMed] [Google Scholar]
- Pollack HA, Pereyra M, Parish CL, et al. , 2014. Dentists’ willingness to provide expanded HIV screening in oral health care settings: results from a nationally representative survey. Am. J. Public Health. 104 (5), 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkow L, Turner L, Lucas E, et al. , 2015. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health. Aff. 34 (3), 484–492. [DOI] [PubMed] [Google Scholar]
- Slavova S, Rock P, Bush HM, et al. , 2020. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug. Alcohol. Depend. 214, 108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2020. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20–07-01–001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/ [Google Scholar]
- Wide-ranging online data for epidemiologic research (WONDER), 2020. National Center for Health Statistics. http://wonder.cdc.gov.
- van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF, 2015. Stigmatisering van patiënten met een verslaving en de gevolgen voor de hulpverlening: een systematisch literatuuronderzoek [Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review]. Tijdschr. Psychiatr. 57 (7), 489–497. [PubMed] [Google Scholar]
- Volkow ND, 2020. Collision of the COVID-19 and addiction epidemics. Ann. Intern. Med 173 (1), 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.