Abstract
While estrogens are involved in normal prostate morphogenesis and function, inappropriate early-life estrogenic exposures, either in type, dose or timing, can reprogram the prostate gland and lead to increased disease risk with aging. This process is referred to as estrogen imprinting or developmental estrogenization of the prostate gland. The present review discusses published and new evidence for prostatic developmental estrogenization that includes extensive research in rodent models combined with epidemiology findings that together have helped to uncover the architectural and molecular underpinnings that promote this phenotype. Complex interactions between steroid receptors, developmental morphoregulatory factors, epigenetic machinery and stem-progenitor cell targets coalesce to hard wire structural, cellular and epigenomic reorganization of the tissue which retains a life-long memory of early-life estrogens, ultimately predisposing the gland to prostatitis, hyperplasia and carcinogenesis with aging.
Keywords: prostate, development, estrogen, estrogenization, reprogramming, HOXB13
Introduction:
The prostate gland is derived embryologically from the endodermal urogenital sinus and its development, growth and function throughout life are under tight androgenic regulation (1,2). Dihydrotestosterone (DHT), derived from intraprostatic metabolism of testosterone, is the primary androgen controlling prostate activity, mediated through the androgen receptor (AR) (3,4). It is also long recognized that estrogens can impact prostate growth, homeostasis and disease throughout life with actions mediated through multiple estrogens receptors (ERs), including ERα, ERβ, GPER and estrogen-related receptors (ERR) that are expressed in a cell-specific manner in the prostate. During development, estradiol-17β plays a physiologic role in modulating prostate branching morphogenesis and epithelial differentiation through ERα and ERβ, respectively (5–8). However, inappropriate estrogenic exposures during development, in terms of dose, type and timing have been shown to reprogram the prostate gland resulting in developmental defects that set the stage for increased disease susceptibility, a phenotype referred to as estrogenic imprinting or developmental estrogenization. This process and implications for adult onset diseases are the focus of the present review.
Estrogen Receptors in the Developing Prostate
Rodent studies have shown robust stromal ERα expression during early-life prostate morphogenesis, which significantly declines with puberty as androgen levels rise, suggesting a specific developmental role (9,10). One study also identified transient epithelial ERα levels in the murine prostate at week 2 of life which thereafter declined (8); however, this was not observed in the neonatal or prepubertal rat ventral, dorsal or lateral lobes (personnel observations). Elegant experiments in the Yeh laboratory using stromal cell-specific deletion of ESR1, the gene that encodes ERα, in murine prostates determined that fibroblast ERα modulates branching morphogenesis whereas smooth muscle ERα regulates stromal cell proliferation and ECM deposition (5,6). In humans, ERα is likewise expressed in stromal cells during fetal development (11,12) whereas at least one study identifies its localization in periurethral prostatic epithelium during mid-to-late gestation where it is associated with squamous metaplasia (12). Furthermore, recent studies have also identified ERα in human prostatic epithelial stem and progenitor cells, where they mediate estrogen-induced stimulation of stem cell self-renewal and progenitor cell proliferation (13–15). This implicates a potential estrogenic role in maintaining prostate epithelial homeostasis through this repopulating stem population. Of particular note, estrogen actions in the stem-progenitor cell pool are mediated through both genomic and membrane-initiated signaling pathways (16).
In contrast to ERα, ERβ expression is localized almost exclusively to prostate epithelial cells with a putative role in differentiation of the luminal epithelium (17). In the rodent prostate, ESR2 expression, the gene that encodes ERβ, steeply increases concomitant with luminal cell differentiation during the postnatal period implicating a role in that process (7). In humans, ERβ is widely expressed in epithelial and stromal cells by gestational week 7 and is maintained throughout gestation and postnatally for several months, suggesting a specific developmental function (11,12). Further, ERβ is found in human prostate stem and progenitor cells, localizing to both the nuclear and membrane compartments where it restrains stem cell symmetric self-renewal and promotes progenitor cell differentiation (14,16)
Estrogen Imprinting of the Prostate Gland
Epidemiologic evidence suggests that inappropriate estrogen exposures during development can lead to abnormal growth of the human prostate with predisposition to diseases with aging such as benign prostatic hyperplasia (BPH) and adenocarcinoma (18,19). Swedish cohort analysis found strong correlations between indicators of high levels of pregnancy estradiol, such as high birth weight and jaundice in the offspring, and increased risk for prostate cancer whereas indicators of low maternal estrogens, like pre-eclampsia/eclampsia, were associated with decreased risk in offspring as they aged (20,21). African-American men have a two-fold higher risk of developing prostate cancer with aging than Caucasian men and evidence indicates this may be linked, in part, to elevated maternal estrogens during the first trimester (22). Further, sons of mothers who used diethylstilbestrol (DES) during pregnancy are predisposed to reproductive tract neoplasia (23) and exhibit excessive epithelial metaplasia (24) and structural abnormalities of the prostatic utricle at 1 month of age with persistent ectasia (25).
In contrast to humans where prostate development is largely completed in utero, the rodent prostate is rudimentary at birth and undergoes branching morphogenesis and cell differentiation postnatally (26). As such, the neonatal rodent prostate serves as a model for evaluating the effects of natural, pharmaceutical and environmental estrogens on prostate development. Studies from multiple laboratories have found that neonatal exposures to high levels of estrogens, modeling pharmaceutical exposures, leads to developmental and differentiation defects of the stroma and epithelium and onset of prostatic diseases with aging that include chronic prostatitis, stromal hyperplasia, epithelial dysplasia, adenomas, prostatic intraepithelial neoplasia (PIN) and over time, onset of adenocarcinoma (27–32). In the rat, lobe-specific effects were observed with the greatest perturbations in the ventral lobe (30) which was similar to that found in the estrogenized mouse prostate (33). These findings have led to the supposition that high-dose estrogenic exposures during development can directly predispose to prostate neoplasia and tumor formation with aging.
Increased concern in recent years regarding early-life exposures to endocrine disrupting chemicals (EDCs) has led to considerable research on prostate gland reprogramming by estrogenic EDCs. Bisphenol A (BPA) has been the most thoroughly investigated and serves as a model estrogenic EDC. The first report that developmental exposures to BPA altered the adult prostate came from the vom Saal laboratory which found that gestational low-dose BPA exposure led to larger dorsolateral prostates in adult mice (34). Underlying mechanisms include altered ERα and AR expression in mesenchymal cells through global epigenetic modifications (35) and elevated CYP19A1 activity and UGS estradiol levels (36). In the Sprague-Dawley rat model used in the Prins laboratory, brief neonatal low-dose BPA exposure was insufficient for inducing prostate pathology or weight changes in adulthood; however, it directly led to epigenetic reprogramming and stem cell modifications in the dorsolateral prostate that resulted in heightened susceptibility to estrogen-driven carcinogenesis with aging (37–41), an observation replicated by an independent group (42). Further, modeling with humanized prostate-like renal grafts in nude mice found similar carcinogenic responses to transient BPA exposures in the human-derived epithelium (15) indicating that the effects seen in rodent models are translatable to the human gland. Evidence with other estrogenic EDCs is limited but also points to prostate aberrations with aging that include hyperplasia and heightened carcinogenic risk (43,44).
Together, the phenotypic data in rodents and humanized models combined with epidemiology studies reveal that developmental exposures to multiple types of estrogens – elevated maternal estradiol, pharmaceutical estrogens, estrogenic EDCs – can increase disease propensity in the adult prostate gland, a concept that reinforces the developmental basis of adult disease paradigm. The following sections will review the mechanistic underpinnings of these processes that include direct changes in steroid receptor expression profiles and activity, interference in morpho-regulatory gene expression resulting in distorted glandular architecture, epigenetic modifications leading to cellular reprogramming and altered cell memory, and aberrant stem cell reprogramming with altered self-renewal and lineage commitment leading to persistent life-long effects and tissue perturbations. Importantly, these mechanisms are not mutually exclusive and work together to drive heightened susceptibility to prostatic diseases with aging.
Altered Steroid Receptor and Developmental Gene Expression
Steroid Receptors
The developing rodent prostate responds to multiple steroidal signals which modulate development through specific steroid receptors (SRs). AR is the dominant receptor and is essential for ontogeny and development of the gland. At the early developmental stages, AR expression is confined to mesenchymal/stromal cells and when liganded, directs glandular morphogenesis and cellular differentiation through stimulation of stromal-derived secretory growth factors (1,45). As the epithelium differentiates, AR expression is induced in luminal cells, between postnatal days (PND) 5–15 in the rat prostate lobes (46), where it controls functional differentiation. As discussed above, ERs also contribute to normal prostate development as do other SRs including retinoid receptors RARs/RXRs whose levels decline post-pubertally implicating a specific developmental role (47–49). Studies in our laboratory have found that exposures to high levels of estrogens during the neonatal critical window (PND 1–5) markedly alter the expression profile of multiple SRs in the rat and mouse prostate glands. Some changes occur immediately (ERα, AR) and directly drive the early estrogenized phenotype whereas other SR changes appear later in development or adulthood (e.g. ERβ). Furthermore, some of the initial SR alterations are transient (ERα, PR) whereas others are permanent (AR, ERβ, RAR/RXR), lasting throughout life. We propose that these estrogen-induced changes in SR expression play a fundamental role in initiating growth and differentiation defects during early development and maintaining these phenotypes throughout life.
Studies with ERαKO and ERβKO mice determined that neonatal estrogenization of the rodent prostate is mediated entirely through ERα in the developing gland (50). Recent studies using membrane-only ERα and nuclear-only ERα mice (H2NES and NOER mice, respectively) revealed that both membrane-localized (mER) and nuclear ER pools are necessary for a full estrogenized phenotype, acting in a cooperative manner (Cooke & Prins, unpublished data). Soon after high-dose estrogen exposure, AR protein is sharply down-regulated in both stromal and epithelial cells (46) through heightened proteasomal degradation (51) and remains low throughout life leading to a reduced activational response to androgens (30,52). The estrogenic exposure leads to a transient up-regulation of ERα in periductal stromal cells which in turn, permits a transient induction of PR is these cells (10,53). In addition, RARs and RXRs and intraprostatic retinoid are immediately and permanently elevated allowing for the amplification of retinoid signaling during development and with aging (48,54). Other investigators have also examined AR and ER levels following early-life estrogen exposure and found similar results (35,55,56). The prostatic steroid receptor changes during development due to early estrogens are summarized in Figure 1, bottom.
Figure 1:
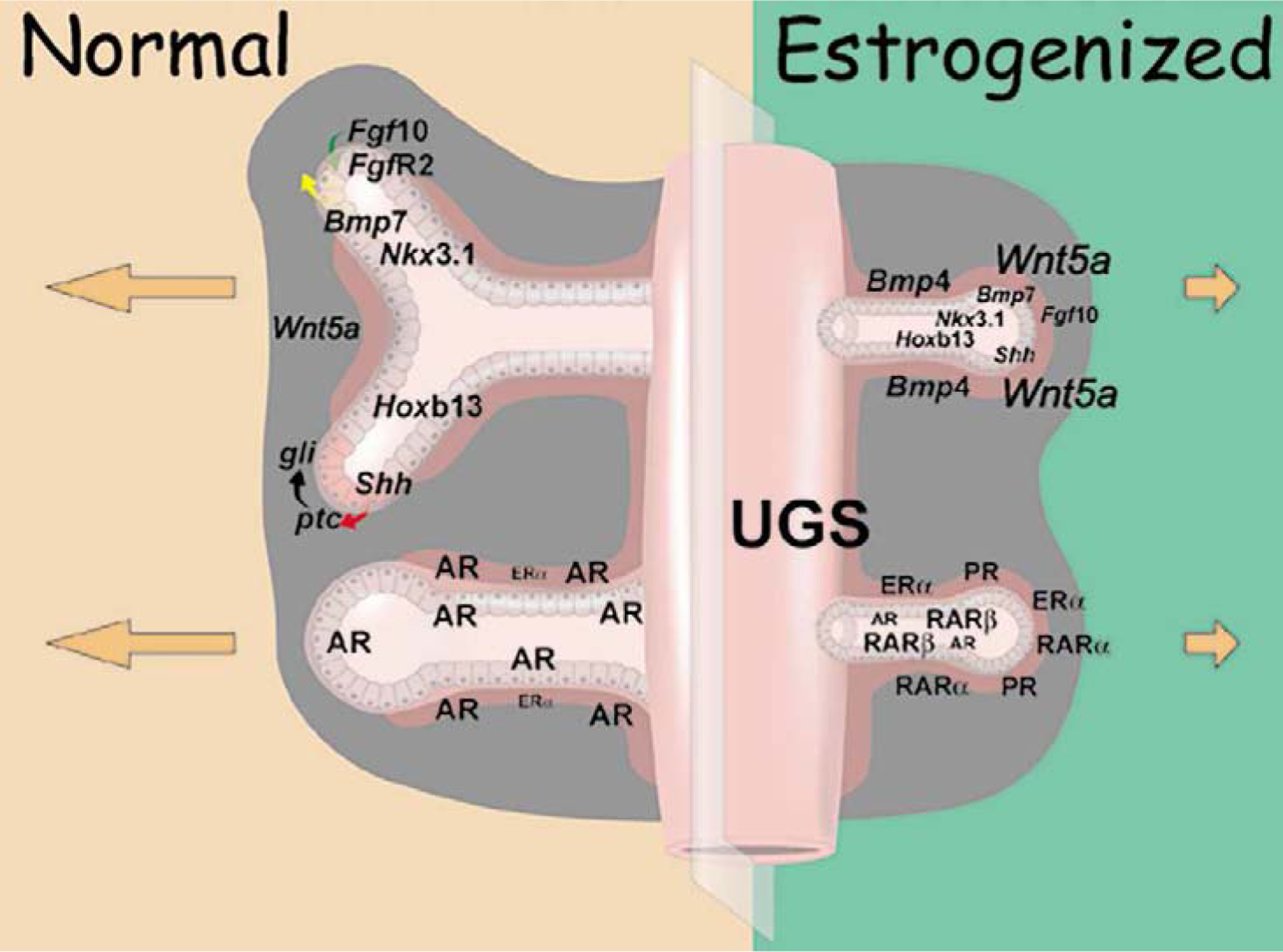
Schematic representation of the normal (left) and estrogenized (right) developing prostate gland that highlight essential morphogens and transcription factors (top) and steroid receptors (bottom) that coordinately regulate morphogenesis and differentiation. Relative gene expression levels are represented by type size. Dysregulation of steroid receptors and morphoregulatory gene expression following early estrogenic exposures results in stunted growth, structural rearrangements and aberrant cell differentiation. See text for details. Reproduced with permission from (57).
In the normal prostate, AR is the dominant SR dictating morphogenesis whereas lower levels of ERα are found in stromal cells surrounding the proximal ducts. Neonatal exposure to high-dose estradiol sharply reduces AR while amplifying ERα in periductal stromal cells along the length of the developing ducts. Additionally there is induction of PR and increased RARα in stromal cells and upregulation of RARβ in epithelial basal cells. Thus the developing prostate is no longer under predominant androgen-AR regulation, but is rather driven by alternate steroids through estrogen-ER, progesterone-PR and retinoid-RAR signaling pathways. We propose the net effect of these changes is that programming and organizational signals that normally dictate and determine prostate development during discreet temporal windows are irretrievably altered.
Developmental Genes
Continuous branching morphogenesis of the prostate during development is dictated by time-specific and region-specific expression of master regulatory genes which form a prostatic code that includes hedgehogs, Wnts, Bmps, Fgfs, Nkx3.1 and posterior Hox genes (see reviews in (2) and (58)). Importantly, there is clear evidence of a role for steroids in regulating developmental genes in hormone-sensitive tissues (59,60), including the prostate gland. Towards this end, our laboratory focused on determining whether neonatal exposure to estrogens altered rat prostate development through SR-induced changes in key developmental genes. Indeed, the expression of a number of prostate morphoregulatory genes, including secreted morphogens, their cognate receptors and developmentally critical transcription factors were either transiently or permanently altered by inappropriate early-life estrogen exposure, contributing to permanent structural reorganization and differentiation abnormalities. These included alterations in expression and/or activities of TGFβ-TGFβR1 (61), SHH-PTC-GLI (62), FGF-10/FGFR2iiib (63), BMP-4 and −7 (54,64), WNT5a (65), NKX3.1 (66), and HOX13 genes (67,68) as schematized in Figure 1, top. In the normal prostate, mesenchymal secretion of FGF-10 acts on epithelial FGFR2iiib to stimulate ductal outgrowth and branching while BMP4 and WNT5a act as localized inhibitors to restrict these events at specific sites. Distal tip epithelial cells secrete SHH which ligands to mesenchymal cell PTC receptors and activates GLI transcription factors that regionally restrict growth. Together these and other factors act in concert to tightly regulate branching morphogenesis. Transcription factors NKX3.1 and HOXB13 are expressed by developing epithelial cells to control differentiation. The alterations in steroidal signaling in response to neonatal estrogens redirect the expression of these morphoregulatory genes (Figure 1, top right). Specifically, BPM4 and WNT5a levels are markedly increased and remain elevated while FGF10/FGFR2iiib levels and signaling are reduced. SHH signaling is heightened and together these factors act to suppress growth and branching of the ducts. Transient postnatal reduction in Nkx3.1 expression, at the time of normal epithelial cell differentiation, combined with permanent Hoxb13 gene suppression initiate differentiation defects that persist throughout life.
It is noteworthy that dysregulation of several of these morphoregulatory genes occurs during prostate carcinogenesis in humans (69–73), thus providing a mechanistic link between developmental reprogramming and tumor formation with aging. One in particular, HOXB13, is of specific interest since a novel HOXB13 G84E variant in humans is associated with increased risk of hereditary prostate cancer (74) and is the topic of intense research for its role in sporadic prostate cancer and metastasis. The Prins laboratory observed over 20 years ago that neonatal estradiol suppressed the Hoxb13 levels in the developing rat ventral prostate which persisted throughout life (67). Further characterization of the rat prostatic Hox code found maximal Hoxb13 expression in the adult rat ventral prostate epithelium, with decreasing expression in anterior direction; i.e. ventral > lateral > dorsal prostate lobes and minimal expression in the coagulating glands (anterior prostate) (68). Using Hoxb13-lentiviral vectors targeted to undifferentiated prostate epithelial cells, an essential role for HOXB13 in initiating and maintaining luminal cell differentiation was directly demonstrated, supporting earlier findings in the murine prostate (75). Prostatic Hoxb13 expression in rats neonatally exposed to 25µg estradiol on PND 1, 3 and 5 was immediately and persistently suppressed in all three lobes with the greatest response noted in the ventral prostate (Figure 2A) (76). Further, immunohistochemistry of HOXB13, luminal cytokeratin 8, basal cytokeratin 5 and the secretory prostate binding protein (PBP) in serial sections of the day 90 ventral lobe revealed that suppressed Hoxb13 levels directly overlapped with aberrant epithelial differentiation and loss of functional activity (Figure 2B). As such, we propose that life-long suppression of HOXB13 contributes to permanent differentiation defects of estrogenized prostate and its predisposition to cancer.
Figure 2:
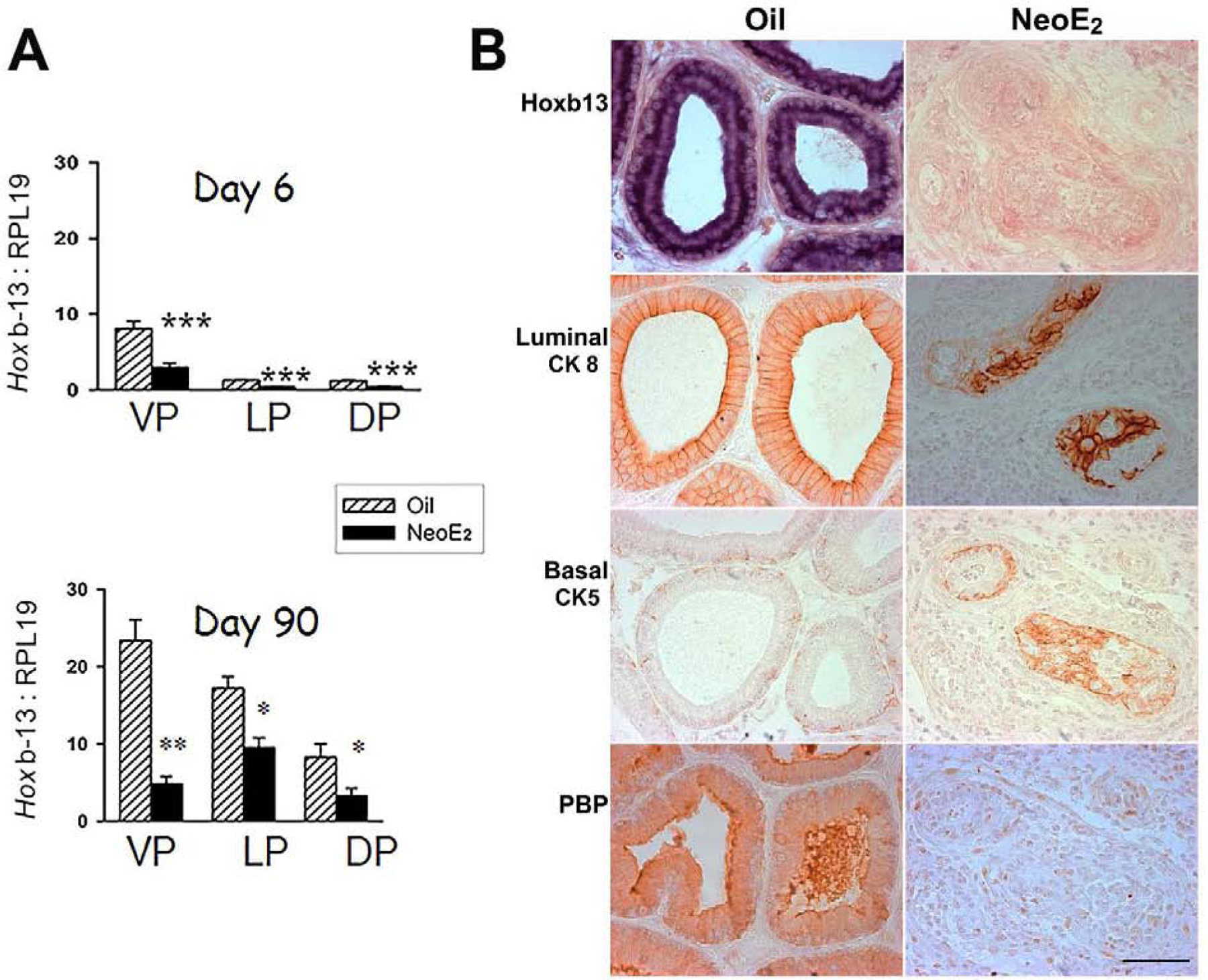
Hoxb13 expression in the developing and adult prostate lobes of rats treated neonatally with oil or 25µg estradiol benzoate (NeoE2) on PND 1, 3 and 5. A: Expression levels of Hoxb13, measured by RT-PCR, in the ventral (VP), lateral (LP) and dorsal (DP) lobes at day 6 and 90 in oil control and neoE2 exposed rats. Expression is the highest in the VP with declining levels in the LP and DP of control prostates. Exposure to NeoE2 immediately suppresses Hoxb13 expression in the developing prostates which is maintained through adulthood. B: Immunohistochemistry in the adult VP for HOXB13, luminal cell CK8/18, basal cell CK5/15 and secretory prostate binding protein (PBP) on serial sections of both oil and NeoE2 exposed prostates. Estrogenized prostates exhibit a disorganized epithelium with loss of luminal HOXB13, defective luminal and basal cell differentiation and orientation, and loss of luminal PBP secretions. See (63,68) for methodologic details.
Structural Reorganization of the Estrogenized Prostate
Tissue development is an einbeinstrasse (one-way street) and as such, the early estrogen-induced reprogramming events lead to permanent alterations in prostate structure and activity throughout life. Prostates of rodents exposed to high-dose estrogen exhibit stunted growth and reconfigured stromal and epithelial organization (30). Normal prostatic ducts have a heterogenous pattern of cell types and functions along the ductal length (77,78) and those exposed to high-dose estrogen retain an exaggerated proximal duct phenotype along the length of the prostatic ducts (79). This proximalized phenotype is characterized by a thick periductal fibroblast layer that extends to the distal tips and prevents interactions (e.g. secreted growth factors) between the central-distal epithelial cells and smooth muscle cells that normally lie adjacent to the basement membrane. Similarly, the epithelial cell organization retains a proximal pattern with a continuous layer of basal cells along the ductal length in contrast to the normal rodent prostate where the basal cell layer is discontinuous in the central-distal regions (46). This is accompanied by permanent shifts in epithelial cell adhesion and gap junction proteins (80) which further alter cell signaling. As such, in young adulthood, estrogenized prostate epithelial cells are disorganized, aberrantly differentiated and their secretory function is markedly compromised (Figure 2B) (52). We hypothesize that the estrogen-driven proximalized prostate phenotype reflects a change in proximal-distal positional identity within prostatic ducts, mediated by altered expression of genes that regulate proximal-distal axis specification.
It is important to note that these structural changes are quite different with low-dose estrogenic exposures, including EDCs where larger glands and hyperplasia in adulthood are reported (81). This underlines an established endocrinologic phenomenon where non-linear and bell-shaped dose response curves occur and stresses the importance of evaluating responses to estrogens based on dose (82).
Epigenetic Reprogramming by Early-life Estrogens
In addition to structural reorganization, prostate stromal and epithelial cells exposed inappropriately to estrogens during development retain a lifelong memory of the event such that they are sensitized to later life exposures which further promote disease. One identified mechanism that enables retained cellular memory is molecular reprogramming of the epigenome, first proposed by McLachlan for early-life estrogen and EDC exposures (83,84). Epigenetic modifications are heritable changes in gene expression not caused by nucleotide alterations in the genome. These include DNA methylation at CpG sites, histone methylation at lysine and arginine residues on histone tails, and noncoding RNAs with downstream epigenetic consequences (reviewed in (85)). Evidence has now emerged that all three of these epigenetic processes are involved in reprogramming of the developmentally estrogenized prostate gland.
The first evidence for epigenetic reprogramming of the prostate came from the Ho laboratory in collaboration with the Prins laboratory which, using genome-wide screening, identified differential methylation of > 50 genes in PND 10, 90 and 200 prostates following neonatal estradiol- or BPA-exposure as compared to controls (37). Persistent DNA hypomethylation resulting in increased gene transcription was detailed for nucleosome binding protein (Nsbp1) which plays a role in chromatin remodeling, phosphodiesterase 4 variant 4 (Pde4d4) which degrades cAMP, and Hippocalcin-like 1 (Hpcal1), involved in cAMP formation (86). Of note, expression of both PDE4D4 and NSBP1 is associated with human prostate cancer cell growth (87,88) The reprogrammed DNA methylome is likely mediated by estrogen/BPA-induced permanent increases in expression of de novo DNA methyltransferases DMNT3a/b and methyl-CpG binding domain proteins (Mbd2/4) that have demethylating activities. In follow-up studies of young adult prostates neonatally exposed to estradiol or BPA, differentially methylated regions were identified for 111 estradiol-associated and 86 BPA-associated genes as compared to controls, with 20 in common for both treatment groups, thus uncovering unique DNA methylation fingerprints for specific estrogenic chemicals (40,89). Pathway analysis of the 20 common genes identified cancer as the top common disease pathway. The methylation status of 7 genes (Pitx3, Wnt10b, Paqr4, Sox2, Chst14, Tpd52, Creb3l4) showed inverse correlation with gene expression in tissue samples thus documenting functional relevance. Significantly, functional connectivity of these 7 genes was linked to embryonic stem cell pluripotency whereas clustering analyses using The Cancer Genome Atlas dataset discovered that expression of this 7 gene set was associated with recurrence-free survival of prostate cancer patients. Together, these results reveal that gene-specific promoter methylation changes resulting from early-life estrogen/EDC exposure in the rat may serve as predictive epigenetic biomarkers of prostate cancer recurrence and raise the possibility that such exposures may impact human disease. Several other research laboratories have similarly identified altered DNA methylation patterns and altered gene expression in the prostate as a function of early-life estrogenic exposures (35,90–92) thus solidifying an epigenetic underpinning of prostatic developmental estrogenization.
The Walker laboratory extended the epigenetic reprogramming pathways involved in estrogenization by examining histone tail methylation marks in rat prostate tissue. They unequivocally demonstrated that brief estrogenic low-dose BPA exposure rapidly activated the mERα-PI3K-pAKT signaling cascade in the PND 6 rat prostates which in turn activated of MLL1, a histone methyl transferase (HMT) component of the COMPASS complex responsible for laying down H3K4me3 marks on specific genes (93). Remarkably, these activational marks on multiple prostatic genes, including several KEGG prostate cancer genes, persisted into adulthood (not in controls) and exaggerated transcriptional responses to an adult estrogen/testosterone challenge. Similarly, DES and BPA activation of mESR1-PI3K-AKT signaling phosphorylated and down repressive H3K27me3 marks. The net result was a reduction in H3K27me3 levels with increased expression of downstream genes (94). Together, these important studies indicate that epigenetic reprogramming of histone methyl marks in the neonatal estrogen-exposed prostate, mediated through rapid mERα signaling, is a key mediator of life-long cellular imprinting and altered cell memory.
Modulation of ncRNAs was also found in human prostate progenitor cells enriched in 3-D prostasphere cultures and exposed to either estradiol or increasing doses of BPA for one week (95). Across all treatment groups, there was suppression of small nucleolar RNAs (SNORDS), a class of ncRNAs that guide methylation of rRNA/tRNA during protein biogenesis and regulate alternate RNA splicing. Notably, SNORD reprogramming was not mediated through DNA methylation but rather through modifications of H3K4me3, H3K9me3 and H3K27me3 histone methyl marks.
As a continuing story of Hoxb13 downregulation in estrogenized rat prostates, our laboratory explored possible epigenetic pathways that mediate its life-long suppression. First, we identified two CpG Islands (CGI) upstream of the rat Hoxb13 promoter and, using Mass Sequenom, evaluated their methylation status in day 90 ventral prostates of rats exposed neonatally to 25µg estradiol or vehicle (Figure 3A). Methylation at both CGIs was relatively low, averaging 6–10% of available CpG sites in the control prostates. While CGI-2 methylation was not influenced by neonatal estradiol exposure, a small but significant increase in methylation (from 6 to 8%) of the proximal CGI-1 was noted. This mapped to a single CpG site that overlapped with an HMX2 binding site, a homeobox gene with yet unknown roles in prostate development. Next, we identified a polycomb group response element (PRE) and trithorax response element (TRE) positioned between CGI-1 and CGI-2 in the Hoxb13 promoter region (Figure 3 B). Inactivating PRC2, which contains EZH2 that trimethylates H3K27, binds to PRE sites through SUZ12 while activating trithorax genes, UTX and JMJD3 which demethylate these sites, bind at TREs. Together, these complexes regulate the methylation status of H3K27. To determine whether neonatal estrogens influenced H3K27 methylation of the Hoxb13 promoter region to silence prostatic expression, we used ChIP-PCR for selected histone methylation marks and for SUZ12 at the PRE and transcription start site (TSS) of the Hoxb13 gene. H3K27me3 and SUZ12 were enriched at the PRE site in estrogen-exposed prostates versus oil controls (Figure 3B). At the Hoxb13 TSS, H3K4me1, H3K4me3 and H3K9me3 were unaffected by estrogen exposure; however, SUZ12 and H3K27di- and tri-methylation were significantly enhanced compared to oil controls (Figure 3B). Together, these data demonstrate that early-life estrogens epigenetically suppress prostate Hoxb13 expression by laying down permanent silencing histone methylation marks as schematized in Figure 3C.
Figure 3:
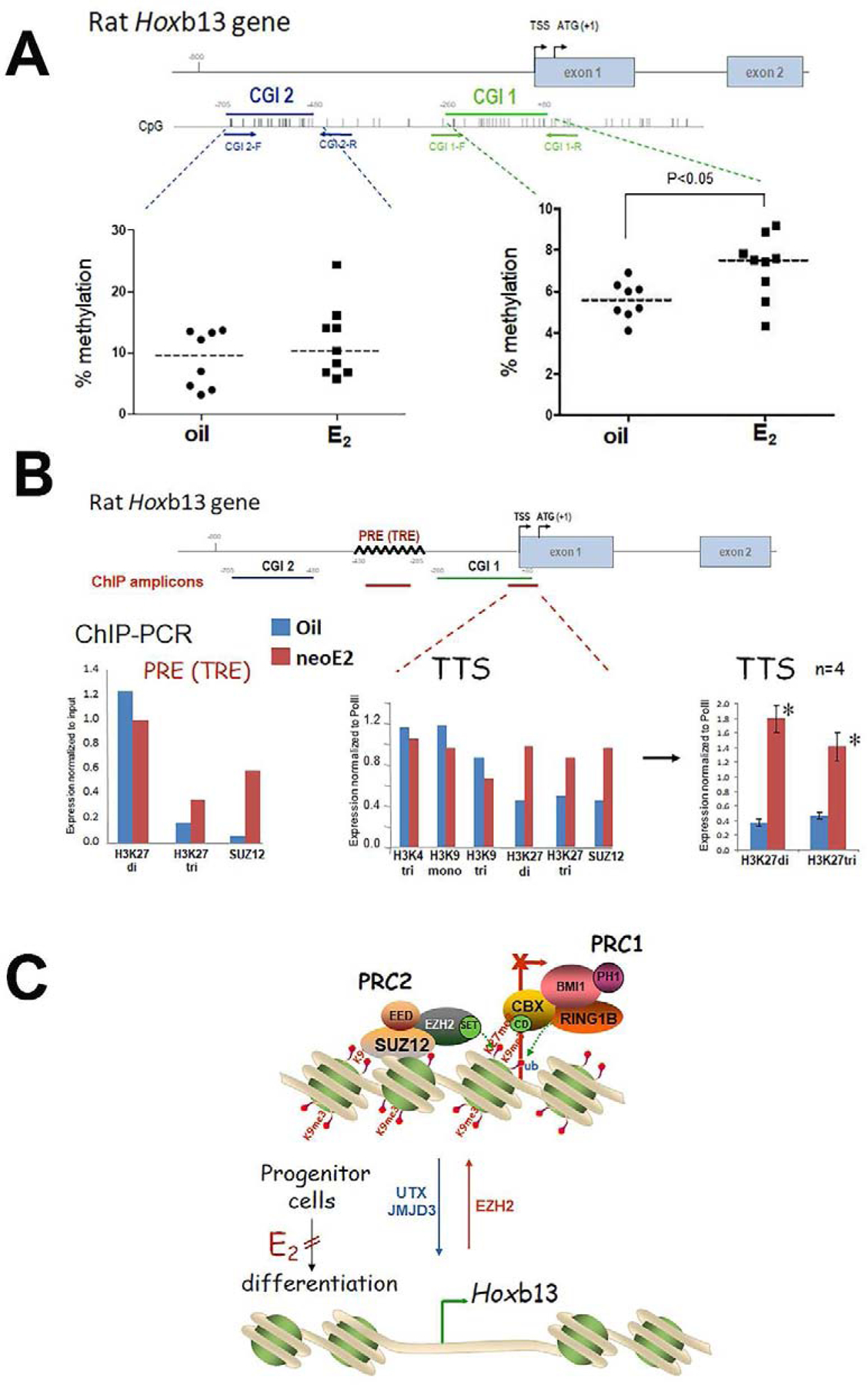
Hoxb13 epigenomic reprogramming in the adult rat ventral prostate by neonatal estradiol (neoE2) exposure. A: Hoxb13 promoter DNA methylation. A proximal CGI-1 and distal CGI-2 were identified in the first 800 bp upstream of the Hoxb13 TSS. Mass Sequenom was used to identify DNA methylation at CpG sites in these CGIs from prostates of neonatal oil and neoE2 treated rats (n=8–9 per treatment group). Average % methylation in CGI-2 was not affected by neoE2 treatment whereas an increase was noted in CG-1 from 6% to 8% (p<0.05). B: Histone methylation in the Hoxb13 promoter. A PRE/TRE was identified between CGI-1 and CGI-2 of the rat Hoxb13 promoter for PRC2 binding. ChIP-PCR was used to assess occupancy of the SUZ12, a PRC2 component, and methylated forms of H3K4, H3K9 and H3K27at the PRE and TSS (amplicons marked in red bar on gene schematic). The PRE was enriched for SUZ12 and H3K27me3 in neoE2 prostates vs oil controls. At the TTS, neoE2 had no effect on H3K4me3, H3K9me1 or H3K9me3 whereas there was an increase in H3K27me2, H3K27me3 and SUZ12 in the estrogenized prostates. The H3K27me2 and H3K27me3 occupancy at the TSS was repeated in 4 tissues per treatment group and the enrichment was highly significant (p<0.01). C: Schematic representation of histone reprogramming of Hoxb13 gene expression. In undifferentiated progenitor cells, PRC2 occupancy at the PRE and TSS permits EZH2 to lay down silencing H3K27me2 and H3K27me3 histone marks and recruit PRC1 to prevent Hoxb13 gene transcription. With development, UTX and JMJD3 demethylases remove the H3K27 silencing methyl marks to permit Hoxb13 transcription and epithelial differentiation. Exposure to neoE2 retains PRC2 occupancy and promoter H3K27 di- and tri-methylation during this critical developmental window, thus permanently blocking epithelial differentiation.
During organogenesis, establishment of DNA and histone methylation marks is a dynamic process that is vulnerable to endogenous and exogenous influences (85). The results summarized herein clearly show that early-life estrogens rewire or reprogram the developing prostate epigenome at multiple levels and in so doing, permanently alter tissue development which retains a memory of this exposure that renders the gland more responsive to subsequent exposures throughout life. It is particularly noteworthy that new research has revealed that prostate cancer reactivates developmental epigenomic programs during metastatic progression (96), affirming that altered epigenomic programs laid down during prostate development by brief estrogenic exposures can be later-life drivers of prostate cancer development and progression.
Prostate Stem-Progenitor Cells as Estrogen Targets
Stem cells are fundamental components of biological organization, responsible for the development and maintenance of tissues and organ systems (97). Embryonic stem cells (eSCs) are pluripotent cells with a robust proliferative capacity and ability to differentiate into the three germ cell layers. These subsequently give rise to unique organs, each with their own tissue-specific stem cell population, including for the prostate gland. Once tissues and organs are formed after morphogenesis, adult tissue-specific stem cells maintain homeostasis within that structure, providing cells for natural tissue turnover and regeneration in response to injury. While prostate stem cells have been identified in both rodents and humans (98,99), there are several unresolved issues including potential differences between stem cells at the developmental stage and adulthood, the lineage hierarchy of prostate stem cells to differentiated progeny, and differences in hierarchical lineage commitment in rodents versus humans. Nonetheless, Figure 4 presents a simplified schematic for the purposes of the present review. The highly plastic state of stem and daughter progenitor cells during development and tissue maintenance permits the needed flexibility for proper tissue formation and repair. Regrettably, this plasticity also provides an opportunity for aberrant cellular reprogramming due to inappropriate signals, both endogenous and exogenous, that can lead to persistent life-long effects and tissue perturbations.
Figure 4:
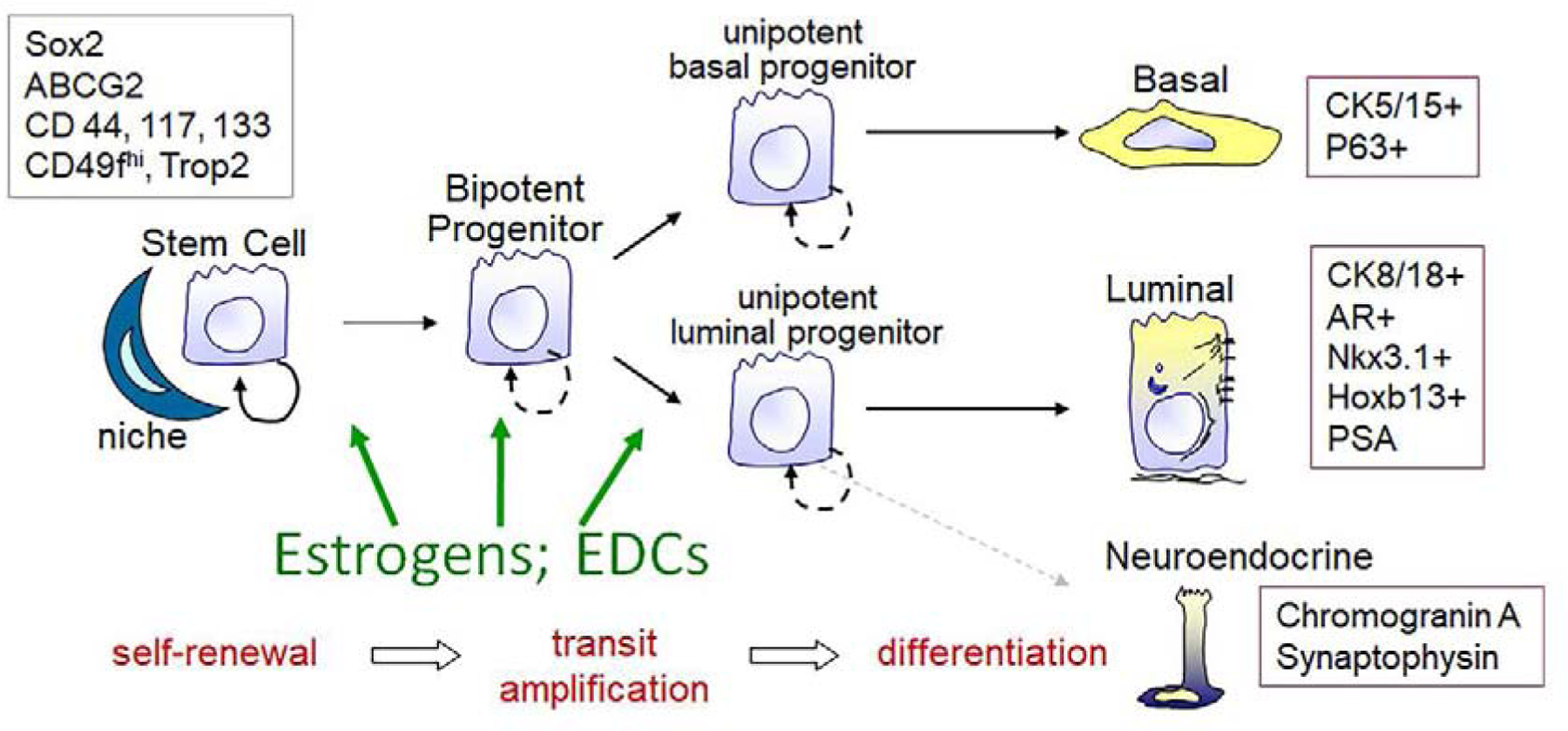
Simplified schematic of prostate lineage hierarchy in the developing prostate. A rare stem cell population resides within the stem cell niche and in response to cues, undergoes asymmetric self-renewal, giving rise to a daughter stem and a daughter bipotent progenitor cell. The progenitor cell undergoes transit amplification and lineage commitment to unipotent basal and luminal progenitor cells that give rise, upon further divisions to differentiated basal, luminal and rare neuroendocrine epithelial cells. Markers for the stem and differentiated cells are shown in the boxes. We propose that estrogens and EDCs target the stem and progenitor cell populations to affect growth and lineage commitment pathways.
While stem cells are tightly regulated by their niche microenvironment, hormonally sensitive tissues, including the prostate, have an additional layer of hormonal influences on the stem-progenitor populations. Recent research from our laboratory has identified human prostate stem and progenitor cells as direct hormone targets through the expression of multiple steroid receptors including ERs, RAR/RXRs, PPARγ and GRs (100). Notably, AR is absent in both the prostate stem and early-stage progenitors thus any androgen actions on their activity must be directed through the niche cells. Regarding ERs and estrogenic control, the human prostate stem and progenitors express ERα and ERβ at differing levels and with opposing actions (13,14,101). Specifically, ERβ levels are relatively higher in the stem compared to the daughter progenitor cells whereas ERα levels are higher in the progenitor cells. As previously mentioned, ERβ restrains stem cell self-renewal and promotes progenitor cell differentiation whereas ERα stimulates stem cell self-renewal and progenitor cell amplification. Further, estrogen actions are mediated through both mERα and mERβ that, through phosphorylation cascades, activate HMTs as well as through classical nuclear-localized signaling that drives gene transcription (16).
To directly interrogate whether early-life estrogenic exposures influence the prostate stem and progenitor cell populations, several approaches were utilized. In the BPA-Consortium Linking Academic and Regulatory Insights on Toxicity (CLARITY), Sprague Dawley rats were exposed from gestation day 6 through six months of age to ethinyl estradiol (0.5 µg/kg BW/day) or BPA (2.5, 25 or 250 µg/kg BW/day) at the FDA laboratories as described (41,102). Prostates were shipped overnight and the stem-progenitor cells were isolated and evaluated for numbers and lineage commitment through gene expression profiling. Both ethinyl estradiol and the 2.5µg BPA dose doubled the total stem cell numbers in the 6 month dorsolateral prostate. Further, ethinyl estradiol and the two higher BPA doses stimulated progenitor cell proliferation and shifted their lineage commitment to basal progenitors at the expense of decreased luminal progenitor cells. These exposures, particularly 2.5µg BPA/kg BW developmental exposure, also led to increased carcinogenic susceptibility in response to adult estradiol plus testosterone treatment in a separate animal group. As such, we propose that stem cell reprogramming by estrogens contribute to heightened carcinogenic susceptibility of the gland.
To directly assess the actions of estrogens on prostate stem and progenitor cells and to provide relevance of these findings to the human prostate gland, we utilized in vitro and in vivo systems using primary cells cultured from prostates of young, disease-free organ donors. In addition to estradiol, several EDCs that utilize ERs likewise stimulated stem cell self-renewal and progenitor proliferation including BPA (15), dioxin (101), and inorganic arsenic (103). Using these normal human stem-progenitor cells cultured in 3-D as prostaspheres, we grew humanized prostate-like structures by combining them with inductive rat embryonic UGS mesenchyme and grafting them under the renal capsule of adult male nude mice. To mimic developmental exposures, mice were fed low-doses of BPA during the first 2 weeks as the prostate-like tissues formed. At one month, the hosts were given testosterone plus estradiol pellets to drive carcinogenesis as described (13). After 3 months, the prostate-like structures were evaluated and results showed that the malignancy incidence in the human prostate epithelium significantly increased from 13% in oil-exposed controls to 33–36% in tissues exposed to BPA during the developmental window. This further increased to 45% cancerous lesions when the spheroids were precultured in BPA for 1 week prior to grafting followed by the in vivo BPA exposure. Together, these findings demonstrate that developmental BPA exposure increases the susceptibility of human prostate epithelium to estrogen-driven carcinogenesis and that the prostate stem-progenitor cells are the direct targets that propel this increased cancer risk.
One caveat of the above study is that young adult prostate stem cells were used which may differ from the developmental stage prostate stem cells. To address this, our laboratory established prostate organoids in vitro from directed differentiation of human eSC using sequential exposure to stage-specific prostate growth factors and steroids (104). Following exposure of the differentiating eSC and prostate organoids to low-dose BPA, their phenotype and stem cell population was examined by immunohistochemistry with confocal microscopy (105). Although differentiation to mature organoids at day 30 was not affected by BPA, there was an increase in focal clusters of resident stem cells in the BPA-exposed organoids that was not observed in controls. Further, organoid expression of prostate stemness genes OCT4, NANOG and CD49f was increased by BPA exposure. This concurs with adult stem cell findings and shows that low-dose BPA exposures stimulate prostate stem cell symmetric self-renewal resulting in stem cell nests that do not properly enter lineage commitment. These results support our hypothesis that the human fetal prostate gland may be reprogrammed by estrogenic chemicals which may heighten carcinogenic risk. Similar conclusions were reached by an independent laboratory using human engrafted human fetal prostate tissue (91).
While discussing eSCs and prostate stem cells, it is critical to note that the epigenetic code is dynamically established in these cells and their progeny early in life, making them particularly vulnerable to epigenetic reprogramming from inappropriate estrogenic exposures. For mouse embryonic stem cells, H3K4me3 and H3K27me3 discriminate genes that are expressed, poised for expression, or repressed and reflect the stem-progenitor cell stage (106). Noteworthy is the discovery that several stem-progenitor cell genes possess bipotent chromatin marks with both H3K4me3 and H3K27me3 at their promoters, serving to poise key developmental genes for lineage-specific activation or repression. In this context, prostate reprogramming can be viewed as a combination of altered DNA methylation and histone modifications that are heritable as stem and progenitor cells self-renew, transmitting altered epigenomic information throughout the lifespan of the individual. Direct evidence that this occurs in prostate progenitor cells has been presented in the previous section where brief exposure of human prostaspheres to estradiol or BPA altered ncRNA (SNORD) expression through changes in H3K4me3, H3K9me3 and H3K27me3. Additionally, BPA-induced reprogramming of the neonatal rat prostate resulted in multiple genes retaining bipotent H3K4me3 and H3K27me3 marks in the adult prostate, perhaps a function of a rewired progenitor cell chromatin state (93). As such, we propose that the prostate epithelial stem-progenitor cells are functional targets of estrogens and environmental chemicals, as highlighted in Figure 4, mediated through coordinate alterations in DNA and histone methylation marks known to control stem and progenitor differentiation patterns during early development. Heritable perturbations in these cells would directly lead to aberrant differentiation. More importantly, continued proliferation of permanently reprogrammed prostate progenitor cells may contribute to heightened sensitivity to hormones throughout life and conceivably increased susceptibility to hormonal carcinogenesis in aging men.
Conclusions
In conclusion, the results summarized herein from decades of research in multiple laboratories including our own has documented the process of developmental reprogramming of the prostate gland by early-life estrogenic exposures that leads to increased disease risk with aging. As development is a stage of high plasticity influenced easily by endogenous and exogenous factors, perturbations set down early in life position the gland on a life-long trajectory of structural and differentiation defects that contribute to heightened disease predisposition. Cellular and molecular underpinnings of developmental estrogenization, aka estrogen imprinting, include structural and epigenetic rearrangements mediated through dysregulated expression of steroid receptors, morphoregulatory genes and the epigenetic machinery that reprogram prostate cells, including the long-lived stem cell population, for life. The prostatic perturbations have dose-dependent trajectories that are typically non-linear, with responses varying between the types of estrogenic exposure. Understanding the developmental factors that contribute to BPH and prostate carcinoma with aging will be fundamental towards uncovering appropriate intervention strategies in future studies.
Acknowledgements
The author acknowledges the work of Lynn Birch, Drs. Jessica Belmonte, Esther Calderon, William Chang, Dan Ping Hu, Wen Yang Hu, Liwei Huang, Doug Luccio-Camelo, Ikena Madueke, Shyama Majumdar, Yong Bing Pu, Oliver Putz, Carl Woodham, Lishi Xie and Shu Hua Ye from the Prins Laboratory who conducted the research summarized in this review. Additional contributors include Andre Balla, MD, PhD (UIC), Chuck Bieberich, PhD (University of Maryland), Shuk-mei Ho, PhD (University of Arkansas), Susan Kasper, PhD (University of Cincinnati), Dominic Smiraglia, PhD (Roswell Park Cancer Institute) and Richard van Breeman, PhD (Oregon State University). This work has been supported by NIH grants DK40890, ES018758, ES015584, ES022071, DK117633 and ES027792 and the Michael Reese Research and Education Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endo Rev. 1987;8(3):338–363. [DOI] [PubMed] [Google Scholar]
- 2.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation; research in biological diversity. 2008;76(6):641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. JCEM. 1974;38(1):113–125. [DOI] [PubMed] [Google Scholar]
- 4.Liao S. Receptors and the mechanism of action of androgens. In: Pasqualini JR, ed. Receptors and Mechanism of Action of Steroid Hormones. Vol 8. New York: Marcell Dekker, Inc.; 1976:Chapter 5: 159–214. [Google Scholar]
- 5.Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology. 2009;150(1):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitkus S, Yeh CR, Lin HH, Hsu I, Yu J, Chen M, Yeh S. Distinct function of estrogen receptor alpha in smooth muscle and fibroblast cells in prostate development. Molecular endocrinology (Baltimore, Md). 2013;27(1):38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139(3):874–883. [DOI] [PubMed] [Google Scholar]
- 8.Omoto Y, Imamov O, Warner M, Gustafsson JA. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilley WD, Horsfall DJ, Skinner JM, Henderson DW, Marshall VR. Effect of pubertal development on estrogen receptor levels and stromal morphology in the guinea pig prostate. The Prostate. 1989;15:195–210. [DOI] [PubMed] [Google Scholar]
- 10.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138(5):1801–1809. [DOI] [PubMed] [Google Scholar]
- 11.Adams JY, Leav I, Lau KM, Ho SM, Pflueger SM. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. The Prostate. 2002;52(1):69–81. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro E, Huang H, Masch RJ, McFadden DE, Wilson EL, Wu XR. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J Urology. 2005;174(5):2051–2053. [DOI] [PubMed] [Google Scholar]
- 13.Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology. 2011;152(6):2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prins GS, Calderon-Gierszal EL, Hu WY. Stem Cells as Hormone Targets That Lead to Increased Cancer Susceptibility. Endocrinology. 2015;156(10):3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155(3):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar S, Rinaldi JC, Malhotra NR, Xie L, Hu DP, Gauntner TD, Grewal HS, Hu WY, Kim SH, Katzenellenbogen JA, Kasper S, Prins GS. Differential Actions of Estrogen Receptor α and β via Nongenomic Signaling in Human Prostate Stem and Progenitor Cells. Endocrinology. 2019;160(11):2692–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson JA. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9375–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zondek T, Zondek LH. The fetal and neonatal prostate. In: Goland M, ed. Normal and Abnormal Growth of the Prostate. Springfield, IL: Thomas C. Thomas; 1975:5–28. [Google Scholar]
- 19.Santti R, Pylkkanen L, Newbhold R, McLachlan JA. Developmental oestrogenization and prostatic neoplasia. International journal of andrology. 1990;13:77–80. [DOI] [PubMed] [Google Scholar]
- 20.Ekbom A, Hsieh CC, Lipworth L, Wolk A, Ponten J, Adami HO, Trichopoulos D. Perinatal characteristics in relation to incidence of and mortality from prostate cancer. British Med J. 1996;313:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekbom A Growing evidence that several human cancers may originate in utero. Cancer Biology. 1998;8:237–244. [DOI] [PubMed] [Google Scholar]
- 22.Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. British journal of cancer. 1988;57(2):216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill WB, Schumacher GFB, Hubby MM, Blough RR. Male genital tract changes in humans following intauterine exposure to diethylstilbestrol. In: Herbst AL, Bern HA, eds. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. New York: Thieme-Stratton, Inc.; 1981:103–119. [Google Scholar]
- 24.Sugimura Y, Cunha GR, Yonemura CU, Kawamura J. Temporal and spatial factors in diethylstilbestrol-induced squamous metaplasia of the developing human prostate. Human Path. 1988;19(2):133–139. [DOI] [PubMed] [Google Scholar]
- 25.Driscoll SG, Taylor SH. Effects of prenatal maternal estrogen on the male urogenital system. Obstet Gynecol. 1980;56(5):537–542. [PubMed] [Google Scholar]
- 26.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biology of reproduction. 1986;34(5):961–971. [DOI] [PubMed] [Google Scholar]
- 27.Rajfer J, Coffey DS. Effects of neonatal steroids on male sex tissues. Invest Urol. 1979;17(1):3–8. [PubMed] [Google Scholar]
- 28.Chung LWK, MacFadden DK. Sex steroid imprinting and prostatic growth. Invest Urol. 1980;17(4):337–342. [PubMed] [Google Scholar]
- 29.Arai Y, Mori T, Suzuki Y, Bern HA. Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. In: Bourne GHaD JF, ed. International Review of Cytology. Vol 84. New York, New York: Academic Press, Inc.; 1983:235–268. [DOI] [PubMed] [Google Scholar]
- 30.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130(6):3703–3714. [DOI] [PubMed] [Google Scholar]
- 31.Pylkkanen L, Makela S, Valve E, Harkonen P, Toikkanen S, Santti R. Prostatic dysplasia associated with increased expression of C-MYC in neonatally estrogenized mice. The Journal of urology. 1993;149:1593–1601. [DOI] [PubMed] [Google Scholar]
- 32.Gilleran JP, Putz O, DeJong M, DeJong S, Birch L, Pu Y, Huang L, Prins GS. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144(5):2046–2054. [DOI] [PubMed] [Google Scholar]
- 33.Pylkkanen L, Santti R, Newbold R, McLachlan J. Regional differences in the prostate of the neonatally estrogenized mouse. The Prostate. 1991;18:117–129. [DOI] [PubMed] [Google Scholar]
- 34.Nagel SC, vom Saal FS, Thayer KA, Dhar MG, Boechler M, Weshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative In Vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Envir Hlth Prospect. 1997;105(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhandari RK, Taylor JA, Sommerfeld-Sager J, Tillitt DE, Ricke WA, Vom Saal FS. Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol. Environmental epigenetics. 2019;5(3):dvz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J, Sugimura Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biology of reproduction. 2011;84(4):734–742. [DOI] [PubMed] [Google Scholar]
- 37.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer research. 2006;66(11):5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reproductive toxicology (Elmsford, NY). 2011;31(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reproductive toxicology (Elmsford, NY). 2016;59:167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prins GS, Ye SH, Birch L, Zhang X, Cheong A, Lin H, Calderon-Gierszal E, Groen J, Hu WY, Ho SM, van Breemen RB. Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis. Environmental health perspectives. 2017;125(7):077007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prins GS, Hu WY, Xie L, Shi GB, Hu DP, Birch L, Bosland MC. Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study. Environmental health perspectives. 2018;126(11):117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong RL, Wang Q, Trevino LS, Bosland MC, Chen J, Medvedovic M, Prins GS, Kannan K, Ho SM, Walker CL. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofkamp L, Bradley S, J. T, Lichtensteiger W, Schumpf M, Timms B. Region-specific growth effects in the developing rat prostate following developmental exposure to estrogenic UV filters. Envir Hlth Prospect. 2008;116:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vezina CM, Lin TM, Peterson RE. AHR signaling in prostate growth, morphogenesis, and disease. Biochemical pharmacology. 2009;77(4):566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Developmental biology. 2003;253:165–174. [DOI] [PubMed] [Google Scholar]
- 46.Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136(3):1303–1314. [DOI] [PubMed] [Google Scholar]
- 47.Aboseif SR, Dahiya R, Narayan P, Cunha GR. Effect of retinoic acid on prostatic development. The Prostate. 1997;31:161–167. [DOI] [PubMed] [Google Scholar]
- 48.Prins GS, Chang WY, Wang Y, van Breemen RB. Retinoic acid receptors and retinoids are up-regulated in the developing and adult rat prostate by neonatal estrogen exposure. Endocrinology. 2002;143(9):3628–3640. [DOI] [PubMed] [Google Scholar]
- 49.Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, Peterson RE. Retinoic acid induces prostatic bud formation. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237(5):1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland in mediated through stromal estrogen receptor a: studies with aERKO and bERKO mice. Canc Res. 2001;61(16):6089–6097. [PubMed] [Google Scholar]
- 51.Woodham C, Birch L, Prins GS. Neonatal estrogen down-regulates prostatic androgen receptor through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144(11):4841–4850. [DOI] [PubMed] [Google Scholar]
- 52.Prins GS, Woodham C, Lepinske M, Birch L. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology. 1993;132(6):2387–2398. [DOI] [PubMed] [Google Scholar]
- 53.Sabharwal V, Putz O, Prins GS. Neonatal estrogen exposure induces progesterone receptor expression in the developing prostate gland. Paper presented at: 95th Annual Meeting of the American Urologic Association; April 29 - May 4, 2000, 2000; Atlanta, GA. [Google Scholar]
- 54.Pu Y, Deng L, Davies PJP, Prins GS. Retinoic acid metabolizing enzymes, binding proteins and RXRs are differentially expressed in the developing and adult rat prostate lobes and are altered by neonatal estrogens in a lobe-specific manner. Paper presented at: The Endocrine Society’s 85th Annual Meeting; June 19–23, 2003, 2003; Philadelphia, PA. [Google Scholar]
- 55.Turner T, Edery M, Mills KT, Bern HA. Influence of neonatal diethylstilbestrol treatment on androgen and estrogen receptor levels in the mouse anterior prostate, ventral prostate and seminal vesicle. J Steroid Beiochem. 1989;32(4):559–564. [DOI] [PubMed] [Google Scholar]
- 56.Un-no T, Hayami S, Nobata S, Sudoko H, Honma S, Fujita K, Ozono S. Neonatal exposure to estrogen in the Wistar rat decreases estrogen receptor-beta and induces epithelial proliferation of the prostate in the adult. Urologia internationalis. 2007;79(4):345–351. [DOI] [PubMed] [Google Scholar]
- 57.Prins GS, Ho SM. Early life estrogens and prostate cancer in an animal model. Journal of Developmental Origins of Health and Disease. 2010;1(6):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prins GS, Lindgren M. Accessory Sex Glands in the Male. Vol 1. Fourth Edition ed: Elsevier, Inc; Academic Press; 2015. [Google Scholar]
- 59.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B(AbdB) hoxa genes: Regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES). Developmental biology. 1998;197:141–154. [DOI] [PubMed] [Google Scholar]
- 60.Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-a knockout mice exhibit resistance to the developmental effects of diethylstilbestrol exposure on the female reproductive tract. Developmental biology. 2001;238(2):224–238. [DOI] [PubMed] [Google Scholar]
- 61.Chang WY, Birch L, Woodham C, Gold LI, Prins GS. Neonatal estrogen exposure alters the transforming growth factor-b signaling system in the developing rat prostate and blocks the transient p21cip1/wafl expression associated with epithelial differentiation. Endocrinology. 1999;140(6):2801–2813. [DOI] [PubMed] [Google Scholar]
- 62.Pu Y, Huang L, Prins GS. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Developmental biology. 2004;273(2):257–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L, Pu Y, Alam S, Birch L, Prins GS. The role of Fgf10 signaling in branching morphogenesis and gene expression in the rat prostate gland: lobe-specific supression by neonatal estrogens. Developmental biology. 2005;278(2):396–414. [DOI] [PubMed] [Google Scholar]
- 64.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Annals of the New York Academy of Sciences. 2006;1089:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo D, Yamaguchi T, Prins GS. The role of Wnt5a in prostate gland development. Developmental biology. 2009;328(2):188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang L, Pu Y, Alam S, Birch L, Prins GS. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. Journal of andrology. 2004;25(3):330–337. [DOI] [PubMed] [Google Scholar]
- 67.Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, Bieberich C. Influence of neonatal estrogens on rat prostate development. Reproduction, fertility, and development. 2001;13(4):241–252. [DOI] [PubMed] [Google Scholar]
- 68.Huang L, Pu Y, Hepps D, Danielpour D, Prins GS. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology. 2007;148(3):1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28(50):4480–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma F, Ye H, He HH, Gerrin SJ, Chen S, Tanenbaum BA, Cai C, Sowalsky AG, He L, Wang H, Balk SP, Yuan X. SOX9 drives WNT pathway activation in prostate cancer. The Journal of clinical investigation. 2016;126(5):1745–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu B, Kyprianou N. Transforming growth factor beta and prostate cancer. Cancer treatment and research. 2005;126:157–173. [DOI] [PubMed] [Google Scholar]
- 72.Clayton NS, Grose RP. Emerging Roles of Fibroblast Growth Factor 10 in Cancer. Frontiers in genetics. 2018;9:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kruithof-de Julio M, Shibata M, Desai N, Reynon M, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. Canonical Wnt signaling regulates Nkx3.1 expression and luminal epithelial differentiation during prostate organogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242(10):1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, Wiley KE, Isaacs SD, Johng D, Wang Y, Bizon C, Yan G, Gielzak M, Partin AW, Shanmugam V, Izatt T, Sinari S, Craig DW, Zheng SL, Walsh PC, Montie JE, Xu J, Carpten JD, Isaacs WB, Cooney KA. Germline mutations in HOXB13 and prostate-cancer risk. The New England journal of medicine. 2012;366(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development (Cambridge, England). 2003;130(2):2061–2069. [DOI] [PubMed] [Google Scholar]
- 76.Chang W, Bieberich C, Bhatia-Gaur R, Tebeau C, Prins GS. Developmental estrogenization of rat prostate is associated with reductions in Hoxb-13 and Nkx3.1 homeobox gene expression. Paper presented at: The Endocrine Society Annual Meeting; June 21–24, 2000, 2000; Toronto, Canada. [Google Scholar]
- 77.Lee C, Sensibar JA, Dudek SM, Hiipakka RA, Liao S. Prostatic ductal system in rats: Regional variation in morphological and functional activities. Biology of reproduction. 1990;43:1079–1086. [DOI] [PubMed] [Google Scholar]
- 78.Prins GS, Cooke PS, Birch L, Donjacour AA, Yalcinkaya TM, Siiteri PK, Cunha GR. Androgen receptor expression and 5a-reductase activity along the proximal-distal axis of the rat prostatic duct. Endocrinology. 1992;130(5):3066–3073. [DOI] [PubMed] [Google Scholar]
- 79.Chang WY, Wilson MJ, Birch L, Prins GS. Neonatal estrogen stimulates proliferation of periductal fibroblasts and alters the extracellular matrix composition in the rat prostate. Endocrinology. 1999;140(1):405–415. [DOI] [PubMed] [Google Scholar]
- 80.Habermann H, Chang WY, Birch L, Mehta P, Prins GS. Developmental exposure to estrogens alters epithelial cell adhesion and gap junction proteins in the adult rat prostate. Endocrinology. 2001;142(1):359–369. [DOI] [PubMed] [Google Scholar]
- 81.vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94(5):2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitamin Horm. 2014;94:129–165. [DOI] [PubMed] [Google Scholar]
- 83.Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactorfferrin gene in mouse uterus. Canc Res. 1997;57(19):4356–4359. [PubMed] [Google Scholar]
- 84.McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocrine reviews. 2001;22(3):319–341. [DOI] [PubMed] [Google Scholar]
- 85.Walker CL. Minireview: Epigenomic Plasticity and Vulnerability to EDC Exposures. Molecular endocrinology (Baltimore, Md). 2016;30(8):848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153(1):42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powers GL, Hammer KD, Domenech M, Frantskevich K, Malinowski RL, Bushman W, Beebe DJ, Marker PC. Phosphodiesterase 4D inhibitors limit prostate cancer growth potential. Molecular cancer research : MCR. 2015;13(1):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang N, Zhou LQ, Zhang XY. Downregulation of the nucleosome-binding protein 1 (NSBP1) gene can inhibit the in vitro and in vivo proliferation of prostate cancer cells. Asian journal of andrology. 2010;12(5):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheong A, Zhang X, Cheung YY, Tang WY, Chen J, Ye SH, Medvedovic M, Leung YK, Prins GS, Ho SM. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016;11(9):674–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Augusto TM, Rosa-Ribeiro R, Carvalho HF. Neonatal exposure to high doses of 17β-estradiol results in inhibition of heparanase-1 expression in the adult prostate. Histochemistry and cell biology. 2011;136(5):609–615. [DOI] [PubMed] [Google Scholar]
- 91.Saffarini CM, McDonnell-Clark EV, Amin A, Huse SM, Boekelheide K. Developmental exposure to estrogen alters differentiation and epigenetic programming in a human fetal prostate xenograft model. PloS one. 2015;10(3):e0122290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kradolfer D, Flöter VL, Bick JT, Fürst RW, Rode K, Brehm R, Henning H, Waberski D, Bauersachs S, Ulbrich SE. Epigenetic effects of prenatal estradiol-17β exposure on the reproductive system of pigs. Molecular and cellular endocrinology. 2016;430:125–137. [DOI] [PubMed] [Google Scholar]
- 93.Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O’Malley BW, Shilatifard A, Walker CL. Reprogramming of the Epigenome by MLL1 Links Early-Life Environmental Exposures to Prostate Cancer Risk. Molecular endocrinology (Baltimore, Md). 2016;30(8):856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho SM, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Molecular cancer research : MCR. 2012;10(4):546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, Chen J, Zhang X, Medvedovic M, Leung YK, Prins GS. Exposure of Human Prostaspheres to Bisphenol A Epigenetically Regulates SNORD Family Noncoding RNAs via Histone Modification. Endocrinology. 2015;156(11):3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pomerantz MM, Qiu X, Zhu Y, Takeda DY, Pan W, Baca SC, Gusev A, Korthauer KD, Severson TM, Ha G, Viswanathan SR, Seo JH, Nguyen HM, Zhang B, Pasaniuc B, Giambartolomei C, Alaiwi SA, Bell CA, O’Connor EP, Chabot MS, Stillman DR, Lis R, Font-Tello A, Li L, Cejas P, Bergman AM, Sanders J, van der Poel HG, Gayther SA, Lawrenson K, Fonseca MAS, Reddy J, Corona RI, Martovetsky G, Egan B, Choueiri T, Ellis L, Garraway IP, Lee GM, Corey E, Long HW, Zwart W, Freedman ML. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nature genetics. 2020;52(8):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. [DOI] [PubMed] [Google Scholar]
- 98.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single cell. Nature. 2008;456(7223):804–808. [DOI] [PubMed] [Google Scholar]
- 99.Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, Sandher R, Pickard RS, Robson CN, Turnbull DM, Taylor RW, Heer R. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. The Journal of pathology. 2011;225(2):181–188. [DOI] [PubMed] [Google Scholar]
- 100.Prins GS, Hu WY. Prostate Stem Cells, Hormones and Development. In: Cramer SD, ed. Stem Cells and Prostate Cancer. Vol VII: Springer LLC; 2013:1–20. [Google Scholar]
- 101.Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Molecular and cellular endocrinology. 2012;354(1–2):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heindel JJ, Belcher S, Flaws JA, Prins GS, Ho SM, Mao J, Patisaul HB, Ricke W, Rosenfeld CS, Soto AM, Vom Saal FS, Zoeller RT. Data integration, analysis, and interpretation of eight academic CLARITY-BPA studies. Reproductive toxicology (Elmsford, NY). 2020. [DOI] [PMC free article] [PubMed]
- 103.Xie L, Hu WY, Hu DP, Shi G, Li Y, Yang J, Prins GS. Effects of Inorganic Arsenic on Human Prostate Stem-Progenitor Cell Transformation, Autophagic Flux Blockade, and NRF2 Pathway Activation. Environmental health perspectives. 2020;128(6):67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calderon-Gierszal E, Prins GS. Directed Differentiation of Prostate Organoids from Embryonic Stem Cells. In: Dacies J, ed. Organoids and Mini-Organs: Elsevier; 2018:89–116. [Google Scholar]
- 105.Calderon-Gierszal EL, Prins GS. Directed Differentiation of Human Embryonic Stem Cells into Prostate Organoids In Vitro and its Perturbation by Low-Dose Bisphenol A Exposure. PloS one. 2015;10(7):e0133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]


