Abstract
Background:
The influence of sex hormone and insulin/insulin-like growth factor (IGF) axis signaling on endometrial cancer recurrence is unknown. We evaluated these pathways in a prospective cohort of Gynecologic Oncology Group (GOG)0210 trial endometrial adenocarcinoma patients.
Methods:
Stage II-IV patients (N=816) were included in this study. Pre-treatment specimens were tested for tumor mRNA and protein expression of IGF1, IGF2, IGF binding proteins (IGFBP)-1 and −3, insulin (IR) and IGF-I receptors (IGF1R), phosphorylated IR/IGF1R (pIGF1R/pIR), and estrogen (ER) and progesterone receptors (PR) using qPCR and immunohistochemistry. Serum concentrations of insulin, IGF-I, IGFBP-3, estradiol, estrone and sex hormone binding globulin were measured. Hazard ratios (HR) and 95% confidence intervals (CI) for progression-free survival were calculated from Cox models adjusting for age, stage and grade.
Results:
Recurrence occurred in 280 (34%) cases during a median of 4.6 years of follow-up. ER-positivity (HR 0.67, 95% CI 0.47–0.95), IR-positivity (HR 0.53, 95% CI 0.29–0.98) and circulating IGF-I (highest versus lowest quartile, HR 0.66, 95% CI 0.47–0.92) were inversely associated with recurrence risk. Circulating estradiol (highest versus lowest tertile, HR 1.55, 95% CI 1.02–2.36) and pIGF1R/pIR positivity (HR 1.40, 95% CI 1.02–1.92) were associated with increased recurrence risk.
Conclusions:
Circulating estradiol and tissue phosphorylated (activated) IGR1R/IR were independently associated with higher risk of recurrence in endometrial cancer patients.
Impact:
This study may inform future clinical trials of endocrine-targeted adjuvant therapies in endometrial cancer patients that could include baseline assessment of serum and tissue biomarkers of estradiol and insulin signaling pathways.
Keywords: endometrial cancer, recurrence, prognosis, insulin, insulin-like growth factor, estrogen
INTRODUCTION
Endometrial cancer is the most common gynecologic cancer worldwide with 382,069 new cases reported in 2018.(1) The most common type, endometrioid adenocarcinoma, accounts for 75% of cases.(2) In the U.S., most women diagnosed with endometrioid adenocarcinoma have a favorable prognosis mainly because a high proportion of cases (~67%) present with localized disease. However, for women who are diagnosed with advanced (stage II or higher) disease, a greater proportion will experience disease recurrence that often results in death.(3) Established clinical predictors of recurrent endometrioid adenocarcinoma include tumor grade, FIGO stage(4), invasion of the myometrium, as well as older patient age and postmenopausal status. Molecular testing offers an important opportunity to identify biomarkers that could be used to understand the biological risk factors for recurrence and identify biomarkers for recurrence risk in high stage patients.
Established risk factors for developing endometrioid adenocarcinoma are obesity (5,6) and exposure to high estrogen levels (that are not simultaneously opposed by progesterone).(7,8) In postmenopausal women, obesity is associated with increased circulating estradiol levels with adipose tissue as the primary site of estrogen production from androgen precursors.(9) Studies focusing on circulating levels of insulin, insulin-like growth factor (IGF)-I and sex hormones have shown that these pathways are dysregulated in obesity as well as in endometrioid adenocarcinoma development(7) and higher levels of insulin and estradiol are associated with endometrial cancer risk.(10) Local tissue levels of IGF-I play an important role in IGF-I receptor (IGF1R) activation, as do IGF binding proteins (IGFBPs) via their regulation of ligand bioavailability. While insulin predominantly signals through the insulin receptor (IR), IGF-I binds to the IGF1R as well as hybrid IGF-I/insulin receptors.(11) A previous study reported upregulation of pIGF1R/pIR in complex atypical endometrial hyperplasia, a putative precursor lesion for endometrioid adenocarcinoma, as well as in endometrial adenocarcinoma (type I) as compared with normal endometrium.(12) Notably, we recently observed that amongst postmenopausal women without cancer, a higher proportion of those with diabetes (86%) versus non-diabetics (28%) had positive pIGF1R/pIR endometrial immunohistochemical (IHC) staining, which could partly explain the link between obesity, diabetes and endometrioid adenocarcinoma risk.(13)
Based on the hypothesis that the molecular risk factors for endometrial cancer development may also increase the likelihood of its progression or recurrence, the current study assessed whether key components in the sex hormone and insulin/IGF axes were associated with risk of recurrent endometrioid adenocarcinoma among participants with stage II-IV disease enrolled in the Gynecologic Oncology Group (GOG) GOG0210 endometrial cancer staging trial.
MATERIALS AND METHODS
Study population
Details of the NRG Oncology/GOG0210 trial NCT00340808 were previously reported.(14,15) Briefly, the study was conducted from September 22, 2003 to December 1, 2011 across 62 US institutions. In 2007 (second enrollment phase), the eligibility criteria was restricted to enrich underrepresented patients or those at high risk of recurrence.(15) Patients were enrolled in the trial between the time of their initial diagnosis (biopsy) and their date of surgery. Prior to surgery, patients completed a self-administered questionnaire. Follow-up information on the development of recurrent endometrioid adenocarcinoma was available through November 1, 2013.
The current study used a selected subpopulation of the GOG0210; specifically women who provided written informed consent for their biological specimens and clinical data to be collected as part of the GOG0210 protocol were eligible. Women had surgical staging including a hysterectomy and bilateral oophorectomy. For the current study, women needed sufficient high quality frozen primary tumor, formalin-fixed and paraffin-embedded (FFPE) primary tumor and/or preoperative serum available, leaving 1129 subjects in the study. Of these subjects, we excluded: primary not endometrial cancer (N=69); did not meet histology criteria (not adenocarcinoma or carcinosarcoma) (N=56); never received treatment (N=54); inadequate material for pathology review (N=45); did not meet eligibility criteria during restricted enrollment period (N=17); withdrew consent or it was subsequently determined that the patient did not meet the eligibility criteria (N=7); benign diagnosis (N=6); improper pre-protocol treatment (N=2); diagnosis of a second primary tumor surgery (N=2); clerical error (N=1); improper surgery (N=1); non-endometrioid tumor histology (N=28); stage I disease (N=25). This left 816 cases with stage II-IV endometrioid adenocarcinoma in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by institutional review boards at the Albert Einstein College of Medicine and Montefiore Medical Center and all participating study centers.
Outcome assessment
The primary endpoint was endometrioid adenocarcinoma recurrence/progression. Recurrence was defined as the discovery of disease that was not previously identified by clinical, radiographic and/or laboratory means. In the case of patients with stage IV disease, the endpoint was an ≥50% increase in the product of any documented lesions. Finally, for a subset of stage IV patients who had no documented measurable disease following primary surgery, recurrence was defined as the development of disease after an interval of at least three months of being disease free.
Blood and tissue collection
Preoperative blood samples were obtained from the participants and the serum was stored at −70°C within four hours. A portion of the blood samples (19.4%) were collected from women who were fasting. Endometrial tumor tissues were collected during hysterectomy surgery and immediately flash frozen and/or fixed in 10% buffered formalin and embedded in paraffin. All tissues were frozen or formalin-fixed within 30–60 minutes of operative removal when possible.
Laboratory assays
Laboratory analyses were performed by investigators blinded to clinical data. IHC staining was carried out for IGF1R, IR, pIGF1R/pIR, ER alpha and PR on FFPE tissues in tissue microarrays (TMAs). Three TMAs were created by the GOG Tissue Bank; TMA slides contained one core of primary endometrioid adenocarcinoma FFPE tissue from 524 independent patients and approximately 30 controls. Methods used to perform IHC staining are detailed in the Supplementary Methods. Positive control and negative control tissue slides were stained in parallel for all IHC staining assays. TMA slides were scored by the study pathologists. For the ER and PR, nuclear staining was scored as the staining intensity and the percentage of cells that stained positive. For the IR, the percentage of nuclear cells that stained positive (<50%, ≥50%) as well as the cytoplasmic and overall staining intensity were scored. For the IGF1R and pIGF1R/pIR, we recorded the percentage of cells with positive membranous staining (<50%, ≥50%) and the cytoplasmic and overall staining intensities. No nuclear staining was observed for IGF1R and pIGF1R/pIR. For the IHC data, the staining intensity in different areas of the section was assessed using standardized ranges and allocated a value of 0 (none), 1 (weak), 2 (moderate) or 3 (strong). For ER and PR, the percentage of cells that stained positive in five 40x fields was estimated, and the percentage of positive cells was multiplied by the intensity value to calculate the histological score (H-score) with a maximum value of 300. A clinical cut-off (H-score ≥75) was used to differentiate between positive and negative ER and PR staining.(16)
Gene expression (mRNA levels) of IGF1, IGF2, IGFBP1 and IGFBP3 were measured with quantitative real time reverse transcription PCR (qPCR) (Supplementary Methods). All of the RNA samples used in qPCR passed rigorous quality control assessment and assays were repeated in triplicate. Preoperative concentrations of serum insulin, total IGF-I, IGFBP-3 were determined in the laboratory of Dr. Herbert Yu. Insulin was measured using an immunoassay from Diagnostic Systems Laboratories (DSL, Webster, Texas, USA; assay sensitivity 0.01ng/mL). Concentrations of total IGF-I and IGFBP-3 were measured by Quantikine ELISAs (R&D Systems, Minneapolis, MN, USA; assay sensitivities for IGF-I, 0.05ng/mL, IGFBP-3, 0.14ng/mL). Serum concentrations of estrone and SHBG were measured in the GCRC Analytic Core Laboratory at the Albert Einstein College of Medicine using ELISAs from ALPCO (Salem, NH, USA; assay sensitivities for estrone, 3pg/mL; SHBG, 0.1nmol/L). Levels of estradiol were measured in the Einstein Department of Pathology laboratory using an electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN, USA; assay sensitivity 5pg/mL). For quality control, 10 blind duplicates (5-pairs) were analyzed for every 100 participant samples. The mean intra-assay coefficients of variation from the duplicate samples were 4.5% for insulin, 7.8% for total IGF-1, 9.7% for IGFBP-3, 8.7% for SHBG, 14.5% for estrone and 16.1% for estradiol.
Statistical analysis
Progression-free survival time was defined as the date from enrollment to the date of evidence of disease recurrence or progression, death or date of last contact, whichever occurred first. If participants died due to other causes (not endometrial cancer), they were censored at their time of death. Multivariable Cox proportional hazards regression models were used to derive hazard ratios (HR) and 95% confidence intervals (CIs) for the association between each marker of interest adjusting for covariates that influence risk of recurrent endometrioid adenocarcinoma: age at enrollment, FIGO stage and grade. Cox proportional hazards assumptions were examined using proportionality tests by adding time varying covariates and no violations were observed. We assessed whether additional adjustment for treatment (adjuvant therapy) or race changed the risk estimates by ≥10%(17), but the estimates were very similar; therefore, these factors were not included in the final models. Data on neoadjuvant chemotherapy were not available; however, neoadjuvant chemotherapy is rarely utilized in the management of endometrioid endometrial cancer. We carried out further sensitivity analyses with stratification by stage. The P-trend was estimated by entering continuous terms into the regression model and using a Wald test. To assess correlations, Pearson’s correlation coefficients were calculated. All statistical tests were two-sided and P-values<0.05 were considered statistically significant. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The study population included 816 women with advanced stage endometrioid adenocarcinoma (588 and 228 cases in phases one and two, respectively), of whom 280 (34%) experienced disease recurrence/progression during follow-up. The median (interquartile range) survival time was 4.6 (2.2–6.4) years. Samples from 808 cases were available for the serologic testing, 524 cases had FFPE samples and were included in the TMA analysis and 358 had a fresh frozen tissue sample that was used for the qPCR analysis. Although efforts were made to collect a complete set of biospecimens (serum, FFPE tumor and fresh frozen tumor) from each patient, this was not always possible (particularly for tumor tissues). This was primarily due to limitations in tissue availability following clinical processing etc. We included all cases for whom we had data in each analysis by biospecimen type, thus we had smaller sample sizes for analyses of tumor tissues. Cox analysis of clinical and demographic characteristics showed that the following factors were associated with an increased risk of recurrence: older age (≥70 versus <55 years, HR 2.82, 95% CI 2.00–3.98); higher grade (grade 3 versus 1, HR 3.31, 95% CI 2.38–4.60), and higher stage (FIGO stage IV versus II, HR 5.47, 95% CI 3.79–7.90) (Table 1). Compared with cases who had invasion of the inner half of the myometrium, those who had invasion of the outer half (HR 1.88, 95% CI 1.41–2.50) or invasion that broke through to the serosa (HR 4.30, 95% CI 2.99–6.19) had a higher risk of recurrence. Factors that were not associated with recurrence risk were race, BMI, diabetes, menopausal status and menopausal hormone therapy use.
Table 1:
Association between clinicopathologic factors and progression-free survival among 816 women with advanced stage endometrioid adenocarcinoma
| Total N | Recurrent N | Recurrent % | HRa | 95% CIa | 95% CIa | |
|---|---|---|---|---|---|---|
| Age at enrollment (years) | ||||||
| <55 | 203 | 47 | 23.2 | 1.00 (Ref) | ||
| 55–59 | 160 | 45 | 28.1 | 1.20 | 0.80 | 1.80 |
| 60–64 | 146 | 42 | 28.8 | 1.29 | 0.85 | 1.96 |
| 65–69 | 111 | 41 | 36.9 | 1.72 | 1.13 | 2.62 |
| ≥70 | 196 | 105 | 53.6 | 2.82 | 2.00 | 3.98 |
| Gradeb | ||||||
| 1 | 231 | 48 | 20.8 | 1.00 (Ref) | ||
| 2 | 310 | 91 | 29.4 | 1.54 | 1.09 | 2.18 |
| 3 | 273 | 141 | 51.7 | 3.31 | 2.38 | 4.60 |
| FIGO stage | ||||||
| II | 264 | 53 | 20.1 | 1.00 (Ref) | ||
| III | 458 | 164 | 35.8 | 2.03 | 1.49 | 2.77 |
| IV | 94 | 63 | 67.0 | 5.47 | 3.79 | 7.90 |
| Depth of myometrial invasion | ||||||
| None | 49 | 8 | 16.3 | 0.71 | 0.34 | 1.48 |
| Inner half (<50% thickness) | 318 | 74 | 23.3 | 1.00 (Ref) | ||
| Outer half (>50% thickness) | 342 | 133 | 38.9 | 1.88 | 1.41 | 2.50 |
| Break through to the serosa | 74 | 49 | 66.2 | 4.30 | 2.99 | 6.19 |
| Not assessed | 9 | 6 | 66.7 | 6.60 | 2.87 | 15.19 |
| Missing | 24 | 10 | 41.7 | 1.89 | 0.98 | 3.66 |
| Adjuvant therapyb,c | ||||||
| CT | 206 | 99 | 48.1 | 3.16 | 2.22 | 4.51 |
| RT | 196 | 45 | 23.0 | 1.00 (Ref) | ||
| CT-RT | 228 | 71 | 31.1 | 1.61 | 1.10 | 2.34 |
| Other | 14 | 2 | 14.3 | 0.69 | 0.17 | 2.83 |
| Race | ||||||
| White | 693 | 235 | 33.9 | 1.00 (Ref) | ||
| Black/African American | 86 | 35 | 40.7 | 1.36 | 0.95 | 1.94 |
| Asian | 17 | 4 | 23.5 | 0.75 | 0.28 | 2.00 |
| American Indian or Alaska Native | 4 | 1 | 25.0 | 1.03 | 0.14 | 7.40 |
| Unknown | 16 | 5 | 31.3 | 1.36 | 0.56 | 3.31 |
| BMI (kg/m2) | ||||||
| Underweight (<18.5) | 10 | 4 | 40.0 | 1.41 | 0.51 | 3.92 |
| Normal (18.5–24.99) | 159 | 51 | 32.1 | 1.00 (Ref) | ||
| Overweight (25–29.99) | 183 | 63 | 34.4 | 1.01 | 0.70 | 1.46 |
| Class I obese (30–35.99) | 175 | 58 | 33.1 | 1.09 | 0.75 | 1.58 |
| Class II obese (36–39.99) | 127 | 52 | 40.9 | 1.31 | 0.89 | 1.92 |
| Class III obese (≥40) | 162 | 52 | 32.1 | 1.06 | 0.72 | 1.56 |
| Diabetesb | ||||||
| No | 541 | 180 | 33.3 | 1.00 (Ref) | ||
| Yes | 152 | 54 | 35.5 | 0.93 | 0.68 | 1.26 |
| Not sure | 9 | 3 | 33.3 | 1.18 | 0.38 | 3.70 |
| Menopausal statusb | ||||||
| Premenopause | 23 | 6 | 26.1 | 0.62 | 0.15 | 2.53 |
| Perimenopause | 74 | 18 | 24.3 | 0.52 | 0.25 | 1.07 |
| Postmenopause | 580 | 212 | 36.6 | 1.00 (Ref) | ||
| Menopausal hormone therapy useb,d | ||||||
| No/not sure | 405 | 151 | 37.3 | 1.00 (Ref) | ||
| Yes | 168 | 57 | 33.9 | 0.85 | 0.63 | 1.15 |
Adjusted for age at enrollment (continuous) with the exception of menopausal status (the crude estimate is shown).
N=2 (0.25%) were missing grade; N=172 (21.1%) were missing adjuvant therapy; N=114 (14.0%) were missing diabetes status; N=139 (17.0%) were missing menopausal status; N=7 (1.2%) were missing information on menopausal hormone therapy.
In general treatments are correlated with disease stage: RT is used for stage II; CT or RT, or CT-RT for stage III; and CT for stage IV.
Restricted to postmenopausal women.
Abbreviations: BMI body mass index, CI confidence interval, CT chemotherapy, HR hazard ratio, RT radiotherapy, CT-RT chemotherapy and radiotherapy.
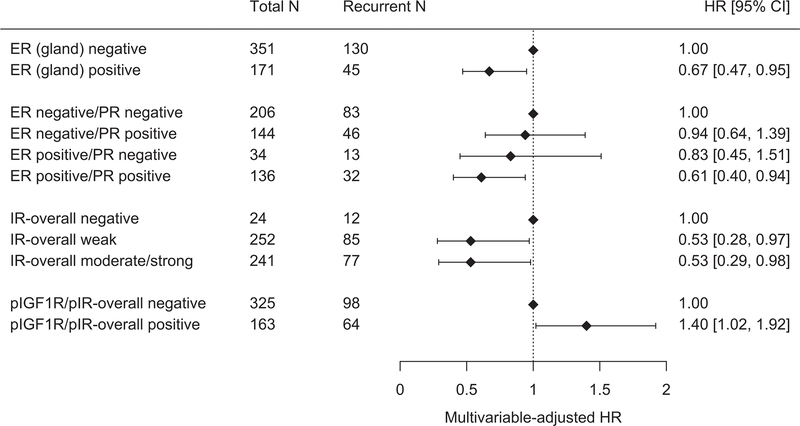
In a multivariable model that included age, stage and grade, cases with ER-positive versus ER-negative tumors had a significantly lower risk of recurrence (HR 0.67, 95% CI 0.47–0.95) (Figure 1, Figure 2A, Supplementary Table S1). Although PR-positivity appeared to be associated with a lower risk of recurrence in the age-adjusted model, this association was no longer significant after adjustment for stage and grade (HR 0.81, 95% CI 0.59–1.11). Compared with tumors that were ER- and PR-negative, a lower risk of recurrence was observed for cases that were positive for both ER and PR (HR 0.61, 95% CI 0.40–0.94). In contrast, there was no association with recurrence risk for tumors that were ER-positive/PR-negative or ER-negative/PR-positive (versus ER-negative/PR-negative).
Figure 1: Forest plots showing hazard ratios (HR) and 95% confidence intervals (95% CI) for the associations between immunohistochemical staining of selected insulin/IGF and sex hormone axis factors in endometrial cancer in relation to risk of recurrence.
Multivariable models presented are adjusted for age at enrollment (continuous), FIGO stage (II [Ref], III, IV) and grade (1, 2, 3 [Ref]).
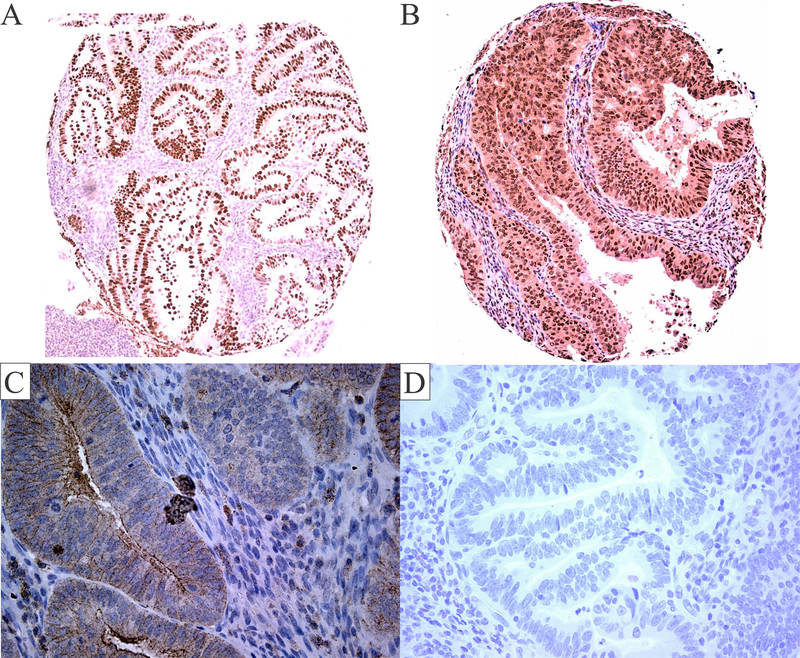
Figure 2: Insulin/IGF and sex hormone axes protein expression in endometrial cancer tissues.
ER glandular nuclear staining is shown in this representative endometrial cancer sample (A). This representative sample shows moderate cytoplasmic staining and nuclear staining for the IR (B). Weak cytoplasmic and membranous staining for the pIGF1R/pIR is shown in this representative sample (C). Negative staining is shown in this representative sample (D). Images (A-B) are 100× magnification, and images (C-D) are 400× magnification.
Testing positive for IR by IHC was significantly inversely associated with cancer recurrence although these data show no additional decrease in risk with higher staining intensity (overall staining, moderate/strong, HR 0.53, 95% CI 0.29–0.98; cytoplasmic staining, moderate/strong, HR 0.47, 95% CI 0.23–0.93; ≥50% positive nuclear staining, HR 0.62, 95% CI 0.42–0.92) (Figure 2B). In contrast, cases who tested positive for pIGF1R/pIR overall staining intensity had a higher risk of recurrence than those who tested negative (HR 1.40, 95% CI 1.02–1.92) (Figure 2C). Expression of the IGF1R protein or gene expression levels of IGF1, IGF2, IGFBP1 and IGFBP3 (Supplementary Table S2) were not associated with risk of recurrence.
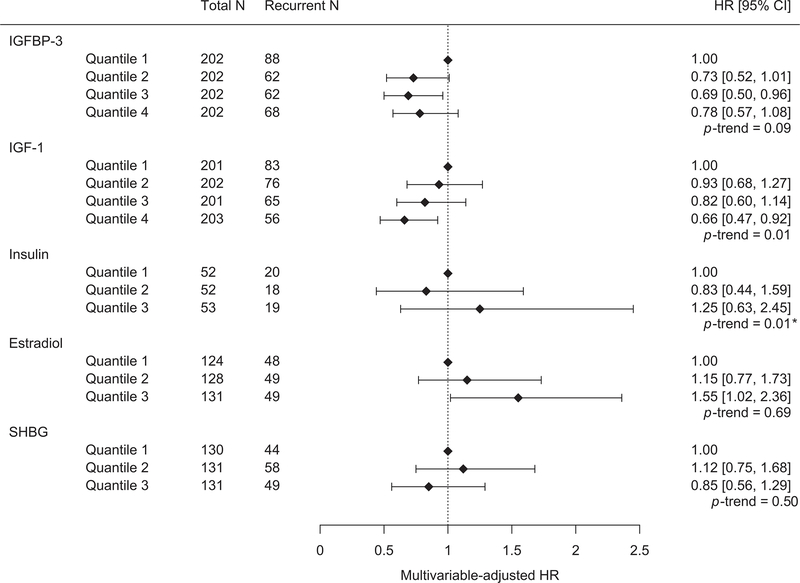
In our evaluation of preoperative circulating concentrations of IGF-I, IGFBP-3, insulin, estradiol, estrone and SHBG, cases who were classified in the highest quartile of circulating IGF-I had a lower risk of recurrence than those in the lowest quartile (Q4 versus Q1, HR 0.66, 95% CI 0.47–0.92) (P-trend=0.01) (Figure 3, Supplementary Table S3). For circulating IGFBP-3, there was no association on comparison of the extreme quartiles (Q4 versus Q1, HR 0.78, 95% CI 0.57–1.08) but there was a suggested lower recurrence risk in the comparison of Q3 versus Q1 (HR 0.69, 95% CI 0.50–0.96) (P-trend=0.09). We compared sex hormone concentrations using tertiles instead of quartiles (due to the smaller number of cases after restricting to postmenopausal women who were not using hormones) and observed that cases who were classified in the highest versus lowest tertile of estradiol had a higher risk of recurrence (T3 versus T1, HR 1.55, 95% CI 1.02–2.36). Compared with postmenopausal women who were classified in the lowest tertile, there was a higher risk of recurrence for women in tertile 2 of circulating estrone (T2 versus T1, HR 1.61, 95% CI 1.08–2.39) but no association for tertile 3. There was no association between serologic SHBG levels and risk of recurrence. When restricting the analysis to cases who were fasting at blood collection (157 cases, 57 recurrences), there was no association with circulating insulin levels categorized into tertiles. We further assessed the associations of the tissue and serologic biomarkers with recurrence stratifying by stage and results were found to be similar and consistent with the overall findings (Supplementary Tables S4 and S5).
Figure 3: Forest plots showing hazard ratios (HR) and 95% confidence intervals (95% CI) for the associations between preoperative circulating levels of insulin/IGF and sex hormone axis components in relation to risk of recurrent endometrioid adenocarcinoma.
Forest plots show results from models adjusted for age at enrollment (continuous), FIGO stage (II [Ref], III, IV) and grade (1, 2, 3 [Ref]). *Following log transformation, the P-trend for circulating insulin was no longer significant.
Overall there was little correlation between serologic levels (insulin, IGF-I, IGFBP-3, estradiol, estrone, SHBG) with tissue gene expression (IGF1, IGF2, IGFBP1, IGFBP3) or IHC staining (IR, IGF1R, pIGF1R/pIR, ER, PR) (Supplementary Tables S6–S7), or between gene and protein expression levels in tissue (Supplementary Table S8). We observed modest correlations for the serologic factors IGF-I and IGFBP-3 (r=0.56) (Supplementary Table S9) and there was little correlation between gene expression levels of IGF1, IGF2, IGFBP1 and IGFBP3 in tissue (Supplementary Table S10). Several of the IHC staining levels showed modest correlations (Supplementary Table S11).
DISCUSSION
Approximately one third of women with endometrioid endometrial adenocarcinoma are diagnosed with advanced (stage II or higher) disease, and >30% of these cases will experience disease recurrence and death.(3) We hypothesized that molecular risk factors for endometrioid adenocarcinoma development, namely markers of sex hormone and insulin/IGF axis components, may also be associated with an increased likelihood of disease progression or recurrence. We observed that endometrioid adenocarcinoma cases whose tumors stained positive for ER or IR had a lower risk of recurrence compared with patients who had negative tumor staining. We also observed that patients who had positive staining for pIGF1R/pIR had a higher risk of recurrence compared with cases whose tumors had negative staining. In our analyses of serologic factors, patients who had higher levels of circulating estradiol and lower levels of IGF-I, had a higher risk of recurrence.
In the current study, high circulating levels of estradiol among postmenopausal women were associated with increased endometrioid adenocarcinoma recurrence risk. This finding is consistent with the established role of higher estrogen levels (that are not simultaneously opposed by progesterone) in promoting endometrial epithelial proliferation that may lead to the development of endometrial cancer.(7,8) On the other hand, ER-positive tumors had a lower risk of recurrence than ER-negative tumors. The better prognosis for ER-positive cases was expected as it was in agreement with earlier studies.(18–22) The current study extends the findings from these earlier investigations as we focused on advanced stage endometrioid adenocarcinoma whereas stage I disease was the most common group in the earlier reports.
We observed that cases with higher circulating IGF-I levels had a lower risk of endometrioid adenocarcinoma recurrence. This finding aligns with previous studies that have shown that higher circulating IGF-I levels confer a lower risk of endometrioid adenocarcinoma development.(10) The possible protective effect of circulating IGF-I levels, in this context, may be explained by the anti-inflammatory properties of serologic IGF-I. For example, serologic IGF-I has been shown to have an inverse correlation with C-reactive protein (CRP) and levels of several inflammatory cytokines, and laboratory studies have found that IGF-I can reduce cytokine levels.(23) In line with this hypothesis, higher serum CRP levels have been reported to be associated with a poor disease-free and overall survival in endometrioid adenocarcinoma patients.(24)
In analyses of tumor markers, we observed that while IR staining was associated with a lower risk of recurrence, there was a positive association between staining for the phosphorylated (activated) pIGF1R/pIR and risk of endometrioid adenocarcinoma recurrence. Activation of either IGF1R or IR as reflected by their phosphorylation status can be measured by IHC, although IHC does not distinguish between the two receptors in their phosphorylated forms. We are not aware of any published studies that have examined the prognostic value of IR or pIGF1R/pIR in endometrioid adenocarcinoma. Two previous studies (including mostly stage I endometrial cancer cases) evaluated whether IGF1R immunostaining was related to recurrence-free survival(25) and/or overall survival(26) and, consistent with the current study, they observed no association after accounting for clinicopathologic factors. The association between pIGF1R/pIR and recurrent endometrioid adenocarcinoma is consistent with the role of this receptor which signals through phosphoinositide 3-kinase (PI3K)-protein kinase B/AKT and various effector pathways to promote cell proliferation and enhance survival.(11)
Very few studies have measured circulating levels of insulin/IGF and sex hormone biomarkers as well as levels in endometrial tumor tissue in the same patient. We note that there was little correlation between serological and local expression of the ligand/hormones with each other or with expression of their cognate receptors in endometrial tumor tissue. This may be due to the fact that serologic levels of these markers predominantly reflect the endocrine activities of the pancreas, liver, and adipose tissue compartments while endometrial tumor levels likely reflect synthesis of some ligands by the tumor itself (e.g. IGF1) as well as production by the local tissue environment.(27,28) These observations suggest that the measurement of both circulating factors as well as local tissue markers and the relevant receptors is likely necessary to capture the influence of both systemic and paracrine/autocrine effects of the insulin/IGF and sex hormone axis on endometrial cancer progression and recurrence. In addition, the assessment of downstream components of the insulin/IGF and sex hormone signal transduction cascade such as PI3K/AKT as well as PTEN may impart additional information on the extent to which activation of these pathways promotes recurrence.
This study had several important strengths. To our knowledge this was the first study to evaluate biomarkers of recurrence among a “high risk” population comprised of endometrioid adenocarcinoma patients with advanced stage disease. Importantly, all patients were treated based on the uniform GOG0210 trial protocol that accounted for clinical features and they underwent a similar tissue and serum collection protocol. Extensive data were available on clinical predictors of recurrence risk (including treatment and grade) as well as important endometrial adenocarcinoma risk factors (including BMI and postmenopausal hormone use) that we accounted for in the analysis. The study was conducted at 62 US institutions and therefore the findings can reasonably be generalized to the nationwide US population of endometrial cancer cases. Limitations of the current study were that the number of recurrences (N=280) that occurred from our population of over 800 endometrioid adenocarcinoma cases was modest. However, our study focused on an important and clinically relevant population and to our knowledge this is the first study to evaluate biomarkers of recurrence exclusively among women with advanced stage disease. Since African American women made up 10.5% of the cases in our study, we were unable to address whether sex hormone and insulin/IGF axis factors may explain the poor prognosis in African American endometrial adenocarcinoma patients.(29) In addition, our analysis of IHC markers used one tumor tissue core per patient in the TMAs; thus, the small amount of tissue assayed in the core may not be representative of the entire tumor. Finally, due to multiple testing some of the significant findings may be due to chance.
In our analyses of circulating markers, we demonstrated an association between higher estradiol levels with elevated risk of endometrial cancer recurrence. These novel findings suggest that endocrine signaling pathways may serve as oncogenic drivers to promote endometrial adenocarcinoma progression and could represent potential therapeutic targets in this disease. To date, clinical trials of endocrine therapy in endometrial adenocarcinoma have met with limited success; however, there is a lack of randomized trial data available to draw firm conclusions.(30) In a previous GOG trial, the efficacy of aromatase inhibitor (AI) therapy (which lowers circulating and tissue estrogen levels) was evaluated in ER-positive recurrent endometrial adenocarcinoma.(31) This study enrolled 23 patients with only four patients deriving clinical benefit (two partial response and two with short-term stable disease). Another area for further work could also be to examine the use of metformin (which normalizes circulating insulin) in combination with endocrine therapy for the treatment of endometrial cancer patients.(30)
Extensive crosstalk exists between the estrogen and insulin signaling pathways. The mitogenic effects of estrogen and insulin are synergistic, and their signaling pathways converge on cyclin D-CDK4/6.(32) Following concomitant estrogen and insulin stimulation, increased expression and stabilization of cyclin D (CCND1) by both signaling pathways enhances CDK4/6 activation, which leads to Rb phosphorylation. Increased phosphorylation of Rb promotes cell cycle entry and progression to S phase, corresponding to increased proliferation in response to estrogen and insulin compared with either mitogen alone. As a convergent node of the estrogen and insulin signaling pathways, CDK4/6 thus represents a rational target for the treatment recurrent/advanced endometrial cancer.
Additional studies are warranted to expand the range of sex hormone and insulin/IGF-axis components examined, and to determine their clinical utility as biomarkers to predict treatment response using currently available and emerging targeted therapeutics. These studies might inform the design of future clinical trials of endocrine-targeted adjuvant therapies that could include baseline assessment of these identified novel biomarkers, as well as the evaluation of the effects of treatment on these biomarkers, which could serve as early indicators of clinical benefit among endometrial cancer patients.
Supplementary Material
Acknowledgements
The authors thank the study pathologists, Mona El-Bahrawy and Nesreen M. Magdy, who carried out the tissue microarray scoring and provided guidance on the interpretation of immunohistochemical staining data. We thank Robin Sgueglia for carrying out the serum estradiol and estrone assays. We are grateful to Jurriaan Brouwer-Visser and Maria Jose Cossio for their assistance with the acquisition of laboratory data for the tissue assays. We thank Dr. Joseph Rothwell for creating the forest plots. We acknowledge the use of the Albert Einstein Cancer Center Shared Resources (Analytical Imaging Facility, Biorepository and Histopathology) which is supported by the Cancer Center Support Grant (National Cancer Institute P30 CA013330), and the Harold and Muriel Block Institute for Clinical and Translational Research (Biomarker Analytic Research Core) which is supported by the CTSA Grant UL1 TR001073, TL1 TR001072, KL2 TR001071 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Ohio State University Comprehensive Cancer Center, Washington University School of Medicine, Women and Infants Hospital, University of Minnesota Medical Center – Fairview, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Case Western Reserve University, Stony Brook University Medical Center, Roswell Park Cancer Institute, University of Pittsburgh Cancer Institute (UPCI), Tacoma General Hospital, University of California at Los Angeles Health System, Women’s Cancer Center of Nevada, University of Texas Southwestern Medical Center, Penn State Milton S Hershey Medical Center, University of North Carolina at Chapel Hill, Cooper Hospital University Medical Center, University of California Medical Center at Irvine-Orange Campus, Gynecologic Oncology of West Michigan PLLC, Duke University Medical Center, University of Iowa Hospitals and Clinics, Gynecologic Oncology Network/Brody School of Medicine, Yale University, Northwestern University, University of Massachusetts Memorial Health Care, Abington Memorial Hospital-Asplundh Cancer Pavilion, Fox Chase Cancer Center, University of Wisconsin Hospital and Clinics, University of Illinois, University of Virginia, University of New Mexico, Moffit Cancer Center and Research Institute, Wayne State University/Kamanos Cancer Institute, Wake Forest University Health Sciences, Mayo Clinic, Cleveland Clinic Foundation, The Hospital of Central Connecticut, Walter Reed National Military Medical Center, University of Arkansas Medical Center, Aurora Women’s Pavilion of Aurora West Allis Medical Center, University of Alabama at Birmingham, University of Mississippi Medical Center, Fred Hutchinson Cancer Research Center, University of Cincinnati, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Wisconsin NCI Community Oncology Research Program, Evanston CCOP-NorthShore University Health System, Delaware/Christiana Care CCOP, Abramson Cancer Center of the University of Pennsylvania, University of Chicago, New York University Medical Center, Tufts-New England Medical Center, North Shore University Hospital, Fletcher Allen Health Care, Michigan Cancer Research Consortium Community Clinical Oncology Program, William Beaumont Hospital and Saint Vincent Hospital.
Financial support: This work was supported by the National Cancer Institute at the National Institutes of Health [1R01CA133010 to M.J.G. as well as intramural funds). G.S.H. was supported by the Reproductive Scientist Development Program through the American Congress of Obstetricians and Gynecologists. This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office and Gynecologic Oncology Group Tissue Bank [U10 CA27469], the Gynecologic Oncology Group Statistical and Data Center [U10 CA180822], the Gynecologic Oncology Group Tissue Bank [U24 CA114796], the NRG Oncology Operations Center and the NRG Oncology Biospecimen Bank-Columbus [U10 CA180868], the NRG Oncology Statistics and Data Management Center [U10 CA180822] and the NRG Oncology Biospecimen Bank-Columbus [U24 CA196067].
Footnotes
Disclosure statement: The authors declare no potential conflicts of interest.
Disclaimer
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer. Lyon, France: 2018. [Google Scholar]
- 2.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014;15:e268–78 [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Endometrial Cancer. 2017. [Google Scholar]
- 4.Zaino RJ. FIGO staging of endometrial adenocarcinoma: a critical review and proposal. Int J Gynecol Pathol 2009;28:1–9 [DOI] [PubMed] [Google Scholar]
- 5.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015;26:1635–48 [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384:755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002;11:1531–43 [PubMed] [Google Scholar]
- 8.Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988;57:205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perel E, Killinger DW. The interconversion and aromatization of androgens by human adipose tissue. J Steroid Biochem 1979;10:623–7 [DOI] [PubMed] [Google Scholar]
- 10.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Manson JE, Li J, et al. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2008;17:921–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak M The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–69 [DOI] [PubMed] [Google Scholar]
- 12.McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res 2006;12:6373–8 [DOI] [PubMed] [Google Scholar]
- 13.Merritt MA, Strickler HD, Einstein MH, Yang HP, Sherman ME, Wentzensen N, et al. Insulin/IGF and sex hormone axes in human endometrium and associations with endometrial cancer risk factors. Cancer Causes Control 2016;27:737–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol 2013;129:277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creasman WT, Ali S, Mutch DG, Zaino RJ, Powell MA, Mannel RS, et al. Surgical-pathological findings in type 1 and 2 endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study on GOG-210 protocol. Gynecol Oncol 2017;145:519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsel LB, Szabo E, Greene GL, Konrath J, Leight GS, McCarty KS Jr. Immunocytochemical analysis of estrogen receptors as a predictor of prognosis in breast cancer patients: comparison with quantitative biochemical methods. Cancer Res 1989;49:1052–6 [PubMed] [Google Scholar]
- 17.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wik E, Raeder MB, Krakstad C, Trovik J, Birkeland E, Hoivik EA, et al. Lack of estrogen receptor-alpha is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res 2013;19:1094–105 [DOI] [PubMed] [Google Scholar]
- 19.Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB. GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol 2008;199:543.e1–7 [DOI] [PubMed] [Google Scholar]
- 20.Jongen V, Briet J, de Jong R, ten Hoor K, Boezen M, van der Zee A, et al. Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol Oncol 2009;112:537–42 [DOI] [PubMed] [Google Scholar]
- 21.Creasman WT, McCarty KS Sr., Barton TK, McCarty KS Jr. Clinical correlates of estrogen- and progesterone-binding proteins in human endometrial adenocarcinoma. Obstet Gynecol 1980;55:363–70 [DOI] [PubMed] [Google Scholar]
- 22.Busch EL, Crous-Bou M, Prescott J, Chen MM, Downing MJ, Rosner BA, et al. Endometrial Cancer Risk Factors, Hormone Receptors, and Mortality Prediction. Cancer Epidemiol Biomarkers Prev 2017;26:727–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eivindson M, Nielsen JN, Gronbaek H, Flyvbjerg A, Hey H. The insulin-like growth factor system and markers of inflammation in adult patients with inflammatory bowel disease. Hormone research 2005;64:9–15 [DOI] [PubMed] [Google Scholar]
- 24.Schmid M, Schneitter A, Hinterberger S, Seeber J, Reinthaller A, Hefler L. Association of elevated C-reactive protein levels with an impaired prognosis in patients with surgically treated endometrial cancer. Obstet Gynecol 2007;110:1231–6 [DOI] [PubMed] [Google Scholar]
- 25.Joehlin-Price AS, Stephens JA, Zhang J, Backes FJ, Cohn DE, Suarez AA. Endometrial Cancer Insulin-Like Growth Factor 1 Receptor (IGF1R) Expression Increases with Body Mass Index and Is Associated with Pathologic Extent and Prognosis. Cancer Epidemiol Biomarkers Prev 2016;25:438–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiro G, Lohse P, Mayr D, Diebold J. Insulin-like growth factor-I receptor and PTEN protein expression in endometrial carcinoma. Correlation with bax and bcl-2 expression, microsatellite instability status, and outcome. Am J Clin Pathol 2003;120:78–85 [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci U S A 2007;104:15847–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol 1998;12:399–406 [DOI] [PubMed] [Google Scholar]
- 29.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014;133:353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: An old dog with new tricks. Gynecol Oncol 2019;153:175–83 [DOI] [PubMed] [Google Scholar]
- 31.Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee RB. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2000;78:212–6 [DOI] [PubMed] [Google Scholar]
- 32.Lai A, Sarcevic B, Prall OW, Sutherland RL. Insulin/insulin-like growth factor-I and estrogen cooperate to stimulate cyclin E-Cdk2 activation and cell Cycle progression in MCF-7 breast cancer cells through differential regulation of cyclin E and p21(WAF1/Cip1). J Biol Chem 2001;276:25823–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.